Abstract
Background
Pulmonary function is increasingly recognized as a key factor in metabolic diseases. However, its link to gout risk remains unclear. The study aimed to investigate the relationship between pulmonary function and the risk of developing gout and the underlying biological mechanisms.
Methods
Our study included 420,002 participants with complete pulmonary function data from the UK Biobank. Logistic regression was used to evaluate gout prevalence among individuals with different pulmonary function statuses. Propensity score matching (PSM) created balanced groups, while Cox regression gauged the risk association between reduced lung capacity and gout compared with normal function. Mendelian randomization (MR) analysis was used to verify causal associations. Non-linear correlations were assessed with restricted cubic spline (RCS) analysis, and mediation analysis was used to explore the role of blood biomarkers. Mediation analyses were used to investigate the potential mediating role of biomarkers in the association.
Results
Cross-sectional analysis revealed a higher prevalence of gout in individuals with preserved ratio of impaired spirometry (PRISm) of 6.31% and chronic obstructive pulmonary disease (COPD) of 6.26% than in those with normal pulmonary function (3.45%). After adjustment for covariates, both PRISm (odds ratio [OR] 1.24, 95% confidence interval [CI] 1.17–1.31) and COPD (OR 1.14, 95% CI 1.07–1.22) were significantly associated with gout. Longitudinal analysis confirmed that impaired pulmonary function significantly increased the risk of developing gout (hazard ratio [HR] 1.32, 95% CI 1.24–1.40). MR further revealed a potential causal effect of decreased pulmonary function on an increased risk of gout. Subgroup analysis revealed significant interactions between impaired pulmonary function and several factors, including body mass index (BMI), levels of physical activity, and diabetes status, in their associations with the risk of gout. RCS analysis showed a nonlinear relationship between pulmonary function indicators and gout incidence, characterized by an inverse S-shaped curve. Mediation analysis revealed that urate levels (49.1% mediation proportion), C-reactive protein (CRP) levels (6.62%), monocyte counts (1.33%), and neutrophil counts (4.85%) significantly mediated the relationship between pulmonary function and the risk of gout.
Conclusions
Our study revealed a significant association between impaired pulmonary function and an increased risk of developing gout. The association might be partially mediated by biomarkers including urate levels, inflammatory markers, and immune cell counts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03836-8.
Keywords: Gout, Pulmonary function, UK Biobank, Spirometry, Mediation analysis, Urate levels, Inflammation, Immune cell counts
Background
Gout is a metabolic disorder characterized by the deposition of monosodium urate crystals in joints, posing a worldwide health concern [1]. According to epidemiological studies, the prevalence of gout worldwide has increased from 1 to 6.8%, with the incidence rising in parallel with the prevalence of obesity and metabolic syndrome [2]. This condition leads to considerable morbidity and reduced quality of life, imposing a substantial economic burden on affected individuals and society [3].
The pathophysiology of gout involves elevated blood uric acid levels resulting from overproduction or underexcretion, which leads to urate crystal formation in the peripheral joints. Urate crystals initiate an NOD-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome-driven inflammatory response and pro-inflammatory cytokines, which are the hallmarks of acute gout attacks [4]. With the evolution of our understanding of uric acid metabolism and gout, it has become clear that the condition is multifactorial and influenced by genetic, environmental, and lifestyle factors.
Pulmonary function impairment, including chronic obstructive pulmonary disease (COPD) and preserved ratio impaired spirometry (PRISm), is known to increase the risk of various chronic diseases. Research has shown that reduced pulmonary function may trigger systemic inflammation, oxidative stress, and altered metabolic profiles, leading to the development of metabolic and immune disorders [5–7]. For example, COPD patients often display increased inflammatory markers and higher circulating uric acid levels, suggesting a potential overlap between respiratory and metabolic dysfunction [8, 9]. Rheumatoid arthritis (RA) was found to be associated with increased odds of both restrictive and obstructive pulmonary patterns [10]. PRISm can influence plasma metabolites, thereby promoting the onset of type 2 diabetes [11]. Impaired pulmonary function is also associated with macrophage activation, potentially accelerating inflammation progression [7]. However, as an inflammatory and metabolic disease, the connection between gout and pulmonary function is poorly understood, and there is a paucity of large-scale longitudinal studies to confirm these potential links.
The UK Biobank, a large-scale database, offers a valuable opportunity to comprehensively investigate this relationship [12, 13]. By leveraging its extensive resources and longitudinal follow-up data, we aimed to elucidate the association between pulmonary function and the risk of gout. Our study employed a robust research design that included spirometry measurements, assessment of clinical outcomes, and analysis of potential mediators via blood biomarkers. Mendelian randomization (MR) analysis was used to verify causal associations [14]. We hope to elucidate the relationship between pulmonary function and gout and identify potential mediators that may link impaired pulmonary function with an increased risk of developing gout.
Methods
Research design and participants
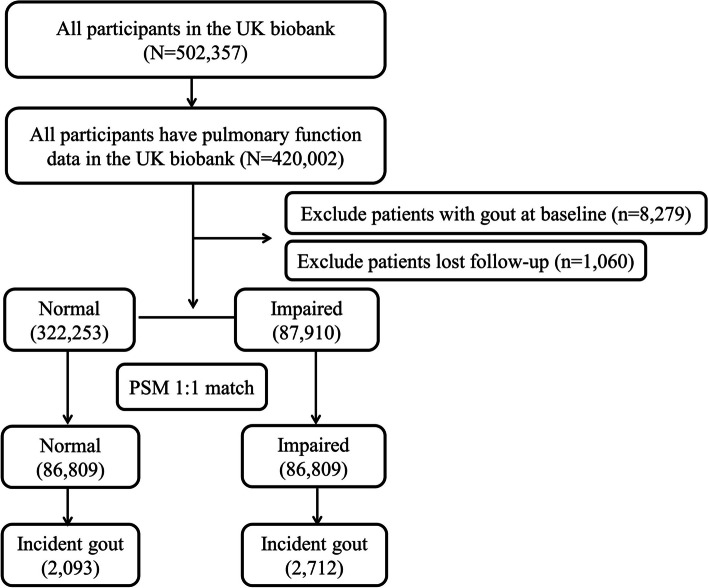
Our study included participants from the UK Biobank (https://www.ukbiobank.ac.uk/), a comprehensive longitudinal cohort study initiated between 2006 and 2010. It includes a wide demographic, enrolling over 500,000 individuals aged between 37 and 73 years from various locations in England. Initially, these participants provided detailed health and lifestyle data through structured questionnaires and participated in physical assessments and biological sampling at one of 22 designated centers [15]. The UK Biobank consistently updates morbidity and mortality data by integrating information from national health registries. We included 420,002 participants whose pulmonary function data were fully documented (Additional file 1: Table S1). The detailed selection process is illustrated in Fig. 1. Ethical approval for this study was granted by the UK Biobank Ethics Committee (approval number 106397), which adheres to the Research Tissue Bank standards.
Fig. 1.
Flow diagram of participants included in the study. PSM: Propensity score matching
Spirometry measurement techniques
Spirometry was conducted by professionally trained personnel using the Vitalograph Pneumotrac 6800 spirometer during the initial data collection phase, adhering to a rigorously standardized protocol [16]. Each participant was required to complete two to three forced exhalations of a minimum 6-s duration within 6 min, with the spirometer calibrated before each session. Only the highest recorded values were considered for further analysis if the first two attempts were within 5% variability; otherwise, the third attempt was conducted. The parameters of forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were calculated as a percentage of the predicted values based on the Global Lung Initiative 2012 norms [17, 18]. Definitions were applied to categorize participants into groups: COPD was defined as an FEV1 less than 80% predicted and an FEV1/FVC ratio less than 0.7; PRISm, a concept assessing pulmonary function impairment, was identified as an FEV1 less than 80% predicted but an FEV1/FVC ratio equal to or greater than 0.7 [19]; and participants with normal spirometry results had FEV1 and FEV1/FVC ratios equal to or exceeding 80% and 0.7, respectively.
Clinical outcomes
Gout status was defined using the International Classification of Diseases (ICD), Tenth Revision, code M10, which was extracted from the first occurrence variables in the UK Biobank. Briefly, gout diagnosis information was obtained predominantly from primary care records, hospital admissions, and self-reported health conditions. The follow-up time was calculated from the baseline interview date to the date of incident gout diagnosis, death, or the end of follow-up on August, 2023, whichever occurred first.
Covariates
We considered various factors, including sociodemographic characteristics, lifestyle factors, dietary habits, and comorbidities, as covariates to address potential confounding. The sociodemographic factors included continuous age, sex (male/female), ethnicity (white, Asian/Asian British, black/black British, mixed, and others), and socioeconomic status. The latter was derived using the Townsend index based on the postcode of residence. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) and categorized according to the World Health Organization criteria: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2). Smoking status was classified into never smoked, ever smoked, or currently smoking. Alcohol intake frequency was assessed using a questionnaire and categorized into three levels from never to daily. Physical activity was measured through total metabolic equivalent task (MET) minutes of all exercise during the previous week and categorized into four levels: none, < 600 MET minutes/week, 600–3000 MET minutes/week, and ≥ 3000 MET minutes/week. Comorbidities were confirmed through a combination of self-reported medical history, review of medication records, and inpatient diagnostic reports. Dietary information was obtained through the food frequency questionnaire (FFQ), which provides a comprehensive overview of participants’ usual dietary intake. Given that fish and meat are primary dietary triggers for gout due to their high purine contents, we focused on these food groups. The intake of meat and fish was scored based on their frequency: oily fish, non-oily fish, processed meat, and unprocessed meat (including poultry, beef, lamb, and pork).
MR analysis
We obtained publicly available genome-wide association study (GWAS) data for lung function indicators and gout from the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/). The specific IEU IDs for the lung function indicators are as follows: ebi-a-GCST90029027-finn-b for FVC, ukb-a-235-finn-b for FEV1, ukb-a-337-finn-b for the predicted percentage of FEV1, and finn-b-GOUT for gout. Instrumental variables for genetic variants of lung function indicators were selected based on a linkage disequilibrium R2 of 0.001, a clumping distance of 10,000 kb, and a P-value threshold of 5e − 08. The main MR analyses were conducted using the random-effects inverse-variance weighted (RE-IVW) approach, which is robust to the presence of heterogeneity in MR settings [20].
Assessment of potential mediators
The exploration of potential mediators was based on blood biomarkers and blood cells as candidates for intermediate variables [11, 21–23]. These biomarkers and blood cells involved in inflammation, metabolism, and liver and kidney function may mediate the relationship between pulmonary function and the risk of gout (Additional file 1: Table S2). Within the UK Biobank initiative, blood tests were conducted on participants who provided informed consent. Approximately 4 ml of blood was drawn from the participants, processed for separation, and then stored at − 80 °C before being analyzed within 24 h using a Beckman Coulter LH750 instrument. Blood biomarkers were subjected to rigorous quality checks and externally validated. Inflammation-related biomarkers included leukocyte, neutrophil, monocyte, and lymphocyte counts and C-reactive protein (CRP) levels. Markers associated with liver function included alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total bilirubin (TBIL), total protein, and albumin. Renal function was assessed using cystatin C, urate, and urea levels.
Statistical analysis
The FEV1% predicted was determined using the Global Lung Initiative 2012 reference values calculated using the RSpiro software package. Participant characteristics were statistically compared across the normal spirometry, PRISm, and COPD groups using the Kruskal–Wallis and chi-squared tests for continuous and categorical variables. Missing data were addressed by including a separate category for incomplete data. [24]. Logistic regression was used for cross-sectional analysis to determine the prevalence of gout across different pulmonary function levels, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to quantify the strength of this association. In this cohort study, we utilized propensity score matching (PSM) to ensure comparability among participants with varying levels of pulmonary function. This method matched participants based on various factors, including demographic features, lifestyle, and comorbidities. The matching was conducted at a 1:1 ratio with a stringent criterion, employing a caliper set to 0.1 standard deviations of the propensity score to ensure precision in the matching process [25, 26]. Three models with increasing adjustments were fitted for PSM: Model 1 was adjusted for age, sex, socioeconomic status, and BMI, and Model 2 was additionally adjusted for lifestyle factors, including activities, smoking, alcohol consumption, and dietary intake of meat and fish. Model 3 was further adjusted for comorbidities, such as hypertension, diabetes, cardiovascular disease (CVD), chronic kidney disease (CKD), and asthma. The association between baseline impaired pulmonary function status and the risk of developing gout was assessed using a stratified Cox model based on the match ID generated by PSM to address the paired samples [27], producing hazard ratios (HRs) and 95% CIs. To assess the impact of unmeasured confounders, we conducted a sensitivity analysis using the E-value method. The E-value estimates the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to explain away the observed association [28]. Subgroup analyses based on various variables were conducted to explore potential effect modifications of the association between pulmonary function and gout across these factors. Furthermore, the dose–response relationship between the percentage of predicted values for FVE1 (% predicted) and FVC (% predicted) and the risk of gout was examined using restricted cubic spline (RCS) regression with four knots (5th, 35th, 65th, and 95th percentiles) of the pulmonary function variable distribution, allowing for the detection of potential linear and nonlinear associations.
Selected blood biomarkers and blood cells could serve as potential mediators based on the following analyses [11, 21–23]. First, to address potential biases caused by missing values of potential mediators, we employed multivariate imputations by chained equations using the Mice R package to impute these variables with a missing percentage of ≥ 1% [29]. Second, we normalized the raw data using a z-score and applied multiple linear regression models to assess the associations between pulmonary function and these biomarkers or blood cells. Next, we used Cox regression models to explore the relationships among pulmonary function, biomarkers, and incident gout. Significant biomarkers or blood cells in these steps were considered potential mediators for subsequent mediation analyses. We estimated the proportion mediated (PM) using the “mediation” package [30], and the non-parametric bootstrap method (with 1000 draws) was employed to calculate 95% CIs for the PM.
All the analyses were conducted using R software (version 4.2.2). A false discovery rate (FDR)-adjusted P-value < 0.05 for analyses involving biomarkers or blood cells was considered statistically significant. A two-tailed P-value < 0.05 was also deemed statistically significant in the prospective association analysis.
Results
Cross-sectional analysis of pulmonary function and gout associations
Our study included 422,002 individuals with pulmonary function data from the UK Biobank database (Fig. 1). Among these individuals, 328,954 (77.95%) exhibited normal pulmonary function, 55,365 (13.11%) were classified as PRISm, and 35,683 (8.46%) were identified as COPD. Compared with those with normal spirometry, participants with PRISm or COPD were more likely to be older, male, and overweight; had a lower likelihood of exercising; and had more comorbidities (P < 0.001, Table 1).
Table 1.
Baseline characteristics of the study population
|
Normal (N = 328,954) |
PRISm (N = 55,365) |
COPD (N = 35,683) |
P-value | |
|---|---|---|---|---|
| Age (y) | 57 (49–62) | 58 (50–63) | 61 (55–65) | < 0.001 |
| Sex (%) | < 0.001 | |||
| Female | 191,079 (58.1%) | 25,594 (46.2%) | 14,704 (41.2%) | |
| Male | 37,875 (41.9%) | 29,771 (53.8%) | 20,979 (58.8%) | |
| BMI (%) | < 0.001 | |||
| Underweight | 111,064 (33.8%) | 11,975 (21.6%) | 11,472 (32.1%) | |
| Normal | 1443 (0.4%) | 225 (0.4%) | 358 (1.0%) | |
| Overweight | 216,213 (65.7%) | 43,043 (77.7%) | 23,798 (66.7%) | |
| Townsend score (%) | < 0.001 | |||
| 1 (least deprived) | 87,743 (26.7%) | 11,432 (20.6%) | 7346 (20.6%) | |
| 2 | 85,857 (26.1%) | 11,955 (21.6%) | 7771 (21.8%) | |
| 3 | 82,741 (25.2%) | 13,548 (24.5%) | 8736 (24.5%) | |
| 4 (most deprived) | 72,197 (21.9%) | 18,354 (33.2%) | 11,794 (33.1%) | |
| Smoking (%) | < 0.001 | |||
| Never | 190,713 (58.0%) | 29,508 (53.3%) | 13,577 (38.0%) | |
| Previous | 110,591 (33.6%) | 18,561 (33.5%) | 13,726 (38.5%) | |
| Current | 26,401 (8.0%) | 6808 (12.3%) | 8078 (22.6%) | |
| Drink (%) | < 0.001 | |||
| Never | 22,300 (6.8%) | 7290 (13.2%) | 3583 (10.0%) | |
| Occasions | 73,079 (22.2%) | 14,354 (25.9%) | 7840 (22.0%) | |
| Frequent | 233,136 (70.9%) | 33,431 (60.4%) | 24,115 (67.6%) | |
| Activity (%) | < 0.001 | |||
| Normal | 40,567 (12.3%) | 8057 (14.6%) | 4612 (12.9%) | |
| Low | 137,464 (41.8%) | 21,274 (38.4%) | 13,582 (38.1%) | |
| High | 80,924 (24.6%) | 11,354 (20.5%) | 8167 (22.9%) | |
| Fish consumption (%) | < 0.001 | |||
| Low | 158,230 (48.1%) | 27,419 (49.5%) | 17,392 (48.7%) | |
| High | 170,512 (51.8%) | 27,801 (50.2%) | 18,226 (51.1%) | |
| Meat consumption (%) | < 0.001 | |||
| Low | 81,702 (24.8%) | 12,251 (22.1%) | 7175 (20.1%) | |
| Medium | 129,673 (39.4%) | 21,271 (38.4%) | 13,747 (38.5%) | |
| High | 116,941 (35.5%) | 21,407 (38.7%) | 14,561 (40.8%) | |
| Comorbidities (%) | ||||
| Hypertension | 158,899 (48.3%) | 33,816 (61.1%) | 21,600 (60.5%) | < 0.001 |
| Diabetes | 10,179 (3.1%) | 5496 (9.9%) | 2321 (6.5%) | < 0.001 |
| CVD | 10,273 (3.1%) | 4278 (7.7%) | 2803 (7.9%) | < 0.001 |
| CKD | 7753 (2.4%) | 1583 (2.9%) | 949 (2.7%) | < 0.001 |
| Asthma | 27,294 (8.3%) | 7286 (13.2%) | 9893 (27.7%) | < 0.001 |
| FVC Pred (%) | 100.39 (92.53–109.31) | 75.79 (69.40–80.65) | 84.17 (74.99–91.30) | < 0.001 |
| FEV1 Pred (%) | 98.19 (90.44–106.90) | 73.58 (67.72–77.24) | 67.57(58.10–74.19) | < 0.001 |
| FEV1/FVC | 0.78 (0.75–0.81) | 0.76 (0.73–0.80) | 0.64 (0.59–0.67) | < 0.001 |
Abbreviations: BMI body mass index, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, PRISm preserved ratio impaired spirometry, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, CKD chronic kidney disease
In the cross-sectional analysis, the prevalence rates of gout in the normal, PRISm, and COPD groups were 3.45% (11,351/328,954), 6.31% (3494/55,365), and 6.26% (2232/35,683), respectively. After adjustment for sociodemographic characteristics, lifestyles, and health-related factors, both PRISm (OR 1.24, 95% CI 1.17–1.31) and COPD (OR 1.14, 95% CI 1.07–1.22) were significantly associated with gout (both P < 0.001, Additional file 1: Table S3).
Longitudinal association between baseline pulmonary function and incident gout after PSM
Our cross-sectional study suggested an association between PRISm, COPD, and gout. Consequently, we combined individuals with PRISm and COPD into a single group, termed the “impaired pulmonary function” group. After individuals with gout at baseline (n = 8279) and those who were lost to follow-up (n = 1060) were removed, PSM was used to adjust for confounding variables, aligning the baseline characteristics between this group and those with normal pulmonary function (Fig. 1). The two groups were closely aligned in terms of demographic profiles, socioeconomic, and psychosocial factors, tobacco use, nicotine dependence, healthcare utilization, and comorbid conditions, with a standardized mean difference (SMD) below 0.1 (Additional file 1: Table S4).
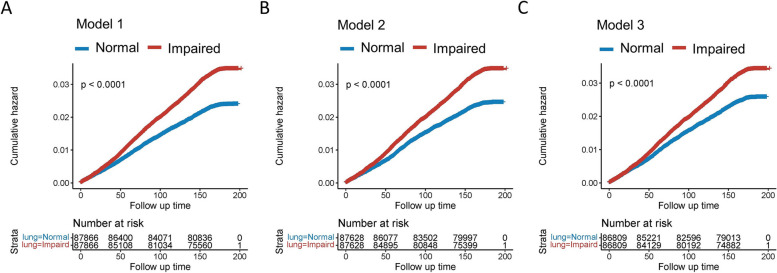
During a median follow-up of 14.41 years, the risk of gout was found to be substantially higher in the impaired pulmonary function group compared to the control group across three different matching models that were sequentially adjusted for basic demographic characteristics (Model 1), lifestyle and dietary factors (Model 2), and comorbid conditions (Model 3, Additional file 1: Fig. S1). The corresponding HRs were 1.45 (95% CI 1.37–1.54), 1.41 (95% CI 1.33–1.50), and 1.32 (95% CI 1.24–1.40), respectively (Additional file 1: Table S5). The Kaplan–Meier curve also revealed a higher risk of incident gout in the impaired pulmonary group compared to the control group (Fig. 2A–C, Additional file 1: Fig. S2A-D).
Fig. 2.
Kaplan–Meier analysis showing the risk of gout in individuals with impaired pulmonary function and those with normal pulmonary function in model 1 (A), model 2 (B), and model 3 (C). Model 1: Propensity matching by age, sex, Townsend score, and body mass index. Model 2: Propensity matching by age, sex, Townsend score, body mass index, smoking status, alcohol consumption, physical activity, fish consumption, and meat consumption Model 3: Propensity matching by age, sex, Townsend score, body mass index, smoking status, alcohol consumption, physical activity, fish consumption, meat consumption, hypertension, diabetes, CKD, CVD, and asthma. CVD: Cardiovascular disease; CKD: Chronic kidney disease
To minimize the potential for reverse causality, we excluded gout patients diagnosed within 2 years of follow-up. The impaired pulmonary function group remained significantly associated with an increased risk of developing gout, with corresponding HRs of 1.49 (95% CI 1.40–1.59), 1.44 (95% CI 1.36–1.54), and 1.38 (95% CI 1.29–1.47) in Model 1–3, respectively (Additional file 1: Table S6). To evaluate the potential impact of unmeasured confounding, we conducted a sensitivity analysis using the E-value method, which yielded a value of 1.97, suggesting that our findings are relatively robust to unmeasured confounding (Additional file 1: Fig. S3). We also performed MR analysis and our results demonstrated a significant connection between lung function indicators and the risk of gout (β < 0 and p < 0.05 by RE-IVW method). The OR values for the FVC, FEV1, and FEV1 predicted percentage were found to be 0.80 (95% CI 0.66–0.96), 0.72 (95% CI 0.55–0.95), and 0.70 (95% CI 0.53–0.93), respectively (Additional file 1: Table S7, Additional file 1: Fig. S4). These findings suggest a potential causal relationship between decreased pulmonary function and an increased risk of gout.
The normal and impaired pulmonary groups were further divided into subgroups based on age, sex, race, BMI, lifestyle, and comorbidities. The detrimental effects of impaired pulmonary function were associated with BMI, activity level, and diabetes status (P for interaction < 0.05, Fig. 3), emphasizing the importance of considering these factors and in the context of pulmonary function impairment.
Fig. 3.
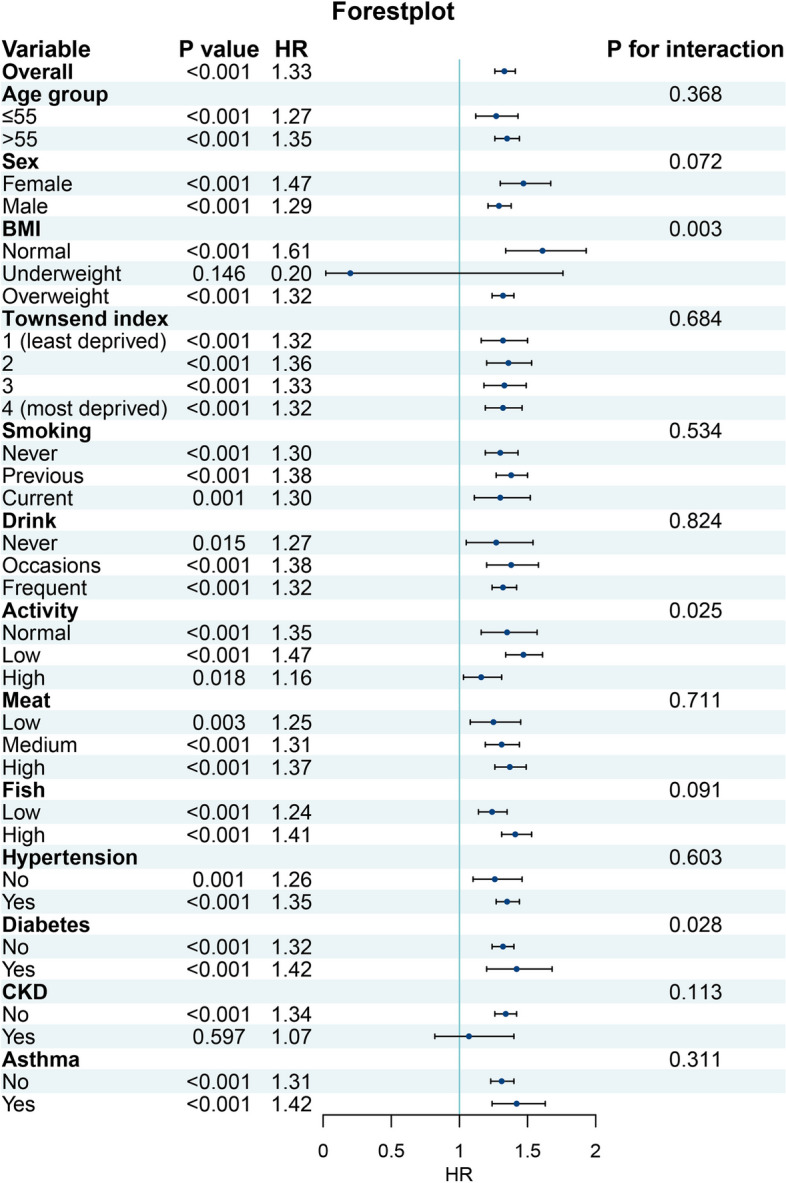
Forest plot showing the subgroup analysis of gout risk exposed to impaired pulmonary function compared to normal pulmonary function. HR: Hazard ratio; CVD: Cardiovascular disease; CKD: Chronic kidney disease; BMI: Body mass index
Detection of nonlinear and linear relationships between pulmonary function indicators and the risk of gout
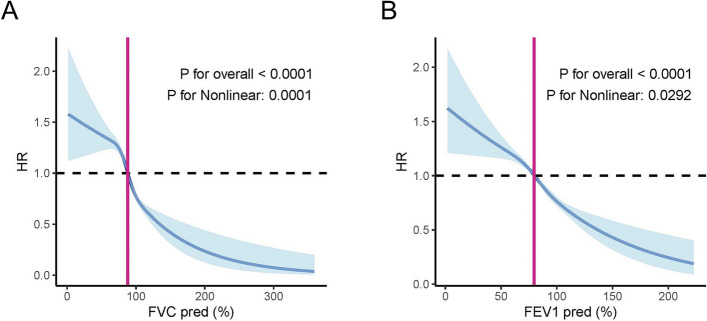
As FEV1 (% predicted) and FCV (% predicted) are the leading indicators defining pulmonary function, we stratified them into four grades to evaluate the risk of gout development. The findings indicated that higher grades of FEV1 (% predicted) and FCV (% predicted) were associated with a lower risk of gout (Additional file 1: Table S8). The RCS method was then used to explore the relationships between FEV1 (% predicted), FCV (% predicted), and the risk of gout. We plotted the RCS curve with four knots at the 5th, 35th, 65th, and 95th percentiles of FEV1 (% predicted) and FCV (% predicted). The RCS curve exhibited an inverse S-shaped pattern, indicating a nonlinear relationship between pulmonary function and gout risk (P for overall association < 0.001 and P for nonlinear association < 0.001). We found that decreased FEV1 (%predicted) and FCV (%predicted) were significantly linked to an elevated risk of gout. Specifically, subjects with FVC (%predicted) below 88% or FEV1 (%predicted) below 79% showed a pronounced increase in the risk of developing gout (Fig. 4A,B). As pulmonary function exceeds these thresholds, the influence of pulmonary function on gout risk shifts in both direction and strength, indicating that enhanced pulmonary function can reduce the risk of gout (Fig. 4A,B).
Fig. 4.
Association between FVC (% predicted) and FEV1 (% predicted) and the risk of gout in populations. A, B The RCS curves illustrating the nonlinear relationship between pulmonary function and FVC (% predicted) (A) and FEV1 (% predicted) (B). Each hazard ratio was computed with an FVC (% predicted) level of 88% (turning point) and FEV1(% predicted) level of 79% (turning point) as the reference. The solid line and red area represent the estimated values and their corresponding 95% CIs. HR: Hazard ratio; RCS: Restricted cubic spline; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity
Mediation analysis of peripheral blood biomarkers in the association between pulmonary function and gout risk
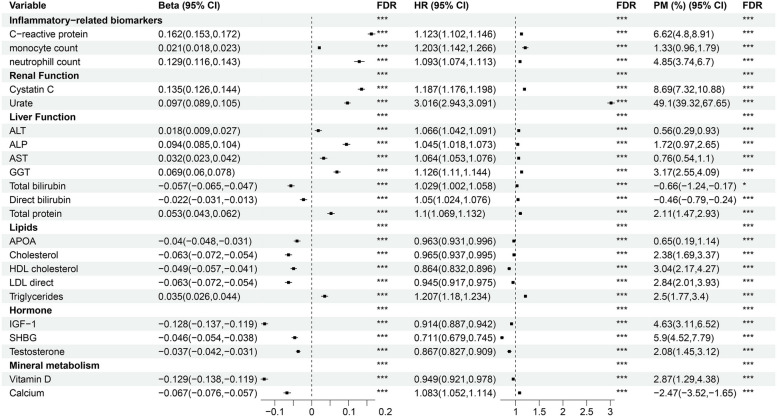
Given that gout is involved in inflammation, uric acid levels, and immune cell function, we conducted a mediation analysis focusing on peripheral blood biomarkers and blood cells as potential mediators to further explore potential mediators (Additional file 1: Table S9). After imputing the mediator variables, the distribution of the data remained consistent with the original values (Additional file 1: Fig. S5-8). We employed multiple linear models to analyze the relationships between impaired pulmonary function and circulating biomarkers, while using Cox regression to examine the associations between these biomarkers and gout events. Additionally, we conducted a mediation analysis to assess the mediating effects of circulating biomarkers on the relationship between impaired pulmonary function and gout. A total of 22 plasma biomarkers showed significant mediating effects. A total of 22 plasma biomarkers showed significant mediating effects. Notably, the urate level exhibited the most significant mediating effect, with an HR of 3.016 (95% CI 2.943–3.091), corresponding to a 49.1% mediation proportion for the risk of gout (Fig. 5). This result indicated that a decline in pulmonary function was associated with a significant increase in uric acid levels, further promoting gout development. Inflammatory markers, such as CRP, also demonstrated substantial mediation effects, with a mediation proportion of 6.62%. Furthermore, immune cell counts, including monocyte and neutrophil counts, were also identified as significant mediators, with mediating proportions of 1.33% and 4.85%, respectively, suggesting that inflammation is intricately involved in gout pathology (Fig. 5). Additional mediators included GGT (3.17%), cystatin C (8.69%), insulin-like growth factor 1(IGF-1, 4.63%), sex hormone-binding globulin (SHBG, 5.9%), vitamin D (2.87%), and calcium (− 2.47%), indicating that systemic metabolism and nutrition may also contribute to gout risk.
Fig. 5.
Associations between pulmonary function and incident gout mediated by blood biomarkers and blood cells. Significance (Sig.): ***FDR < 0.001, **FDR < 0.01, *FDR < 0.05; FDR: False discovery rate; HR: Hazard ratio; CI: Confidence interval; PM: Proportion mediated
Discussion
The present study utilized a large sample from the UK Biobank and revealed a significant association between impaired pulmonary function and gout. Our findings indicate that individuals with PRISm and COPD have a higher prevalence of gout compared to those with normal spirometry function. A longitudinal analysis further confirmed that impaired pulmonary function is a potential risk factor for the incidence of gout. Pulmonary function indicators, as measured by FEV1 (% predicted) and FCV (% predicted), are not only associated with a higher risk of gout but also follow a nonlinear pattern. Mediation analysis revealed that urate levels, inflammatory markers, and immune cell counts significantly mediated this relationship.
Effective gas exchange relies on normal pulmonary function, which is crucial for maintaining the body’s normal physiological metabolism. Recent studies have focused on the interplay between pulmonary function and a spectrum of diseases, notably rheumatic and metabolic disorders. Prisco et al. [10] conducted a cross-sectional analysis of a large UK Biobank cohort, revealing a robust association between RA and the presence of restrictive and obstructive pulmonary patterns. Studies by Ahn et al. [8] and Yang et al. [9] have both identified a link between hyperuricemia and diminished pulmonary capacity, suggesting a potential metabolic basis for respiratory impairment. However, contrasting results have emerged from studies by Luo et al. [31] and Kang et al. [32], who used the National Health and Nutrition Examination Survey dataset to report an inverse relationship between hyperuricemia and pulmonary function in the U.S. adult population. These discrepancies may be due to variations in study design, population demographics, or methodologies used to assess pulmonary function and metabolic health. Our study included a large cohort for broader generalizability, utilized stringent pulmonary function testing for accuracy, and employed PSM to adjust for confounding variables, thereby enhancing the robustness of our findings regarding the association between gout and pulmonary function. Our study also extends the current understanding by examining the relationship between PRISm and gout, which has been less explored in the literature [11, 33–35]. The inclusion of PRISm in our analysis adds depth to the existing literature by highlighting that even a preserved ratio of pulmonary function can be associated with gout, which challenges the traditional focus on COPD alone.
Previous studies on pulmonary function and adverse health outcomes have shown significant nonlinear associations [36, 37]. Our study confirms these findings and provides novel evidence supporting a nonlinear association between pulmonary function indicators and gout. Stratified analyses suggested that the association between pulmonary function and gout was influenced by BMI, physical activity level, and diabetes status. These findings highlight the necessity of integrating these factors into the clinical management of pulmonary function impairment. Future research should aim to elucidate the mechanistic connections between these variables to guide the development of preventive strategies for individuals with impaired pulmonary function.
We also examined potential mediators, which shed further light on the link between impaired pulmonary function and an increased risk of gout. Our results showed that urate levels had the highest mediating effect, accounting for 49.1% of the mediation proportion in the risk of gout, highlighting the pivotal role of uric acid in the association between pulmonary function and gout. Decreased pulmonary function may precipitate localized increases in CO2 within the microenvironment, triggering metabolic changes and hypoxia, which can lead to reduced renal clearance of uric acid and localized pH changes in tissues. These alterations potentially lower the solubility of urate salts, facilitating the crystallization of monosodium urate and the onset of gout [38]. Chronic systemic inflammation, driven by pulmonary dysfunction, further amplifies this risk by impairing metabolic homeostasis and immune regulation. Notably, elevated counts of monocytes and neutrophils, which we identified as significant mediators in our study, increase the production of proinflammatory cytokines, such as IL-1β, a critical factor in the initiation of gout flares [21, 39]. Consequently, this can result in elevated levels of inflammatory cells and cytokines in the bloodstream, contributing to the development of gout. Additionally, neutrophils play a key role in the formation of neutrophil extracellular traps (NETs), which are actively involved in crystal-induced inflammation [40]. These NETs can exacerbate the inflammatory response in gout by promoting monosodium urate crystal deposition [41]. Additionally, a MR study supported a causal relationship between pulmonary diseases and renal function [42]. Remarkably, the relationship between renal dysfunction and gout has been well established [43].
Although our study benefited from a large sample size and rigorous methodology, several limitations should be acknowledged. First, the cross-sectional nature of spirometry data may not fully capture dynamic changes in pulmonary function over time. Longitudinal assessments may help provide deeper insights into the progressive effects of pulmonary decline on gout development and strengthen causal inferences. Second, the diagnosis of gout in the UK Biobank relied on ICD-10 codes rather than objective clinical examinations, which could have resulted in underreporting or misclassification. Primary care records cover less than half of the participants, potentially leading to underreported mild gout cases and misclassification, leading to the underreporting of mild gout cases, potentially resulting in misclassification. Future studies are expected to use more objective diagnostic criteria to validate our findings. Third, although we adjusted for numerous confounders, the possibility of unmeasured confounding factors cannot be entirely dismissed. Fourth, the generalizability of our findings may be constrained by the predominantly Caucasian demographics of the UK Biobank cohort. As genetic predispositions, environmental exposures, and lifestyle factors differ across populations, our findings may have limited generalizability to non-Caucasian or geographically distinct groups. Diverse population studies are necessary to increase the generalizability of the findings. Investigating the biological mechanisms linking pulmonary function to gout using experimental models may provide more definitive evidence for these associations.
Conclusions
Based on a large-scale prospective cohort, our research revealed a significant association between impaired pulmonary function and an increased risk of gout. This association might be partially mediated by biomarkers including urate levels, inflammatory markers, and immune cell counts. Pulmonary function tests might be considered a supplementary tool for individuals at risk of gout. Closer collaboration between pulmonologists and rheumatologists may enhance patient management.
Supplementary information.
Supplementary Information
Additional file 1: Table S1. Extraction of data variables from the UK Biobank. Table S2. Selection of biomarkers as potential mediators between pulmonary function and incident gout. Table S3. Cross-sectional association between pulmonary function and gout. Table S4. Baseline characteristics of the study population after PSM. Table S5. Risk of gout exposed to impaired pulmonary function compared to normal pulmonary function. Table S6. Sensitivity analyses for the association between impaired pulmonary function and gout risk, excluding cases diagnosed within the first two years of follow-up. Table S7. Effects of genetically predicted lung function indicators on the risk of gout in the MR analysis. Table S8. Incidence of gout according to baseline pulmonary indicators. Table S9. Selection of biomarkers as potential mediators between pulmonary function and incident gout. Fig. S1. The forest plot illustrating the outcomes of a multivariable Cox regression analysis. Fig. S2. Kaplan–Meier analysis for risk of gout in males (A), females (B), age ≤ 55 individuals (C) and age ≤ 55 (D) individuals with impaired pulmonary function compared to normal pulmonary function. Fig. S3. Sensitivity analysis using E-value estimation for unmeasured confounding. Fig. S4. Scatter plots of genetic associations between lung function indicators and gout. Fig. S5. Distribution of inflammatory-related and renal function biomarkers pre- and post-imputation. Fig. S6. Distribution of liver function biomarkers pre- and post-imputation. Fig. S7. Distribution of lipids and glucose pre- and post-imputation. Fig. S8. Distribution of hormone and mineral metabolism biomarker pre- and post-imputation.
Acknowledgements
The authors express their sincere gratitude to all participants and professionals who have contributed to the UK Biobank. This research was conducted using the UK Biobank Resource under Application Number 106397.
Abbreviations
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CI
Confidence interval
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- FDR
False discovery rate
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- FFQ
Food frequency questionnaire
- GGT
Gamma-glutamyl transferase
- GWAS
Genome-wide association study
- HR
Hazard ratio
- ICD
International Classification of Diseases
- IGF-1
Insulin-like growth factor 1
- MET
Metabolic equivalent task
- MR
Mendelian randomization
- NETs
Neutrophil extracellular traps
- NLRP3
NOD-like receptor pyrin domain-containing protein 3
- OR
Odds ratio
- PM
Proportion mediated
- PRISm
Preserved ratio of impaired spirometry
- PSM
Propensity score matching
- RA
Rheumatoid arthritis
- RCS
Restricted cubic spline
- RE-IVW
Random-effects inverse-variance weighted
- SMD
Standardized mean difference
- TBIL
Total bilirubin
Authors’ contributions
S-MD and QT conceived the idea and designed the study. CZ extracted the data. ZK analyzed the data and wrote the manuscript. JZ, CZ, YZ and HJ discussed the data and helped refine the manuscript. S-MD and QT critically reviewed the manuscript and were responsible for its overall content as guarantors. All authors read and approved the final manuscript.
Funding
ZK was supported by the Postdoctoral Fellowship Program of CPSF under Grant Number 2023M742331 and Pujiang Rheumatism Young Physicians Training Program under Grant Number SPROG2302. CZ was supported by the 2115 Talent Development Program of China Agricultural University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The UK Biobank has obtained ethical approval from the North West Multi-centre Research Ethics Committee (Reference number: 11/NW/0382, https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics). Informed consent was collected from all study participants via electronic signature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zijian Kang, Jianzheng Zhang, and Chen Zhu contributed equally to this work.
Contributor Information
Qiang Tong, Email: jasontong1985@outlook.com.
Sheng-Ming Dai, Email: shengmingdai@163.com.
References
- 1.Tao H, Mo Y, Liu W, Wang H. A review on gout: Looking back and looking ahead. Int Immunopharmacol. 2023;117: 109977. 10.1016/j.intimp.2023.109977. [DOI] [PubMed] [Google Scholar]
- 2.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16:380–90. 10.1038/s41584-020-0441-1. [DOI] [PubMed] [Google Scholar]
- 3.He Q, Mok T-N, Sin T-H, Yin J, Li S, Yin Y, et al. Global, Regional, and National Prevalence of Gout From 1990 to 2019: Age-Period-Cohort Analysis With Future Burden Prediction. JMIR public Heal Surveill. 2023;9: e45943. 10.2196/45943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28:51. 10.1186/s11658-023-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamodu OA, Wu S-M, Feng P-H, Sun W-L, Lin C-W, Chuang H-C, et al. lnc-IL7R Expression Reflects Physiological Pulmonary Function and Its Aberration Is a Putative Indicator of COPD. Biomedicines 2022;10. 10.3390/biomedicines10040786 [DOI] [PMC free article] [PubMed]
- 6.Xuan L, Han F, Gong L, Lv Y, Wan Z, Liu H, et al. Association between chronic obstructive pulmonary disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 2018;17:263. 10.1186/s12944-018-0904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines KJ, Backer V, Gibson PG, Powel H, Porsbjerg CM. Impaired lung function is associated with systemic inflammation and macrophage activation. Eur Respir J. 2015;45:557–9. 10.1183/09031936.00187514. [DOI] [PubMed] [Google Scholar]
- 8.Ahn K-M, Lee S-Y, Lee S-H, Kim S-S, Park H-W. Lung function decline is associated with serum uric acid in Korean health screening individuals. Sci Rep. 2021;11:10183. 10.1038/s41598-021-89678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Wang Z, Xiao S, Dai C, Wen X, Wu F, et al. Association Between Serum Uric Acid and Lung Function in People with and without Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2022;17:1069–80. 10.2147/COPD.S356797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prisco L, Moll M, Wang J, Hobbs BD, Huang W, Martin LW, et al. Relationship Between Rheumatoid Arthritis and Pulmonary Function Measures on Spirometry in the UK Biobank. Arthritis Rheumatol (Hoboken, NJ). 2021;73:1994–2002. 10.1002/art.41791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Jankowich MD, Lu Y, Wu L, Shao L, Ke C. Preserved Ratio Impaired Spirometry, Metabolomics, and the Risk of Type 2 Diabetes. J Clin Endocrinol Metab. 2023;108:E769–78. 10.1210/clinem/dgad140. [DOI] [PubMed] [Google Scholar]
- 12.Bešević J, Lacey B, Conroy M, Omiyale W, Feng Q, Collins R, et al. New Horizons: the value of UK Biobank to research on endocrine and metabolic disorders. J Clin Endocrinol Metab. 2022;107:2403–10. 10.1210/clinem/dgac407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn P, Greenland P. Contributions of the UK biobank high impact papers in the era of precision medicine. Eur J Epidemiol. 2020;35:5–10. 10.1007/s10654-020-00606-7. [DOI] [PubMed] [Google Scholar]
- 14.Davies NM, Holmes MV, Davey SG. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362: k601. 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12: e1001779. 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J 2019;54. 10.1183/13993003.02140-2018 [DOI] [PubMed]
- 17.Langhammer A, Johannessen A, Holmen TL, Melbye H, Stanojevic S, Lund MB, et al. Global lung function initiative 2012 reference equations for spirometry in the Norwegian population. Eur Respir J. 2016;48:1602–11. 10.1183/13993003.00443-2016. [DOI] [PubMed] [Google Scholar]
- 18.Diao JA, He Y, Khazanchi R, Nguemeni Tiako MJ, Witonsky JI, Pierson E, et al. Implications of Race Adjustment in Lung-Function Equations. N Engl J Med Published Online First: May 2024. 10.1056/NEJMsa2311809 [DOI] [PMC free article] [PubMed]
- 19.Brusasco V, Pellegrino R. On the Assessment of Preserved Ratio Impaired Spirometry. Am J Respir Crit Care Med. 2023;208:1343–4. 10.1164/rccm.202309-1701LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48:728–42. 10.1093/ije/dyy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu W, Liu BP, Jia CX. Association and biological pathways between lung function and incident depression: a prospective cohort study of 280,032 participants. BMC Med. 2024;22:1–11. 10.1186/s12916-024-03382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L, et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PLoS Med. 2023;20: e1004135. 10.1371/journal.pmed.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y-H, Shen L-X, Li Y-Z, Leng Y, Yang L, Chen S-D, et al. Lung function and risk of incident dementia: A prospective cohort study of 431,834 individuals. Brain Behav Immun. 2023;109:321–30. 10.1016/j.bbi.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Groenwold RHH, White IR, Donders ART, Carpenter JR, Altman DG, Moons KGM. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184:1265–9. 10.1503/cmaj.110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong Q, Du Y, Cui R, Chen M, Ing S, Cheng J, et al. Risk of erectile dysfunction in male patients with gout treated with febuxostat or allopurinol : a propensity score ‑ matched cohort study. 2022;:1717–26. 10.1007/s40265-022-01816-x [DOI] [PubMed]
- 26.Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014;179:226–35. 10.1093/aje/kwt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer New York 2005. https://books.google.com/books?id=jS2Cy0lezJIC
- 28.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167:268–74. 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 29.Shah AD, Bartlett JW, Carpenter J, Nicholas O, Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol. 2014;179:764–74. 10.1093/aje/kwt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw 2014;59:1–38. 10.18637/jss.v059.i05
- 31.Luo W, Wang C, Wang W, Yao X, Lu F, Wu D, et al. Serum uric acid is inversely associated with lung function in US adults. Sci Rep. 2024;14:1300. 10.1038/s41598-024-51808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang T, Xi Y, Lu S, Qian T, Du M, Shi X, et al. Association between serum uric acid levels and lung function in the NHANES cohort (2007–2012): A cross-sectional analysis of a diverse American population. Int J Rheum Dis. 2024;27: e15043. 10.1111/1756-185X.15043. [DOI] [PubMed] [Google Scholar]
- 33.Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA. 2021;326:2287–98. 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade RC, Wells JM. Preserved Ratio With Impaired Spirometry: The Lung’s Contribution to Metabolic Syndrome. Chest. 2023;164:1075–6. 10.1016/j.chest.2023.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Kawatoko K, Washio Y, Ohara T, Fukuyama S, Honda T, Hata J, et al. Risks of dementia in a general Japanese older population with preserved ratio impaired spirometry: The Hisayama Study. J Epidemiol Published Online First: December 2023. 10.2188/jea.JE20230207 [DOI] [PMC free article] [PubMed]
- 36.Zhou L, Yang H, Zhang Y, Li H, Zhang S, Li D, et al. Association of impaired lung function with dementia, and brain magnetic resonance imaging indices: a large population-based longitudinal study. Age Ageing 2022;51. 10.1093/ageing/afac269 [DOI] [PubMed]
- 37.Li G, Lu Y, Qiao Y, Hu D, Ke C. Role of Pulmonary Function in Predicting New-Onset Cardiometabolic Diseases and Cardiometabolic Multimorbidity. Chest. 2022;162:421–32. 10.1016/j.chest.2021.12.663. [DOI] [PubMed] [Google Scholar]
- 38.Chhana A, Lee G, Dalbeth N. Factors influencing the crystallization of monosodium urate: a systematic literature review. BMC Musculoskelet Disord. 2015;16:296. 10.1186/s12891-015-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klück V, Liu R, Joosten LAB. The role of interleukin-1 family members in hyperuricemia and gout. Jt bone spine. 2021;88: 105092. 10.1016/j.jbspin.2020.105092. [DOI] [PubMed] [Google Scholar]
- 40.Ma Q, Steiger S. Neutrophils and extracellular traps in crystal-associated diseases. Trends Mol Med. 2024;30:809–23. 10.1016/j.molmed.2024.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Chen T, Zhou J, Dang W. Mechanism of neutrophil extracellular traps in the pathogenesis of gout. Clin Exp Rheumatol Published Online First: June 2024. 10.55563/clinexprheumatol/ezzfbt [DOI] [PubMed]
- 42.Park S, Lee S, Kim Y, Cho S, Kim K, Kim YC, et al. Kidney function and obstructive lung disease: a bidirectional Mendelian randomisation study. Eur Respir J 2021;58. 10.1183/13993003.00848-2021 [DOI] [PubMed]
- 43.Stamp LK, Farquhar H, Pisaniello HL, Vargas-Santos AB, Fisher M, Mount DB, et al. Management of gout in chronic kidney disease: a G-CAN Consensus Statement on the research priorities. Nat Rev Rheumatol. 2021;17:633–41. 10.1038/s41584-021-00657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Extraction of data variables from the UK Biobank. Table S2. Selection of biomarkers as potential mediators between pulmonary function and incident gout. Table S3. Cross-sectional association between pulmonary function and gout. Table S4. Baseline characteristics of the study population after PSM. Table S5. Risk of gout exposed to impaired pulmonary function compared to normal pulmonary function. Table S6. Sensitivity analyses for the association between impaired pulmonary function and gout risk, excluding cases diagnosed within the first two years of follow-up. Table S7. Effects of genetically predicted lung function indicators on the risk of gout in the MR analysis. Table S8. Incidence of gout according to baseline pulmonary indicators. Table S9. Selection of biomarkers as potential mediators between pulmonary function and incident gout. Fig. S1. The forest plot illustrating the outcomes of a multivariable Cox regression analysis. Fig. S2. Kaplan–Meier analysis for risk of gout in males (A), females (B), age ≤ 55 individuals (C) and age ≤ 55 (D) individuals with impaired pulmonary function compared to normal pulmonary function. Fig. S3. Sensitivity analysis using E-value estimation for unmeasured confounding. Fig. S4. Scatter plots of genetic associations between lung function indicators and gout. Fig. S5. Distribution of inflammatory-related and renal function biomarkers pre- and post-imputation. Fig. S6. Distribution of liver function biomarkers pre- and post-imputation. Fig. S7. Distribution of lipids and glucose pre- and post-imputation. Fig. S8. Distribution of hormone and mineral metabolism biomarker pre- and post-imputation.
Data Availability Statement
No datasets were generated or analysed during the current study.