Abstract
Background
Taenia spp. and Toxoplasma gondii are foodborne parasites affecting humans and pigs. The magnitude of the burden of these parasites in pigs in Burundi is not known. Therefore, this study aimed to estimate the prevalence of Taenia spp. infections in pigs by meat inspection, partial carcass dissection and molecular confirmation and estimate the prevalence of Toxoplasma gondii infection in pigs by serology. A cross-sectional study was conducted in pig slaughter slabs located in Bujumbura city, Kayanza and Ngozi provinces. Multisampling strategies were used to sample 576 pigs. Upon pig slaughter, blood samples were collected to perform indirect ELISA for detecting antibodies against the T. gondii P30 protein in the serum. Routine meat inspection was carried out to detect T. solium and T. hydatigena cysticerci. The tongue, heart and masseter muscles were dissected by making slices less than 5 mm thick to estimate the intensity and stages of T. solium cysticerci. A subset of cysticerci and suspected lesions per infected pig were examined using PCR-RFLP to differentiate Taenia spp.
Results
Of the 576 pigs, 14 (2.4%) were positive for T. solium cysticercosis by meat inspection and 67 (11.6%) by partial carcass dissection. After molecular analysis, 66 (11.5%) samples were confirmed to be T. solium infections. The average of T. solium cysticerci in the dissected organs was estimated at 80 cysticerci. Most cysticerci (76.1%) were counted in the masseter muscles, followed by the tongue (18.8%) and the heart (5.1%). The majority of cysticerci (88.3%) were viable, 6.4% were calcified and 5.3% were degenerated. Approximately 69% of the pigs infected with T. solium had light infections, 13.4% had moderate infections and 17.9% had heavy infections. Thirty-two out of 576 pigs (5.5%) were suspected of being infected with T. hydatigena by meat inspection, but 24 pigs (4.2%) were confirmed molecularly to be positive for T. hydatigena infection. The seroprevalence of T. gondii infection in pigs was 17.7%.
Conclusions
This study indicates that T. solium and T. gondii parasites are endemic in Burundi and provides evidence of potential public health risks for the local population. Effective control strategies, including improved pig farming practices, better hygiene and sanitation, increased meat inspection, monitoring of infected pigs, risk-free culinary practices, and treatment of tapeworm carriers, should be implemented to avoid the perpetual contamination of pigs and humans with these zoonotic parasites.
Keywords: Occurrence, Taenia solium, Taenia hydatigena, Toxoplasma gondii, Pigs, Burundi
Background
Taenia spp. and Toxoplasma gondii are among the foodborne parasites contributing to a high societal and health burden [1]. Taenia solium is a zoonotic parasite with a complex two-host life cycle, including humans as definitive hosts, and pigs and accidentally humans as intermediate hosts [2]. It is endemic in low- and middle-income countries, including those in sub-Saharan Africa, South and Central America, Southeast Asia, and the Western Pacific [1]. Humans acquire T. solium taeniasis by eating raw or undercooked pork containing metacestode larval stages (cysticerci), which results in the development of an adult pork tapeworm in the small intestine [2]. Most patients with pork tapeworms remain asymptomatic, though they may experience abdominal pain, diarrhoea, bloating, and nausea [2]. Humans can get accidentally infected by ingesting fruits, vegetables, and water contaminated with eggs from adult tapeworm (T. solium) carriers, leading to (neuro)cysticercosis, a major cause of acquired epilepsy in sub-Saharan Africa [3, 4]. Patients with neurocysticercosis may develop symptoms several years after infection, including epilepsy, hydrocephalus, severe headaches, stroke, and dementia, with epilepsy being the most common neurological disorder [5]. Pigs develop T. solium cysticercosis by ingesting human faeces, feed, and water contaminated with eggs from adult tapeworms, resulting in the development of cysticerci in muscles and organs [6]. Pigs infected with T. solium typically do not show clinical signs. Still, in rare cases, they may develop myositis in locomotion, somnolence, chewing disorders, seizures in heavily infected pigs, and loss of consciousness [7, 8]. Pigs can also get infected with Taenia hydatigena, a non-zoonotic cestode whose metacestode larval stages develop in the organs and membranes of the thoracic and abdominal cavities, while dogs and other canids act as definitive hosts, harbouring the adult tapeworm in their small intestines [9]. Toxoplasma gondii is a cosmopolitan zoonotic parasite that infects all warm-blooded species, including pigs and humans as intermediate hosts, and cats and other felids as definitive hosts [10]. Humans acquire toxoplasmosis through ingestion of undercooked meat containing T. gondii cysts and oocysts from water, soil, or food contaminated with cat faeces [11]. They can get infected through transplacental transmission, blood transfusion or organ transplantation, and accidental inoculation of tachyzoites [11]. Most cases of acquired human toxoplasmosis are asymptomatic, but congenital toxoplasmosis can cause abortion, stillbirth, or result in newborns developing neurological and ocular disorders such as encephalitis, hydrocephalus, chorioretinitis, intracranial calcifications, and mental retardation [12, 13]. Pigs, like other livestock and wild animals, become infected through ingestion of oocysts from environmental contamination with cat faeces; ingestion of cysts in the tissues of infected birds, rodents, or cannibalism, and by vertical transmission [10]. Toxoplasma gondii infection in pigs is asymptomatic, but can lead to abortions during pregnancy [14].
Epidemiological studies on T. solium and T. gondii infections have been conducted in many countries around the world and have shown a high prevalence of these infections in Africa [15–18]. Higher prevalences for T. solium and T. gondii infections were usually observed in pigs and humans in countries with inadequate hygiene and sanitation, traditional pig farming practices, and inadequate meat inspection [1, 10]. In Burundi, pig farming is a major source of income for resource-poor farmers, where risk factors for Taenia spp. and T. gondii transmission are abundant [19]. A prevalence of 15.5% by tongue palpation and 31.5% by antibody enzyme-linked immunosorbent assay (Ab-ELISA) was reported for T. solium cysticercosis in pigs and humans, respectively [19, 20]. The prevalence of toxoplasmosis was 44.1% by Ab-ELISA and indirect immunofluorescence test for humans, whereas no data is currently available for toxoplasmosis in pigs [21]. Although some epidemiological data on T. solium cysticercosis are available in Burundi, updated data using more optimal diagnostic techniques are needed. Full carcass dissection with meat slices less than 5 mm thick is considered the gold standard diagnostic technique for estimating T. solium occurrence, cysticerci stages and infection intensity levels in endemic areas [22]. Partial carcass dissection including only the heart, tongue and masseter muscles showed satisfactory performance results, with a sensitivity estimated at 81% [23]. Considering the labour-intensive process and high costs of purchasing pigs for full carcass dissection, researchers are recommended to use partial carcass dissection to save time, labour, and costs [22, 23]. A study unravelling the occurrence and burden of Taenia spp. and T. gondii in pigs is needed to provide evidence-based results and to advocate for the development and implementation of control measures against these parasites in the future. Therefore, this study aimed to (i) estimate the prevalence of Taenia spp. infections based on meat inspection, partial carcass dissection, and molecular confirmation and (ii) estimate the prevalence of T. gondii infection in pigs using serology.
Results
Pig slaughter slab facilities and sample description
Of all the pig slaughter slabs visited, only the national slaughterhouse in Bujumbura city adhered to basic standards including appropriate facilities and hygienic standards (Fig. 1). In contrast, other pig slaughter slabs, such as in Gikoma in Bujumbura city and those in Kayanza and Ngozi provinces, had low levels of hygiene and cleanliness, with pigs being slaughtered outdoors on wooden or cemented floors, or even in the bush (Fig. 2). A total of 576 pigs were sampled in different slaughter slabs. More than half of the pigs (320/576, 55.6%) were females. The age of the pigs ranged from 6 months to 36 months with an average age of 14 months. Sixty percent of the pigs (60%) were cross breeds (large white), while 40% were local breeds (black pigs).
Fig. 1.

Pigs slaughtered at the national slaughterhouse in Bujumbura city
Fig. 2.

An example of a pig slaughter slab in the study area (Gikoma, Kayanza and Ngozi)
Prevalence of Taenia spp. infections using meat inspection, partial carcass dissection and molecular confirmation
By meat inspection, 14 out of 576 pigs (2.4%, 95% CI: 1.3-4.0) were positive while by partial carcass dissection 67 out of 576 pigs (11.6%, 95% CI: 9.1–14.5) were positive for T. solium cysticercosis, respectively (Table 1). Of the 67 pigs positive by partial carcass dissection, 66 (98.5%) were confirmed molecularly, leading to a prevalence based on molecular confirmation of 11.5% (95% CI: 9-14.4), including 26 pigs (9.0%, 95% CI: 6–13) in Bujumbura city, 24 pigs (16.7%, 95% CI: 11-23.8) in Kayanza province and 16 pigs (11.1%, 95% CI: 6.5–17.4) in Ngozi province. Significant differences in prevalence were observed in pig breed and origin (Table 1). The cysticerci intensity in the dissected organs and muscles of pigs infected with T. solium ranged from 1 to 1449 cysticerci, with an average of 80 cysticerci. Most cysticerci (76.1%) were found and counted in the masseter muscles, followed by the tongue (18.8%) and the heart (5.1%). The number of masseter muscle cysticerci was significantly higher than in the tongue and heart (p < 0.05) (Table 2). In organs and muscles, the majority of cysticerci were viable (88.3%) (Figs. 3, 4 and 5). Six-point-four percent (6.4%) were calcified cysticerci and 5.3% degenerated. No significant differences were observed in cysticerci intensity by province, sex, age and breed (Table 3). Based on infection intensities, 46 pigs (68.7%) infected with T. solium had light infections, 9 pigs (13.4%) had moderate infections, and 12 pigs (17.9%) had heavy infections (Table 4).
Table 1.
Distribution of the prevalence of Taenia solium cysticercosis by meat inspection and partial carcass dissection
| Variables | N | P MI | MI % (95% CI) | P PCD | PCD % (95% CI) | χ2PCD | p-value |
|---|---|---|---|---|---|---|---|
| Provinces | |||||||
| Bujumbura city | 288 | 5 | 1.7 (0.6-4) | 26 | 9.0 (6–13) | 5.46 | 0.065 |
| Kayanza | 144 | 7 | 4.9 (2-9.8) | 24 | 16.7 (11-23.8) | ||
| Ngozi | 144 | 2 | 1.4 (0.2–4.9) | 17 | 11.8 (7.0-18.2) | ||
| Slaughter slabs | |||||||
| National slaughterhouse | 144 | 1 | 0.7 (0.0-3.8) | 14 | 9.7 (5.4–15.8) | 5.93 | 0.431 |
| Gikoma | 144 | 4 | 2.8 (0.8-7) | 12 | 8.3 (4.4–14.1) | ||
| Kayanza | 104 | 6 | 5.8 (2.2–12.1) | 18 | 17.3 (10.6–26) | ||
| Muhanga | 40 | 1 | 2.5 (0.1–13.2) | 6 | 15 (5.7–29.8) | ||
| Ngozi | 79 | 1 | 1.3 (0.0-6.9) | 10 | 12.7 (6.2–22.1) | ||
| Busiga | 34 | 1 | 2.9 (0.1–15.3) | 4 | 11.8 (3.3–27.5) | ||
| Gashikanwa | 31 | 0 | 0.0 | 3 | 9.7 (2-25.8) | ||
| Sex | |||||||
| Male | 256 | 4 | 1.6 (0.4-4) | 23 | 9 (5.8–13.2) | 2.69 | 0.101 |
| Female | 320 | 10 | 3.1 (1.5–5.7) | 44 | 13.8 (10.2–18) | ||
| Age | |||||||
| 6–12 months | 285 | 4 | 1.4 (0.4–3.6) | 29 | 10.2 (6.9–14.3) | 0.90 | 0.343 |
| ≥ 13 months | 291 | 10 | 3.4 (1.7–6.2) | 38 | 13.1 (9.4–17.5) | ||
| Breed | |||||||
| Local | 228 | 9 | 4 (1.8–7.4) | 39 | 17.1 (12.5–22.6) | 10.14 | 0.001* |
| Crossed | 348 | 5 | 1.4 (0.5–3.3) | 28 | 8.1 (5.4–11.4) | ||
| Origin of pigs | |||||||
| Bujumbura | 55 | 0 | 0.0 | 4 | 7.3 (2-17.6) | 11.54 | 0.042* |
| Gitega | 15 | 1 | 6.7 (0.2–32) | 4 | 26.7 (7.8–55.1) | ||
| Karusi | 67 | 0 | 0.0 | 2 | 3 (0.4–10.4) | ||
| Kayanza | 221 | 8 | 3.6 (1.6-7.0) | 31 | 14.0 (9.7–19.3) | ||
| Kirundo | 28 | 3 | 10.7 (2.3–28.2) | 5 | 17.9 (6.1–36.9) | ||
| Ngozi | 190 | 2 | 1.1 (0.1–3.8) | 21 | 11.1 (7-16.4) | ||
| Total | 576 | 14 | 2.4 (1.3-4.0) | 67 | 11.6 (9.1–14.5) | - | - |
N: Number of examined pigs, P: Number of infected pigs, MI: Meat inspection, PCD: Partial carcass dissection, CI: confidence interval, χ2: chi-square, *significant
Table 2.
Taenia solium cysticerci intensity and stages in organs and muscles
| Organs and muscles | Infected pigs (%) | Viable | Degenerated | Calcified | Total | % | p-value |
|---|---|---|---|---|---|---|---|
| Heart | 22 (32.8) | 205 | 2 | 64 | 271 | 5.1 | < 0.001 (Ref) |
| Tongue | 39 (58.2) | 891 | 28 | 82 | 1001 | 18.8 | 0.091 |
| Masseter | 40 (59.7) | 3610 | 254 | 194 | 4058 | 76.1 | < 0.001* |
| Total | - | 4706 (88.3%) | 284 (5.3%) | 340 (6.4%) | 5330 | 100 | |
| Only tongue | 20 (29.8) | 24 | 0 | 5 | 29 | 0.6 | |
| Only heart | 6 (9) | 2 | 0 | 5 | 7 | 0.1 | |
| Only masseter | 20 (29.8) | 100 | 5 | 8 | 113 | 2.1 | |
| Tongue + heart | 1 (1.5) | 0 | 0 | 5 | 5 | 0.1 | |
| Tongue + masseter | 5 (7.5) | 591 | 228 | 53 | 872 | 16.4 | |
| Heart + masseter | 2 (3) | 23 | 0 | 5 | 28 | 0.5 | |
| Tongue + heart + masseter | 13 (19.4) | 3966 | 51 | 259 | 4276 | 80.2 | |
| Total | 67 (100) | 4706 (88.3%) | 284 (5.3%) | 340 (6.4%) | 5330 | 100 |
*Significant
Fig. 3.
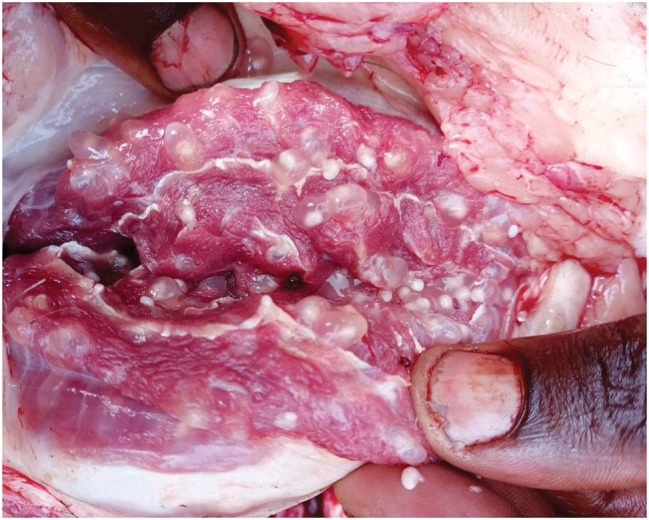
An example of a masseter muscle heavily infected with Taenia solium cysticerci
Fig. 4.

An example of a heart heavily infected with Taenia solium cysticerci
Fig. 5.
An example of a tongue heavily infected with Taenia solium cysticerci
Table 3.
Taenia solium cysticerci intensity in infected pigs by province, sex, age and breed
| Variables | Infected pigs | Total number and mean of cysticerci per infected pig | % of all cysticerci | p-value |
|---|---|---|---|---|
| Provinces | ||||
| Bujumbura city | 26 | 1738 (66.8) | 32.6 | < 0.001 (Ref) |
| Kayanza | 24 | 2809 (117) | 52.7 | 0.317 |
| Ngozi | 17 | 783 (46.1) | 14.7 | 0.546 |
| Sex | ||||
| Female | 44 | 3052 (69.4) | 57.3 | < 0.001 (Ref) |
| Male | 23 | 2278 (99) | 42.7 | 0.487 |
| Age | ||||
| 6–12 months | 29 | 1353 (46.7) | 25.4 | < 0.001(Ref) |
| ≥ 13 months | 38 | 3977 (104.7) | 74.6 | 0.097 |
| Breed | ||||
| Crossed | 28 | 1708 (61) | 32.0 | < 0.001 (Ref) |
| Local | 39 | 3622 (92.9) | 68 | 0.394 |
Table 4.
Proportion of Taenia solium infection levels in infected pigs per province
| Infection intensity level | Bujumbura city | Kayanza | Ngozi | Total | % | Mean of cysticerci per infected pig |
|---|---|---|---|---|---|---|
| Light (1–10 cysticerci) | 19 | 13 | 14 | 46 | 68.7 | 2 |
| Moderate (11–100 cysticerci) | 3 | 6 | 0 | 9 | 13.4 | 49 |
| Heavy (101–1449 cysticerci) | 4 | 5 | 3 | 12 | 17.9 | 402 |
| Total | 26 | 24 | 17 | 67 | 100 | 80 |
| Light (diagnosed by MI) | 0 | 0 | 0 | 0/46 | 0.00 | - |
| Moderate (diagnosed by MI) | 1 | 3 | 0 | 4/9 | 44.4 | - |
| Heavy (diagnosed by MI) | 4 | 4 | 2 | 10/12 | 83.3 | - |
| Total | 5 | 7 | 2 | 14/67 | 20.9 | - |
MI: Meat inspection
Regarding T. hydatigena infection, 32 out of 576 pigs (5.5%, 95% CI: 3.8–7.8) were suspected of being infected during the inspection, including 7 pigs (1.2%, 95% CI: 0.3–2.1) having large T. hydatigena cysticerci in the liver and mesentery and 25 pigs (4.3%, 95% CI: 2.8–6.3) with small cysticerci or lesions in the liver (Figs. 6, 7 and 8). Of those 32 pigs suspected of T. hydatigena infection, 24 (7 with large cysticerci in the liver and mesentery and 17 with small cysticerci or lesions in the liver) were confirmed molecularly to be positive for T. hydatigena, and one with a small cysticercus in the liver was confirmed molecularly to be positive for T. solium. The overall prevalence of T. hydatigena infection was 4.2% (95% CI: 2.7–6.1), including 12 pigs (4.2%, 95% CI: 2.2–7.2) in Bujumbura city, 6 pigs (4.2%, 95% CI: 1.5–8.9) in Kayanza province and 6 pigs (4.2%, 95% CI: 1.5–8.9) in Ngozi province. The pig with the small cysticercus in the liver, which was confirmed positive for T. solium cysticercosis, also tested positive during both partial carcass dissection and molecular analyses. In addition, 2 pigs were co-infected with T. solium and T. hydatigena. The first pig had T. solium cysticerci in the masseter muscles and T. hydatigena cysticerci in the mesentery. The second pig had T. solium cysticerci on the tongue and T. hydatigena cysticerci in the liver. Seven negative samples at PCR-RFLP from liver suspected lesions were also negative for Echinococcus spp. and Sarcocystis spp. using multiplex PCR.
Fig. 6.

Liver infected with Taenia hydatigena cysticerci
Fig. 7.

Large Taenia hydatigena cysticerci found in the abdominal cavity (mesentery)
Fig. 8.

Liver with a suspected lesion (small white nodule)
Prevalence of T. gondii infection in pigs based on serological analyses
Of the 576 pig sera, antibodies against T. gondii were detected in 102 pigs (17.7%, 95% CI: 14.7–21.1) (Table 5). Toxoplasma gondii seropositivity was significantly associated with sex, location of slaughter slabs, and origin of pigs (Table 5).
Table 5.
Distribution of the prevalence of toxoplasmosis in pigs in Burundi using indirect ELISA
| Variables | Examined pigs | Infected pigs | Prevalence % (95% CI) | Chi-square | p-value |
|---|---|---|---|---|---|
| Provinces | |||||
| Bujumbura city | 288 | 52 | 18.1 (13.9–23) | 0.43 | 0.807 |
| Kayanza | 144 | 23 | 16 (10.4–23) | ||
| Ngozi | 144 | 27 | 18.8 (12.7–26.1) | ||
| Slaughter slabs | |||||
| National slaughterhouse | 144 | 17 | 11.8 (7.0-18.2) | 16.45 | 0.012* |
| Gikoma | 144 | 35 | 24.3 (17.6–32.2) | ||
| Kayanza | 104 | 15 | 14.4 (8.3–22.7) | ||
| Muhanga | 40 | 8 | 20 (9.1–35.7) | ||
| Ngozi | 79 | 21 | 26.6 (17.3–37.7) | ||
| Busiga | 34 | 4 | 11.8 (3.3–27.5) | ||
| Gashikanwa | 31 | 2 | 6.5 (0.8–21.4) | ||
| Sex | |||||
| Male | 256 | 35 | 13.7 (9.7–18.5) | 4.67 | 0.031* |
| Female | 320 | 67 | 20.9 (16.6–25.8) | ||
| Age | |||||
| 6–12 months | 285 | 48 | 16.8 (12.7–21.7) | 0.18 | 0.667 |
| ≥ 13 months | 291 | 54 | 18.6 (14.3–23.5) | ||
| Breed | |||||
| Local | 228 | 44 | 19.3 (14.4–25.0) | 0.49 | 0.486 |
| Crossed | 348 | 58 | 16.7 (12.9–21.0) | ||
| Origin of pigs | |||||
| Bujumbura | 55 | 20 | 36.4 (23.8–50.4) | 23.76 | < 0.001* |
| Gitega | 15 | 1 | 6.7 (0.2–32) | ||
| Karusi | 67 | 4 | 6 (1.7–14.6) | ||
| Kayanza | 221 | 32 | 14.5 (10.1–19.8) | ||
| Kirundo | 28 | 5 | 17.9 (6.1–36.9) | ||
| Ngozi | 190 | 40 | 21.1 (15.5–27.5) | ||
| Total | 576 | 102 | 17.7 (14.7–21.1) | - | - |
CI: Confidence interval, *significant
Discussion
This study is the first to report the occurrence of T. hydatigena and T. gondii in pigs in Burundi. The prevalence of T. solium cysticercosis by partial carcass dissection was almost five times higher than that detected by meat inspection. This could be explained by the fact that partial carcass dissection is more sensitive (81%) compared to meat inspection (22.1%), particularly for light infections [23, 24], which represented the majority in our study (68.7%). Meat inspection, the routinely implemented technique in Burundi, detected only heavy and some moderate infections and failed to detect all light infections, which could lead to significant public health problems due to the consumption of these infected carcasses, especially as most detected cysts were viable. In this study, the prevalence based on meat inspection was low. Similar low prevalences were reported in Kenya (1.8%), India (1.4%), Uganda (0.6%), Madagascar (4.6%) and South Africa (5%) [25–29]. This low prevalence in Burundi could be due to tongue palpation carried out before the sale or slaughter of pigs, which led to the clandestine and home slaughter of infected pigs. This is a common practice in Burundi to avoid carcass condemnation in slaughter slabs [19]. In a study conducted on pig farms in Burundi, a high prevalence of T. solium cysticercosis (15.5%) in pigs based on tongue palpation was reported [19]. Despite the low sensitivity of tongue palpation in light infections (21%) [24], it is still a rapid and cheap diagnostic tool used by pig traders and butchers in Burundi to ensure they bring healthy pigs to the slaughter slabs. However, this practice can lead to huge losses for farmers when a pig is found infected with cysticerci on the tongue, as the selling price is discounted up to 80% for an infected pig [19, 30]. This can have significant implications for public health because infected pigs do not reach slaughter slabs for meat inspection [19]. They are either slaughtered at home for family and neighbourhood consumption or they undergo clandestine slaughter by butchers for public sale at various outlets, thus posing a high risk for T. solium taeniasis. This practice of consuming infected pork from clandestine or home slaughter ensures sustained local transmission, as often pig farmers will get infected with pork tapeworms, resulting in greater environmental contamination with Taenia eggs and an increased risk of pig infection within the communities.
Partial carcass dissection, an alternative technique to full carcass dissection (the gold standard), was used to estimate T. solium cysticerci intensities and stages in organs and muscles [23]. Similar prevalences based on partial carcass dissection including tongue, heart, and masseter muscles were estimated at 12.1% in Peru and 17.6% in Cameroon [23]. With a sensitivity of 81%, partial carcass dissection is probably the better technique under research conditions, due to cost savings in purchasing pigs when the sample size is large, as well as the reduced labour and time required to dissect the entire carcass [22, 23]. Although this technique is more sensitive than meat inspection, implementing this technique routinely at pig slaughter slabs is impossible due to its laborious and time-consuming nature, coupled with the loss of value in dissected meat and potential unfamiliarity among meat inspectors.
In this study, local breed pigs were more infected than cross breeds and pigs from Gitega and Kirundo were more infected than those from other provinces. This could probably be explained by the fact that cross breeds were mainly found on large farms with improved husbandry practices, regular deworming schedules, and commercial feeds, in contrast to black pigs kept on small-scale farms with traditional farming practices, including free-ranging. Furthermore, in the densely populated provinces of Burundi (Gitega, Kirundo, Kayanza, Muyinga, and Ngozi), pigs are typically raised on small-scale farms using traditional methods intended to generate household income with minimal inputs [19, 31]. This approach may increase the risk of exposing pigs in these areas to T. solium eggs. Based on infection intensity, 65.4% of pigs infected with cysticerci in Tanzania and 76% in Zambia had light to moderate infections, consistent with our results [22, 32]. It was demonstrated that free-range pigs were likely to have cysticercosis with moderate or heavy infections due to the ingestion of human faeces released during open defecation, which might contain large quantities of pork tapeworm eggs [33]. In addition, light infections in pigs could result from a low dose of pork tapeworm eggs, potentially ingested by roaming in the field, herbs brought into the pig pens, or consuming contaminated water [33]. All these factors could explain the three levels of cysticercosis infection in Burundi associated with pig farming systems, including pigs raised in pens, partially penned pigs and free-range pigs [19]. Findings in Cameroon corroborated the heavier infections in masseter muscles compared to the tongue and heart [34]. Given the high T. solium cysticerci intensities in masseter muscles and the tongue, the Ministry in charge of livestock must enforce the inspection of these muscles, which are currently absent from the meat inspection policy [35]. In this study, all pigs with light and moderate infections and most pigs with heavy infections entered the food chain. This could be attributed to a loophole in the meat inspection policy, which allows pigs with light and moderate infections to be delivered for human consumption [35]. Additionally, a lack of vigilance among meat inspectors during the inspection period was noted, which could lead to infected carcasses reaching pork sales outlets. Thus, rigorous meat inspection and good decisions at the slaughter slabs need to be made to prevent moderately and heavily infected pork from reaching consumers. Moreover, it is crucial to carefully manage pig carcasses with light infections that are not detected during routine meat inspections. These pig carcasses could pose a potential risk for taeniasis as they appear safe, leading consumers to underestimate the risk [33]. Hence awareness of culinary and consumption practices to limit the transmission of the parasite should be raised. Moreover, education campaigns on improved pig husbandry practices, hygiene, sanitation, and treatment of pork tapeworm carriers would be important for preventing T. solium cysticercosis contamination in pigs and humans [36].
Molecular analyses confirmed the presence of the non-zoonotic tapeworm T. hydatigena with a prevalence of 4.2% in Burundi, which is consistent with the prevalence reported in Africa [37]. The presence of this parasite in pigs in Burundi could significantly indicate the presence of tapeworm in dogs, which could get infected by visiting slaughter slabs, consuming entrails, and subsequently contaminating the environment with their faeces [9]. In agreement with this study, co-infections with T. hydatigena and T. solium were also found in Tanzania, Cameroon, and Zambia [22, 34, 38]. Considering that dogs could have access to all slaughter slabs or home slaughters, proper management of offal that may contain T. hydatigena cysticerci, combined with improved pig husbandry practices, should be implemented to control the transmission of this parasite in Burundi.
This study revealed the presence of T. gondii in pigs in Burundi. The findings of this study corresponded to the seroprevalence in pigs reported in Africa, which ranges from 17 to 34%, with an average of 25% [39]. Higher seropositivity was observed in Kenya (34.5%), Ethiopia (32.1%), South Africa (33.6%), and Ghana (39%) [40–43]. In addition, similar seropositivity figures were found in South America and Asia, but they were higher than those in Europe [39]. This difference in seropositivity between countries could be associated with variations in pig management systems, climatic conditions (temperature, rainfall, humidity), hygienic conditions, and the density of cats and rodents on pig farms [10]. Although this indirect ELISA for detecting antibodies against the T. gondii P30 protein in serum demonstrates better sensitivity and specificity [44, 45], it does not imply that all seropositive pigs had viable T. gondii cysts, as it detects antibodies and cannot distinguish between past and active infections. No cross-reactions with other apicomplexan parasites have yet been reported in pigs using this ELISA kit [44, 45]. It was shown that pigs raised in extensive systems had a higher probability of ingesting sporulated oocysts during free-roaming, as well as animal tissues from birds or rodents and even contaminated water, in contrast to pigs raised in intensive systems [10, 46]. In Burundi, cats are usually kept to control rodents within households, and their access to pig housing, feed, and water can explain the transmission of the parasite to penned pigs. A study in the United States of America showed that a prevalence of less than 1% was observed in confined pigs without access to cats and rodents, highlighting the need to strengthen control methods to prevent their introduction to pig farms [47]. As a zoonotic parasite, this seropositivity in Burundi indicated that pork consumers could be highly exposed to this parasite via undercooked pork consumption. There is no way to prevent infected carcasses from reaching the food chain, as routine meat inspections cannot detect T. gondii cysts in meat tissues [48]. Pork consumers need to ensure that pork is well cooked, fried, or roasted to avoid contamination by this parasite. These good practices could also be applied to T. solium cysticercosis to help reduce the risk of human infection. Cooking meat at 67 °C for more than 3 min was recommended as an effective way to kill T. gondii cysts [49]. In addition, community awareness about pig management practices, cat keeping, pork preparation and consumption, hygiene, and sanitation should be implemented to prevent animal and human toxoplasmosis infections [50].
Conclusions
The study’s findings indicate that two foodborne zoonotic parasites, T. solium and T. gondii, are endemic in Burundi. These results show evidence of potential public health risks for the local population due to the consumption of pig carcasses infected with these parasites. The prevalence of T. solium cysticercosis in this study could probably be underestimated because pigs found to be infected with cysticerci during tongue palpation in pig farms could not reach official slaughter slabs and the sensitivity of partial carcass dissection is not 100%. Effective control strategies should be implemented to avoid the perpetual contamination of pigs and humans with these zoonotic parasites. Possible control strategies to be considered tackling both parasites are improved pig farming practices, better hygiene and sanitation, increased meat inspection and monitoring of infected pigs, risk-free culinary practices, and treatment of tapeworm carriers.
Methods
Study area
The study was conducted in slaughter slabs located in urban (Bujumbura city, economic capital) and rural areas (Ngozi and Kayanza provinces) of Burundi (Fig. 9). Bujumbura city was chosen due to the high number of pigs from the countryside that are slaughtered and marketed in the city. Kayanza and Ngozi provinces were selected because they are densely populated, and extensive pig farming systems are a major source of income for pig farmers [51].
Fig. 9.

Map of Burundi showing the study area
Study design and sample size
A cross-sectional study including field and laboratory work was conducted from October 2023 to March 2024. The number of pigs slaughtered per day was an inclusion criterion when selecting slaughter slabs for the study. Thus, the national slaughterhouse of Bujumbura, the slaughter slab of Gikoma in Bujumbura city; slaughter slabs located in Kayanza and Muhanga communes in Kayanza province; and slaughter slabs located in Ngozi, Gashikanwa and Busiga communes in Ngozi province were included in this study. When more than 10 pigs were available for slaughter per day, a random sampling of pigs was applied. If less than 10 pigs were available, all the available pigs meeting the inclusion criteria were selected. Pigs were selected according to their sex, age, breed, and origin. Only apparently healthy pigs older than 5 months were included in the study.
The sample size of pigs was determined using the formula N = Z2pq/L2 [52]. A prevalence of porcine cysticercosis estimated at 15.5% by tongue palpation in Burundi [19] and a seroprevalence of toxoplasmosis in pigs estimated at 25% in Africa [39] were considered to calculate the required sample size. Since porcine cysticercosis and toxoplasmosis were assessed on the same pigs at the same slaughter slabs, a sample size of 288 pigs was considered. Considering both urban and rural areas, a total of 576 pigs were sampled, including 288 pigs from Bujumbura city and 288 pigs from Kayanza and Ngozi provinces.
Field data collection
Pigs of pig traders and butchers who agreed to offer their pigs for the study were included. Information on the age, sex, breed, and origin of each pig was recorded. Upon pig slaughter, jugular vein blood samples were collected into 50 mL Falcon tubes. After pig slaughter, the meat inspector (veterinarian) conducted the meat inspection according to the procedures in Burundi [35]. Meat inspection regulations for T. solium cysticercosis in Burundi include incising of thigh muscles, abdominal wall, psoas, diaphragm, intercostal muscles, larynx and heart [35]. In the slaughter slabs, pig carcasses were sometimes inspected by making a deep incision with a knife in the muscles of the fore and hind limbs, as well as the heart, to search for T. solium cysticerci. In the national slaughterhouse of Bujumbura, the deep incision of the forelimb muscles, heart and tongue was conducted on each pig carcass for searching T. solium cysticerci. In addition, during the pig carcass inspection the pigs’ abdominal cavity, particularly the liver and mesentery, was inspected for T. hydatigena cysticerci. Taenia hydatigena cysticerci were identified macroscopically if they were moderately large (≥ 2 cm), translucent, filled with clear fluid, and had a visible white spot indicating a long-necked scolex [53]. Small cysticerci and other unclear lesions in the liver showing cysticerci stages were considered suspected lesions for either T. hydatigena or T. solium. Large T. hydatigena cysticerci found in the liver and mesentery were collected separately in 50 mL Falcon tubes containing 70% ethanol, while suspected liver lesions were collected separately in 2 mL cryovials containing 70% ethanol for further molecular analysis. After meat inspection, the tongue, heart, and masseter muscles from each inspected pig were purchased from butchers and pig traders and packaged in small bags. The blood and meat samples were labelled and placed in cool boxes and transported to the National Veterinary Laboratory of Bujumbura for serological analysis and partial carcass dissection. Samples for molecular analysis were sent to the laboratory of the Institute of Tropical Medicine in Antwerp, Belgium and the Laboratory of Foodborne Parasitic Zoonoses at Ghent University, Belgium for further analyses. All the results from the meat, partial carcass dissection and molecular analysis were recorded.
Laboratory analyses
Partial carcass dissection
The tongue, heart, and masseter muscles were dissected by making slices less than 5 mm thick and inspecting for T. solium cysticerci [54]. The cysticerci were counted and classified as viable, degenerated and calcified based on their macroscopic appearance. The cysticerci were classified as viable if they had a translucent fluid with visible whitish protoscolices; degenerated if they had damaged viscous walls or absence of cystic fluid; and calcified if they had solid caseous masses [22]. Infection intensities were estimated based on the number of cysticerci: light (1–10 cysticerci), moderate (11–100 cysticerci), and heavy (> 100 cysticerci). A maximum of 5 cysticerci from the collected heart, tongue and masseter muscles from each infected pig carcass were collected and stored together in 2 mL cryovials with 70% ethanol for molecular analysis.
Serological analysis
The blood tubes were allowed to clot overnight at 4 °C to obtain serum. The serum was aliquoted, transferred to 2 ml cryovials, and stored at -20 °C until analysis. Serum samples were tested by an indirect ELISA for the detection of antibodies against the T. gondii P30 protein in serum, plasma, and meat juice from multiple species (ID Screen® Toxoplasmosis Indirect Multi-species). This ELISA was performed following the manufacturer’s recommendations.
Molecular analysis
All collected cysticerci and suspected lesions were sent to and analysed in the Helminthology Laboratory of the Institute of Tropical Medicine in Antwerp, Belgium. The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) to identify and differentiate Taenia species was adopted. Genomic DNA from all samples was extracted using the DNeasyBlood and Tissue Extraction kit according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). PCR was used to amplify a mitochondrial 12S rDNA gene fragment with the primer set ITM TnR-TaenF and nTAE [55]. Subsequently, RFLP was used to differentiate Taenia spp. using restriction enzymes, including DdeI, HinfI, and HpaI [55, 56]. Therefore, the PCR-RFLP results were interpreted by analysing the band sizes specific to T. solium and T. hydatigena. Negative genomic DNA samples from liver suspected lesions were then tested with the multiplex PCR [57] for Echinococcus spp. and Sarcocystis spp. in the Laboratory of Foodborne Parasitic Zoonoses at Ghent University, Belgium.
Data analysis
Data from the field and laboratory analyses were entered in Microsoft Excel and then exported to R Software, version 4.3.3 [58]. Descriptive statistical analyses including frequencies, means, proportions, and 95% confidence intervals (CI) were performed. Associations between disease prevalence and province, age, sex, breed, and origin of pigs were estimated using the Chi-square test. In addition, Poisson regression and negative binomial regression models were used to assess the significance of cysticerci intensity counted in the heart, tongue and masseter muscles and the effect of age, sex, breed, and province on the cysticerci intensity in organs/muscles. The variable was statistically significant when the p-value was less than 0.05.
Acknowledgements
The authors would like to thank the veterinarians and provincial officers in Kayanza and Ngozi provinces, as well as the staff of the national slaughterhouse and Gikoma slaughter slab in Bujumbura city, for facilitating the completion of this study. We are grateful to the National Veterinary Laboratory for the permission to do laboratory analyses. We would also like to extend special thanks to Ndayikeza E., Iradukunda C., Mbazumutima M. (National Veterinary Laboratory, Burundi), de Jong T., Linda P. (Institute of Tropical Medicine, Belgium), and Vangeenberghe S. (Ghent University, Belgium) for their support with laboratory analyses. Additionally, we thank Habarugira H. and Nkurunziza E. (Department of Biology, University of Burundi) for their logistical support.
Abbreviations
- CI
Confidence interval
- ELISA
enzyme-linked immunosorbent assay
- MI
Meat inspection
- PCD
Partial carcass dissection
- PCR
RFLP-Polymerase chain reaction-restriction fragment length polymorphism
Author contributions
SM, JBN, AG, CT and SG conceived and designed the study. SM, PN, ES and AL performed the data collection. SM, CT and SG analysed and interpreted the data. SM drafted the manuscript. SM, ES, AL, PN, JBN, AG, CT and SG reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Directorate General for Development Cooperation (DGD), Belgium, through the individual sandwich PhD scholarship programme (Salvator Minani) of the Institute of Tropical Medicine, Antwerp, Belgium.
Data availability
The data supporting the conclusions of this article are included within the article. The raw datasets are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The research protocol was approved by the Institutional Review Board of the Institute of Tropical Medicine (ITM) in Belgium (IRB/RR/AC/091 Ref 1595/22) and the National Ethics Committee in Burundi (CNE/25/2022). Permission and verbal consent were obtained from local administration, slaughter slab managers, butchers, and pig traders to collect pig blood and meat samples at the slaughter slabs.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chiara Trevisan and Sarah Gabriël contributed equally to this work.
References
- 1.FAO/WHO. Multicriteria-based ranking for Risk Management of Food-Borne parasites. Microbiol Risk Assess Ser 23, 2014.
- 2.García HH, Gonzalez AE, Evans CAW, Gilman RH. Taenia solium cysticerccysticercosisrcosis working group in Peru. Lancet. 2003;362:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahanty S, Garcia HH. Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog Neurobiol. 2010;91:172–84. [DOI] [PubMed] [Google Scholar]
- 4.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrell K, Dorny P, Flisser A, Geerts S, Kyvsgaard N, McManus D et al. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cysticercosis. 2005.
- 7.Trevisan C, Mkupasi EM, Ngowi HA, Forkman B, Johansen MV. Severe seizures in pigs naturally infected with Taenia solium in Tanzania. Vet Parasitol. 2016;220:67–71. 10.1016/j.vetpar.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mkupasi EM, Ngowi HA, Sikasunge CS, Leifsson PS, Johansen MV. Distribution and histopathological changes induced by cysts of Taenia solium in the brain of pigs from Tanzania. J Helminthol. 2015;89:559–64. [DOI] [PubMed] [Google Scholar]
- 9.Saari S, Näreaho A, Nikander S. Cestoda (Tapeworms). Canine Parasites Parasit Dis. 2019;55–81.
- 10.Stelzer S, Basso W, Benavides Silván J, Ortega-Mora LM, Maksimov P, Gethmann J et al. Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 2019;15. [DOI] [PMC free article] [PubMed]
- 11.Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39:877–82. 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections. Parasitology. 2021;1–11. [DOI] [PMC free article] [PubMed]
- 13.Weiss ML, Dubey PJ. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 2009;39:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Hill D, Yang Y, et al. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet Parasitol. 2020;288:109185. [DOI] [PubMed] [Google Scholar]
- 15.Gulelat Y, Eguale T, Kebede N, Aleme H, Fèvre EM, Cook EAJ. Epidemiology of Porcine Cysticercosis in Eastern and Southern Africa: systematic review and Meta-analysis. Front Public Heal. 2022;10:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coral-Almeida M, Gabriël S, Abatih EN, Praet N, Benitez W, Dorny P. Taenia solium human cysticercosis: A systematic review of sero-epidemological data from endemic zones around the world. PLoS Negl Trop Dis. 2015;9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019;36:898–925. [PubMed] [Google Scholar]
- 18.Zulu G, Stelzle D, Mwape KE, Welte TM, Strømme H, Mubanga C, et al. The epidemiology of human Taenia solium infections: a systematic review of the distribution in Eastern and Southern Africa. PLoS Negl Trop Dis. 2023;17(3):e0011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minani S, Dorny P, Trevisan C. Prevalence and risk assessment of porcine cysticercosis in Ngozi Province, Burundi. Vet Parasitol Reg Stud Rep. 2021;23:100514. 10.1016/j.vprsr.2020.100514. [DOI] [PubMed] [Google Scholar]
- 20.Nsengiyumva G, Druet-Cabanac M, Ramanankandrasana B, Bouteille B, Nsizabira L, Preux PM. Cysticercosis as a major risk factor for epilepsy in Burundi, East Africa. Epilepsia. 2003;44:950–5. [DOI] [PubMed] [Google Scholar]
- 21.Excler JL, Pretat E, Pozzetto B, Charpin BGJ. Sero-epidemiological survey for toxoplasmosis in Burundi. Trop Med Parasitol. 1988;39:139–41. [PubMed] [Google Scholar]
- 22.Chembensofu M, Mwape KE, Van Damme I, Hobbs E, Phiri IK, Masuku M, et al. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasites Vectors. 2017;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lightowlers MW, Assana E, Jayashi CM, Gauci CG, Donadeu M. Sensitivity of partial carcass dissection for assessment of porcine cysticercosis at necropsy. Int J Parasitol. 2015;45:815–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL, Brandt J, et al. A bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–76. [DOI] [PubMed] [Google Scholar]
- 25.Mwabonimana M, Macharia A, Inyagwa CM, Shakala K, Bebe BO. Prevalence of porcine cysticercosis among scavenging pigs in western Kenya. Afr J Infect Dis. 2020;14:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh SP, Singh BB, Kalambhe DG, Pathak D, Aulakh RS, Dhand NK. Prevalence and distribution of Taenia solium cysticercosis in naturally infected pigs in Punjab, India. PLoS Negl Trop Dis. 2018;12(11):e0006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kungu JM, Afayoa M, Dione MM. Taenia solium cysticercosis survey at a slaughterhouse in Kampala, Uganda. Rev d’Elevage Med Vet des Pays Trop. 2020;73:277–81. [Google Scholar]
- 28.Porphyre V, Rasamoelina-Andriamanivo H, Rakotoarimanana A, Rasamoelina O, Bernard C, Jambou R, et al. Spatio-temporal prevalence of porcine cysticercosis in Madagascar based on meat inspection. Parasites Vectors. 2015;8:1–8. 10.1186/s13071-015-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sithole MI, Bekker JL, Tsotetsi-Khambule AM, Mukaratirwa S. Ineffectiveness of meat inspection in the detection of Taenia solium cysticerci in pigs slaughtered at two abattoirs in the Eastern Cape Province of South Africa. Vet Parasitol Reg Stud Rep. 2019;17:100299. 10.1016/j.vprsr.2019.100299. [DOI] [PubMed] [Google Scholar]
- 30.Minani S, Devleesschauwer B, Gasogo A, Ntirandekura JB, Gabriël S, Dorny P, et al. Assessing the burden of Taenia solium cysticercosis in Burundi, 2020. BMC Infect Dis. 2022;22:1–13. 10.1186/s12879-022-07849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministère de l’Agriculture et de l’Elevage (MINAGRIE). Rapport National sur l’état des Ressources Génétiques Animales au Burundi. 2003;57 pp.
- 32.Kabululu ML, Ngowi HA, Mlangwa JED, Mkupasi EM, Braae UC, Trevisan C, et al. Endemicity of Taenia solium cysticercosis in pigs from Mbeya Rural and Mbozi districts, Tanzania. BMC Vet Res. 2020;16:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabululu ML, Johansen MV, Lightowlers M, Trevisan C, Braae UC, Ngowi HA. Aggregation of Taenia solium cysticerci in pigs: implications for transmission and control. Parasite Epidemiol Control. 2023;22:e00307. 10.1016/j.parepi.2023.e00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assana E, Awah-Ndukum J, Djonmaïla JD, Zoli AP. Prevalence of porcine Taenia solium and Taenia Hydatigena cysticercosis in Cameroon. Prev Vet Med. 2019;169:104690. 10.1016/j.prevetmed.2019.104690. [DOI] [PubMed] [Google Scholar]
- 35.Ministère de l’Environnement de l’Agriculture et de l’Elevage (MINEAGRIE). Ordonnance ministérielle relative à l’examen des animaux d’abattage et à l’inspection sanitaire véterinaire des viandes et produits à base de viande au Burundi. 2019. p. 11.
- 36.Sarti E, Rajshekhar V. Measures for the prevention and control of Taenia solium taeniosis and cysticercosis. Acta Trop. 2003;87:137–43. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen MTT, Gabriël S, Abatih EN, Dorny P. A systematic review on the global occurrence of Taenia hydatigena in pigs and cattle. Vet Parasitol. 2016;226:97–103. 10.1016/j.vetpar.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Braae UC, Kabululu M, Nørmark ME, Nejsum P, Ngowi HA, Johansen MV. Taenia hydatigena cysticercosis in slaughtered pigs, goats, and sheep in Tanzania. Trop Anim Health Prod. 2015;47:1523–30. [DOI] [PubMed] [Google Scholar]
- 39.Foroutan M, Fakhri Y, Riahi SM, Ebrahimpour S, Namroodi S, Taghipour A, et al. The global seroprevalence of Toxoplasma gondii in pigs: a systematic review and meta-analysis. Vet Parasitol. 2019;269:42–52. 10.1016/j.vetpar.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Chepyatich D, Sentamu DN, Bor N, Onono J, Gathura PB, Akoko JM, et al. Seroprevalence of Toxoplasma gondii in Slaughtered pigs in Kiambu, Kenya. Zoonotic Dis. 2023;3:301–6. [Google Scholar]
- 41.Gebremedhin EZ, Kebeta MM, Asaye M, Ashenafi H, Di Marco V, Vitale M. First report on seroepidemiology of Toxoplasma gondii infection in pigs in Central Ethiopia. BMC Vet Res. 2015;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tagwireyi WM, Etter E, Neves L. Seroprevalence and associated risk factors of Toxoplasma gondii infection in domestic animals in southeastern South Africa. Onderstepoort J Vet Res. 2019;86:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arko-Mensah J, Bosompem KM, Canacoo EA, Wastling JM, Akanmori BD. The seroprevalence of toxoplasmosis in pigs in Ghana. Acta Trop. 2000;76:27–31. [DOI] [PubMed] [Google Scholar]
- 44.Liyanage KLDTD, Wiethoelter A, Hufschmid J, Jabbar A. Descriptive comparison of ELISAs for the detection of Toxoplasma gondii Antibodies in animals: a systematic review. Pathogens. 2021;10:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Ureña NM, Calero-Bernal R, Vázquez-Calvo Á, Sánchez-Sánchez R, Ortega-Mora LM, Álvarez-García G. A comparative study of serological tests used in the diagnosis of Toxoplasma gondii infection in small ruminants evidenced the importance of cross-reactions for harmonizing diagnostic performance. Res Vet Sci. 2023;165:105052. [DOI] [PubMed] [Google Scholar]
- 46.Dubey JP. Toxoplasmosis in pigs-the last 20 years. Vet Parasitol. 2009;164:89–103. [DOI] [PubMed] [Google Scholar]
- 47.De Berardinis A, Paludi D, Pennisi L, Vergara A. Toxoplasma gondii, a Foodborne Pathogen in the Swine Production Chain from a European perspective. Foodborne Pathog Dis. 2017;14:637–48. [DOI] [PubMed] [Google Scholar]
- 48.Kuruca L, Belluco S, Vieira-Pinto M, Antic D, Blagojevic B. Current control options and a way towards risk-based control of Toxoplasma gondii in the meat chain. Food Control. 2023;146:109556. 10.1016/j.foodcont.2022.109556. [Google Scholar]
- 49.Dubey JP, Kotula AW, Sharar A, Andrews CD, Lindsay DS. Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. J Parasitol. 1990;76:201–4. [PubMed] [Google Scholar]
- 50.Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG. Control of human toxoplasmosis. Int J Parasitol. 2021;51:95–121. 10.1016/j.ijpara.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Ministère de l’Environnement de l’Agriculture et de l’Elevage (MINEAGRIE). Enquête Nationale Agricole du Burundi. 2018;1-131 p.
- 52.Martin S, Meek A, Willeberg P, editors. Veterinary epidemiology: principles and methods. Ames: Lowa State Univ.; 1987. p. 343. [Google Scholar]
- 53.Kabululu ML, Johansen MV, Mlangwa JED, Mkupasi EM, Braae UC, Trevisan C, et al. Performance of Ag-ELISA in the diagnosis of Taenia solium cysticercosis in naturally infected pigs in Tanzania. Parasites Vectors. 2020;13:1–7. 10.1186/s13071-020-04416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phiri IK, Dorny P, Gabriel S, Willingham AL, Sikasunge C, Siziya S, et al. Assessment of routine inspection methods for porcine cysticercosis in Zambian village pigs. J Helminthol. 2006;80:69–72. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Hidalgo R, Geysen D, Benítez-Ortiz W, Geertst S, Brandt J. Comparison of conventional techniques to differentiate between Taenia solium and Taenia saginata and an improved polymerase chain reaction-restriction fragment length polymorphism assay using a mitochondrial 12S rDNA fragment. J Parasitol. 2002;88:1007–11. [DOI] [PubMed] [Google Scholar]
- 56.Devleesschauwer B, Aryal A, Tharmalingam J, Joshi DD, Rijal S, Speybroeck N, et al. Complexities in using sentinel pigs to study Taenia solium transmission dynamics under field conditions. Vet Parasitol. 2013;193:172–8. 10.1016/j.vetpar.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez LM, Montero E, Harrison LJS, Parkhouse RME, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol. 2000;38:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. https://www.r-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. The raw datasets are available from the corresponding author upon reasonable request.



