Abstract
Background
Proton therapy (PRT) is an innovative radiotherapeutic modality for the treatment of cancer with unique ballistic properties. The depth-dose distribution of a proton beam reduces exposure of healthy tissues to radiations, compared with photon-therapy (XRT). To date, only few indications for proton-therapy, like pediatric cancers, chordomas, or intra-ocular neoplasms, are reimbursed by Health systems. There is no published or recruiting prospective study evaluating the impact of proton-therapy or conventional irradiation on neurocognitive function for meningioma patients. Notably, long-term cognitive or ocular impact of these modern irradiation schemes remains poorly known. Yet, these patients had a long life-expectancy, and are at risk of developing long-term sequelae. Thus, according to its ballistic advantage, an improvement of patient functional outcomes and a reduction of neurocognitive long-term toxicity are expected if tissue sparing proton-therapy is used .Randomized trial seems crucial to further assess proton-therapy indication for patients with cavernous sinus meningioma.
Methods
COG-PROTON-01 is the first worldwide randomized phase III prospective study evaluating long-term toxicity of these two irradiation modalities (PRT and XRT)for the treatment of cavernous sinus meningioma. Primary objective is to compare long-term cognitive and/or functional (visual, hearing, neurological and/or endocrinological) deterioration between patients treated by fractionated proton-therapy (PRT) or photon radiotherapy (XRT), 5 years after the end of irradiation. The primary endpoint is based on the individual neurocognitive test scores (grouped into five cognitive domains: attention, executive functioning, verbal memory, working memory, information processing speed) and on visual, hearing, endocrinological and neurological evaluations, five years after radiotherapy. Eligible patients with low-grade cavernous sinus meningioma will be 1:1 randomised, with stratification on age, sex, MoCA score. Overall, the inclusion of 160 patients is planned (80 in each arm). To be considered as positive, asumming that 47% of patients will not develop long-term cognitive disabilities deficits after XRT radiotherapy,, thus at least 70% of the patients treated with PRT should not develop functional impairment. First inclusions started on September 2023 (NCT05895344 ).
Trial registration
The study was registered on clinicaltrials.gov on June 8, 2023 with the following number: NCT 05895344.
Keywords: Meningioma, Skull base, Protontherapy, Cognition, Irradiation
Background
Meningiomas are slow-growing tumors that arise from the arachnoid cap cells of the central nervous system (CNS). It is the most frequent primary CNS tumors. Approximately 80% of the tumors are benign. Neurosurgery is often the treatment of choice. However, for some localization such as cavernous sinus meningioma, surgery may expose patients to brain hemorrhage or cranial nerve paralyses. Thus, cavernous sinus meningiomas are generally treated by exclusive radiation (HAS-2011).
Most of the time, fractionated photonic irradiation (1.8–2 Gy per fraction, for a total dose of 50.4–54 Gy), with or without stereotactic deliverance, according to tumoral volume and distances from organs at risk, is used. An excellent ten-year local control rate comprised between 90 and 100% is observed [1, 2], with similar tumor control and clinical outcomes between radiation modalities [3]. Modern photonic modalities (Tomotherapy, volumetric arc therapy…) offer excellent conformation to the target volume. However, there is a “price” to pay for this conformation. These approaches increase the integral dose to the surrounding normal tissues such as the pituitary gland, hippocampi, cochlea or cranial nerves. Such unwanted spread-out dose is known to increase the risk of carcinogenicity, to impair neurocognitive functions, and to alter visual, endocrinal or hearing functions [4]. Patients could be exposed to severe alteration of quality of life.
Prospective data about long-term toxicity of irradiation for patients with benign meningioma is very poor, but publications about long-term survivors of low-grade glioma (LGG) are available and can be exploited. Indeed, similar long-term side effects of irradiation are expected in both diseases.
According to a large retrospective study, long-term survivors of low grade glioma who did not have radiotherapy had stable radiological and cognitive status [5, 6]. By contrast, patients with low-grade glioma who received radiotherapy showed a progressive decline in attentional functioning, even those who received fraction doses that are regarded as safe. In this population, until 50% of irradiated patients developed cognitive impairment 10 years after conventional photonic irradiation. Recent data with proton therapy (PRT) showed more encouraging results than conventional irradiation [7]. Twenty patients with LGG were enrolled in a prospective study and received PRT dose of 54 Gy (RBE) in 30 fractions. Comprehensive baseline and longitudinal assessments of toxicity, neurocognitive and neuroendocrine function, quality of life, and survival outcomes were performed up to 5 years following treatment. Authors did not observe any overall decline in any of the tested domains. Most of LGG patients who received PRT maintain neurocognitive and neuroendocrine function and compare favorably to results of photon studies in terms of treatment-related morbidity. For example, radiation dose dependent neuroendocrine deficiencies were likely reduced by superior dosimetry with protons (30% deficiency versus 38–41% for X-Ray irradiation).
Thus, for potential long-term survivors, tissue sparing irradiation modality such as proton beam irradiation may be preferred but warrants better level of proof.
Proton therapy is an innovative radiotherapeutic modality for the treatment of cancer with unique ballistic properties. The depth-dose distribution of a proton beam reduces exposure of healthy tissues to radiations, compared with photon-therapy. To date, only few indications for proton-therapy, like pediatric cancers, chordomas, or intra-ocular neoplasms, are reimbursed by the French Health System. Recently, the American society for radiation oncology added benign meningioma to the Group 1 indications, corresponding to the clinical scenarii that frequently support the use of proton-therapy based on published clinical data. However, none of these publications are based on prospective or comparative data confronted to photon-irradiation. To date, only nine retrospective studies have been published with excellent outcomes [8–16].
Hypothesis and expected clinical outcomes
Cavernous sinus meningiomas are close to optic nerve, pituitary gland, cranial nerve, and hippocampi.
The doses delivered to these structures are crucial and radiotherapy of cavernous sinus meningiomas exposes patients to late secondary effects (pituitary deficit, nerve palsy, cognitive impairment…). In 2012, Gondi et al. reported that a dose given to 40% of the bilateral hippocampi greater than 7.3 Gy is associated with a long-term impairment in list-learning delayed recall after FSRT for benign or low-grade adult brain tumors [17].
There is no published or recruiting prospective study evaluating the impact of proton-therapy or conventional irradiation on neurocognitive function for meningioma patients. Notably, long-term cognitive or ocular impact of these modern irradiation schemes remains poorly known. Yet, these patients had a long life-expectancy, and are at risk of developing long-term sequelae. Thus, according to its ballistic advantage, an improvement of patient functional outcomes and a reduction of neurocognitive long-term toxicity are expected if tissue sparing proton-therapy is used.
In this context, a randomized prospective study evaluating long-term toxicity of these two irradiation modalities (PRT and XRT) seems crucial to further assess proton-therapy indication for these patients.
Although literature reports excellent outcomes for intracranial meningioma patients treated by proton-therapy, none of the eight retrospective studies found in literature used an accurate and full evaluation of long-term toxicity [8, 10–18].
Methods
The COG-PROTON-01 study is an open-label multicenter prospective comparative phase 3 randomized 1:1 trial.
Primary objectives and endpoints
The primary objective is to compare long-term cognitive and/or functional (visual, hearing, neurological and/or endocrinological) deterioration among patients with cavernous sinus meningioma treated by fractionated proton-therapy (PRT) or photon radiotherapy (XRT), 5 years after the end of irradiation. The primary endpoint is based on the individual neurocognitive test scores (grouped into five cognitive domains: attention, executive functioning, verbal memory, working memory, information processing speed) and on visual, hearing, endocrinological and neurological evaluations, five years after radiotherapy.
Radiation-induced functional impairment is defined as a clinically significant deterioration, in comparison with baseline evaluation (before irradiation) in one or more of these functions:
Neurocognitive: deterioration is defined as the occurrence of cognitive impairment (a total of ≥ 5 impaired z-scores, as considered in Douw et al. [5]) among patients who were not cognitively impaired at baseline, or the worsening of cognitive impairment (≥ 1 supplementary impaired z-scores) among patients who were already cognitively impaired at baseline. Scores will be converted to z-scores by subtracting mean and dividing by standard deviation (SD) of normative data of healthy controls. A z-score of at least 2 SD below normative data is defined as impaired.
Visual: deterioration is defined as the occurrence of an eye disorder on the same side as the lesion which necessitates a medical intervention (≥ Grade II according to NCI CTCAE V5.0 criteria) or impacts patient’s quality of life (the patient reports a symptom: photophobia, phosphenes….), or a worsening of the symptomatology according to NCI CTCAE V5.0 criteria.
Hearing: deterioration is defined as the apparition of a ≥ grade I hearing impairment according to NCI CTCAE v5.0 or a worsening of the symptomatology according to NCI CTCAE V5.0 criteria or the need of a hearing aid.
Endocrinological: deterioration is defined as the occurrence of hypopituitarism (deficiency of one or more pituitary hormones) with the necessity of medical supplementation or intervention ( ≥grade II according to NCI CTCAE V5.0 criteria).
Neurological: deterioration is defined as the occurrence of a nerve palsy symptomatology, on the same side as the lesion (≥grade I-II according to NCI CTCAE V5.0 criteria), or a worsening of the symptomatology according to NCI CTCAE V5.0 criteria. Vascular strokes, if they occur in the vascular territory of the homolateral carotid could be considered as a complication related to irradiation.
Secondary objectives and endpoints
The secondary objectives are:
• At the patient level,
°To evaluate in each group:
-the delay from randomization to cognitive or functional deterioration.
-the specific impact of irradiation on visual pathways, hearing function, pituitary axis and cranial nerves.
-the rate of clinical symptomatology improvement post-irradiation.
-the evolution of health-related quality-of-life post-irradiation.
-the local control post-irradiation and the progression-free survival according to RANO criteria.
-the professional reintegration for working-age patients.
-the consumption of medical care and medical goods within 5 years post-randomization.
°To quantify, per individual, and per irradiation modality, the evolution of cognitive function over time.
°To describe, in the control group, the cognitive and/or functional deterioration over time, according to delivering XRT in stereotactic conditions or not.
• With regard to radiotherapy parameters:
°To evaluate in each group:
-the dosimetric gain on organ-at-risk (OAR) with proton-therapy modality.
-The correlation between late dosimetric data and the occurrence of cognitive deterioration.
-To build tumoral control probability (TCP) and normal tissue complication probability (NTCP) on the basis of the prospective evaluations.
• With regard to brain MRI parameters:
°To evaluate, in each group,
-Alterations of cortical, hippocampi, sub-ventricular area and white matters after irradiation on brain MRI and their correlation with cognitive deterioration.
-The impact of LET variations on MRI changes using the FROG facility.
°To correlate cognitive deterioration with MRI changes.
•To determine the cost-effectiveness alongside the clinical trial following standard guidelines through a within-trial cost utility analysis using standard methods, focusing only on interventions directly evaluated in the trial from the societal perspective (See “Medico-economic study” section).
•To constitute a biological collection to further explore potential biomarkers predictive of radio-induced toxicities.
The secondary endpoints are:
-The delay between randomization and date of first functional or cognitive impairment (as defined for the primary endpoint).
-The proportion of patients with cognitive impairment for each cognitive domains over time after irradiation.
-The proportion of patients with visual, endocrinological, auditive, and neurologic impairment over time after irradiation.
-The proportion of patients with clinical symptomatology improvement over time after irradiation, as compared to baseline.
-Patient reported outcomes: Scores of Quality-of-life according to EORTC QLQ-C30 and specific BN-20 module, FACT-Cog, HADS, MFI.
-The proportion of patients with local control of disease according to RANO responses recommendations for meningioma [19].
-The progression free survival, defined as the time from randomization to the date of disease progression or death from any cause.
-Among working-age patients, the conditions and modalities for resuming work or initiating redeployment procedures, the proportion of patients who return to work, the delay from randomization to resuming work.
-Mean percentage of dose decrease per organ at risk (Dmax, V20, V30…) function or irradiation modality.
-Volumetric anatomical modifications on MRI sequences: % of thickening, atrophy of hippocampi, grey and white matter.
-Diffusivity alteration on MRI sequences.
-Correlation between LET and MRI signal changes.
-Correlation between the dose delivered to the OAR, and clinical dysfunction.
-Quality-adjusted life years (QALYs) and cumulative costs, discounted.
-Costs for all healthcare use.
Eligibility criteria
Patients have to fulfil the following main inclusion criteria:
-Cavernous sinus meningioma for which clinical target volume is larger than 3 centimeters.
-Anterior skull base meningioma, invading by contiguity the cavernous sinus can be included.
-Histologic proven Grade I meningioma.
-Meningioma for which biopsy is not safely achievable and for which growing and imaging criteria are in favour of grade I meningioma can be included.
-Age > 18 years and < 60 years.
-Indication of irradiation validated by a pluridisciplinary meeting.
-Adjuvant or exclusive irradiation is allowed.
-Use of conventional fractionation: 1.8 Gy (RBE)/fraction.
-Signed informed consent form.
-WHO Performance status equal to 0 or 1.
-Patient affiliated to the French social health insurance.
-MoCA score ≥ cut-off of GRECOGVASC normative data [20].
-Patient whose neuropsychological abilities allow to follow the requirements of the protocol.
All of the following exclusion criteria must be met:
-Patient with mutation in a known predisposition gene (NF-2, SMARCE-1…).
-Cerebrovascular pathology, presence of other tumors of the nervous system, congenital malformations of the nervous system, multiple sclerosis, Parkinson’s disease and other dementias, organic psychosis (other than dementia), schizophrenia, and neurodegenerative disease.
-Radiosurgery, hypofractionated regimen.
-Other localization than cavernous sinus.
-Histologic proven Grade II or III meningioma.
-Patient with unadjusted antiepileptic drug.
-Contraindication to MRI.
-Patient with a history of brain irradiation.
-Patient with a history of cancer in the last five years (excluding skin baso-cellular carcinoma).
-Pregnant/breastfeeding woman.
-Any geographical conditions, social and associated psychopathology that may compromise the patient’s ability to participate in the study.
-Participation in a therapeutic trial for less than 30 days.
-Patient deprived of freedom or under guardianship.
Trial schedule
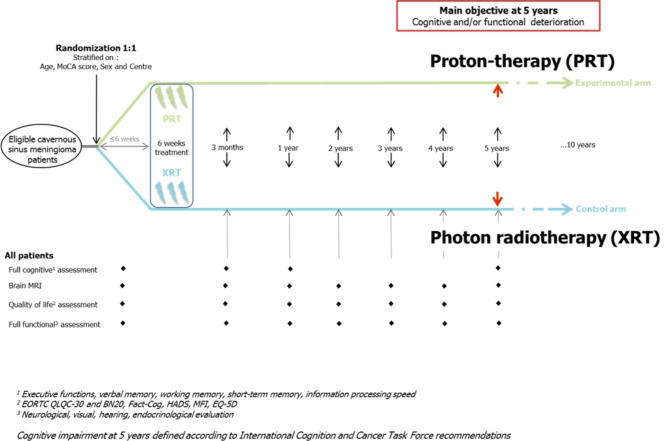
COG PROTON is a multicenter prospective comparative randomized (ratio 1:1) phase 3 trial.
Eligible patients with low-grade cavernous sinus meningioma will be 1:1 randomised, with stratification on age, sex, MoCA score and centers between:
PRT arm: Proton pencil-beam-scanning (or double scattering) irradiation (50,4 Gy (RBE) in 28 fractions).
XRT arm: Intensity modulated radiotherapy (50,4 Gy in 28 fractions).
Radiotherapy will be initiated within 6 weeks post-randomization and will last 6 weeks.
Overall, the inclusion of 160 patients is planned. They will be assigned by randomization (ratio 1:1) between:
PRT arm: 80 patients who will be treated by Proton pencil-beam-scanning irradiation.
XRT arm: 80 patients who will be treated by Intensity modulated radiotherapy.
The overall duration of the project is estimated to 8 years and 6 months, including 3 years and 6 months of inclusion and around 5 years of participation. Beyond 5 years of follow-up for main objective, the follow-up is expected to be pursued provided additional funding is obtained.
Fig. 1.
Study Schedule
Statistical design overview
The primary endpoint is the proportion of patients without any deterioration in cognitive or functional parameters 5 years after irradiation of cavernous sinus meningioma. According to Douw et al. [5], almost 47% of patients did not develop long-term cognitive disabilities deficits after XRT radiotherapy for low-grade glioma. For patients receiving proton-therapy, we thus assume:
P (XRT) = 47%.
P (PRT) = 70%.
with P being the proportion of patients without any clinically significant deterioration in cognitive or functional parameters 5 years after radiation.
A total of 71 assessable patients per arm are required to detect such a difference as significant with a Chi-squared test comparing two independent groups (two-sided alpha risk = 0.05, power = 80% ratio 1:1).
To anticipate around 11% of non-assessable patients, we plan to enroll 160 (80/80) patients.
All outcomes will be analyzed based on intention-to-treat principle, and a sensitivity analysis will be done on the per-protocol population for the main objective, including all patients eligible for the study (no major deviation from the protocol in relation to inclusion and non-inclusion criteria’s) and who have received at least.
Primary objective
The proportion of patients without any deterioration in cognitive or functional parameters 5 years after irradiation of cavernous sinus meningioma will be estimated with a 95% confidence interval, and compared through a Chi-squared test between arms (PRT or XRT).
Secondary objectives
Descriptive statistics will be summarized by using quartiles and range for quantitative variables (mean and standard deviation for Gaussian-like variables), and by using numbers and proportions for qualitative variables. Comparisons between groups will be done by the Chi-squared test (or Fisher exact test) for qualitative variables and by the Student test (or non-parametric Wilcoxon-Mann Whitney test) for quantitative variables. Progression-free survival will be estimated by the Kaplan-Meier method, and compared between arms through the log-rank test. Longitudinal analyses including time as a covariate will be used in order to describe how cognition but also quality of life are impacted in the proton versus photon therapy arms, by the mean of mixed effects models.
All adverse events will be described and categorized according to NCI CTCAE v 5.0 terminologies. For the main above selected toxicities, the most important grade of the same adverse event observed in the same patient will be considered. Cumulative risk as well as longitudinal conditional risk will be estimated using survival data analysis methods and proportional hazards to take into account the time and grade of each of the toxicities.
Study sites
Six French centers are actually recruiting patients (Table 1). Others study sites are expected in France, during 2024 or 2025.
Table 1.
Study sites
| Center | Town | Country | Treatment available on site |
|---|---|---|---|
| Centre François Baclesse | Caen | France | PRT &XRT |
| Centre Antoine Lacassagne | Nice | France | PRT &XRT |
| Institut Curie | Paris | France | PRT &XRT |
| ICANS | Strasbourg | France | XRT |
| IUCT Oncopole | Toulouse | France | XRT |
| APHP Pitié Salpetrière | Paris | France | XRT |
Study procedures
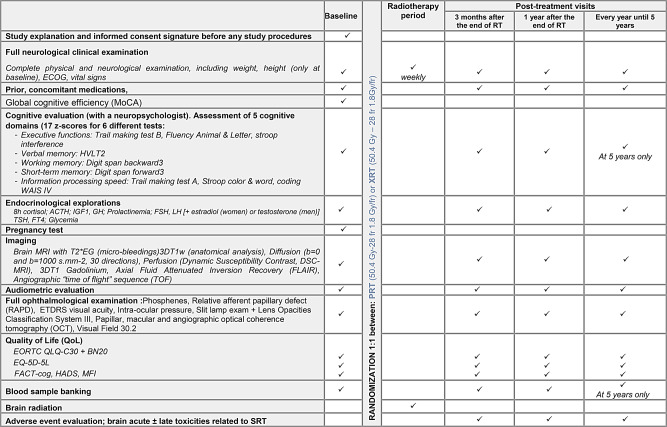
Procedures are summed up in Table 2.
Table 2.
Study procedures
Irradiation
All patients have to complete their irradiation schedule in less than 45 days, from the first day of irradiation to the last day. Treatment should not be protracted over more than 45 days.
Target volume delineation and dosimetry dummy run will be a prerequisite for each participating center before first inclusion.
An independent central review of delineation and dosimetry is planned. Data of baseline imaging tumor assessments as well as dosimetric data (RTdose, RTstruct files and dosimetric CT-scanners and, if relevant, other images) will be anonymised for a centralized review.
Similarly, an independent central review of MRIs will be also performed to confirm imaging abnormalities from baseline.
Proton Therapy (PRT).
Patients will receive a dose of 50.4 Gy (RBE) in 28 fractions of 1.8 Gy (RBE) with proton irradiation, considering the consensual value of RBE of 1.1 delivered on consecutive weekdays.
Pencil beam scanning or double scattering technical will be accepted.
It will be possible to use intensity modulated proton-therapy (IMPT), if implemented in routine.
Two or three beams will be used. It will not be authorized to treat patients with a single beam.
Clinicians and medical physicists should pay attention to avoid proton beam angles with critical organs at risk directly distal to the tumor.
According to each proton therapy center habits, dosimetry could be optimized on a classical PTV, or on the CTV in case of robust optimization. In case of pencil beam irradiation, robust optimization should be preferred.
Intensity modulated radiotherapy (XRT):
Patients will receive a dose of 50.4 Gy in 28 fractions of 1.8 Gy with intensity modulated radiotherapy delivered on consecutive weekdays.
Irradiation could be delivered with or without stereotactic positioning, depending on tumoral volume and localization and distance from organs at risk, according to local practices and at the discretion of the investigator.
Non coplanar irradiation will be authorized.
Dosimetry will be optimized on a PTV.
Follow up after irradiation
Evaluations will be realized at 3 months, 1 year and every year until 5 years after the end of radiotherapy (Table 2).
Patient withdrawal
The treatment will be interrupted at any time under the following circumstances:
-Treatment failure/confirmed disease progression.
-Major protocol violation.
-Intolerable toxicity.
-Concomitant disease or other reason requiring the discontinuation of treatment.
-Patient request (withdrawal of consent for further treatment).
-Investigator’s request (with detailed documentation of reasoning).
-Non-compliance of patient.
-Trial termination by the sponsor.
-Pregnancy
-Death
Any patient who prematurely withdraws from the study treatment only will continue to be followed, unless she withdraws from the study.
Medico economic study
Cost-effectiveness will be determined alongside the clinical trial following standard guidelines. We will conduct a within-trial cost utility analysis using standard methods, focusing only on interventions directly evaluated in the trial from the societal perspective. Outcomes will be reported as quality-adjusted life years (QALYs) and cumulative costs, both discounted at 2.5% (following French guidelines).
The costs for all healthcare will be estimated using national costs (hospital admissions), the social health-insurance (services), and purchase prices (drugs and services), including : proton-therapy or photon radiotherapy; physician services; hospitalisation, emergency visits and day surgery; outpatient diagnostic tests; drugs, including drug costs unrelated to cancer; home care; long-term care; health-related out-of-pocket costs; productivity costs (the mean per capita GDP). Overall quality-of-life will be assessed at each follow up visit using the self-administered standardized EQ-5D, this measure will be used for the economic evaluation, to compute QALYs. The EQ-5D-5 L self-questionnaire consists of 2 pages: the EQ-5D descriptive system and the EQ visual analogue scale (EQ VAS). The descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ VAS records the patient’s self-rated health on a vertical visual analogue scale. The VAS can be used as a quantitative measure of health outcome that reflect the patient’s own judgement.
Cumulative costs and QALYs for each trial arm will be estimated and compared in order to calculate the incremental cost-utility ratio.
Ancillary studies
Biomarkers
The blood sample banking will be performed for further analyses aiming at evaluating neurotoxicity and associated elements such as DNA damage, vascular damage, hypoxic/oxidative stress or neuro-inflammation.
LET Cartography
LET cartography is not available on commercial TPS. Thus, we will use the FROG tools [23, 24] (Fast Recalculation on GPU), a unique graphics processing unit (GPU) based software architecture allowing rapid and robust dose calculation and enabling comparative analysis of different models for estimation of physical and biological effective dose in 3D). To access deterioration of normal tissues due to irradiation, we will use multi parametric MRI and unexpected toxicity recorded by investigators.
Thus, we aim to correlate High LET area with MRI signal modification, and/or with unexpected toxicity. Ultimately, it could change our practices, and make us use LET optimization dosimetry in routine, to avoid radiation induce toxicity.
Discussion
Given the excellent prognosis of skull base meningioma, every effort should be done to avoid radiation induced long-term toxicities such as cognitive impairment or visual deficiency. If the dosimetric advantage of protonbeam irradiation is no more discussed, the clinical benefit of protontherapy in comparison with modern radiotherapy remains debated. We described here the first worldwide randomized study comparing radiotherapy to proton therapy for the treatment of skull base meningioma, with an innovative and composite judgment criterion based on cognitive, auditive, ophthalmologic, neurologic and endocrinological assesments. First inclusions started on August 2023. Final results are expected between 2030 and 2035.
Acknowledgements
We thank the Cancer & Cognition platform and the ANOCEF/IGCNO intergroup fort their support. We also Thank the cancer research patients committee of the French league against cancer for their re-readings of the patient information form.
Abbreviations
- ACTH
Adreno CorticoTropic Hormone
- CNS
Central Nervous System
- Dmax
Maximal Dose
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FSH
Follicle Stimulating Hormone
- FSRT
Fractionated Stereotactic Radiotherapy
- GH
Growth Hormone
- Gy
Gray
- HAS
Haute Autorité de Santé
- IGF1
Insulin-like Growth Factor 1
- IMPT
Intensity Modulated Proton Therapy
- ITT
Intention To Treat
- LET
Linear Energic Transfer
- LGG
Low Grade Glioma
- LH
Luteinizing Hormone
- MRI
Magnetic Resonance Imaging
- NF2
Neuro Fibromatosis type 2
- OAR
Organ At Risk
- OCT
Optical Coherence Tomography
- PP
Per Protocol
- PRT
Proton Therapy
- QALY
Quality-Adjusted Life Year
- RANO
Response Assessment in Neuro-Oncology
- RBE
Relative Biologic Effectiveness
- TSH
Thyroid Stimulating Hormone
- T4
Tétraiodothyronine
- SAP
Statistical Analyses Plan
- SD
Standard Deviation
- V20
Volume receiving 20 Gy
- WHO
World Health Organization
- XRT
Xray Radiation Therapy
Author contributions
PL, IL, BC, JL, JMG, BL , CH wrote the study protocol.IL and JL were in charge of the statistics analysisDS, LF, GN, DR, JB, NG develop the best schedule to assess main hypothesis.IZD was responsible of medico economics analysis.MC was responsible of pharmacovigilanceML and AC develop the neuropsychologic evaluationSV develop the imaging protocolAll Authors reviewed the manuscript.
Funding
PHRC-Cancer 2020 (PHRC-K 20–110, INCa-DGOS_15471). The funding agencies were not involved in the design and conduct of the study, nor in the collection, management, analysis, and interpretation of the data. They were not involved in the writing of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
This study has received ethical approval from the Comité de protection des personnes Ouest V and from National agency for medical and health products safety (Ref IDRCB 2023-A00401-44) in. All patients will give their written informed consent before any study relative assessment start.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Metellus P, Batra S, Karkar S, Kapoor S, Weiss S, Kleinberg L, et al. Fractionated conformal radiotherapy in the management of cavernous sinus meningiomas: long-term functional outcome and tumor control at a single institution. Int J Radiat Oncol Biol Phys. 2010;78:836–43. [DOI] [PubMed] [Google Scholar]
- 2.Correa SFM, Marta GN, Teixeira MJ. Neurosymptomatic carvenous sinus meningioma: a 15-years experience with fractionated stereotactic radiotherapy and radiosurgery. Radiat Oncol. 2014;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroy H-A, Tuleasca C, Reyns N, Levivier M. Radiosurgery and fractionated radiotherapy for cavernous sinus meningioma: a systematic review and meta-analysis. Acta Neurochir (Wien). 2018;160:2367–78. [DOI] [PubMed] [Google Scholar]
- 4.Arvold ND, Niemierko A, Broussard GP, Adams J, Fullerton B, Loeffler JS, et al. Projected second tumor risk and dose to neurocognitive structures after proton versus photon radiotherapy for benign meningioma. Int J Radiat Oncol Biol Phys. 2012;83:e495–500. [DOI] [PubMed] [Google Scholar]
- 5.Douw L, Klein M, Fagel SS, Heuvel J, van den, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8. [DOI] [PubMed] [Google Scholar]
- 6.Taphoorn MJB, Schiphorst AK, Snoek FJ, Lindeboom J, Wolbers JG, Karim ABMF et al. Cognitive functions and quality of life in patients with low-grade gliomas: The impact of radiotherapy. Annals of Neurology. 1994. https://onlinelibrary.wiley.com/doi/abs/10.1002/ana.410360111. Accessed 16 Aug 2019. [DOI] [PubMed]
- 7.Tabrizi S, Yeap BY, Sherman JC, Nachtigall LB, Colvin MK, Dworkin M, et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiother Oncol. 2019;137:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slater JD, Loredo LN, Chung A, Bush DA, Patyal B, Johnson WD, et al. Fractionated proton radiotherapy for benign cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2012;83:e633–637. [DOI] [PubMed] [Google Scholar]
- 9.Vlachogiannis P, Gudjonsson O, Montelius A, Grusell E, Isacsson U, Nilsson K, et al. Hypofractionated high-energy proton-beam irradiation is an alternative treatment for WHO grade I meningiomas. Acta Neurochir (Wien). 2017;159:2391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halasz LM, Bussière MR, Dennis ER, Niemierko A, Chapman PH, Loeffler JS, et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys. 2011;81:1428–35. [DOI] [PubMed] [Google Scholar]
- 11.Vernimmen FJ, Harris JK, Wilson JA, Melvill R, Smit BJ, Slabbert JP. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2001;49:99–105. [DOI] [PubMed] [Google Scholar]
- 12.Weber DC, Lomax AJ, Rutz HP, Stadelmann O, Egger E, Timmermann B, et al. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71:251–8. [DOI] [PubMed] [Google Scholar]
- 13.Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, et al. Spot scanning-based Proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2012;83:865–71. [DOI] [PubMed] [Google Scholar]
- 14.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S et al. Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol. 2018;13. [DOI] [PMC free article] [PubMed]
- 15.Gudjonsson O, Blomquist E, Nyberg G, Pellettieri L, Montelius A, Grusell E, et al. Stereotactic irradiation of skull base meningiomas with high energy protons. Acta Neurochir (Wien). 1999;141:933–40. [DOI] [PubMed] [Google Scholar]
- 16.Murray FR, Snider JW, Bolsi A, Lomax AJ, Walser M, Kliebsch U, et al. Long-term clinical outcomes of Pencil Beam scanning Proton Therapy for Benign and Non-benign Intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2017;99:1190–8. [DOI] [PubMed] [Google Scholar]
- 17.Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83:e487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesueur P, Calugaru V, Nauraye C, Stefan D, Cao K, Emery E, et al. Proton therapy for treatment of intracranial benign tumors in adults: a systematic review. Cancer Treat Rev. 2019;72:56–64. [DOI] [PubMed] [Google Scholar]
- 19.Huang RY, Bi WL, Weller M, Kaley T, Blakeley J, Dunn I, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussel M, Godefroy O. La batterie GRECOGVASC. Evaluation et diagnostic des troubles neurocognitifs vasculaires avec ou sans contexte d’accident vasculaire cérébral. deboeck supérieur ed. Paris; 2016.
- 21.Rieu D, Bachoud-Levi AC, Laurent A, Jurion E, Dalla BG. [French adaptation of the Hopkins Verbal Learning Test]. Rev Neurol (Paris). 2006;162:721–8. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. WAIS-IV nouvelle version de l’échelle d’intelligence de wechsler pour adultes - Quatrième édition. Paris: ECPA; 2011. [Google Scholar]
- 23.Mein S, Choi K, Kopp B, Tessonnier T, Bauer J, Ferrari A, et al. Fast robust dose calculation on GPU for high-precision 1H, 4He, 12 C and 16O ion therapy: the FRoG platform. Sci Rep. 2018;8:14829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mein S, Kopp B, Vela A, Dutheil P, Lesueur P, Stefan D, et al. How can we consider variable RBE and LETd prediction during clinical practice? A pediatric case report at the Normandy Proton Therapy Centre using an independent dose engine. Radiat Oncol. 2022;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.