Abstract
Substantial evidence supports that delay of surgery after breast cancer diagnosis is associated with increased mortality risk, leading to the introduction of a new Commission on Cancer quality measure for receipt of surgery within 60 days of diagnosis for non-neoadjuvant patients. Breast cancer subtype is a critical prognostic factor and determines treatment options; however, it remains unknown whether surgical delay-associated breast cancer-specific mortality (BCSM) risk differs by subtype. This retrospective cohort study aimed to assess whether the impact of delayed surgery on survival varies by subtype (hormone [HR] + /HER2 −, HR −/HER2 −, and HER2 +) in patients with loco-regional breast cancer who received surgery as their first treatment between 2010 and 2017 using the SEER-Medicare database. Exposure of this study was continuous time to surgery from diagnostic biopsy (TTS; days) in reference to TTS = 30 days. BCSM were evaluated as flexibly dependent on continuous time (days) to surgery from diagnosis (TTS) using Fine and Gray competing-risk regression models, respectively, by HR status. Inverse propensity score-weighting was adjusted for demographic, clinical, and treatment variables impacting TTS. Adjusted BCSM risk grew with increasing TTS across all subtypes; however, the pattern and extent of the association varied. HR + /HER2 − patients exhibited the most pronounced increase in BCSM risk associated with TTS, with approximately exponential growth after 42 days, with adjusted subdistribution hazard ratios (sHR) of 1.21 (95% CI: 1.06–1.37) at TTS = 60 days, 1.79 (95% CI: 1.40–2.29) at TTS = 90 days, and 2.83 (95% CI: 1.76–4.55) at TTS = 120 days. In contrast, both HER2 + and HR −/HER2 − patients showed slower, approximately linear growth in sHR, although non-significant in HR −HER2 −.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01949-9.
Keywords: Surgical delay, Breast cancer-specific mortality, SEER-Medicare, Tumor subtype, Hormone-receptor, HER2
Introduction
Breast cancer is the most commonly diagnosed malignancy among women in the United States [1]. Due to improvement in early detection and successful implementation of screening programs [2], 66% of breast cancer cases are diagnosed at localized stage when the tumor is still relatively small and surgically resected first [3]. However, 15-year survival rates for early-stage breast cancer range from 81% [4], with considerable recurrence rates.
A recent meta-analysis of 34 studies on 17 cancers (n = 1,272,681) found that treatment delay is a critical factor contributing to mortality risk in multiple types of solid tumors, and indicated a 6–8% increased risk of death for each 4-week delay in treatment [7]. In breast cancer specifically, across 6 retrospective observational comparison studies published from 2013 to 2020 (RE model: Q = 84.79; df = 5, p = 0.001; I2 = 94.1%), surgery delay was associated with decreased overall survival every four weeks at a Hazard ratio of 1.08 (95% CI; 1.03–1.13). Extended time to surgery from diagnostic biopsy (TTS), in particular, has negative survival implications in breast cancer [2–6], and studies have noted that both the frequency and length of delay are increasing [8–10]. Thus, in 2022, the Commission on Cancer (CoC) introduced a new quality measure for accredited facilities of receipt of surgery within 60 days of diagnosis for Stage I–III patients in the non-neoadjuvant setting [11] in order to address the negative survival impact of surgical delay. The breast cancer subtype is a critical prognostic factor that provides critical therapy-relevant information since the underlying biological differences reflect tumor behavior as well as treatment options [12]. Thus, questions remain as to whether all patients are predisposed to an equal level of mortality risk posed by extended TTS or whether risk differs with intrinsic properties such as subtypes. Only a few studies have explored outcomes in relation to TTS by subtype [13, 14]. In a retrospective study of 351,087 Stage I–III breast cancer patients, Mateo et al. reported a consistent 10% increase in the risk of overall mortality each subsequent month after the first 30 days post-diagnosis in a cohort of 351,087 Stage I–III patients that did not change based on subtype [13]. In contrast, in a cohort of 90,405 T1N0 breast cancer patients who received breast-conserving surgery, Hills et al. found that the risk of TTS-associated disease progression was confined to only hormone receptor (HR) + disease, with 18% and 47% higher likelihood of tumor size progression for patients who waited between 61 and 90 days and over 90 days, respectively [14]. While research thus far has shed critical light on the steady increase of surgery delay among breast cancer patients [8–10] and the risk it presents for mortality outcomes [7, 15, 16], the commonly adopted approach of examining TTS as fixed monthly or bi-monthly increments hinders vital understanding of how breast cancer-specific mortality (BCSM) risk may flexibly change with increasing TTS by subtype. Thus, the key objective of this study was to gain a comprehensive picture of whether TTS differentially impacts BCSM by subtype through flexible modeling of daily estimates of risk in women with loco-regional breast cancer in the non-neoadjuvant setting using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Methods
Cohort: A retrospective cohort of women diagnosed by needle or incisional biopsy with loco-regional invasive, non-inflammatory breast cancer between 2010 and 2017 in the SEER-Medicare database who received surgery as their first treatment was selected. The SEER-Medicare linked database combines Medicare Parts A and B claims with clinical and outcome data from SEER cancer registries [17]. Medicare is a federal health insurance program available for individuals 65 and older and some individuals younger than 65 with specific disabilities or conditions [18]. All data were de-identified and met the criteria for exempt review by the University of Oklahoma Health Sciences Center Institutional Review Board (IRB7446). Patients younger than 66 were excluded due to the bias presented by the enrollment criteria for those under 65 and lack of claims history for patients who qualified for enrollment based on age in the same year as their diagnosis. Patients who had HMO coverage or did not have continuous Part A and B coverage for at least one year prior through one year after diagnosis were excluded due to the inability to accurately ascertain existing comorbidities at the time of diagnosis or full claims for the primary course of treatment. Patients who received surgery within 7 days of diagnostic biopsy were excluded since the time required for pathologic molecular diagnosis commonly takes up to one week [19]. Additionally, patients that did not receive surgery until over ≥ 120 days after diagnosis, had a time of death less than one year after surgery, SEER reported follow-up shorter than TTS, non-definitive initial surgery (i.e., re-excisions), prior cancer diagnosis, non-locoregional disease (i.e., in situ, regional direct extension, or distant metastatic spread), or missing information were excluded (Fig. 1).
Fig. 1.
Cohort exclusion scheme
Exposure: The primary exposure, time-to-surgery (TTS), was defined as the days from the date of diagnostic biopsy to the date of surgery.
Outcome: Breast cancer-specific mortality (BCSM) in the presence of competing events (i.e., death from other causes) was assessed, and survival times were calculated from the date of surgery to death or last contact (censored).
Definitions: Loco-regional breast cancer (see Appendix) is defined by the SEER summary as a localized disease confined to breast tissue and fat, including the nipple and/or areola; regional lymph node involvement is defined as axillary (levels I–III), infraclavicular (subclavicular), internal mammary, intramammary, or other regional lymph nodes not otherwise specified. The cohort was stratified by hormone receptor (i.e., estrogen and/or progesterone receptor; [HR]) and HER2 status into 3 groups: HR + /HER2 −, HR −/HER2 −, and HR + or HR −/HER2 + [HER2 +]. Age at the time of diagnosis was categorized in 5-year intervals (i.e. < 70, 70–74, 75–79, 80–84, and ≥ 85 years old). Race/ethnicity was categorized as non-Hispanic Black (Black), other (Asian, Hispanic, Pacific Islander, American Indian/Eskimo/Aleutian, and other non-specified race or ethnicity), or non-Hispanic White (White). The Charlson Comorbidity Index was calculated for each patient using the SEER-Medicare developed Comorbidity SAS Macro [20] (2021 version) to search for relevant claims in the year prior to diagnosis, and classified as 0, 1, or ≥ 2. Education (% of residents without high school degree) and residential median income were based on census tract level information from the 2010 U.S. Census and the patient’s census tract of residence at the time of diagnosis. Histology was categorized as ductal, lobular, or other by ICD-O-3 codes (Appendix 1). The HCPCS, ICD-9, and ICD-10 codes used to classify diagnosis, surgery, and adjuvant therapies in Medicare claims are listed in Appendix 2. The surgery type was classified as breast-conserving, mastectomy, or mastectomy with immediate reconstruction.
Statistical methods: Time to death as a function of TTS was analyzed separately by subtype using Fine-Gray competing risk models for BCSM. All models were adjusted using inverse propensity score weights (IPW) to account for potential imbalances in covariates associated with TTS [21–23]. Covariate balancing propensity scores were computed using the R package “CBPS” with socio-demographic (age at diagnosis, race/ethnicity, census tract median income, and census tract % without a high school degree) and clinical factors (Charlson Comorbidity Index, year of diagnosis, SEER combined summary stage, histology grade, type of surgery, histology) as predictors and log-transformed TTS as the response variable [23]. Pre-/post-weighting balance was assessed for each model using Love plots. Final survival models were adjusted by normalized IPW, with extreme weights beyond the 95th percentile winsorized, along with receipt of adjuvant radiation or systemic therapy, comorbidity score, and, in HER2 + patients, hormone receptor status. B-splines were used to flexibly model the subdistribution hazard of mortality as nonparametric functions of TTS. Subdistribution hazard ratio (sHR) estimates were calculated using TTS = 30 days as the reference point since it is commonly used as the upper limit of the reference in categorical TTS studies [13, 15, 24]. Simultaneous 95% confidence intervals (CI) at each TTS point were computed using the Scheffe method. The association between TTS and sHR was considered significant when the simultaneous 95% CI did not include a subdistribution hazard ratio of 1. To provide estimates of the BCSM incidence at TTS of 30, 60, 90, and 120 days, the adjusted cumulative incidence function was derived from the Fine-Gray model conditioned on the subgroup of patients with the most common characteristics. The Wilcoxon rank sum test was used to compare the median TTS differences between race groups. The Jonckheere trend test [25] was used to test the increasing trend in median TTS by diagnosis year. All statistical analyses were conducted using SAS (version 9.4; Cary, NC) and R software (version 4.0.4), and graphs were generated using JMP Pro 15.2.0 (SAS; Cary, NC).
Results
Cohort characteristics. Following exclusions (Fig. 1), 34,248 loco-regional breast cancer patients diagnosed in 2010–2017 who received surgery as their first treatment (i.e., non-neoadjuvant) were selected from the SEER-Medicare database. The median age at diagnosis was 73 years old (range: 66–100 years old; first quartile Q1:69, third quartile Q3:78), and median follow-up time after surgery was 4.2 years (range: 0 days to 8.9 years; Q1: 2.4 years, Q3: 6.3 years). Approximately 82.7% (n = 28,332) of patients were HR + /HER2 −, 9.4% (n = 3,226) were HER2 + , and 7.9% (n = 2,690) were HR −/HER2 − (Table 1). Median TTS was the same across subtypes (29 days) but ranged by clinical and demographic characteristics. Black patients had longer median TTS than White in HR + /HER2 − (34 vs. 29 days; p < 0.001) and HR −/HER2 − (36 vs. 28 days; p < 0.001). Additionally, median TTS increased steadily with a year of diagnosis from 2010 to 2017 in all subtypes (p < 0.001). Notably, the largest difference in median TTS was observed for patients who received mastectomy with immediate reconstruction, respectively HR + /HER2-, 44 days in HER2 + , and 41 days in HR −/HER2 −; Table 1).
Table 1.
Distribution of TTS by cohort demographic and clinical characteristics within subtypes
| HR + /HER2- | HER2 + | HR-/HER2- | ||||
|---|---|---|---|---|---|---|
| n (%) | Median (Q1–Q3) | n (%) | Median (Q1–Q3) | n (%) | Median (Q1–Q3) | |
| 28,332 (100) | 29 (21–42) | 3,226 (100) | 29 (20–41) | 2,690 (100) | 29 (20–42) | |
| Age | ||||||
| < 70 | 7,766 (27.4) | 31 (21–44) | 919 (28.5) | 29 (20–42) | 637 (23.7) | 29 (20–42) |
| 70- 74 | 8,678 (30.6) | 29 (21–42) | 914 (28.3) | 29 (20–41) | 782 (29.1) | 29 (20–42) |
| 75- 79 | 6,254 (22.1) | 29 (21–42) | 693 (21.5) | 28 (20–40) | 595 (22.1) | 29 (20–43) |
| 80–84 | 3,687 (13.0) | 28 (20–41) | 439 (13.6) | 29 (20–41) | 383 (14.2) | 28 (20–41) |
| 85 + | 1,947 (6.9) | 28 (20–42) | 261 (8.1) | 28 (20–40) | 293 (10.9) | 29 (21–40) |
| Race/ethnicity | ||||||
| White | 25,206 (89.0) | 29 (21–42) | 2,775 (86.0) | 28 (20–41) | 2,234 (83) | 28 (20–41) |
| Black | 1,526 (5.4) | 34 (23–48) | 230 (7.1) | 31 (22–51) | 320 (11.9) | 36 (23–51) |
| Other | 1,600 (5.6) | 33 (22–44) | 221 (6.9) | 30 (21–43) | 136 (5.1) | 29 (20–43) |
| Charlson comorbidity index | ||||||
| 0 | 16,102 (56.8) | 29 (21–42) | 1,738 (53.9) | 28 (19–40) | 1,411 (52.5) | 28 (20–41) |
| 1 | 6,820 (24.1) | 29 (21–42) | 794 (24.6) | 29 (20–41) | 650 (24.2) | 28 (20–42) |
| 2 + | 5,410 (19.1) | 31 (21–45) | 694 (21.5) | 32 (22–44) | 629 (23.4) | 31 (21–45) |
| Year of diagnosis | ||||||
| 2010 | 3,021 (10.7) | 27 (19–38) | 370 (11.5) | 25 (17–37) | 348 (12.9) | 28 (19–40) |
| 2011 | 3,173 (11.2) | 28 (19–40) | 367 (11.4) | 27 (18–36) | 338 (12.6) | 27 (19–41) |
| 2012 | 3,407 (12.0) | 29 (20–41) | 397 (12.3) | 28 (18–38) | 371 (13.8) | 27 (19–37) |
| 2013 | 3,553 (12.5) | 29 (20–42) | 397 (12.3) | 28 (20–42) | 324 (12.0) | 29 (20–43) |
| 2014 | 3,564 (12.6) | 29 (21–42) | 424 (13.1) | 30 (21–42) | 345 (12.8) | 28 (21–42) |
| 2015 | 3,780 (13.3) | 31 (22–43) | 463 (14.4) | 29 (21–42) | 311 (11.6) | 32 (22–43) |
| 2016 | 3,998 (14.1) | 32 (22–44) | 417 (12.9) | 31 (22–43) | 321 (11.9) | 31 (21–45) |
| 2017 | 3,836 (13.5) | 33 (22–47) | 391 (12.1) | 32 (24–46) | 332 (12.3) | 31(22–43) |
| SEER Stage | ||||||
| Local | 22,874 (80.7) | 29 (21–42) | 2,342 (72.6) | 28 (20–41) | 2,152 (80.0) | 29 (20–42) |
| Regional lymph node involvement | 5,458 (19.3) | 30 (21–44) | 884 (27.4) | 29 (20–42) | 538 (20.0) | 29 (20–44) |
| Histology | ||||||
| Ductal | 21,086 (74.4) | 29 (21–42) | 2,849 (88.3) | 28 (20–41) | 2,312 (86.0) | 29 (20–42) |
| Lobular | 5,736 (20.2) | 32 (22–44) | 282 (8.7) | 33 (23–49) | 104 (3.9) | 29 (19–45) |
| Other | 1,510 (5.3) | 29 (21–41) | 95 (2.9) | 30 (18–43) | 274 (10.2) | 31 (21–44) |
| Grade | ||||||
| 1 | 9,615 (33.9) | 29 (21–42) | 240 (7.4) | 28 (20–40.5) | 86 (3.2) | 28 (19–43) |
| 2 | 14,745 (52.1) | 30 (21–43) | 1,244 (38.6) | 30(20–42) | 661 (24.6) | 31 (21–43) |
| 3 or 4 | 3,972 (14.0) | 28 (20–42) | 1,742 (54) | 28 (20–41) | 1,943 (72.2) | 28 (20–41) |
| Type of surgery | ||||||
| Breast conserving | 19,730 (69.6) | 29 (21–41) | 1,775 (55) | 28 (20–40) | 1,602 (59.6) | 28 (21–41) |
| Mastectomy | 7,179 (25.4) | 29 (20–43) | 1,265 (39.2) | 28 (20–41) | 982 (36.5) | 29 (19–43) |
| Mastectomy w/reconstruction | 1,423 (5.0) | 45 (33–61) | 186 (5.8) | 44 (32–60) | 106 (3.9) | 41 (27–60) |
| Chemo/targeted therapy | ||||||
| No | 22,354 (78.9) | 29 (21–42) | 1,030 (31.9) | 29 (21–42) | 1,171 (43.5) | 30 (21–43) |
| Yes | 5,978 (21.1) | 29 (20–42) | 2,196 (68.1) | 29 (20–41) | 1,519 (56.5) | 28 (20–41) |
| Radiation | ||||||
| No | 9,714 (34.3) | 31 (21–46) | 1,258 (39) | 30 (20–45) | 903 (33.6) | 30 (20–44) |
| Yes | 18,618 (65.7) | 29 (21–41) | 1,968 (61) | 28 (20–40) | 1,787 (66.4) | 29 (20–41) |
| HR status | ||||||
| Negative | 1,200 (37) | 29 (20–42) | 2,690 (100) | |||
| Positive | 28,332 (100) | 2,026 (63) | 28 (20–41) | |||
| Cause of death | ||||||
| Breast cancer | 900 (3.2) | 28 (20–44) | 210 (6.5) | 29 (20–41) | 342 (12.7) | 29 (19–42) |
| Other | 2,800 (9.9) | 28 (20–42) | 334 (10.4) | 30 (20–42) | 324 (12.0) | 28 (20–43) |
| Censored | 24,632 (86.9) | 30 (21–42) | 2,682 (83.1) | 29 (20–41) | 2,024 (75.3) | 29 (21–42) |
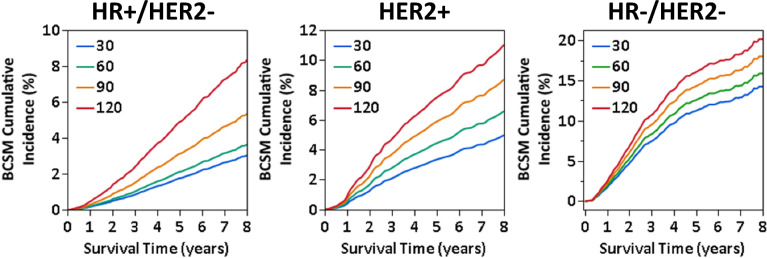
The pattern of BSCM risk associated with TTS varies by subtype. After inverse propensity score weight adjustment, BCSM risk grew across all subtypes with increasing TTS, yet differing extent and patterns were noted by subtype (Fig. 2; summarized at weekly time-points in Table 2). In HR + /HER2 −, the sHR exhibited approximately exponential growth starting at TTS = 42 days, equivalent to 10% higher risk each week relative to the one prior (Supplemental Fig. 1), with adjusted sHR reached 1.21 (95% CI: 1.06–1.37) at TTS = 60 days, 1.79 (95% CI: 1.40–2.29) at TTS = 90 days, and 2.83 (95% CI: 1.76–4.55) at TTS = 120 days compared to the reference of TTS = 30 days (Fig. 2). Adjusted 5-year BCSM cumulative incidence was 0.4% (2.1%; 95% CI: 1.8–32.5%) at TTS = 60 days, 1.4% at TTS = 90 (3.1%; 95% CI: 2.4–4.1%), and 3.1% (4.9%; 95% CI: 3.0–8.1%) higher at TTS = 120 compared to TTS = 30 days (Fig. 3).
Fig. 2.
Adjusted risk (subdistribution hazard ratio [sHR]) of breast cancer-specific mortality (BCSM) associated with continuous TTS in HR + /HER2 −, HER2 + , and HR −/HER2 − locoregional breast cancer patients in a SEER-Medicare cohort
Table 2.
Adjusted risk of breast cancer-specific mortality at weekly points of TTS by subtype
| HR + /HER2- | HER2 + | HR-/HER2- | ||||
|---|---|---|---|---|---|---|
| sHR | 95% CI | sHR | 95% CI | sHR | 95% CI | |
| TTS (days) | ||||||
| 14 | 1.15 | (0.99–1.31) | 1.06 | (0.79–1.42) | 1.07 | (0.85–1.34) |
| 21 | 1.05 | (0.97–1.13) | 0.97 | (0.84–1.13) | 1.00 | (0.89–1.13) |
| 28 | 1.00 | (0.99–1.02) | 0.99 | (0.96–1.02) | 1.00 | (0.98–1.02) |
| 35 | 1.00 | (0.97–1.03) | 1.04 | (0.97–1.12) | 1.01 | (0.96–1.07) |
| 42 | 1.03 | (0.96–1.11) | 1.11 | (0.96–1.28) | 1.04 | (0.93–1.16) |
| 49 | 1.08 | (0.98–1.19) | 1.2 | (0.98–1.46) | 1.07 | (0.92–1.25) |
| 56 | 1.15 | (1.02–1.29) | 1.28 | (1.02–1.64) | 1.11 | (0.91–1.34) |
| 63 | 1.24 | (1.08–1.42) | 1.38 | (1.02–1.87) | 1.14 | (0.91–1.44) |
| 70 | 1.36 | (1.16–1.59) | 1.48 | (1.02–2.13) | 1.18 | (0.89–1.56) |
| 77 | 1.49 | (1.25–1.80) | 1.59 | (1.00–2.51) | 1.22 | (0.86–1.72) |
| 84 | 1.64 | (1.32–2.02) | 1.69 | (0.97–2.94) | 1.26 | (0.83–1.90) |
| 91 | 1.83 | (1.42–2.37) | 1.8 | (0.92–3.53) | 1.30 | (0.79–2.14) |
| 98 | 2.03 | (1.50–2.74) | 1.92 | (0.85–4.35) | 1.34 | (0.73–2.45) |
| 105 | 2.26 | (1.59–3.22) | 2.04 | (0.78–5.35) | 1.38 | (0.68–2.82) |
| 112 | 2.50 | (1.67–3.78) | 2.15 | (0.71–6.51) | 1.42 | (0.63–3.21) |
| 119 | 2.79 | (1.75–4.45) | 2.71 | (0.64–8.06) | 1.46 | (0.58–3.71) |
Time points in this table at which the simultaneous 95% CI did not include a sHR of 1 in comparison to TTS=30 days are noted by bold font
Fig. 3.
Adjusted cumulative incidence function (CIF) of breast cancer-specific mortality (BCSM) in HR + /HER2 −, HER2 + , and HR −/HER2 − locoregional breast cancer patients at specified TTS points (30, 60, 90, and 120 days) in a SEER-Medicare cohort
For HER2 + patients, the sHR increased approximately linearly by 0.10 each week after TTS = 30 days (Supplemental Fig. 1), with the sHR relative to TTS = 30 days were 1.34 (95% CI: 1.02–1.76) at TTS = 60 days, 1.78 (95% CI: 0.92–3.44) at TTS = 90 days, and 2.29 (95% CI: 0.63–8.31) at TTS = 120 days (Fig. 2). Adjusted BCSM cumulative incidence difference at 5 years was 4.1% (TTS = 30 days: 3.3%, 95% CI: 2.4–4.5%; TTS = 120 days: 7.4%, 95% CI: 2.0–27.8%; Fig. 3). For HR −/HER2 − patients, the estimated BCSM risk showed a much smaller linear increase in sHR of approximately 0.04 weekly (Supplemental Fig. 1), with sHR estimates not significantly different. (Fig. 2). Approximately 1.6% difference in 5-year adjusted BCSM cumulative incidence was observed between each 30-day point in TTS, or a 4.8% total difference between TTS = 30 days (11.3%, 95% CI: 9.1–614.0%) and TTS = 120 (16.1%, 95% CI: 6.6–39.1%), and 2.0% (TTS = 30 days: 14.2%, 95% CI: 11.6–17.5%; TTS = 120 days: 20.2%, 95% CI: 9.5–42.7%; Fig. 3).
Discussion
Our study is the first to provide dynamic insight into subtype-specific differential patterns of BCSM risk associated with TTS. Through a robust statistical approach, capturing flexible daily estimates of BCSM risk rather than broadly grouped, discrete TTS intervals [13–15, 24], we found that patients with HR + /HER2 − breast cancer experienced a rapid exponential trajectory of TTS-associated mortality risk, as opposed to the slower linear growth seen for patients with HER2 + and HR −/HER2 − breast cancer. The adjusted TTS-associated BCSM cumulative incidence in HR + /HER2 − patients was reflected by increasingly larger gaps, with approximately 5% higher 8-year mortality in patients with TTS = 120 days compared to 30 days (3.0% vs. 8.4%). This is important, especially since patients with TTS beyond 60 days are continually exposed to growing risk across the follow-up period. Our data showed growing BCSM risk in all subtypes with increasing TTS, albeit non-significant in HR −/HER2 − patients, emphasizing the benefit of timely surgery after biopsy diagnosis, consistent with the CoC’s recent quality measure for surgery within 60 days of diagnosis for non-neoadjuvant Stage I–III patients [11].
The observed differences in mortality pattern by subtype were unexpected based on the prevailing view of a favorable prognosis for HR + /HER2 − disease and a less favorable prognosis for HER2 + and HR −/HER2 − breast cancer [26]. Death from breast cancer is primarily the result of metastatic outgrowth of disseminated cells in distant organs, thus provoking the question as to whether metastatic dissemination occurs differently by subtype during TTS. Breast cancer dissemination is proposed to occur by two key mechanisms: (1) linear progression in which cancer cells and the tumor microenvironment gradually acquire a phenotype conducive to metastasis or (2) parallel progression through early dissemination of inherently metastatic cancer cells in response to an angiogenic switch [27, 28]. Mathematical simulation indicates that the natural breast tumor growth rate is relatively slow, taking approximately 1.7 years for a 1 cm tumor to double in size [29]. Thus, tumor size upstaging of T1N0M0 patients [14] and exponential BCSM risk in HR + /HER2 − after a brief 42-day period following diagnosis may suggest the possibility of accelerated disease progression beyond the rate of natural linear progression after diagnosis [14]. We have provided possible biological explanations for such a rapid disease progression by demonstrating that needle biopsy of breast tumors leaves an unhealed wound that provokes pro-metastatic changes such as epithelial-to-mesenchymal transition and angiogenesis, processes critical for disease dissemination, using two independent mouse models of ER + breast cancer [30]. In this light, TTS may provide extra time for progressive phenotypic or microenvironment changes, especially in predominantly epithelial-like, well-differentiated ER + breast cancers, instead of generally poorly differentiated TN and HER2 subtypes. Since TTS-associated mortality could reflect an intrinsic metastatic subpopulation within the highly heterogeneous HR + /HER2 − subtype, both Oncotype recurrence risk score and percentage of estrogen receptor/progesterone receptor positivity, which contribute to substantial prognostic diversity even in early-stage disease [31–33], should be further investigated to determine their role in TTS-associated outcomes. Along similar lines, adjuvant chemotherapy may impact TTS-associated BCSM risk by attenuating the likelihood of disseminated cancer cell survival during the TTS period. Indeed, only 21% of HR + /HER2- patients in our cohort received systemic chemotherapy due to expected insufficient response [34], as compared to 68% and 56% in HER2 + and HR −/HER2 − breast cancer, respectively (Table 1). In this respect, we could not disentangle the potential effects of adjuvant chemotherapy and subtype on TTS-associated mortality patterns in the current study.
The primary strength of this study lies in robust statistical modeling to identify the dynamic nature of mortality risk associated with TTS. Integrating the novel approach for propensity score calculation for non-parametric continuous variables developed by Imai and colleagues [21–23] to adjust for socio-demographic and clinical characteristics, Fine-Gray competing risk survival analysis delineated non-linear TTS-associated risk of BCSM in HR + /HER2 − patients, which may not be visible when fixed effect sizes across TTS monthly or bi-monthly increments are compared. Limitations of this study include the potential for confounders outside the scope of, or with incomplete reporting in, the databases (e.g., clinical staging, Ki67 status). Additionally, the cohort is composed of an elderly population with Medicare coverage and the sample size of patients with HER2 + or HR −/HER2 − subtype, which typically makes up a greater proportion of younger patients [35], is limited, resulting in large confidence bounds. Despite the strength of examining BCSM in the competing risk models, there is the potential for the primary cause of death to be misattributed based on death certificate records [36]. Thus, further studies of differences in TTS-associated BCSM risk by subtype in younger women and across more diverse socioeconomic statuses are recommended. Further study to elucidate the underlying reasons for TTS-associated mortality risk, including delay of adjuvant therapies, biologic changes [30], or natural disease progression, is warranted.
Conclusion
This study identified that the association between surgical delay and BCSM risk varies by tumor subtype, with a rapid exponential increase in risk in HR + /HER2 − patients and lesser linear increases in patients with HER2 + or HR −/HER2 − breast cancer. Prevention of surgical delays holds the potential to improve survival outcomes for patients with locoregional breast cancer.
Supplementary Information
Supplementary file 1. ICD-O-2 codes used to classify tumor histology.
Supplementary file 2. HCPCS, ICD-9, and ICD-10 codes used to classify breast cancer surgery and treatment types.
Supplementary file 3. Approximated equations for breast cancer-specific mortality risk relative to TTS as a spline function, calculated on the log-subdistribution hazard scale in HR+/HER2- patients and subdistribution hazard scale in HER2+ and HR-/HER2-
Acknowledgements
The study was partly supported by the National Cancer Institute Cancer Center Support Grant P30CA225520 and the Oklahoma Tobacco Settlement Endowment Trust contract awarded to the University of Oklahoma Stephenson Cancer Center.
Abbreviations
- HR +
Hormone receptor-positive
- HR −
Hormone receptor-negative
- HER2
Human epidermal growth factor receptor 2
- TTS
Time from biopsy to surgery
- BCSM
Breast cancer specific mortality
- HR
Hazard ratio
- sHR
Subdistribuition hazard ratio
- IPW
Inverse propensity score weights
- SEER
Surveillance, epidemiology, and end results
Author contributions
Conception and design: TT, MLS, IC, and HR Financial support: TT Collection and assembly of data: MLS, RP, and IC Data analysis and interpretation: MLS, IC, TT, WD, and HR Manuscript writing: All authors Final approval of manuscript: All authors Accountable for all aspects of the work: All authors.
Funding
This study was supported by the Department of Defense (TT: HT9425-23-1-0710 from 2023 to 2026).
Data availability
The data that support this study's findings are available from the NCI SEER program, but restrictions apply to their availability. These data were used under license for the current study and are not publicly available. However, data are available from the authors upon reasonable request and with permission of the SEER program.
Code availability
The underlying code for this study is available in the Appendix.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Inna Chervoneva, Email: Inna.Chervoneva@jefferson.edu.
Takemi Tanaka, Email: takemi-tanaka@ouhsc.edu.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 3.Breastcancer.org. Breast Cancer Facts and Statistics. 2024. https://www.breastcancer.org/facts-statistics
- 4.Society AC. Breast cancer facts & figures 2024–2025. American Cancer Society 2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/2024/breast-cancer-facts-and-figures-2024.pdf
- 5.Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed]
- 6.Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed]
- 7.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–85. 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 9.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209(2):180–7. 10.1016/j.jamcollsurg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulvat M, Sandalow N, Rademaker A, Helenowski I, Hansen NM. Time from diagnosis to definitive operative treatment of operable breast cancer in the era of multimodal imaging. Surgery. 2010;148(4):746–50. 10.1016/j.surg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 11.NCDB announces new breast quality measure 2022. https://www.facs.org/for-medical-professionals/news-publications/news-and-articles/acs-brief/july-12-2022-issue/ncdb-announces-new-breast-quality-measure/. Accessed 6 October 2022.
- 12.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 13.Mateo AM, Mazor AM, Obeid E, et al. Time to surgery and the impact of delay in the non-neoadjuvant setting on triple-negative breast cancers and other phenotypes. Ann Surg Oncol. 2020;27(5):1679–92. 10.1245/s10434-019-08050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hills N, Leslie M, Davis R, et al. Prolonged time from diagnosis to breast-conserving surgery is associated with upstaging in hormone receptor-positive invasive ductal breast carcinoma. Ann Surg Oncol. 2021;28(11):5895–905. 10.1245/s10434-021-09747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–9. 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson L, Bergh J, Humphreys K, Warnberg F, Tornberg S, Czene K. Time from breast cancer diagnosis to therapeutic surgery and breast cancer prognosis: a population-based cohort study. Int J Cancer. 2018. 10.1002/ijc.31411. [DOI] [PubMed] [Google Scholar]
- 17.Institute NC. SEER-Medicare: Brief Description of the SEER-Medicare Database. Updated 16 May, 2019. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed 20 August 2021.
- 18.Services UDoHaH. Who's eligible for Medicare? 2024. https://www.hhs.gov/answers/medicare-and-medicaid/who-is-eligible-for-medicare/index.html
- 19.Dressman LA. Navigator identification of breast biopsy results: a time analysis of biopsy results and variance in turnaround time. presented at: academy of oncology nurse & patient navigators seventh annual conference; 2016; Las Vegas, NV. Accessed 14 October 2022. https://www.jons-online.com/jons-categories?view=article&artid=1521:18-navigator-identification-of-breast-biopsy-results-a-time-analysis-of-biopsy-results-and-variance-in-turnaround-time&catid=87
- 20.Institute NC. SEER-Medicare: Comorbidity SAS Macros. Updated April 19, 2024. https://healthcaredelivery.cancer.gov/seermedicare/considerations/calculation.html
- 21.Imai K, van Dyk DA. Causal inference with general treatment regimes: generalizing the propensity score. J Am Stat Assoc. 2004;99(467):854–66. 10.1198/016214504000001187. [Google Scholar]
- 22.Imai KRM. Covariate balancing propensity score. J Roy Stat Soc. 2014;76(1):243–63. [Google Scholar]
- 23.Ratkovic M, Imai, K. and Fong, C. Cbps: R package for covariate balancing propensity score.
- 24.Prakash I, Thomas SM, Greenup RA, et al. Time to surgery among women treated with neoadjuvant systemic therapy and upfront surgery for breast cancer. Breast Cancer Res Treat. 2021;186(2):535–50. 10.1007/s10549-020-06012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41(1/2):133–45. 10.2307/2333011. [Google Scholar]
- 26.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–26. 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 27.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–12. 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 28.Gui P, Bivona TG. Evolution of metastasis: new tools and insights. Trends Cancer. 2022;8(2):98–109. 10.1016/j.trecan.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10(3):R41. 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kameyama H, Dondapati P, Simmons R, et al. Needle biopsy accelerates pro-metastatic changes and systemic dissemination in breast cancer: implications for mortality by surgery delay. Cell Rep Med. 2023;4(12):101330. 10.1016/j.xcrm.2023.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138. 10.1186/s12885-015-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaafsma E, Zhang B, Schaafsma M, Tong CY, Zhang L, Cheng C. Impact of oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. 2021;23(1):74. 10.1186/s13058-021-01453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVeigh TP, Kerin MJ. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer (Dove Med Press). 2017;9:393–400. 10.2147/BCTT.S109847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;23(3):749–56. 10.1245/s10434-015-4921-5. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute: Surveillance E, and End Results Program,. Cancer Stat Facts: Female Breast Cancer Subtypes. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed 14 October 2022.
- 36.National cancer institute: surveillance E, and end results program SEER cause-specific death classification. 2024. https://seer.cancer.gov/causespecific/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. ICD-O-2 codes used to classify tumor histology.
Supplementary file 2. HCPCS, ICD-9, and ICD-10 codes used to classify breast cancer surgery and treatment types.
Supplementary file 3. Approximated equations for breast cancer-specific mortality risk relative to TTS as a spline function, calculated on the log-subdistribution hazard scale in HR+/HER2- patients and subdistribution hazard scale in HER2+ and HR-/HER2-
Data Availability Statement
The data that support this study's findings are available from the NCI SEER program, but restrictions apply to their availability. These data were used under license for the current study and are not publicly available. However, data are available from the authors upon reasonable request and with permission of the SEER program.
The underlying code for this study is available in the Appendix.