Abstract
Background
The genus Lithocarpus is a species-rich dominant woody lineage in East Asian evergreen broad-leaved forests. Despite its ecological and economic significance, the plastome structure and evolutionary history of the genus remain poorly understood. In this study, we comprehensively analyzed the 34 plastomes representing 33 Lithocarpus species. Of which, 21 were newly assembled. The plastome-based phylogenomic tree was reconstructed to reveal the maternal evolutionary patterns of the genus.
Results
The Lithocarpus plastomes exhibit a typical quadripartite structure, ranging in length from 161,010 to 161,476 bp, and containing 131 genes, including 86 protein-coding genes, 8 rRNA genes, and 37 tRNA genes. Remarkably, the infA gene was identified as a pseudogene in 17 species. Significant variability was observed in simple sequence repeats (SSRs) as well as in the boundary regions between the two single-copy regions and the inverted repeat region (SC/IR) across the plastomes. Additionally, four genes (accD, atpF, rpl32, and rps8) were found to be under positive selection. The monophyletic status of Lithocarpus was strongly supported by plastome-based phylogeny; however, the phylogenetic tree topology showed a significant difference from that obtained by the nuclear genome-based phylogeny.
Conclusions
The plastome of Fagaceae is generally conserved. Nevertheless, genes related to metabolism, photosynthesis, and energy were under strong positive selection in Lithocarpus, likely driven by environmental pressures and local adaptation. The plastome-based phylogeny confirmed the monophyletic status of Lithocarpus and revealed a phylogeographic pattern indicating limited seed-mediated gene flow in the ancestral lineage. The prevalence of cytonuclear discordance in Lithocarpus and other Fagaceae genera suggests that ancient introgression, incomplete lineage sorting, and asymmetrical seed- and pollen-mediated geneflow might contribute to this discordance. Future studies are essential to test these hypotheses and further elucidate the divergence patterns of this unique Asian evergreen lineage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05874-z.
Keywords: Phylogenomics, Plastome, Evolution, Positive selection
Background
The plastome, a crucial uniparentally inherited organelle in plant cells, is responsible for photosynthesis and other metabolic processes essential for plant adaptation to their environment [1, 2]. Although nuclear genome datasets have become increasingly prominent in phylogenetic and plant genome analyses, plastomes remain indispensable for tracing the maternal evolutionary history of angiosperm taxa, offering critical insights that complement nuclear data [3, 4]. One key advantage of plastomes in phylogenetic reconstruction is their conserved gene content and structure, which provide a high degree of homology across diverse plant lineages [3, 5]. Moreover, the low or no recombination rates within plastomes enhance the reliability of phylogenetic inferences by preserving the evolutionary signature of maternal lineages, facilitating the resolution of ambiguous phylogenetic relationships [4]. Plastome-based phylogenetic approaches are thus powerful tools in plant phylogenetics and evolutionary research, enabling the detection of introgression events such as plastome capture [e.g., 6, 7], monitoring seed-mediated gene flow in spatial population genetic studies [e.g., 8], and examining the structure, diversity, and evolution of organellar genomes [e.g., 9].
The genus Lithocarpus Blume, commonly known as tanoak or stone oak, includes approximately 330 to 347 species, making it the second-largest genus in the family Fagaceae [10]. The northern Indo-China and Malaysian regions are two important species diversity centers of the genus [11–14]. These species are usually dominant trees in evergreen broadleaved forests and play a critical role in maintaining regional microclimate and biodiversity [15, 16]. They also provide important ecological services to society as sources of starches [17, 18], timber [18], and important sugar substitutes in the therapy of type two diabetes [18, 19]. However, Asian Fagaceae species have faced severe population reductions and habitat degradation in recent decades. Estimates indicate that Asian evergreen forests have experienced severe population declines, habitat loss, and habitat degradation [20, 21]. According to an International Union for Conservation of Nature (IUCN) primary assessment, about one-third of Asian Fagaceae species may be endangered [22]. These evergreen fagaceous lineages in the Asian tropics and subtropics are particularly vulnerable to intense human disturbance, driven by the high productivity and biodiversity of these regions [20, 21, 23, 24]. Understanding the genomics of Lithocarpus is crucial for characterizing biodiversity and enhancing conservation efforts among these species.
As a species-rich dominant lineage in evergreen broadleaved forests, Lithocarpus has been the focus of several phylogenetic studies in recent years. Sanger-based chloroplast DNA (cp.DNA) barcode sequencing has been widely applied to infer the phylogenetic structure and population genetic structure of fagaceous plants [25–29]. Cannon and Manos [26] resolved two main clades within Southeast Asian Lithocarpus using cp.DNA, corresponding to Borneo versus other widespread East Asian regions. Their research revealed a strong geographical structure, high genetic diversity, and endemism of cp. DNA haplotypes within Lithocarpus, likely owing to limited migration and extinction events in Southeast Asia [26].
Phylogenetic inferences based on the atpB -rbcL spacer (cp.DNA) and internal transcribed spacer (ITS) (nuclear ribosomal DNA; nrDNA) reveal an incongruent tree topology [29]. These findings suggest a complex evolutionary history of Lithocarpus, influenced by East Asian geographic events and ancient introgression [29–31]. However, the tree topology inferred from these Sanger-sequencing-based markers only received medium to low support.
Next-generation sequencing (NGS) technologies offer powerful tools to resolve phylogenetic relationships not only at evolutionarily deep nodes, but also among closely related species characterized by recent interspecific gene flow within Fagaceae [32–34]. Recent phylogenomic reconstructions of Fagaceae using genomic DNA resequencing [30] and hybrid enrichment sequencing (Hyb-Seq) [31] yielded similar tree topologies for the main lineages in Fagaceae and the species phylogeny within Lithocarpus specifically. These studies resolved a sister relationship between Chrysolepis and Lithocarpus [30, 31], whereas the phylogenetic tree inferred from plastome sequences showed Lithocarpus as the sister group to Castanopsis + Castanea [30, 31]. Such notable discordance between the plastome- and nuclear genome-based trees may be the result of incomplete lineage sorting, horizontal transfer, or ancient gene flow among the ancestral lineages [30, 31]. The accumulated findings of phylogenomic studies on Fagaceae offer significant insights into the evolutionary history of Lithocarpus and related taxa. However, these studies have included only a limited number of Lithocarpus species. Meanwhile, phylogenetic studies of Lithocarpus based on NGS data remain scarce, with only sporadic reports of plastomes published to date [e.g., 35–40]. Moreover, the monophyletic status of Asian Lithocarpus has not been consistently resolved. Some studies indicated that the plastome-based phylogeny was polyphyletic in Lithocarpus [41], in contrast to the monophyly inferred by Zhou et al. [30]. These studies provide valuable insights into the evolution of Lithocarpus, but also highlight substantial uncertainties in its plastome evolutionary history. Therefore, the evolutionary history within Lithocarpus warrants further investigation.
In recent years, the gradual sequencing of plastomes from Fagaceae species has substantially enhanced our understanding of the structure and divergence patterns of plastomes within this family [e.g., 6, 42, 43]. These studies have shown that the plastome structure of the family Fagaceae is relatively conserved in structure in terms of size (158,163–161,419 bp), GC content (36.8–37.1%), and gene order [6, 42, 43]. Despite this, molecular signatures of adaptive evolution have been observed in certain protein-coding genes in Fagaceae [42–47], though these previous studies have mainly focused on the genera Quercus [43, 48], Castanea [46], Fagus [44, 47], and Castanopsis [49]. Despite Lithocarpus accounting for approximately one-third of the species diversity within the family Fagaceae, investigations of Lithocarpus plastomes have remained limited. Most studies either report the plastome structure of a single species or plastomes of Lithocarpus were only used as molecular evidence for the classification of new species [e.g., 37, 39, 40]. No comprehensive study has ever been conducted on the plastome structure, gene function, or molecular signatures of the adaptive evolution of Lithocarpus.
In this study, we newly sequenced and assembled the plastomes of 21 species representing the main morphological groups of the genus Lithocarpus. Utilizing these new plastomes in combination with the previously reported Lithocarpus plastomes, we analyzed the plastome structure and sequence divergence pattern and reconstructed the plastome-based phylogenomic tree, aiming to (1) examine the plastome structure and sequence divergence patterns of Lithocarpus and (2) infer the maternal evolutionary history of Lithocarpus. This study also provides important insights into the evolution and adaptation of this unique East Asian fagaceous lineage.
Materials and methods
Plant material and DNA extraction
Twenty-one species of Lithocarpus from East Asia (including Tibet, Yunnan, Hunan, Hainan, and Guangdong provinces of China, among other areas) were sequenced, representing five of the thirteen subgenera (i.e., Synaedrys, Pachybalanus, Gymnobalanus, Pseudosynaedrys, and Pasania) proposed by Camus [11]. Genomic DNA was isolated using an optimized CTAB method as described by Doyle and Doyle [50]. The quality of the genomic DNA was checked by 1% agarose gel electrophoresis, and the DNA concentration was measured using a Qubit® 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA) and then adjusted to 20 ng/uL for library construction. Detailed collection information and voucher specimens for each species are summarized in Table 1. The herbarium voucher specimens were identified by Dr. Min Deng, a Fagaceae taxonomy expert. The voucher specimens were deposited in the Herbarium of Yunnan University (YUKU), Kunming, China.
Table 1.
Collection information and Genbank accessions of the plant materials used for this study
| Taxa | subgenus | Locality | Voucher information | Herbarium information | GenBank Accession |
|---|---|---|---|---|---|
| Lithocarpus amygdalifolius (Skan) Hayata | Pachybalanus | Nanning, Guangxi, China | DM26426 | Yunnan University | PQ276669 |
| L. areca (Hickel et A. Camus) A. Camus | Pasania | Chongzuo, Guangxi, China | DM26599 | Yunnan University | PQ276671 |
| L. brevicaudatus (Skan) Hayata | Pasania | Wuzhishan, Hainan, China | DM24894 | Yunnan University | PQ276666 |
| L. cleistocarpus (Seemen) Rehder et E. H. Wilson | Pachybalanus | Zhaotong, Yunnan, China | DM23979 | Yunnan University | OM112296 |
| L. dealbatus (J. D. Hooker et Thomson ex Miquel) Rehder | Pasania | Kunming, Yunnan, China | DM25628 | Yunnan University | PQ276668 |
| L. elizabethiae (Tutcher) Rehder | Pasania | Kunming, Yunnan, China | DM25627 | Yunnan University | PQ276667 |
| L. fenestratus (Roxburgh) Rehd | Pasania | Yuxi, Yunnan, China | DM24080 | Yunnan University | OM112300 |
| L. fenzelianus A. Camus | Pachybalanus | Wuzhishan, Hainan, China | DM24905 | Yunnan University | OM388302 |
| L. fohaiensis (Hu) A. Camus | Pachybalanus | Xishuangbanna, Yunnan, China | DM22706 | Yunnan University | PQ276656 |
| L. glaber (Thunb.) Nakai | Pasania | Zhuzhou, Hunan, China | DM24713 | Yunnan University | OM388303 |
| L. grandifolius (D. Don) S. N. Biswas | Pasania | Nanchuan, Chongqing, China | DM24500 | Yunnan University | PQ276659 |
| L. gymnocarpus A. Camus | Gymnobalanus | Honghe, Yunnan, China | DM24655 | Yunnan University | PQ276663 |
| L. konishii (Hayata) Hayata | Gymnobalanus | Shenzhen, Guangdong, China | DM24770 | Yunnan University | PQ276665 |
| L. longzhouicus (C. C. Huang & Y. T. Chang) J. Q. Li & L. Chen | Nanning, Guangxi, China | DM26595 | Yunnan University | PQ276670 | |
| L. obscurus C. C. Huang et Y. T. Chang | Motuo, Tibet, China | DM23454 | Yunnan University | OM112297 | |
| L. pachylepis A.Camus | Synaedrys | Honghe, Yunnan, China | DM24588 | Yunnan University | PQ276660 |
| L. sp. | Wenshan, Yunnan, China | DM24476 | Yunnan University | PQ276657 | |
| L. sphaerocarpus (Hickel & A.Camus) | Wenshan, Yunnan, China | DM24498 | Yunnan University | PQ276658 | |
| L. tabularis Y.C.Hsu & H.Wei Jen | Honghe, Yunnan, China | DM24606 | Yunnan University | PQ276662 | |
| L. uvariifolius (Hance) Rehd. | Synaedrys | Shenzhen, Guangdong, China | DM24743 | Yunnan University | PQ276664 |
| L. xylocarpus (Kurz) Markgraf | Pseudosynaedrys | Honghe, Yunnan, China | DM24591 | Yunnan University | PQ276661 |
Illumina sequencing, assembly, and annotation
High-quality DNA was used to build genomic libraries. The paired-end (PE) read library was constructed using TruSeq DNA sample preparation kits (Illumina, San Diego, CA, USA). Sequencing was performed using 150-bp paired-end reads on the Illumina HiSeq2500 platform with an insert size of approximately 400 bp. Raw reads were filtered and trimmed to remove the low-quality reads using Fastp [51] with default parameters. Approximately 2 GB of clean data were generated per library. All sequencing was conducted by Biomarker Technologies Inc. (Beijing, China). Additionally, twelve sets of raw reads of Fagaceae whole-genome sequencing data, including seven Lithocarpus species and five species from other genera of Fagaceae, reported by Zhou et al. [30], were downloaded and used for subsequent analyses. Detailed GenBank accession information for the data is summarized in Table S1.
High-quality clean data were assembled using GetOrganelle v1.7.2 [52] with the following parameter settings: ‘-R 10 -k 21,45,65,85,105,115,127 -F embplant_pt”, utilizing Lithocarpus balansae (GenBank accession number, KP299291) as the reference genome. Genome annotation was performed using CPGAVAS [53] and confirmed with DOGMA (http://dogma.ccbb.utexas.edu/) [54] and BLAST [55]. Additionally, tRNAs were identified using tRNAscan-SE [56]. The manual correction was made to locate the start and stop codons and exon-intron boundaries using Geneious Prime [57], with L. hancei (MW375417) and L. balansae (KP299291) as reference genomes. Complete plastome maps were generated using OGDRAWv1.2 (Draw Organelle Genome Maps, http://ogdraw.mpimp-golm.mpg.de/) [58]. All annotated plastome sequences have been deposited in GenBank, under the accession numbers listed in Table 1. Complete plastome sequences of seven Lithocarpus species reported in previous studies [30] and six whole plastomes of Lithocarpus species (L. hancei, L. dealbatus, L. balansae, L. polystachyus, L. longinux, and L. litseifolius) downloaded from NCBI (Table S1), along with the newly sequenced and assembled Lithocarpus plastomes. In total 34 plastomes representing 33 species were used for subsequent analyses.
Genomic feature analyses
Relative synonymous codon usage (RSCU) values were calculated by dividing the observed frequency by the expected frequency (RSCU > 1 indicates higher than expected codon use and RSCU < 1 indicates lower use [59]. Using MEGA X software (https://www.megasoftware.net/) [60], we determined the RSCU values for 34 plastomes of Lithocarpus (21 newly obtained and 13 previously published) [60], revealing variations in synonymous codon usage. The RSCU cluster diagram was created using the R package pheatmap (https://CRAN.R-project.org/package=pheatmap) [61].
Simple sequence repeats (SSR) within the 34 complete plastomes of Lithocarpus were detected using MISA (http://pgrc.ipk-gatersleben.de/misa) with motif sizes ranging from one to six nucleotides [62]. Thresholds for the minimum number of repeat units were set as follows: 10 for mono-nucleotide SSRs, 5 for di-nucleotide SSRs, 4 for tri-nucleotide SSRs, and 3 for tetra-nucleotide, penta-nucleotide, and hexa-nucleotide SSRs, respectively.
Comparative genomic analyses and sequence divergence
The boundaries of the large single-copy (LSC), small single-copy (SSC), and inverted repeat (IR) regions of the Lithocarpus plastomes, along with those of other Fagaceae species, were drawn using the IRscope online tool (https://irscope.shinyapps.io/irapp/) [63]. The mVISTA program in Shuffle-LAGAN mode was used to compare the 34 complete plastomes of Lithocarpus with the annotation of L. balansae (KP299291) used as the reference [64]. The synonymous substitution rate (Ks), nonsynonymous substitution rate (Ka), and the Ka/Ks ratio of coding sequences (CDS) in the whole plastome regions were calculated among the 34 Lithocarpus plastomes using KaKs Calculator 2.0 [65]. The Ka/Ks values were visualized with a boxplot generated using the R package ggplot2 (https://cran.r-project.org/package=ggplot2) [66]. A one-sample t-test with µ = 1 was performed for each gene to evaluate statistical significance.
Phylogenetic analyses
To infer the phylogenetic structure of Fagaceae, 61 plastomes, including 34 individuals of Lithocarpus, 2 individuals of Notholithocarpus, 12 species of Quercus, 3 species of Castanopsis, 3 species of Castanea, 2 species of Chrysolepis, 2 species of Trigonobalanus, and 2 species of Fagus, with Betula pendula as an outgroup, were used to reconstruct the plastome-based phylogenomic tree. Of these, 21 plastomes were newly obtained in this study, and 40 were downloaded from NCBI. Betula pendula of the family Betulaceae was used as an outgroup to root the tree [30, 67]. GenBank accession numbers of the plastomes are provided in Supplementary Tables 1 and Table S1.
Maximum-likelihood (ML) and Bayesian inference (BI) phylogenetic analyses were conducted using three data partitions: (1) the whole plastome sequences, (2) protein-coding exons, and (3) introns and intergenic spacers. All sequences were aligned using MAFFT 7.0 [68] with default parameters. The best-fit nucleotide substitution model (TVM + F + R10) was identified by ModelFinder [69]. The ML tree was reconstructed using IQ-tree v2.0 [70] with 1000 ultrafast bootstrap replicates and default settings. The BI tree was reconstructed using MrBayes v3.2.6 [71]. Markov Chain Monte Carlo (MCMC) analysis was performed over 10,000,000 generations, with tree sampling every 1000 generations. The MrBayes output was inspected using Tracer ver.1.7.1 [72] to ensure proper convergence and mixing (effective sample sizes all > 200), and a maximum clade credibility tree was generated after a 20% burn-in. The result was visualized and edited with Figtree v1.4 (https://github.com/rambaut/figtree/releases) [73]. Phylogenetic trees were plotted on the world map using the R package phytools v2.0 (https://cran.r-project.org/web/packages/phytools/index.html) [74]. In addition, we utilized the R package ape v5.8 (10.1093/bioinformatics/bty633) [75] to trim the nuclear phylogenetic tree obtained by Liu et al. [31] and our plastome tree to illustrate the discordance between the plastome and nuclear genomes.
Morphological traits divergence pattern
We gathered the leaf epidermal trichome (Bubble Trichome Group [BBT]; Appressed parallel tufts Group [APT]; Glabrous Group [Glabrous]) [76, 77], and acorn type (Acorn fruit [AR]; Enclosed receptacle fruit [ER]) [78, 79] characteristics of Lithocarpus reported in previous studies, then mapped these traits at the tips of the phylogenetic tree to illustrate the morphological divergence pattern to these key taxonomic significant characteristics.
Results
Plastome features in Lithocarpus species
Illumina sequencing generated 2 to 5 G of raw data for each sampled species library. After sequencing, trimming, and quality control of reads, 21 high-quality plastomes were newly assembled (Fig. S1), and 13 additional Lithocarpus plastomes were reassembled and compared. GenBank accession numbers and the sources of the plastomes used in this study are provided in Supplementary Tables S1 and S2.
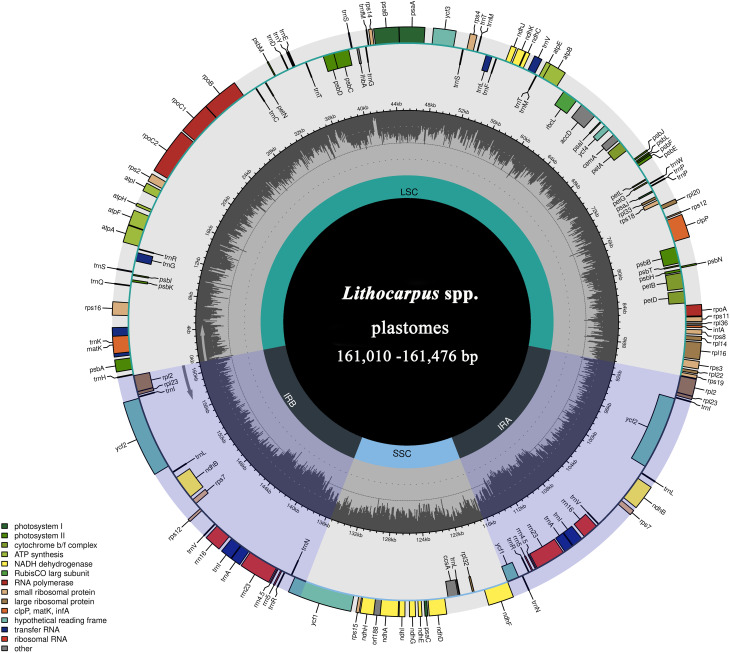
The sizes of plastomes of the 34 Lithocarpus individuals ranged from 161,010 (L. pachylepis) to 161,476 bp (L. dealbatus) (Table 2). The plastome of Lithocarpus is a typical single-circular molecule with a four-segment structure, comprising a large single-copy region (LSC) (90,394–90,731 bp), a small single-copy region (SSC) (18,933–19,255 bp), and the two inverted repeat regions (IRA and IRB, respectively) (25,632–25,911 bp) (Fig. 1; Table 2). All of the 34 plastomes showed a similar total GC content, ranging from 36.7 to 36.8%. The GC contents of the LSC and SSC regions were 34.50–34.60% and 30.70–31.00%, respectively, while the IR regions had a higher GC content of 42.70–42.80% (Table 2).
Table 2.
Characteristics of the 34 complete plastomes in Lithocarpus
| Species | Size (bp) | LSC (bp) | IR | SSC (bp) | GC content (%) | LSC GC content (%) | IR GC content (%) | SSC GC content (%) | Total genes | CDS | tRNAs | rRNAs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lithocarpus amygdalifolius | 161,283 | 90,538 | 25,883 | 18,979 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. areca | 161,144 | 90,427 | 25,871 | 18,979 | 36.7 | 34.6 | 42.7 | 30.9 | 131 | 86 | 37 | 8 |
| L. balansae | 161,020 | 90,596 | 25,632 | 19,160 | 36.7 | 34.5 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. brevicaudatus | 161,208 | 90,462 | 25,893 | 18,960 | 36.8 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. calophyllus | 161,288 | 90,510 | 25,904 | 18,970 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. cleistocarpus | 161,178 | 90,558 | 25,762 | 19,096 | 36.7 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. corneus | 161,112 | 90,543 | 25,676 | 19,217 | 36.8 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. dealbatus | 161,118 | 90,460 | 25,781 | 19,096 | 36.8 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. dealbatus | 161,476 | 90,731 | 25,879 | 18,987 | 36.8 | 34.6 | 42.7 | 30.9 | 131 | 86 | 37 | 8 |
| L. elizabethiae | 161,273 | 90,619 | 25,779 | 19,096 | 36.7 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. fenestratus | 161,184 | 90,524 | 25,804 | 19,052 | 36.7 | 34.5 | 42.7 | 30.8 | 131 | 86 | 37 | 8 |
| L. fenzelianus | 161,148 | 90,421 | 25,893 | 18,941 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. fohaiensis | 161,370 | 90,615 | 25,911 | 18,933 | 36.8 | 34.6 | 42.7 | 31 | 131 | 86 | 37 | 8 |
| L. glaber | 161,302 | 90,556 | 25,883 | 18,980 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. grandifolius | 161,193 | 90,510 | 25,797 | 19,089 | 36.7 | 34.5 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. gymnocarpus | 161,295 | 90,541 | 25,875 | 19,004 | 36.7 | 34.6 | 42.7 | 30.8 | 131 | 86 | 37 | 8 |
| L. haipinii | 161,289 | 90,537 | 25,880 | 18,992 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. hancei | 161,228 | 90,509 | 25,880 | 18,959 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. konishii | 161,374 | 90,614 | 25,893 | 18,974 | 36.7 | 34.6 | 42.7 | 30.8 | 131 | 86 | 37 | 8 |
| L. litseifolius | 161,322 | 90,551 | 25,897 | 18,977 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. longanoides | 161,281 | 90,539 | 25,883 | 18,976 | 36.7 | 34.5 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. longinux | 161,420 | 90,409 | 25,878 | 19,255 | 36.8 | 34.6 | 42.7 | 31 | 131 | 86 | 37 | 8 |
| L. longipedicellatus | 161,408 | 90,615 | 25,897 | 18,999 | 36.8 | 34.6 | 42.7 | 30.9 | 131 | 86 | 37 | 8 |
| L. longzhouicus | 161,143 | 90,399 | 25,888 | 18,978 | 36.8 | 34.6 | 42.7 | 30.9 | 131 | 86 | 37 | 8 |
| L. obscurus | 161,349 | 90,616 | 25,882 | 18,969 | 36.8 | 34.6 | 42.7 | 30.9 | 131 | 86 | 37 | 8 |
| L. pachylepis | 161,010 | 90,563 | 25,715 | 19,017 | 36.8 | 34.6 | 42.7 | 30.8 | 131 | 86 | 37 | 8 |
| L. polystachyus | 161,217 | 90,491 | 25,879 | 18,968 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. sp. | 161,291 | 90,636 | 25,780 | 19,095 | 36.7 | 34.5 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. sphaerocarpus | 161,283 | 90,632 | 25,772 | 19,107 | 36.7 | 34.5 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. tabularis | 161,131 | 90,512 | 25,776 | 19,067 | 36.7 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
| L. tephrocarpus | 161,233 | 90,465 | 25,899 | 18,970 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. truncatus | 161,368 | 90,575 | 25,893 | 19,007 | 36.7 | 34.6 | 42.7 | 30.8 | 131 | 86 | 37 | 8 |
| L. uvariifolius | 161,155 | 90,394 | 25,902 | 18,957 | 36.7 | 34.6 | 42.7 | 30.7 | 131 | 86 | 37 | 8 |
| L. xylocarpus | 161,239 | 90,613 | 25,765 | 19,096 | 36.8 | 34.6 | 42.8 | 30.8 | 131 | 86 | 37 | 8 |
Fig. 1.
Plastomes map of Lithocarpus species. The transcription of genes seen outside the outer layer circle is done clockwise, whereas the transcription of genes inside is done counterclockwise. Different functional groups of genes are color-coded. The GC content of the plastome is indicated by the dark gray area in the inner circle. LSC, large single-copy region; SSC, small single-copy region; IRa, IRb, inverted repeat A and B, respectively
A total of 131 genes were annotated in the plastome of the 21 newly assembled Lithocarpus species, including 86 protein-coding genes (PCGs), 37 tRNA genes, and 8 rRNA genes. These genes were categorized based on their functions as being related to photosynthesis, self-replication, and other functions (Table 3). Among these genes, 18 contained introns, including 12 protein-coding genes and 6 tRNA genes. Most genes had a single intron, while the ycf3 clpP and rps12 genes contained two introns (Table 3). Additionally, the infA gene was identified as a pseudogene in 17 Lithocarpus plastomes (Fig. 2).
Table 3.
Gene composition within the plastomes of Lithocarpus species
| Category of Genes | Group of gene | Name of gene |
|---|---|---|
| Self‒replication | Ribosomal RNA genes | rrn4.5 × 2, rrn5 × 2, rrn16 × 2, rrn23 × 2 |
| Transfer RNA genes |
trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-GUG, trnK-UUU*, trnL-UAA, trnL-UAG, trnM-CAU, trnP-UGG, trnQ-UUG, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-UAC, trnW-CCA, trnY-GUA, trnA-UGC*×2, trnI-CAU×2, trnL-CAA×2, trnI-GAU*×2, trnV-GAC×2, trnR-ACG×2, trnN-GUU×2 |
|
|
Ribosomal protein (small subunit) |
rps11, rps12*×2, rps14, rps15, rps16*, rps18, rps19, rps2, rps3, rps4, rps7 × 2, rps8 |
|
|
Ribosomal protein (large subunit) |
rpl14, rpl16*, rpl2*×2, rpl20, rpl22, rpl23 × 2, rpl32, rpl33, rpl36 |
|
| RNA polymerase | rpoA, rpoB, rpoC1*, rpoC2 | |
| Translational initiation factor | infA# | |
| Genes for photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF*, atpH, atpI | |
| Large subunit of Rubisco | rbcL | |
|
Subunits of NADH Dehydrogenase |
ndhA*, ndhB*×2, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Other genes | Maturase | matK |
| Envelope membrane protein | cemA | |
| Subunit of acetyl‒CoA | accD | |
| Synthesis gene | ccsA | |
| ATP‒dependent protease | clpP** | |
| Genes of unknown function | ycf1, ycf2 × 2, ycf3**, ycf4 |
Notes Gene*: Gene with one introns; Gene**: Gene with two introns; #Gene: Pseudo gene; Gene (×2): Number of copies of multi-copy genes
Fig. 2.
Example of infA pseudogenes in the plastomes of Lithocarpus. “*” in the black boxes showing the termination codons
Codon usage
Sixty-four codons encoding 20 amino acids were detected in the 34 Lithocarpus plastomes. Lithocarpus longipedicellatus had the highest number of codons, with a total of 25,091 codons, while L. balansae had the fewest, with 24,886 codons. The two most frequently used amino acids in Lithocarpus species were leucine (Leu) and isoleucine (Ile), whereas cysteine (Cys) was the least common amino acid based on codons. There were six synonymous codons for Leu, serine (Ser), and arginine (Arg), but only one codon each for methionine (Met) and tryptophan (Try) (L. glaber is shown as an example in Fig. 3). Among the 64 codons, RSCU values of 30 codons were greater than 1.00, with 29 codons ending in A/U. Conversely, 31 out of 34 codons with RSCU values less than 1.00 ended with G/C (Table S2). Approximately half of the codons were used more frequently, as indicated by RSCU values exceeding 1 (Fig. 4). Notably, the codon usage of Lithocarpus plastomes showed a clear bias towards A/U at the third codon position. The codon usage and RSCU of the plastomes of 34 Lithocarpus individuals are summarized in Table S2.
Fig. 3.
Comparative analysis of plastome codon usage bias of Lithocarpus glaber. The colors indicate different codes, and the RSCU value frequency is illustrated as height in the upper diagram
Fig. 4.
The heat map of codon usage bias in the plastomes of Lithocarpus. The red color indicates higher RSCU values and the blue color indicates lower RSCU values
Analysis of SSRs
The number of SSRs observed in Lithocarpus species was high within the range of the family Fagaceae, comparable to that found in Quercus (Fig. 5B). The 34 Lithocarpus plastomes SSRs ranged in numbers of repeated from 117 (L. dealbatus) to 133 (L. tephrocarpus) (Fig. 5A). Among these, the mononucleotide repeats were the most abundant (62.02–68.22%), particularly A/T repeats (56.59–65.57%), followed by dinucleotide repeats (12.88–15.87%) (Fig. 5A, Table S3). Most of the SSRs (69%) were distributed in the intergenic spacer (IGS) region across all plastomes (Fig. 5C). However, a few SSRs with specific repeat units were unique to different Lithocarpus species. As shown in Table S3, the SSRs ACT/AGT, AAAC/GTTT, AACTC/AGTTG, AAATAT/ATATTT, and AATATC/ATATTG, among others, were unique to L. fohaiensis, L. grandifolius, L. elizabethiae, L. longipedicellatus, and L. gymnocarpus, respectively.
Fig. 5.
Analyses of simple sequence repeats (SSRs) in Lithocarpus plastomes. (A) Types and numbers of SSRs in the plastomes of Lithocarpus; (B) Types and numbers of SSRs in the plastomes of Fagaceae; (C) Distribution of SSRs in the intergenic spacer (IGS), coding sequences (CDS), intron, and Transfer RNA (tRNA) regions of the plastomes of Lithocarpus
Expansion and contraction of IR region in the Lithocarpus plastome
The LSC/IRb, SSC/IRa, and IRa/LSC boundaries among the 34 Lithocarpus plastomes were generally conserved. The LSC/IRb boundary genes were rps19 and rpl2, the SSC/IRa boundary was located within the ycf1 gene, and the IRa/LSC boundary genes were rpl2 and trnH. In contrast, the IRb/SSC junctions of plastomes within Lithocarpus were significantly variable and could be categorized into two distinct types. The expansion of the IR into the ndhF gene (type I) was observed in 16 Lithocarpus species, while the expansion into the ycf1 gene (type II) occurred in the remaining Lithocarpus species (Fig. S2). Notably, type I was found only in Lithocarpus and Fagus species (Fig. S3).
Plastome comparison and evolution
The gene arrangement of the 34 Lithocarpus plastomes was conserved (Fig. S4). Based on the comparison of Lithocarpus plastomes using mVISTA (Fig. S5), it is also evident that the plastomes of Lithocarpus exhibit a high degree of similarity. Overall, non-coding and single-copy (SC) regions exhibited more nucleotide divergence than coding and inverted repeats (IRs) (Fig. S5).
Our study showed that Ka or Ks values of certain genes of Lithocarpus plastomes are zero, rendering the calculation of Ka/Ks ratios unfeasible. After excluding these anomalous values, we assessed the selection pressure on 56 protein-coding genes by calculating the ratios of Ka to Ks substitutions. Most Ka/Ks ratios were less than 1 or undefined. A high Ka/Ks ratio (> 1) was observed in four genes (accD, atpF, rpl32, and rps8) with a p-value < 0.01, indicating these genes might be under a positive selection (Fig. 6).
Fig. 6.
Boxplots of Ka/Ks values among every shared protein-coding gene of Lithocarpus. Statistics analysis was performed using the one-sample t-test and the resulting probabilities (P-values *<0.05, **< 0.01, ***< 0.001) are shown
Plastome-based phylogeny
The phylogenetic trees reconstructed by ML and BI searching methods based on the three data partitions of the plastome (complete plastome, protein-coding exons, and non-coding regions) show the identical tree topology on the main clades, except for minor difference at the terminal tips (Fig. 7, S6, S7). Among these, the protein-coding tree inferred by the Bayesian inference method received the highest support values for the main clades (Fig. 7). Of which, the monophyly of most Fagaceae genera was strongly supported (bootstrap values ranging [BS] = 100, BI posterior probability [PP] = 1.00), except for Quercus, which exhibited polyphyly. Fagus and Trigonobalanus were resolved as a grade in two successive divergences. Notholithocarpus and Chrysolepis were nested within the Quercus clade, forming sister groups with Quercus subgenus Quercus and subgenus Lobatae, respectively. Castanopsis and Castanea were inferred to be sister taxa (BS = 93, PP = 1.00), while three Quercus species were determined to be sister taxa to Lithocarpus (BS = 98, PP = 1.00). Within Lithocarpus, two clades (Clade I and Clade II) were resolved. Clade I included L. obscurus (mainly distributed in Mêdog, Tibet) and L. dealbatus (from India). Clade II primarily comprised species from the southern Qinling Mountains of China, with two main subclades: Clade II-1, consisting of species from Southwest China, and Clade II-2, comprising species from the central-east China–Japan region (Fig. 8).
Fig. 7.
Phylogenetic relationships of Fagaceae inferred from ML and BI analyses based on the plastome protein-coding regions. The numbers near each node are bootstrap support values and posterior probability, respectively
Fig. 8.
Cladogram and geographic distribution of Lithocarpus. The map was created using the open-source R package phytools v2.0. The dots on the map show the sampling sites and the dot color indicates the main clades in Lithocarpus illustrated in Fig. 7
Leaf epidermal trichome and acorn morphology divergence pattern
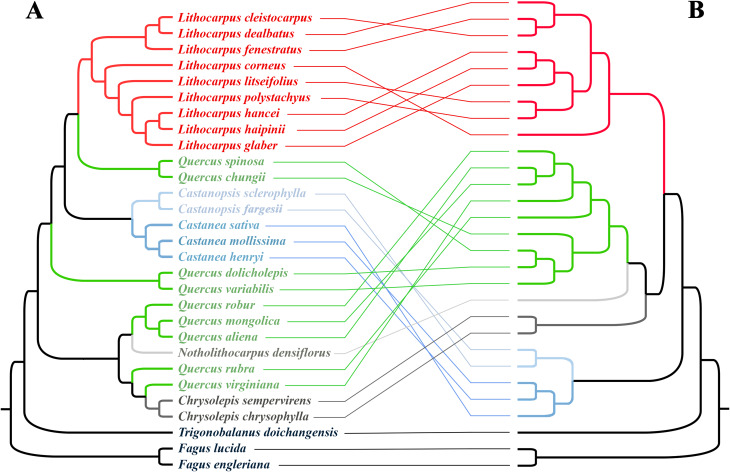
The Clade I and Clade II inferred by the plasma-based phylogenetic tree were not supported by either the leaf trichome features or acorn type (Fig. 7). The only species with BBT trichome and ER acorn―L. corneus was inferred as a basal group in Lithocarpus, but ATP and Glabrous leaf trichome types and ER and AR fruit types are paraphyletic on the trimmed nuclear-based phylogenetic tree of Liu et al. [31] (Fig. 9).
Fig. 9.
Conflicts between the plastome (A) and nuclear (B, modified from the nuclear tree of Liu et al. [31]) species trees, visualized using the R package ape v5.8
Discussion
Plastome characteristics and adaptive evolution of Lithocarpus
The plastome structure across the 34 Lithocarpus samples was largely conserved, though variations in gene content, SSRs, and SC/IR boundaries were observed. Such variation is similar to that reported among other Fagaceae genera [42, 43, 46, 47]. The conservation of plastome structure is likely constrained by the need to maintain the stability of plastome functionality [80].
The protein-coding infA gene encoding translation initiation factor 1, which aids in the assembly of the translation initiation complex [81], was found to be a pseudogene in 17 Lithocarpus species. The loss and pseudogenization of infA have been previously documented in various genera of Fagaceae [42, 43, 82] and other flowering plant families [83, 84]. In some seed plant lineages, the infA gene has been identified as translocated from the plastome to the nuclear genome [80], possibly as a consequence of relaxed purifying selection on the plastome.
Typically, IR regions of angiosperm plastomes begin near the rps19 gene and end consistently downstream of either trnN-GUU or the truncated ycf1 gene [85]. While IR expansion has been documented in specific lineages, usually within the LSC region, the IR/SSC junctions are considered relatively stable [85]. However, this study discovered obvious IR region expansion at the IR/SSC boundaries in Lithocarpus (Fig. S2). A similar IR expansion was also detected in the early derived taxa of Fagaceae (i.e., Fagus) (Fig. S3). These findings suggest that IR expansions are independent events in the Fagaceae.
An analysis of different SSR repeat types revealed that mononucleotide repeats, particularly A/T repeats, were the most prevalent, while the remaining SSRs exhibited high A/T content. Such an A/T bias has been widely reported among Fagaceae plastomes [45–47, 86] and many other plant plastomes [87, 88]. These findings align with the proposition that the plastome not only exhibits abundant A/T content but also harbors a considerable number of short polyadenine (polyA) and polythymine (polyT) repeats [89]. Although the numbers of repeat motifs are similar across Lithocarpus, certain motifs are species-specific. These loci may contain crucial information that may be used to untangle the intraspecific genetic structure of Lithocarpus.
Our study identified four genes that underwent positive selection, including those involved in energy storage (accD), photosynthesis (atpF), and protein synthesis (rpl32, and rps8). Previous studies have shown that the accD gene, which encodes the β-carboxyl transferase subunit of acetyl-CoA carboxylase, plays a role in leaf growth [90, 91], leaf longevity [92], and fatty acid biosynthesis [93, 94]. These processes enhance photosynthesis and reserve energy, helping plants cope with seasonal resource constraints and defense responses [95, 96]. The accD gene has also exhibited signatures of positive selection in some evergreen angiosperm lineages in the tropics and subtropics, e.g., Alpinia [97], Pterocarpus [98], and some species of Araceae [99]. The wide distribution of Lithocarpus in heterogeneous environments of East Asia may boost genetic divergence.
The gene atpF encodes a subunit of H+-ATP synthase, which is essential for chloroplast electron transport, photorespiration, and stress resistance in plants [100]. ATP synthase is crucial for providing the energy required for photosynthesis [101]. Notably, the atpF gene is also associated with deciduous versus evergreen habits in oaks, showing stronger signatures of positive selection in the subalpine sclerophyllous oak Quercus aquifolioides compared to deciduous oaks from mesic habitats [45]. Similarly, the positive selection detected in the atpF gene in Lithocarpus may reflect its role in photosynthesis and in maintaining energy for year-round leaf retention in these species.
Additionally, rpl32 and rps8 encode ribosomal proteins L32 and S8, respectively [102]. Environmental stress can cause oxidative damage, affecting the translation system, particularly in systems of prokaryote origin such as the chloroplast [103]. These ribosomal protein-coding genes have undergone strong selection, possibly driven by regional adaptation. These genes likely help Lithocarpus maintain its leaves year-round, enhancing the species’ ability to acclimatize and cope with the hot and humid conditions in the tropics and subtropics.
Phylogenetic analysis
Our plastome-based phylogenetic trees showed similar topologies using three distinctly different data partitions, with only minor differences among the tips. The phylogenetic tree of protein-coding exons received the highest credibility support (Fig. 7, S6, S7). Generally, regions under relatively relaxed selection, such as introns, exhibit higher polymorphism than exons [104]. Consequently, coding regions typically show higher sequence homology than non-coding regions, as they contain fewer conflicting phylogenetic signals [105], often leading to higher resolution.
Compared to previous phylogenetic studies based on single or multiple locus DNA sequences [106, 107], our plastome tree provides a robust phylogenetic framework for the family Fagaceae, with major nodes showing strong support (i.e., PP = 1.00 and BS ≥ 93). The results further confirmed that whole plastome sequencing can enhance the phylogenetic resolution within a given lineage [108, 109]. Most fagaceous genera were resolved as monophyletic based on the plastome sequence data, e.g., Lithocarpus, Castanopsis, and Castanea, consistent with the results of previous studies [30, 31, 38], except for Quercus, which was inferred to be polyphyletic on the plastome tree in our study. This result is consistent with that obtained by Zhou et al. [30], suggesting possible a widespread ancient gene flow in the ancestral lineage [30, 31].
This study is the first to analyze the maternal evolutionary history of the genus Lithocarpus based on a large dataset of plastomes. Notably, our plastome-based phylogenomic tree resolved two main clades in Lithocarpus with high credibility support. One clade, composed of two species from the southern Himalaya lowlands, formed a sister group to the rest of the Lithocarpus species. The latter clade includes two subclades corresponding to the two geographical regions of Southwest China (Clade II-1) versus China–Japan (Clade II-2), suggesting possible phylogeographic structure in the plastome of Lithocarpus (Fig. 8). These findings are consistent with previous biogeographic studies using cp. DNA sequences on Lithocarpus by Cannon & Manos [26] and Yang et al. [29]. Non-recombining genetic units, such as plastomes, can show significant divergence even within continuous populations, owing to limited seed dispersal capabilities [110]. Lithocarpus species show the highest acorn morphological diversity within the Fagaceae family, and their acorns are generally large [106, 111]. Fagaceous acorns are primarily dispersed by gravity and/or by hoarding animals (e.g., rodents and jays) with limited dispersal abilities, meaning they are typically dispersed within 50 m of the maternal tree [112–114]. Additionally, fagaceous seeds are highly sensitive to moisture loss [115, 116], resulting in high mortality at the post-dispersal stage [117, 118]. The phylogeographic structure detected in the plastome-based phylogenetic tree of Lithocarpus in this study may indicate restricted seed-mediated gene flow in ancestral lineages.
Compared to previous phylogenetic reconstruction using nuclear DNA datasets [29–31], there is a noticeable discordance in tree topologies between the nuclear- and plastome-based datasets from Lithocarpus (Fig. 9). Similar discrepancies have also been observed in the deep nodes of other fagaceous lineages, especially Quercus [6, 30, 31]. Four hypotheses may explain this inconsistency: (1) convergent evolution of plastome sequences [119]; (2) introgressive hybridization [7, 120]; (3) incomplete lineage sorting [121]; and (4) different rates of pollen- and seed-mediated gene flow [122, 123].
In the present study system, the probability of sequence convergence across an entire plastome was low, given the large size of the plastome used in this study. Therefore, convergent evolution is unlikely to explain the observed incongruence. Hybridization and introgression commonly occur among oaks with sympatric distributions [124–126]. When interspecific gene flow is asymmetric, one parental species may experience assimilation of its nuclear genome, while its maternal plastome is retained in the populations. This phenomenon is commonly referred to as the introgression-induced chloroplast capture [127, 128]. Introgression-induced chloroplast capture has been identified as a mechanism that can distort phylogenetic relationships, often resulting in geographic clustering of introgressed taxa [129]. In the Fagaceae family, natural hybridization and introgression are commonly observed among the species within the same Sect. [126] and sometimes even among more distantly related species [124, 125]. Recently, phylogenetic work on Fagaceae has revealed a secondary increase in the speciation rate of Lithocarpus during the Oligocene and Miocene [30], suggesting that interspecific hybridization may have occurred during the early stages of its diversification. Incomplete lineage sorting among taxa is often associated with radiations [130, 131]. Previous research has suggested that Lithocarpus might have experienced such radiation during the Neogene [30]. Accordingly, the possibility of incomplete lineage sorting causing cytonuclear discordance cannot be totally discounted. Furthermore, pollen and seed dispersal are critical determinants of gene flow [132]. Gene flow via pollen is significantly greater than that occurring via seeds, leading to broader genetic exchange for the nuclear genome compared to the plastome [133]. Differences between seed- and pollen-mediated gene flow can result in cytonuclear discordance in phylogenetic studies, as previously observed in Quercus [122] and Carya [123]. In nature, Lithocarpus species are primarily pollinated by insects [134], and the long-distance transmission of pollen enhances gene flow among populations. In contrast, the seed dispersal of these species with large recalcitrant seeds is more limited [122, 123]. The contrasting patterns of pollen- and seed-mediated gene flow among the ancestral populations could contribute to the cyto-nuclear discordance observed in Lithocarpus. All these hypotheses should be tested in future studies.
Furthermore, the plastome-based phylogenetic framework does not align with key taxonomical groupings based on acorn morphology (e.g., enclosed receptacle fruit, ER; and acorn fruit, AC) [106], nor with leaf epidermis characteristics (e.g., Bubble Trichome Group, BBT; Appressed parallel tufts Group, APT; and Glabrous Group) [77] in Lithocarpus (Fig. 7). In contrast, the phylogenomic tree inferred from nuclear DNA appears to be more consistent with species groupings based on leaf epidermal features [29, 31], indicating that these fine morphoanatomical traits are phylogenetically informative within lower taxonomical ranks in the genus Lithocarpus. The paraphyletic pattern of the acorn traits on these nuclear trees may indicate that these traits have multiple independent origins, possibly as a consequence of convergent adaptation to cope with animal predation [111].
Conclusions
Lithocarpus plastomes are conserved in terms of their genome structure, size, gene arrangement, and codon bias, but they show variation in gene content, SSRs, and the borders of SC/IR sequences. Most of the plastome genes in Lithocarpus are under purifying selection, while four genes related to metabolizing, photosynthesis, and energy storage (accD, atpF, rpl32, and rps8) underwent positive selection, indicating their roles in adaptation to diverse environments. The monophyletic status of Lithocarpus was strongly supported by the present plastome-based phylogenies. The plastome tree topologies revealed geographically structured relationships that conflict with previous nDNA phylogenies. Cytonuclear discordance, observed in Lithocarpus and other genera within the Fagaceae, may result from ancient introgression, incomplete lineage sorting, and asymmetric seed- and pollen-mediated gene flow. Future investigations, including whole-genome high-throughput sequencing to compare plastome and nuclear evolutionary histories, are essential to test these hypotheses and understand the drivers of such discordance, shedding light on the patterns and processes of diversification among fagaceous species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Lin Lin, Yu Tu, Li Li, Yan Luo, Lu Tang, Wu Sun, Gengchang Li, and Qingping Li for their help in sample collection. The field work was assited by Asian Elephant Yunnan Field Scientific Observation and Research Station, Yunnan Asian Elephant Field Scientific Observation and Research Station of the Ministry of Education (Kunming 650504, China).
Abbreviations
- AC
Acorn fruit
- APT
Appressed parallel tufts group
- BBT
Bubble trichome group
- BI
Bayesian inference
- bp
Base pair
- BS
Bootstrap value
- CDS
Coding sequences
- cpDNA
Chloroplast DNA
- CTAB
Cetyl trimethylammonium bromide
- ER
Enclosed receptacle fruit
- Hyb
Seq-Hybrid enrichment sequencing
- IGS
Intergenic spacer region
- IR
Inverted repeat region
- ITS
Internal transcribed spacer
- IUCN
International Union for Conservation of Nature
- LSC
Large single copy region
- ML
Maximum Likelihood
- NGS
Next-generation sequencing
- nrDNA
Nuclear ribosomal DNA
- PE
Paired-end
- PP
Posterior probability
- PCGs
Protein-coding genes
- rRNA
Ribosomal RNA
- RSCU
Relative synonymous codon usage
- SSC
Small single copy region
- SSR
Simple sequence repeat
- tRNA
Transfer RNA
- YUKU
Herbarium of Yunnan University
Author contributions
M. D., XL.J., and SJ. Z. designed the research. CY. W. and LF. Y. collected and analyzed the data; LF.Y., XL. J., and M. D. prepared and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Foundation for National Key Research and Development Program of China [Grant number 2023YFF1305001], National Natural Science Foundation of China [Grant number 32460060 & 31972858] and Yunnan Key Laboratory for Integrative Conservation of Plant Species with Extremely Small Populations [Grant number PSESP2021F01] and Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences [Grant number Y4ZK111B01]. The funders were not involved in the design of the research, collection, analysis, interpretation of data, and manuscript preparation.
Data availability
The 21 newly sequenced plastomes have been submitted to NCBI with accession numbers: OM112296-OM112297, OM112300, OM388302-OM388303, and PQ276656-PQ276671. The resulting DNA alignments and trees are available on GitHub (github.com/yanglifang116/Lithocarpus_plastomes).
Declarations
Ethics approval and consent to participate
The collection of all samples fully complied with national and local legislation. The plant samples used in this study were not listed as national key protected species and were not collected from national parks or nature reserves. In accordance with national and local laws, no specific permits were required for the collection of these plants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaolong Jiang, Email: xiaolongjiang1@gmail.com.
Min Deng, Email: dengmin@ynu.edu.cn.
References
- 1.Zhao CC, Wang YY, Chan KX, Marchant DB, Franks PJ, Randall D, et al. Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proc Natl Acad Sci USA. 2019;116:5015–20. 10.1073/pnas.1812092116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dopp IJ, Yang XD, Mackenzie SA. A new take on organelle-mediated stress sensing in plants. New Phytol. 2021;230:2148–53. 10.1111/nph.17333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gitzendanner MA, Soltis PS, Yi TS, Li DZ, Soltis DE. Plastome phylogenetics: 30 years of inferences into plant evolution. Advances in Botanical Research. Elsevier; 2018. pp. 293–313.
- 4.Daniell H, Jin S, Zhu XG, Gitzendanner MA, Soltis DE, Soltis PS. Green giant—a tiny chloroplast genome with mighty power to produce high-value proteins: history and phylogeny. Plant Biotechnol J. 2021;19:430–47. 10.1111/pbi.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DR. Mutation rates in plastid genomes: they are lower than you might think. Genome Biol Evol. 2015;7:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YY, Qu XJ, Zhang R, Stull GW, Yi TS. Plastid phylogenomic analyses of Fagales reveal signatures of conflict and ancient chloroplast capture. Mol Phylogenet Evol. 2021;163:1–11. 10.1016/j.ympev.2021.107232. [DOI] [PubMed] [Google Scholar]
- 7.Acosta MC, Premoli AC. Evidence of chloroplast capture in South American Nothofagus (Subgenus Nothofagus, Nothofagaceae). Mol Phylogenet Evol. 2010;54:235–42. 10.1016/j.ympev.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Hodel RGJ, Knowles LL, McDaniel SF, Payton AC, Dunaway JF, Soltis PS, et al. Terrestrial species adapted to sea dispersal: differences in propagule dispersal of two Caribbean mangroves. Mol Ecol. 2018;27:4612–26. 10.1111/mec.14894. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey AJ, Mandel JR. When one genome is not enough: Organellar heteroplasmy in plants. Annual Plant Reviews online. John Wiley & Sons, Ltd; 2019. pp. 619–58.
- 10.POWO. Plants of the world online. Facilitated by the Royal Botanic Gardens, Kew. 2024. https://powo.science.kew.org/. Accessed 5 Aug 2024.
- 11.Camus A, Les, Chênes. Monographie Du genre Quercus and Monographie Du genre Lithocarpus. Paris: Paul Lechevalier; 1952–4.
- 12.Soepadmo E. Fagaceae. In: Van Steenis CGGJ, editor. Flora Malesiana. Leiden: Noordhoff International Publishing; 1972. pp. 265–403. [Google Scholar]
- 13.Govaerts R, Frodin DG. World checklist and bibliography of Fagales: Betulaceae, Corylaceae, Fagaceae and Ticodendraceae. Richmond: Royal Botanic Gardens, Kew; 1998. [Google Scholar]
- 14.Huang CJ, Zhang YT, Bartholomew B. Fagaceae. In: Wu ZY, Raven PH, editors. Flora of China (volume 4). Beijing and St. Louis: Science Press and Missouri Botanical Garden; 1999. pp. 380–400. [Google Scholar]
- 15.Webb CO, Cannon CH, Davies SJ. Ecological organization, biogeography, and the phylogenetic structure of tropical forest tree communities. Trop for Community Ecol. 2008;6:79–97. [Google Scholar]
- 16.Petit RJ, Carlson J, Curtu AL, Loustau ML, Plomion C, González-Rodríguez A, et al. Fagaceae trees as models to integrate ecology, evolution and genomics. New Phytol. 2013;197:369–71. 10.1111/nph.12089. [DOI] [PubMed] [Google Scholar]
- 17.Xie BX, Xie T. Exploitation study of acorn resources in China. J Cent South Univ Forestry Technol. 2002;22:37–41. [Google Scholar]
- 18.Zhou W, Wu BC, Song CF, Liu QX. Resources and exploitation of Lithocarpus (Fagaceae) in China. Chin Wild Plant Resour. 2016;35:60–2. [Google Scholar]
- 19.Liu X, Chang EM, Liu JF, Jiang ZP. Comparative analysis of the complete chloroplast genomes of six white oaks with high ecological amplitude in China. J Res. 2021;32:2203–18. 10.1007/s11676-020-01288-3. [Google Scholar]
- 20.Miettinen J, Shi C, Liew SC. Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob Change Biol. 2011;17:2261–70. 10.1111/j.1365-2486.2011.02398.x. [Google Scholar]
- 21.Estoque RC, Ooba M, Avitabile V, Hijioka Y, DasGupta R, Togawa T, et al. The future of Southeast Asia’s forests. Nat Commun. 2019;10:1–12. 10.1038/s41467-019-09646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrero C, Jerome D, Beckman E, Byrne A, Coombes A, Deng M, et al. The red list of oaks 2020. Illinois: The Morton Arboretum; 2020. [Google Scholar]
- 23.Hughes AC. Understanding the drivers of southeast Asian biodiversity loss. Ecosphere. 2017;8:1–33. 10.1002/ecs2.1624.29552374 [Google Scholar]
- 24.Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–3. 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 25.Cannon CH. Morphological and molecular diversity in Lithocarpus (Fagaceae) of Mount Kinabalu. Sabah Parks Nat J. 2001;4:45–69. [Google Scholar]
- 26.Cannon CH, Manos PS. Phylogeography of the southeast Asian stone oaks (Lithocarpus): Phylogeography of Lithocarpus. J Biogeogr. 2003;30:211–26. 10.1046/j.1365-2699.2003.00829.x. [Google Scholar]
- 27.Manos PS, Cannon CH, Oh SH. Phylogenetic relationships and taxonomic status of the paleoendemic Fagaceae of western North America: recognition of a new genus. Notholithocarpus Madroño. 2008;55:181–90. 10.3120/0024-9637-55.3.181. [Google Scholar]
- 28.Oh SH, Manos PS. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 2008;57:434–51. 10.2307/25066014. [Google Scholar]
- 29.Yang CK, Chiang YC, Huang BH, Ju LP, Liao PC. Nuclear and chloroplast DNA phylogeography suggests an early Miocene southward expansion of Lithocarpus (Fagaceae) on the Asian continent and islands. Bot Stud. 2018;59:1–17. 10.1186/s40529-018-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou BF, Yuan S, Crowl AA, Liang YY, Shi Y, Chen XY, et al. Phylogenomic analyses highlight innovation and introgression in the continental radiationscof Fagaceae across the Northern Hemisphere. Nat Commun. 2022;13:1–14. 10.1038/s41467-022-28917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu SY, Yang YY, Tian Q, Yang ZY, Li SF, Valdes PJ, et al. An integrative framework reveals widespread gene flow during the early radiation of oaks and relatives in Quercoideae (Fagaceae). J Integr PlantBiol. 2024;00:1–23. 10.1111/jipb.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng M, Jiang XL, Hipp AL, Manos PS, Hahn M. Phylogeny and biogeography of east Asian evergreen oaks (Quercus section Cyclobalanopsis; Fagaceae): insights into the cenozoic history of evergreen broad-leaved forests in subtropical Asia. Mol Phylogenet Evol. 2018;119:170–81. 10.1016/j.ympev.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Jiang XL, Hipp AL, Deng M, Su T, Zhou ZK, Yan MX. East Asian origins of European holly oaks (Quercus section Ilex Loudon) via the Tibet-Himalaya. J Biogeogr. 2019;46:2203–14. 10.1111/jbi.13654. [Google Scholar]
- 34.Hipp AL, Manos PS, Hahn M, Avishai M, Bodénès C, Cavender-Bares J, et al. Genomic landscape of the global oak phylogeny. New Phytol. 2020;226:1198–212. 10.1111/nph.16162. [DOI] [PubMed] [Google Scholar]
- 35.Li YQ, Guo W, He P, Yu LH. The complete chloroplast genome of sweet tea (Lithocarpus Polystachyus). Mitochondrial DNA Part B-Resources. 2019;4:2489–90. 10.1080/23802359.2019.1638841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin L, Liu JJ, Xiao TW, Li QM, Lin LX, Shao XN, et al. Community phylogenetics require phylogenies reconstructed from plastid genomes. Mol Ecol Resour. 2021;1:1–43. 10.22541/au.161834751.14170237/v1. [Google Scholar]
- 37.Ma CX, Yan HF, Ge XJ. The complete chloroplast genome of Lithocarpus hancei (Benth.) Rehd (Fagaceae) from Zhejiang, China. Mitochondrial DNA Part B-Resources. 2021;6:2022–3. 10.1080/23802359.2021.1935357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelke R, Banerjje R, Joshi B, Singh PP, Tiwari GJ, Adhikari D, et al. Chloroplast genome of Lithocarpus dealbatus: identification of hotspots for sequence variability and phylogeny analysis in Quercoideae, a sub-family of Fagaceae. Res Square. 2022;12:1–29. 10.21203/rs.3.rs-1361496/v1. [Google Scholar]
- 39.Wu CY, Lin L, Yao KP, Yang RJ, Deng M. The complete chloroplast genome sequence of Lithocarpus longinux (Fagaceae). Mitochondrial DNA Part B-Resources. 2022;7:1229–31. 10.1080/23802359.2022.2093664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Zhang XH, Shi S, Chen BH. Lithocarpus dahuensis (Fagaceae), a new species from Fujian Province based on morphology and genomic data. PhytoKeys. 2023;222:1–18. 10.3897/phytokeys.222.99370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Li JQ, Wang HC, Li XW, Peng YS. LithocLongzhouicusouicus comb. nov. (Fagaceae) from China: based on morphological and molecular data. Nord J Bot. 2009;27:90–6. 10.1111/j.1756-1051.2008.00313.x. [Google Scholar]
- 42.Yang YC, Zhu J, Feng L, Zhou T, Bai GQ, Yang J, et al. Plastid genome comparative and phylogenetic analyses of the key genera in Fagaceae: highlighting the effect of codon composition bias in phylogenetic inference. Front Plant Sci. 2018;9:1–13. 10.3389/fpls.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YC, Zhou T, Qian ZQ, Zhao GF. Phylogenetic relationships in Chinese oaks (Fagaceae, Quercus): evidence from plastid genome using low-coverage whole genome sequencing. Genomics. 2021;113:1438–47. 10.1016/j.ygeno.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Yang JY, Takayama K, Youn JS, Pak JH, Kim SC. Plastome characterization and phylogenomics of east Asian beeches with a special emphasis on Fagus multinervis on Ulleung Island. Korea Genes. 2020;11:1–16. 10.3390/genes11111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin KQ, Zhang Y, Li YJ, Du FK. Different natural selection pressures on the atpF gene in evergreen sclerophyllous and deciduous oak species: evidence from comparative analysis of the complete chloroplast genome of Quercus aquifolioides with other oak species. Int J Mol Sci. 2018;19:1–15. 10.3390/ijms19041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou HJ, Gao XX, Woeste K, Zhao P, Zhang SX. Comparative analysis of the complete chloroplast genomes of four chestnut species (Castanea). Forests. 2021;12:1–14. 10.3390/f12070861. [Google Scholar]
- 47.Liang DQ, Wang HY, Zhang J, Zhao YX, Wu F. Complete chloroplast genome sequence of Fagus longipetiolata Seemen (Fagaceae): genome structure, adaptive evolution, and phylogenetic relationships. Life. 2022;12:1–17. 10.3390/life12010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simeone MC, Grimm GW, Papini A, Vessella F, Cardoni S, Tordoni E, et al. Plastome data reveal multiple geographic origins of Quercus Group Ilex. PeerJ. 2016;4:1–31. 10.7717/peerj.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye XM, Hu DN, Guo YP, Sun RX. Complete chloroplast genome of Castanopsis sclerophylla (Lindl.) Schott: Genome structure and comparative and phylogenetic analysis. Plos One. 2019;14:1–14. 10.1371/journal.pone.0212325 [DOI] [PMC free article] [PubMed]
- 50.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–3. 10.2307/4119796. [Google Scholar]
- 51.Chen SF, Zhou YQ, Chen YR, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90. 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin JJ, Yu WB, Yang JB, Song Y, DePamphilis CW, Yi TS, et al. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21:1–31. 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, Shi LC, Zhu YJ, Chen HM, Zhang JH, Lin XH, et al. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genomics. 2012;13:1–7. 10.1186/1471-2164-13-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–5. 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 55.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36 suppl2:W5–9. 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33 suppl2:W686–9. 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:W575–81. 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharp PM, Li WH. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–95. 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Statist. 1996;5:299–314. 10.1080/10618600.1996.10474713. [Google Scholar]
- 62.Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33:2583–5. 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amiryousefi A, Hyvönen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34:3030–1. 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 64.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32 suppl2:W273–9. 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang DP, Zhang YB, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteom Bioinf. 2010;8:77–80. 10.1016/S1672-0229(10)60008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-; 2016. https://cran.r-project.org/package=ggplot2. [Google Scholar]
- 67.Salojrvi J, Smolander OP, Nieminen K, Rajaraman S, Kangasjrvi J. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat Genet. 2017;49:904–17. 10.1038/ng.3862. [DOI] [PubMed] [Google Scholar]
- 68.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–6. 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9. 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74. 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67:901–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rambaut A. FigTree 1.4. 2 software. 2014. http://tree.bio.ed.ac.uk/software/fgtree/
- 74.Revell LJ. Phytools 2.0: an updated R ecosystem for phylogenetic comparative methods (and other things). Peerj. 2024;12:1–75. https://cran.r-project.org/web/packages/phytools/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paradis E, Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–8. 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 76.Zhou W, Xia NH. Leaf epidermal features of Lithocarpus (Fagaceae) from China and their systematic significance. Bot J Linn Soc. 2012;168:216–28. 10.1111/j.1095-8339.2011.01196.x. [Google Scholar]
- 77.Deng M, Li QS, Yang ST, Liu YC, Xu J. Comparative morphology of leaf epidermis in the genus Lithocarpus and its implication in leaf epidermal feature evolution in Fagaceae. Plant Syst Evol. 2013;299:659–81. 10.1007/s00606-012-0751-0. [Google Scholar]
- 78.Chen X, Kohyama TS, Cannon CH. Associated morphometric and geospatial differentiation among 98 species of stone oaks (Lithocarpus). PLoS ONE. 2018;13:1–18. 10.1371/journal.pone.0199538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen CL, Ruhfel BR, Li JL, Wang ZF, Zhang LS, Zhang L, et al. Phylotranscriptomics of Swertiinae (Gentianaceae) reveals that key floral traits are not phylogenetically correlated. J Integr Plant Biol. 2023;65:1490–504. 10.1111/jipb.13464. [DOI] [PubMed] [Google Scholar]
- 80.Jansen RK, Ruhlman TA. Plastid genomes of seed plants. In: Bock R, Volker K, editors. Genomics of chloroplasts and mitochondria. Dordrecht: Springer Science & Business Media; 2012. pp. 103–26. [Google Scholar]
- 81.Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76:273–97. 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kremer A, Abbott AG, Carlson JE, Manos PS, Plomion C, Sisco P, et al. Genomics of Fagaceae. Tree Genet Genomes. 2012;8:583–610. 10.1007/s11295-012-0498-3. [Google Scholar]
- 83.Millen RS, Olmstead RG, Adams KL, Palmer JD, Lao NT, Heggie L, et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 2001;13:645–58. 10.1105/tpc.13.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jansen RK, Cai Z, Raubeson LA, Daniell H, Depamphilis CW, Leebens-Mack J, et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc Natl Acad Sci. 2007;104:19369–74. 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu AD, Guo WH, Gupta SS, Fan WS, Mower JP. Evolutionary dynamics of the plastid inverted repeat: the effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016;209:1747–56. 10.1111/nph.13743. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Wang TR, Kozlowski G, Liu MH, Yi LT, Song YG. Complete chloroplast genome of an endangered species Quercus litseoides, and its comparative, evolutionary, and phylogenetic study with other Quercus section Cyclobalanopsis species. Genes. 2022;13:1–16. 10.3390/genes13071184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zavala-Páez M, Vieira L, de do N, Baura VA, Balsanelli E, de Souza EM, Cevallos MC et al. Comparative plastid genomics of neotropical Bulbophyllum (Orchidaceae; Epidendroideae). Front Plant Sci. 2020;11:1–15. 10.3389/fpls.2020.00799 [DOI] [PMC free article] [PubMed]
- 88.Liu J, Lindstrom AJ, Gong X. Towards the plastome evolution and phylogeny of Cycas L. (Cycadaceae): molecular-morphology discordance and gene tree space analysis. BMC Plant Biol. 2022;22:1–15. 10.1186/s12870-022-03491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuang DY, Wu H, Wang YL, Gao LM, Lu L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): implication for DNA barcoding and population genetics. Genome. 2011;54:663–73. 10.1139/g11-026. [DOI] [PubMed] [Google Scholar]
- 90.Wakasugi T, Tsudzuki T, Sugiura M. The genomics of land plant chloroplasts: gene content and alteration of genomic information by RNA editing. Photosynth Res. 2001;70:107–18. 10.1023/A:1013892009589. [DOI] [PubMed] [Google Scholar]
- 91.Kode V, Mudd EA, Iamtham S, Day A. The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 2005;44:237–44. 10.1111/j.1365-313X.2005.02533.x. [DOI] [PubMed] [Google Scholar]
- 92.Madoka Y, Tomizawa KI, Mizoi J, Nishida I, Nagano Y, Sasaki Y. Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol. 2002;43:1518–25. 10.1093/pcp/pcf172. [DOI] [PubMed] [Google Scholar]
- 93.Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–70. 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasaki Y, Nagano Y. Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci Biotechnol Biochem. 2004;68:1175–84. 10.1271/bbb.68.1175. [DOI] [PubMed] [Google Scholar]
- 95.Okazaki Y, Saito K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014;79:584–96. 10.1111/tpj.12556/. [DOI] [PubMed] [Google Scholar]
- 96.Lim GH, Singhal R, Kachroo A, Kachroo P. Fatty acid- and lipid-mediated signaling in plant defense. Annu Rev Phytopathol. 2017;55:505–36. 10.1146/annurev-phyto-080516-035406. [DOI] [PubMed] [Google Scholar]
- 97.Yang HY, Wang LQ, Chen HM, Jiang M, Wu WW, Liu SY, et al. Phylogenetic analysis and development of molecular markers for five medicinal Alpinia species based on complete plastome sequences. BMC Plant Biol. 2021;21:1–16. 10.1186/s12870-021-03204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong Z, Wu ZQ, Zhao KK, Yang ZJ, Zhang NN, Guo J, et al. Comparative analyses of five complete chloroplast genomes from the genus Pterocarpus (Fabacaeae). Int J Mol Sci. 2020;21:1–18. 10.3390/ijms21113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li BC, Liu T, Ali A, Xiao Y, Shan N, Sun JY, et al. Complete chloroplast genome sequences of three Aroideae species (Araceae): lights into selective pressure, marker development and phylogenetic relationships. BMC Genomics. 2022;23:1–16. 10.1186/s12864-022-08400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hudson GS, Mason JG. The chloroplast genes encoding subunits of the H+-ATP synthase. Photosynth Res. 1988;45:565–82. 10.1007/BF00042985. [DOI] [PubMed] [Google Scholar]
- 101.Westhoff P, Alt J, Nelson N, Herrmann RG. Genes and transcripts for the ATP synthase CF0 subunits I and II from spinach thylakoid membranes. Mol Gen Genet. 1985;199:290–9. 10.1007/BF00330271. [Google Scholar]
- 102.Subramanian AR. Molecular genetics of chloroplast ribosomal proteins. Trends Biochem Sci. 1993;18:177–81. 10.1016/0968-0004(93)90110-9. [DOI] [PubMed] [Google Scholar]
- 103.Smirnoff N. Plant resistance to environmental stress. Curr Opin Biotechnol. 1998;9:214–9. 10.1016/S0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- 104.Oruç Ö. An application of information theoretical measures for DNA structure. Turk Klinikleri J Biostat. 2011;3:1–7. [Google Scholar]
- 105.Wiens JJ. Missing data, incomplete taxa, and phylogenetic accuracy. Syst Biol. 2003;52:528–38. 10.1080/10635150390218330. [DOI] [PubMed] [Google Scholar]
- 106.Cannon CH, Manos PS. Combining and comparing morphometric shape descriptors with a molecular phylogeny: the case of fruit type evolution in Bornean Lithocarpus (Fagaceae). Syst Biol. 2001;50:860–80. 10.1080/106351501753462849. [DOI] [PubMed] [Google Scholar]
- 107.Manos PS, Zhou ZK, Cannon CH. Systematics of Fagaceae: phylogenetic tests of reproductive trait evolution. Int J Plant Sci. 2001;162:1361–79. 10.1086/322949. [Google Scholar]
- 108.Moore MJ, Bell CD, Soltis PS, Soltis DE. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proc Natl Acad Sci. 2007;104:19363–8. 10.1073/pnas.0708072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc Natl Acad Sci. 2010;107:4623–8. 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Irwin DE. Phylogeographic breaks without geographic barriers to gene flow. Evolution. 2002;56:2383–94. 10.1111/j.0014-3820.2002.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 111.Chen X, Qin YY, Jia DR. The fruit morphometric variation and fruit type evolution of the stone oaks (Fagaceae, Lithocarpus). BMC Plant Biol. 2023;23:1–19. 10.1186/s12870-023-04237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moran EV, Clark JS. Between-site differences in the scale of dispersal and gene flow in red oak. PLoS ONE. 2012;7:1–15. 10.1371/journal.pone.0036492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scofield DG, Alfaro VR, Sork VL, Grivet D, Martinez E, Papp J, et al. Foraging patterns of acorn woodpeckers (Melanerpes formicivorus) on valley oak (Quercus lobata Née) in two California oak savanna-woodlands. Oecologia. 2011;166:187–96. 10.1007/s00442-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramos-Palacios CR, Badano EI, Flores J, Flores-Cano JA, Flores-Flores JL. Distribution patterns of acorns after primary dispersion in a fragmented oak forest and their consequences on predators and dispersers. Eur J Res. 2014;133:391–404. 10.1007/s10342-013-0771-5. [Google Scholar]
- 115.Walters C, Berjak P, Pammenter N, Kennedy K, Raven P. Preservation of recalcitrant seeds. Science. 2013;339:915–6. 10.1126/science.1230935. [DOI] [PubMed] [Google Scholar]
- 116.Xia K, Daws MI, Peng LL. Climate drives patterns of seed traits in Quercus species across China. New Phytol. 2022;234:1629–38. 10.1111/nph.18103. [DOI] [PubMed] [Google Scholar]
- 117.Suszka B, Tylkowski T, Suszka B, Tylkowski T. Storage of acorns of the English oak Quercus robur over 1 5 winters. Arboretum Kornickie. 1980;25:199–230. [Google Scholar]
- 118.Chen X, Luo YJ, Wang R, Du FK. The distinct fruit size and physical defense promote divergent secondary seed dispersal strategies of three oak species. Ecol Manage. 2023;529:1–7. 10.1016/j.foreco.2022.120642. [Google Scholar]
- 119.Zhang C, Li SQ, Xie HH, Liu JQ. Comparative plastid genome analyses of Rosa: insights into the phylogeny and gene divergence. Tree Genet Genomes. 2022;18:1–17. 10.1007/s11295-022-01549-8. [Google Scholar]
- 120.Degnan JH, Rosenberg NA. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol. 2009;24:332–40. 10.1016/j.tree.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 121.Jakob SS, Blattner FR. A chloroplast genealogy of Hordeum (Poaceae): long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Mol Biol Evol. 2006;23:1602–12. 10.1093/molbev/msl018. [DOI] [PubMed] [Google Scholar]
- 122.Xu J, Song YG, Deng M, Jiang XL, Zheng SS, Li Y. Seed germination schedule and environmental context shaped the population genetic structure of subtropical evergreen oaks on the Yun-Gui Plateau, Southwest China. Heredity. 2020;124:499–513. 10.1038/s41437-019-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu LL, Yu RM, Lin XR, Zhang BW, Li N, Lin K, et al. Different rates of pollen and seed gene flow cause branch-length and geographic cytonuclear discordance within Asian butternuts. New Phytol. 2021;232:388–403. 10.1111/nph.17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bacon JR, Spellenberg R. Hybridization in two distantly related Mexican black oaks Quercus conzattii and Quercus eduardii (Fagaceae: Quercus: section Lobatae). SIDA Contrib Bot. 1996;17:17–41. 10.2307/41960947. [Google Scholar]
- 125.Scareli-Santos C, Herrera-Arroyo ML, Sánchez-Mondragon ML, González-Rodríguez A, Bacon J, Oyama K. Comparative analysis of micromorphological characters in two distantly related Mexican oaks, Quercus Conzattii and Q. eduardii (Fagaceae), and their hybrids. Brittonia. 2007;59:37–48. 10.1663/0007-196X(2007)59[37:CAOMCI]2.0.CO;2. [Google Scholar]
- 126.Kremer A, Hipp AL. Oaks: an evolutionary success story. New Phytol. 2020;226:987–1011. 10.1111/nph.16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallace LE, Culley TM, Weller SG, Sakai AK, Kuenzi A, Roy T, et al. Asymmetrical gene flow in a hybrid zone of hawaiian Schiedea (Caryophyllaceae) species with contrasting mating systems. PLoS ONE. 2011;6:1–12. 10.1371/journal.pone.0024845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCauley DE, Stevens JE, Peroni PA, Raveill JA. The spatial distribution of chloroplast DNA and allozyme polymorphisms within a population of Silene alba (Caryophyllaceae). Am J Bot. 1996;83:727–31. 10.1002/j.1537-2197.1996.tb12761.x. [Google Scholar]
- 129.Zhao TT, Wang GX, Ma QH, Liang LS, Yang Z. Multilocus data reveal deep phylogenetic relationships and intercontinental biogeography of the eurasian-north American genus Corylus (Betulaceae). Mol Phylogenet Evol. 2020;142:1–14. 10.1016/j.ympev.2019.106658. [DOI] [PubMed] [Google Scholar]
- 130.Enard W, Paabo S. Comparative primate genomics. Annu Rev Genomics Hum Genet. 2004;5:351–78. 10.1146/annurev.genom.5.061903.180040. [DOI] [PubMed] [Google Scholar]
- 131.Pollard DA, Iyer VN, Moses AM, Eisen MB. Widespread discordance of gene trees with species tree in Drosophila: evidence for incomplete lineage sorting. Plos Genet. 2006;2:1634–47. 10.1371/journal.pgen.0020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu XS, He FL. Seed and pollen flow in expanding a species’ range. J Theor Biol. 2006;240:662–72. 10.1016/j.jtbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG. Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol. 2005;14:689–701. 10.1111/j.1365-294X.2004.02410.x. [DOI] [PubMed] [Google Scholar]
- 134.Nixon KC. Origins of Fagaceae. In: Crane PR, Blackmore S, editors. Evolution, systematics, and fossil history of the Hamamelidae. Oxford: Clarendon; 1989. pp. 23–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 21 newly sequenced plastomes have been submitted to NCBI with accession numbers: OM112296-OM112297, OM112300, OM388302-OM388303, and PQ276656-PQ276671. The resulting DNA alignments and trees are available on GitHub (github.com/yanglifang116/Lithocarpus_plastomes).