Abstract
Background
Major depressive disorder (MDD) is a highly prevalent mental disorder with devastating consequences that often first manifest during adolescence. Anhedonia has emerged as one of the most promising symptoms of adolescent MDD, which means a longer time to remission, fewer depression-free days, and also increased risk of suicide ideas or actions. Research has shown that at least two-thirds of depressed adolescents have significant sleep-onset or sleep-maintenance problems. However, the association between sleep disorder and anhedonia, and the potential mediators are less understood.
Methods
This is a cross-sectional study that includes 200 adolescents suffered from MDD between the ages of 12–17. We use Spearman’s test to explore the relationship among main variables. To evaluate the mediating effects of stress, we applied regression models and used bootstrap method to validate the significance of effects.
Results
Significant correlation exists among sleep disorder, stress, and anhedonia (P<0.05).The direct effect of sleep disorder on anhedonia was 0.214 (95% CI: 1.5235, 6.2073), while the total effect was 0.295 (95% CI: 2.9683, 7.6924). The indirect effect of sleep disorder on anhedonia mediated by stress was 0.081 (95% CI: 0.5842, 2.5268). Robustness of the regression analysis results has been verified by bootstrap test.
Conclusions
Our finding suggested a positive correlation between sleep disturbance and anhedonia in adMDD. Stress partially mediated the relationship between sleep disorder and anhedonia. Due to the deleterious effects of anhedonia on depressed adolescents, these findings provide impetus to investigate further the causal relationship between sleep problems and anhedonia.
Trial registration
ChiCTR2200060176(Registration Date: 21/05/2022).
Keywords: Major depression disorder, Adolescent, Anhedonia, Sleep disorder, Stress, Cross-sectional study
Introduction
Adolescent major depressive disorder (adMDD) is a common health concern with high prevalence [1], estimated in a recent study to be 16.9% among the U.S. adolescent population [2]. Current research suggests that adolescent-onset MDD is more heritable than preadolescent-onset MDD, which is already more chronic and recurrent than adult-onset MDD, with common symptoms such as emotion dysregulation, cognitive dysfunction, and behavioral problems [3]. Moreover, patients with adMDD tend to face more severe social and educational problems like interpersonal relationships, physical and mental health, and general well-being [4]. Anhedonia is one of the two core symptoms of MDD and is generally accepted as a decrease in an individual’s ability to experience pleasure or a loss of interest in previously rewarding stimuli [5]. For adMDD patients, the severity of anhedonia is associated with greater overall disease severity, longer depression episode duration, and more frequent episodes of MDD [6]. Moreover, the impact of anhedonia in adMDD patients is particularly prominent which means a higher risk of non-suicidal self-injury behaviors and even ideas/attempts of suicide [7]. In addition, adMDD patients with anhedonia could not achieve complete remission despite sufficient doses of antidepressant treatment compared with patients without anhedonia [8]. Therefore, improving anhedonia symptom in adMDD has become a priority for better prognosis of adolescence depression.
Evidence has suggested that the association between sleep and positive affect is particularly robust [9]. What’s more, sleep-related circadian rhythms of melatonin have also been demonstrated to be closely correlated with anhedonia [10]. Sleep disorders potentially influenced fatigue and a sense of listlessness among individuals, thereby diminishing their inclination and actual participation in social activities, which means decreased social anhedonia [11]. More than 90% of MDD patients have reported several types of sleep problems, such as insomnia, difficulty in falling asleep, more times of awakenings and early morning awakenings [12]. Furthermore, many clinical trials have reported that insufficient sleep is prevalent globally among adolescents. An extensive cohort study indicates that the proportion of adolescents regularly sleeping for > 7 h per night has decreased to less than 40% in the past 20 years [13]. Previous research also shows that at least two-thirds of adMDD patients suffer from severe sleep disturbances [14]. A more recent literature review suggests that the presence of these sleep problems is associated with a more unfavorable disease course of MDD, increased symptom severity, more frequent and increased risk of relapses, poorer therapeutic response, more treatment resistance, and increased suicide risk in patients with MDD [15]. However, the relationship between sleep disorder and anhedonia in patients with adMDD is still unclear.
Stress has been found closely correlated with sleep-related disorders. Several studies have identified the moderating role of sleep in the effects of stress [16–18]. In particular, family stress, peer victimization and parental conflict have been more strongly associated with internalizing symptoms among adolescents and tended to have lower sleep quality and efficiency and shorter sleep duration comparing with those better sleepers [19]. What’s more, another study has highlighted the importance of recording the fluctuations in night sleep affecting next-day stress and its impact on daytime functioning, and also demonstrated that poor sleep quality or short sleep duration is predictive of higher next-day stress, especially the role of slow wave sleep and rapid eye movement sleep in regulating the perception of stress [20]. However, the details about the relevance in stress, sleep disturbance and anhedonia in adMDD patients still need further study.
Previous research has confirmed that the relationship between perceived stress and anhedonia during psychotherapy treatment exists. More precisely, reducing the level of perceived stress by early treatment was able to make changes in hedonic functioning during mid-late treatment. Therefore, what is especially vital for us is to evaluate new interventions for anhedonia to measure stress levels repeatedly [21]. Stress also is a well-validated predictor for the occurrence and relapse of depression [22, 23], and depression has been confirmed by numerous experiments that reward-related function always changes [24]. Increased level of inflammation caused by stress is an important mechanism bridging stress and reward dysfunction. After confronting with chronic stress or acute stressors, the responsiveness of neural and behavioral reward tend to reduce in humans [25], which also been found in animal [26]. In addition, a study which included a large sample of 8 to 14 years old has demonstrated that adolescents who experienced stressful life events or sleep problems are at an increased risk of developing depressive symptoms if they characterized by a decreased reward response [27]. Previous study has already demonstrated the worse performance during reward-related task, when participants have poorer sleep quality, longer awakening and shorter sleep duration [28].
Therefore, we hold the view that adMDD with sleep disorders are more prone to the impacts of external stress events. Even comparatively trifling stress events have the potential to instigate substantial emotional swings and stress reactions. These adolescents experience difficulties in wholeheartedly participating in a diverse range of activities, which in turn diminishes the probability of deriving pleasure from such undertakings. The protracted absence of these activities will further intensify the symptoms of anhedonia. This study is designed to explore the relationship between sleep disorder, stress, and anhedonia in adMDD. The main hypotheses of our study encompass the following three:
Hypothesis 1
Anhedonia is influenced by sleep disorder.
Hypothesis 2
Stress is influenced by sleep disorder.
Hypothesis 3
Anhedonia is influenced by stress.
Methods
Study design and participants
In this cross-sectional study, we collected data from 200 patients diagnosed with adMDD to explore the relationship between anhedonia and sleep disorder. The definition of adolescence was set from 12 to 17, similar to the article by Renee D. Goodwin [2]. These adolescents were diagnosed with MDD based on the diagnostic criteria defined in Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), and informed consent was obtained from them and their legal guardians at the beginning of the study. Exclusion criteria were set to exclude those who used antidepressants and/or sedative and hypnotic drugs within the past two weeks, had any other comorbid psychiatric disorders, a history of alcohol or drug use disorder and acute poisoning, communication disorders (language barrier, severe visual or auditory impairment), neurology diseases such as epilepsy, or had participated in other clinical trials. Our study was conducted according to the 1975 Declaration of Helsinki, and all participants provided written consent.
Sample size
We estimated the sample size by using the software of G*Power 3.1. There were totally 14 variables in this study. Assuming that the effect size of this study was 0.15, the two-tailed alpha level was 0.05, and the power value was 0.8, we calculated that the sample size for this study was at least 135. Considering 20% of invalid questionnaires, the minimum sample size for this study was determined to be 169.
Instruments
This study was an investigation study, and three scale tools were utilized as follows. The primary aim was to explore the relationship between the symptoms of anhedonia evaluated by the Snaith-Hamilton Pleasure Scale (SHAPS) scores and sleep disorders. According to the scores of the Pittsburgh Sleep Quality Index (PSQI), participants were divided into two different groups, which were sleep disorder (PSQI > 7) and no sleep disorder (PSQI ≤ 7) [29–32]. PSQI was used to evaluate subjects’ sleep quality in the past month. The final score consisted of 18 self-evaluation items divided into 7 factors, each scoring 0–3 points. The total PSQI score was the cumulative scores of each factor, with a total score range of 0–21 points. The higher the score obtained, the poorer the sleep quality a participant would have. In this study, the SHAPS was used to evaluate symptoms of anhedonia by assessing subjects’ pleasure experiences in four aspects: interest/entertainment, social interaction, sensory experience, and food/beverage [33]. The total score on this scale was 14–56 points, with higher scores indicating greater losses in pleasure.
The Stressors Scale for Middle School Students (SSMSS) was applied to assess related stressors of adolescents. Stressor was measured by the 39-item SSMSS (Chinese version). We used this scale to reflect the stressful life events that high school students often encounter in their daily life. It consists of seven subscales: Learning stress, Teacher stress, Family environment stress, Parenting style stress, Classmates and friends stress, Social and culture stress, Physical and psychological stress. Items were measured on a 5point Likert scale (0 refers to “none”,1 refers to “slight”, 2 refers to “moderate”, 3 refers to “severe”, 4 refers to “extremely”). The reliability and validity of this scale has been validated [34].
Several covariates were also included in this study, such as age, gender, height, weight, place of residence, one child or not, grade of education, academic performance, living status in the past year and parental education level.
Procedures
Researchers screened 640 adolescent patients who came to the outpatient department of the Department of Psychiatry in Xijing Hospital from May 2022 to February 2023. After being screened and evaluated by researchers based on the inclusion and exclusion criteria, qualified adMDD patients were included in the trial. All participants firstly signed informed consent forms by themselves and their legal guardians. Researchers involved in this study has been strictly trained. Participants were asked to independently complete three paper-version of self-assessment scales in a quiet location without discussion or collaboration. It took approximately l5 to 20 min to complete all three self-assessment scales. To protect patients’ privacy, participants completed these questionnaires anonymously and their responses would be kept strictly confidential by researchers. Researchers carefully examined each one for completeness after collecting the questionnaires.
Ethical approval
All procedures followed the principles of the Declaration of Helsinki and were approved by the Ethics Committee of Xijing Hospital of the Fourth Military Medical University (KY20222058-F-1). We completed registration at the Chinese Clinical Trial Registration Center before we included the first outpatients (ChiCTR2200060176; Registration Date: 21/05/2022).
Statistical analysis
Data analysis was performed using IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, USA) and its macro, PROCESS. As for the analysis of baseline data, participants were classified into two groups: “with sleep disorder” and “without sleep disorder,” which was based on whether the PSQI score was higher than 7. Categorical variables were presented in terms of frequencies and percentages, while continuous variables were described by means and standard deviation (SD). Kolmogorov-Smirnov tests were performed for data normality; two-sample t-tests were used for continuous variables that conform to the normal distribution, χ2 test or Fisher’s exact test was used by categorical variables, and P value was calculated. Spearman correlation analysis was applied to evaluate correlations among sleep disorder, anhedonia and stress. What’s more, to analyze the relationship between sleep disorder, anhedonia and stress, we took the recommendations of Judd and Kenny into consideration, and we applied Baron and Kenny’s causal step approach in current study. Thus, we establish three regression models to estimate the ensuing effects. Firstly, we confirm the effect of sleep disorder on anhedonia. Next, we test the effect of sleep disorder on stress. Then, we verify the effect of stress on anhedonia when sleep disorder is controlled. Lastly, we validate the effect of sleep disorder on anhedonia when stress is controlled. To mitigate the potential effect of confounders and improve the accuracy of model estimation, we included covariates in all regression analyses. In addition, we verified these effects by bootstrapping with 5000 samples and we also show the 95% confidence interval. Statistical significance level was set at P < 0.05 (two-tailed).
Results
Descriptive statistics
From May 2022 to February 2023, 640 adolescent patients were screened. Of these, 405 failed to meet the inclusion criteria, 12 declined to take part, 5 opted to discontinue filling out the scale midway, and 18 had severe logical flaws in their scale data. Ultimately, 200 adolescent patients with MDD who met the inclusion and exclusion criteria were included in this study and asked to complete the questionnaires. For continuous variables that follow a normal distribution, such as age, height and weight, a two-sample t-test was performed. In contrast, a χ2 or Fisher’s exact test was conducted for categorical variables. The results showed that (a) Among adMDD patients, the prevalence of sleep disorder is 85.5% (171/200); (b) Baseline data of the two groups of patients, including age, gender, weight, place of residence, whether they were the only child, academic level, and parental education level, were balanced (all P > 0.05). As illustrated in Table 1.
Table 1.
Characteristics of the study population
| Variables | Total (n = 200) |
No sleep disorder (n = 29) |
Sleep disorder (n = 171) |
P |
|---|---|---|---|---|
| Age (y), Mean ± SD | 14.9 ± 1.5 | 14.8 ± 1.5 | 14.9 ± 1.5 | 0.693 |
| Gender, n (%) | 0.541 | |||
| Male | 65 (32.5) | 8 (27.6) | 57 (33.3) | |
| Female | 135 (67.5) | 21 (72.4) | 114 (66.7) | |
| Height (cm), Mean ± SD | 166.4 ± 7.9 | 163.4 ± 6.9 | 166.9 ± 7.9 | 0.028 |
| Weight (kg), Mean ± SD | 56.4 ± 12.0 | 54.6 ± 12.8 | 56.7 ± 11.9 | 0.386 |
| Household registration, n (%) | 0.740 | |||
| Urban | 161 (80.5) | 24 (82.8) | 137 (80.1) | |
| Rural | 39 (19.5) | 5 (17.2) | 34 (19.9) | |
| One child, n (%) | 0.550 | |||
| No | 121 (60.5) | 19 (65.5) | 102 (59.6) | |
| Yes | 79 (39.5) | 10 (34.5) | 69 (40.4) | |
| Grade, n (%) | 0.789 | |||
| Middle school | 97 (48.5) | 13 (44.8) | 84 (49.1) | |
| High school | 98 (49.0) | 16 (55.2) | 82 (48.0) | |
| Professional school | 5 (2.5) | 0 (0.0) | 5 (2.9) | |
| Academic performance, n (%) | 0.174 | |||
| Bad | 49 (24.5) | 4 (13.8) | 45 (26.3) | |
| Moderate | 126 (63.0) | 23 (79.3) | 103 (60.2) | |
| Good | 25 (12.5) | 2 (6.9) | 23 (13.5) | |
| Living arrangement, n (%) | ||||
|
All members (including grandparents) |
23(11.5) | 2(6.9) | 21(12.3) | 0.000 |
| With parents | 149(74.5) | 25(86.2) | 124(72.5) | |
| Single parent | 14(7.0) | 2(6.9) | 12(7.0) | |
| Others | 14(7.0) | 0(0.0) | 14(8.2) | |
| Father’s degree, n (%) | 0.678 | |||
| Primary or middle school | 93 (46.5) | 15 (51.7) | 78 (45.6) | |
| High school | 60 (30.0) | 9 (31.0) | 51 (29.8) | |
| University or above | 47 (23.5) | 5 (17.2) | 42 (24.6) | |
| Mother’s degree, n (%) | 0.464 | |||
| Primary or middle school | 97 (48.5) | 17 (58.6) | 80 (46.8) | |
| High school | 63 (31.5) | 8 (27.6) | 55 (32.2) | |
| University or above | 40 (20.0) | 4 (13.8) | 36 (21.1) | |
| SHAPS, Mean ± SD | 35.6 ± 6.4 | 31.1 ± 5.8 | 36.3 ± 6.2 | 0.000 |
| SSMSS, Mean ± SD | 51.7 ± 23.2 | 36.1 ± 15.4 | 54.3 ± 23.2 | 0.000 |
Note: Data were expressed as n/N (%) or mean ± SD
Correlations of sleep disorder, stress and anhedonia
As shown in Table 2, the results of Spearman correlation analysis showed that correlations among anhedonia, stress, and sleep disorder were notable, respectively (P < 0.01). Specifically, sleep disorder was positively associated with anhedonia (r = 0.282, P < 0.01) and stress (r = 0.289, P < 0.01). Sleep disorder was positively associated with stress (r = 0.289, P < 0.01).
Table 2.
Bivariate correlation matrix for anhedonia, stress and sleep disorder
| Variables | Anhedonia | Stress | Sleep disorder |
|---|---|---|---|
| Anhedonia | 1 | ||
| Stress | 0.415** | 1 | |
| Sleep disorder | 0.282** | 0.289** | 1 |
Note: **P < 0.01
Mediating effect analysis
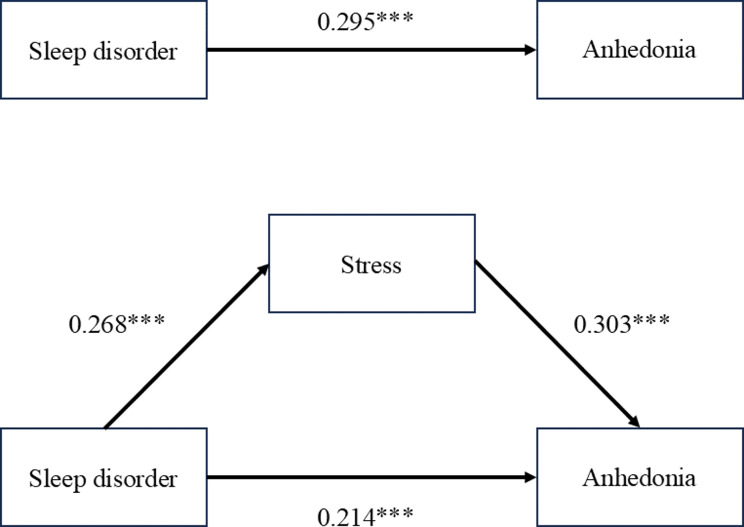
We applied regression analysis to explore the mediating effect of stress. For every regression model, we firstly calculate the variance inflation factor (VIF), and the corresponding VIF of all the variables are < 3. Therefore, excessive multicollinearity problem didn’t exist. The results of the mediation analysis of stress showed in Table 3; Fig. 1, which has controlled related covariates. Sleep disorder was a significant predictor of anhedonia (β = 0.295, P < 0.01) in the total effects regression. This relationship remained significant when stress was considered (β = 0.214, P < 0.01). Moreover, sleep disorder showed a significant effect on stress (β = 0.268, P < 0.01), whereas stress exerted a significant effect on anhedonia (β = 0.303, P < 0.01).
Table 3.
The mediating role of stress between anhedonia and sleep disorder
| Variables | Anhedonia | Stress | Anhedonia |
|---|---|---|---|
| Sleep disorder |
0.295*** (2.968,7.692) |
0.268*** (8.766,26,429) |
0.214*** (1.524,6.207) |
| Age |
0.113 (-0.444,1.399) |
0.015 (-3.214,3.677) |
0.108 (-0.420,1.336) |
| Height |
0.036 (-0.139,0.197) |
0.189 (-0.072,1.185) |
-0.022 (-0.179,0.144) |
| Weight |
-0.148 (-0.166,0.010) |
-0.128 (-0.577,0.084) |
-0.109 (-0.142,0.027) |
| Gender |
0.105 (-0.919,3.766) |
0.093 (-4.147,13.370) |
0.077 (-1.199,3.278) |
| Household registration |
0.096 (-0.769,3.855) |
-0.006 (-9.010,8.282) |
0.098 (-0.630,3.777) |
| One child or not |
0.034 (-1.351,2.229) |
-0.017 (-7.500,5.886) |
0.039 (-1.200,2.212) |
| Grade |
-0.190 (-4.618,0.186) |
-0.149 (-15.283,2.680) |
-0.145 (-3.992,0.609) |
| Academic performance |
-0.134* (-2.814, -0.035) |
-0.230*** (-14.104, -3.715) |
-0.064 (-2.046,0.681) |
| Living arrangement |
0.099 (-0.305, 2.168) |
0.069 (-6.992,2.254) |
0.120 (-0.053,2.310) |
| Father’s degree |
-0.029 (-1.872, 1.412) |
-0.022 (-6.780,5.499) |
-0.022 (-1.742,1.388) |
| Mother’s degree |
-0.181 (-3.185,0.217) |
-0.129 (-10.199,2.522) |
-0.142 (-2.791,0.0463) |
| Stress |
0.303*** (0.046,0.120) |
||
| Constant |
24.75 (-3.757, 53.208) |
-31.110 (-137.608,75.389) |
27.315* (0.150,54.480) |
| Samples | 200 | 200 | 200 |
| R-squared | 0.224 | 0.127 | 0.250 |
Note 95% confidence intervals in brackets; *P < 0.05, **P < 0.01, ***P < 0.005
Fig. 1.
Relationship between sleep disorder, stress and anhedonia
To validate the effect of different paths, we applied the method of bootstrap tests. As shown in Table 4, the results indicated that the direct effect of sleep disorder on anhedonia was 0.214 (95% CI: 1.5235, 6.2073), while the total effect was 0.295 (95% CI: 2.9683, 7.6924). The indirect effect of sleep disorder on anhedonia mediated by stress was 0.081 (95% CI: 0.5842, 2.5268). The effect of all paths was prominent, which suggested robustness of the mediation analysis.
Table 4.
Bootstrap tests for mediation models
| Paths | Observed Coefficient | Bootstrap Standard Error | P | LLCI | ULCI |
|---|---|---|---|---|---|
| Indirect effect | 0.081 | 0.4953 | 0.5842 | 2.5268 | |
| Direct effect | 0.214 | 1.1871 | 0.0013 | 1.5235 | 6.2073 |
| Total effect | 0.295 | 1.1973 | 0.000 | 2.9683 | 7.6924 |
Note: LLCI, lower level for confidence interval; ULCI, upper level for confidence interval
Discussion
The current study demonstrated a positive correlation between sleep disorder and anhedonia in adMDD patients. Stress partially mediated the relationship between sleep disorder and anhedonia. Robustness of our results has been validated by the Bootstrap methods.
Sleep disorders are among the most prevalent symptoms in patients with MDD, with 92% reporting sleep complaints [12]. In the current study, we used PSQI, a well-validated self-report questionnaire that has been widely used in many sleep-related studies [29, 35]. The adMDD patients enrolled in this study were categorized into “no sleep disorder” (PSQI ≤ 7) and “sleep disorder” (PSQI > 7) groups, based on previous studies [29]. We found that 85.5% (171/200) of adMDD patients had sleep disorder. Although there is a significant difference in the number of individuals between the two groups, this proportion is consistent with what has been reported in previous literature. Moreover, in another large-scale national community sample study that defined sleep disorders based on the DSM-IV diagnostic criteria, the prevalence of sleep disorders in patients with MDD was found to be 83.5% [36], further validating that this study effectively distinguished sleep disorders using PSQI.
The present study has confirmed that sleep disorder has a positive predictive effect on anhedonia in adMDD, which supports hypothesis 1. There are several prospective mechanisms that form the basis of this relationship. Accumulating evidence has suggested that the association between sleep and positive affect is particularly robust [9]. For instance, recently published research including 79 good sleeping adults eventually found that trait positive affect could protect persons from inflammatory activation following sleep loss, with the potential to mitigate the adverse health consequences of sleep disorder [37]. What’s more, only 1.5–2 h shorter-than-usual sleep duration for 3 consecutive nights in their own home was enough to make subjects become more impulsive and experienced reduced positive affect in the morning [38, 39]. Thus, sleep disorder not only affects generalized levels of positive affect, but also changes emotion responsivity to pleasurable or rewarding experiences. Noticeably, reward system dysfunction tends to be implicated in the clinical experience of anhedonia or low positive affect used in many trials [40]. A number of studies has demonstrated the link between sleep disorder and reward processing. Researchers found that protracted short sleep obviously influenced food reward process in adolescents, which greatly increased the appeal and reinforcing value of a variety of foods and therefore increased the risk of obesity [41, 42]. Adolescences showed worse performance in monetary reward task when they had sleep problems such as insomnia. At the same time, the result of functional magnetic resonance imaging scan also indicated some changes in prefrontal cortex which means a disrupting response to reward anticipation [39]. Later sleep onset time and lower sleep quality in healthy adolescence also indicated a lower score during reward anticipation and outcome and less activation in the caudate in a guessing task with monetary rewards [43]. Therefore, accumulating research has confirmed that neural activity in response to reward processing has robust association with sleep, which also indicated the link between sleep disorder and anhedonia.
Sleep disorder is associated with an increased stress level in adMDD, confirming hypothesis 2. Firstly, adMDD with sleep disorder maybe more sensitivity to stressors. Previous study has demonstrated that worse sleep quality was relevant to higher post-recovery negative emotion, whereas worse sleep efficiency was associated with lower post-recovery positive emotion. The results suggest that poor sleep is connected with delayed affective recovery from a stressful event [44]. In addition, research on animals has demonstrated that increased non-rapid eye movement sleep is a positive response to social-defeat stress that plays a vital role in promoting resilience [45]. What’s more, cortisol rhythm also is an neglectable mechanism linked with both daily variations in sleep behaviors and ongoing sleep disorder [46]. Last but not least, researchers also confirmed that mouse exposed to sleep deprivation experienced the deterioration of mitochondria, suggesting that oxidative stress can be provoked by sleep deprivation, which maybe another proof of the relationship between sleep disorder and stress [47].
Stress is highly relevant to anhedonia in adMDD, supporting hypothesis 3. Considerable progress has been made to clarify the plausible mechanisms bridging the occurrence of stress and the onset of anhedonia. In the first, stress can modify mesolimbic reward-related processing, especially dopamine system, which are closely linked to anhedonic behavior [48]. Stress can disturb the interaction between medial prefrontal cortex (mPFC) and mesolimbic by remodeling dendritic in mPFC. For example, chronic restraint stress cause dendritic shrinkage in mPFC [49]. What’s more, stress can increase the risk of several mood disorders, such as MDD and post-traumatic stress disorder, which all involve the symptom of anhedonia [50]. Neuroendocrine stress responses are vital to keep healthy. Corticotropin-releasing factor (CRF) activity in the mesolimbic pathway plays an important role in reward motivation following stress [51]. By injecting CRF antagonist into ventral tegmental area, motivation to work for food reward obviously increase after restraint stress [52]. What’s more, clinical researches have demonstrated association between stress, especially early life stress, and anhedonia [53, 54].
From the results of Bootstrap analysis, we found that stress played a partially role to mediate the relationship between sleep disorder and anhedonia in adMDD. The direct effect of sleep disorder on anhedonia was 0.214, the total effect was 0.295, and the indirect effect mediated by stress was 0.081. Consequently, we can’t neglect that sleep disorder for adMDD is potentially able to accelerate the deterioration of the stress level regardless of being objective or subjective, thereby increasing the risk of anhedonia in the adMDD population. Therefore, we should not only focus on the sleep of adMDD, but also pay great attention to the stressful life event they experienced. Outlining an anhedonic phenotype based on etiology is essential for psychiatric research. This phenotype can assist in enhancing homogeneity in clinical research samples. Moreover, the consequent increase in statistical power can make it simpler to identify crucial vulnerability factors for chronic and severe anhedonia. Consequently, recognizing vulnerability elements and proximate causes of anhedonia may improve prognosis and find novel treatment objectives.
Our research also has several limitations. Firstly, current study is cross-sectional, which means that patients may experience sleep disorders and anhedonia symptoms simultaneously. Therefore, we could only analyze the correlation relationship and further longitudinal studies are needed to determine whether there is a causal relationship. Secondly, although the PSQI has been repeatedly validated and widely used to assess patients’ sleep quality, there is still some subjectivity in defining patients’ sleep status through the scale. The primary outcome definition uses the scale score as a categorical variable, which may lead to the omission of some information. In future studies, applying polysomnography may better clarify the relationship between sleep disorders and anhedonia in patients with adMDD. Thirdly, although we have collected as many confounding variables as possible, it is still likely that some have been omitted, such as family income status and family history. These possible confounding factors could be collected and incorporated into the analysis in future studies. Last but not least, the Stressor Scale for Middle School Students used in this study may not fully capture the breadth of stressors experienced by adolescents. Future research is better to adopt more comprehensive assessment tools to better reflect the complexities of adolescent stress.
In summary, Hypothesis 1 pinpoints the effect of sleep disorders on anhedonia in adMDD. Subsequently, Hypothesis 2 ascertains the influence of sleep disorders on the stress levels of such patients. Eventually, Hypothesis 3 substantiates the indirect mediating function of stress factors. Hence, it is our contention that sleep disorders not only have an impact on an individual’s emotional condition but also heighten the sense of stress. Prolonged exposure to sleep disorders leads to feelings of fatigue, anxiety, and depression, accompanied by a diminished engagement in social activities. Given that social activities represent a crucial source of enjoyment and contentment, protracted social isolation serves to exacerbate anhedonia symptoms. Moreover, in the event of insufficient sleep, the brain’s attentional capacity is restricted, rendering it difficult to wholeheartedly participate in diverse activities. This, in turn, curtails the likelihood of deriving pleasure therefrom and further compounds the anhedonia symptoms.
Conclusion
Current study clarified the relationship among sleep disorder, stress and anhedonia in adMDD. Our findings showed significant correlations among sleep disorder, stress and anhedonia in adMDD. Stress was found to partly mediate the relationship between sleep disorder and anhedonia. Therefore, we should not only focus on the problem of sleep of adMDD, but also pay great attention to the stressful life event they experienced. By this way, we could probably improve the symptom of anhedonia and eventually the prognosis of adolescence depression.
Acknowledgements
We want to express sincere appreciation to adolescents and their parents who participated in our survey.
Abbreviations
- MDD
Major depressive disorder
- adMDD
Adolescent major depressive disorder
- SHAPS
Snaith-Hamilton Pleasure Scale
- PSQI
Pittsburgh Sleep Quality Index
- SD
Standard Deviation
- SSMSS
Stressors Scale for Middle School Students
- VIF
Variance Inflation Factor
- LLCI
Lower Level for Confidence Interval
- ULCI
Upper Level for Confidence Interval
- CRF
Corticotropin-Releasing Factor
- mPFC
Medial Prefrontal Cortex
Author contributions
MC, GCZ and HNW provide research ideas and designs; XXZ, WML, WJW are responsible for data collection; NL, LS, CL and XXZ modify and supervise the knowledge content of statistical analysis; HZM and XXM revise the writing of manuscript; HLD provide funding acquisition and projection administration. All authors reviewed the manuscript.
Funding
This study was supported by the Key Program of National Natural Science of China (Grant No. 82030038 to Hailong Dong), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. 82221001 to Hailong Dong) and the National Natural Science Foundation of China (No. 82271211 to Guangchao Zhao) and Shaanxi Province Science and Technology New Star Project (No. 2023KJXX-024 to Min Cai).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of the Ethics Committee of Xijing Hospital of the Fourth Military Medical University (KY20222058-F-1) and registered in Chinese Clinical Trial Registration Center (ChiCTR2200060176; Registration Date: 21/05/2022). All participants and their legal guardians gave informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guangchao Zhao, Email: gczhao0518@hotmail.com.
Min Cai, Email: mincai8787@hotmail.com.
References
- 1.Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: a systematic review and meta-analysis. Br J Clin Psychol. 2021;61(2):287–305. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RD, Dierker LC, Wu M, Galea S, Hoven CW, Weinberger AH. Trends in U.S. Depression Prevalence from 2015 to 2020: the Widening Treatment Gap. Am J Prev Med. 2022;63(5):726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379(9820):1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators GMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289. [DOI] [PubMed] [Google Scholar]
- 6.Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol. 2015;25(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuba T, Yakushi T, Fukuhara H, Nakamoto Y, Singeo ST Jr., Tanaka O, Kondo T. Suicide-related events among child and adolescent patients during short-term antidepressant therapy. Psychiatry Clin Neurosci. 2011;65(3):239–45. [DOI] [PubMed] [Google Scholar]
- 8.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51(4):404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wild-Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, van Os J, Simons CJP. Day-to-day associations between subjective sleep and affect in regard to future depressionin a female population-based sample. Br J Psychiatry. 2018;202(6):407–12. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Yu J, Jiang S, Fang L, Li Y, Ma S, Kong H, Qin X, Zhu D. Circadian rhythms of melatonin and its relationship with anhedonia in patients with mood disorders: a cross-sectional study. BMC Psychiatry 2024;24(1). [DOI] [PMC free article] [PubMed]
- 11.Palmer CA, Powell SL, Deutchman DR, Tintzman C, Poppler A, Oosterhoff B. Sleepy and secluded: Sleep disturbances are Associated with connectedness in early adolescent Social Networks. J Res Adolescence. 2021;32(2):756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geoffroy PA, Hoertel N, Etain B, Bellivier F, Delorme R, Limosin F, Peyre H. Insomnia and hypersomnia in major depressive episode: prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord. 2018;226:132–41. [DOI] [PubMed] [Google Scholar]
- 13.Keyes KM, Maslowsky J, Hamilton A, Schulenberg J. The great sleep recession: changes in sleep duration among US adolescents, 1991–2012. Pediatrics. 2015;135(3):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XBD, Gentzler AL. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep. 2007;30(1):83–90. [DOI] [PubMed] [Google Scholar]
- 15.Maruani J, Boiret C, Leseur J, Romier A, Bazin B, Stern E, Lejoyeux M, Geoffroy PA. Major depressive episode with insomnia and excessive daytime sleepiness: a more homogeneous and severe subtype of depression. Psychiatry Res 2023;330:115603. 10.1016/j.psychres.2023.115603 [DOI] [PubMed]
- 16.Yip T. The effects of Ethnic/Racial discrimination and sleep quality on depressive symptoms and self-esteem trajectories among Diverse adolescents. J Youth Adolesc. 2014;44(2):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemola S, Schwarz B, Siffert A. Interparental conflict and early adolescents’ aggression: is irregular sleep a vulnerability factor? J Adolesc. 2011;35(1):97–105. [DOI] [PubMed] [Google Scholar]
- 18.Bakth FN, Chen M, Wang Y. Adolescents’ experiences of peer ethnic/racial victimization and school engagement in everyday life: sleep as a moderator. Sleep Health. 2023;9(3):322–30. [DOI] [PubMed] [Google Scholar]
- 19.Chiang JJ, Kim JJ, Almeida DM, Bower JE, Dahl RE, Irwin MR, McCreath H, Fuligni AJ. Sleep efficiency modulates associations between Family stress and adolescent depressive symptoms and negative affect. J Adolesc Health. 2017;61(4):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap Y, Tung NYC, Collins J, Phillips A, Bei B, Wiley JF. Daily relations between stress and electroencephalography-assessed sleep: a 15-Day intensive Longitudinal Design with Ecological momentary assessments. Ann Behav Med. 2022;56(11):1144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips R, Walsh E, Jensen T, Nagy G, Kinard J, Cernasov P, Smoski M, Dichter G. Longitudinal associations between perceived stress and anhedonia during psychotherapy. J Affect Disord. 2023;330:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeMoult J, Humphreys KL, Tracy A, Hoffmeister J-A, Ip E, Gotlib IH. Meta-analysis: exposure to early life stress and risk for Depression in Childhood and Adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59(7):842–55. [DOI] [PubMed] [Google Scholar]
- 23.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. A neurocomputational account of how inflammation enhances sensitivity to punishments Versus rewards. Biol Psychiatry. 2016;80(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress — Meta-analyses on deactivations and activations of the neural correlates of stress reactions. NeuroImage. 2015;119:235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle CC, Stanton AL, Eisenberger NI, Seeman TE, Bower JE. Effects of stress-induced inflammation on reward processing in healthy young women. Brain Behav Immun. 2020;83:126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burani K, Klawohn J, Levinson AR, Klein DN, Nelson BD, Hajcak G. Neural response to rewards, stress and sleep interact to prospectively predict depressive symptoms in adolescent girls. J Clin Child Adolesc Psychol. 2021;50(1):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieman ST, Arditte Hall KA, MacDonald HZ, Gallagher MW, Suvak MK, Rando AA, Liverant GI. Relationships among Sleep disturbance, reward system functioning, Anhedonia, and depressive symptoms. Behav Ther. 2022;53(1):105–18. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Xie L, Xu Y, Yu S, Yao B, Xiang D. Sleep disturbances among medical workers during the outbreak of COVID-2019. Occup Med (Lond). 2020;70(5):364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian H, Wang L, He Q, Xu X, Zhang Y, Yang J, Ye H, Jiang L. Association between sleep quality and cardiovascular disease in maintenance hemodialysis patients: a prospective cohort study. Ren Fail 2023;45(2). [DOI] [PMC free article] [PubMed]
- 31.Zhang W, Sun Q, Chen B, Basta M, Xu C, Li Y. Insomnia symptoms are associated with metabolic syndrome in patients with severe psychiatric disorders. Sleep Med. 2021;83:168–74. [DOI] [PubMed] [Google Scholar]
- 32.Niu J, Han H, Wang Y, Wang L, Gao X, Liao S. Sleep quality and cognitive decline in a community of older adults in Daqing City, China. Sleep Med. 2016;17:69–74. [DOI] [PubMed] [Google Scholar]
- 33.Martino I, Santangelo G, Moschella D, Marino L, Servidio R, Augimeri A, Costabile A, Capoderose G, Cerasa A. Assessment of Snaith-Hamilton pleasure scale (SHAPS): the dimension of anhedonia in Italian healthy sample. Neurol Sci. 2018;39(4):657–61. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Liu T, Luo J, Ren S. Data for teenagers’ stressor, mental health, coping style, social support, parenting style and self-efficacy in South China. Data Brief 2020;29:105202. 10.1016/j.dib.2020.105202 [DOI] [PMC free article] [PubMed]
- 35.Bergmann M, Tschiderer L, Stefani A, Heidbreder A, Willeit P, Högl B. Sleep quality and daytime sleepiness in epilepsy: systematic review and meta-analysis of 25 studies including 8,196 individuals. Sleep Med Rev 2021;57:101466. 10.1016/j.smrv.2021.101466 [DOI] [PubMed]
- 36.Stewart RBA, Bebbington P. Insomnia Comorbidity and Impact and hypnotic use by Age Group in a National Survey Population aged 16 to 74 years. Sleep. 2006;29(11):1391–7. [DOI] [PubMed] [Google Scholar]
- 37.Hunt CA, Smith MT, Mun CJ, Irwin MR, Finan PH. Trait positive affect buffers the association between experimental sleep disruption and inflammation. Psychoneuroendocrinology 2021;129:105240. 10.1016/j.psyneuen.2021.105240 [DOI] [PMC free article] [PubMed]
- 38.Saksvik-Lehouillier I, Saksvik SB, Dahlberg J, Tanum TK, Ringen H, Karlsen HR, Smedbøl T, Sørengaard TA, Stople M, Kallestad H et al. Mild to moderate partial sleep deprivation is associated with increased impulsivity and decreased positive affect in young adults. Sleep 2020;43(10). [DOI] [PMC free article] [PubMed]
- 39.Casement MD, Keenan KE, Hipwell AE, Guyer AE, Forbes EE. Neural reward Processing mediates the relationship between insomnia symptoms and Depression in Adolescence. Sleep. 2016;39(2):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corral-Frías NS, Nadel L, Fellous J-M, Jacobs WJ. Behavioral and self-reported sensitivity to reward are linked to stress-related differences in positive affect. Psychoneuroendocrinology. 2016;66:205–13. [DOI] [PubMed] [Google Scholar]
- 41.Duraccio KM, Krietsch KN, Zhang N, Whitacre C, Howarth T, Pfeiffer M, Beebe DW. The impact of short sleep on food reward processes in adolescents. J Sleep Res 2020;30(2). [DOI] [PMC free article] [PubMed]
- 42.Duraccio KM, Whitacre C, Krietsch KN, Zhang N, Summer S, Price M, Saelens BE, Beebe DW. Losing sleep by staying up late leads adolescents to consume more carbohydrates and a higher glycemic load. Sleep 2022;45(3). [DOI] [PMC free article] [PubMed]
- 43.Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in Pre/Early Pubertal and Mid/Late Pubertal adolescents. J Adolesc Health. 2009;45(4):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leger KA, Charles ST. Affective recovery from stress and its associations with sleep. Stress Health. 2020;36(5):693–9. [DOI] [PubMed] [Google Scholar]
- 45.Bush BJ, Donnay C, Andrews E-JA, Lewis-Sanders D, Gray CL, Qiao Z, Brager AJ, Johnson H, Brewer HCS, Sood S et al. Non-rapid eye movement sleep determines resilience to social stress. eLife 2022, 11. [DOI] [PMC free article] [PubMed]
- 46.Tell D, Mathews HL, Janusek LW. Day-to-Day dynamics of associations between Sleep, Napping, fatigue, and the Cortisol Diurnal Rhythm in women diagnosed as having breast Cancer. Psychosom Med. 2014;76(7):519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sin NL, Wen JH, Klaiber P, Buxton OM, Almeida DM. Sleep duration and affective reactivity to stressors and positive events in daily life. Health Psychol. 2020;39(12):1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollon NG, Burgeno LM, Phillips PEM. Stress effects on the neural substrates of motivated behavior. Nat Neurosci. 2015;18(10):1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS, Morrison John H. The brain on stress: vulnerability and plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehavioral Reviews. 2015;51:189–204. [DOI] [PubMed] [Google Scholar]
- 51.Wook Koo J, Labonté B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han M-H, et al. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress–Induced depressive behaviors. Biol Psychiatry. 2016;80(6):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanat MJ, Bonci A, Phillips PEM. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci. 2013;16(4):383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, Tyrka AR. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, Cross C, Baram TZ, Mahler SV. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress. 2018;8:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.