Abstract
Background
Epilepsy, as a chronic noncommunicable disease with recurrent seizures, may be a marker of deterioration or alteration in other underlying neurological diseases. This study aimed to investigate the relationship of epilepsy with brain function, other common brain disorders, and their underlying mechanisms.
Methods
The study was based on clinical diagnostic and test data from 426,527 participants in the UK Biobank, of whom 3,251 were diagnosed with epilepsy at baseline. Multiple linear and Cox regression models were used to explore the association between epilepsy, brain function, and other brain disorders.
Results
This study demonstrated consistent deleterious effects of epilepsy on cognitive and motor function and mental health. The risk of neurological diseases and psychiatric disorders was significantly elevated in the epilepsy population during the 17-year follow-up period, according to the longitudinal analysis. We also identified several brain regions associated with epilepsy, including the pallidum, hippocampus, and precentral regions. Mediation analyses revealed mediating effects of peripheral markers and proteins (e.g., GGT, HDL, ACE2, and GDF15), suggesting that liver function and lipid metabolism may be involved in the development of other brain disorders in individuals with epilepsy.
Conclusions
Our study provides robust evidence of the association between epilepsy and poor brain health, underscoring the importance of early intervention for epilepsy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-06006-9.
Keywords: Epilepsy, Cognitive, Mental health, Dementia, Depression, Mechanism
Introduction
As the global population ages, the surge in the incidence of neurological diseases and psychiatric disorders (e.g., dementia, stroke, anxiety, and depression) is becoming increasingly evident [1, 2]. There is a growing academic and public interest in human brain health, and even the World Health Organization has declared it a key priority [3]. Brain disorders, which are impairments of brain health characterized by damage to the structure or function of the brain, cause 9 million deaths each year and are the leading cause of death and disability in the world [4]. However, the lack of effective treatments to reverse the brain damage caused by most diseases makes primary prevention a key strategy. Various modifiable risk factors such as personality traits, physical activity, sleep regulation, and hypertension have been covered in previously proposed key strategies for prevention [5–7]. Therefore, it has become particularly important to identify potential influencing factors that may adversely affect brain health. Recently, the Intersectoral Global Action Plan (IGAP) noted that epilepsy could be used as an entry point for other neurological diseases and to conduct in-depth research on epilepsy and brain health [8].
IGAP proposed for most countries to have a program to promote brain health and prevent brain disorders by 2031 and called for a public health approach to epilepsy [3, 9]. To a certain extent, it demonstrated the strong and almost equal importance of the relationship between epilepsy and brain health. Epilepsy is the second most prevalent neurological disease characterized by unprovoked paroxysms, affecting approximately 50 million people worldwide [10–13]. Seizures can be a manifestation of other disorders, including infection and metabolic imbalance, or a sign of deterioration or alteration of underlying neurological diseases. Evidence suggests that recurrent seizures in epilepsy can lead to declines in memory, attention, executive functions, and overall cognitive abilities [14]. Additionally, comorbidities in people with epilepsy represent a huge burden. Compared to the general population, people with epilepsy suffer eight times more from other brain disorders such as dementia, depression, and anxiety [15, 16]. Identifying the underlying mechanisms linking epilepsy to other brain disorders could help develop targeted interventions to reduce the burden of neurological diseases, thereby improving global brain health.
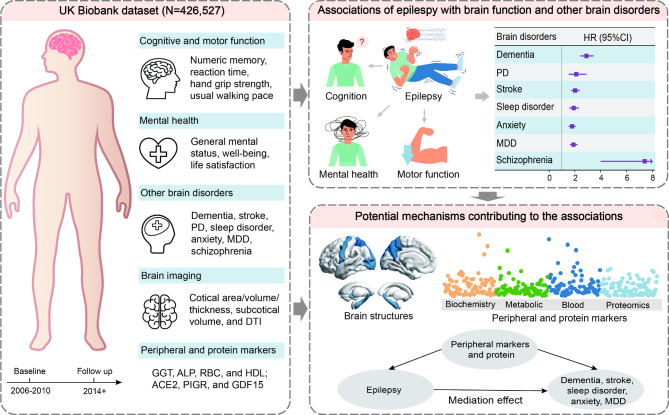
Our study aimed to assess the impact of epilepsy on brain health within a prospective UK Biobank (UKB) cohort (Fig. 1). First, we investigated the impact of epilepsy on brain function. Next, employing the Cox proportional hazards model, we explored the longitudinal relationships between epilepsy and other prevalent neurobehavioral disorders. Neuroimaging data allowed us to examine the relationship between epilepsy and brain structures, including the white matter, and cortical and subcortical regions. Finally, utilizing mediation analyses, we investigated the potential mechanisms of the association between epilepsy and other common brain disorders through peripheral and protein markers. Our study aims to investigate the relationship between epilepsy and various brain health outcomes, identifying future research priorities to minimize the burden on individuals with epilepsy.
Fig. 1.
Guideline of the study. Left, UK Biobank data used in the study included brain function, other brain disorders, brain imaging, peripheral markers, and protein biomarkers. Top right, the associations between epilepsy and brain function, and the associations of epilepsy with neurological diseases and psychiatric disorders. Bottom right, possible mechanisms for the link between epilepsy and 5 studied brain disorders. MDD, major depressive disease, PD, Parkinson’s disease, HR, hazard ratio, CI, confidence interval
Materials and methods
Participants
The current study was based on data from the UKB, a large prospective cohort. The cohort included more than 500,000 participants aged 37–73 who attended one of 22 assessment centers between 2006 and 2010 [17]. The assessment consisted of touchscreen questionnaires and verbal interviews, covering health and lifestyle information, cognitive tests, physical measurements, biological samples, imaging and genotyping. In addition, primary care, hospital inpatient, death and cancer registry data were also covered in the database. The UKB was approved by the North West Multicenter Research Ethical Committee (https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics). The study utilized the UKB resource, application number 19,542. All participants signed a written informed consent form. The following data were used in this study: demographic and behavioral assessments, epilepsy diagnoses, brain function assessments (cognitive tests, grip strength tests, general health, and neuropsychiatric questionnaires), brain structural imaging, other brain disorders diagnoses, death data, blood biochemistry markers, blood cell counts, metabolomics and proteomics data.
The epilepsy diagnoses were defined as individuals with First Reported Occurrences (field identification [ID] 131048, 131050, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code G40, G41). Firstly, we included 502,307 participants with available epilepsy information from the UKB. We excluded participants with self-reported epilepsy only (N = 725), participants diagnosed with epilepsy after baseline (N = 3,644), and participants with dementia, Parkinson’s disease (PD), sleep disorder, stroke, anxiety, bipolar disorder, major depressive disorder (MDD), and schizophrenia at baseline (N = 71,411). Finally, there were 426,527 participants included in the primary analysis.
Brain function
We constructed brain function phenotypes [18, 19] based on cognitive tests, motor function, and behavioral and psycho-behavioral symptoms at baseline. Cognitive test results were collected at baseline via a touchscreen interface, including numeric memory, fluid intelligence, prospective memory, and reaction time. Motor function phenotypes include hand grip strength (left and right), usual working pace, and falls in the last year. Nine mental health symptom-related phenotypes were extracted and constructed through the touchscreen questionnaire on psychological factors and mental health, including general mental status, MDD, anxiety, life satisfaction, subjective well-being, unusual and psychotic experiences, and traumatic events. Life satisfaction and subjective well-being were scored as the higher the score the more dissatisfied and unhappy. The fields and original questions used for cognitive function phenotypes, motor function phenotypes, and psycho-behavioral symptoms are shown in Additional file 1 Table 1.
Neurological disease and psychiatric disorder
The selected brain disorders included neurological diseases (dementia, PD, stroke, and sleep disorder) and psychiatric disorders (anxiety, bipolar disorder, MDD, and schizophrenia). These disorders were identified according to the International Classification of Disease (ICD) codes (Additional file 1 Table 2). In addition, we used disease diagnosis data obtained through an algorithmic combination of coded information collected from the UKB baseline assessment data and linked data from hospital admissions and death registries. It is based on algorithms developed by the UKB outcome adjudication group, aiming to classify disease outcomes with high positive predictive value (i.e. a high probability that people classified as being positive for a health-related event have indeed experienced that event). Data on deaths due to neurological diseases (field 40001, codes G00-G99) are available through a link to the Death Register (https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=40001). The baseline date is the date of initial enrollment in the assessment center (field 53). Follow-up dates were as of the earliest date of any brain disease diagnosis, the date of death (field 40,000), loss to follow-up, or the last available date in hospital inpatient data (fields 41280–41281) or primary care data (field 42040), whichever was recorded earliest.
Assessment of covariates
Relevant covariates were collected through questionnaires and registers at baseline, including age (field 21022), sex (field 31), ethnicity (field 21000), qualification (field 6138), body mass index (field 21001), socioeconomic (field 22189, townsend deprivation index at recruitment), smoking status (field 20116), alcohol drinker status (field 20117), diastolic blood pressure (DBP, field 4079), systolic blood pressure (SBP, field 4080), imaging scanning sites (field 54) and total intracranial volume (TIV, field 26521). Covariates used in different analyses in detail can be seen in Additional file 1 Table 3. Additionally, we collected the history of anti-seizure medications (ASMs, field 20003, Additional file 1 Table 4) used to analyze the association between epilepsy and some significant peripheral blood marker levels. We categorized ASMs obtained in UKB into 4 groups [20–22]: enzyme-inducing ASMs (EIASMs, including carbamazepine, oxcarbazepine, phenobarbitone, phenytoin, primidone, and topiramate) and non-enzyme-active ASMs (nEAASMs, including gabapentin, lamotrigine, levetiracetam, pregabalin, and vigabatrin); old-generation ASMs (carbamazepine, ethosuximide, phenobarbitone, phenytoin) and new-generation ASMs (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate and vigabatrin).
Brain imaging
We extracted brain magnetic resonance imaging data in UKB, which were obtained using a standard Siemens Skyra 3T scanner equipped with a 32-channel head coil. Specific sequences and parameters can be accessed and queried from the open-source documentation available on the UKB official website (https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/brain_mri.pdf).
T1-weighted neuroimaging data underwent quality control and segmentation using FreeSurfer. Cortical brain regions were extracted using the FreeSurfer aparc (ID = 192) atlas tool, while subcortical brain structures were extracted using the FreeSurfer aseg (ID = 190) atlas tool. The output brain region data from FreeSurfer underwent automatic checks using the Qoala-T method, supplemented by manual inspection of outputs approaching threshold levels.Ultimately, we chose whole-brain gray matter volume (field 26518), whole-brain subcortical gray matter volume (field 26517), area of total surface (field 26721, 26822), mean thickness (field 26755, 26856), and cortex volume (field 26552, 26583), as well as surface area, volume, and thickness phenotypes for 68 key cortical brain regions, and volume phenotypes for 16 subcortical brain structures. Of the 426,527 participants, only 43,076 had structural brain data.
Diffusion tensor imaging (DTI) neuroimaging data underwent preprocessing and analysis using the FMRIB Software Library (FSL). The FSL diffusion toolbox was employed for modeling and tractography, resulting in 27 white matter fiber tracts. The DTIFIT toolbox was used to compute the fractional anisotropy (FA) and mean diffusivity (MD) for each fiber tract. Out of a total of 426,527 participants, DTI data was successfully obtained for 31,060 individuals.
Peripheral markers
Blood count data (category 100081) and blood biochemistry data (category 17518) were derived from the results of tests performed by UKB on blood samples collected at the Assessment Centre. Detailed information on the blood sample process can be available at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100080. There were 31 blood counts and 30 blood biochemistry markers were collected from the UKB. In addition, we derived 4 ratios from blood cell counts (neutrophils, lymphocytes, platelets, and monocytes) with reference to the study by Zhang et al. [6], including LMR (lymphocytes/monocytes), NLR (neutrophils/lymphocytes), PLR (platelets/lymphocytes) and SII (neutrophils × platelets/lymphocytes). These ratios are important biomarkers of inflammation and immune status [23–26], and they play an important role in the development of disease. Blood count data and blood biochemistry data were available for the 426,527 participants included.
A high-throughput nuclear magnetic resonance-based metabolic biomarker profiling platform was utilized to measure 251 metabolic biomarkers (category 220) in randomly selected ethylenediaminetetraacetic acid plasma samples from approximately 280,000 UKB participants. Detailed information on the metabolic biomarker measurement process can be accessible at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=220. Then we grouped these markers into broad categories based on different metabolic pathways, including amino acids, apolipoproteins, cholesterol, cholesteryl esters, fatty acids, fluid balance, free cholesterol, glycolysis related metabolites, inflammation, ketone bodies, lipoprotein particle concentrations/sizes, lipoprotein subclasses, other lipids, phospholipids, relative lipoprotein lipid concentrations, and total lipids. Metabolic marker data were available for all 426,527 participants included. Detailed information and categorization of the peripheral markers are provided in Additional file 1 Tables 5, 6 and 7.
Proteomics
Protein biomarkers data (category 1839) were accessed from the normalized protein expression (NPX) data made available in the Olink data table via the Data Portal from the UKB. The process for assessing the protein biomarkers was available at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=1839. We included a total of 2,923 protein biomarkers and divided them into 4 categories, including cardiometabolic, inflammation, neurology, and oncology (Additional file 2). Among the 426,527 participants included, plasma protein biomarker test data were available in 46,326 participants.
Statistical analysis
Linear regression model
Linear regression models were used to test associations of epilepsy with cognitive function, motor function and mental health, adjusting for covariates of age, sex, ethnicity, qualification, body mass index (BMI), socioeconomic status, smoking status, and alcohol drinker status. The model was also used to investigate the association between epilepsy and brain structural imaging, with additional adjustments for the imaging scanning sites and TIV. The two-sided P values were calculated using t-tests in linear regression, and false discovery rate correction (FDR-Q) were additionally presented.
Cox proportional hazard regression model
Cox regression models were utilized to explore the association of epilepsy with other brain disorders and mortality. Hazard ratios (HRs) and confidence intervals (CIs) were calculated in regression analyses. Data with missing covariates were automatically removed from the analysis. For stroke, the model adjusted for covariates such as age, sex, ethnicity, SBP, and DBP. For other brain disorders and mortality, the model adjusted for age, sex, ethnicity, and qualification. The Kaplan-Meier (K-M) method and log-rank test were used to plot survival curves and assess survival differences [27, 28]. None of the neurological diseases and psychiatric disorders except bipolar disorder violated the log-rank hypothesis test. In addition, we further investigated whether the association of epilepsy with other brain disorders morbidity risk and mortality differed by gender via sex-stratified analysis. For sensitivity analyses of age at onset of epilepsy, we matched the populations of the epilepsy and non-epilepsy groups in a 1:4 ratio using propensity score matching.
Mediation analysis
The mediation analyses were used to detect the role of peripheral markers and protein biomarkers in the correlation between epilepsy and 5 common neurological diseases and psychiatric disorders. First, linear regression was utilized to investigate the associations of epilepsy with peripheral markers and protein biomarkers, adjusting for age, sex, ethnicity, qualification, BMI, socioeconomic status, smoking status, and alcohol drinker status. Then we assessed the relationship between peripheral markers, protein biomarkers, and other brain disorders by using the Cox regression model adjusted for age, sex, ethnicity, and qualification. For stroke, the model adjusted for age, sex, ethnicity, SBP, and DBP. The top 4 peripheral markers and protein biomarkers related to both epilepsy and studied neurological diseases and psychiatric disorders were selected to evaluate the mediation effect on the association between epilepsy and brain diseases, respectively. Mediation analyses of variables related to epilepsy as well as neurological diseases and psychiatric disorders were performed through the R package “Mediation“ [29].
R statistical software (http://www.r-project.org/) was used for statistical analysis. Statistical significance was based on a two-sided P value of less than 0.05. FDR correction was applied for multiple comparisons.
Results
Population characteristics
In the primary analysis, a total of 426,527 participants were enrolled in the study at baseline (mean age 56.52 years, 53.59% female). A total of 3,251 participants were diagnosed with epilepsy at baseline, with an average age at diagnosis of approximately 39.92 years, and most of them were concentrated in the 20–40 age range (Additional file 1 Fig. 1). The number and basic characteristics of epilepsy and non-epilepsy diagnosed individuals are shown in Table 1. Of the 3,251 patients with a baseline diagnosis of epilepsy, 152 suffered from dementia, 44 from PD, 123 from sleep disorders, 209 from stroke, 231 from anxiety, 4 from bipolar disorder, 241 from MDD, and 13 from schizophrenia during the follow-up (Additional file 1 Table 8). Descriptive statistics were calculated using mean (s.d.) for continuous variables and number (percentage) for categorical variables.
Table 1.
Demographic characteristics of participants at baseline
| Characteristics | Overall (N = 426,527) |
Epilepsy (N = 3,251) |
No epilepsy (N = 423,276) |
P value |
|---|---|---|---|---|
| Age at enrollment, mean (s.d.) | 56.52 (8.12) | 56.49 (8.13) | 56.52 (8.12) | 0.838 |
| Sex (%) | < 0.001 | |||
| Male | 197,963 (46.41) | 1,641 (50.48) | 196,322 (46.38) | |
| Female | 228,564 (53.59) | 1,610 (49.52) | 226,954 (53.62) | |
| Ethnicity (%) | < 0.001 | |||
| White | 400,231 (93.83) | 3,126 (96.16) | 397,105 (93.82) | |
| Others | 23,950 (5.62) | 102 (3.14) | 23,848 (5.63) | |
| Education level (%) | < 0.001 | |||
| College degree | 200,532 (47.02) | 1,244 (38.27) | 199,288 (47.08) | |
| Others | 217,175 (50.92) | 1,911 (58.78) | 215,264 (50.86) | |
| Smoking status (%) | < 0.001 | |||
| Current smoking | 42,079 (9.87) | 422 (12.98) | 41,657 (9.84) | |
| Previous smoking | 145,473 (34.11) | 1,083 (33.31) | 144,390 (34.11) | |
| Never smoking | 236,498 (55.45) | 1,714 (52.72) | 234,784 (55.47) | |
| Alcohol drinker status (%) | < 0.001 | |||
| Current drinking | 393,422 (92.24) | 2,713 (83.45) | 390,709 (92.31) | |
| Previous drinking | 13,203 (3.10) | 273 (8.40) | 12,930 (3.05) | |
| Never drinking | 18,544 (4.35) | 240 (7.38) | 18,304 (4.32) | |
| Other brain disorders (%) a | ||||
| Dementia | 7,224 (1.69) | 152 (4.68) | 7,072 (1.67) | < 0.001 |
| PD | 2,815 (0.66) | 44 (1.35) | 2,771 (0.65) | < 0.001 |
| Sleep disorders | 8,465 (1.98) | 123 (3.78) | 8,342 (1.97) | < 0.001 |
| Stroke | 14,311 (3.36) | 209 (6.43) | 14,102 (3.33) | < 0.001 |
| Anxiety | 17,677 (4.14) | 231 (7.11) | 17,446 (4.12) | < 0.001 |
| Bipolar | 300 (0.07) | 4 (0.12) | 296 (0.06) | 0.420 |
| MDD | 16,979 (3.98) | 241 (7.41) | 16,738 (3.95) | < 0.001 |
| Schizophrenia | 239 (0.06) | 13 (0.40) | 226 (0.05) | < 0.001 |
Note: Data presented as mean (SD) for continuous variables and number (%) for categorical variables.Ethnicity was categorized as white or others. Education level was categorized as college/university degree or other degree. a Diagnosed with other brain disorders during follow-up. PD, Parkinson’s disease; MDD, major depressive disorder
Associations of epilepsy with brain function
There were significant associations of epilepsy with 4 cognitive scores, 4 physical measures, and 7 mental health symptoms after FDR correction. The results showed that the participants with epilepsy presented a lower level of numeric memory, fluid intelligence, prospective memory, hand grip strength, and usual walking pace (Table 2). In addition, the participants with epilepsy presented a higher level of numeric memory reaction time, falls in the last year, general mental status, the Personal Health Questionnaire-4 (PHQ-4) score, the PHQ-9 score, the Generalized Anxiety Disorder-7 (GAD-7) score, life satisfaction, subjective well-being, and traumatic events (Table 2).
Table 2.
Linear associations between epilepsy and brain function
| Brain function | Beta | P | FDR-Q |
|---|---|---|---|
| Cognitive function | |||
| Numeric memory | −0.217 | < 0.001 | < 0.001 |
| Fluid intelligence | -0.173 | < 0.001 | < 0.001 |
| Prospective memory | −0.157 | < 0.001 | < 0.001 |
| Reaction time | 0.271 | < 0.001 | < 0.001 |
| Motor function | |||
| Hand grip strength (left) | −0.190 | < 0.001 | < 0.001 |
| Hand grip strength (right) | −0.202 | < 0.001 | < 0.001 |
| Usual walking pace | −0.203 | < 0.001 | < 0.001 |
| Falls in the last year | 0.370 | < 0.001 | < 0.001 |
| Mental health | |||
| General mental status | 0.128 | < 0.001 | < 0.001 |
| Depression (PHQ−4 score) | 0.184 | < 0.001 | < 0.001 |
| Depression (PHQ−9 score) | 0.242 | < 0.001 | < 0.001 |
| Depression (CIDI score) | 0.058 | 0.365 | 0.411 |
| Anxiety (GAD−7 score) | 0.107 | 0.003 | 0.005 |
| Life satisfaction | 0.213 | < 0.001 | < 0.001 |
| Subjective well-being | 0.115 | 0.002 | 0.003 |
| Unusual and psychotic experiences | 0.120 | 0.418 | 0.418 |
| Traumatic events | 0.120 | 0.023 | 0.030 |
Note: The model was adjusted for age, sex, ethnicity, qualification, BMI, socioeconomic status, smoking status, alcohol drinker status. FDR-Q is the association P-value adjusted for the false discovery rate. PHQ-4, Personal Health Questionnaire-4; CIDI, Composite International Diagnostic Interview; GAD−7: Generalized Anxiety Disorder−7
Association of epilepsy with other brain disorders
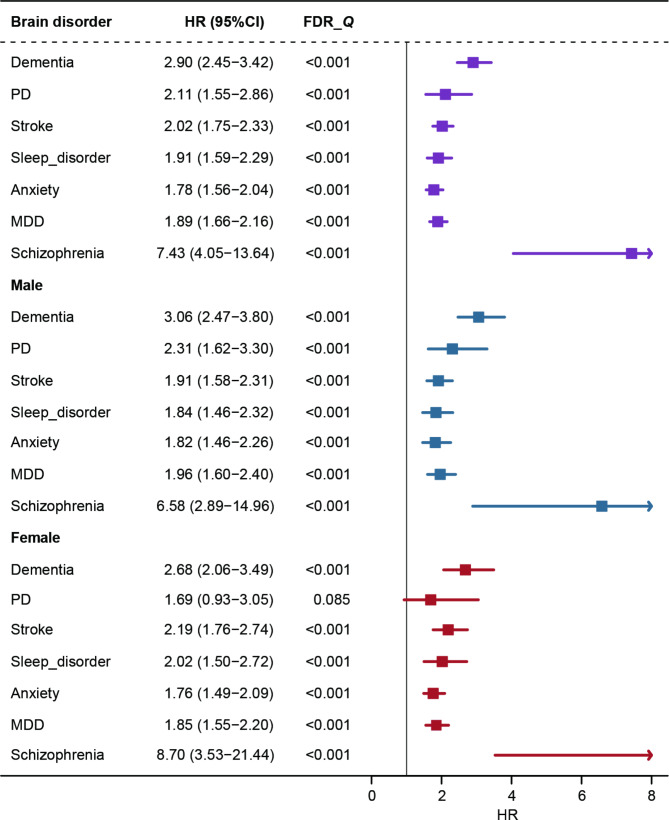
Cox regression models were utilized to investigate the association of epilepsy with the risk of other brain disorders and specific death. The proportional hazards hypothesis was tested using the K-M test and the log-rank test. As the association between epilepsy and bipolar disorder was found to violate the hypothesis test (Additional file 1 Fig. 2), we performed survival analyses for only the remaining 4 neurological diseases (dementia, PD, sleep disorder, and stroke) and 3 psychiatric disorders (anxiety, MDD, and schizophrenia). After FDR correction for associations between epilepsy and studied brain disorders, we observed that the risks conferred by epilepsy remained significant. Strong evidence supported that epilepsy was significantly associated with an increased risk of developing dementia, PD, stroke, sleep disorder, anxiety, MDD, and schizophrenia (Fig. 2). Subsequent sex-stratified analyses showed that men with epilepsy had a higher susceptibility to the development of these brain disorders. However, among females, epilepsy was associated with only 6 studied brain diseases besides PD (FDR-Q = 0.085) (Fig. 2).
Fig. 2.
The association of epilepsy with other neurological diseases and psychiatric disorders in total and sex-specific populations. The model for stroke was adjusted for age at baseline, sex, ethnicity, systolic blood pressure, and diastolic blood pressure. The model for other disorders was adjusted for age at baseline, sex, ethnicity, and qualification. Data are presented as HR ± 95% CI for Cox regression. FDR-Q is the association P-value adjusted for the false discovery rate. PD, Parkinson’s disease, MDD, major depressive disorder, l, left, r, right
We also categorized epilepsy based on the age of onset to delve deeper into the potential effects of varying ages of onset on the development of other brain disorders. We found that the risk of other brain disorders was increased in both those with epilepsy onset age < 40 years and ≥ 40 years compared to a control group without epilepsy. However, no significant risk disparities were observed between the two age-at-onset subgroups (Additional file 1 Table 9). In a sensitivity analysis examining the use of epilepsy medications, we did not find significant associations between epilepsy-taking different types of ASM and other brain disorders (Additional file 1 Table 10). In addition, we examined the association between epilepsy and the risk of death due to neurological diseases. It was found that the association between epilepsy and death from neurological diseases did not violate the log-rank test (Additional file 1 Fig. 3), and a significant positive correlation (3.78, 2.88–4.97) was present. This positive association persisted in both male and female populations (Additional file 1 Fig. 4).
Association between epilepsy and brain structures
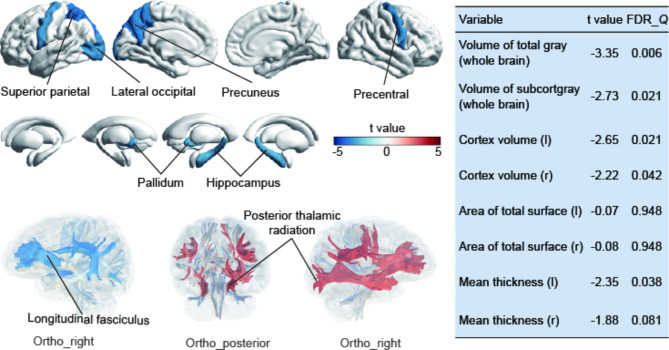
To learn more about the potential mechanisms underlying epilepsy and brain health, we assessed the correlation between epilepsy and brain structure among 43,076 participants with available neuroimaging data. Overall, the observed correlations aligned with expectations, indicating that epilepsy was significantly associated with reductions in cortical thickness, volume, and subcortical volume (Fig. 3). Specifically, cortical thickness affected included the superior parietal cortex (t value=-3.63, FDR-Q = 0.019), precuneus (t value=-3.37, FDR-Q = 0.026), lateral occipital cortex (t value=-2.99, FDR-Q = 0.038), and precentral cortex (t value=-3.00, FDR-Q = 0.038), while subcortical structures encompassed the pallidum (t value=-3.04, FDR-Q = 0.037), and hippocampus (t value=-2.72, FDR-Q = 0.037). More details are available in Additional file 1 Table 11, and 12. Regarding the white matter, individuals with epilepsy exhibited lower FA and higher MD values in specific regions, including the cingulate gyrus, anterior and posterior thalamic radiation, inferior and superior longitudinal fasciculus, and so on (Additional file 1 Table 13).
Fig. 3.
Association of epilepsy with brain structures. Cortical regions, subcortical structures, and white matter tracts associated with epilepsy. Linear regression model was adjusted for age, sex, ethnicity, BMI, socioeconomic status, smoking status, alcohol drinker status, neuroimaging scanning sites, and the intracranial volume (FDR-Q < 0.05)
The mediating role of peripheral markers in the associations of epilepsy and other brain disorders
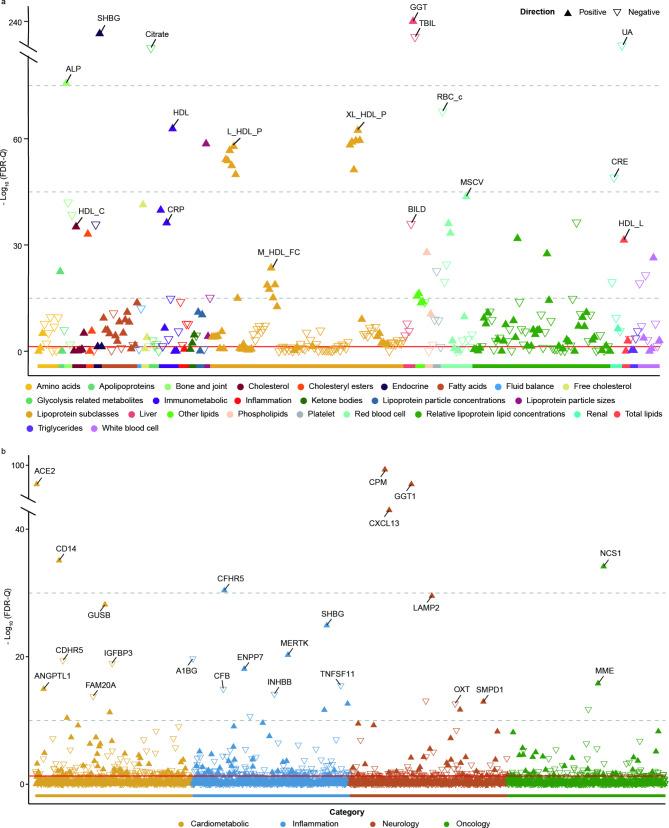
To identify the potential markers mediating the role of peripheral markers in the associations of epilepsy and other brain disorders, we first investigated the epilepsy associated markers. The linear regression analyses showed that there were 226 peripheral markers (including 26 blood count markers, 28 biochemistry markers, and 172 metabolites) significantly associated with epilepsy after FDR correction (Fig. 4a, Additional file 3). We discovered that gamma-glutamyltransferase (GGT) (β = 0.746, FDR-Q = 0) was the most significant among peripheral markers. Blood and metabolic markers significantly associated with epilepsy are more concentrated in the lipoprotein and lipid metabolism subclasses, such as high-density lipoprotein (HDL) (β = 0.275, FDR-Q = 1.56 × 10− 63), concentration of very large HDL particles (XL_HDL_P, β = 0.355, FDR-Q = 4.41 × 10− 63). Then, we explored the markers related to other brain disorders. Cox analyses showed that out of 316 peripheral markers, 207 were associated with dementia, 158 with PD, 285 with sleep disorders, 268 with stroke, 252 with anxiety, 275 with MDD, and 117 with schizophrenia (Additional file 4).
Fig. 4.
Association of peripheral markers and protein biomarkers with epilepsy. (a) Associations between epilepsy and peripheral markers. Full results for each association can be found in Additional file 3. (b) Associations between epilepsy and protein biomarkers. Full results of each protein can be found in Additional file 6. The model adjusted for age at baseline, sex, ethnicity, qualification, BMI, socioeconomic status, smoking status, and alcohol drinker status. FDR-Q is the association P-value adjusted for the false discovery rate
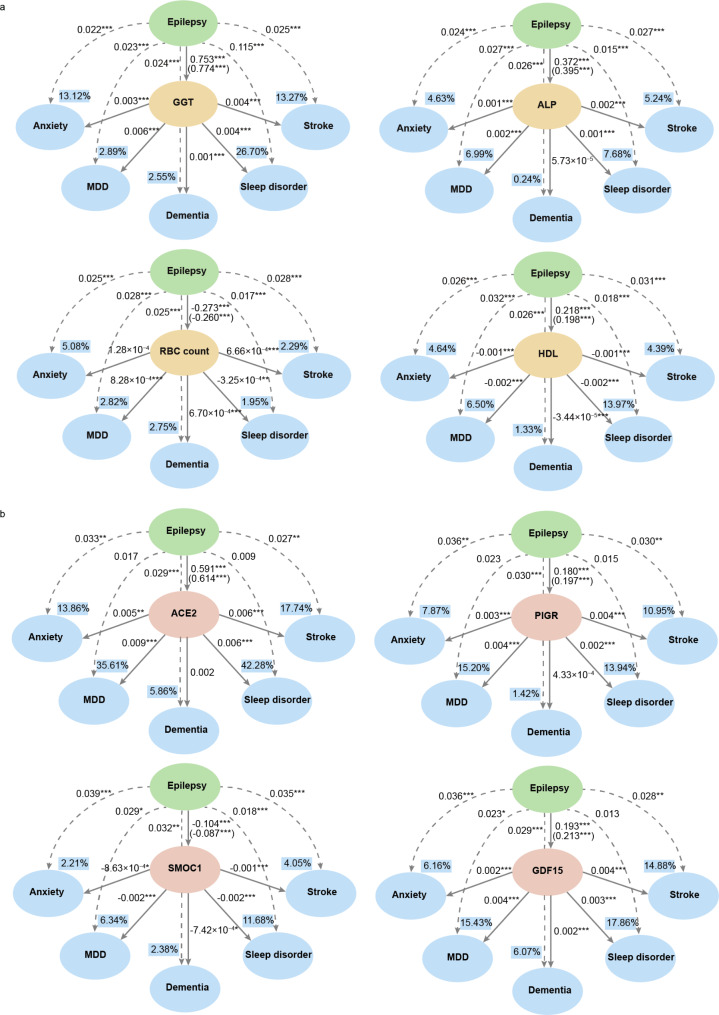
In a total, there were 111 peripheral markers (Additional file 5) simultaneously associated with epilepsy and 5 included disorders (dementia, stroke, sleep disorder, anxiety, and MDD). The mediation analyses were utilized to further explore the potential mechanisms. PD and schizophrenia were not included in the analysis because of the lower incidence risk (cases < 100). We selected the top 4 peripheral markers (GGT, alkaline phosphatase (ALP), red blood cell (RBC) count, and HDL) of these markers that were most relevant to epilepsy to investigate their mediation effects. The results revealed that GGT and HDL mediated the link between epilepsy and 5 included disorders, while ALP and RBC count mediated the correlation between epilepsy and MDD, sleep disorders, and stroke (Fig. 5a, Additional file 1 Table 14). Additionally, since blood metabolic markers are easily affected by medication, we analyzed the association between using ASMs and these 4 markers. We found significant differences between these 4 markers in the plasma of epilepsy patients taking ASMs and those not taking ASMs. There was a strong correlation between this significant difference and the type of ASMs, e.g. plasma GGT, ALP, and HDL levels were higher in epileptic patients using EIASMs and old-generation ASMs than using nEAASMs and new-generation ASMs (Additional file 1 Table 15).
Fig. 5.
The mediating effect of peripheral markers and protein biomarkers in the association between epilepsy and other brain disorders. (a) Mediation of the top 4 peripheral markers simultaneously associated with epilepsy and 5 studied brain disorders. (b) Mediation of the top 4 proteins simultaneously associated with epilepsy and 5 studied brain disorders. The model adjusted for age, sex, ethnicity, and qualification. Values in parentheses are effect values between epilepsy and stroke adjusted for age, sex, ethnicity, systolic blood pressure, and diastolic blood pressure. Dashed and solid lines indicate direct and causally mediated effects, respectively. Value in the box indicates the proportion of mediation. *P < 0.05, **P < 0.01, ***P < 0.001. GGT, gamma-glutamyltransferase, ALP, alkaline phosphatase, RBC count, red blood cell count, HDL, high-density lipoprotein
The mediating role of protein markers in the associations of epilepsy and other brain disorders
We surveyed epilepsy-related markers to identify potential mediator markers of protein markers in epilepsy and other brain disorders related to epilepsy. After FDR correction, there were significant associations of 507 protein biomarkers with epilepsy (Fig. 4b, Additional file 6). Among the protein markers, CPM (FDR-Q = 1.72 × 10− 98), ACE2 (FDR-Q = 7.56 × 10− 78), and GGT1 (FDR-Q = 7.56 × 10− 78) showed the strongest correlation with epilepsy. The study also found significant associations of protein markers with other brain disorders, with 361 proteins associated with dementia, 303 proteins with PD, 1,239 proteins with sleep disorders, 798 proteins with stroke, 542 proteins with anxiety, 1,244 proteins with MDD, and 109 proteins with schizophrenia (Additional file 7).
Ultimately, there were 22 proteins associated with both epilepsy and 5 included disorders (Additional file 8). The top 4 proteins (ACE2, PIGR, SMOC1, and GDF15) were selected to assess the mediation effects between epilepsy and included brain disorders. We discovered that only SMOC1 was simultaneously involved in mediating the association between epilepsy and 5 studied brain disorders. ACE2 and PIGR mediated the correlation of epilepsy with anxiety and stroke, while the mediating effect of GDF15 was strong for studied neurological diseases and psychiatric disorders except sleep disorders (Fig. 5b, Additional file 1 Table 16).
Discussion
This is the first study to explore the relationship between epilepsy and brain health using multimodal data from a large, prospective cohort. Our study demonstrated that epilepsy negatively impacted brain function and other brain disorders such as dementia, stroke, and anxiety. We found that epilepsy was associated with a reduction in cortical and subcortical structures as well as reduced white matter bundle connectivity, which partially explained the impact of epilepsy on poor brain health. Furthermore, this study illustrated the important mediating role of GGT, HDL, and GDF15 in the association between epilepsy and some other brain disorders.
Our results indicated that patients with epilepsy had a significantly increased risk of other brain disorders, yielding findings consistent with previous cohort studies [30–32]. Gowers first proposed the concept of epileptic dementia, implying a close relationship between epilepsy and dementia [33]. Höller et al. found a higher incidence of mild cognitive impairment among patients with temporal lobe epilepsy [34]. Although the risk of stroke rose with advancing age, a previous cohort study indicated that within epilepsy cases, the risk of stroke increased at a more rapid pace [35]. The prevalence of sleep disorders was notably high among epilepsy patients [36], including excessive daytime sleepiness, obstructive sleep apnea, and insomnia, which may be due to epilepsy, medication effects, or comorbid conditions. Epilepsy was also often comorbid with psychiatric disorders such as anxiety and MDD [37, 38]. Notably, a bidirectional relationship between epilepsy and MDD has become increasingly recognized in recent years [39, 40]. Epidemiologic studies have demonstrated that individuals with epilepsy are at a higher risk for developing MDD, while those diagnosed with primary MDD also face an elevated risk of experiencing epilepsy [32]. Hence, our study further validates and highlights that rigorous control and monitoring of epilepsy-related comorbidities are crucial for enhancing the overall care and management of epilepsy.
To further explore the mechanisms through which epilepsy affects poor brain health, we conducted analyses of brain structural changes in epilepsy patients. It demonstrated that the alteration in brain structure stands as a seemingly plausible mechanism through which epilepsy influences brain health. Specifically, our study showed associations between epilepsy and reduced thickness in the superior parietal cortex, precuneus, lateral occipital cortex, and precentral cortex. These regions suggested potential effects on cognitive control, emotional regulation, episodic memory and motor function in epilepsy individuals. Notably, reduced gray matter thickness and dysfunction in these cortical regions above were frequently associated with various brain disorders such as schizophrenia, Alzheimer’s disease, and MDD [41–44]. Previous study demonstrated the key role of the pallidum in the circuitry of focal epileptic seizures [45]. These findings were consistent with our results about brain structures.
There was growing evidence that recurrent or prolonged epileptic seizures could permanently change neural circuits, inducing pathological changes in inflammation and metabolism, ultimately worsening epilepsy and triggering other brain disorders [46, 47]. Mediation analysis was used to explore the potential mechanisms between epilepsy, peripheral and protein markers, and other brain disorders. The significant mediation effects of GGT and HDL indicated that liver function and lipid metabolism might be involved in the association between epilepsy and other brain disorders. EIASMs are known to elevate GGT levels [48–50] and impact blood lipid levels, leading to increases in HDL and total cholesterol levels [51–53], which are consistent with our findings. Notably, many previous studies have confirmed that patients with liver function impairment and lipid metabolism abnormalities might be more susceptible to dementia, stroke, MDD, schizophrenia [54–57]. Given that epilepsy patients are often on long-term ASM therapies, future treatment strategies should ideally start with a newer, noninducing ASM unless there is a strong indication for one of the EIASMs. Older or inducing ASMs should be reserved when necessary [58]. Additionally, it is crucial to prevent alterations in related liver function and lipid metabolism indicators caused by ASMs, thereby reducing the occurrence of epilepsy-related comorbidities.
In addition, it is worth noting that our findings were the first to identify significantly reduced levels of SMOC1 in patients with epilepsy. We also found that SMOC1 was involved in mediating the association between epilepsy and other brain disorders. This may be associated with disturbances of glucose metabolism observed in individuals with epilepsy [46]. SMOC1 is a glucose-reactive hepatokine and glucose homeostasis regulator that decreases gluconeogenic gene expression and inhibits hepatic glucose output [59]. Previous studies have shown that abnormalities in glucose metabolism have been linked to various brain disorders [60–63]. Furthermore, GDF15 was also an important protein that played a mediation effect between epilepsy and other brain disorders. It serves as a biomarker of aging [64], mediating inflammatory responses and participating in the regulation of weight and appetite [65, 66]. A large-scale cross-sectional study in multiple European populations found that elevated plasma GDF15 concentrations were significantly linked to cognitive impairment and depressive states [67]. Additionally, a prior Mendelian randomization analysis indicated an increased risk of dementia associated with high GDF15 levels [68].
Our study has some strengths. This is a pioneering exploration into the association between epilepsy and brain health. It is a prospective study based on a large sample population with long term follow up and different outcomes of neurological diseases and psychiatric disorders. This study additionally utilized data on brain structure as well as peripheral and protein markers to explore potential mechanisms of the impact of epilepsy on neurological diseases and psychiatric disorders. We also acknowledge several limitations. First, the data in UKB was collected primarily from Caucasians, making the findings potentially not broadly applicable in other populations. Second, we have only studied epilepsy prevalence and not further research based on different subtypes. Future research could analyze different subtypes of epilepsy to gain a more nuanced understanding. Third, only 43,076 individuals had brain imaging data, and these data were obtained four years after enrollment, thus the interpretation of the correlation between epilepsy and brain structure should be more rigorous. Moreover, schizophrenia and PD cases with low case numbers were not included in our mediation analysis, which may have limited the comprehensiveness of our study. Finally, our study lacks validation in other cohort populations due to the difficulty of obtaining comprehensive data on epilepsy, brain disorders, brain imaging, peripheral markers, and proteomes in the other cohort. Future studies should consider further validating our results in other cohorts, deeply exploring the association between epilepsy and other brain disorders.
Conclusions
In conclusion, our study conducted a comprehensive investigation of the association between epilepsy and poor brain health. The study revealed that the underlying mechanisms of these associations may be related to immune inflammation and metabolism. Our study emphasized the importance of early intervention in the diagnosis and treatment of epilepsy for brain health, and provided new insights to improve the quality of survival of patients with epilepsy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- IGAP
The Intersectoral Global Action Plan
- UKB
UK Biobank
- PD
Parkinson’ s disease
- MDD
Major depressive disorder
- ICD
The International Classification of Disease
- DBP
Diastolic blood pressure
- SBP
Systolic blood pressure
- ASMs
Anti-seizure medications
- EIASMs
Enzyme-inducing ASMs
- nEAASMs
Non-enzyme-active ASMs
- TIV
Total intracranial volume
- DTI
Diffusion tensor imaging
- FSL
FMRIB Software Library
- FA
Fractional anisotropy
- MD
Mean diffusivity
- LMR
Lymphocytes/Monocytes
- NLR
Neutrophils/Lymphocytes
- PLR
Platelets/Lymphocytes
- SII
Neutrophils × Platelets/Lymphocytes
- NPX
Normalized protein expression
- BMI
Body mass index
- FDR-Q
False discovery rate correction
- HRs
Hazard ratios
- CIs
Confidence intervals
- K-M test
Kaplan-Meier test
- PHQ-4
Personal Health Questionnaire-4
- GAD-7
Generalized Anxiety Disorder-7
- GGT
Gamma-glutamyltransferase
- HDL
High-density lipoprotein
- XL_HDL_P
Concentration of very large HDL particles
- ALP
Alkaline phosphatase
- RBC count
Red blood cell count
Author contributions
JT Yu, JF Feng, and W Cheng designed the study. DD Zhang and ZY Wang conducted the main analyses and drafted the manuscript. W Zhang, PY Gao, Y Fu, HC Chi, YJ Ge, LY Ma, XY He, and J You contributed to data collection and analyses. L Tan, YR Zhang, and JT Yu were involved in revising the article. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), National Natural Science Foundation of China (82071201, 82071997), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), Research Start-up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), Shanghai Talent Development Funding for The Project (2019074), Shanghai Rising-Star Program (21QA1408700), 111 Project (B18015), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Shanghai Center for Brain Science and Brain-Inspired Technology, Fudan University.
Data availability
The primary data used in this study were obtained from the publicly available UK Biobank, application number 19542. Packages including “survival” and “mediation” in R version 4.2.2 were used to perform Cox regression model and mediation analysis, respectively.
Declarations
Ethics approval and consent to participate
The study was conducted following the Declaration of Helsinki. The UK Biobank has research tissue bank approval from the North West Multi-Center Research Ethics Committee (11/NW/0382). Written informed consent was obtained from all participants. The present study was approved by UK Biobank under application number 19542.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan-Dan Zhang, Zi-Yi Wang and Ya-Ru Zhang contributed equally to this work.
Contributor Information
Lan Tan, Email: dr.tanlan@163.com.
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
References
- 1.Hou Y, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–81. [DOI] [PubMed] [Google Scholar]
- 2.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. [DOI] [PubMed] [Google Scholar]
- 3.Samarasekera U. Making brain health a global priority. Lancet Neurol. 2023;22(4):297–8. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Pan Y, Li H. What is brain health and why is it important? BMJ. 2020;371:m3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endo H, et al. A machine learning-based Approach to discrimination of tauopathies using [(18) F]PM-PBB3 PET images. Mov Disord. 2022;37(11):2236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y-R, et al. Personality traits and brain health: a large prospective cohort study. Nat Mental Health. 2023;1(10):722–35. [Google Scholar]
- 7.Li Y, et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat Aging. 2022;2(5):425–37. [DOI] [PubMed] [Google Scholar]
- 8.World Health O. Intersectoral global action plan on epilepsy and other neurological disorders 2022–2031. Geneva: World Health Organization; 2023. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S et al. Neurosurgery and the World Health Organization Intersectoral Global Action Plan for Epilepsy and Other Neurological disorders 2022–2031. Neurosurgery, 2024. [DOI] [PubMed]
- 10.Kotloski RJ, et al. Epilepsy and aging. Handb Clin Neurol. 2019;167:455–75. [DOI] [PubMed] [Google Scholar]
- 11.Beghi E, Giussani G. Aging and the epidemiology of Epilepsy. Neuroepidemiology. 2018;51(3–4):216–23. [DOI] [PubMed] [Google Scholar]
- 12.Ngugi AK, et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiest KM, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinter K et al. [Cognitive deficits in epilepsy]. Ugeskr Laeger, 2022. 184(26). [PubMed]
- 15.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–15. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Liew D, Kwan P. Excess mortality and hospitalized morbidity in newly treated epilepsy patients. Neurology. 2016;87(7):718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer LJ. UK Biobank: bank on it. Lancet. 2007;369(9578):1980–2. [DOI] [PubMed] [Google Scholar]
- 18.deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9(9):720–9. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–27. [DOI] [PubMed] [Google Scholar]
- 20.Borghs S, et al. Health care cost associated with the use of enzyme-inducing and non-enzyme-active antiepileptic drugs in the UK: a long-term retrospective matched cohort study. BMC Neurol. 2017;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephson CB, et al. Association of enzyme-inducing Antiseizure Drug Use with Long-Term Cardiovascular Disease. JAMA Neurol. 2021;78(11):1367–74. [DOI] [PubMed] [Google Scholar]
- 23.Buonacera A et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the Immune System and diseases. Int J Mol Sci, 2022. 23(7). [DOI] [PMC free article] [PubMed]
- 24.Li XT, et al. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J (Engl). 2020;133(4):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai G, et al. Platelet-lymphocyte ratio as a new predictor of in-hospital mortality in cardiac intensive care unit patients. Sci Rep. 2021;11(1):23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninla-aesong P, et al. Relative value of novel systemic immune-inflammatory indices and classical hematological parameters in predicting depression, suicide attempts and treatment response. Sci Rep. 2024;14(1):19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350(17):1713–21. [DOI] [PubMed] [Google Scholar]
- 28.Jager KJ, et al. The analysis of survival data: the Kaplan-Meier method. Kidney Int. 2008;74(5):560–5. [DOI] [PubMed] [Google Scholar]
- 29.Tingley D et al. mediation: R Package for Causal Mediation Analysis. 2014. 59: pp. 1–38.
- 30.Schnier C, et al. A nationwide, retrospective, data-linkage, cohort study of epilepsy and incident dementia. Neurology. 2020;95(12):e1686–93. [DOI] [PubMed] [Google Scholar]
- 31.Sundelin HEK, et al. Pediatric Ischemic Stroke and Epilepsy: a Nationwide Cohort Study. Stroke. 2021;52(11):3532–40. [DOI] [PubMed] [Google Scholar]
- 32.Bølling-Ladegaard E, et al. Directionality of the Association between Epilepsy and Depression: a Nationwide Register-based Cohort Study. Neurology. 2023;100(9):e932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucy PC. Epilepsy and other Chronic Convulsive diseases - their causes symptoms and treatment. J Neurosurg. 1965;22(2):220–. [Google Scholar]
- 34.Höller Y, Trinka E. What do temporal lobe epilepsy and progressive mild cognitive impairment have in common? Front Syst Neurosci. 2014;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wannamaker BB, et al. Stroke after adult-onset epilepsy: a population-based retrospective cohort study. Epilepsy Behav. 2015;43:93–9. [DOI] [PubMed] [Google Scholar]
- 36.Zanzmera P, et al. Markedly disturbed sleep in medically refractory compared to controlled epilepsy - a clinical and polysomnography study. Seizure. 2012;21(7):487–90. [DOI] [PubMed] [Google Scholar]
- 37.Fiest K.M., Patten S.B., Jetté N. Screening for depression and anxiety in Epilepsy. Neurol Clin. 2016;34(2):351–61. vii-viii. [DOI] [PubMed]
- 38.Błaszczyk B, Czuczwar SJ. Epilepsy coexisting with depression. Pharmacol Rep. 2016;68(5):1084–92. [DOI] [PubMed] [Google Scholar]
- 39.Gnanavel S. Epilepsy and Depression: a bidirectional relationship. J Neurosci Rural Pract. 2017;8(Suppl 1):S5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Josephson CB, et al. Association of Depression and treated Depression with Epilepsy and Seizure outcomes: a Multicohort Analysis. JAMA Neurol. 2017;74(5):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messina A, et al. Clinical anatomy of the precuneus and pathogenesis of the schizophrenia. Anat Sci Int. 2023;98(4):473–81. [DOI] [PubMed] [Google Scholar]
- 42.Moussavi Z. Repetitive TMS applied to the precuneus stabilizes cognitive status in Alzheimer’s disease. Brain. 2022;145(11):3730–2. [DOI] [PubMed] [Google Scholar]
- 43.Rubart AK, et al. Precuneus connectivity and symptom severity in chronic depression(). Psychiatry Res Neuroimaging. 2022;322:111471. [DOI] [PubMed] [Google Scholar]
- 44.Maller JJ, et al. Occipital bending in depression. Brain. 2014;137(Pt 6):1830–7. [DOI] [PubMed] [Google Scholar]
- 45.Brodovskaya A, Kapur J. Circuits generating secondarily generalized seizures. Epilepsy Behav. 2019;101(Pt B):106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rho JM, Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022;18(6):333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vezzani A, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadzagic-Catibusic F, et al. Effects of Carbamazepine and Valproate on serum aspartate aminotransferase, Alanine Aminotransferase and Gamma - Glutamyltransferase in Children. Med Arch. 2017;71(4):239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu J, et al. Effects of Sodium Valproate Monotherapy on blood liver enzyme levels in patients with Epilepsy: a Meta-analysis. Horm Metab Res. 2021;53(7):425–34. [DOI] [PubMed] [Google Scholar]
- 50.Lippi G, et al. Influence of stable, long-term treatment with phenobarbital on the activity of serum alanine aminotransferase and gamma-glutamyltransferase. Br J Biomed Sci. 2008;65(3):132–5. [DOI] [PubMed] [Google Scholar]
- 51.Vyas MV, et al. Antiepileptic drug use for treatment of epilepsy and dyslipidemia: systematic review. Epilepsy Res. 2015;113:44–67. [DOI] [PubMed] [Google Scholar]
- 52.Schwaninger M, et al. Elevated plasma concentrations of lipoprotein(a) in medicated epileptic patients. J Neurol. 2000;247(9):687–90. [DOI] [PubMed] [Google Scholar]
- 53.Mintzer S, et al. Conversion from enzyme-inducing antiepileptic drugs to topiramate: effects on lipids and C-reactive protein. Epilepsy Res. 2012;98(1):88–93. [DOI] [PubMed] [Google Scholar]
- 54.Gao PY, et al. Associations of liver dysfunction with incident dementia, cognition, and brain structure: a prospective cohort study of 431 699 adults. J Neurochem. 2024;168(1):26–38. [DOI] [PubMed] [Google Scholar]
- 55.Wu M, et al. Non-alcoholic fatty liver disease and stroke: a mendelian randomization study. Eur J Neurol. 2022;29(5):1534–7. [DOI] [PubMed] [Google Scholar]
- 56.Ntona S, et al. Impact of nonalcoholic fatty liver disease-related metabolic state on depression. Neurochem Int. 2023;163:105484. [DOI] [PubMed] [Google Scholar]
- 57.Kroll J. Liver dysfunction and schizophrenia. Lancet. 1965;1(7388):763–4. [DOI] [PubMed] [Google Scholar]
- 58.Mintzer S, Mattson RT. Should enzyme-inducing antiepileptic drugs be considered first-line agents? Epilepsia. 2009;50:42–50. [DOI] [PubMed] [Google Scholar]
- 59.Montgomery MK et al. SMOC1 is a glucose-responsive hepatokine and therapeutic target for glycemic control. Sci Transl Med, 2020. 12(559). [DOI] [PubMed]
- 60.Yang Y, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirvalidze M, et al. The role of glucose in cognition, risk of dementia, and related biomarkers in individuals without type 2 diabetes mellitus or the metabolic syndrome: a systematic review of observational studies. Neurosci Biobehav Rev. 2022;135:104551. [DOI] [PubMed] [Google Scholar]
- 62.Minbay M, et al. Sex-specific associations between circadian-related genes and depression in UK Biobank participants highlight links to glucose metabolism, inflammation and neuroplasticity pathways. Psychiatry Res. 2024;337:115948. [DOI] [PubMed] [Google Scholar]
- 63.Chourpiliadis C, et al. Metabolic Profile and Long-Term risk of Depression, anxiety, and stress-related disorders. JAMA Netw Open. 2024;7(4):e244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conte M, et al. GDF15, an emerging key player in human aging. Ageing Res Rev. 2022;75:101569. [DOI] [PubMed] [Google Scholar]
- 65.Herpich C et al. The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women. Nutrients, 2022. 14(19). [DOI] [PMC free article] [PubMed]
- 66.Tsai VW, et al. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS ONE. 2013;8(2):e55174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kochlik B et al. Associations of circulating GDF15 with combined cognitive frailty and depression in older adults of the MARK-AGE study. Geroscience, 2023. [DOI] [PMC free article] [PubMed]
- 68.Zonneveld MH, et al. Exploring the possible causal effects of cardiac blood biomarkers in dementia and cognitive performance: a mendelian randomization study. Geroscience. 2023;45(6):3165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary data used in this study were obtained from the publicly available UK Biobank, application number 19542. Packages including “survival” and “mediation” in R version 4.2.2 were used to perform Cox regression model and mediation analysis, respectively.