Abstract
Background
The detection of tumor localization is difficult in robotic surgery because surgeons have no sense of touch and rely on visual information. This study aimed to evaluate the efficacy of preoperative CT-guided dye marking of lung nodules prior to robotic surgery.
Methods
Patients undergoing CT-guided dye marking prior to robotic surgery at our hospital between September 2019 and April 2024 were retrospectively analyzed.
Results
Thirty lung nodules from 29 patients were analyzed. The dye marking procedure was successfully completed. Indigo carmine and indocyanine green were used for 20 and 10 pulmonary nodules, respectively. Slight pneumothorax was the most common complication and occurred in 6 patients (20.7%), none of whom required chest tube placement. Dye marking was visualized in 29/30 (96.7%) nodules and one nodule had poor intraoperative visualization due to severe adhesions. One patient underwent open thoracotomy because of difficulty ventilating one lung. Fourteen patients underwent wide wedge resection and 16 patients underwent segmentectomy for the target nodules. All target nodules were successfully resected with negative margins.
Conclusions
CT-guided dye marking of small pulmonary nodules prior to robotic surgery appears feasible and safe. This procedure can facilitate the performance of robotic sublobar resection.
Keywords: Lung cancer, Robotic surgery, Ground glass opacity, Localization
Background
The number of cases of subloblar resection for small lung cancer has been increasing due to two recent large clinical studies that have shown that subloblar resection is associated with an equivalent or higher survival rate to standard lobectomy in patients with small lung cancers [1, 2]. This increase is also due to the improvement in CT performance. Robotic surgery has been reported to have advantages over open thoracotomy in terms of pain and shorter hospital stay [3]. Accordingly, the number of robotic surgeries has recently increased. Therefore, the number of robot-assisted sublobar resections are expected to increase. However, it is important to detect the tumor location and achieve the resection margins in robotic surgery because surgeons do not have a sense of touch and must rely on visual information. To achieve adequate resection margins, tumor localization is crucial in robotic surgery. Moreover, various methods for intraoperative tumor localization have been reported for open thoracotomy and video-assisted thoracoscopic surgery (VATS); however, there are few reports on robot-assisted thoracoscopic surgery (RATS). We present our CT-guided dye marking procedure, which is performed prior to RATS, and evaluate the results of this procedure.
Methods
Patients who underwent percutaneous CT-guided dye marking prior to robot assisted thoracoscopic surgery at Sakai City Medical Center Hospital from September 2019 to April 2024 were analyzed. It was suspected that the pulmonary lesions would be difficult to identify intraoperatively during robotic surgery because of their small size, deep location, and the presence of pure GGOs without pleural changes. The surgical indications for small lung lesions included persistence of a nodule with interval growth and, development of solid component in a nodule with ground-glass opacity (GGO), confirmation of the diagnosis (i.e., metastatic lesion, multiple primary lung cancer, or some other benign tumor). All patients who underwent wide wedge resection and were eligible for CT-guided dye marking included cases of synchronous ipsilateral multiple lung lesions. Robotic anatomical lung resection was planned for the main lesion. Lesions eligible for wide wedge resection after CT-guided dye marking included 2nd smaller lesions, including tumors with a maximum diameter of < 2.0 cm, GGO dominant tumor, and tumors located in the outer one-third where wide wedge resection was possible. In segmentectomy cases, CT-guided dye marking was indicated in cases in which the margin distance between the tumor and intersegmental plane was expected to be less than the maximum tumor diameter on preoperative thin slice CT or 3D-CT using Synapse Vincent (Fujifilm CO., Tokyo, Japan). The tumor margin we aimed was relative to tumor size, so it was set to be greater than the maximum tumor diameter [4]. Tumor margins were confirmed macroscopically during surgery. The patient data were retrospectively collected, and this retrospective study was approved by the Institutional Review Board (Control Number 24-440). Written informed consent for the procedures was obtained from each patient.
Computed tomography fluoroscopy guided color marking procedure
All dye-marking procedures were performed on the day of robotic surgery. Dye marking was performed using CT-fluoroscopy according to the Japanese Society of Interventional Radiology guidelines [5]. Before the procedure, 5 mL of a 4:1 mixture of dye (indigo carmine or indocyanine green) and iopamidol was prepared. After reviewing prior CT images, the patient was placed in a position that was consistent with the planned needle path. First, chest CT was performed to confirm the location of the lung lesion. Local anesthesia was performed with 1% lidocaine using a 6-cm 23-gauge needle (Terumo Cattelan Needle, Terumo Corp, Tokyo, Japan). The needle was gradually advanced by the target lung nodule as closely as possible, and 5 mL of the mixture was injected into the lung parenchyma while withdrawing the needle until iopamidol accumulation reached the pleural surface on real-time CT fluoroscopy (Fig. 1a, b). Chest CT was repeatedly performed after the procedure to evaluate the marking position and complications. The patients were then transferred to the operating room.
Fig. 1.
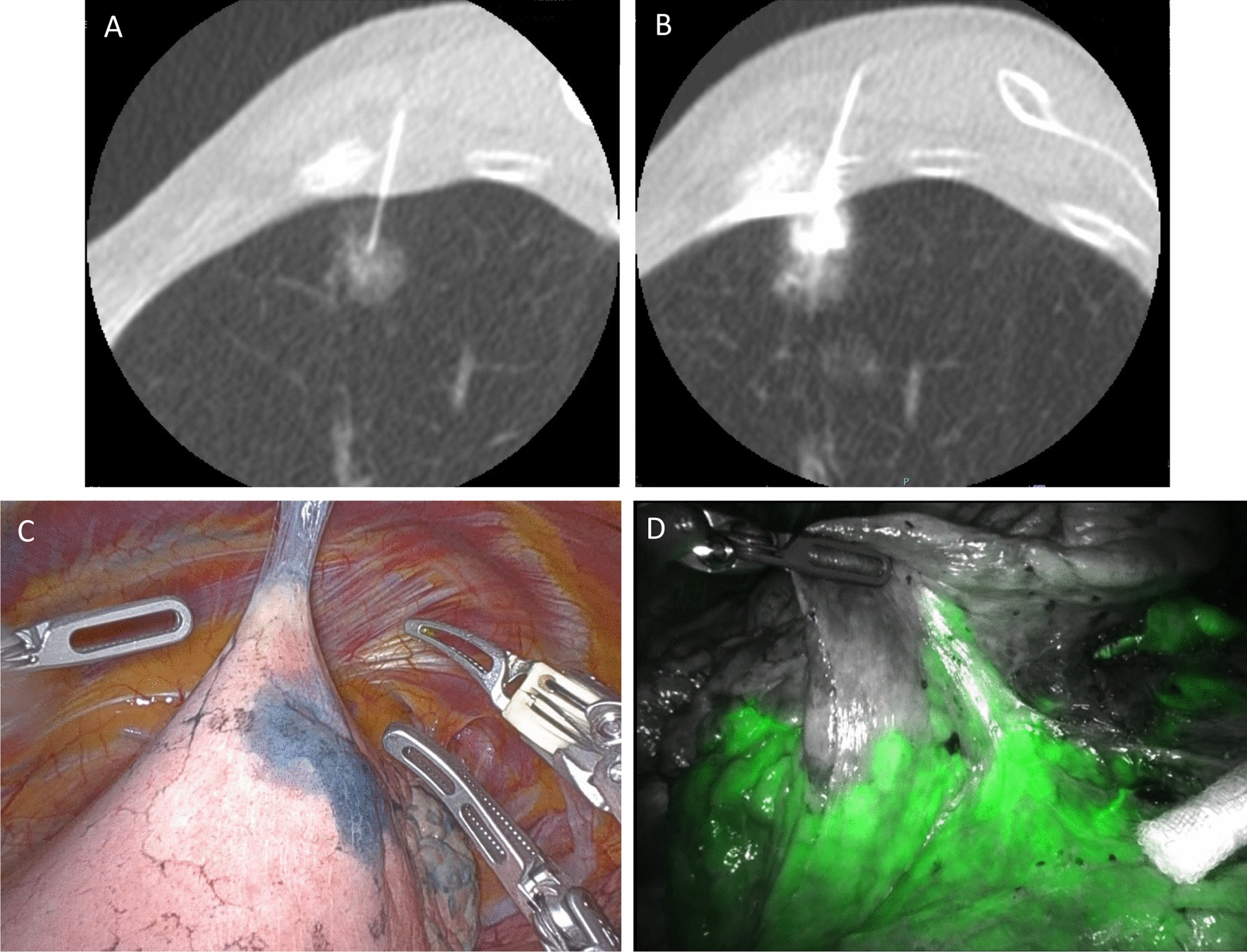
A 77-year-old-woman with a part-solid ground-glass nodule that was diagnosed as adenocarcinoma by computed tomography-guided biopsy underwent robotic left upper segmentectomy. a A 23G needle was inserted into the lung parenchyma as close as possible to the nodule. b Five milliliters of a 4:1 mixture of dye indigo carmine and iopamidol was injected. c Images taken during robotic- surgery, showing 1 area in the left upper lobe that was clearly stained after computed tomography fluoroscopy-guided dye localization. d Intravenous indocyanine green delineation of the intersegmental plane between the lingula and left upper lobe segments illuminated by the Firefly
Robotic-assisted thoracoscopic surgery procedure
All surgeries were performed under robot-assisted thoracoscopic guidance, with the patient placed in a lateral decubitus position under general anesthesia with one-lung ventilation using a double-lumen endobronchial tube. The Da Vinci Xi system (Intuitive Surgical, Sunnyvale, CA) was used, and surgical procedures were performed using complete robotic portal pulmonary resection with 4 robotic arms and a CO2 insufflation system at 8 mmHg. Port placement in the 8th and 9th intercostal space was adopted, and an assistant port was placed in the 10th intercostal space. The robotic instruments included a Cadiere, Long bipolar Grasper and Tip-Up Fenestrated Grasper. A vessel-sealing system (e.g., Vessel Sealer Extend) was frequently used to cut and seal thick tissues and small blood vessel branches. Da Vinci staplers (e.g., EndoWrist Staplers and SureForm) were used to staple the pulmonary arteries, veins, and bronchus, and to divide the lung parenchyma.
Results
From our data, we identified 30 lung nodules in 29 patients who underwent CT-guided dye marking prior to robotic surgery. The clinical and nodule characteristics and surgical outcomes of the patients are summarized in Table 1. Five nodules were solid, 18 were pure ground-glass, and 7 were part-solid ground-glass.
Table 1.
Clinical information, lung nodule characteristics and surgical outcomes in patients who received CT-guided dye localization before RATS
| Variable | N |
|---|---|
| Patient (n = 29) | |
| Age, median (year, range) | 69 (42–79) |
| Sex | |
| Female | 19 |
| Male | 10 |
| Smoking status | |
| Current smoker | 2 |
| Ex-smoker | 12 |
| Never smoker | 15 |
| BrinkmanIndex, median (range) | 0 (0–2150) |
| No. of patients with | |
| 1 nodule | 28 |
| 2 nodules | 1 |
| Nodule (n = 30) | |
| Tumor characteristics | |
| Solid | 5 |
| Part solid | 7 |
| Pure GGO | 18 |
| Tumor size, median (mm, range) | 8 (3–21) |
| Depth from pluera, median (mm, range) | 14 (4–45) |
| Location | |
| Rt S2 | 3 |
| Rt S3 | 1 |
| Rt S6 | 2 |
| Rt S8 | 5 |
| Rt S9 | 5 |
| Lt S1 + 2 | 7 |
| Lt S6 | 6 |
| Lt S10 | 1 |
| Dye material | |
| Indocyanine green | 10 |
| Indio carmine | 20 |
| Marking time, median (mim, range) | 12 (4–26) |
| Complications, yes | 6 (pneumothorax) |
| Type of resection for marked lesion | |
| segmentectomy | 16 |
| wide wedge resection | 14 |
| Conversion to thoracotomy, yes | 1 |
| Histopathology | |
| AAH | 3 |
| AIS | 15 |
| IA | 4 |
| MIA | 3 |
| Lymph node | 3 |
| Fibrous nodule | 1 |
| Epithelial granulomas | 1 |
All 30 dye marking procedures in 29 patients, were all successfully completed. The median time of the marking procedure (from injection of local anesthesia to confirmation CT after color marking) was 12 min (range 4–26). Slight pneumothorax was the most common complication and occurred in 6 patients (20.7%); however, no patients required chest tube placement. No complications requiring intervention or procedure-related deaths occurred. The median time from the end of marking to the start of surgery was 57 min (range: 43–158 min). One patient who had previously undergone a left upper lobectomy and wedge resection of the left lower lobe required conversion to thoracotomy because the patient was unable to ventilate the differential lung. During surgery, the dye-marked area was visualized in 29/30 (96.7%) lesions. There was one lesion involving intraoperative total adhesion, in which the dye-marked area could not be visualized. All the target lesions were successfully resected. Wide-wedge resection was performed in 14 patients, and segmentectomy was performed in 16 patients with marked lesions. In all marked lesions, surgical margins were confirmed with greater than the maximum tumor diameter during surgery. The pathological diagnoses included 25 lung cancers and 5 benign tumors and the surgical margins were pathologically negative in all marked lesions.
Discussion
The present study demonstrated that CT fluoroscopy-guided dye marking prior to robotic pulmonary resection was associated with high technical and surgical success rates.
The most important advantage of this method is the simplicity and ease of tumor localization during robotic surgery. This procedure can facilitate the performance of robotic sublobar resection. In robotic surgery, surgeons have no tactile sense and rely solely on visual information. The surgeon found a lung nodule along the trace of the dye at the lung surface, and resection of the lung nodule was performed smoothly suing robotic surgery. Our surgical success rate was as high as that reported in previous studies [6, 7]. In addition, this method does not require additional devices during surgery. Thus far, various techniques such as Lipiodol mixture, hook wire, microcoil, ultrasound radiotracer, and radiofrequency identification (RFID) have been developed for the localization of lung nodules during VATS; however, there are few reports on robot-assisted surgery. There is a risk of dislodgement of the hook-wire before surgery, and higher complication rates have been reported [8, 9]. Lipiodol localization requires an intraoperative fluoroscopic c-arm which cannot be performed while the robot is docking [10]. Radiotracer and RFID marking require the insertion of specialized probes through the assistant port, which may limit manipulation because the assistant port is located relatively more caudally than in VATS [11, 12]. Intraoperative ultrasound also requires a skilled patient-side assistant [11]. In comparison to these techniques, dye marking is simple and easy to use to identify the tumor location and facilitates the performance of robotic surgery without requiring additional devices.
The disadvantages of dye localization include rapid diffusion and poor visualization in cases with intraoperative adhesion. The dye diffuses and is absorbed over time; thus, dye marking procedures must be performed on the day of robotic surgery, and the surgery should then be performed as soon as possible after marking. Even during surgery, the dye fads over time. When necessary, we intraoperatively performed suture marking at the dye-area at the start of the console. In this study, 1 lesion exhibited poor visualization of dye marking. This was a case in which full adhesion was observed intraoperatively, in which the visceral pleura was relatively thick. Wide-wedge resection was planned for the marked lesion; however, dye marking could not be visualized after detachment of the adhesions. Because the puncture site on the thoracic wall could be identified, the lesion could be resected as planned. If marking fails, the target nodules can be detected by examining the thoracic wall. Moreover, the rates of complications requiring intervention was reported to be 0–2% after CT-guided marking with dye materials [7, 13]. Our results are similar to these reports. There were no severe complications, such as air embolism, that could have suspended the surgery and led to a fatal result. To avoid the potential risk of air embolism, patients were not allowed to assume a head-up position. Furthermore, iopamidol, a water-soluble material, was used as a contrast agent and relatively small-gauge needles were used in CT-guided dye localization, which minimizes the risk of complications.
Several dyeing materials (e.g., indocyanine green [ICG], methylene blue, indigo carmine, and patent blue V) have been reported [7, 13–16]. Although these are widely used, there are concerns regarding allergies [16]. No side effects related to indocyanine green (ICG) and indigo carmine were observed. In segmentectomy, the corresponding vessels and bronchus are resected, and the lung parenchyma is divided using a stapler. During division of the lung parenchyma, surgeons must identify the intersegmental plane while achieving an appropriate resection margin. In the da Vinci Xi robotic system, the robotic camera is equipped with near-infrared ray technology (Firefly, Intuitive Surgical, Sunnyvale, CA) which illuminates ICG- perfused tissue after intravenous administration. The intravenous administration of ICG and Firefly help to detect the intersegmental plane after resection of the corresponding segmental arteries and veins [14, 15]. Considering these findings, we propose that dye materials other than ICG are preferable for tumor localization marking when using Firefly because the use of ICG for both tumor localization and detection of the intersegmental plane could be confusing. For these reasons, we select indigo carmine for identifying tumor localization in segmentectomy cases, suggesting that tumor localization and intersegmental plane need to be in a different color. Appropriate resection margins can be ensured by identifying the tumor localization with indigo carmine (Fig. 1c) and the intersegmental plane with the intravenous administration of ICG and Firefly (Fig. 1d). Therefore, the dye material should be carefully selected according to the type of surgery.
In recent years, the detection of multiple GGOs or multiple primary lung cancers has increased owing to the advent of CT technology and lung cancer screening programs. However, the standard surgical management or appropriate surgical procedure has not been established [17, 18]. Our treatment strategy for ipsilateral multiple lesions is to perform a single-stage operation and preserve the lung function while ensuring curability. In cases of ipsilateral multiple lung lesions, we performed CT-guided dye marking in cases where robotic anatomical pulmonary resection was planned for the primary lesion and wide wedge resection was planned for the secondary lesion. Moreover, CT-guided dye marking allows the administration of dyes at multiple locations [19]. We experienced one case of CT-guided dye marking of two lung lesions and performed S8 segmentectomy and S2 wide wedge resection. Both tumors were successfully resected, with negative tumor margins. Therefore, CT-guided dye marking prior to robotic surgery could be an option when performing surgery for ipsilateral multiple lung lesions.
The present study was associated with several limitations. First, it was a retrospective, single-institutional study, and a selection bias could not be avoided. Second, sample size was small. Third, our dye-marking procedures were performed by highly experienced interventional radiologists. Further studies should prospectively examine more cases, and including those of interventional radiologists.
Conclusions
In conclusion, CT-guided dye marking of small lung nodules prior to robotic surgery appears feasible and safe. This procedure can facilitate the performance of robotic sublobar resection.
Abbreviations
- CT
Computed tomography
- VATS
Video-assisted thoracoscopic surgery
- RATS
Robot-assisted thoracoscopic surgery
- CO2
Carbon dioxide
- RFID
Radiofrequency identification
- ICG
Indocyanine green
- GGO
Grand glass opacity
- Rt
Right
- Lt
Left
- AAH
Atypical adenomatous hyperplasia
- AIS
Adenocarcinoma in situ
- IA
Invasive adenocarcinoma
- MIA
Minimally invasive adenocarcinoma
Author contributions
YY: Data curation, Data analysis, Manuscript writing, NI: Data curation, Supervision, MN: Data curation
Availability of data and materials
All the data used in the present study are preserved in Department of General Thoracic Surgery, Sakai City Medi cal Center and are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The patient data were retrospectively collected, and this retrospective study was approved by the Institutional Review Board (control number 24 440). Wri tten informed consent for the procedures was obtained from each patient.
Consent for publication
This study has no information by which an individual could be identified. Then,written consent for publication was waived. All the patients have the right to opt out of being included in research studies, way of to do so is written in the webpage of our Department.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-seed peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-lable, phase 3, randomized, controlled, non-inferiority trial. Lancet. 2022;399:1607–17. [DOI] [PubMed] [Google Scholar]
- 2.Altorki NK, Wang X, Kozono D, Watt C, Landreneau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiolfi A, Nosotti M, Micheletto G, Khor D, Bonitta G, Perali C, et al. Pulmonary lobectomy for cancer: systemic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery. 2021;169:436–46. [DOI] [PubMed] [Google Scholar]
- 4.Sawabata N, Ohta M, Matsumura A, Nakagawa K, Hirano H, Maeda H, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2004;77:415–20. [DOI] [PubMed] [Google Scholar]
- 5.Japanese society of interventional radiology guideline. https://www.jsir.or.jp/docs/guideline/CT_seiken2006.pdf (Japanese)
- 6.Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151:316–28. [DOI] [PubMed] [Google Scholar]
- 7.Vadoni RE, Cuttat JF, Wichy S, Suter M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14:265–70. [DOI] [PubMed] [Google Scholar]
- 8.Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K, et al. Preoprative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002;225:511–8. [DOI] [PubMed] [Google Scholar]
- 9.Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del Maschio A, Zannini P. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardio Thorac Surg. 2004;25:429–33. [DOI] [PubMed] [Google Scholar]
- 10.Kawanaka K, Nomori H, Mori T, et al. Marking of small pulmonary nodules before thoracoscopic resection. Acad Radiol. 2009;16:39–45. [DOI] [PubMed] [Google Scholar]
- 11.Udelsman BV, Blasberg JD. Using the robotic platform in the therapy of multifocal ground glass opacities. J Surg Oncol. 2023;127:262–8. [DOI] [PubMed] [Google Scholar]
- 12.Yutaka Y, Sato T, Hidaka Y, Kato T, Kayawake H, Tanaka S, et al. Electromagnetic navigation bronchoscopy-guided radiofrequency identification marking in wedge resection for fluoroscopically invisible small lung lesions. Eur J Cardiothorac Surg. 2022;63:ezad006. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Kuroda H, Sato Y, Matsuo K, Sakata S, Yashiro H, et al. The utility of indigo carmine and lipiodol mixture for preoperative pulmonary nodule localization before video-assisted thoracic surgery. J Vasc Interv Radiol. 2019;30:446–52. [DOI] [PubMed] [Google Scholar]
- 14.Voulaz E, Giudici VM, Lanza E, Bottoni E, Cariboni U, Crepaldi A, et al. Percutaneous computed tomography-guided localization with indocyanine green for the thoracoscopic resection of small pulmonary nodules. J Clin Med. 2023;12:6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari-Light D, Geraci TC, Sasankan P, Cerfolio RJ. The utility of near-infrared fluorescence and indocyanine green during robotic pulmonary resection. Front Surg. 2019;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JR, Tseng YH, Lin MW, Chen HM, Chen YC, Chen MC, et al. Safety and efficacy of computed tomography-guided dye localization using patent blue V for single lung nodule for video-assisted thoracoscopic surgery: a retrospective study. Ann Transl Med. 2019;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa Y, Nakayama H, Ito H, Yokose T, Tsuboi M, Nishii T, et al. Surgical treatment for synchronous primary lung adenocarcinomas. Ann Thorac Surg. 2014;98:1983–8. [DOI] [PubMed] [Google Scholar]
- 18.Yu YC, Hsu PK, Yeh YC, Huang CS, Hsieh CC, Chou TY, et al. Surgical results of synchronous multiple primary lung cancers similar to the stage matched solitary primary lung cancers? Ann Thorac Surg. 2013;96:1966–74. [DOI] [PubMed] [Google Scholar]
- 19.Lin CY, Chang CC, Huang LT, Chung TJ, Liu YS, Yen YT, et al. Computed tomography-guided methylene blue localization: single versus multiple lung nodules. Front Med. 2021;8:661956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used in the present study are preserved in Department of General Thoracic Surgery, Sakai City Medi cal Center and are available from the corresponding author on reasonable request.


