Abstract
Greater than 90% of lung infections in cystic fibrosis (CF) patients are caused by Pseudomonas aeruginosa, and the majority of these patients subsequently die from lung damage. Current therapies are either targeted at reducing obstruction, reducing inflammation, or reducing infection. To identify potential therapeutic agents for the CF lung, 150 antimicrobial peptides consisting of three distinct structural classes were screened against mucoid and multidrug-resistant clinical isolates of P. aeruginosa, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Staphylococcus aureus. Five peptides that retained potent antimicrobial activities in physiological salt and divalent cation environment were further characterized in vivo using a rat chronic lung infection model. All animals were inoculated intratracheally with 104 P. aeruginosa mucoid PAO1 cells in agar beads. Three days following inoculation treatment was initiated. Animals were treated daily for 3 days with 100 μl of peptide solution (1 mg/ml) in 10 mM sodium citrate, which was deposited via either intratracheal instillation or aerosolization. Control animals received daily exposure to vehicle alone. At the end of the treatment the lungs of the animals were removed for quantitative culture. Four peptides, HBCM2, HBCM3, HBCPα-2, and HB71, demonstrated significant reduction in Pseudomonas bioburden in the lung of rats. Further in vivo studies provided direct evidence that anti-inflammatory activity was associated with three of these peptides. Therefore, small bioactive peptides have the potential to attack two of the components responsible for the progression of lung damage in the CF disease: infection and inflammation.
Cystic fibrosis (CF) is an autosomal-recessive genetic disease due to a defect in the CF transmembrane conductance regulator (CFTR) gene of chromosome 7 encoding the CFTR chloride channel protein (30). It is the most common life-shortening genetic disease among caucasians and affects approximately 60,000 people worldwide. The major morbidity and mortality in CF are caused by the progressive loss of pulmonary function that results from a cycle of inflammation and infection. Patients with cystic fibrosis typically harbor multiple morphologically distinct microorganisms. By 18 years of age, 80% of patients become chronically infected with Pseudomonas aeruginosa (1, 25). P. aeruginosa is a difficult organism to treat in most human infections due to its high intrinsic antibiotic resistance, due primarily to low outer membrane permeability bolstered by effective multidrug efflux and an inducible class C β-lactamase. In the setting of the CF lung this issue appears to be exacerbated, and further development of cross-resistance to ciprofloxacin and ofloxacin is regularly seen while resistance to aminoglycosides is also a problem in CF patients with chronic P. aeruginosa infections (12, 22). Other well-characterized CF pathogens include Staphylococcus aureus and nontypeable Haemophilus influenzae, which are of significance primarily during the first decade of life (26). In the past two decades, intrinsically antibiotic-resistant, gram-negative organisms such as Burkholderia cepacia, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans are newly emerging microorganisms isolated with increased frequencies from CF lungs (9, 23, 26). The inadequacy and deficiency of effective antibiotics for CF has led medical professionals to reconsider the value of conventional antibiotics, even when they do not have strong anti-pseudomonal activity. For example, the macrolide antibiotic azithromycin has been evaluated in patients with chronic Pseudomonas lung infection (14). However, this approach has also failed to demonstrate success and furthermore put patients under selective pressure promoting the carriage of macrolide-resistant bacteria. Chronic inflammation complicates therapy in the CF airways leading, for example, to high concentrations of DNA and neutrophil elastase (10). As a consequence of inflammation, the immune response in the CF lungs appears to be dysfunctional. Therefore, there is a vital need to develop new effective anti-pseudomonal as well as anti-inflammatory therapeutics.
Antimicrobial cationic peptides are ubiquitous in nature. They are a key component of the innate immune system acting as the first line of defense against infectious agents. The properties of these peptides make them extremely attractive candidates for development as therapeutics for CF. Not only are they fast acting, bactericidal, and active against multidrug-resistant bacteria, but certain peptides exhibit anti-inflammatory, immunomodulatory, and wound healing activities in addition to antimicrobial activity (11, 13, 18, 21, 34). Although lung epithelial cells secrete cationic antimicrobial peptides and proteins, most endogenous peptides such as β-defensins and LL-37 are in very low concentrations in the lung and are salt sensitive in vitro and thus are presumably ineffective in the high-salt environment created on the apical side of CF epithelial cells (3, 15). A promising therapeutic approach would be to exogenously apply antimicrobial cationic peptides with combined antimicrobial and anti-inflammatory activity to the lung against P. aeruginosa infections and inflammation via aerosol formulation (18). The rationale for use of such peptides in CF therapy is further supported by work demonstrating that the overexpression of LL-37 by viral gene transfer results in the augmentation of innate host defenses against P. aeruginosa in a bronchial xenograft model of cystic fibrosis (4).
In this study, we took the approach of screening for peptides with activity against CF pathogens in the presence of factors that mimic the physiological environment of the CF lung. Peptides with good anti-inflammatory as well as antimicrobial activity were then tested in animal models for in vivo efficacy. Our data suggest that the lead peptides significantly reduced the numbers of viable bacteria in the infected lungs of rats as well as demonstrating good anti-inflammatory activity in mice.
MATERIALS AND METHODS
Peptide synthesis.
All peptides were synthesized using standard Fmoc chemistry on an Advanced ChemTech (Louisville, KY) Apex 396 Multiple Peptide Synthesizer. Primary sequence confirmation and preparative purification to >95% purity was accomplished using a liquid chromatography-tandem mass spectrometry system (ABI API2000).
Bacterial strains and culture conditions.
Bacterial strains included in this study are listed in Table 1. P. aeruginosa, S. aureus, and Candida species were grown in Mueller Hinton (MH; Difco, BD Biosciences, MD) agar plates and broth at 37°C unless otherwise indicated. Bacteria from frozen stock were subcultured onto freshly made MH agar plates prior to susceptibility testing. P. aeruginosa clinical isolates, multidrug-resistant isolates, and methicillin-resistant S. aureus (MRSA) were all from laboratory stocks at the University of British Columbia, Vancouver, Canada. Tobramycin, rifampin, chloramphenicol, calf thymus DNA, and mucin were purchased from Sigma Chemical Company (St. Louis, MO).
TABLE 1.
Bacterial strains used in this study
| Bacterium | Description |
|---|---|
| Escherichia coli UB1005 | Wild type; F−metB1 relA1 spoT1 gyrA216 lamB |
| Salmonella enterica serovar Typhimurium 14028s | Wild type |
| P. aeruginosa PAO1 | Wild type |
| P. aeruginosa H374 | nfxBa mutant of strain PAO1 |
| P. aeruginosa H744 | nalBb mutant of strain PAO1 |
| P. aeruginosa H401 | Mucoid clinical isolate |
| P. aeruginosa H403 | Mucoid clinical isolate |
| P. aeruginosa 105663 | Tobramycin resistant |
| P. aeruginosa 100609 | Tobramycin resistant |
| P. aeruginosa M76 | β-Lactam resistant |
| P. aeruginosa M917 | β-Lactam and aminoglycoside resistant |
| P. aeruginosa M1251 | Imipenem and aminoglycoside resistance |
| P. aeruginosa M1426 | β-Lactam resistant |
| P. aeruginosa R70 | Imipenem resistant |
| P. stutzeri | Sputum |
| P. fluorescens | Sputum |
| P. pickettii | Sputum |
| Stenotrophomonas maltophilia | ATCC13637 |
| Achromobacter xylosoxidans | ATCC27061 |
| Staphlococcus aureus SAP0017 | Methicillin resistant; MRSA |
| Candida albicans 105 | Environmental isolate |
MexCD-OprJ efflux pump overexpressing mutant.
MexAB-OprM efflux pump overexpressing mutant.
MIC.
The MIC of each peptide was determined using a modified (to prevent peptide binding to microtiter trays) NCCLS microtiter broth dilution assay (29) and an inoculum of 105 to 106 CFU/ml. The MIC was taken as the lowest peptide concentration at which more than 90% of bacterial growth was inhibited after 15 to 20 h of incubation at 37°C.
To test peptide activity in the presence of DNA or mucin, approximately 2 × 105 CFU/ml of bacteria was added to 1.2% hog gastric mucin or 0.8 mg/ml of calf thymus DNA in saline and dispensed (80 μl) into each well of a 96-well plate, containing 10 μl of 2-fold serial diluted peptides. After incubation at 37°C for 2 h, the cell viability was determined by spotting 10 μl of the reaction mix onto fresh MH agar plates and subsequent incubation at 37°C overnight. The MIC was taken as the lowest concentration at which no colonies were observed.
Activity of peptides in sputum.
Individual sputum samples from CF patients were pooled and diluted 10-fold with 10 mM phosphate buffer (pH 7.0). The suspensions were thoroughly mixed and then centrifuged at 1,000 rpm for 10 min (Mini Centrifuge C1200; National Labnet Co., Woodbridge, NJ). Supernatants were saved and used in killing and stability tests. For killing assays, about 5 × 107 CFU/ml of P. aeruginosa were added to a 10% diluted sputum supernatant, and peptide was added to a final concentration of 100 μg/ml. The mixture was incubated at 37°C for 1 h and then diluted and plated on freshly made MH agar plates.
For stability tests, individual peptides were added to the 10% diluted sputum supernatant to a final concentration of 100 μg/ml, and the mixture was incubated at 37°C for various time intervals, after which the reaction was stopped by the addition of 10 μl of 10% HCl. Residual peptide concentrations were determined with a reversed-phase high-performance liquid chromatography system.
Rat CF lung model.
The chronic rat lung model of agar bead coated P. aeruginosa infection was used to assess the effects of peptides on the bioburden in the lungs (16). Rats (5 animals in each group) were inoculated intratracheally with approximately 104 CFU of P. aeruginosa strain PAO1 in agar beads. Treatment was conducted by either intratracheal instillation or nebulization. For intratracheal instillation of peptides, treatment was initiated 3 days after inoculation and deposited intratracheally. Peptides (100 μg in 100 μl sodium citrate buffer) were administered intratracheally to anesthetized animals using a laryngoscope introduced into the oral cavity while the incisors were suspended over a holding bar of an instrument that permitted observation of the entrance to the trachea. A 22-gauge gavage needle was then introduced into the oral cavity between the visible vocal folds, allowing the solution to be introduced into the left lung. Control animals (group 1) received daily exposure to saline, and treatment groups received daily exposure to one of five different formulations of peptides (groups 2 to 6). Animals were sacrificed on day 3, and the lungs of animals removed for quantitative culture.
For nebulization studies, rats were exposed to aerosol preparations using an Aero-tech II nebulizer (CIS-US, Bedford, MA) operated at 45 lb/in2, with a flow rate of 10 liters/min and containing 10 ml of the preparation to be aerosolized. The 10-ml volume was dispensed in 25 to 30 min, during which rats were inhaling the drug aerosol. Animals (5 in each group) were treated once daily for 3 days; control animals received daily exposure to 10 ml of 10 mM sodium citrate (pH 7.0); one treatment group received daily exposure to 10 ml of 10 mM sodium citrate (pH 7.0) containing 5 mg/ml of HBCM3; one treatment group received daily exposure to 10 ml of 10 mM sodium citrate (pH 7.0) containing 5 mg/ml of HBCPα2. Animals were sacrificed on day 3, and the lungs removed for quantitative culture. The amount of compound delivered to the lung in this model was estimated to be approximately 10 to 15 μg per treatment based on analogous studies with similar doses of the polycationic aminoglycoside antibiotic amikacin (D. E. Woods, unpublished data).
In vivo anti-inflammation assay using an ear edema model in mice.
Inflammation assays were perform in mice by CEREP (Paris, France) using the technology described by Chang et al. (8). Twenty-five CD-1 male mice weighing 21 to 25 g were used. Thirty minutes after topical application of the test peptide (20 μg/ear), an irritant, phorbyl myristoyl acetate (PMA; 5 μg in 20 μl of ethanol) was topically applied to the inner and outer surface of the right ear. Thirty minutes later a second dose of the test peptide was topically applied on the ear. Ear weight was measured 6 h after PMA application. The mean ear weight, expressed in milligrams, was calculated as the difference between the right and left ear weight. Bethamethasone was used as a positive control at 0.3 mg/ear.
Resistance.
The MIC of peptides for P. aeruginosa was assessed as described above. The cells treated with the concentration of peptide equivalent to one half the MIC were diluted in saline and used as an inoculum in the next round of MIC assessment. These procedures were repeated 30 times. Chloramphenicol and rifampin were used as antibiotic controls. Cross-resistance was tested after 30 passages for a panel of peptides.
RESULTS
Antimicrobial activity of peptides.
A total of 155 peptides were tested for their antimicrobial activity against bacteria and yeast. Table 2 shows some representative peptides with distinctive activities. A group of peptides represented by HBCM2, HBCM3, HBCPα2, (formerly termed CM2, CM3 and CPα2, respectively [28]), HB12, HB71, and HB46 exhibited selective activity against gram-negative bacteria, although HB71 and HB46 also showed moderate antifungal activity against Candida albicans (Table 2). Certain peptides were more antibacterial than antifungal, e.g., HB25, HB300, and HB301. In contrast, both HB77 and HB401 were yeast selective and ineffective against bacteria (Table 2). Most peptides, represented by HB35, HB43, HB55, HB146, HB153, and HBPM4, formerly termed PV-7 (34), displayed a broad spectrum activity with MICs ranging from 1 to 16 μg/ml against both bacteria and yeast (Table 2).
TABLE 2.
Activity of peptides against representative microorganisms, including clinical isolates and other gram-negative pathogens associated with CFa
| Peptide | Sequence | MIC (μg/ml)
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.c. | S.t. |
Pseudomonas aeruginosa
|
S.a. | C.a. | P.s. | P.f. | P.p. | S.m. | A.x. | ||||||||||
| PAO1 | Mu | Tb-r | M76 | MD | M917 | M1251 | M1426 | R70 | |||||||||||
| HBCM2 | KWKSFIKKLTKAAKKVVTTAKKPLIV-NH2 | 2 | 4 | 4 | 8 | —b | 4 | 2 | 32 | 2 | 4 | 2 | >128 | >128 | 1 | 2 | 2 | 1 | 4 |
| HBCM3 | KWKKFIKSLTKSAAKTVVKTAKKPLIV-NH2 | 1 | 3 | 4 | — | — | — | 2-4 | — | — | — | — | >64 | — | — | — | — | — | — |
| HB12 | MPKWKVFKKIEKVGRNIRNGIVKAGPAIAVLGEAKALG-NH2 | 1 | 4 | 16 | 4 | 8 | 8 | 8 | 16 | 4 | 8 | 4 | >128 | >128 | 4 | 8 | 8 | 2 | 32 |
| HB71 | FAKKLAKKLKKLAKKLAK-COOH | 2 | 8 | 8 | 16 | 16 | 8 | 4 | 32 | 8 | 8 | 2 | >128 | 32 | 2 | 8 | 4 | 1 | 8 |
| HB25 | FALALKALKKLLKKLKKLAKKAL-NH2 | 4 | 8 | 8 | 16 | 8 | 32 | 8 | 128 | 16 | 16 | 16 | 8 | 64 | 2 | 8 | 4 | 4 | 32 |
| HB35 | FAKKLAKLAKKLAKLAL-NH2 | 2 | 4 | 4 | 2 | 4 | 2 | 4 | 8 | 2 | 4 | 2 | 8 | 32 | 4 | 8 | 4 | 2 | 16 |
| HB43 | FAKLLAKLAKKLL-NH2 | 4 | 4 | 8 | 4 | 8 | 4 | 8 | 16 | 4 | 8 | 4 | 4 | 8 | 4 | 8 | — | 2 | 64 |
| HB55 | FAKLLAKALKKLL-NH2 | 4 | 4 | 8 | 8-16 | 8-16 | 16 | 8 | 32 | 8 | 8 | 4 | 4 | 16 | 8 | 16 | 16 | 2 | 64 |
| HB146 | KYKKALKKLAKLL-NH2 | 4 | 4 | 4 | 8 | 8 | 4 | 2 | 16 | 2 | 16 | 2 | 16 | 8 | 4 | 8 | 4 | 2 | 8 |
| HB153 | FALKALKKLKKALKKAL-NH2 | 4 | 8 | 8 | 8 | 8 | 16 | 4 | 16 | 8 | 16 | 4 | 8 | 16 | 4 | 4 | 4 | 4 | 128 |
| HBCPα2 | KWKKFIKKIGIGAVLKVLTTGLPALKLTKK-NH2 | 2 | 2 | 4 | — | — | — | 1 | — | — | — | — | 16 | — | — | — | — | — | — |
| HB504 | VAKKLAKLAKKLAKLALAL-NH2 | 64 | 64 | 32 | 8 | 8-16 | 8 | 8 | 16 | 8 | 8 | 8 | 32 | 16 | 4 | 8 | 8 | 4 | 64 |
| HBPM4 | RRWCFRVCYKGFCRYKCR-CONH2 | 4 | 4 | 8 | 32 | — | 8 | — | 64 | 4 | 8 | 4 | 4 | 64 | 4 | 2 | 4 | 0.25 | 64 |
| Tb | — | 1 | 2 | 8 | 1-2 | 16-32 | 0.13 | — | 4 | 0.06 | 0.25 | 0.06 | 8 | 8 | 1 | 1 | 8 | 2 | 128 |
E.c., E. coli UB1005; S.t., S. enterica serovar Typhimurium; PAO1, P. aeruginosa; Mu, mucoid P. aeruginosa; MD, multidrug-resistant P. aeruginosa; Tb-r, tobromycin-resistant P. aeruginosa; Ef., efflux mutants of P. aeruginosa H374 and H744 (Table 1); M76, M917, M1251, M1426, R70, multidrug-resistant P. aeruginosa clinical isolates; S.a., S. aureus; C.a., C. albicans; P.s., P. stutzri; P.f., P. fluorescens; P.p., P. pickettii; S.m., Stenotrophomonas maltophilia; A.x., Achromobacter xylosoxidans.
—, Not done.
Activity of selected peptides against mucoid and multidrug-resistant clinical isolates of Pseudomonas and other gram-negative pathogens associated with CF.
The clinical deterioration of chronic, progressive lung disease of CF has been associated with the appearance of the mucoid phenotype of P. aeruginosa. In addition, hospital isolates frequently demonstrate multidrug resistance (MDR) phenotypes as a consequence of the constant selective pressure of chronic antibiotic use (1). In addition, alternative gram-negative pathogens, including S. maltophilia and A. xylosoxidans, have become the most frequently isolated bacteria after P. aeruginosa from the lungs of CF patients (9, 23).
Table 2 also shows the results for the same peptides against clinical isolates of CF pathogens. The mean MIC of peptides against two mucoid clinical isolates of P. aeruginosa ranged from 2 to 32 μg/ml, which was comparable to the MIC of the same peptides against their nonmucoid isogenic counterparts. The two efflux pump overexpressing mutants, that were resistant to 5,000 μg/ml nalidixic acid, retained susceptibility to antimicrobial peptides with MICs of 2 to 8 μg/ml. Similarly, strains resistant to 16 to 32 μg/ml of tobramycin generally also showed similar susceptibility to antimicrobial peptides with MICs ranging from 8 to 16 μg/ml. Among the multidrug-resistant clinical isolates of P. aeruginosa that were resistant to β-lactams aminoglycosides and imipenem, represented by M76, M917, M1251, M1426, and R70, all peptides were highly effective, although elevated MICs were observed for M917.
All tested peptides were highly active against other Pseudomonas species isolated from sputum, including P. stutzeri, P. fluorescens, and P. pickettii, with MICs of 2 to 16 μg/ml. They also were demonstrated potent activity against S. maltophilia. Some peptides, including HBCM2, HB71, HB35, HB146, and HB301, were more active than others against Achromobacter xylosoxidans, which possesses intrinsic resistance to macrolide antibiotics as well as resistance to 128 μg/ml tobramycin.
Activity of peptides in the presence of salt, DNA, and mucin.
It has been speculated that the NaCl concentration may be elevated in CF airway surface liquid, resulting in ineffective defense of some endogenous antimicrobial peptides such as defensins and LL37 against bacteria (4, 16), although it is still disputed as to whether the ionic composition of the airway surface fluid is different between CF and healthy individuals (33, 35). It has been reported that the epithelial environment of CF lung has an NaCl concentration of 120 to 150 mM (33, 35) and a divalent cation concentration around 1 to 2 mM (2, 17). Another important factor in the CF lung is dead neutrophils present at concentrations around 1,000-fold higher than those observed for healthy individuals (10). Indeed, it has been proposed that the release of long, viscous DNA strands and glycoproteins from such dead cells are a principal cause of airway clogging and damage. Such polyanionic polymers which are found in CF sputum would also be anticipated to antagonize the activity of polycationic antibiotics due to electrostatic binding.
We therefore tested the effects of salts, DNA, and mucin on the activity of several peptides. Most peptides retained activity in salt, divalent cations, and DNA, with moderate two- to fourfold increases in MIC. In contrast, none of the peptides tested here were effective in the presence of 1% gastric mucin (Table 3). Tobramycin, however, was less antagonized by DNA or mucin than physiological salt concentrations (Table 3).
TABLE 3.
Activity of lead peptides against P. aeruginosa and S. aureus in the presence of salts, DNA (1 mg/ml), and 1% mucin
| Peptide | MIC (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
P. aeruginosa
|
S. aureus
|
|||||||||
| Normal | 150 mM NaCI | 1.7 mM CaCl2 | DNA | 1% Mucin | Normal | 150 mM NaCl | 1.7 mM CaCl2 | DNA | 1% Mucin | |
| HBCM2 | 4 | 16 | 16 | 32 | >128 | >128 | —a | — | — | — |
| HB12 | 16 | >128 | — | 64 | >128 | 4 | — | — | — | — |
| HB35 | 4 | 8 | 16 | 8 | >128 | 8 | 16 | 16 | 16 | >128 |
| HB43 | 8 | 16 | — | 16 | >128 | 4 | 4 | — | 8 | >128 |
| HB55 | 8 | 16 | 16 | 16 | >128 | 4 | 4 | 8 | 4 | >128 |
| HB71 | 8 | 64 | — | 128 | >128 | >128 | — | — | — | — |
| HB146 | 4 | 16 | 32 | 64 | >128 | 16 | >128 | >128 | >128 | >128 |
| HB153 | 8 | 16 | 32 | 16 | >128 | 8 | 128 | 32 | >128 | >128 |
| HB504 | 8 | 16 | — | 16 | >128 | 4 | 8 | — | 8 | >128 |
| HBPM4 | 8 | 16 | 16 | >128 | >128 | 8 | 16 | 16 | >128 | >128 |
| Tobramycin | 2 | 128 | — | 0.25 | 8 | >128 | >128 | — | >128 | >128 |
—, Not done.
HB71 and its D-amino acid isomer, HB71D, showed only marginal activity at 100 μg/ml against P. aeruginosa in 10% CF sputum, reducing bacterial counts by 50%. In contrast, in our hands the histatin-derivative P113D, that was considered for use in cystic fibrosis, caused less than 10% decrease in counts. Since HB71D (and P113D) was stable and resistant to protease degradation (data not shown), a result that was consistent with data published for D-isomers (27), we assume that this reflects antagonism by mucous. Using undiluted sputum none of these three peptides were active.
In vivo efficacy of antimicrobial peptides in a chronically infected rat lung model.
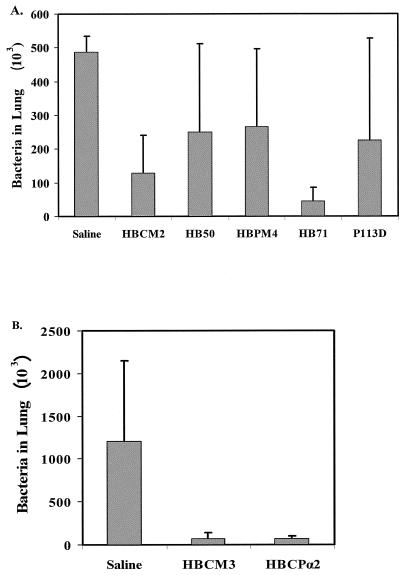
Intratracheal instillation of peptide allows for the use of significantly lower concentrations of antibiotic and is thus particularly useful in preliminary efficacy studies prior to aerosolization studies (24). Using such a delivery procedure, quantitative bacterial counts indicated that animals that received treatments of 100 μg of either HBCM2 or HB71 once daily for 3 days demonstrated, respectively, 74% (P < 0.05) and 91% (P < 0.01) reductions in total P. aeruginosa counts from 4.9 × 104 to 1.3 × 104 and 4.5 × 103, respectively, compared to the control group that received saline only during the same period of time (Fig. 1A). This compares favorably with the results of Omri et al. (24) who, using the same model, delivered, by intratracheal installation, a 500-μg dose of polymyxin B to the lungs of rats, leading to a therapeutic (87%) reduction in P. aeruginosa bioburden. In contrast, rats receiving HB50 and HBPM4 showed no significant reduction in cell counts in the lung (Fig. 2A).
FIG. 1.
In vivo efficacy assessment in rat chronic lung infection model. A. P. aeruginosa bioburden in the lungs of rats after three consecutive daily treatments via intratracheal instillation of peptide. Shown is the percentage of the number of CFU per milliliter of bronchoalveolar lavage recovered from the lungs treated daily with 100 μg of the named peptide relative to that for control animals treated with saline alone. B. P. aeruginosa bioburden in the lungs of rats after three consecutive daily treatments via nebulization of peptide.
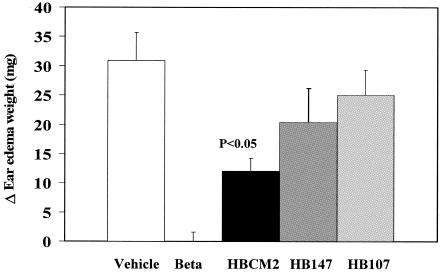
FIG. 2.
Effect of topical application of peptides in chronic phobol-ester-induced ear edema in mice. Anti-inflammatory activity is shown as reduction in milligram of ear edema weight in mice treated with peptides in comparison to a vehicle group. Bethamethasone (Beta), as a positive control, was applied at 0.3 mg/ear; peptides were applied at 20 μg/ear.
Nebulization, on the other hand, is more costly and requires larger amounts of peptide but is arguably more relevant to clinical applications. Peptides were delivered to the lung via nebulization in 3 daily doses following infection, resulting in an estimated lung delivery of 10 to 15 μg per dose. Compared to the control group, treated only with saline, which had lung P. aeruginosa cell counts of 1.2 × 106 ± 0.95 × 106, treatment with HBCM3 and HBCPα2 resulted in significant (P < 0.001 by unpaired Student t test) 94% reductions in bacterial load to 7.4 × 104 ± 6.7 × 104 and 6.8 × 104 ± 3.6 × 104, respectively (Fig. 1B).
Anti-inflammatory activity in an ear edema mouse model.
A major factor in CF lung disease is inflammation which appears to be responsible for a considerable amount of lung damage. It was previously demonstrated that a variety of peptides, including HBCM2, HBCM3, HBCPα2, and HBPM4 (28, 34), demonstrated activity in neutralizing endotoxin, which causes a clinical syndrome analogous to extreme inflammation. Therefore, we examined here if peptides have anti-inflammatory activity. Three peptides were selected for the mouse ear edema model. HBCM2 was chosen because previous work suggested that cecropin:melittin hybrid peptides have anti-endotoxic activity and can modulate innate immune responses (6, 28). HB146 was an alternative antimicrobial α-helical peptide, while HB107 was a nonantimicrobial α-helical peptide. As shown in Fig. 2, HBCM2 at 2 μg per ear significantly reduced phorbol-ester-induced edema. To a lesser extent, HB107 and HB147 appeared to marginally reduce inflammation.
Resistance induction in vitro.
P. aeruginosa was serially transferred daily in the presence of sub-MIC concentrations of HB50, HB153, HBPM4, and HB71. After 30 passages (Table 4), mean MICs were increased to a panel of 8 peptides by no more than fourfold. However, such resistance appeared to be transient, since a single passage of each resistant strain in the absence of peptide lead to reversion to near susceptible levels with less than twofold alterations in MIC compared to the original parent strain (data not shown). In contrast, serial transfers on chloramphenicol or rifampin had little effect on susceptibility to peptides while increasing resistance to the utilized drug by 8- to 16-fold.
TABLE 4.
Induction of cross-resistance measured as MIC (μg/ml) of each peptide against strain H187 treated for 30 passages with one-half the MIC of various peptides or antibiotics
| Peptide | MIC (μg/ml) for strain H187 trained on sub-MIC concentrations
|
||||||
|---|---|---|---|---|---|---|---|
| Untreated | HB50 trained | HB71 trained | HB153 trained | HBPM4 trained | CMa trained | Rifampin trained | |
| HB35 | 8 | 16 | 8 | 8 | 8 | 8 | 4 |
| HB64 | 16 | 64 | 32 | 64 | 32 | 32 | 16 |
| HB71 | 8-16 | 32 | 32 | 32 | 32 | 4 | 4 |
| HB146 | 4 | 16 | 16 | 32 | 16 | 8 | 4 |
| HB153 | 8 | 16 | 16 | 32 | 16 | 8 | 4 |
| HBCM2 | 8 | 32 | 16 | 4 | 16 | 8 | 8 |
| HBPM4 | 8 | 32 | 32 | 16 | 64 | 32 | 16 |
CM, chloramphenicol.
DISCUSSION
During the past decade, no new antibiotics have been approved for the treatment of pathogens in the CF patient lung. Thus, researchers have been exploring novel applications of currently available agents including aerosolized aminoglycosides and liposomally encapsulated drugs (5) as well as macrolides that lack activity against multidrug-resistant CF pathogens such as P. aeruginosa, B. cepacia, S. maltophilia, and A. xylosoxidans (26). In this study, in an attempt to develop therapeutics for CF, we screened a range of proprietary peptide molecules. The lead candidates were selected based upon optimal MICs against P. aeruginosa clinical isolates and other organisms involved in CF lung pathogenesis. The in vitro antimicrobial coverage of most peptides (Table 2) was superior to most conventional antibiotics (data not shown). In addition to bactericidal activity towards multiple microorganisms, some peptides also possessed potent anti-gram-positive and anti-Candida activity, an advantage, since those pathogens can be present in the CF lungs, and other antibiotics used in CF therapy, such as tobramycin, often lack useful gram-positive and fungal coverage.
Our animal model studies on selected peptides revealed the greater effectiveness, in accelerating bacterial clearance from the airways during chronic P. aeruginosa lung infection in rats, of certain gram-negative selective peptides, including HBCM2, HB71 (by intratracheal therapy), HBCM3, and HBCPα2 (by nebulization therapy), when compared to a broad-spectrum peptides such as HBPM4 (Fig. 2), despite similar MICs for P. aeruginosa. The extent of decrease in colony counts for the successful peptides compares favorably to the levels of protection afforded by the nonliposomally encapsulated polycationic antibiotics polymyxin B (24) and aminoglycosides (5 and D. E. Woods, unpublished data). The animal model employed is one of the most utilized animal models developed to study the pathogenesis of chronic P. aeruginosa infections and has been used to test the efficacy of a number of anti-pseudomonal antibiotics prior to clinical trials (5, 7, 24, 32). It involves the incorporation of P. aeruginosa into agar beads followed by intratracheal deposition into the lungs of rats to ensure a chronic infection, and animals have been studied up to 1 year following inoculation and remain chronically infected (32). For cationic peptides it represents a particularly stringent model as the anionic polysaccharide that makes up agar would be anticipated to antagonize the activity of the peptides. Indeed, it is well known that most peptides have no activity on solid medium-based MIC assays unless agarose is substituted for agar. The successful reduction of bioburden observed in this model indicates that either the biological half-life of peptides at the lung surface is sufficient to accelerate bacterial clearance despite the prospective susceptibility of peptides in general to degradation by high levels of active neutrophil elastase and other proteases present in the rat lungs, or that the peptides work in some other way, such as via immune enhancement (6). Indeed, with respect to the alternative mechanisms, other animal model data demonstrated that the peptides had anti-inflammatory activities. Thus, given that CF is complicated by a destructive inflammatory response that is associated with chronic infection by P. aeruginosa, the anti-inflammatory activity represents a major asset of such peptides.
The main method of combating lung infections in CF patients is antibiotic treatment (19). With the increasing life expectancy of such patients due to improved care, more courses of antibiotics are used for longer terms and at higher doses, with the adverse consequence of increasing antimicrobial resistance. The observation of lack of potential resistance and/or cross-resistance makes antimicrobial peptides extremely interesting candidates for use in CF therapy. After 30 passages in the presence of sub-MIC concentrations, changes in mean MICs of selected peptides against P. aeruginosa were only two- to fourfold greater than the initial MICs for most peptides. Also, no cross-resistance was observed to different peptides. This was inconsistent with the finding that resistance to the cationic lipopeptide colistin (a mixture of peptides polymyxins E1 and E2) is seldom seen, despite daily selective pressure in patients receiving colistin by inhalation (20, 31).
Our own data with P113D, a D-isomer of histatin 5, disagreed with an earlier study (27) that had suggested that while it did not possess activity in broth against P. aeruginosa, it was highly effective in sputum against this bacterium. In our hands, P113D, like HB71 and HB71D, when added at final concentration of 1 mg/ml to a pooled of undiluted sputum containing 107 CFU/ml of P. aeruginosa, was unable to effectively reduce bacterial counts. Reducing the sputum concentration to 10% and the peptide concentration to 100 μg/ml led to modest killing by HB71 and HB71D, but again P113D was completely ineffective. Our results were perhaps not surprising, since sputum contains as much as 12% mucin, and we observed that most peptides were ineffective in vitro in 1% of gastric mucin (Table 3). However, the lack of in vitro activity of peptides in sputum raises an interesting question as to whether this assay is clinically relevant, since we were able to demonstrate here that four such peptides were efficacious in vivo.
Acknowledgments
R.E.W.H. and D.E.W. were financially supported by the SPARx program of the Canadian Cystic Fibrosis Foundation and the Canadian Bacterial Diseases Network, and each are the recipients of a Canada Research Chair.
REFERENCES
- 1.Alonso, A., F. Rojo, and J. L. Martinez. 1999. Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ. Microbiol. 1:421-430. [DOI] [PubMed] [Google Scholar]
- 2.Baconnais, S., R. Tirouvanziam, J. M. Zahm, S. de Bentzmann, B. Peault, G. Balossier, and E. Puchelle. 1999. Ion composition and rheology of airway liquid from cystic fibrosis fetal tracheal xenografts. Am. J. Respir. Cell Mol. Biol. 20:605-611. [DOI] [PubMed] [Google Scholar]
- 3.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Investig. 103:1113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaulac, C., S. Sachetelli, and J. Lagace. 1999. Aerosolization of low phase transition temperature liposomal tobramycin as a dry powder in an animal model of chronic pulmonary infection caused by Pseudomonas aeruginosa. J. Drug Targeting 7:33-41. [DOI] [PubMed] [Google Scholar]
- 6.Bowdish, D. M. E., D. J. Davidson, and R. E. W. Hancock. 2004. The role of host defence and related peptides in immunity. Curr. Prot. Peptide Sci. 6:35-51. [DOI] [PubMed] [Google Scholar]
- 7.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 8.Chang, J., E. Blazek, M. Skowronek, L. Marinari, and R. P. Carlson. 1987. The antiinflammatory action of guanabenz is mediated through 5-lipoxygenase and cyclooxygenase inhibition. Eur. J. Pharmacol. 142:197-205. [DOI] [PubMed] [Google Scholar]
- 9.Clermont, D., C. Harmant, and C. Bizet. 2001. Identification of strains of Alcaligenes and Agrobacterium by a polyphasic approach. J. Clin. Microbiol. 39:3104-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duranton, J., D. Belorgey, J. Carrere, L. Donato, T. Moritz, and J. G. Bieth. 2000. Effect of DNase on the activity of neutrophil elastase, cathepsin G and proteinase 3 in the presence of DNA. FEBS Lett. 473:154-156. [DOI] [PubMed] [Google Scholar]
- 11.Finlay, B. B., and R. E. W. Hancock. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497-504. [DOI] [PubMed] [Google Scholar]
- 12.Ford, A. S., A. L. Baltch, R. P. Smith, and W. Ritz. 1993. In-vitro susceptibilities of Pseudomonas aeruginosa and Pseudomonas spp. to the new fluoroquinolones clinafloxacin and PD 131628 and nine other antimicrobial agents. J. Antimicrob. Chemother. 31:523-532. [DOI] [PubMed] [Google Scholar]
- 13.Gallo, R. L., M. Murakami, T. Ohtake, and M. Zaiou. 2002. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 110:823-831. [DOI] [PubMed] [Google Scholar]
- 14.Gaylor, A. S., and J. C. Reilly. 2002. Therapy with macrolides in patients with cystic fibrosis. Pharmacotherapy 22:227-239. [DOI] [PubMed] [Google Scholar]
- 15.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, M. Zasloff, and J. M. Wilson. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 16.Grimwood, K., M. To, H. R. Rabin, and D. E. Woods. 1989. Subinhibitory antibiotics reduce Pseudomonas aeruginosa tissue injury in the rat lung model. J. Antimicrob. Chemother. 24:937-945. [DOI] [PubMed] [Google Scholar]
- 17.Halmerbauer, G., S. Arri, M. Schierl, E. Strauch, and D. Y. Koller. 2000. The relationship of eosinophil granule proteins to ions in the sputum of patients with cystic fibrosis. Clin. Exp. Allergy 30:1771-1776. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E. W., and A. Patrzykat. 2002. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug. Targets Infect. Disord. 2:79-83. [DOI] [PubMed] [Google Scholar]
- 19.Hodson, M. E. 1995. Maintenance treatment with antibiotics in cystic fibrosis patients. Sense or nonsense? Neth. J. Med. 46:288-292. [DOI] [PubMed] [Google Scholar]
- 20.Jensen, T., S. S. Pedersen, S. Garne, C. Heilmann, N. Hoiby, and C. Koch. 1987. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J. Antimicrob. Chemother. 19:831-838. [DOI] [PubMed] [Google Scholar]
- 21.Koczulla, A. R., and R. Bals. 2003. Antimicrobial peptides: current status and therapeutic potential. Drugs 63:389-406. [DOI] [PubMed] [Google Scholar]
- 22.Lester, A., and J. J. Andreasen. 1988. In vitro susceptibility of Pseudomonas aeruginosa from bacteremic and fibrocystic patients to four quinolones and five other antipseudomonal antibiotics. Scand. J. Infect. Dis. 20:525-529. [DOI] [PubMed] [Google Scholar]
- 23.Minkwitz, A., and G. Berg. 2001. Comparison of antifungal activities and 16S ribosomal DNA sequences of clinical and environmental isolates of Stenotrophomonas maltophilia. J. Clin. Microbiol. 39:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omri, A., Z. E. Suntres, and P. N. Shek. 2002. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 64:1407-1413. [DOI] [PubMed] [Google Scholar]
- 25.Rajan, S., and L. Saiman. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47-56. [DOI] [PubMed] [Google Scholar]
- 26.Saiman, L., and J. Siegel. 2003. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am. J. Infect. Control. 31:S1-S62. [PubMed] [Google Scholar]
- 27.Sajjan, U. S., L. T. Tran, N. Sole, C. Rovaldi, A. Akiyama, P. M. Friden, J. F. Forstner, and D. M. Rothstein. 2001. P-113D, an antimicrobial peptide active against Pseudomonas aeruginosa, retains activity in the presence of sputum from cystic fibrosis patients. Antimicrob. Agents Chemother. 45:3437-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, M. G., H. Yan, and R. E. W. Hancock. 1999. Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect. Immun. 67:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trezise, A. E., C. Szpirer, and M. Buchwald. 1992. Localization of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) in the rat to chromosome 4 and implications for the evolution of mammalian chromosomes. Genomics 14:869-874. [DOI] [PubMed] [Google Scholar]
- 31.Unertl, K., G. Ruckdeschel, H. K. Selbmann, U. Jensen, H. Forst, F. P. Lenhart, and K. Peter. 1987. Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med. 13:106-113. [DOI] [PubMed] [Google Scholar]
- 32.Woods, D. E., P. A. Sokol, L. E. Bryan, D. G. Storey, S. J. Mattingly, H. J. Vogel, and H. Ceri. 1991. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J. Infect. Dis. 163:143-149. [DOI] [PubMed] [Google Scholar]
- 33.Zabner, J., J. J. Smith, P. H. Karp, J. H. Widdicombe, and M. J. Welsh. 1998. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell. 2:397-403. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, L., M. G. Scott, H. Yan, L. D. Mayer, and R. E. W. Hancock. 2000. Interaction of polyphemusin I and structural analogs with bacterial membranes, lipopolysaccharide, and lipid monolayers. Biochemistry 39:14504-14514. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Y., and J. F. Engelhardt. 1999. Airway surface fluid volume and Cl content in cystic fibrosis and normal bronchial xenografts. Am. J. Physiol. 276:C469-C476. [DOI] [PubMed] [Google Scholar]