Abstract
Background
This study aimed to construct, evaluate, and validate nomograms for breast cancer-specific survival (BCSS) and overall survival (OS) prediction in patients with HER2- overexpressing (HER2+) metastatic breast cancer (MBC).
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was used to select female patients diagnosed with HER2 + MBC between 2010 and 2015. These patients were distributed into training and validation groups (7:3 ratio). Variables were screened using univariate and multivariate Cox regression analyses, and BCSS and OS nomograms were constructed to determine one-, three-, and five-year survival probabilities. The nomograms were evaluated and validated using the concordance index (C-index), time-dependent receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis. Stratification was evaluated using Kaplan–Meier curves and log-rank tests based on optimal total score cut-off values. We published web-based versions of these nomograms for clinical use.
Results
A total of 2,151 eligible patients were randomized into training (n = 1,505) and validation (n = 646) groups. Independent prognostic factors of BCSS and OS included: age; marital status; race; oestrogen receptor status; surgery; chemotherapy; and bone, brain, liver, and lung metastases. The C-indices for the BCSS and OS training groups were 0.707 and 0.702, respectively. The ROC, calibration, and decision curves demonstrated the strength of the nomograms. According to cut-off values, patients were categorized into low-, intermediate-, and high-risk groups, with significant differences in survival outcomes between them.
Conclusion
We constructed predictive nomograms and stratified risk to assess the prognosis of patients with HER2 + MBC, which could help inform therapeutic decisions.
Trial registration
Not applicable.
Keywords: HER2-positive breast cancer, Metastatic, Nomogram, Breast cancer-specific survival, Overall survival, SEER database
Background
As one of the most frequent malignant tumours in women today, breast cancer has the highest incidence rate (31%) and the second highest mortality rate (15%) among all malignancies [1]. Breast cancer is classified into subtypes based on oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. Approximately 15–20% of primary breast cancers overexpress HER2; this cancer subtype (HER2+) is invasive and has a high risk of recurrence compared with subtypes that do not overexpress HER2, resulting in a poorer prognosis [2, 3]. Furthermore, distant metastases occur in approximately 29.4% of patients with HER2 + breast cancer, with visceral metastasis being the most common [4]. HER2-targeted drugs are in development, including monoclonal antibodies, tyrosine kinase inhibitors, and antibody-coupled drugs, which have significantly improved patient prognosis [5]. However, HER2 + metastatic breast cancer (MBC) currently has no cure, and resistance to HER2 therapy substantially decreases survival time [6]. Therefore, a prognostic model for patients with HER2 + MBC is crucial.
A nomogram is a common clinical predictive tool in the cancer field and is used to visualize predictive models that accurately assess the prognosis of individual patients by integrating various disease characteristics [7]. Various nomograms that predict the prognosis of MBC have been developed for other subtypes, such as HER2-negative and triple-negative MBC [8, 9]. However, a prognostic nomogram for individuals with HER2 + MBC does not exist. As a result, we developed a prognostic model to forecast breast cancer-specific survival (BCSS) and overall survival (OS) in patients with HER2 + MBC using clinical data from the Surveillance, Epidemiology, and End Results (SEER) database, aiming to provide a convenient Web-based version of the program for easy use by clinicians.
Materials and methods
Source of data
Patient data were gathered from the SEER database (http://seer.cancer.gov/), the official source of information on cancer incidence and survival in the United States. This database provides demographic, clinicopathological, and survival data. We utilised the SEER Research Data, 17 Registries (2000–2020) version released in November 2022 and collected data from patients diagnosed with breast cancer using SEER*Stat software (version 8.4.2). The 17 cancer registries cover approximately 26.5% of the U.S. population, with a broad geographic distribution representing the East and West coasts as well as the Midwest and South regions of the U.S. The patient population is remarkably heterogeneous, encompassing a wide range of races and ethnicities, genders, ages, socioeconomic statuses, and healthcare accessibility.
Patients
This retrospective analysis evaluated data from patients with breast cancer diagnosed between 2010 and 2015. The criteria for inclusion comprised the following: (1) female sex; (2) age ≥ 20 years; (3) diagnosed with breast cancer based on site and morphology (site recode ICD-O-3/WHO 2008); (4) microscopically confirmed cancer; (5) breast cancer was the first primary and only malignant cancer identified; (6) HER2+; and (7) with distant metastasis. The exclusion criteria were: (1) those with unavailable information; (2) breast cancer was confirmed by death certificate or autopsy only; and (3) patients with stage T0, Tx, and Nx.
Patients who fit these requirements were enrolled, and a 7:3 randomization process was used to classify them into training and validation groups. Figure 1 illustrates the study screening procedures. The SEER database is openly accessible; thus, this study did not require informed consent.
Fig. 1.
The flowchart of patient selection
Predictors
We extracted and reclassified variables from the SEER database, including age; marital status; race; histological type; grade; ER and PR status; American Joint Committee on Cancer (AJCC) 7th edition tumour (T) and node (N) stages; surgery; radiotherapy; chemotherapy; and bone, brain, liver, and lung metastases.
Outcomes
The primary outcomes were BCSS and OS. The period from diagnosis to breast cancer-related death was defined as BCSS and that from diagnosis to either all-cause death or the date of the last follow-up as OS.
Statistical analyses
The training and validation groups were randomly assigned (7:3 ratio) using R software (version 4.3.2; R Core Team, Vienna, Austria), and the two groups’ baseline characteristics were compared using the chi-squared test. To determine independent prognostic factors influencing BCSS and OS, sixteen variables were analysed using univariate Cox regression; those with p-values < 0.05 were included in the multivariate Cox analysis.
Subsequently, using the ‘rms’ and ‘survival’ packages in R, we constructed nomograms based on the independent prognostic factors for predicting one-, three-, and five-year BCSS and OS for HER2 + MBC. The concordance indices (C-index) and time-dependent receiver operating characteristic (ROC) curves with the areas under the curves (AUCs) were employed for assessment of discrimination capacity; a higher AUC value indicated better discrimination [10]. Model-predicted survival was compared with observational survival using calibration curves [11]. Decision curve analysis (DCA) was applied to evaluate the clinical practicability of each model.
We also utilized the ‘nomogramFormula’ package in R to calculate the total points for all patients. The optimal cut-off values of the total points were then calculated using X-tile software (version 3.6.1) (https://medicine.yale.edu/lab/rimm/research/software/), and the patients were stratified into low-, intermediate-, and high-risk groups depending on this value. Kaplan–Meier survival curves and log-rank tests were performed to evaluate the nomograms’ capacity to stratify BCSS and OS risk. Lastly, we used the ‘DynNom’ package in R and shinyapps.io (https://www.shinyapps.io/) to develop and publish our Web-based dynamic nomograms. All statistical analyses were performed using R software, and p-values < 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 2,151 patients were assessed and randomly assigned to training (n = 1,505) or validation (n = 646) groups. The two groups did not differ when considering their baseline characteristics (Table 1). Most patients were 40–59 years old (47.00%), followed by the 60–79 years (34.03%), 20–39 years (12.65%), and > 80 years (6.32%) groups. Furthermore, 46.35% and 22.04% of the patients were married and single, respectively, and 1,578 (73.36%) were Caucasian. Invasive ductal carcinoma was the predominant pathological type (85.68%), and histological grades I–IV accounted for 2.00%, 33.84%, 63.88%, and 0.28%, respectively. Overall, 61.60% and 43.65% of the tumours were ER- and PR-positive, respectively. Stages T1 to T4 accounted for 10.55%, 33.38%, 18.92%, and 37.15% of cases, respectively. Stages N0 to N3 accounted for 17.62%, 49.42%, 13.39%, and 19.57% of cases, respectively. Bone, brain, liver, and lung metastases occurred in 56.35%, 7.67%, 39.66%, and 32.91% of patients, respectively. Finally, 40.40%, 34.40%, and 80.33% underwent surgery, radiotherapy, and chemotherapy, respectively.
Table 1.
Baseline characteristics of the selected patients diagnosed with HER2-positive metastatic breast cancer
| Characteristics | Total (n = 2151) |
Training group (n = 1505) |
Validation group (n = 646) |
P |
|---|---|---|---|---|
| Age, n(%) | 0.954 | |||
| 20–39 | 272 (12.65) | 191 (12.69) | 81 (12.54) | |
| 40–59 | 1011 (47.00) | 710 (47.18) | 301 (46.59) | |
| 60–79 | 732 (34.03) | 507 (33.69) | 225 (34.83) | |
| 80+ | 136 (6.32) | 97 (6.45) | 39 (6.04) | |
| Marital status, n(%) | 0.169 | |||
| Married | 997 (46.35) | 682 (45.32) | 315 (48.76) | |
| Single | 474 (22.04) | 329 (21.86) | 145 (22.45) | |
| Widowed/divorced/other | 680 (31.61) | 494 (32.82) | 186 (28.79) | |
| Race, n(%) | 0.676 | |||
| Black | 356 (16.55) | 253 (16.81) | 103 (15.94) | |
| White | 1578 (73.36) | 1096 (72.82) | 482 (74.61) | |
| Other | 217 (10.09) | 156 (10.37) | 61 (9.44) | |
| Histologic type, n(%) | 0.675 | |||
| IDC | 1843 (85.68) | 1285 (85.38) | 558 (86.38) | |
| ILC | 61 (2.84) | 45 (2.99) | 16 (2.48) | |
| Mixed | 80 (3.72) | 60 (3.99) | 20 (3.10) | |
| Other | 167 (7.76) | 115 (7.64) | 52 (8.05) | |
| Grade, n(%) | 1.000 | |||
| I | 43 (2.00) | 30 (1.99) | 13 (2.01) | |
| II | 728 (33.84) | 510 (33.89) | 218 (33.75) | |
| III | 1374 (63.88) | 961 (63.85) | 413 (63.93) | |
| IV | 6 (0.28) | 4 (0.27) | 2 (0.31) | |
| ER Status, n(%) | 0.243 | |||
| Positive | 1325 (61.60) | 915 (60.80) | 410 (63.47) | |
| Negative | 826 (38.40) | 590 (39.20) | 236 (36.53) | |
| PR Status, n(%) | 0.776 | |||
| Positive | 939 (43.65) | 654 (43.46) | 285 (44.12) | |
| Negative | 1212 (56.35) | 851 (56.54) | 361 (55.88) | |
| T stage, n(%) | 0.432 | |||
| 1 | 227 (10.55) | 158 (10.50) | 69 (10.68) | |
| 2 | 718 (33.38) | 506 (33.62) | 212 (32.82) | |
| 3 | 407 (18.92) | 296 (19.67) | 111 (17.18) | |
| 4 | 799 (37.15) | 545 (36.21) | 254 (39.32) | |
| N stage, n(%) | 0.935 | |||
| 0 | 379 (17.62) | 268 (17.81) | 111 (17.18) | |
| 1 | 1063 (49.42) | 747 (49.63) | 316 (48.92) | |
| 2 | 288 (13.39) | 198 (13.16) | 90 (13.93) | |
| 3 | 421 (19.57) | 292 (19.40) | 129 (19.97) | |
| Bone metastasis, n(%) | 0.635 | |||
| Yes | 1212 (56.35) | 843 (56.01) | 369 (57.12) | |
| No | 939 (43.65) | 662 (43.99) | 277 (42.88) | |
| Brain metastasis, n(%) | 0.922 | |||
| Yes | 165 (7.67) | 116 (7.71) | 49 (7.59) | |
| No | 1986 (92.33) | 1389 (92.29) | 597 (92.41) | |
| Liver metastasis, n(%) | 0.205 | |||
| Yes | 853 (39.66) | 610 (40.53) | 243 (37.62) | |
| No | 1298 (60.34) | 895 (59.47) | 403 (62.38) | |
| Lung metastasis, n(%) | 0.082 | |||
| Yes | 708 (32.91) | 478 (31.76) | 230 (35.60) | |
| No | 1443 (67.09) | 1027 (68.24) | 416 (64.40) | |
| Surgery, n(%) | 0.925 | |||
| Yes | 869 (40.40) | 609 (40.47) | 260 (40.25) | |
| No | 1282 (59.60) | 896 (59.53) | 386 (59.75) | |
| Radiotherapy, n(%) | 0.862 | |||
| Yes | 740 (34.40) | 516 (34.29) | 224 (34.67) | |
| No/Unknown | 1411 (65.60) | 989 (65.71) | 422 (65.33) | |
| Chemotherapy, n(%) | 0.809 | |||
| Yes | 1728 (80.33) | 1207 (80.20) | 521 (80.65) | |
| No/Unknown | 423 (19.67) | 298 (19.80) | 125 (19.35) |
Univariate and multivariate Cox analyses for prognosis
In the training group, 12 variables—including age; marital status; race; ER status; PR status; T stage; surgery; chemotherapy; and bone, brain, liver, and lung metastases—were significantly correlated with both BCSS and OS in the univariate Cox analysis (all p < 0.05); radiotherapy only correlated with OS. Subsequently, ten variables were identified as independent prognostic factors in the multivariate Cox analysis, including age; marital status; race; ER status; surgery; chemotherapy; and bone, brain, liver, and lung metastases (Table 2).
Table 2.
Univariate and multivariate Cox regression analyses of BCSS and OS in the training group
| Variables | Univariate COX analysis | Multivariate COX analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCSS | OS | BCSS | OS | |||||||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |||||
| Age | ||||||||||||||||
| 20–39 | Ref | Ref | Ref | Ref | ||||||||||||
| 40–59 | 1.456 | 1.150–1.843 | 0.002 | 1.418 | 1.134–1.773 | 0.002 | 1.249 | 0.980–1.584 | 0.073 | 1.221 | 0.973–1.533 | 0.085 | ||||
| 60–79 | 2.102 | 1.654–2.671 | < 0.001 | 2.119 | 1.690–2.657 | < 0.001 | 1.699 | 1.322–2.183 | < 0.001 | 1.735 | 1.369–2.199 | < 0.001 | ||||
| 80+ | 4.573 | 3.352–6.238 | < 0.001 | 4.576 | 3.411–6.138 | < 0.001 | 2.791 | 1.965–3.964 | < 0.001 | 2.864 | 2.054–3.994 | < 0.001 | ||||
| Marital status | ||||||||||||||||
| Married | Ref | Ref | Ref | Ref | ||||||||||||
| Single | 1.229 | 1.032–1.464 | 0.021 | 1.247 | 1.057–1.471 | 0.009 | 1.133 | 0.946–1.358 | 0.176 | 1.147 | 0.967–1.361 | 0.116 | ||||
| Widowed/divorced/other | 1.637 | 1.411-1.900 | < 0.001 | 1.623 | 1.408–1.870 | < 0.001 | 1.250 | 1.068–1.462 | 0.005 | 1.240 | 1.068–1.440 | 0.005 | ||||
| Race | ||||||||||||||||
| Black | Ref | Ref | Ref | Ref | ||||||||||||
| White | 0.770 | 0.650–0.911 | 0.002 | 0.733 | 0.625–0.859 | < 0.001 | 0.722 | 0.606–0.861 | < 0.001 | 0.686 | 0.583–0.809 | < 0.001 | ||||
| Other | 0.712 | 0.549–0.924 | 0.010 | 0.660 | 0.515–0.846 | 0.001 | 0.800 | 0.614–1.041 | 0.097 | 0.735 | 0.571–0.946 | 0.017 | ||||
| Histologic type | ||||||||||||||||
| IDC | Ref | Ref | Ref | Ref | ||||||||||||
| ILC | 1.350 | 0.957–1.904 | 0.087 | 1.250 | 0.892–1.754 | 0.195 | / | / | / | / | / | / | ||||
| Mixed | 0.885 | 0.625–1.255 | 0.494 | 0.846 | 0.603–1.186 | 0.331 | / | / | / | / | / | / | ||||
| Other | 1.052 | 0.820–1.352 | 0.687 | 1.075 | 0.850–1.359 | 0.547 | / | / | / | / | / | / | ||||
| Grade | ||||||||||||||||
| I | Ref | Ref | Ref | Ref | ||||||||||||
| II | 1.063 | 0.660–1.712 | 0.801 | 1.080 | 0.687–1.697 | 0.739 | / | / | / | / | / | / | ||||
| III | 1.209 | 0.757–1.933 | 0.427 | 1.192 | 0.764–1.860 | 0.440 | / | / | / | / | / | / | ||||
| IV | 2.461 | 0.833–7.276 | 0.103 | 2.210 | 0.755–6.467 | 0.148 | / | / | / | / | / | / | ||||
| ER Status | ||||||||||||||||
| Positive | Ref | Ref | Ref | Ref | ||||||||||||
| Negative | 1.337 | 1.171–1.528 | < 0.001 | 1.336 | 1.177–1.517 | < 0.001 | 1.419 | 1.193–1.688 | < 0.001 | 1.442 | 1.223–1.701 | < 0.001 | ||||
| PR Status | ||||||||||||||||
| Positive | Ref | Ref | Ref | Ref | ||||||||||||
| Negative | 1.257 | 1.100-1.436 | 0.001 | 1.243 | 1.095–1.411 | 0.001 | 1.110 | 0.936–1.316 | 0.231 | 1.092 | 0.929–1.284 | 0.286 | ||||
| T stage | ||||||||||||||||
| T1 | Ref | Ref | Ref | Ref | ||||||||||||
| T2 | 1.143 | 0.889–1.470 | 0.296 | 1.143 | 0.901–1.450 | 0.273 | 1.226 | 0.951–1.579 | 0.115 | 1.225 | 0.964–1.558 | 0.097 | ||||
| T3 | 1.206 | 0.921–1.579 | 0.173 | 1.202 | 0.931–1.552 | 0.159 | 1.143 | 0.870–1.500 | 0.337 | 1.139 | 0.880–1.474 | 0.324 | ||||
| T4 | 1.758 | 1.377–2.244 | < 0.001 | 1.742 | 1.382–2.196 | < 0.001 | 1.507 | 1.177–1.929 | 0.001 | 1.477 | 1.169–1.867 | 0.001 | ||||
| N stage | ||||||||||||||||
| N0 | Ref | Ref | Ref | Ref | ||||||||||||
| N1 | 0.878 | 0.734–1.051 | 0.156 | 0.872 | 0.735–1.033 | 0.112 | / | / | / | / | / | / | ||||
| N2 | 0.846 | 0.667–1.074 | 0.169 | 0.851 | 0.680–1.066 | 0.160 | / | / | / | / | / | / | ||||
| N3 | 0.928 | 0.751–1.147 | 0.491 | 0.911 | 0.745–1.114 | 0.365 | / | / | / | / | / | / | ||||
| Bone metastasis | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No | 0.854 | 0.747–0.976 | 0.020 | 0.864 | 0.761–0.981 | 0.024 | 0.726 | 0.630–0.836 | < 0.001 | 0.743 | 0.649–0.851 | < 0.001 | ||||
| Brain metastasis | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No | 0.534 | 0.427–0.667 | < 0.001 | 0.554 | 0.447–0.687 | < 0.001 | 0.545 | 0.435–0.684 | < 0.001 | 0.582 | 0.465–0.727 | < 0.001 | ||||
| Liver metastasis | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No | 0.693 | 0.607–0.791 | < 0.001 | 0.733 | 0.646–0.831 | < 0.001 | 0.625 | 0.545–0.717 | < 0.001 | 0.661 | 0.580–0.753 | < 0.001 | ||||
| Lung metastasis | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No | 0.661 | 0.576–0.759 | < 0.001 | 0.648 | 0.570–0.739 | < 0.001 | 0.721 | 0.625–0.832 | < 0.001 | 0.704 | 0.614–0.806 | < 0.001 | ||||
| Surgery | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No | 1.959 | 1.701–2.254 | < 0.001 | 1.941 | 1.699–2.218 | < 0.001 | 1.450 | 1.251–1.681 | < 0.001 | 1.484 | 1.284–1.717 | < 0.001 | ||||
| Radiotherapy | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No/Unknown | 1.140 | 0.992–1.311 | 0.065 | 1.180 | 1.033–1.348 | 0.015 | / | / | / | 0.927 | 0.803–1.070 | 0.301 | ||||
| Chemotherapy | ||||||||||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||||||
| No/Unknown | 2.683 | 2.307–3.120 | < 0.001 | 2.621 | 2.268–3.028 | < 0.001 | 2.318 | 1.952–2.752 | < 0.001 | 2.219 | 1.882–2.615 | < 0.001 | ||||
Nomogram construction and validation
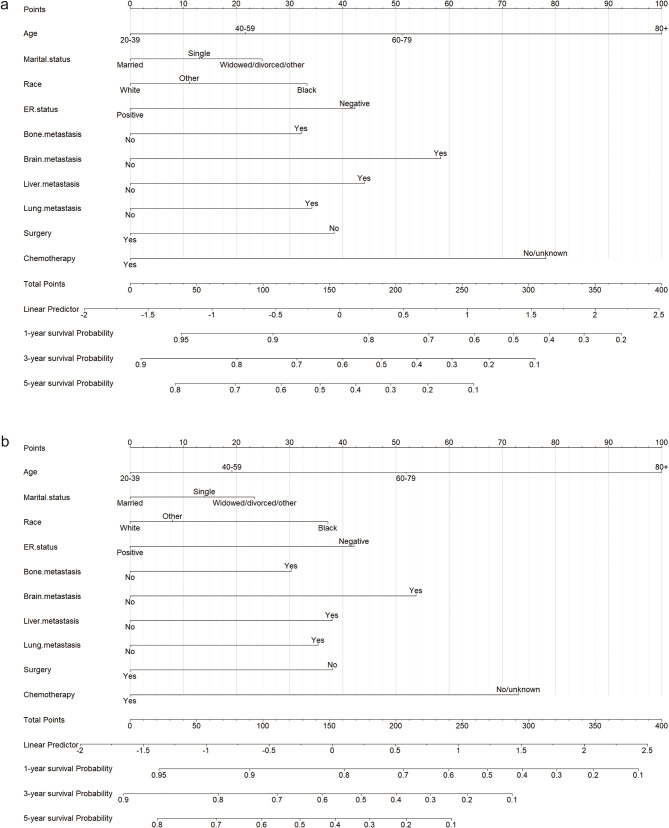
Based on the variables determined above, nomograms were constructed for patients with HER2 + MBC to predict one-, three-, and five-year BCSS and OS (Fig. 2). Take the following case as an example: a 36-year-old married white patient with ER-positive (ER+) HER2 + MBC that had metastasized to the bone but not to the brain, liver, or lung was treated with chemotherapy but not surgery. In this case, the total nomogram scores for BCSS and OS were 70.57 and 68.34, respectively. Thus, the one-, three-, and five-year BCSS probabilities were 93%, 82%, and 72%, respectively, and the OS probabilities were 92%, 80%, and 69%, respectively.
Fig. 2.
Nomograms for predicting survival in patients with HER2 + MBC. The nomograms for predicting one-, three-, and five-year BCSS (a) and OS (b). HER2 + MBC, human epidermal growth factor receptor 2-overexpressing metastatic breast cancer; BCSS, breast cancer-specific survival; OS, overall survival
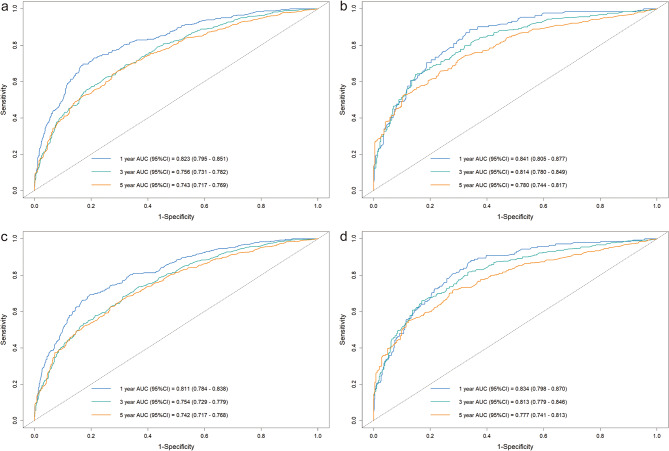
The BCSS and OS C-indices in the training group were 0.707 (95% confidence interval [CI]: 0.690–0.724) and 0.702 (95% CI: 0.686–0.718), respectively; in the validation group, they were 0.731 (95% CI: 0.707–0.755) and 0.725 (95% CI: 0.725–0.748), respectively. We also calculated the AUC values to assess the models’ discrimination abilities. For the BCSS nomogram, the one-, three-, and five-year BCSS AUCs in the training group were 0.823, 0.756, and 0.743, respectively; in the validation group, they were 0.841, 0.814, and 0.780, respectively (Fig. 3a, b). For the OS nomogram, the one-, three-, and five-year OS AUCs in the training group were 0.811, 0.754, and 0.742, respectively; in the validation group, they were 0.834, 0.813, and 0.777, respectively (Fig. 3c, d).
Fig. 3.
Time-dependent ROC curves of the nomograms. ROC curves of the nomogram for predicting one-, three-, and five-year BCSS in the training group (a) and validation group (b). ROC curves of the nomogram for predicting one-, three-, and five-year OS in the training group (c) and validation group (d). ROC, receiver operating characteristic; BCSS, breast cancer-specific survival; OS, overall survival
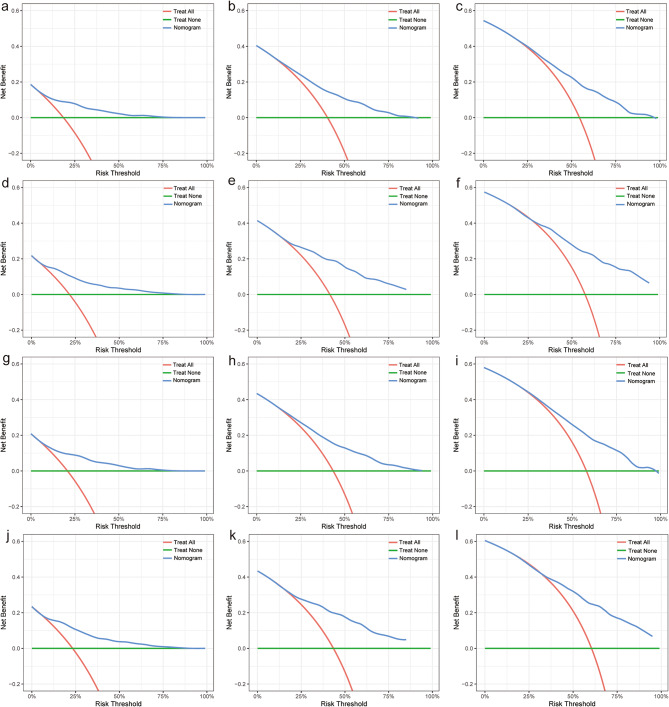
Furthermore, both the training and validation groups’ calibration curves comparing the one-, three-, and five-year predicted and observed BCSS and OS showed a high degree of consistency (Fig. 4). The DCA enables clinicians to select the optimal model. The benefit curves of the BCSS and OS nomograms were greater than those of the reference strategies, indicating that the models have practical clinical applications (Fig. 5).
Fig. 4.
Calibration curves of the nomograms. Calibration curves for predicting one- (a, d), three- (b, e) and five-year (c, f) BCSS in the training and validation groups, respectively. Calibration curves for predicting one- (g, j), three- (h, k) and five-year (i, l) OS in the training and validation groups, respectively. BCSS, breast cancer-specific survival; OS, overall survival
Fig. 5.
DCA of the nomograms. DCA of the nomogram for predicting one- (a, d), three- (b, e) and five-year (c, f) BCSS in the training and validation groups, respectively. DCA of the nomogram predicting one- (g, j), three- (h, k) and five-year (i, l) OS in the training group and validation group, respectively. DCA, decision curve analysis; BCSS, breast cancer-specific survival; OS, overall survival
Risk stratification and web-based dynamic nomogram development
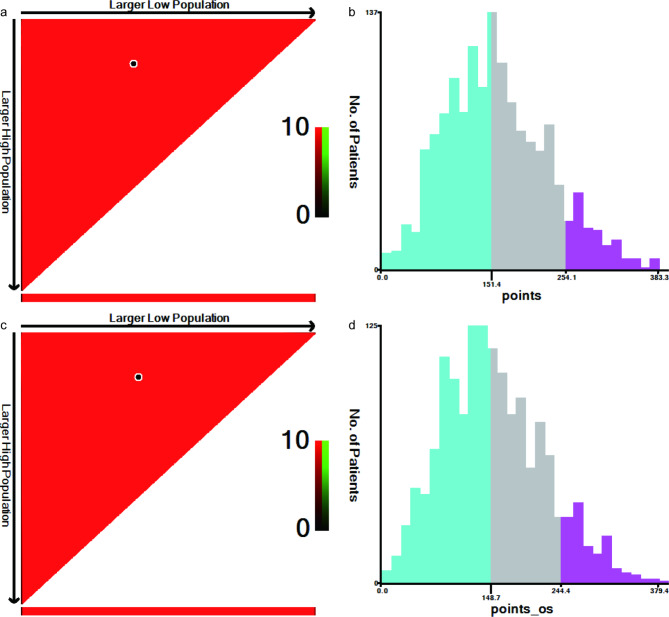
To stratify risk, the optimal cut-off values of the patients’ total nomogram scores were calculated with X-tile software. The BCSS total score cut-off values were 151.4 and 254.1 for categorizing patients into low-risk (score ≤ 151.4), intermediate-risk (151.4 < score ≤ 254.1), and high-risk (score > 254.1) groups (Fig. 6a, b). The OS cut-off values were 148.7 and 244.4 for categorizing patients into low-risk (score ≤ 148.7), intermediate-risk (148.7 < score ≤ 244.4), and high-risk (score > 244.4) groups (Fig. 6c, d).
Fig. 6.
The optimal cut-off values for the nomograms. Optimal cut-off values for the patients’ total scores based on the BCSS and OS nomograms using X-tile analysis (a, c). The histograms for describing the distribution of patients based on the cut-off values (b, d). BCSS, breast cancer-specific survival; OS, overall survival
Kaplan–Meier survival curves were created based on the above results. The median BCSS for the low-, intermediate-, and high-risk groups in the training group were 95, 33, and 5 months, respectively, and 95, 26, and 5 months in the validation group, respectively (Fig. 7a, b). The median OS for the low-, intermediate-, and high-risk groups in the training group were 82, 28, and 4 months, respectively, and 78, 24, and 6 months in the validation group, respectively (Fig. 7c, d). The survival outcomes significantly differed in the Kaplan–Meier survival curves for both BCSS and OS (p < 0.001).
Fig. 7.
Kaplan–Meier survival curves after risk stratification. Kaplan–Meier survival curves of low-, intermediate-, and high-risk groups for the BCSS nomogram in the training (a) and validation groups (b). Kaplan–Meier survival curves of low-, intermediate-, and high-risk groups for the OS nomogram in the training (c) and validation groups (d). BCSS, breast cancer-specific survival; OS, overall survival
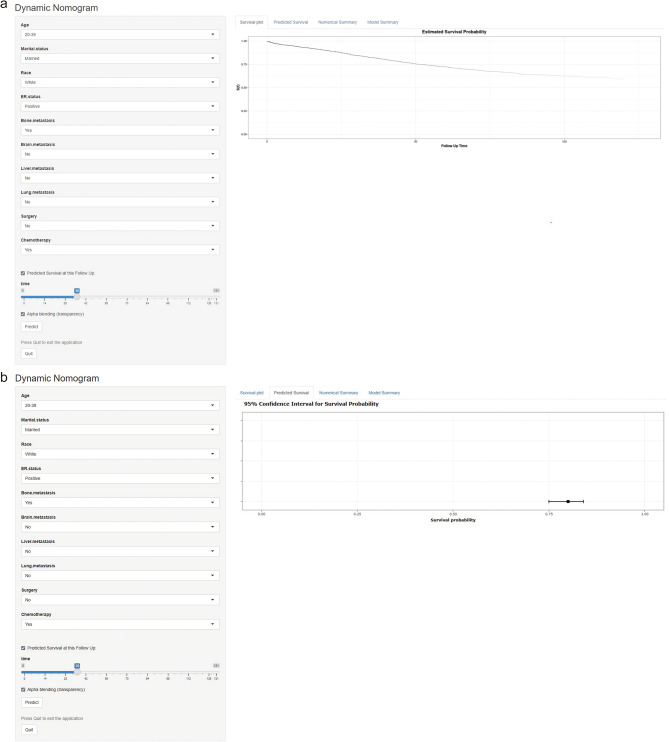
Finally, we established and published Web-based dynamic nomograms for BCSS (Fig. 8a) and OS (Fig. 8b) for patients with HER2 + MBC, accessible at:
Fig. 8.
A screenshot of the Web-based dynamic nomograms. The nomograms for BCSS (a) and OS (b) in patients with HER2 + MBC. HER2 + MBC, human epidermal growth factor receptor 2-overexpressing metastatic breast cancer; BCSS, breast cancer-specific survival; OS, overall survival
1) (https://her2mbcnomo.shinyapps.io/DynNomapp/); and.
Discussion
Patients with a HER2 + status commonly have a poor prognosis owing to the high invasiveness and recurrence rate associated with this cancer subtype. HER2 + MBC remains incurable; therefore, a reliable prognostic model is crucial. The study by Wang et al., including 1174 patients, demonstrated that the histological subtype was a key factor in HER2 + MBC prognosis but did not construct a model that could individualise and accurately predict BCSS and OS [12]. The cohort study by Fan et al. included 34,819 patients with HER2 + breast cancer and reported a nomogram to forecast BCSS. Although the C-index of this nomogram was as high as 0.853 (95% CI: 0.845–0.861), it did not explicitly target the high-risk MBC group [13]. Lyu et al. constructed a BCSS prognostic model based on a subgroup of 1204 HER2 + patients with breast cancer with bone metastases, which possessed strong accuracy, with C-indexes of 0.74 and 0.77 for the training and validation groups, respectively. However, this nomogram excluded other distant metastases and, therefore, was not applicable to all metastatic cases [14]. Lin et al. included 1680 patients with HER2 + MBC as a training cohort and constructed the first nomogram for predicting the probability of OS based on independent prognostic factors of demographic and clinicopathologic characteristics. In the training and validation cohorts, the C-index was 0.70 (95% CI, 0.68–0.72) and 0.68 (95% CI, 0.65–0.72), respectively [15]. In our study, 1505 patients were included in the training group, and a total of 10 demographic and clinicopathologic characteristics and treatment modalities were demonstrated as independent prognostic factors. Based on these findings, we developed nomograms for BCSS and OS that had higher predictive accuracy and better clinical applicability than the previous models. Furthermore, based on the nomograms’ total scores, we identified the optimal cut-off values for low-, intermediate-, and high-risk stratification, which could help clinicians accurately recognize high-risk patients and promptly adjust their therapeutic schedule. Finally, we published easy-to-use Web versions of the nomograms for convenience and clinical application.
Age, marital status, and race were key demographic variables used in the models in this study. Age was a major predictor of survival in patients with MBC, consistent with previous studies [16]. Younger patients with breast cancer are more likely to have larger tumour diameters, more axillary lymph node involvement, poorer pathologic staging, and more distant metastases than older patients, leading to a poorer prognosis, which is contrary to our study’s results [17, 18]. We found that increased age negatively affected BCSS and OS, possibly because older individuals often undergo nonstandard treatments owing to their poor physical condition that results in a lower tolerance for surgery, chemotherapy, and radiotherapy [19]. Furthermore, immune senescence, defined as a decline in immunity with age, can lead to poor prognosis in older adults owing to decreased defences against tumours, even if tumour aggressiveness decreases with age [20–22]. Consequently, age remains an important independent outcome predictor for patients with HER2 + MBC.
Breast cancer is a systemic disease, and the influence of sociopsychological factors, such as marital status, on its prognosis cannot be underestimated. Similar to previous studies, we found that married patients exhibited an increased survival rate compared with single and widowed patients. Married patients, with the support of their spouses and children, are potentially more accepting of surgery, chemotherapy, and radiation therapy and adhere more strictly to their clinicians’ treatment strategies. Moreover, the severity of depressive symptoms is significantly associated with elevated levels of circulating pro-inflammatory factors. Married patients may receive social support from their families, reducing the risk of depressive symptoms and improving their prognosis [23–25]. We also identified race as an independent prognostic predictor of HER2 + MBC. Black race has been associated with poor prognosis and low survival of MBC [26, 27]. The primary reasons for racial differences are socioeconomic support and tumour characteristics [27].
Clinicopathological features, such as ER status and bone, brain, liver, and lung metastases, are closely related to prognosis. Our study found that ER positivity was a protective prognostic factor, consistent with previous studies that suggested an interaction between ER-negative (ER–) and HER2 + statuses, leading to increased breast cancer invasiveness. Thus, patients with ER + and HER2 + cancer have a less-invasive subtype and a wider range of therapeutic options, such as hormonal therapies [28, 29]. The ER status also affects the metastasis site. According to Cletus et al., patients with ER + breast cancer had a higher rate of bone metastasis than those with ER– subtypes, and patients with ER– breast cancers had a higher probability of visceral metastasis, especially liver metastasis. Consistent with these results, we identified bone metastasis as an independent prognostic factor, although it had the least impact on patient prognosis compared with the other identified factors. In contrast, patients with brain metastasis had a substantial decrease in BCSS and OS [26, 30]. Lastly, the Tumour Node Metastasis staging system is widely used for disease risk assessment, and the T stage was a meaningful prognostic factor in this study. However, it was not included in the models because we discovered that the prognosis for stage T2 was shown to be worse than that of stage T3, inconsistent with previous studies [16].
We found that surgery and chemotherapy prolonged BCSS and OS in patients with HER2 + MBC. The National Comprehensive Cancer Network guidelines recommend targeted therapy combined with chemotherapy for HER2 + metastatic disease; however, surgical treatment of patients with MBC is controversial. Surgery improves the prognosis initially by reducing the tumour load via primary tumour resection, blocking the source of distant tumour metastasis [31, 32]. Moreover, surgical resection of tumour lesions decreases the number of immunosuppressive factors released by the tumour, restores immune activity, and assists in systemic therapy [33]. However, surgery dramatically reduces the levels of anti-angiogenic factors and growth factor inhibitors, accelerating tumour recurrence, and postoperative complications and delays in adjuvant therapy can affect prognosis [34–36]. In contrast, the MF07-01 study reported improved 40-month survival in patients with stage IV breast cancer treated with locoregional therapy, and a French multicentre retrospective study found that locoregional treatment prolonged OS in patients with MBC, especially those with HER + cancer [37, 38]. Furthermore, a randomized controlled trial in India and a prospective phase III trial (ABCSG-28) demonstrated that surgery improved OS in patients with stage IV breast cancer [39, 40]. In this study, surgical treatment improved BCSS and OS.
The advent of anti-HER2 therapy has dramatically improved the prognosis of patients with HER2 + MBC, with treatment options including monoclonal antibodies against HER2, tyrosine kinase inhibitors, and antibody-drug conjugates (ADCs) [41]. The CLEOPATRA study validated dual HER2-blocking therapy with docetaxel combined with pertuzumab and trastuzumab as the preferred first-line treatment option for HER2 + MBC [42]. The PHENIX and PHOEBE studies together validated the superior efficacy of the second-line pyrotinib and capecitabine combination [43, 44]. Lapatinib also showed a trend toward better outcomes in patients with brain metastases when no new anti-HER2 agents were available [45]. The EMILIA study established trastuzumab emtansine (T-DM1) as the second-line treatment for HER2 + MBC [46]. Moreover, the DESTINY-03 study showed trastuzumab deruxtecan (T-DXd) significantly improve progression-free survival compared with T-DM1, making it a preferred option for second-line treatment [47]. However, the SEER database mainly provides clinical characteristics and survival data and does not include detailed information on HER2-targeted therapies and specific chemotherapeutic regimens, which may limit the accuracy and completeness of our model. While current studies suggest that trastuzumab and pertuzumab in combination with chemotherapy are commonly preferred first-line treatment options for patients with HER2 + MBC [48, 49], it is worth noting that patients receiving chemotherapy does not mean that they also receive HER2-targeted therapy. Therefore, it is crucial to obtain relevant treatment data regarding anti-HER2 agents. We expect that future experiments will further refine relevant explorations in this area.
There are some limitations to our study. First, this study adopted a retrospective design, relying on the strength of the SEER database, which potentially led to selection bias. Second, the SEER database contains insufficient information regarding factors potentially affecting prognosis, such as HER2-targeted treatment plans, detailed chemotherapy plans, and Ki-67 expression. Third, the SEER database only provided information on distant metastatic sites and molecular types since 2010, and partial treatment data are still incomplete after 2015. Therefore, only data from 2010 to 2015 were selected for our analyses, so the most recent treatment regimen may have had an impact on patient outcomes. Lastly, an independent database was not used to externally validate the nomograms. Further large prospective studies and the integration of more relevant variables from real-world patient data, especially systemic treatment regimens, are required to enhance and refine our predictive models.
Conclusion
In summary, we constructed two nomograms to predict individual survival among female patients with distant metastatic HER2 + breast cancer using risk stratification. Moreover, we published the models online for general use by clinicians to accurately predict one-, three-, and five-year BCSS and OS and distinguish high-risk patients in this population to optimize clinical decision-making.
Acknowledgements
The authors express gratitude to the Surveillance, Epidemiology, and End Results Program for supplying the data.
Abbreviations
- ADC
Antibody-drug conjugate
- AJCC
American Joint Committee on Cancer
- AUC
Areas under the curves
- BCSS
Breast cancer-specific survival
- CI
Confidence interval
- C-index
Concordance index
- DCA
Decision curve analysis
- ER
Oestrogen receptor
- ER–
Oestrogen receptor-negative
- ER+
Oestrogen receptor-positive
- HER2
Human epidermal growth factor receptor 2
- HER2+
Human epidermal growth factor receptor 2-overexpressing
- MBC
Metastatic breast cancer
- N stage
Node stage
- OS
Overall survival
- PR
Progesterone receptor
- ROC
Receiver operating characteristic
- SEER
Surveillance, Epidemiology, and End Results
- T-DM1
Trastuzumab emtansine
- T-DXd
Trastuzumab deruxtecan
- T stage
Tumour stage
Author contributions
YC contributed to the conceptualization of the study, methodology, visualization, validation of the data, and writing the original draft. YQ contributed to the conceptualization of the study, methodology, validation of the data, and writing the original draft. HS was responsible for the software and visualization. SY was responsible for the software and visualization. JL contributed to data curation. WW contributed to the review and editing of the manuscript, supervision, and funding acquisition. All authors have approved the final version of the manuscript. Authors YC and YQ share first authorship.
Funding
This research was funded by the Ningbo Clinical Medical Research Center for Thoracic Malignancies (grant number 2021L002) and The Fourth Round of Ningbo Medical Key Disciplines Construction Plan (grant number 2022-F03).
Data availability
The SEER database (http://seer.cancer.gov/), which is publicly accessible, provided the data analyzed in this study.
Declarations
Ethics approval and consent to participate
Our study was approved by the Ethics Review Board of The Affiliated Lihuili Hospital, Ningbo University, Ningbo (registration number: KY2024ML045). Informed consent was not required as the data were collected from a publicly available repository.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Chen and Yu Qiu share first authorship.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. Cancer J Clin. 2024;74(1):12–49. [DOI] [PubMed]
- 2.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524 – 41. [DOI] [PubMed]
- 3.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biology Int. 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, et al. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat. 2015;150(3):621–9. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes CL, Silva DJ, Mesquita A. Novel HER-2 targeted therapies in breast Cancer. Cancers. 2023;16(1). [DOI] [PMC free article] [PubMed]
- 6.Soleja M, Rimawi MF. Metastatic human epidermal growth factor receptor 2-positive breast cancer: management, challenges, and future directions. Curr Probl Cancer. 2016;40(2–4):117–29. [DOI] [PubMed] [Google Scholar]
- 7.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26(8):1364–70. [DOI] [PubMed] [Google Scholar]
- 8.Duan F, Song C, Ma Y, Jiang K, Xu F, Bi X et al. Establishment of Prognostic Nomograms for Predicting the Survival of HR-Positive, HER2-Negative Metastatic Breast Cancer Patients Treated with Everolimus. Drug design, development and therapy. 2021;15:3463-73. [DOI] [PMC free article] [PubMed]
- 9.Wang Z, Wang H, Sun X, Fang Y, Lu SS, Ding SN, et al. A risk stratification model for Predicting overall survival and Surgical Benefit in Triple-negative breast Cancer patients with de novo distant metastasis. Front Oncol. 2020;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users’ guides to the Medical Literature. JAMA. 2017;318(14):1377–84. [DOI] [PubMed] [Google Scholar]
- 11.Tong Y, Cui Y, Jiang L, Pi Y, Gong Y, Zhao D. Clinical characteristics, prognostic factor and a Novel Dynamic Prediction Model for overall survival of Elderly patients with Chondrosarcoma: a Population-based study. Front Public Health. 2022;10:901680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liang Y, Ye F, Luo D, Jin Y, Li Y, et al. Histologic heterogeneity predicts patient prognosis of HER2-positive metastatic breast cancer: a retrospective study based on SEER database. Cancer Med. 2023;12(18):18597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Wang Y, He L, Imani S, Wen Q. Clinical features of patients with HER2-positive breast cancer and development of a nomogram for predicting survival. ESMO open. 2021;6(4):100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyu X, Luo B. Prognostic factors and survival prediction in HER2-positive breast cancer with bone metastases: a retrospective cohort study. Cancer Med. 2021;10(22):8114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H, Wu Y, Liang G, Chen L. Establishing a predicted model to evaluate prognosis for initially diagnosed metastatic Her2-positive breast cancer patients and exploring the benefit from local surgery. PLoS ONE. 2020;15(11):e0242155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19(1):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida M, Shimizu C, Fukutomi T, Tsuda H, Kinoshita T, Akashi-Tanaka S, et al. Prognostic factors in young Japanese women with breast cancer: prognostic value of age at diagnosis. Jpn J Clin Oncol. 2011;41(2):180–9. [DOI] [PubMed] [Google Scholar]
- 18.Zabicki K, Colbert JA, Dominguez FJ, Gadd MA, Hughes KS, Jones JL, et al. Breast cancer diagnosis in women < or = 40 versus 50 to 60 years: increasing size and stage disparity compared with older women over time. Ann Surg Oncol. 2006;13(8):1072–7. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Xie Z, Xiao Y, Wang B, Zhang P. Prognostic nomogram for female patients suffering from non-metastatic Her2 positive breast cancer: a SEER-based study. Medicine. 2022;101(40):e30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–65. [DOI] [PubMed] [Google Scholar]
- 22.de Kruijf EM, Bastiaannet E, Rubertá F, de Craen AJ, Kuppen PJ, Smit VT, et al. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol. 2014;8(5):1014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu S, Lei C. Association between marital status and all-cause mortality of patients with metastatic breast cancer: a population-based study. Sci Rep. 2023;13(1):9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brothers BM, Andersen BL. Hopelessness as a predictor of depressive symptoms for breast cancer patients coping with recurrence. Psycho-oncology. 2009;18(3):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, et al. Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast Cancer. Psychosom Med. 2016;78(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yardley DA, Tripathy D, Brufsky AM, Rugo HS, Kaufman PA, Mayer M, et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer. 2014;110(11):2756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren JX, Gong Y, Ling H, Hu X, Shao ZM. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat. 2019;173(1):225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park YH, Lee S, Cho EY, Choi YL, Lee JE, Nam SJ, et al. Patterns of relapse and metastatic spread in HER2-overexpressing breast cancer according to estrogen receptor status. Cancer Chemother Pharmacol. 2010;66(3):507–16. [DOI] [PubMed] [Google Scholar]
- 29.Blanchette PS, Desautels DN, Pond GR, Bartlett JMS, Nofech-Mozes S, Yaffe MJ, et al. Factors influencing survival among patients with HER2-positive metastatic breast cancer treated with trastuzumab. Breast Cancer Res Treat. 2018;170(1):169–77. [DOI] [PubMed] [Google Scholar]
- 30.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Annals Oncology: Official J Eur Soc Med Oncol. 2008;19(12):2012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, et al. NCCN Guidelines® insights: breast Cancer, Version 4.2023. J Natl Compr Cancer Network: JNCCN. 2023;21(6):594–608. [DOI] [PubMed] [Google Scholar]
- 32.Wood WC. Breast surgery in advanced breast cancer: local control in the presence of metastases. Breast (Edinburgh Scotland). 2007;16(Suppl 2):S63–6. [DOI] [PubMed] [Google Scholar]
- 33.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205–11. [DOI] [PubMed] [Google Scholar]
- 34.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncology: J Eur Soc Surg Oncol Br Association Surg Oncol. 1999;25(3):231–43. [DOI] [PubMed] [Google Scholar]
- 35.Retsky M, Demicheli R, Hrushesky W. Premenopausal status accelerates relapse in node positive breast cancer: hypothesis links angiogenesis, screening controversy. Breast Cancer Res Treat. 2001;65(3):217–24. [DOI] [PubMed] [Google Scholar]
- 36.Ruiterkamp J, Ernst MF. The role of surgery in metastatic breast cancer. Eur J cancer (Oxford England: 1990). 2011;47(Suppl 3):S6–22. [DOI] [PubMed] [Google Scholar]
- 37.Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast Cancer at Presentation: protocol MF07-01. Ann Surg Oncol. 2018;25(11):3141–9. [DOI] [PubMed] [Google Scholar]
- 38.Pons-Tostivint E, Kirova Y, Lusque A, Campone M, Geffrelot J, Mazouni C, et al. Survival impact of Locoregional Treatment of the primary tumor in De Novo metastatic breast cancers in a large Multicentric Cohort Study: a propensity score-matched analysis. Ann Surg Oncol. 2019;26(2):356–65. [DOI] [PubMed] [Google Scholar]
- 39.Fitzal F, Bjelic-Radisic V, Knauer M, Steger G, Hubalek M, Balic M, et al. Impact of breast surgery in primary metastasized breast Cancer: outcomes of the prospective Randomized Phase III ABCSG-28 POSYTIVE Trial. Ann Surg. 2019;269(6):1163–9. [DOI] [PubMed] [Google Scholar]
- 40.Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16(13):1380–8. [DOI] [PubMed] [Google Scholar]
- 41.Di Maio M, Bighin C, Schettini F, Ruelle T, Marandino L, Fabi A, et al. Evolving treatments and outcomes in HER2-Positive metastatic breast cancer: data from the GIM14/BIOMETA study. Breast (Edinburgh Scotland). 2023;72:103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–30. [DOI] [PubMed] [Google Scholar]
- 43.Yan M, Bian L, Hu XC, Zhang QY, Ouyang QC, Feng JF et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Translational Breast Cancer Res. 2020;1.
- 44.Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–60. [DOI] [PubMed] [Google Scholar]
- 45.Blondeaux E, Ferreira AR, Poggio F, Puglisi F, Bighin C, Sottotetti F et al. Clinical outcomes of patients with breast cancer relapsing after (neo)adjuvant trastuzumab and receiving trastuzumab rechallenge or lapatinib-based therapy: a multicentre retrospective cohort study. ESMO open. 2020;5(4). [DOI] [PMC free article] [PubMed]
- 46.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for breast Cancer. N Engl J Med. 2022;386(12):1143–54. [DOI] [PubMed] [Google Scholar]
- 48.Avelino ARM, Pulipati S, Jamouss K, Bhardwaj PV. Updates in Treatment of HER2-positive Metastatic Breast Cancer. Current treatment options in oncology. 2024. [DOI] [PubMed]
- 49.Miles D, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Campone M, et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Annals Oncology: Official J Eur Soc Med Oncol. 2021;32(10):1245–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The SEER database (http://seer.cancer.gov/), which is publicly accessible, provided the data analyzed in this study.