Abstract
Rapid multiplex molecular syndromic panels (RMMSP) (3 or more pathogens and time-to-results < 6 h) allow simultaneous detection of multiple pathogens and genotypic resistance markers. Their implementation has revolutionized the clinical landscape by significantly enhancing diagnostic accuracy and reducing time-to-results in different critical conditions. The current revision is a comprehensive but not systematic review of the literature. We conducted electronic searches of the PubMed, Medline, Embase, and Google Scholar databases to identify studies assessing the clinical performance of RMMSP in critically ill patients until July 30, 2024. A multidisciplinary group of 11 Spanish specialists developed clinical questions pertaining to the indications and limitations of these diagnostic tools in daily practice in different clinical scenarios. The topics covered included pneumonia, sepsis/septic shock, candidemia, meningitis/encephalitis, and off-label uses of these RMMSP. These tools reduced the time-to-diagnosis (and therefore the time-to-appropriate treatment), reduced inappropriate empiric treatment and the length of antibiotic therapy (which has a positive impact on antimicrobial stewardship and might be associated with lower in-hospital mortality), may reduce the length of hospital stay, which could potentially lead to cost savings. Despite their advantages, these RMMSP have limitations that should be known, including limited availability, missed diagnoses if the causative agent or resistance determinants are not included in the panel, false positives, and codetections. Overall, the implementation of RMMSP represents a significant advancement in infectious disease diagnostics, enabling more precise and timely interventions. This document addresses relevant issues related to the use of RMMSP on different critically ill patient profiles, to standardize procedures, assist in making management decisions and help specialists to obtain optimal outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05224-3.
Keywords: Infection; Critically ill patient; Syndromic molecular diagnosis; Multidrug resistant pathogens; Septic shock, sepsis, bloodstream infection
Introduction
Inadequate empirical antimicrobial therapy in critically ill patients has been associated with worse outcomes, including increased in-hospital mortality and morbidity, prolonged hospital stays, and higher healthcare costs [1, 2]. Co-morbidities and the infecting pathogens significantly impact infection severity, leading to severe outcomes [1]. Optimal antibiotic use in ICUs is essential, as 30–60% of antibiotics prescribed are unnecessary or inappropriate [2], and at least 20% of patients experience adverse effects [3]. While timely treatment is vital for sepsis survival, bacterial resistance complicates effectiveness, contributing to higher mortality [4, 5]. The rise of multi- and pan-drug-resistant pathogens complicates empirical antibiotic selection, often leading to combinations that increase toxicity, costs, and resistance [6, 7]. Since the FDA approved the first respiratory syndromic panel in 2011 [8], molecular syndromic panels have been used to improve diagnosis and reduce unnecessary antibiotic use by guiding targeted therapy [9, 10]. Although molecular testing can improve pathogen identification and treatment tailoring and occasionally may replace conventional culture procedures in gastrointestinal infections, it must not replace conventional culture procedures in other scenarios, particularly when phenotypic susceptibility testing is needed. Interpreting molecular syndromic panel results can be complex, as detecting a target does not confirm it as the causative agent, and genotypic resistance markers may not always reflect in vivo expression, causing discrepancies with phenotypic susceptibility [11, 12]. The SARS-CoV-2 pandemic led to widespread use of these panels in clinical microbiology, especially for critically ill patients, where their use should be justified. This document reviews commercially available FDA and/or CE-marked Rapid Multiplex Molecular Syndromic Panels (RMMSP), capable of detecting multiple pathogens (at least five), including bacteria, viruses, fungi, and/or parasites most frequently associated with specific clinical syndromes (e.g., respiratory infections, meningitis, or gastrointestinal diseases), and for some platforms, genotypic markers of antimicrobial resistance with results provided in less than 6 h [13]. In addition, due to the general self-limiting nature of infectious gastrointestinal disease in the majority of cases, these were not included in our evaluation. The goal was to provide updated, practical information on their use, diagnostic timing, and management of infections in critically ill patients, including pneumonia, bacteremia, meningitis, and off-label applications of syndromic panels.
Methods
On January 29, 2024, an expert-panel meeting was held to discuss the need for a comprehensive review on the use of RMMSP in diagnosing infectious diseases in critically ill patients. Panel members were selected based on their expertise from the Study Group on Infection in the Critically Ill Patient (GEIPC-SEIMC) and the Infectious Diseases and Sepsis Task Force (GTEIS-SEMICYUC) of the Spanish Society of Clinical Microbiology and Infectious Diseases and the Spanish Society of Critical Intensive Care Medicine and Coronary Care Units. The panel identified key topics concerning the indications and limitations of RMMSP in daily clinical practice.
Search strategy
A systematic literature review was conducted on July 30, 2024, utilizing databases such as PubMed, EMBASE, Web of Science, and the Cochrane Library. The search included the keywords [“Molecular syndromic platforms” OR “molecular syndromic testing” OR “Syndromic panels”] AND [“Critically ill patients” OR “Severe infections” OR “Multidrug resistance” OR “septic shock” OR “sepsis”]. Filters were applied to include studies on humans and publications in English, French, Italian, Portuguese, or Spanish, and to limit the review to articles published within the past 10 years. The search was limited to studies published in the past 10 years, as most commercially available rapid tests began to emerge around 2012 [14]. However, some older references were also included due to their significance in the initial development and implementation of this technology.
To ensure a comprehensive review, reference lists from the included studies were manually searched for additional relevant publications not captured in the initial search. The selection process involved two phases: screening titles and abstracts, followed by a full-text review to confirm eligibility. Studies that assessed the use of molecular syndromic platforms in diagnosing severe infections, particularly focusing on multidrug resistance, sepsis, or septic shock in critically ill patients, were included. Animal models, in vitro studies, editorials, and articles without clinical data applicable to patient care were excluded. The study selection was conducted by two independent reviewers to minimize bias, with disagreements resolved by consensus. Covidence software was used for deduplication, organization, and management of the articles [15].
Results
Molecular syndromic panels to diagnose infectious diseases in critically ill patients: general considerations
Time until microbiological diagnosis and minimum diagnostic standards for therapeutic adequacy.
The key point of a rapid diagnosis is administering the correct antibiotic treatment at the right time, through the appropriate route, and with the necessary dose to control the infection and symptoms. Additionally, it involves adjusting or discontinuing treatment when it is no longer needed [8].
While traditional culture-based techniques remain the standard, molecular methods, such as nucleic acid amplification, are rapid and can provide results in a few hours [16–19]. However, its implementation requires adequate technical and organizational resources.
Although clinical microbiology laboratories have maintained their fundamental mission, they have undergone significant transformation, largely driven by molecular diagnostics [20, 21]. Indeed, RMMSP have revolutionized the management of infectious diseases, expanding their impact from routine cases in everyday practice to rare conditions handled by specialists [22–24].
Syndromic molecular panels
Introduction of RMMSP in daily practice capability facilitates timely clinical management decisions, such as hospital admission, isolation, and initiation or avoidance of antimicrobial treatment [25–27].
These panels are tailored to screen for the most common microorganisms associated with specific clinical syndromes such as bloodstream infection, meningitis/encephalitis, gastrointestinal infection, respiratory tract infection, joint infection, urinary tract infection, and sexually transmitted diseases [26–29].
Some RMMSP, like those for bloodstream infection and lower respiratory tract infection, have the capability to detect genotypic markers of β-lactam resistance, such as genes encoding main carbapenemases (blaKPC, blaNDM, blaVIM, blaOXA-48), extended spectrum β-lactamases (ESBL) from the blaCTX-M group, methicillin resistance in Staphylococcus aureus (mecA), or glycopeptide resistance in Enterococcus spp. (van A/vanB) [30, 31].
Commercially available RMMSP typically offer turnaround times of 1–4.5 h. Overall, they enhance microbial detection compared to standard procedures like bacterial culture, potentially speeding up pathogen identification and treatment initiation [8, 20, 21, 26–29]. RMMSP, when combined with antimicrobial stewardship efforts, play a crucial role in clinical decision-making, however, although some recent publications have shown promising results, their impact on patient clinical and economical outcomes requires further investigation through studies with large sample sizes and preferably clinical trial designs [21, 32].
RMMSP offer mainly qualitative results, though some provide (semi)quantitative values for specific bacterial targets, and some are suitable for point-of-care testing [33].
Proper interpretation of PCR detection results is challenging due to the test’s ultra sensitivity. This precision enables detection of pathogens or resistance mechanisms, even when present only as colonizers, which may lead to unnecessary treatments. Literature reports overdiagnosis rates of 49% for the mecA gene, 27% for ESBL, and 15–38% for carbapenem resistance genes unrelated to clinical conditions [18].
Careful interpretation of positive results from RMMSP is crucial, as they may detect colonization or molecular remnants rather than active infection, particularly in non-sterile fluids. This highlights the importance of diagnostic stewardship to differentiate between infection and colonization [31–33]. Commercial RMMSPs are standardized, encompassing nucleic acid extraction, amplification, detection, and reporting, which minimizes the need for direct specimen handling [8, 20, 21, 26–29, 31–33].
Effective use of these results demands specialized training, encompassing test interpretation, result integration, and patient management. Collaborative protocols and strong partnerships between microbiologists and intensivists are essential. Crucially, PCR findings must be contextualized with pretest probabilities, culture results, and the patient's clinical status.
Table 1 outlines clinical situations where the interpretation of molecular test results may be limited due to insufficient data or clinical biases.
Table 1.
Clinical situations in which the rapid multiplex molecular syndromic panels (RMMSP) result may not be interpreted as significant due to technical limitations, insufficient data, or clinical bias. [34]
Modified from Walker et al.
| Challenging clinical situations limiting RMMSP interpretation |
|---|
| Immunosuppressed patients (in this group of patients, there may be other clinically significant pathogens not included in the panel) |
| Poor quality sample (BAS G5 or BAL would be recommended) [35, 36] |
| Absence of clinical context justifying the test (tracheobronchitis, absence of IDSA criteria for pneumonia requiring admission to ICU) [37] |
| Patient with long stay in ICU, suffering worsening [18]* |
*The pretest predictive value could be high and detect false positives in resistance mechanisms of bacteria colonizing the bronchial tree. Indication of test, interpretation of results, and therapeutic attitude should be individualized, in consensus between the microbiologist and the intensivist, including surveillance microbiological studies and local ecology
BAS Bronchioaspirate, BAS G5: < 10 buccal squamous epithelial cells and > 25 leukocytes/field 100 × magnification; IDSA: Infectious Diseases Society of America; ICU: Intensive care unit
Currently available European Community (EC)-marketed RMMSP and their most relevant characteristics are displayed in Table 2.
Table 2.
Relevant characteristics of European Community (EC)-marketed rapid multiplex molecular syndromic panels (RMMSP)
| NAAT platform | Characteristics |
|---|---|
| BD MAX system (BD Diagnostics) | An automated real-time PCR platform utilizing TaqMan hydrolysis probes for pathogen detection |
| The BD MAX instrument integrates extraction reagents and a real-time microfluidic cartridge, automating all sample handling and PCR processes | |
| Results are generated within a 3-h turnaround time | |
| ePLex (GenMark Diagnostics) | A fully automated system that employs eSensor technology, utilizing electrochemical detection of ferrocene-labeled PCR amplicons via capture probes immobilized on gold-plated electrodes |
| Results are available within 30 to 90 min, depending on the specific system (ePLex, ePlex NP, or eSensor XT-8) | |
| Biofire Filmarray system (BioFire Diagnostics) | Fully automated platform performing nucleic acid extraction, real-time PCR detection and high-resolution melting for target identification |
| Turnaround time less than 2 h | |
| Unyvero System (Curetis USA) | Fully automated platform that includes a sample lysis device and a PCR panel analyzer |
| The turnaround time is 4–5 h | |
| VERIGENE system (Luminex Corporation) | Fully automated for nucleic acid extraction, purification, target amplification and hybridization of the target amplicons to a glass detection array in the test cartridge |
| NanoGrid technology is used to identify target molecules. Turnaround time of around 3 h | |
| xTAG technology (Luminex Corporation) | Multiplexed PCR coupled to bead-hybrization with a bead-specific fluorescent reporters that require offline nucleic acid extraction |
| Turnaround time of 5 h |
NAAT Nucleic acid amplification tests; PCR Polymerase chain reaction
Microbiological diagnostic stewardship concept and complement to classical microbiological tests
Microbiological diagnostic stewardship programs aim to optimize diagnostic techniques, supporting appropriate, cost-effective clinical, therapeutic, and preventive decision-making [38–40]. Their implementation can reduce overdiagnosis, promote correct antimicrobial use, and enhance patient safety and care [41–43]. Effective stewardship requires actions such as developing a service portfolio, establishing multidisciplinary committees, utilizing a laboratory information system, implementing quality assurance, conducting cost-effectiveness assessments, providing education programs for nurses and technicians, and continuously evaluating the program [44].
Advantages and disadvantages of implementing a molecular platform for diagnosis of critically ill patients.
Multiplex testing, which enables simultaneous detection of multiple pathogens and antimicrobial resistance (AMR) genes, has become integral to routine diagnostics, offering rapid results to guide patient management [27–29, 45, 46]. However, these panels are more costly than single-plex tests, and the clinical relevance of some targets has been questioned [11, 47, 48]. There are concerns about broad screening, especially for patients with community-acquired pneumonia, where the relevance of molecular techniques and prognosis remains unclear [49, 50]. While multiplex panels offer high sensitivity, specificity, and negative predictive value, they should not replace blood cultures and conventional testing [51–53]. Their limitations include the inability to detect all pathogens, incomplete antibiotic susceptibility data, high costs, and potential false results, with debates about their impact on clinical outcomes [37, 54, 55]. Although they can improve diagnostic efficiency, their cost-effectiveness remains uncertain [56], highlighting the need for careful evaluation before integration into clinical workflows.
The integration of molecular diagnostic platforms for critically ill patients offers notable advantages, particularly in achieving rapid and precise pathogen identification. However, their diagnostic impact is subject to variability influenced by factors such as local prescribing practices, clinical guidelines, and regional epidemiological trends. Furthermore, considerations such as associated costs, technical complexities, and inherent limitations of these platforms must be critically evaluated to ensure optimal implementation and utility [57, 58].
The Table 3 summarizes the main advantages and disadvantages of the RMMSP.
Table 3.
Overview of the main advantages and disadvantages of implementing a molecular platform for diagnosis of critically ill patients
| Main advantages and disadvantages of Implementing RMMSP | |
|---|---|
| Advantages | Disadvantages |
| Rapid Results | Cost |
| Molecular platforms can provide fast turnaround times, allowing for timely diagnosis and treatment initiation in critically ill patients | Molecular platforms can be expensive to purchase and maintain, requiring significant initial investment and ongoing expenses |
| High Sensitivity and Specificity | Complexity |
| Molecular tests typically exhibit high sensitivity and specificity, enabling accurate detection of pathogens even at low concentrations | Molecular testing may require specialized equipment and trained personnel, increasing complexity compared to traditional methods |
| Multiplexing Capability | Technical Challenges |
| Many molecular platforms offer multiplexing, allowing for simultaneous detection of multiple pathogens and antimicrobial resistance genes in a single test, which is beneficial for critically ill patients with complex infections | Molecular tests may be susceptible to technical issues such as sample contamination or inhibition, missing detections due to genetic variants, or lack of correlation between genotype and phenotype for the AMR genes, which can affect test accuracy |
| Reduced Hands-on Time | Limited Coverage |
| Automation in molecular platforms reduces hands-on time for laboratory staff, freeing up time for other tasks | While molecular platforms offer broad pathogen coverage, they may not detect all pathogens relevant to a specific clinical scenario, potentially leading to missed diagnoses |
| Potential for Point-of-Care Testing | Interpretation Challenges |
| Some molecular platforms are suitable for point-of-care testing, enabling rapid diagnosis at the bedside, which is advantageous for critically ill patients requiring immediate intervention | Molecular test results may be complex to interpret, especially in cases of co-infections or detection of commensal organisms, requiring careful clinical correlation |
RMMSP Rapid multiplex molecular syndromic panels; AMR: Antimicrobial resistance
Syndromic Polymerase chain reaction (PCR) panels for the diagnosis and management of multidrug-resistant bacteria (MDR) high-risk severe community acquired pneumonia, hospital-acquired pneumonia and ventilator associated pneumonia
Two CE-marked molecular syndromic panels, BioFire® FilmArray® Pneumonia (PN) panel (FA-PNp) and the Unyvero Hospitalised Pneumonia Panel (HPN), are designed for diagnosing hospital-acquired (HAP), ventilator-associated (VAP), and severe community-acquired (CAP) pneumonia in high-risk patients for MDR bacteria [59–73] (Table 4).
Table 4.
BioFire® FilmArray® Pneumonia Panel plus multiplex (FDA-approved and CE-Marked) and the Unyvero pneumonia panel (CE-Marked) multiplex PCR platforms. The information to build this table has been extracted from references [59–73]
| Characteristic | BioFire® FilmArray® Pneumonia Panel plus | Curetis Unyvero Hospitalised Pneumonia Panel (HPN) |
|---|---|---|
| Number of targets | 34 | 36 |
| Analytical design | Automated sample preparation and and nucleic acid extraction and nested PC (melting curve analysis) | Automated sample preparation and nucleic acid extraction and multiplex PCR and microarray detection of targets |
| Turnaround time | Around 1.5 h | Around 5 h |
| Results reporting | Quantitative (binned values: 104, 105, 106, ≥ 107) for bacterial targets, excluding atypical bacteria | Semiquantitative (+ / + + / + + +) for bacterial and fungal targets |
| Bacterial targets |
Acinetobacter baumannii complex Citrobacter freundii Enterobacter cloacae complex Escherichia coli Haemophilus influenzae Klebsiella aerogenes Klebsiella oxytoca Klebsiella pneumoniae Klebsiella variicola Moraxella catarrhalis Morganella morganii Proteus spp. Pseudomonas aeruginosa Serratia marcescens Stenotrophomonas maltophilia Streptococcus pneumoniae Chlamydophila pneumoniae Legionella pneumophila Mycoplasma pneumoniae |
Acinetobacter calcoaceticus/baumannii complex Enterobacter cloacae complex Escherichia coli Haemophilus influenzae Klebsiella aerogenes Klebsiella oxytoca Klebsiella pneumoniae Moraxella catarrhalis Proteus spp. Pseudomonas aeruginosa Serratia marcescens Streptococcus agalactiae Streptococcus pneumoniae Streptococcus pyogenes Chlamydophila pneumoniae Legionella pneumophila Mycoplasma pneumoniae |
| Virus targets |
Adenovirus Coronaviruses OD43, NL63, HKU1 and 229E Coronavirus del síndrome respiratorio de Oriente Medio (MERS-CoV) Human metapneumovirus Human rhinovirus/ enterovirus Influenza A Influenza B Parainfluenza virus Respiratory syncytial virus |
None |
| Fungal targets | None | Pneumocystis jirovecii |
| Antimicrobial resistance genes |
blaKPC blaNDM blaOXA-48 like bla VIM blaIMP blaCTX-M mecA/C and MREJ |
ermB mecA mecC blaTEM blaSHV blaIMP blaKPC blaNDM blaOXA-23 blaOXA-24/40 blaOXA-48 blaOXA-58 blaVIM sul1 gyrA83 gyrA87 |
| Results of the AMR targets unavailable if bacterial targets are below the limit of detection | Yes | Yes |
| PPV | 63.0–96.2% | 71.6–100.0% |
| NPV | 92.0–98.1% | 97.9–99.8% |
|
A similar panel, featuring a reduced number of antimicrobial resistance genes, has US FDA clearance | ||
AMR Antimicrobial resistance; FDA Food and Drud Administration; PCR Polymerase chain reaction; PPV Positive predictive value; NPV negative predictive value
The F A-PNp panel displays a positive percentage agreement of 96.2% and a negative percentage agreement of 98.1% for identifying bacterial targets compared to routine culture [72]. Sensitivity varies depending on specimen type, with lower sensitivity for sputum-like specimens. For deep respiratory samples, sensitivity and specificity are high [74].
Enne et al. [75] evaluated, by Bayesian latent class analysis, the clinical efficacy of FA-PNp, HPN, and standard microbiological techniques in 652 lower respiratory tract samples from critically ill patients. Compared to traditional methods, RMMSP detected pathogens in a significantly higher proportion of samples (74.2% for FA-PNp and 60.4% for HPN). For common HAP/VAP pathogens, FA-PNp demonstrated a sensitivity of 91.7–100.0% and a specificity of 87.5–99.5%, while HPN exhibited a sensitivity of 50.0–100.0% and a specificity of 89.4–99.0%. Conversely, conventional methods showed low sensitivity, ranging from 27.0 to 69.4%, in comparison to RMMSP. The INHALE WP1 study had several limitations. Its findings may not have been applicable to all healthcare settings due to variations in diagnostic infrastructure and patient populations. The study compared PCR results with routine microbiology, which has lower sensitivity, potentially overstating the benefits of PCR. Although PCR improved pathogen detection, its clinical impact on patient outcomes and antibiotic stewardship remained uncertain, and the study lacked a cost-effectiveness analysis. Additionally, differences in sensitivity and specificity between the PCR platforms tested may have complicated their routine clinical adoption.
There appears to be certain discrepancy between the results of conventional methods and RMMSP, particularly in cases where antimicrobial therapy exposure is present. [74, 76]. Regarding the HPN panel, studies have reported varying sensitivity and specificity for different targets, with antibiotic resistance marker positive predictive values (PPVs) ranging from 79.7 to 100% [63, 64, 66].
Potential clinical impact of the use of syndromic pneumonia molecular panels on patients with HAP/VAP and MDR-CAP patients
Several observational studies suggested that the use of syndromic pneumonia molecular panels in patients with HAP/VAP and MDR organisms-CAP increased the diagnostic yield, led to a reduction in the time to appropriate antimicrobial therapy and decreases antibiotic consumption [62, 68, 69, 71]. Currently available clinical trials have supported this assumption. Poole et al. [77] showed that time to results-directed therapy was 2.3 h in the FA-FNp (mPOCT) group and 46.1 h in the control group (standard microbiological procedures). Similarly, Firezein et al. in ventilated pediatric intensive care unit patients, observed that the length of the time to identification of organism was significantly shortened from 67 to 5 h after implementing the use of FA-FNp [77]. Regarding the duration of antibiotic therapy, Poole et al. [77] reported that 42% of patients in mPOCT group had antibiotics safely de-escalated compared with 8 (8%) of 98 in the control group. Nevertheless, there was no major difference in antibiotic duration or in clinical or safety outcomes between the two groups [77]. Additionally,
Firezein et al. found a significant reduction in the length of antibiotic therapy from 9 to 5 days after introducing the FA-FNp [78].
Darie et al. [79] showed that multiplex bacterial PCR examination of bronchoalveolar lavage (by HPN) decreases the duration of inappropriate antibiotic therapy of patients admitted to hospital with pneumonia and at risk of Gram-negative rod infection. The study had several limitations. Its findings, based on data obtained at two Swiss tertiary centers, may not be generalizable to other healthcare settings. The lack of physician blinding introduced potential bias in antibiotic decisions, while the small sample size (208 patients) limited the strength of subgroup analyses. Additionally, the focus on Gram-negative bacteria excluded other significant pathogens, emphasizing the need for further research to validate its relevance across diverse clinical contexts [79].
Moreover, Markussen et al. [80], in a randomized clinical trial, demonstrated that molecular testing by the FA-PNp significantly increased the proportion of hospitalized patients with suspected CAP who received pathogen-directed treatment and reduced the median time to pathogen-directed treatment by 9.4 h compared with the standard of care.
It has been recently published a single-center, open-label randomized controlled trial that assessed the impact of the BioFire FilmArray pneumonia panel on antibiotic management in hospitalized patients with suspected pneumonia [81]. Participants were randomized to receive diagnostics with the BioFire panel plus conventional culture or conventional culture alone. Among 1152 patients analyzed, the intervention group showed significantly reduced median times to antibiotic escalation for Gram-positive (10.3 vs. 24.6 h, p = 0.044) and Gram-negative organisms (17.3 vs. 27.2 h, p = 0.010). Median time to Gram-positive antibiotic de-escalation was also shorter (20.7 vs. 27.8 h, p = 0.015). However, the authors recommended the need of further research for optimizing Gram-negative antibiotic de-escalation in lower respiratory infections.
What patient profile would benefit from being tested by a pneumonia syndromic PCR Platform including genotypic resistance marker targets?
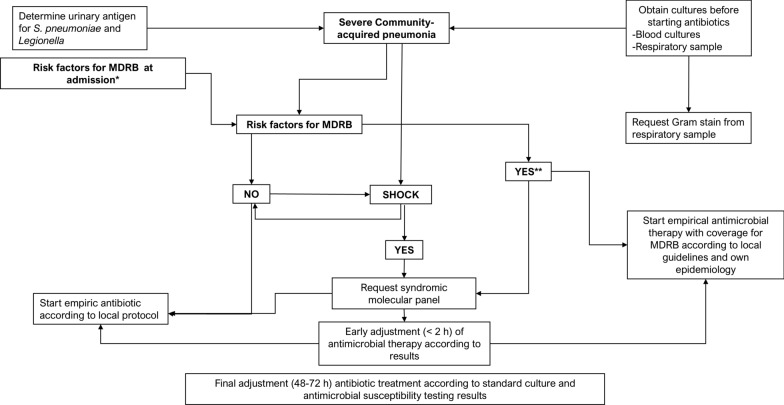
RMMSP may facilitate early adjustment of empirical antimicrobial therapy (EAT) or targeted antimicrobial therapy. In severe CAP patients at high risk for MDR bacteria involvement or with shock, syndromic panels should be requested to guide antimicrobial therapy [21, 82].
For patients with HAP or VAP, testing with syndromic panels is recommended regardless of the risk for MDR bacteria involvement or shock risk factors (Fig. 1). Broad-spectrum empirical treatment should be initiated and adjusted based on panel results [18, 52, 83–86]. Early treatment algorithms may be proposed for CAP and HAP among patients with MDR risk factors. These strategies should be guided by the clinical presentation, regional epidemiological trends, and prevailing patterns of pathogen resistance [23]. Final adjustments to antimicrobial therapy should be made based on standard culture and antimicrobial susceptibility testing results.
Fig. 1.
Severe Community-acquired pneumonia. Adapted from Martin-Loeches et al. [87]. *Risk factors for Multiresistant drug bacteria (MDRB) at admission: Prior contact or hospital admission; stay in chronic health center; Chronic obstructive pulmonary disease; Cystic fibrosis; Bronchiectasis; Immuno-depression; Previous antibiotic treatment; Underweight; High severity illness; Endemic areas; Diabetes mellitus; Chronic alcoholism; Prior hospitalization; Prior colonization; Intravenous drug use; Post influenza or Severe-acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. **With or without shock. MDRB Multiresistant drug bacteria
The challenges in analyzing colonization without limiting the scope to diagnosed patients are exemplified by RMMSP panels' high sensitivity (98.55%) but lower specificity (69%), which detects organisms missed by culture methods. Discordances often reflect early infection or host reaction, as evidenced by increased white blood cells and neutrophil counts in PCR-positive, culture-negative samples. Biomarkers like C-reactive protein and procalcitonin may aid clinical interpretation, akin to optimizing C. difficile PCR in high pre-test probability cases [34].
Experts’ opinion 1: Molecular syndromic panels may be considered as valuable tools for the diagnosis and therapeutic management of HAP, VAP, and CAP when MDR bacteria are suspected. These panels can aid in guiding the adjustment of empirical antimicrobial therapy and facilitating quasi-targeted antimicrobial interventions.
May antimicrobial therapy be withheld until the results of the syndromic PCR panel are available (early targeted antimicrobial therapy)?
In cases where shock is absent, physicians may opt for front-line targeted antimicrobial therapy based on syndromic panel results. The high negative predictive value of RMMSP for all panel targets enables the avoidance of antimicrobials directed at undetectable targets (e.g., linezolid in the absence of methicillin-resistant Staphylococcus aureus [MRSA]).
Experts’ opinion 2: In patients with hemodynamic stability (no shock), antimicrobial therapy could be delayed until molecular syndromic panel results are available (no longer than 3 h thereafter).
What specimens should be run on pneumonia syndromic PCR panels?
Both, bronchoalveolar lavages (BAL) and high-quality (as evaluated microscopically) sputum-like specimens (sputa and endotracheal aspirates) may be used.
Experts’ opinion 3: High quality lower respiratory tract specimens, as assessed microscopically, should be used for testing.
How should the results of the syndromic PCR panel be informed (qualitative vs. quantitative for the filmarray panel)?
When feasible (use of the FA-PNp) results should be reported in a quantitative fashion.
Experts’ opinion 4: Molecular syndromic panel results should be reported quantitatively, when possible. Quantitative results (in copies/ml) should be interpreted on an individual basis considering factors such as patient clinical condition, receipt of antimicrobials at the time of sampling, bacterial load, and number of detected targets.
How should results be interpreted?
The FA-PNp provides quantitative results (genomic copies/ml) in binned values (log10 increments from 104 to ≥ 107 genomic copies/ml) for 15 bacterial targets, excluding atypical bacteria. High bacterial burdens (106 or ≥ 107) generally indicate causality. However, lower genomic copies/ml (104 or 105) may also indicate causality, particularly for certain microorganisms as Pseudomonas aeruginosa, Acinetobacter baumannii, and MRSA, especially in patients receiving appropriate antimicrobial therapy.
Interpreting the detection of more than two targets should be approached cautiously, especially when only qualitative results are available. However, high bacterial burdens (106 or ≥ 107), regardless of the number of co-detected microorganisms, may indicate.
The results of genotypic resistance markers offer valuable insights, particularly for Enterobacterales and MRSA. However, it is crucial to interpret these markers in conjunction with the clinical context, local resistance patterns, and antimicrobial stewardship guidelines.
Should pneumonia syndromic PCR Panels replace standard microbiological procedures?
The use of Pneumonia Syndromic molecular panels must not replace conventional methods, but rather serve as a complementary tool for improving the management of patients and decrease the selection of MDR bacteria.
Regarding FA-PNp assay, it demonstrated strong concordance with standard-of-care diagnostics for included species, with no false positives compared to clinical symptoms or cultures. FA-PNp assay reduced the median time to clinical interpretation, potentially improving patient outcomes. However, the assay missed clinically important pathogens, including H. parainfluenza and fungal species such as Aspergillus. While the assay may expedite diagnosis and treatment for certain bacterial and viral infections, it cannot replace standard culture techniques, especially in lung transplant recipients [88].
For fungal species detection, combining RMMSP with next-generation sequencing (NGS) offers the potential for enhanced accuracy, thereby improving its overall clinical utility [89].
Experts’ opinion 5: Molecular syndromic panel must not replace conventional microbiological procedures.
Summary
The use of syndromic pneumonia molecular panels enhances diagnostic yield, reduces the time to appropriate antimicrobial therapy, and may decrease antibiotic consumption [59–73]. However, challenges remain, including the need for further studies on clinical outcomes, cost-effectiveness, and the involvement of antimicrobial stewardship groups. While molecular diagnostics offer promising benefits for antibiotic stewardship, concerns persist about the necessity of initiating broad-spectrum antibiotics to protect unstable patients [90]. Staying updated with guidelines and ensuring multidisciplinary collaboration are crucial for the effective implementation and cost-effectiveness of these technologies in patient care.
Molecular syndromic panels for the diagnosis and management of sepsis and bloodstream infections
Molecular syndromic panels for the diagnosis and management of bloodstream infections
Bloodstream bacterial and fungal infections are common and lead to significant morbidity and mortality in critically ill patients [91, 92]. Rapid initiation of appropriate therapy is crucial, as delays increase mortality by approximately 10% per hour [93–96]. RMMSP testing enables quicker identification of pathogens and distinguishes between infectious agents and contaminants, aiding in the reduction of unnecessary antibiotic use [97, 98]. The Surviving Sepsis Campaign recommends initiating adequate antimicrobial therapy within the first hour for patients in shock and within three hours for patients with sepsis [99].
While blood cultures remain the standard diagnostic method, they are limited by slow growth, particularly in candidemia, and inefficiency in patients on antibiotics or those with fungal or slow-growing infections [91, 92]. RMMSP testing enhances pathogen detection and improves treatment timing [100–103]. Studies show that inappropriate empirical antibiotic therapy increases mortality in sepsis and septic shock, while timely appropriate therapy improves survival rates [3–5, 104–108].
The EUROBACT-2 study identified common ICU pathogens, including carbapenem-resistant strains, and found that only 51.5% of patients received adequate antimicrobial therapy within 24 h [109]. Delayed antibiotic administration beyond 6 h increases mortality, but immediate initiation is not necessary in all cases, especially for stable surgical patients [93, 110–114].
Invasive fungal infections, including candidemia, are rising in ICU, oncology, and transplant patients due to factors like complex surgeries and prolonged antibiotic use [115–118]. Candida spp. are responsible for up to 10% of hospital bloodstream infections [109]. Tools like Candida scores and beta-delta-glucan tests help rule out invasive candidiasis, and timely antifungal treatment, including echinocandins, improves outcomes [119–123].
Can we recommend the use of syndromic panels directly from blood? Which patients could benefit from their use?
Different RMMSP are available for diagnosing and phenotyping bacteremia, sepsis, or candidemia in critically ill patients (see Table 5).
Table 5.
Overview of the rapid multiplex molecular syndromic platforms that can be used in bacteremia, candidemia or sepsis. Sensitivity–specificity and predictive values
| Name | Bacteria | Fungi | Resistance determinants | Performance | Sample | Time to results | Tecnology | Reference |
|---|---|---|---|---|---|---|---|---|
|
Eazyplex BloodScreen GN CE-IVD |
E.coli K.pneumoniae K.oxytoca P.mirabilis P.aeruginosa |
None | blaCTX-M-1, blaCTX-9 |
Se: 95.5–100% Sp: 99.8–100% PPV: 84.6–100% PNV: 99.5–100% |
Positive Blood culture | < 20 min | LAMP | [124] |
| Eazyplex BloodScreen GP |
E.faecalis Enterococcus spp S.pneumoniae Streptococcus spp |
None |
vanA vanB |
Se: 95.2–100% Sp: 93–98% PPV: 92.3–100% PNV: 96.4–100% |
Positive Blood culture | < 20 min | LAMP | [125] |
| Eazyplex superbug CRE | None | None |
blaCTX-M-1, blaCTX-M-9 VIM, NDM, OXA-48, KPC |
S: 95–100% E: 97.9% |
Positive Blood culture Urine |
< 20 min | LAMP | [126] |
| ePlex BCID GN |
21 Gram-negative bacterial genera or species Pan grampositive Pan Candida |
None | blaCTX-M, blaOXA, blaVIM, blaNDM, blaKPC, blaIMP |
Se: 75–99% Sp: 97–100% PPV: 96% |
Positive Blood culture | 90 min | Multiplex real-time PCR | [127–129] |
| ePlex BCID GP |
20 Gram-positive bacterial genera or species Pan gramnegative Pan Candida |
None |
mecA, mecC, vanA, vanB |
Se: 97–100% Sp: 97–100% PPV: 99% |
Positive Blood culture | 90 min | Multiplex real-time PCR | [127, 128, 130] |
| ePlex BCID FD | None | 15 fungal genera or species | None |
Se: 100% Sp: 100% |
Positive Blood culture | 90 min | Multiplex real-time PCR | [127] |
| Biofire BCID 2 | 26 Gram-positive and Gram-negative genera or species (including “enterobacterales”) | Candida albicans, C. auris, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, Cryptococcus (C. neoformans/C. gattii) |
mecA/C mecA/C and MREJ (MRSA) vanA/vanB blaKPC, blaIMP, blaNDM, blaOXA-48-like, blaVIM mcr−1 blaCTX-M |
Grampositives: Se: 97% Sp: 99% Gramnegatives: Se: 100% Sp: 100% Fungi: Se: 99% Sp: 100% |
Positive Blood culture | About 1 h | Multiplex real-time PCR | [131, 132] |
|
VERIGENE® Gram-Positive Blood Culture Test (BC-GP) |
13 Gram-positive bacterial genera or species |
None |
mecA, vanA, vanB |
PPA (positive percent agreement): 85.7- > 93.1% NPA (negative percent agreement: 81.5- > 98.2% |
Positive Blood culture | 2,5 h |
PCR + gold nanoparticle probe chemistry (NanoGrid) |
[133] |
| VERIGENE® Gram-Negative Blood Culture Test (BC-GN) | 9 Gram-negative bacterial genera or species | None |
blaKPC, blaIMP, blaNDM, blaOXA, blaVIM blaCTX-M |
PPA: 92.9–100% NPA: 99.5–99.9% |
Positive Blood culture | 2 h | PCR + gold nanoparticle probe chemistry (NanoGrid) | [133] |
| Sepsis Flow Chip |
12 Gram-positive bacterial genera or species |
Candida albicans | mecA, vanA/B, blaCTX, blaSHV, blaSME, blaKPC, blaNMC/IMI, blaGES, blaIMP, blaGIM, blaVIM, blaSPM, blaSIM, blaNDM, blaOXA-23, blaOXA-24, blaOXA-48, blaOXA-51 and blaOXA-58 |
For bacterial identification: S: 93.3% Sp: 100% For resistance markers: S: 93.6% Sp: 100% |
Positive Blood culture | 4 h | Multiplex real-time PCR | [134] |
| T2Bacteria® panel |
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli |
None | None |
Se: 90% Sp: 96–98% PNV: 91–99.7% PPV: 48.9% |
Blood | 4–7 h | PCR + miniaturized magnetic resonance | [135, 136] |
| T2Candida® panel | None |
C. albicans/C. tropicalis, C. glabrata/C. krusei, C.parapsilosis |
None |
Se: 78–91% Sp: 98% VPP: 50% (67% ICU patients) VPN: 99% |
Blood | 3–5 h | PCR + miniaturized magnetic resonance | [136, 137] |
| T2Resistance® panel | None | None |
blaCTX-M 14, blaCTX-M 15, blaCMY, blaDHA, blaKPC, blaOXA-48, blaNDM, blaVIM, blaIMP, vanA\B, mecA\C |
PPA:80% NPA:69.2% PPV:42.9% NPV:92.3% |
Blood | 3–5 h | PCR + miniaturized magnetic resonance | [135] |
| Fungiplex® Candida | None |
Candida spp. (C. albicans, C. parapsilosis, C. dubliniensis, C. tropicalis), C. glabrata, C. krusei |
Se: 100% Sp: 94.1% |
Blood | 3 h | Multiplex real-time PCR | [138] | |
|
Yeast Traffic Light PNA FISH® |
None |
C. albicans/C. parapsilosis, C. tropicalis, C. glabrata/C. krusei |
Se: 92.3% (C.g/C.k) −100% Sp: 94.8% (C.g/C.k) −100% |
Positive Blood culture | 90 min | FISH | [139] | |
| Magicplex™ Sepsis |
73 Gram positives, 12 Gram negatives |
C.albicans C. tropicalis C. parapsilosis C. glabrata C. krusei A. fumigatus |
mecA, van A/B |
S: 29–47% Sp: 66–95% PPV: 23% NPV: 87% |
Blood | 3–5 h | Multiplex real-time PCR | [140, 141] |
| Micro-Dx | 200 bacterial genera | 65 fungal genera | None | - | Blood | 7 h | Targeted metagenomics (PCR 16S/18S) | [142] |
| Hybcell Pathogens DNA | 56 species and 11 genera | 19 species and 5 genera | vanA/B, mecA/C, blaCTX-M, blaKPC, blaOXA-48, blaNDM, blaIMP |
S: 63% Sp: 83% |
Blood | 3 h |
Targeted metagenomics (PCR 16S/18S) |
[143] |
RMMSP, directly analyzing blood samples, when applied to sepsis and candidemia, reduce the time to reach an etiological diagnosis and often enhance the sensitivity of conventional blood cultures.
T2 assays, utilizing miniaturized magnetic resonance technology, enable the identification of microorganisms directly in blood samples. These diagnostic panels are not universally accessible and are currently limited in their ability to detect a narrow spectrum of bacterial and fungal pathogens. A critical challenge lies in identifying appropriate patient populations for testing to optimize cost-effectiveness and maximize clinical utility. They demonstrate higher sensitivity than blood culture in detecting pathogens, especially in patients with ongoing antimicrobial treatment or localized infections causing intermittent bacteremia [144–146]. this system enables the detection of intact cells rather than free- deoxyribonucleic acid (DNA) [144].
Persistent positivity on this platform indicates persistent infection, aiding in identifying patients at risk of poor control or metastatic infection. While it has a high negative predictive value for the microorganisms included in the trial, its coverage is limited [135, 144–147].
T2 panels are also effective in diagnosing candidemia and can predict complicated cases when positivity persists on the 5th day [137, 148, 149]. Combining them with classic markers like [1, 3]-β-D-glucan improves diagnosis, particularly in non-hematological critical patients in the ICU [149].
Can we recommend the use of syndromic panels in grown blood cultures? Which patients could benefit from their use? How and when to use syndromic panels in grown blood cultures based on the characteristics of each hospital?
Most commercialized systems for detecting microbial genomes in positive blood cultures showed high sensitivity and specificity for the included targets [124–143]. New diagnostic techniques, such as mass spectrometry (MALDI TOF), rapid tests for resistance determinants, and RMMSP, significantly enhanced diagnostic accuracy [150]. RMMSP, in particular, provided rapid and precise identification of common pathogens and multidrug-resistant bacteria, supporting treatment decisions. However, their clinical utility heavily depended on optimizing the entire diagnostic process. This included ensuring proper pre-analytical procedures (e.g., collecting adequate blood volumes and preventing contamination), conducting analytical processes in a 24/7 microbiology system, and ensuring that results promptly reached clinicians for treatment adjustments [102, 151, 152].
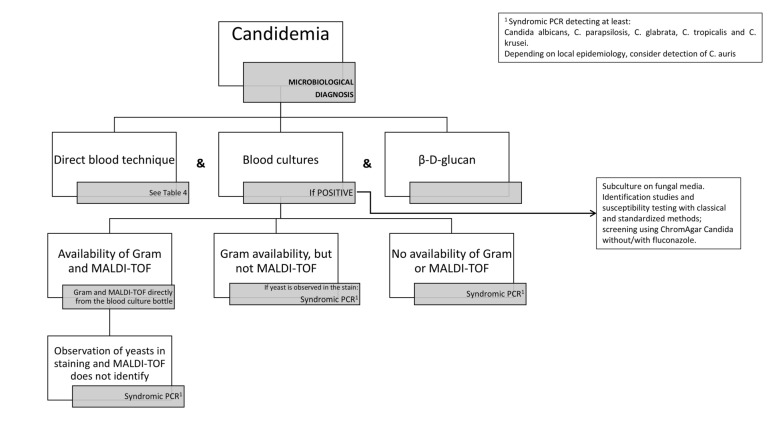
Rapid identification of bacteremia and candidemia etiology, along with key resistance determinants, remained crucial due to the severity of these infections. RMMSP proved especially useful when MALDI-TOF was unavailable or inconclusive, such as in cases of polymicrobial bacteremia, Gram-positive microorganisms, or yeast infections. Additionally, RMMSP was beneficial when rapid resistance gene detection methods were lacking. The protocols are detailed in Figs. 2 and 3.
Fig. 2.
Algorithm for the use of the rapid multiplex molecular syndromic platforms in bacteremia. 1Syndromic PCR panels that detects at least: Streptococcus pyogenes, Streptococcus pneumoniae, Genus Streptococcus, Enterococcus faecalis, Enterococcus faecium, vanA/vanB. 2Perform directly from the blood culture sample. Assess techniques to be carried out according to microorganism and local epidemiology. As a guide, the following instructions can be followed: Escherichia coli: Colorimetric test for the detection of 3rd generation cephalosporin hydrolyzing enzymes. If it is negative and there is clinical suspicion of infection with an extended-spectrum beta-lactamase-producing strain or the patient is very serious/vulnerable: immunochromatography or PCR to detect CTX-M production/gene. Klebsiella spp: Colorimetric test for the detection of enzymes hydrolyzing 3rd generation cephalosporins and carbapenemases. If they are negative and there is clinical suspicion of multidrug-resistant strain infection or the patient is very serious/vulnerable: immunochromatography or PCR for detection of CTX-M production/gene and immunochromatography or PCR for detection/production of genes VIM, NDM, OXA-48, and KPC. Proteus spp: Colorimetric test for the detection of 3rd generation cephalosporin hydrolyzing enzymes. If it is negative and there is clinical suspicion of infection with an extended-spectrum beta-lactamase-producing strain or the patient is very serious/vulnerable: immunochromatography or PCR to detect CTX-M production/gene. Enterobacter spp: Colorimetric test for the detection of carbapenemases. If they are negative and there is clinical suspicion of infection with a multidrug-resistant strain or the patient is very serious/vulnerable: immunochromatography or PCR for detection of production/genes of VIM, NDM, OXA-48 and KPC. Pseudomonas aeruginosa: According to local epidemiology, colorimetric test for the detection of carbapenemases, immunochromatography or PCR for detection of VIM and NDM production/genes. Acinetobacter baumanii: According to local epidemiology, colorimetric test for detection of carbapenemases, immunochromatography or PCR for detection of VIM and NDM production/genes (ideally include OXA-23, OXA-24 and OXA-58). 3Syndromic PCR panels that detects at least: Species-level identification of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumanii and the resistance genes blaCTX-M, blaVIM, blaNDM, blaOXA-48 and blaKPC. 4Syndromic PCR panles that has targets to detect at least: Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Genus Streptococcus, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumanii, Listeria spp, mecA/C, vanA/vanB, blaCTX-M, blaVIM, blaNDM, blaOXA −48 and blaKPC. *See corresponding sections of the current Experts’ opinion Document. **If polymicrobial infection: directly perform syndromic PCR
Fig. 3.
Algorithm for the use of the rapid multiplex molecular syndromic platforms in Candida infection. 1Syndromic PCR that detects at least: Candida albicans, C. parapsilosis, C. glabrata, C. tropicalis and C. krusei. According to local epidemiology, consider C. auris detection
Experts’ opinion 6: Diagnostic tools that allow rapid identification of the microorganism causing bacteriemia/candidemia, as well as the main determinants of resistance, should always be used in critically ill patients. RMMSP on grown blood cultures would be recommended, but they should be used when other procedures are not available, due to their high cost.
Limitations of syndromic panels and considerations regarding the determination of resistance genes. May antimicrobial therapy be withheld until the results of the RMMSP panel are available (early targeted antimicrobial therapy)?
Molecular diagnostic techniques, while offering benefits such as quicker treatment initiation, have certain limitations [124–143]. They may not identify all microorganisms in polymicrobial bacteremia or rare pathogens. Detection of anaerobic bacteria is limited, and they mainly identify beta-lactam drug resistance mechanisms due to beta-lactamases. Presence of a resistance gene does not always imply its expression, causing genotype–phenotype discrepancies during susceptibility testing [124–143]. Specific limitations in detecting determinants of resistance in gram-positive and gram-negative microorganisms are detailed in Table S1.
Despite limited evidence, these techniques generally reduce the time to appropriate treatment, though they do not significantly impact mortality rates, largely due to the complexity of managing critically ill patients [153–155]. Empirical treatments remain vital, and knowledge of local epidemiology, particularly regarding multidrug-resistant bacteria, is crucial [156–158]. Future integration of clinical and microbiological data, along with advancements in artificial intelligence and next-generation sequencing, is expected to improve infection management further [159–161].
Experts’ opinion 7: RMMSP allow a rapid initiation of targeted antibiotic therapy. However, some limitations must be considered, mainly referring to the targets included, both in identification and in resistance genes. Therefore, knowledge of local epidemiology is essential so that their impact on decision-making is optimal.
Rapid multiplex molecular syndromic panels in meningitis and encephalitis
What are the rapid multiplex molecular syndromic panels available in meningitis and encephalitis?
RMMSP have recently been introduced for diagnosing meningitis and encephalitis. The most widely used panels, FilmArray ME and QIAstat-Dx Meningitis/Encephalitis, require a small sample volume (200 µl) and provide rapid results (around one hour). They detect various pathogens, including bacteria like E. coli K1, H. influenzae, L. monocytogenes, N. meningitidis, S. agalactiae, S. pneumoniae, several viruses and Cryptococcus neoformans/C.gattii [162, 163]. While both panels detect almost the same pathogens, QIAstat-Dx Meningitis/Encephalitis includes additional pathogens like M. pneumoniae and S. pyogenes but do not include cytomegalovirus [164, 165]. Both systems can provide information on the threshold cycle (Ct) value and amplification curves, in the case of FilmArray ME through an additional application (Fireworks) [166].
What is the accuracy of the available rapid multiplex molecular syndromic panels in meningitis/encephalitis?
The FilmArray ME panel has been evaluated in several studies. Sensitivity and specificity were high, although they varied among targets. Some viruses, such as enterovirus and herpesvirus, exhibited lower sensitivity [162, 164, 165, 167]. In cases of negative results with high clinical suspicion, it has been recommended to perform monoplex PCR [167]. Interpretation of results for herpesvirus 6 should be cautious due to possibility of its DNA being integrated into chromosomes (1% of human population): in this sense, performing a viral load in peripheral blood can help the interpretation of the results and diagnosis. In bacteria, there were high sensitivity and specificity rates, although false positives were observed for certain species due to contamination. Correct material handling is crucial [168, 169].
A meta-analysis evaluating the clinical performance of the FilmArray ME panel for all bacterial targets found combined sensitivity/specificity ranging from 89.5–92.1% to 97.4–99.2%, respectively, depending on the reference test [154]. A higher proportion of false positives has been observed for S. pneumoniae, H. influenzae and S. agalactiae [163, 170], possibly due to contamination during the procedure [163], emphasizing the importance of proper material handling and cleaning. Both false positives and false negatives have been reported for S. agalactiae [162].
Cryptococcal meningitis diagnosis requires cerebrospinal fluid (CSF) culture and cryptococcal antigen detection, along with syndromic panels. A multicenter study found FilmArray ME panel sensitivity/specificity at 96.4%/99.6% versus culture and 83.8%/99.9% versus antigen [171]. Recent comparisons of FilmArray ME and QIAstat-Dx Meningitis/Encephalitis showed similar diagnostic values [163, 172]. Nosocomial central nervous system (CNS) infections, like those from ventricular catheters, may demand alternative strategies beyond these panels (see 4.4.)
Which patient profile would benefit from the use of syndromic panels in meningitis/encephalitis?
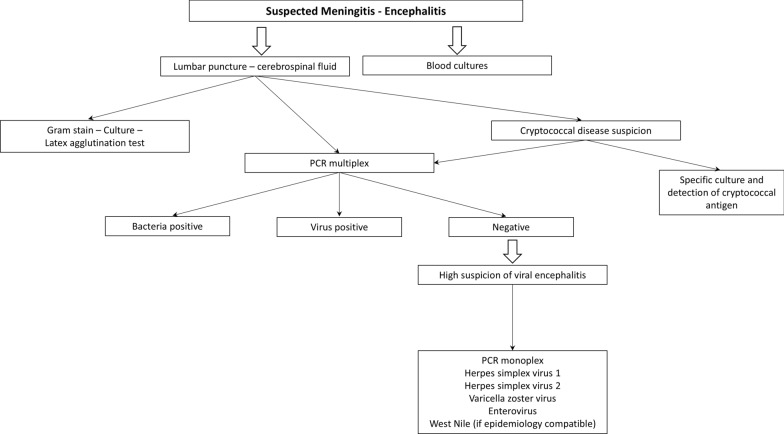
Meningoencephalitis is a severe neurologic syndrome associated with high mortality and disability rate. One in two will require admittance in an intensive care unit. Early antimicrobial therapy is likely to improve the prognosis [173]. RMMSP are of particular interest to antimicrobial stewardship programs, with the aim of placing patients on optimized therapy as soon as possible and discontinuing unnecessary antimicrobials [174]. Diagnosis is based on blood cultures, CSF analysis, Gram stain, culture, molecular tests (PCR) and, in special cases, latex agglutination test (Fig. 4) [175]. However, diagnosis by culture can be delayed and give false negatives. In viral infections, traditional protocols may not be available in laboratories with a smaller number of samples or only with a morning sift schedule [176].
Fig. 4.
Diagnostic algorithm for meningoencephalitis. PCR Polymerase chain reaction
In this context, if the etiology is not clear in the first microbiological evaluation, a syndromic panel should be performed in all patients if meningitis or encephalitis is threatened. RMMSP does not replace the other standard microbiological diagnostics and attempt to identify the pathogen in culture and determination of antibiotic susceptibility [175].
Experts’ opinion 8: We suggest performing a RMMSP in all patients when meningitis or encephalitis is suspected, particularly in those patients with admittance criteria in an intensive care unit.
Rapid multiplex molecular syndromic panels: other applications and “Off-label” use
What are the most relevant off-label applications of syndromic panels so far?
In critically ill patients, early diagnosis of intra-abdominal infections is essential. Syndromic panels, such as T2Bacteria and T2Candida, can significantly improve the diagnostic process in just 3–5 h, identifying microorganisms that are often not detected in blood cultures [177, 178]. Additionally, other samples, such as peritoneal fluid and bile, can be used in conjunction with molecular techniques like syndromic panels for a more rapid diagnosis. This strategy offers new possibilities, as discussed as follows.
RMMSP, although licensed for specific clinical uses, show off-label applications documented in the literature, particularly FilmArray™ Blood Culture Identification Panel (BCID). Its detection limit can vary, being lower than other panels, because it is designed for use with positive blood cultures [179–181]. The reliability of the "off-label" result varies depending on the series and type of sample (Table 6). A negative result does not rule out infection by the microorganisms included (or detected) in/by the panel.
Table 6.
Rapid multiplex molecular syndromic panels used in different microbiological samples (off-label uses)
| Samples | System | Comparator | Results | Reference |
|---|---|---|---|---|
|
Ascitic/peritoneal fluid intra-abdominal abscess |
FilmArray™ BCID | Culture, metagenomics |
Sens: 25–90.5% Spec: 100% |
[178, 182] |
| Bile fluid | Verigene system assays | Culture | Sens: 35.7% | [183] |
| Pleural fluid |
FilmArray™ BCID, FilmArray ME |
Culture |
Sens: 25% Spec: 100% |
[169, 184, 185] |
| Joint fluid |
FilmArray™ BCID Biofire Joint infection panel |
Culture | Se: 57–90% | [169, 186–188] |
| CSF (Nosocomial CNS infections) | FilmArray™ BCID | Culture |
Sens: 77.4% Spec: 100% With melting curve analysis: Sens: 83.9%, Spec: 98.3% |
[180, 184] |
| CSF (community and shunt-associated meningitis) | FilmArray™ BCID | Culture |
Sens: 50% Spec: 91.7% PPV: 80% NPV: 73.3% |
[179] |
| CSF (community meningitis) | FilmArray™ BCID | Culture |
Sens: 73% Spec: 100% |
[169] |
| Aspirates from abscesses and cellulitis | FilmArray™ BCID | Culture |
Sens: 89% Spec: 100% |
[169, 184] |
| Bone and tissue biopsies | FilmArray™ BCID | Culture |
Concordance 95–100% |
[184] |
| Lymph node aspirates | FilmArray™ BCID | Culture | Concordance 100% | [184] |
Sens Sensitivity; Spec Specificity; CSF Cerebrospinal fluid; CNS Central Nervous System; PPV positive predictive value; NPV negative predictive value
RMMSP, which now include resistance markers, are being also used to study rectal colonization in septic patients without an etiological diagnosis [189–191]. Rectal cultures, biomarkers, and risk scales have proven effective in guiding empirical therapy. RMMSP now offer rapid results within an hour, a significant improvement over the 24–48 h required for conventional surveillance cultures [189–191]. However, its high cost and lack of detection of some resistance mechanisms are challenges [192, 193].
New applications of syndromic panels are being explored, such as the detection of Cryptosporidium spp. in respiratory samples and adenovirus in serum using panels designed for other samples [186, 194].
Experts’ opinion 9: It is not possible to establish a general opinion on the off-label use of syndromic panels. However, their use in critically ill patients could be considered in the clinical settings described above to obtain indicative microbiological information while awaiting the results of conventional methods.
Conclusions
An overview of the main subjects and essential conceptual insights is shown in Table 7.
Table 7.
Concluding directives: synopsis of principal matters and essential conceptual insights
| Key-points | Essential conceptual insights |
|---|---|
| Item 1 | These new diagnostic tools (including RMMSP) do not substitute or replace traditional microbiology; instead, they complement and reinforce it |
| Item 2 | The value, qualification, translation, and impact of the results of the "expert system" must be validated and endorsed by a microbiologist with experience and clinical judgment |
| Item 3 | The principles of precise, accurate, reliable, agile, and rapid diagnosis, based on their predictive values, should guide the indication of new diagnostic tools to minimize errors, inadequacy, or ineffectiveness of empirical antibiotic treatment in an MDR context |
| Item 4 | It is not advisable to periodically repeat these diagnostic procedures (without a justified cause) in those patients who do not experience significant clinical changes over the follow-up, which justifies or makes necessary their use |
| Item 5 | These diagnostic tools are not indicated for patients who have recently had a positive and clinically value microbiological test, simply for ruling out other potential pathogens |
| Item 6 | The results of these diagnostic tools should be binding (unless the patient has been diagnosed with septic shock). Tests results should impact on antimicrobial optimization and decision-making, either escalation, de-escalation, or stop the antibiotic regime |
| Item 7 | Although in real-clinical practice these diagnostic tools have been used in "off-label" clinical scenarios, their use will always be customized (following a patient tailored approach) according to microbiologist criteria and agreed with the clinician. Their results cannot be extrapolated to other patients |
| Item 8 | It should be noted that the projection and use of these diagnostic tools may be subject to different nuances and implications in critically ill patients, compared to other complex but not severely infected patients |
| Item 9 | All these microbiological diagnostic tools need to be progressively evaluated in terms of cost–benefit, influence on morbidity and mortality, and health-related outcomes, in order to select the best diagnostic panel for each patient |
| Item 10 | All these considerations and recommendations must be considered within a dynamic context and should be continually reassessed to implement emerging discoveries and future evidence as they become available |
RMMSP rapid multiplex molecular syndromic panels; MDR multi-drug resistant
Inadequate antibiotic therapy can lead to treatment failure, prolonged illness, and the spread of antibiotic-resistant bacteria. When antibiotics are prescribed without identifying the pathogen or selecting ineffective drugs, infections may persist and facilitate the emergence of MDR bacteria. Therefore, accurate diagnosis and proper antibiotic selection are crucial for managing critically ill patients and controlling antibiotic resistance.
RMMSP offer a promising approach to expedite the diagnosis of infectious diseases by simultaneously testing for multiple pathogens associated with a particular clinical syndrome. These panels utilize molecular techniques, such as PCR, to detect the presence of bacterial, viral, and fungal pathogens directly from clinical specimens like blood, urine, respiratory secretions, or sterile fluids.
RMMSP provide rapid results and comprehensive pathogen detection, potentially supporting precision medicine, improving patient outcomes, and contributing to public health surveillance. However, their clinical performance in specific scenarios and their impact on antibiotic use remain areas of ongoing debate. Therefore, they should always be used from a clinical perspective and within the appropriate clinical context.
Furthermore, RMMSP, combined with NGS, have revolutionized diagnostic workflows by providing precise pathogen identification and resistance profiling. This integration significantly reduces turnaround times, enhancing clinical decision-making and patient outcomes, particularly in time-sensitive scenarios such as respiratory and central nervous system infectious diseases.
In conclusion, while syndromic panels provide rapid, comprehensive diagnostics for infectious diseases, their use must be balanced with clinical judgment and antibiotic stewardship to optimize care and reduce risks like inadequate therapy and antibiotic resistance. Future advancements may include integrating RMMSP with predictive artificial intelligence, further enhancing diagnostic accuracy and refining the management of critically ill, infected patients.
Supplementary Information
Acknowledgements
Medical writing and Editorial assistant services have been provided by Antonio Martínez (MD) from Ciencia y Deporte S.L.
Abbreviations
- AMR
Antimicrobial resistance
- AWMF
Association of the scientific medical societies
- BAL
Bronchoalveolar lavages
- BCID
Blood culture identification panel
- CAP
Community-acquired
- CFU
Colony-forming unit
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DNA
Deoxyribonucleic acid
- DTR
Difficult-to-treat resistance
- EAT
Empirical antimicrobial therapy
- EC
European community
- ESBL
Extended spectrum β-lactamases
- FA-PNp
BioFire® FilmArray® Pneumonia (PN) panel
- FDA
Food and drug administration
- GEIPC-SEIMC
Spanish society of clinical microbiology and infectious diseases
- GTEIS-SEMICYUC
Spanish society of critical intensive care medicine and coronary care units
- HAP
Hospital-acquired
- HPN
Unyvero hospitalized pneumonia panel
- ICU
Intensive care unit
- MALDI TOF
Matrix-assisted laser desorption/ionization time-of-flight
- MDR
Multi-drug resistant
- MRSA
Methicillin-resistant Staphylococcus aureus
- PCR
Polymerase chain reaction
- PPV
Positive predictive value
- RMMSP
Rapid multiplex molecular syndromic panels.
- SARS-CoV-2
Severe acute respiratory syndrome-coronavirus type 2.
- VAP
Ventilator-associated
Author contributions
All the authors have made substantial contributions to the conception; design of the study; acquisition, review, and interpretation of the data; and have drafted the paper or substantively revised it. All authors read and approved the final manuscript.
Funding
Medical writing services has been provided by bioMérieux.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Francisco Javier Candel has received (in the last three years) honoraria for lectures and advisory boards from Advanz Pharma, Angelini, Biomerieux, Gilead, Meiji, Menarini, MSD, Pfizer, Shionogi, and Viatris. Miguel Salavert Lletí has received (in the last three years) honoraria for lectures and advisory boards from Advanz Pharma, Angelini, Janssen, Menarini, MSD, Pfizer, Shionogi, and Viatris, and educational grants from Gilead and Tedec-Meiji. Rafael Cantón has participate in educational programs sponsored by BioMeieux, Pfizer, Menarini, MSD, Shionogi, and Tedec-Meiji and participate in research studies funded by BD, BioMerieux, Cepheid, MSD, and Shionogi. Jose Luis del Pozo has received honoraria for lectures and advisory boards from Advanz Pharma, Angelini, Menarini, MSD, Pfizer, Shionogi, and GSK, and educational grants from Pfizer. Fátima Galán-Sánchez has received honoraria for lectures and advisory boards from MSD, Pfizer, Shionogi and bioMerieux, and grants from Pfizer and Menarini. Montserrat Rodríguez-Aguirregabiria has conducted consulting work for Viatris and Shionogi. MR-A has served as a speaker for Pfizer, Gilead Sciences, and Shionogi. Rafael Zaragoza has received (in the last three years) honoraria for lectures and advisory boards from Advanz Pharma, BiomeMeieux, Gilead science, MSD, Mundipharma, Pfizer, Shionogi, and Viatris, David Navarro; Alejandro Rodríguez; Juan Carlos Rodríguez;L and Borja Suberviola declare that they have no competing interests. Jose Luis del Pozo has received honoraria for lectures and advisory boards from Advanz Pharma, Angelini, Menarini, MSD, Pfizer, Shionogi, and GSK, and educational grants from Pfizer. Fátima Galán-Sánchez has received honoraria for lectures and advisory boards from MSD, Pfizer, Shionogi and bioMerieux, and grants from Pfizer and Menarini. Montserrat Rodríguez-Aguirregabiria has conducted consulting work for Viatris and Shionogi. MR-A has served as a speaker for Pfizer, Gilead Sciences, and Shionogi. Rafael Zaragoza has received (in the last three years) honoraria for lectures and advisory boards from Advanz Pharma, BiomeMeieux, Gilead science, MSD, Mundipharma, Pfizer, Shionogi, and Viatris, David Navarro; Alejandro Rodríguez; Juan Carlos Rodríguez;L and Borja Suberviola declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haque M, Sartelli M, McKimm J, Abu BM. Health care-associated infections—an overview. Infect Drug Resist. 2018;15(11):2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luyt CE, Bréchot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Cooperative antimicrobial therapy of septic shock database research group. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–48. [DOI] [PubMed] [Google Scholar]
- 5.Rhee C, Kadri SS, Dekker JP, Danner RL, Chen HC, Fram D, et al. CDC prevention epicenters program. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depuydt PO, Vandijck DM, Bekaert MA, Decruyenaere JM, Blot SI, Vogelaers DP, et al. Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Crit Care. 2008;12(6):R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. 2016;30(2):377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dien Bard J, McElvania E. Panels and syndromic testing in clinical microbiology. Clin Lab Med. 2020;40(4):393–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentilotti E, De Nardo P, Cremonini E, Górska A, Mazzaferri F, Canziani LM, et al. Diagnostic accuracy of point-of-care tests in acute community-acquired lower respiratory tract infections. A systematic review and meta- analysis. Clin Microbiol Infect. 2022;28(1):13–22. [DOI] [PubMed] [Google Scholar]
- 10.Adams J, Ferguson K, Hirschy R, Konopka E, Meckel J, Benanti G, et al. Antimicrobial stewardship techniques for critically ill patients with pneumonia. Antibiotics (Basel). 2023;12(2):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen R, Babushkin F, Finn T, Geller K, Alexander H, Datnow C, et al. High rates of bacterial pulmonary co-infections and superinfections identified by multiplex PCR among critically Ill COVID-19 Patients. Microorganisms. 2021;9(12):2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karolyi M, Pawelka E, Hind J, Baumgartner S, Friese E, Hoepler W, et al. Detection of bacteria via multiplex PCR in respiratory samples of critically ill COVID-19 patients with suspected HAP/VAP in the ICU. Wien Klin Wochenschr. 2022;134(9–10):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apisarnthanarak A, Bin Kim H, Moore LSP, Xiao Y, Singh S, Doi Y, et al. Utility and applicability of rapid diagnostic testing in antimicrobial stewardship in the Asia-Pacific region: a Delphi consensus. Clin Infect Dis. 2022;74(11):2067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos LM, Bruning AHL, Reitsma JB, Schuurman R, Riezebos-Brilman A, Hoepelman AIM, et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis. 2019;69(7):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellermeyer L, Harnke B, Knight S. Covidence and Rayyan. J Med Libr Assoc. 2018;106(4):580–3. [Google Scholar]
- 16.Peker N, Couto N, Sinha B, Rossen JW. Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: recent developments in molecular approaches. Clin Microbiol Infect. 2018;24(9):944–55. [DOI] [PubMed] [Google Scholar]
- 17.Opota O, Jaton K, Greub G. Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin Microbiol Infect. 2015;21(4):323–31. [DOI] [PubMed] [Google Scholar]
- 18.Dessajan J, Timsit JF. Impact of multiplex PCR in the therapeutic management of severe bacterial pneumonia. Antibiotics (Basel). 2024;13(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doppalapudi S, Adrish M. Community-acquired pneumonia: The importance of the early detection of drug-resistant organisms. World J Crit Care Med. 2024;13(2):91314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz JE, Stratton CW, Persing DH, Tang YW. Forty years of molecular diagnostics for infectious diseases. J Clin Microbiol. 2022;60(10): e0244621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchcock MM, Gomez CA, Pozdol J, Banaei N. Effective approaches to diagnostic stewardship of syndromic molecular panels. J Appl Lab Med. 2024;9(1):104–15. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez A, Gómez F, Sarvisé C, Gutiérrez C, Giralt MG, Guerrero-Torres MD, Pardo-Granell S, et al. Clinical and microbiological impact of implementing a decision support algorithm through microbiologic rapid diagnosis in critically Ill patients: an epidemiological retrospective pre-/post-intervention study. Biomedicines. 2023;11(12):3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clari MÁ, Carbonell N, Albert E, Navarro D. Proposal for antimicrobial therapy stewardship of lower respiratory tract infection in mechanically-ventilated patients based upon the Biofire® Filmarray® Pneumonia Plus panel results. Enferm Infecc Microbiol Clin (Engl Ed). 2023;41(8):521–3. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Loeches I, Pereira JG, Teoh TK, Barlow G, Dortet L, Carrol ED, Olgemöller U, Boyd SE, Textoris J. Molecular antimicrobial susceptibility testing in sepsis. Future Microbiol. 2024;19:61–72. [DOI] [PubMed] [Google Scholar]
- 25.Buchan BW, Ledeboer NA. Emerging technologies for the clinical microbiology laboratory. Clin Microbiol Rev. 2014;27(4):783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2017;31(1):e00024-e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumkow LE, Worden LJ, Rao SN. Syndromic diagnostic testing: a new way to approach patient care in the treatment of infectious diseases. J Antimicrob Chemother. 2021;76:iii4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MB. Opinion on syndromic panel-based testing in clinical microbiology. Clin Chem. 2020;66(1):42–4. [DOI] [PubMed] [Google Scholar]
- 29.Cassidy H, Van Genne M, Lizarazo-Forero E, Gard L, Niesters HGM. A discussion of syndromic molecular testing for clinical care. J Antimicrob Chemother. 2021;76(Suppl 3):iii58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couturier MR, Bard JD. Direct-from-specimen pathogen identification: evolution of syndromic panels. Clin Lab Med. 2019;39(3):433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewinski MA, Alby K, Babady NE, Butler-Wu SM, Bard JD, Greninger AL, et al. Exploring the utility of multiplex infectious disease panel testing for diagnosis of infection in different body sites: a joint report of the association for molecular pathology, american society for microbiology, infectious diseases society of america, and pan american society for clinical virology. J Mol Diagn. 2023;25(12):857–75. [DOI] [PubMed] [Google Scholar]
- 32.Rader TS 4th, Stevens MP, Bearman G. Syndromic multiplex polymerase chain reaction (mPCR) testing and antimicrobial stewardship: current practice and future directions. Curr Infect Dis Rep. 2021;23(4):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Relich RF, Abbott AN. Syndromic and point-of-care molecular testing. Clin Lab Med. 2022;42(4):507–31. [DOI] [PubMed] [Google Scholar]
- 34.Walker AM, Timbrook TT, Hommel B, Prinzi AM. Breaking boundaries in pneumonia diagnostics: transitioning from tradition to molecular frontiers with multiplex PCR. Diagnostics (Basel). 2024;14(7):752. 10.3390/diagnostics14070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50(6):339–44. [PubMed] [Google Scholar]
- 36.Cano S, de Michelena P, Clari MÁ, Liñan J, Olea B, Torres I, Carbonell N, et al. Impact of the microscopic quality of endotracheal aspirates on the performance of the Filmarray® pneumonia plus panel in intensive care unit patients with suspected lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 2024. 10.1007/s10096-024-04967-9. [DOI] [PubMed] [Google Scholar]
- 37.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia an official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;2007:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–20. [DOI] [PubMed] [Google Scholar]
- 39.Miller MB, Atrzadeh F, Burnham CA, Cavalieri S, Dunn J, Jones S, et al. ASM clinical and public health microbiology committee and the ASM corporate council. Clinical utility of advanced microbiology testing tools. J Clin Microbiol. 2019;57(9):e00495-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantón R, de la Gómez G, Pedrosa E. Economic impact of rapid diagnostic methods in clinical microbiology: price of the test or overall clinical impact. Enferm Infecc Microbiol Clin. 2017;35(10):659–66. [DOI] [PubMed] [Google Scholar]
- 41.Dyar OJ, Moran-Gilad J, Greub G, Pulcini C. ESGMD executive Committee and the ESGAP Executive committee diagnostic stewardship: are we using the right term? Clin Microbiol Infect. 2019;25(3):272–3. [DOI] [PubMed] [Google Scholar]
- 42.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship-leveraging the laboratory to improve antimicrobial use. JAMA. 2017;318(7):607–8. [DOI] [PubMed] [Google Scholar]
- 43.Coffey KC, Morgan DJ, Claeys KC. Diagnostic stewardship: what impacts antibiotics use? Curr Opin Infect Dis. 2023;36(4):270–5. [DOI] [PubMed] [Google Scholar]
- 44.Bou G, Cantón R, Martínez-Martínez L, Navarro D, Vila J. Fundamentals and implementation of microbiological diagnostic stewardship programs. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39(5):248–51. [DOI] [PubMed] [Google Scholar]
- 45.Huang HS, Tsai CL, Chang J, Hsu TC, Lin S, Lee CC. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta- analysis. Clin Microbiol Infect. 2018;24(10):1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eichinger A, Hagen A, Meyer-Bühn M, Huebner J. Clinical benefits of introducing real-time multiplex PCR for cerebrospinal fluid as routine diagnostic at a tertiary care pediatric center. Infection. 2019;47(1):51–8. [DOI] [PubMed] [Google Scholar]
- 47.Poole S, Clark TW. Rapid syndromic molecular testing in pneumonia: the current landscape and future potential. J Infect. 2020;80(1):1–7. 10.1016/j.jinf.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burillo A, Candel FJ, Canut-Blasco A. Value of syndromic panels in the management of severe community-acquired pneumonia. Rev Esp Quimioter. 2022;35(Suppl 1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Decaro JD, Green NM, Godwin HA. Critical evaluation of FDA-approved respiratory multiplex assays for public health surveillance. Expert Rev Mol Diagn. 2018;18(7):631–43. 10.1080/14737159.2018.1487294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abelenda-Alonso G, Calatayud L, Rombauts A, Meije Y, Oriol I, Sopena N, et al. Multiplex real-time PCR in non-invasive respiratory samples to reduce antibiotic use in community-acquired pneumonia: a randomised trial. Nat Commun. 2024;15(1):7098. 10.1038/s41467-024-51547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanella MC, Meylan P, Kaiser L. Syndromic panels or the panels’ syndrome? A perspective through the lens of respiratory tract infections: author’s response. Clin Microbiol Infect. 2020;26(8):1107–8. 10.1016/j.cmi.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babady NE, England MR, Jurcic Smith KL, He T, Wijetunge DS, Tang YW, et al. Multicenter evaluation of the ePlex respiratory pathogen panel for the detection of viral and bacterial respiratory tract pathogens in nasopharyngeal Swabs. J Clin Microbiol. 2018;56(2):e01658-e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Candel FJ, Salavert M, Estella A, Ferrer M, Ferrer R, Gamazo JJ, et al. Ten issues to update in nosocomial or hospital-acquired pneumonia: an expert review. J Clin Med. 2023;12(20):6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson KE, Azar MM, Banerjee R, Chou A, Colgrove RC, Ginocchio CC, et al. Molecular testing for acute respiratory tract infections: clinical and diagnostic recommendations from the idsa’s diagnostics committee. Clin Infect Dis. 2020;71(10):2744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serigstad S, Ritz C, Faurholt-Jepsen D, Markussen D, Ebbesen MH, Kommedal Ø, CAPNOR study group, et al. Impact of rapid molecular testing on diagnosis, treatment and management of community-acquired pneumonia in Norway: a pragmatic randomised controlled trial (CAPNOR). Trials. 2022;23(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abelenda-Alonso G, Rombauts A, Gudiol C, García-Lerma E, Pallarés N, Ardanuy C, et al. Effect of positive microbiological testing on antibiotic de-escalation and outcomes in community-acquired pneumonia: a propensity score analysis. Clin Microbiol Infect. 2022;28(12):1602–8. [DOI] [PubMed] [Google Scholar]
- 57.Britt NS, Khader K, He T, Willson TM, Effiong A, Timbrook TT, et al. Examining the clinical impact of rapid multiplex polymerase chain reaction-based diagnostic testing for bloodstream infections in a national cohort of the Veterans Health Administration. Pharmacotherapy. 2023;43(1):24–34. [DOI] [PubMed] [Google Scholar]
- 58.Cartuliares MB, Rosenvinge FS, Mogensen CB, Skovsted TA, Andersen SL, Østergaard C, et al. Evaluation of point-of-care multiplex polymerase chain reaction in guiding antibiotic treatment of patients acutely admitted with suspected community-acquired pneumonia in Denmark: a multicentre randomised controlled trial. PLoS Med. 2023;20(11): e1004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.BioFire® FilmArray® Pneumonia (PN) panel summary of product characteristics. Available in: BFDX-MKT-0373-Pneumonia-Panel-Procedure-in-CLSI.docx (live.com) Last accessed September 7, 2024.
- 60.Unyvero Hospitalised Pneumonia Panel summary of product characteristics. Available in: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170047.pdf Last accessed September 7, 2024.
- 61.Buchan BW, Windham S, Balada-Llasat JM, Leber A, Harrington A, Relich R, et al. Practical comparison of the BioFire FilmArray pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol. 2020;58(7):e00135-e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stafylaki D, Maraki S, Vaporidi K, Georgopoulos D, Kontoyiannis DP, Kofteridis DP, et al. Impact of molecular syndromic diagnosis of severe pneumonia in the management of critically Ill patients. Microbiol Spectr. 2022;10(5): e0161622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozongwu C, Personne Y, Platt G, Jeanes C, Aydin S, Kozato N, et al. The Unyvero P55 “sample-in, answer-out” pneumonia assay: a performance evaluation. Biomol Detect Quantif. 2017;13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gadsby NJ, McHugh MP, Forbes C, MacKenzie L, Hamilton SKD, Griffith DM, et al. Comparison of Unyvero P55 Pneumonia Cartridge, in-house PCR and culture for the identification of respiratory pathogens and antibiotic resistance in bronchoalveolar lavage fluids in the critical care setting. Eur J Clin Microbiol Infect Dis. 2019;38(6):1171–8. [DOI] [PubMed] [Google Scholar]
- 65.Collins ME, Popowitch EB, Miller MB. Evaluation of a novel multiplex PCR panel compared to quantitative bacterial culture for diagnosis of lower respiratory tract infections. J Clin Microbiol. 2020;58(5):e02013-e2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peiffer-Smadja N, Bouadma L, Mathy V, Allouche K, Patrier J, Reboul M, et al. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit Care. 2020;24(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy CN, Fowler R, Balada-Llasat JM, Carroll A, Stone H, Akerele O, et al. Multicenter evaluation of the BioFire FilmArray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol. 2020;58(7):e00128-e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein M, Bacher J, Barth S, Atrzadeh F, Siebenhaller K, Ferreira I, et al. Multicenter evaluation of the unyvero platform for testing bronchoalveolar lavage fluid. J Clin Microbiol. 2021;59(3):e02497-e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ginocchio CC, Garcia-Mondragon C, Mauerhofer B, Rindlisbacher C; and the EME Evaluation Program Collaborative. Multinational evaluation of the BioFire® FilmArray® Pneumonia plus Panel as compared to standard of care testing. Eur J Clin Microbiol Infect Dis. 2021;40(8):1609–22. [DOI] [PMC free article] [PubMed]
- 70.Posteraro B, Cortazzo V, Liotti FM, Menchinelli G, Ippoliti C, De Angelis G, et al. Diagnosis and treatment of bacterial pneumonia in critically Ill patients with COVID-19 using a multiplex PCR assay: a large Italian hospital’s five-month experience. Microbiol Spectr. 2021;9(3): e0069521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrer J, Clari MÁ, Giménez E, Carbonell N, Torres I, Blasco ML, et al. The Biofire® Filmarray® Pneumonia Plus panel for management of lower respiratory tract infection in mechanically-ventilated patients in the COVID-19 era: a diagnostic and cost-benefit evaluation. Diagn Microbiol Infect Dis. 2023;105(2): 115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Søgaard KK, Hinic V, Goldenberger D, Gensch A, Schweitzer M, Bättig V, et al. Evaluation of the clinical relevance of the Biofire© FilmArray pneumonia panel among hospitalized patients. Infection. 2024;52(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tellapragada C, Giske CG. The Unyvero Hospital-Acquired pneumonia panel for diagnosis of secondary bacterial pneumonia in COVID-19 patients. Eur J Clin Microbiol Infect Dis. 2021 Dec;40(12):2479–85. Erratum in: Eur J Clin Microbiol Infect Dis. 2021;40(12):2487–8. [DOI] [PMC free article] [PubMed]
- 74.Moy AC, Kimmoun A, Merkling T, Berçot B, Caméléna F, Poncin T, et al. PCR Multiplex Study group (PMS group). Performance evaluation of a PCR panel (FilmArray® Pneumonia Plus) for detection of respiratory bacterial pathogens in respiratory specimens: A systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2023;42(6):101300. [DOI] [PubMed] [Google Scholar]
- 75.Enne VI, Aydin A, Baldan R, Owen DR, Richardson H, Ricciardi F, et al; INHALE WP1 Study Group. Multicentre evaluation of two multiplex PCR platforms for the rapid microbiological investigation of nosocomial pneumonia in UK ICUs: the INHALE WP1 study. Thorax. 2022;77(12):1220–8. [DOI] [PubMed]
- 76.Fratoni AJ, Roberts AL, Nicolau DP, Kuti JL. Effects of clinically achievable pulmonary antibiotic concentrations on the recovery of bacteria: in vitro comparison of the BioFire FILMARRAY Pneumonia Panel versus conventional culture methods in bronchoalveolar lavage fluid. J Clin Microbiol. 2024;62(1): e0113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poole S, Tanner AR, Naidu VV, Borca F, Phan H, Saeed K, Grocott MPW, Dushianthan A, Moyses H, Clark TW. Molecular point-of-care testing for lower respiratory tract pathogens improves safe antibiotic de-escalation in patients with pneumonia in the ICU: Results of a randomised controlled trial. J Infect. 2022;85(6):625–33. [DOI] [PubMed] [Google Scholar]
- 78.Fireizen Y, Babbitt CJ, Adams S, Tricia Morphew T, Ferro ET, I Randhawa I. The Impact of Penumonia PCR Panel Testing in the PICU: A Quality Improvement. J Pediatr Intensive Care 2022. Available in: 10.1055/s-0042-1743178 Last accessed September 7, 2024. [DOI] [PMC free article] [PubMed]
- 79.Darie AM, Khanna N, Jahn K, Osthoff M, Bassetti S, Osthoff M, et al. Fast multiplex bacterial PCR of bronchoalveolar lavage for antibiotic stewardship in hospitalised patients with pneumonia at risk of Gram-negative bacterial infection (Flagship II): a multicentre, randomised controlled trial. Lancet Respir Med. 2022;10(9):877–87. [DOI] [PubMed] [Google Scholar]
- 80.Markussen DL, Serigstad S, Ritz C, Knoop ST, Ebbesen MH, Faurholt-Jepsen D, et al. Diagnostic stewardship in community-acquired pneumonia with syndromic molecular testing: a randomized clinical trial. JAMA Netw Open. 2024;7(3): e240830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Virk A, Strasburg AP, Kies KD, Donadio AD, Mandrekar J, Harmsen WS, et al. Rapid multiplex PCR panel for pneumonia in hospitalised patients with suspected pneumonia in the USA: a single-centre, open-label, pragmatic, randomised controlled trial. Lancet Microbe. 2024;16: 100928. 10.1016/S2666-5247(24)00170-8. [DOI] [PubMed] [Google Scholar]
- 82.Rodríguez A, Moreno G, Bodi M, Martín-Loeches I. Antibiotics in development for multiresistant gram-negative bacilli. Med Intensiva (Engl Ed). 2022;46(11):630–40. [DOI] [PubMed] [Google Scholar]
- 83.Jitmuang A, Puttinad S, Hemvimol S, Pansasiri S, Horthongkham N. A multiplex pneumonia panel for diagnosis of hospital-acquired and ventilator-associated pneumonia in the era of emerging antimicrobial resistance. Front Cell Infect Microbiol. 2022;12: 977320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verroken A, Favresse J, Anantharajah A, Rodriguez-Villalobos H, Wittebole X, Laterre PF. Optimized antibiotic management of critically Ill patients with severe pneumonia following multiplex polymerase chain reaction testing: a prospective clinical exploratory trial. Antibiotics (Basel). 2024;13(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong J, Yang J, Liu L, Chen X, Yang G, He Y, et al. Evaluation and clinical practice of pathogens and antimicrobial resistance genes of BioFire FilmArray Pneumonia panel in lower respiratory tract infections. Infection. 2024;52(2):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cartuliares MB, Rosenvinge FS, Mogensen CB, Skovsted TA, Andersen SL, Østergaard C, et al. Evaluation of point-of-care multiplex polymerase chain reaction in guiding antibiotic treatment of patients acutely admitted with suspected community-acquired pneumonia in Denmark: a multicenter randomised controlled trial. PLoS Med. 2023;20(11): e1004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur Respir J. 2023;61(4):2200735. [DOI] [PubMed] [Google Scholar]
- 88.Hoover J, Mintz MA, Deiter F, Aminian E, Chen J, Hays SR, et al. Rapid molecular detection of airway pathogens in lung transplant recipients. Transpl Infect Dis. 2021;23(4): e13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osborn LJ, Fissel J, Gomez S, Mestas J, Flores-Vazquez J, Lee J, et al. Development of an automated amplicon-based next-generation sequencing pipeline for rapid detection of bacteria and fungi directly from clinical specimens. J Clin Microbiol. 2024;62(5): e0174923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart SF, Pandolfo AM, Moon Z, Jani Y, Brett SJ, Brealey D, et al. UK clinicians’ attitudes towards the application of molecular diagnostics to guide antibiotic use in ICU patients with pneumonias: a quantitative study. J Antimicrob Chemother. 2024;79(1):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie P, Wang W, Dong M. Long-term mortality predictors of ICU fungaemia. Epidemiol Infect. 2021;149: e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munro C, Zilberberg MD, Shorr AF. Bloodstream infection in the intensive care unit: evolving epidemiology and microbiology. Antibiotics (Basel). 2024;13(2):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. [DOI] [PubMed] [Google Scholar]
- 94.She RC, Bender JM. Advances in rapid molecular blood culture diagnostics: healthcare impact, laboratory implications, and multiplex technologies. J Appl Lab Med. 2019;3(4):617–30. [DOI] [PubMed] [Google Scholar]
- 95.Kollef MH, Shorr AF, Bassetti M, Timsit JF, Micek ST, Michelson AP, et al. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18(6):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vazquez-Guillamet C, Scolari M, Zilberberg MD, Shorr AF, Micek ST, Kollef M. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of outcome in severe sepsis and septic shock. Crit Care Med. 2014;42(11):2342–9. [DOI] [PubMed] [Google Scholar]
- 99.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wagner JL, Markovich KC, Barber KE, Stover KR, Biehle LR. Optimizing rapid diagnostics and diagnostic stewardship in Gram-negative bacteremia. Pharmacotherapy. 2021;41(8):676–85. [DOI] [PubMed] [Google Scholar]
- 101.Caméléna F, Péan de Ponfilly G, Pailhoriès H, Bonzon L, Alanio A, Poncin T, et al. Multicenter evaluation of the FilmArray blood culture identification 2 panel for pathogen detection in bloodstream infections. Microbiol Spectr. 2023;11(1):e0254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López-Pintor JM, Sánchez-López J, Navarro-San Francisco C, Sánchez-Díaz AM, Loza E, Cantón R. Real life clinical impact of antimicrobial stewardship actions on the blood culture workflow from a microbiology laboratory. Antibiotics (Basel). 2021;10(12):1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCrink KA, DeRonde KJ, Jimenez A, Rosello G, Natori Y, Claeys KC, et al. Impact of a real-time diagnostic and antimicrobial stewardship workflow on time to appropriate therapy for infections caused by multidrug-resistant Gram-negative organisms. Int J Antimicrob Agents. 2023;61(6): 106811. [DOI] [PubMed] [Google Scholar]
- 104.Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123(5):1615–24. [DOI] [PubMed] [Google Scholar]
- 105.Valles J, Fontanals D, Oliva JC, Martínez M, Navas A, Mesquida J, et al. Trends in the incidence and mortality of patients with community-acquired septic shock 2003–2016. J Crit Care. 2019; 5346–52. [DOI] [PubMed]
- 106.Zasowski EJ, Bassetti M, Blasi F, Goossens H, Rello J, Sotgiu G, et al. A systematic review of the effect of delayed appropriate antibiotic treatment on the outcomes of patients with severe bacterial infections. Chest. 2020;158(3):929–38. [DOI] [PubMed] [Google Scholar]
- 107.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31(12):2742–51. [DOI] [PubMed] [Google Scholar]
- 108.Rüddel H, Thomas-Rüddel DO, Reinhart K, Bach F, Gerlach H, Lindner M, MEDUSA study group, et al. MEDUSA study group. Adverse effects of delayed antimicrobial treatment and surgical source control in adults with sepsis: results of a planned secondary analysis of a cluster-randomized controlled trial. Crit Care. 2022;26(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT, EUROBACT-2 Study Group, et al. EUROBACT-2 Study Group, ESICM, ESCMID ESGCIP and the OUTCOMEREA network. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49(2):178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borges M, Candel F, Ferrer R, Vidal P, Zaragoza R. Documento de Consenso: Código Sepsis [internet]. Madrid, España: IMC; 2015. [Internet]. 2015. Available in: https://www.seguridaddelpaciente.es/resources/documentos/2016/SEPSIS-DOCUMENTO-DE-CONSENSO.pdf Last accessed September 16, 2024.
- 111.Barie PS, Hydo LJ, Shou J, Larone DH, Eachempati SR. Influence of antibiotic therapy on mortality of critical surgical illness caused or complicated by infection. Surg Infect (Larchmt). 2005 Spring;6(1):41–54. [DOI] [PubMed]
- 112.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–55. [DOI] [PubMed] [Google Scholar]
- 113.Hranjec T, Rosenberger LH, Swenson B, Metzger R, Flohr TR, Politano AD, et al. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis. 2012;12(10):774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Heuverswyn J, Valik JK, Desirée van der Werff S, Hedberg P, Giske C, Nauclér P. Association between time to appropriate antimicrobial treatment and 30-day mortality in patients with bloodstream infections: a retrospective cohort study. Clin Infect Dis. 2023;76(3):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morace G, Borghi E. Fungal infections in ICU patients: epidemiology and the role of diagnostics. Minerva Anestesiol. 2010;76(11):950–6. [PubMed] [Google Scholar]
- 116.Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis. 2024;S1473–3099(23):00692–8. [DOI] [PubMed] [Google Scholar]
- 117.Burki T. WHO publish fungal priority pathogens list. Lancet Microbe. 2023;4(2): e74. 10.1016/S2666-5247(23)00003-4. [DOI] [PubMed] [Google Scholar]
- 118.Fisher MC, Denning DW. The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol. 2023;21(4):211–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Posteraro B, Tumbarello M, De Pascale G, Liberto E, Vallecoccia MS, De Carolis E, et al. (1,3)-β-d-Glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: an observational study. J Antimicrob Chemother. 2016;71(8):2262–9. [DOI] [PubMed] [Google Scholar]
- 120.León C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, EPCAN Study Group. A bedside scoring system ("Candida score") for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–7. [DOI] [PubMed]
- 121.Zaragoza R, Pemán J. Invasive fungal infections in critically ill patients: different therapeutic options and a uniform strategy. Rev Iberoam Micol. 2006;23(2):59–63. [DOI] [PubMed] [Google Scholar]
- 122.Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, et al; CANDIPOP Project; GEIH-GEMICOMED (SEIMC); REIPI. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20(4):O245–54. [DOI] [PubMed]
- 123.Zaragoza R, Maseda E, Pemán J. Tratamiento antifúngico individualizado en el paciente crítico con infección fúngica invasora [Individualized antifungal therapy in critically ill patients with invasive fungal infection]. Rev Iberoam Micol. 2021;38(2):68–74. [DOI] [PubMed] [Google Scholar]
- 124.Bach K, Edel B, Höring S, Bartoničkova L, Glöckner S, Löffler B, et al. Performance of the eazyplex® BloodScreen GN as a simple and rapid molecular test for identification of Gram-negative bacteria from positive blood cultures. Eur J Clin Microbiol Infect Dis. 2022;41(3):489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rödel J, Bohnert JA, Stoll S, Wassill L, Edel B, Karrasch M, et al. Evaluation of loop-mediated isothermal amplification for the rapid identification of bacteria and resistance determinants in positive blood cultures. Eur J Clin Microbiol Infect Dis. 2017;36(6):1033–40. [DOI] [PubMed] [Google Scholar]
- 126.Sękowska A, Bogiel T. The evaluation of Eazyplex® SuperBug CRE assay usefulness for the detection of ESBLs and carbapenemases genes directly from urine samples and positive blood cultures. Antibiotics (Basel). 2022;11(2):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bryant S, Almahmoud I, Pierre I, Bardet J, Touati S, Maubon D, et al. Evaluation of microbiological performance and the potential clinical impact of the ePlex® blood culture identification panels for the rapid diagnosis of bacteremia and fungemia. Front Cell Infect Microbiol. 2020;10: 594951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang TD, Melnik E, Bogaerts P, Evrard S, Glupczynski Y. Evaluation of the ePlex blood culture identification panels for detection of pathogens in bloodstream infections. J Clin Microbiol. 2019;57(2):e01597-e1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolk DM, Young S, Whitfield NN, Reid JL, Thornberg A, Carroll KC, et al. A multicenter clinical study to demonstrate the diagnostic accuracy of the GenMark Dx ePlex blood culture identification gram-negative panel. J Clin Microbiol. 2021;59(9): e0248420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carroll KC, Reid JL, Thornberg A, Whitfield NN, Trainor D, Lewis S, et al. Clinical performance of the novel GenMark Dx ePlex blood culture ID gram-positive panel. J Clin Microbiol. 2020;58(4):e01730-e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peri AM, Ling W, Furuya-Kanamori L, Harris PNA, Paterson DL. Performance of BioFire Blood Culture Identification 2 Panel (BCID2) for the detection of bloodstream pathogens and their associated resistance markers: a systematic review and meta-analysis of diagnostic test accuracy studies. BMC Infect Dis. 2022;22(1):794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang M, Tao C. Diagnostic efficiency of the FilmArray blood culture identification (BCID) panel: a systematic review and meta-analysis. J Med Microbiol. 2023. 10.1099/jmm.0.001608. [DOI] [PubMed] [Google Scholar]
- 133.Dunbar SA, Gardner C, Das S. Diagnosis and management of bloodstream infections with rapid, multiplexed molecular assays. Front Cell Infect Microbiol. 2022;12: 859935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Merino E, Gimeno A, Alcalde M, Coy J, Boix V, Molina-Pardines C, et al. Impact of sepsis flow chip, a novelty fast microbiology method, in the treatment of bacteremia caused by Gram-negative bacilli. Rev Esp Quimioter. 2021;34(3):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vrettou CS, Douka E, Perivolioti EP, Vassiliou AG, Sarri A, Giannopoulou V, et al. Accuracy of T2 magnetic resonance assays as point-of-care methods in the intensive care unit. J Hosp Infect. 2023;139:240–8. [DOI] [PubMed] [Google Scholar]
- 136.Clancy CJ, Nguyen MH. T2 magnetic resonance for the diagnosis of bloodstream infections: charting a path forward. J Antimicrob Chemother. 2018. 10.1093/jac/dky050. [DOI] [PubMed] [Google Scholar]
- 137.O’Donnell M, Shields RK, Marini RV, Groetzinger LM, Potoski BA, Falcione BA, et al. Stewardship-guided T2Candida testing shortens time to antifungal treatment and reduces antifungal usage among medical intensive care unit patients with septic shock. Open Forum Infect Dis. 2023;10(11):ofad538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fuchs S, Lass-Flörl C, Posch W. Diagnostic performance of a novel multiplex PCR assay for candidemia among ICU patients. J Fungi (Basel). 2019;5(3):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stone NR, Gorton RL, Barker K, Ramnarain P, Kibbler CC. Evaluation of PNA- FISH yeast traffic light for rapid identification of yeast directly from positive blood cultures and assessment of clinical impact. J Clin Microbiol. 2013;51(4):1301–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zboromyrska Y, Cillóniz C, Cobos-Trigueros N, Almela M, Hurtado JC, Vergara A, et al. Evaluation of the magicplex™ Sepsis real-time test for the rapid diagnosis of bloodstream infections in adults. Front Cell Infect Microbiol. 2019;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ziegler I, Fagerström A, Strålin K, Mölling P. Evaluation of a commercial multiplex PCR assay for detection of pathogen DNA in blood from patients with suspected sepsis. PLoS ONE. 2016;11(12): e0167883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marbjerg LH, Holzknecht BJ, Dargis R, Dessau RB, Nielsen XC, Christensen JJ. Commercial bacterial and fungal broad-range PCR (Micro-Dx™) used on culture-negative specimens from normally sterile sites: diagnostic value and implications for antimicrobial treatment. Diagn Microbiol Infect Dis. 2020;97(2): 115028. [DOI] [PubMed] [Google Scholar]
- 143.Knabl L, Mutschlechner W, Orth-Höller D. Evaluation of a multiplex OnSpot primer-extension PCR assay in the diagnosis of sepsis. J Microbiol Methods. 2016;120:91–3. [DOI] [PubMed] [Google Scholar]
- 144.Peri AM, O’Callaghan K, Rafiei N, Graves B, Sinclair H, Brischetto A, et al. Persistence of detectable pathogens by culture-independent systems (T2 Magnetic Resonance) in patients with bloodstream infection: prognostic role and possible clinical implications. Clin Infect Dis. 2024;78(2):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nguyen MH, Clancy CJ, Pasculle AW, Pappas PG, Alangaden G, Pankey GA, et al. Performance of the T2Bacteria panel for diagnosing bloodstream infections: a diagnostic accuracy study. Ann Intern Med. 2019;170(12):845–52. [DOI] [PubMed] [Google Scholar]
- 146.Kalligeros M, Zacharioudakis IM, Tansarli GS, Tori K, Shehadeh F, Mylonakis E. In-depth analysis of T2Bacteria positive results in patients with concurrent negative blood culture: a case series. BMC Infect Dis. 2020;20(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, et al. The microbiology of bloodstream infection: 20-Year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7):e00355-e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muñoz P, Vena A, Machado M, Gioia F, Martínez-Jiménez MC, Gómez E, et al; T2MadRid study group. T2Candida MR as a predictor of outcome in patients with suspected invasive candidiasis starting empirical antifungal treatment: a prospective pilot study. J Antimicrob Chemother. 2018;73(suppl_4):iv6-iv12. [DOI] [PubMed]
- 149.Rouzé A, Estella Á, Timsit JF. Is (1,3)-β-D-glucan useless to guide antifungal therapy in ICU? Intensive Care Med. 2022;48(7):930–2. [DOI] [PubMed] [Google Scholar]
- 150.Peri AM, Harris PNA, Paterson DL. Culture-independent detection systems for bloodstream infection. Clin Microbiol Infect. 2022;28(2):195–201. [DOI] [PubMed] [Google Scholar]
- 151.Walker SV, Steffens B, Sander D, Wetsch WA. Implementation of antibiotic stewardship improves the quality of blood culture diagnostics at an intensive care unit of a university hospital. J Clin Med. 2022;11(13):3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fabre V, Klein E, Salinas AB, Jones G, Carroll KC, Milstone AM, et al. A diagnostic stewardship intervention to improve blood culture use among adult nonneutropenic inpatients: the distribute study. J Clin Microbiol. 2020;58(10):e01053-e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verroken A, Despas N, Rodriguez-Villalobos H, Laterre PF. The impact of a rapid molecular identification test on positive blood cultures from critically ill with bacteremia: a pre-post intervention study. PLoS ONE. 2019;14(9): e0223122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mo Y. Rapid diagnostics for antibiotic resistance: urgent need for strong clinical evidence. Clin Infect Dis. 2022;75(12):2076–8. [DOI] [PubMed] [Google Scholar]
- 155.D’Onofrio V, Salimans L, Bedenić B, Cartuyvels R, Barišić I, Gyssens IC. The clinical impact of rapid molecular microbiological diagnostics for pathogen and resistance gene identification in patients with sepsis: a systematic review. Open Forum Infect Dis. 2020;7(10):ofaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goodman KE, Baghdadi JD, Magder LS, Heil EL, Sutherland M, Dillon R, et al. Patterns, predictors, and intercenter variability in empiric gram-negative antibiotic use across 928 United States hospitals. Clin Infect Dis. 2023;76(3):e1224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Horner C, Mushtaq S, Allen M, Longshaw C, Reynolds R, Livermore DM. Are resistance rates among bloodstream isolates a good proxy for other infections? Analysis from the BSAC Resistance Surveillance Programme. J Antimicrob Chemother. 2021;76(7):1822–31. [DOI] [PubMed] [Google Scholar]
- 158.El-Sokkary R, Uysal S, Erdem H, Kullar R, Pekok AU, Amer F, et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: an international ID-IRI survey. Eur J Clin Microbiol Infect Dis. 2021;40(11):2323–34. [DOI] [PubMed] [Google Scholar]
- 159.Leitl CJ, Stoll SE, Wetsch WA, Kammerer T, Mathes A, Böttiger BW, et al. Next-Generation sequencing in critically Ill COVID-19 patients with suspected bloodstream infections: a retrospective cohort study. J Clin Med. 2023;12(4):1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhavani SV, Lonjers Z, Carey KA, Afshar M, Gilbert ER, Shah NS, et al. The development and validation of a machine learning model to predict bacteremia and fungemia in hospitalized patients using electronic health record data. Crit Care Med. 2020;48(11):e1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murri R, De Angelis G, Antenucci L, Fiori B, Rinaldi R, Fantoni M, Damiani A, Patarnello S, Sanguinetti M, Valentini V, Posteraro B, Masciocchi C. A machine learning predictive model of bloodstream infection in hospitalized patients. Diagnostics (Basel). 2024;14(4):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonneville R, de Montmollin E, Contou D, Ferrer R, Gurjar M, Klouche K, et al; EURECA Investigator Study Group. Clinical features, etiologies, and outcomes in adult patients with meningoencephalitis requiring intensive care (EURECA): an international prospective multicenter cohort study. Intensive Care Med. 2023;49(5):517–29. [DOI] [PubMed]
- 163.Klein M, Abdel-Hadi C, Bühler R, Grabein B, Linn J, Nau R, et al. German guidelines on community-acquired acute bacterial meningitis in adults. Neurol Res Pract. 2023;5(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, et al. Multicenter evaluation of biofire filmarray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54(9):2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sundelin T, Bialas J, de Diego J, Hermanowski M, Leibhan H, Ponderand L, et al. Evaluation of the QIAstat-Dx meningitis/encephalitis panel, a multiplex PCR platform for the detection of community-acquired meningoencephalitis. J Clin Microbiol. 2023;61(10): e0042623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.BIOFIRE® FIREWORKS™ Summary of product characteristics. Available in: https://www.biofiredx.com/products/biofire-fireworks/ Last accessed September 16, 2024.
- 167.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–84. [DOI] [PubMed] [Google Scholar]
- 168.Liesman RM, Strasburg AP, Heitman AK, Theel ES, Patel R, Binnicker MJ. Evaluation of a commercial multiplex molecular panel for diagnosis of infectious meningitis and encephalitis. J Clin Microbiol. 2018;56(4):e01927-e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trujillo-Gómez J, Tsokani S, Arango-Ferreira C, Atehortúa-Muñoz S, Jimenez-Villegas MJ, Serrano-Tabares C, et al. Biofire FilmArray Meningitis/Encephalitis panel for the aetiological diagnosis of central nervous system infections: a systematic review and diagnostic test accuracy meta- analysis. EClinicalMedicine. 2022;44: 101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schnuriger A, Vimont S, Godmer A, Gozlan J, Gallah S, Macé M, et al. Differential performance of the FilmArray meningitis/encephalitis assay to detect bacterial and viral pathogens in both pediatric and adult populations. Microbiol Spectr. 2022;10(2): e0277421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pandey U, Greninger AL, Levin GR, Jerome KR, Anand VC, Dien BJ. Pathogen or bystander: clinical significance of detecting human herpesvirus 6 in pediatric cerebrospinal fluid. J Clin Microbiol. 2020;58(5):e00313-e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Green DA, Pereira M, Miko B, Radmard S, Whittier S, Thakur K. Clinical significance of human herpesvirus 6 positivity on the FilmArray meningitis/encephalitis panel. Clin Infect Dis. 2018;67(7):1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tansarli GS, Chapin KC. Diagnostic test accuracy of the BioFire® FilmArray® meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(3):281–90. [DOI] [PubMed] [Google Scholar]
- 174.Van TT, Kim TH, Butler-Wu SM. Evaluation of the Biofire FilmArray meningitis/encephalitis assay for the detection of Cryptococcus neoformans/gattii. Clin Microbiol Infect. 2020;26(10):1375–9. [DOI] [PubMed] [Google Scholar]
- 175.Humisto A, Antikainen J, Holma T, Jarva H, Toivonen A, Loginov R, et al. Evaluation of the novel CE-IVD-marked multiplex PCR QIAstat-Dx meningitis/encephalitis panel. Microbiol Spectr. 2023;11(3): e0514422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alqarni A, Kantor E, Grall N, Tanaka S, Zappella N, Godement M, Ribeiro- Parenti L, Tran-Dinh A, Montravers P. Clinical characteristics and prognosis of bacteraemia during postoperative intra-abdominal infections. Crit Care. 2018;22(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krifors A, Ullberg M, Castegren M, Petersson J, Sparrelid E, Özenci V, et al. Combining T2bacteria and T2Candida panels for diagnosing intra-abdominal infections: a prospective multicenter study. J Fungi (Basel). 2022;8(8):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Micó M, Navarro F, de Miniac D, González Y, Brell A, López C, et al. Efficacy of the FilmArray blood culture identification panel for direct molecular diagnosis of infectious diseases from samples other than blood. J Med Microbiol. 2015;64(12):1481–8. [DOI] [PubMed] [Google Scholar]
- 179.Leitner E, Hoenigl M, Wagner B, Krause R, Feierl G, Grisold AJ. Performance of the FilmArray Blood culture identification panel in positive blood culture bottles and cerebrospinal fluid for the diagnosis of sepsis and meningitis. GMS Infect Dis. 2016;4: Doc06. [DOI] [PMC free article] [PubMed]
- 180.López-Amor L, García-Prieto E, Fernández-Suárez J, Escudero D, Vázquez F, Fernández J. Evaluation of a commercial multiplex PCR for diagnosis of central nervous system (CNS) nosocomial infections. J Microbiol Methods. 2020;171: 105865. [DOI] [PubMed] [Google Scholar]
- 181.Spoto S, Daniel Markley J, Valeriani E, Abbate A, Argemi J, Markley R, et al. Active surveillance cultures and procalcitonin in combination with clinical data to guide empirical antimicrobial therapy in hospitalized medical patients with sepsis. Front Microbiol. 2022;13: 797932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kakizaki N, Asai K, Kuroda M, Watanabe R, Kujiraoka M, Sekizuka T, et al. Rapid identification of bacteria using a multiplex polymerase chain reaction system for acute abdominal infections. Front Microbiol. 2023;14:1220651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Watanabe R, Asai K, Kuroda M, Kujiraoka M, Sekizuka T, Katagiri M, et al. Quick detection of causative bacteria in cases of acute cholangitis and cholecystitis using a multichannel gene autoanalyzer. Surg Today. 2021;51(12):1938–45. [DOI] [PubMed] [Google Scholar]
- 184.Messacar K, Hamilton SL, Prinzi AM, Mitchell JC, Beil ED, Dowell EB, et al. Rapid identification of nonblood sterile site broth cultures using the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2019;93(1):22–3. [DOI] [PubMed] [Google Scholar]
- 185.Cobo F, Borrego J, Rodríguez-Granger J, Puertas A, Sampedro A, Navarro-Marí JM. Detection of bacterial pathogens in sterile fluids with the FilmArray Meningitis/Encephalitis identification system. Rev Esp Quimioter. 2019;32(1):85–6. [PMC free article] [PubMed] [Google Scholar]
- 186.Esteban J, Salar-Vidal L, Schmitt BH, Waggoner A, Laurent F, Abad L, et al. Multicenter evaluation of the BIOFIRE Joint Infection Panel for the detection of bacteria, yeast, and AMR genes in synovial fluid samples. J Clin Microbiol. 2023;61(11): e0035723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Salar-Vidal L, Chaves C, Dianzo-Delgado IT, Favier P, Giner-Almaraz S, Gómez-Gómez MJ, et al. Multicenter evaluation of BioFire JI panel related to improved microbiological diagnostics on acute osteoarticular infections. Int J Med Microbiol. 2023;313(6): 151588. [DOI] [PubMed] [Google Scholar]
- 188.Gaillard T, Dupieux-Chabert C, Roux AL, Tessier E, Boutet-Dubois A,Courboulès C, et al. A prospective multicentre evaluation of BioFire® Joint Infection Panel for the rapid microbiological documentation of acute arthritis. Clin Microbiol Infect. 2024:S1198–743X(24)00153–8. [DOI] [PubMed]
- 189.Cohen R, Babushkin F, Cohen S, Afraimov M, Shapiro M, Uda M, et al. A prospective survey of Pseudomonas aeruginosa colonization and infection in the intensive care unit. Antimicrob Resist Infect Control. 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Emmanuel Martinez A, Widmer A, Frei R, Pargger H, Tuchscherer D, Marsch S, et al. ESBL-colonization at ICU admission: impact on subsequent infection, carbapenem-consumption, and outcome. Infect Control Hosp Epidemiol. 2019;40(4):408–13. [DOI] [PubMed] [Google Scholar]
- 191.Jin X, Zhang H, Wu S, Qin X, Jia P, Tenover FC, et al. Multicenter evaluation of Xpert carba-R assay for detection and identification of the carbapenemase genes in rectal swabs and clinical isolates. J Mol Diagn. 2021;23(1):111–9. [DOI] [PubMed] [Google Scholar]
- 192.Protonotariou E, Meletis G, Papadopoulou D, Kachrimanidou M, Toptsi L, Skoura L. Evaluation of the “AMR Direct Flow Chip Kit” DNA microarray for detecting antimicrobial resistance genes directly from rectal and nasopharyngeal clinical samples upon ICU admission. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39(6):276–8. [DOI] [PubMed] [Google Scholar]
- 193.Kriger O, Gefen-Halevi S, Belausov N, Sherman G, Adam E, Rubinstein O, et al. Respiratory cryptosporidiosis detected by commercial multiplex-PCR in immunosuppressed pediatric patients. Diagn Microbiol Infect Dis. 2023;107(2): 116033. [DOI] [PubMed] [Google Scholar]
- 194.Sakakura A, Akashi Y, Shiigai M, Isono H, Suzuki H, Hirose Y. A case of severe pneumonia with viremia caused by adenovirus B7 identified by off-label use of a multiplex PCR system. IDCases. 2020;23: e01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.