Abstract
Antimicrobial peptides have been evaluated in vitro and in vivo as alternatives to conventional antibiotics. Apart from being antimicrobial, the native human cathelicidin-derived peptide LL-37 (amino acids [aa] 104 to 140 of the human cathelicidin antimicrobial peptide) also binds and neutralizes bacterial lipopolysaccharide (LPS) and might therefore have beneficial effects in the treatment of septic shock. However, clinical trials have been hampered by indications of toxic effects of LL-37 on mammalian cells and evidence that its antimicrobial effects are inhibited by serum. For the present study, LL-37 was compared to two less hydrophobic fragments obtained by N-terminal truncation, named 106 (aa 106 to 140) and 110 (aa 110 to 140), and to a previously described more hydrophobic variant, the 18-mer LLKKK, concerning antimicrobial properties, lipopolysaccharide neutralization, toxicity against human erythrocytes and cultured vascular smooth muscle cells, chemotactic activity, and inhibition by serum. LL-37, fragments 106 and 110, and the 18-mer LLKKK inhibited the growth of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans in a radial diffusion assay, inhibited lipopolysaccharide-induced vascular nitric oxide production, and attracted neutrophil granulocytes similarly. While fragments 106 and 110 caused less hemolysis and DNA fragmentation in cultured cells than did LL-37, the 18-mer LLKKK induced severe hemolysis. The antibacterial effect of fragments 106 and 110 was not affected by serum, while the effect of LL-37 was reduced. We concluded that the removal of N-terminal hydrophobic amino acids from LL-37 decreases its cytotoxicity as well as its inhibition by serum without negatively affecting its antimicrobial or LPS-neutralizing action. Such LL-37-derived peptides may thus be beneficial for the treatment of patients with sepsis.
Sepsis is an infection with bacteria, viruses, or fungi that causes an overwhelming inflammatory host response which can lead to multiple organ dysfunction and, ultimately, death (3). The treatment of this complex condition has been confined to antibiotics, surgery, and the support of failing vital functions. The inflammatory response during sepsis is triggered by bacterial components such as lipopolysaccharides (LPS) released from gram-negative bacteria (3). Thus, agents that are able to limit the effects of LPS may be of clinical benefit in the treatment of gram-negative sepsis. Furthermore, progressive antibiotic resistance in both gram-negative and gram-positive pathogens requires improved targeted therapies.
Antimicrobial peptides have been evaluated in vitro and in some in vivo trials as alternatives to conventional antibiotics. The human cationic antimicrobial protein of 18 kDa (hCAP-18) belongs to the class of cathelicidins. It is released from activated neutrophil granulocytes (10, 23). After release, the 37-amino-acid α-helical C-terminal end is cleaved off, forming the functional antimicrobial peptide LL-37 (6, 23). Apart from being antimicrobial, LL-37 also binds LPS, and it was previously shown that this binding reduces LPS-induced nitric oxide release from the rat aorta (2) and protects mice from LPS lethality (10). LL-37 has also been found to have immunomodulatory and chemotactic activities mediated via the formyl peptide receptor FPRL1 (1, 21, 29).
However, it has been observed that LL-37 causes hemolysis (18) and is toxic to human leukocytes and the T-lymphocyte MOLT cell line (8), probably due to hydrophobic interactions with the eukaryotic cell membrane (18). LL-37 has also been shown to induce apoptosis in vascular smooth muscle cells (2). The cytotoxic effects of LL-37 liberated into the circulation are inhibited by its binding to plasma proteins, e.g., apolipoprotein A-I, but unfortunately, the antimicrobial effects are also inhibited by this binding (8, 24, 28). Thus, the use of native LL-37 to treat septic patients would either not have beneficial effects due to binding of the peptide to plasma proteins or, if the plasma binding capacity were exceeded, be harmful due to the cytotoxicity of the peptide. In fact, the results of a recent study suggested that LL-37 was toxic at high doses when given in an attempt to treat experimental sepsis in neonatal rats (5). The present study was designed to test the hypothesis that the removal of hydrophobic amino acids from the N-terminal end of LL-37 would decrease its cytotoxicity and plasma protein binding, leaving the antimicrobial and LPS-binding capacities unchanged. We also evaluated a previously described variant of LL-37, the 18-mer LLKKK (16), which has enhanced hydrophobicity and cationicity, in this respect.
MATERIALS AND METHODS
Peptides.
Peptides were synthesized by AgriSera AB, Vännäs, Sweden, by 9-fluorenylmethoxy carbonyl chemistry. The purity of the peptides (>95%) was confirmed by mass spectrometry. The amino acid sequences of all peptides used for this study are presented in Table 1.
TABLE 1.
Amino acid sequences of the peptides used for this studya
| Peptide | Amino acid sequence (N terminal to C terminal) |
|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| Fragment 106 | GDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| Fragment 110 | RKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| 18-mer LLKKK | KLFKRIVKRILKFLRKLV |
| BMAP-27 | GRFKRFRKKFKKLFKKLSPVIPLLHL-am |
Note that the bovine cathelicidin BMAP-27 is amidated at the C-terminal end. The underlined amino acids of the 18-mer LLKKK have been changed compared to the native peptide, LL-37.
Antimicrobial testing by radial diffusion assay.
Escherichia coli (4), Pseudomonas aeruginosa (15159), Staphylococcus aureus (F18), and Candida albicans (ATCC 90028) isolates were grown for 18 h at 37°C in 10 ml (3% [wt/vol]) of Trypticase soy broth (TSB; Becton Dickinson Europe). To obtain mid-logarithmic-phase organisms, we inoculated 200 μl of each culture into 10 ml of fresh TSB and incubated it for an additional 2 h (except for P. aeruginosa, which was grown overnight) at 37°C. The bacteria were centrifuged at 900 × g for 10 min and washed once, followed by resuspension in 10 ml of cold 10 mM Tris buffer (pH 7.4). The optical density of the solution was measured at 620 nm. A radial diffusion assay was performed as follows and as described previously (12). One percent (wt/vol) low-electroendosmosis-type agarose (Sigma-Aldrich, St. Louis, Mo.), with or without 150 mM NaCl and a final concentration of 0.02% (vol/vol) Tween 20 (Sigma-Aldrich) in 0.05% TSB, was brought to ebullition, cooled to 50°C, mixed with a bacterial suspension (4 × 106 CFU in 5 ml for all bacteria, except C. albicans, for which 3.3 × 106 CFU was used), and poured into a 10-cm petri dish. A series of wells (4-mm diameter) were punched in the plate after the agarose had solidified. Six microliters of peptide sample, dissolved and diluted in sterile distilled water to a concentration of 0, 0.5, 1, 2.5, 5, 10, 20, or 40 μM, was applied to each well, and the plates were incubated for 3 h at 37°C. An overlay agar composed of 6% TSB and 0.5% (wt/vol) low-electroendosmosis-type agarose was then poured over each plate, and the plates were incubated upside down for 18 h in 37°C to allow for visible growth of bacterial colonies. Antibacterial activity was indicated by a clear zone corresponding to a lack of bacterial growth around the well. The diameter of the clear zone surrounding the wells was measured with a metric scale scribed in 0.1-mm increments. The gels were stained with a Coomassie brilliant blue solution containing 2 mg Coomassie blue R-250 (Merck, Darmstadt, Germany), 27 ml of methanol, and 15 ml of 37% formaldehyde (Sigma-Aldrich) in 63 ml of water for 24 h. The staining solution was replaced with distilled water, and the gels were washed for 24 h and dried for permanent recording of the results.
Measurement of nitric oxide production from rat aortas.
The Institutional Review Board for the Care of Animal Subjects approved this study, and the care and handling of animals were done in accordance with National Institutes of Health guidelines. Seven male Sprague-Dawley rats (250 g of body weight) were anesthetized with isoflurane (Abbott Scandinavia, Solna, Sweden) and bled to death. The thoracic aorta was removed, cleaned of adherent fat, and cut into 3-mm-long cylindrical segments. The segments were incubated for 24 h at 37°C, with or without LPS (1 ng ml−1; from E. coli strain O111:B4) (Difco Laboratories, Detroit, Mich.) and together with either LL-37, fragment 106, fragment 110, the 18-mer LLKKK, BMAP-27, or the classical LPS binder polymyxin B (0, 0.2, and 2 μM), in 1 ml of Dulbecco's modified Eagle's medium without phenol red (DMEM, Gibco, N.Y.) saturated with a gas mixture containing 8% CO2 in oxygen. The DMEM contained l-arginine (1 mM) (4), penicillin (2,000 U ml−1), and streptomycin (0.2 mg ml−1) (all from Sigma-Aldrich). After incubation, the aorta segments were removed, briefly blotted on a paper cloth, and weighed. NO is rapidly oxidized to nitrate and nitrite (9). NO release from the segments was therefore reflected in the accumulation of nitrate and nitrite in the incubation medium. The incubation medium was centrifuged at 11,000 × g for 5 min at room temperature. All nitrate in 100 μl of the supernatant was reduced to nitrite with nitrate reductase (2 mU) and NADPH (20 nmol) (both from Sigma-Aldrich) in 70 μl of 20 mM potassium phosphate buffer (pH 7.40) containing glucose-6-phosphate dehydrogenase (8 mU) and glucose-6-phosphate (40 nmol) (both from Sigma-Aldrich) for 2 h at room temperature (2). After reduction, 100 μl of Griess reagent (40 mg ml−1; Sigma-Aldrich) was added and the optical density was measured at 550 nm. Standard curves were constructed by analyzing DMEM with known concentrations of sodium nitrate (Sigma-Aldrich). To exclude any direct interference of the peptides with the assay, we added the peptides (2 μM) to DMEM with a 1.5 μM nitrate standard in separate experiments. The assay was performed as described above, and no interference was detected.
Hemolysis assay.
Blood was drawn from the antecubital veins of seven healthy donors into plastic tubes containing EDTA (2 mg ml−1). After centrifugation at 800 × g for 10 min, the plasma and buffy coat were removed. The erythrocytes were rinsed three times by centrifugation for 10 min at 800 × g and resuspension in 5% (vol/vol) phosphate-buffered saline (pH 7.4). Next, 400 μl of the erythrocyte suspension was incubated for 1 h at 37°C with gentle end-over-end rotation in the presence of LL-37, fragment 106 or 110, the 18-mer LLKKK, or BMAP-27 at a concentration of either 0 (negative control), 0.6, 2, 6, 20, or 60 μM. Triton X-100 at 2% (Sigma-Aldrich) served as a positive control. After incubation, the samples were centrifuged at 800 × g for 10 min. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540 nm and is expressed as a percentage of the value for Triton X-100-induced hemolysis.
DNA fragmentation assay.
Confluent human aortic vascular smooth muscle cells (CC-2571; BioWhittaker, Walkersville, Md.) from passages 5 to 7 were cultured in 24-well plates and incubated in serum-free DMEM for 16 h in the absence (control) or presence of either LL-37, fragment 106, or fragment 110 at a concentration of either 2, 6, or 20 μM. Internucleosomal DNA fragmentation was measured by use of a Cell Death Detection ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. In short, the cells were lysed in the culture wells and the DNA fragments in the lysate were bound to a microtiter plate coated with monoclonal antihistone antibodies. The bound DNA fragments were then detected with peroxidase-conjugated monoclonal anti-DNA antibodies and 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]. The optical density was measured at 415 nm and is expressed as the increase (fold) in absorbance over that of untreated controls.
Chemotaxis assay.
Twenty milliliters of blood was drawn from the antecubital veins of four healthy donors into plastic tubes containing EDTA (2 mg ml−1). Polymorphonuclear cells were isolated by centrifugation over Polymorphprep (Axis-Shield PoC, Oslo, Norway) according to the manufacturer's instructions. The cells were washed and resuspended in RPMI 1640 containing l-glutamine (Gibco) to achieve 2 × 106 cells ml−1. LL-37, fragments 106 and 110, the 18-mer LLKKK (all diluted in RPMI to 0.01, 0.1, 1, or 10 μM), and N-formyl-Met-Leu-Phe (fMLP; 0.1 μM) (Sigma-Aldrich) were added to the lower wells of a 48-well microchemotaxis chamber (AP48; Neuro Probe Inc., Gaithersburg, Md.). Fifty microliters of the cell suspension was added to the upper chamber, which was separated from the lower chamber by a polycarbonate membrane with 5-μm pores. The chamber was incubated for 1 h at 37°C in a humidified gas mixture containing 5% CO2 in air. After incubation, cells that had not migrated were wiped off the upper face of the membrane. After fixation in methanol and drying, the membrane was stained with MGG quick stain (Bio-Optica, Milan, Italy) according to the manufacturer's instructions, dried, and mounted under coverslips on microscope slides by using Pertex medium (Histolab Products AB, Gothenburg, Sweden). The number of transmigrated cells was counted in at least three 0.03-mm2 fields with a light microscope at a magnification of ×1,000 and is expressed as the number of cells per mm2.
Effects of serum on antimicrobial activity.
Blood was drawn from the antecubital veins of five healthy donors into glass tubes without additives and left to coagulate for 1 hour at room temperature. Sera were collected after centrifugation for 10 min at 2,000 × g. LL-37 or fragment 106 or 110 was diluted in sterile distilled water. Serum was added to achieve a peptide concentration of 20 μM in 0, 40, or 99% serum. The peptide-serum mixtures were applied to the wells of a plate in a radial diffusion assay using E. coli (4, 37), as described above.
Statistics.
When a peptide was found to be active against the pathogen in the radial diffusion assay, the diameter of the wells was subtracted from the diameters of the clear zones. For experiments with several peptide concentrations, the resulting values were plotted against the log10 peptide concentration, and a linear relationship was always found. The x-axis intercept of the regression line corresponds to the log10 minimal effective concentration, which was calculated with linear regression software (Sigma Plot 8.0; SPSS Inc., Chicago, Ill.). The minimal effective concentration was used as an estimation of antimicrobial potency. One- or two-way repeated-measurement analysis of variance (ANOVA), followed by post hoc testing using the Holm-Sidak method when appropriate, and Student's paired t test were used as indicated in the figure legends. Significance was accepted at P values of <0.05. The data are reported as means ± standard errors of the means (SEM). “n” equals the number of independent experiments for experiments with cultured prokaryotic or eukaryotic cells or the number of rats or humans.
RESULTS
Radial diffusion assay.
All of the peptides tested were antimicrobial. The minimal effective concentration values are presented in Table 2. The minimal effective concentration values for E. coli, P. aeruginosa, and S. aureus at low salt concentrations were similar between the different peptides, while the shorter fragments 106 and 110 as well as the 18-mer LLKKK and the bovine cathelicidin BMAP-27 were more active against C. albicans than was LL-37. It has been found that the antimicrobial activity of LL-37 against gram-positive bacteria and C. albicans is reduced at physiological salt concentrations (26, 27). We found that the presence of NaCl at 150 mM did not affect the antimicrobial activities of the peptides for E. coli and P. aeruginosa to any major extent, with the only exception being LL-37, which appeared to lose some of its potency against E. coli. NaCl reduced the activity against S. aureus for LL-37 and fragments 106 and 110. In the presence of 150 mM NaCl, all of the peptides lost their activity against C. albicans at the concentrations tested. We concluded that the N-terminal truncation of LL-37 does not affect its antimicrobial activity negatively.
TABLE 2.
Minimal effective concentrations obtained by a radial diffusion assay for LL-37, fragments 106 and 110, the 18-mer LLKKK, and BMAP-27 for gram-negative and -positive bacteria and C. albicansa
| Pathogen | NaCl concn (mM) | Minimal effective concn (μM)
|
||||
|---|---|---|---|---|---|---|
| LL-37 | Fr 106 | Fr 110 | 18-mer | BMAP-27 | ||
| E. coli | 0 | 0.26 | 0.40 | 0.33 | 0.33 | 0.14 |
| 150 | 1.2 | 0.42 | 0.47 | 0.66 | 0.50 | |
| P. aeruginosa | 0 | 0.18 | 0.26 | 0.18 | 0.29 | 0.20 |
| 150 | 0.19 | 0.22 | 0.15 | 0.06 | 0.10 | |
| S. aureus | 0 | 1.5 | 0.86 | 0.8 | 1.2 | 0.67 |
| 150 | 4.6 | 2.6 | 2.4 | 0.39 | 0.45 | |
| C. albicans | 0 | 10 | 5.1 | 2.4 | 2.2 | 0.36 |
| 150 | >40 | >40 | >40 | >40 | >40 | |
The values were determined at a low (0 mM) or physiological (150 mM) concentration of NaCl. Each value is based on data from three independent experiments.
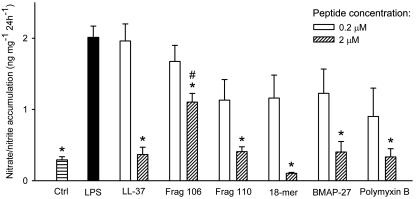
Nitrate/nitrite accumulation.
Next, we wanted to see if the peptides could inhibit LPS-induced NO production in isolated rat aortas, leading to a subsequent decrease in the accumulation of nitrate/nitrite in the incubation medium. LPS induced a more than fivefold increase in nitrate/nitrite accumulation (Fig. 1). All peptides tested inhibited nitrate/nitrite accumulation with similar potencies, except fragment 106, which at 2 μM was significantly less efficient than LL-37. The incubation of aortic segments with the peptides in the absence of LPS did not affect the baseline nitrate/nitrite accumulation (not shown). This suggests that the peptides do not have any proinflammatory effects in this model. We concluded that the N-terminal truncation of LL-37 does not affect LPS binding and neutralization to any major extent.
FIG. 1.
Production of nitrate/nitrite in segments of rat aortas during 24 h of incubation, as measured with Griess reagent. LPS alone (filled bar) increased the amount of nitrate/nitrite production compared to the control (Ctrl). Compared to that induced by LPS alone, the production of nitrate/nitrite was lower in segments coincubated with the peptides at 2 μM (*). However, fragment 106 was less effective at inhibiting nitrate/nitrate production than LL-37 at 2 μM (#). The data were analyzed by one-way repeated-measurement ANOVA followed by post hoc testing by the Holm-Sidak method (P < 0.05). Values are means + SEM (n = 7).
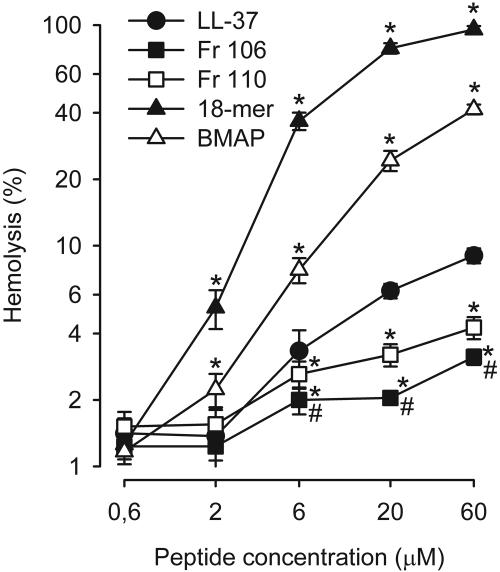
Hemolysis.
All peptides induced a concentration-dependent hemolysis (Fig. 2). The hemolysis induced by the 18-mer LLKKK and BMAP-27 was significantly more pronounced than that induced by LL-37, indicating the severe cytotoxicity of these peptides. Fragments 106 and 110 caused significantly less hemolysis than LL-37. Fragment 106 was found to be the least cytotoxic peptide to erythrocytes.
FIG. 2.
Concentration-response curves of the hemolytic activities of the peptides towards human erythrocytes. The 18-mer LLKKK (▴) and BMAP-27 (▵) induced significantly more (*) hemolysis, while fragments 106 (▪) and 110 (□) induced significantly less hemolysis, than LL-37 (•). Fragment 106 induced significantly less hemolysis than fragment 110 (#). The data were analyzed by two-way repeated-measurement ANOVA for the factors of different peptides and peptide concentrations followed by post hoc testing by the Holm-Sidak method (P < 0.05). Values are means ± SEM (n = 7). Note the log scales on both the x and y axes.
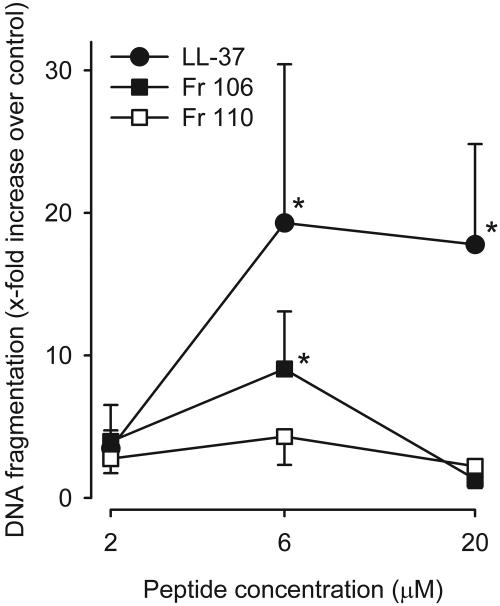
DNA fragmentation.
Having ruled out the 18-mer LLKKK and BMAP-27 as potential therapeutic agents due to their severe, rapidly developing cytotoxicities, we compared the toxicities of the remaining fragments, 106 and 110, with that of LL-37 after a longer exposure time. As shown in Fig. 3, LL-37 at concentrations of 6 and 20 μM induced significant DNA fragmentation in human vascular smooth muscle cells compared to the control, confirming the previously found toxicity at these concentrations (2). Fragment 106 also induced significant DNA fragmentation, while fragment 110 did not. Thus, the removal of the N-terminal hydrophobic amino acids reduces the cytotoxicity of LL-37.
FIG. 3.
DNA fragmentation in cultured human vascular smooth muscle cells after 16 h of incubation with LL-37 (•) or fragment 106 (▪) or 110 (□). LL-37 at 6 and 20 μM as well as fragment 106 at 6 μM induced significant DNA fragmentation compared to the control (*). The data were analyzed by two-way repeated-measurement ANOVA for the factors of different peptides and peptide concentrations followed by post hoc testing by the Holm-Sidak method (P < 0.05). DNA fragmentation is expressed as the fold increase in absorbance over that of the control. Values are means ± SEM (n = 8).
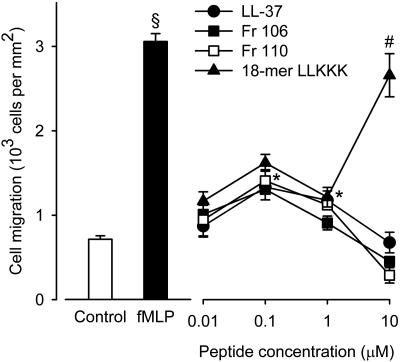
Chemotactic activity.
LL-37 and its N-terminally truncated analogs, fragments 106 and 110, all displayed similar concentration-dependent chemotactic activities on granulocytes (Fig. 4). The concentration-response curves were biphasic, with a maximum effect at 0.1 μM amounting to nearly half that achieved with the classical chemoattractant formyl peptide, fMLP, which was used as a positive control at a single concentration of 0.1 μM. The concentration-response curve for the 18-mer LLKKK was similar to that for LL-37 at concentrations up to 1 μM. At 10 μM, there was a pronounced increase in chemotactic activity, probably due to a loss of selectivity of this compound resulting in an agonistic activity on chemoattractant receptors other than FPRL1.
FIG. 4.
Chemotaxis of human neutrophils in response to LL-37, fragments 106 and 110, the 18-mer LLKKK, and the classical chemoattractant formyl peptide, fMLP. fMLP (0.1 μM) displayed a statistically significant chemoattractant activity (filled bar, §) compared to the control (open bar) (Student's paired t test; P < 0.05). The concentration-response curve for LL-37 was biphasic, with statistically significant chemoattractant activities at 0.1 and 1 μM but not at 10 μM (•,*). The concentration-response curves for fragments 106 (▪) and 110 (□) as well as that for the 18-mer LLKKK (▴) were similar to that of LL-37, except that at 10 μM, the 18-mer LLKKK induced a larger chemotactic response than that induced by LL-37 (#). The data were analyzed by two-way repeated-measurement ANOVA for the factors of different peptides and peptide concentrations followed by post hoc testing by the Holm-Sidak method (P < 0.05). Values are means ± SEM (n = 4).
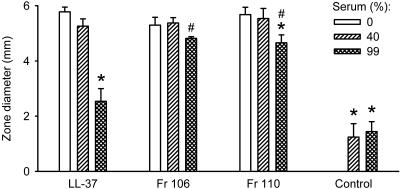
Effect of serum on antibacterial activity.
LL-37 and fragments 106 and 110 inhibited the growth of E. coli to the same extent as indicated by the similar sizes of the clear zones around the wells containing the peptides (Fig. 5). Although serum alone was weakly antibacterial, it markedly decreased the antibacterial activity of LL-37 and, to a significantly smaller extent, the activity of fragment 110, while the activity of fragment 106 was not affected. These results indicate that the N-terminal truncation of LL-37 decreases its binding and neutralization by plasma proteins.
FIG. 5.
Inhibition of antibacterial activity by serum, as assessed by a radial diffusion assay using E. coli. In the absence of serum (open bars), LL-37 and fragments 106 and 110 (all at 20 μM) inhibited bacterial growth to the same extent. Although serum alone was weakly antibacterial (*), it markedly decreased the antibacterial activity of LL-37 and, to a smaller extent, the activity of fragment 110 (*). The activity of fragment 106 was not significantly affected. The data were analyzed by two-way repeated-measurement ANOVA for the factors of different peptides and serum concentrations followed by post hoc testing by the Holm-Sidak method (P < 0.05). *, statistically significantly different from the value in the absence of serum; #, statistically significantly different from the value for LL-37 in the presence of serum at the same concentration. Values are means + SEM (n = 5).
DISCUSSION
We tested the antimicrobial effects of LL-37 and derivative peptides with some pathogens known to cause sepsis. The antimicrobial potency of LL-37 found in the present study was within the range that was previously reported (11, 15, 17, 26, 27). LL-37 was also found to bind and neutralize the effects of LPS as described in the literature, with a potency similar to that of the well-characterized LPS-binder polymyxin B (2, 10, 20, 27). However, we also confirmed earlier observations of short- and long-term cytotoxic effects of the peptide (2, 8, 18). This precludes any therapeutic use of native LL-37. LL-37 has an abundance of hydrophobic amino acids at its N terminus. Oren and colleagues found that the removal of four N-terminal amino acids from LL-37 reduces its hemolytic activity (18). A corresponding removal of the hydrophobic C-terminal tails of the bovine cathelicidins BMAP-27 and BMAP-28 also markedly reduced their hemolytic activities (22). We chose to reduce the hydrophobicity of LL-37 in a stepwise fashion by removing the first two N-terminal leucines (fragment 106) and the first six amino acids, including two leucines, one glycine, and two phenylalanines (fragment 110). This N-terminal truncation did not seem to reduce the antimicrobial potency of LL-37 at a low or physiological salt concentration. Interestingly, it was recently found that a peptide identical to fragment 110 is formed naturally on the skin, where it may protect against microbial colonization (15). The N-terminal truncation of LL-37 did not eliminate its ability to inhibit LPS-induced NO production in the rat aorta, a model which is relevant to gram-negative septic shock. In these experiments, fragment 110 was equipotent with LL-37.
As expected, fragments 106 and 110 were less cytotoxic to human cells than the parent peptide LL-37. Interestingly, this did not seem to be due to a reduction of nonspecific toxicity to eukaryotic cells, since truncation in fact increased the suppressive effect on the growth of C. albicans. This suggests that the mechanisms underlying the antifungal action of LL-37-derived peptides are complex and require further investigation. We found that the activities of all of the peptides against C. albicans decreased profoundly in the presence of a physiological salt concentration. The present results do not provide any explanation for this. It has previously been demonstrated that the antibacterial activity of LL-37 correlates with the formation of a helical structure of the molecule, which in turn is affected by the presence of anions such as Cl− (8). It seems reasonable to assume that the salt-dependent loss of activity against C. albicans is also due to conformational changes of the peptide molecules.
Nagaoka and colleagues have shown that peptides derived from the structure of the middle portion of the LL-37 molecule retain the LPS-neutralizing ability (16). Of several such peptides tested, a variant with an increased hydrophobicity and positive charge, named the 18-mer LLKKK, was found to be the most powerful at protecting mice from a lethal dose of endotoxin and was put forward as a candidate drug for the treatment of gram-negative endotoxic shock. Our results show that the antimicrobial effects of this peptide compared to those of LL-37 are retained as well. However, although it was reported to not be toxic to the murine macrophage cell line RAW 264.7 at concentrations below 4 μM (16), we found it to be far more hemolytic than LL-37 itself already at 2 μM.
The bovine cathelicidin BMAP-27 was included in this study as a positive control for cathelicidin-induced cytotoxicity (19, 22). We found it to be a potent LPS neutralizer, which to our knowledge has not been reported before. However, due to its cytotoxicity, confirmed by the present results, it would not be suitable for the treatment of endotoxemia.
The chemotactic function of neutrophil granulocytes is decreased in septic patients, but the pathophysiological implications of this remain to be elucidated (25). However, it seems reasonable to assume that the migration of immune cells into infectious foci is decreased, thereby impairing the clearance of the underlying infection. Infusion of the chemoattractant interleukin-8 or fMLP into rabbits causes a loss of the ability of neutrophil granulocytes to migrate into tissues, partly due to an inhibition of adhesion to the endothelium (7, 13). Furthermore, the pretreatment of neutrophil granulocytes with chemotactic mediators decreases their migration through endothelial monolayers in vitro (14). We found that the chemotactic activity of LL-37 was not affected by N-terminal truncation. This indicates that the N-terminally truncated analogs of LL-37 are chemoattractant receptor agonists, and it cannot be excluded that they will, to some degree, inhibit the chemotactic function of neutrophil granulocytes when given as a systemic treatment to sepsis patients. Further studies are needed to identify the chemotactic domains of the LL-37 analogs and to explore the effects of amino acid substitutions in this region on their chemotactic activity.
It has been demonstrated that LL-37 binds to the plasma protein apolipoprotein A-I and that this binding inhibits its antimicrobial and cytotoxic effects (28). We found that serum did not inhibit the antimicrobial action of LL-37 after the removal of hydrophobic N-terminal amino acids. This suggests that these amino acids are important for binding to apolipoprotein A-I, probably due to the hydrophobic nature of the binding, as suggested by Sørensen and colleagues (24). The fact that the actions of fragments 106 and 110 were only marginally inhibited by serum must be regarded as fundamental if they are to be used as a systemic treatment for sepsis patients.
In conclusion, the removal of N-terminal hydrophobic amino acids from LL-37 decreases its cytotoxicity for human cells as well as its inhibition by serum without negatively affecting its antimicrobial or LPS-neutralizing action. While fragment 110 inhibits bacterial growth and neutralizes LPS at least as effectively as LL-37, it is significantly less hemolytic and cytotoxic in the long term. We believe that our results will facilitate the development of novel peptide-based strategies for the treatment of sepsis.
Acknowledgments
This work was supported by Swedish Research Council grants 2002-6270, 2004-3874, and 13471, by the Medical Faculty of Lund University, by Lund University Hospital Research funds, by the Region Skåne Research Council, and by the Royal Physiographical Society.
We thank Emma Andersson Nordahl and Adrian Ionescu for scientific discussions and advice regarding the radial diffusion assay and the staining of cells, respectively, and Pia Andersson and Tyberiusz Moska for their excellent technical assistance.
REFERENCES
- 1.Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jörnvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. [PubMed] [Google Scholar]
- 2.Ciornei, C. D., A. Egesten, and M. Bodelsson. 2003. Effects of human cathelicidin antimicrobial peptide LL-37 on lipopolysaccharide-induced nitric oxide release from rat aorta in vitro. Acta Anaesthesiol. Scand. 47:213-220. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 4.Fleming, I., G. A. Gray, G. Julou-Schaeffer, J. R. Parratt, and J. C. Stoclet. 1990. Incubation with endotoxin activates the l-arginine pathway in vascular tissue. Biochem. Biophys. Res. Commun. 171:562-568. [DOI] [PubMed] [Google Scholar]
- 5.Fukomoto, K., I. Nagaoka, A. Yamataka, H. Kobayashi, T. Yanai, Y. Kato, and T. Miyano. 2005. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr. Surg. Int. 121:20-24. [DOI] [PubMed] [Google Scholar]
- 6.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 7.Hechtman, D. H., M. I. Cybulsky, H. J. Fuchs, J. B. Baker, and M. A. Gimbrone, Jr. 1991. Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J. Immunol. 147:883-892. [PubMed] [Google Scholar]
- 8.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 9.Kelm, M. 1999. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta 1411:273-289. [DOI] [PubMed] [Google Scholar]
- 10.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larrick, J. W., M. Hirata, J. Zhong, and S. C. Wright. 1995. Anti-microbial activity of human CAP18 peptides. Immunotechnology 1:65-72. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer, R. I., M. Rosenman, S. S. S. L. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 13.Ley, K., J. B. Baker, M. I. Cybulsky, M. A. Gimbrone, Jr., and F. W. Luscinskas. 1993. Intravenous interleukin-8 inhibits granulocyte emigration from rabbit mesenteric venules without altering L-selectin expression or leukocyte rolling. J. Immunol. 151:6347-6357. [PubMed] [Google Scholar]
- 14.Luu, N. T., G. E. Rainger, and G. B. Nash. 2000. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J. Immunol. 164:5961-5969. [DOI] [PubMed] [Google Scholar]
- 15.Murakami, M., B. Lopez-Garcia, M. Braff, R. A. Dorschner, and R. L. Gallo. 2004. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 172:3070-3077. [DOI] [PubMed] [Google Scholar]
- 16.Nagaoka, I., S. Hirota, F. Niyonsaba, M. Hirata, Y. Adachi, H. Tamura, S. Tanaka, and D. Heumann. 2002. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin. Diagn. Lab. Immunol. 9:972-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 18.Oren, Z., J. C. Lerman, G. H. Gudmundsson, B. Agerberth, and Y. Shai. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 19.Risso, A., M. Zanetti, and R. Gennaro. 1998. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell. Immunol. 189:107-115. [DOI] [PubMed] [Google Scholar]
- 20.Schindler, M., and M. J. Osborn. 1979. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry 18:4425-4430. [DOI] [PubMed] [Google Scholar]
- 21.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 22.Skerlavaj, B., R. Gennaro, L. Bagella, L. Merluzzi, A. Risso, and M. Zanetti. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375-28381. [DOI] [PubMed] [Google Scholar]
- 23.Sørensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 24.Sørensen, O., T. Bratt, A. H. Johnsen, M. T. Madsen, and N. Borregaard. 1999. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J. Biol. Chem. 274:22445-22451. [DOI] [PubMed] [Google Scholar]
- 25.Tavares-Murta, B. M., M. Zaparoli, R. B. Ferreira, M. L. Silva-Vergara, C. H. B. Oliveira, E. F. C. Murta, S. H. Ferreira, and F. Q. Cunha. 2002. Failure of neutrophil chemotactic function in septic patients. Crit. Care. Med. 30:1056-1061. [DOI] [PubMed] [Google Scholar]
- 26.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, Jr., R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Y., B. Agerberth, A. Löthgren, A. Almstedt, and J. Johansson. 1998. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 273:33115-33118. [DOI] [PubMed] [Google Scholar]
- 29.Yang, D., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]