Abstract
Background
Spontaneous pneumomediastinum (SPM) and subcutaneous emphysema (SE) are rare, severe, and potentially life-threatening complications associated with asthma exacerbation. Most of these conditions are benign and self-limiting. However, the overlapping symptoms between asthma exacerbation and pneumomediastinum (PM) may delay diagnosis. These conditions can usually be managed through conservative treatment, although unfamiliarity with this presentation may lead some physicians to consider surgical intervention.
Case presentation
We report a unique case involving a 9-year-old patient experiencing a severe bronchial asthma attack and right lobe atelectasis complicated by PM and severe SE that extended to his left eye. The condition was successfully treated conservatively, with aggressive management of asthma exacerbation and close monitoring in the intensive care unit.
Conclusion
This case highlights the effectiveness of conservative management of PM and SE with appropriate asthma exacerbation treatment. Early diagnosis and management can lead to a favorable prognosis and a relatively brief hospital stay.
Clinical trial number
Not applicable.
Keywords: Pneumomediastinum, Subcutaneous emphysema, Spontaneous pneumomediastinum, Bronchial asthma, Children
Introduction
Spontaneous pneumomediastinum (SPM) and subcutaneous emphysema (SE) are rare complications of asthma exacerbation that can become life-threatening if not recognized early and properly managed. These conditions are uncommon in children and are typically associated with obstructive lung diseases and lower airway infections. Bronchial asthma is a well-known risk factor for SPM, with studies indicating that 34% of patients with SPM have a history of bronchial asthma, and 68% exhibit wheezing at the time of presentation. The most common clinical symptoms include acute substernal chest pain, cough, dyspnea, and facial swelling. Diagnosis may be delayed because of overlapping symptoms of asthma exacerbation and pneumomediastinum (PM) [1]. Most cases are resolved with conservative, supportive management without major issues, as these conditions resolve on their own and tend to follow a benign course [2]. However, early recognition remains important to prevent rare, severe complications where invasive procedures may be considered. This case report describes a 9-year-old boy who developed severe PM and SE that extended to his left eye as a complication of acute asthma exacerbation. The patient responded well to supportive management and treatment for bronchial asthma.
Case presentation
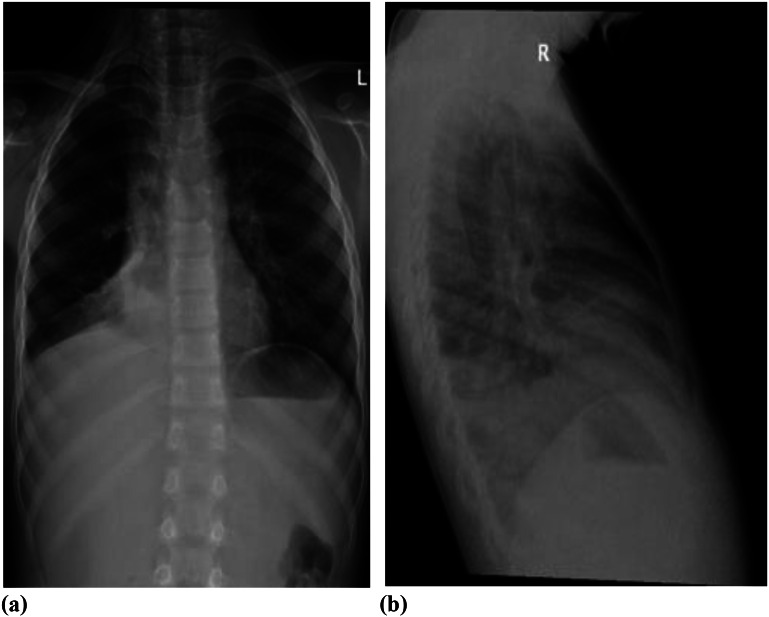
A nine-year-old boy who was diagnosed with bronchial asthma was treated with a regular fluticasone metered-dose inhaler (MDI) and PRN salbutamol MDI at home. He presented to the Emergency Department (ED) with symptoms of a dry cough and difficulty breathing, which began two days before admission. He had also experienced one episode of vomiting. An initial chest X-ray showed right lower lobe collapse, air trapping, and very mild SE, as illustrated in Fig. 1.
Fig. 1.
a & b: right lower lobe collapse, air-trapping and very mild subcutaneous emphysema
He was started on salbutamol and ipratropium bromide nebulization and systemic corticosteroids. Subsequently, his condition worsened, and he began to experience intense chest pain. Swelling then appeared in the upper chest and upper arms, spreading rapidly to his face, left eye, and neck.
The patient was in respiratory distress with tachypnea (respiratory rate of 44/min) and tachycardia (heart rate of 147/min). His oxygen saturation was 85% in room air, his blood pressure was 116/66 mm Hg, and his body temperature was 38 °C. A chest examination revealed suprasternal and intercostal recessions, with palpable crepitus across the chest, reduced air entry bilaterally, and inspiratory and expiratory wheezing. Mild epigastric tenderness was also noted. Other systemic examinations were unremarkable.
The laboratory workup revealed a venous blood gas pH of 7.41, a PCO2 of 32 mm Hg, a PaO2 of 53 mm Hg, and an HCO3 of 22 mEq/L. A complete blood count revealed a white blood cell (WBC) count of 13.4 × 103/ml (lymphocytes 2%, neutrophils 96.6%), a red blood cell (RBC) count of 5.06 × 105/ml, hemoglobin of 13.3 g/dl, hematocrit of 37.1%, and platelet count of 293 × 103/ml. Renal and liver function tests were within normal ranges. A chest X-ray (Fig. 2) revealed right lower lobe atelectasis with obscuration of the right heart border and extensive SE from the chest wall to the neck and arms.
Fig. 2.
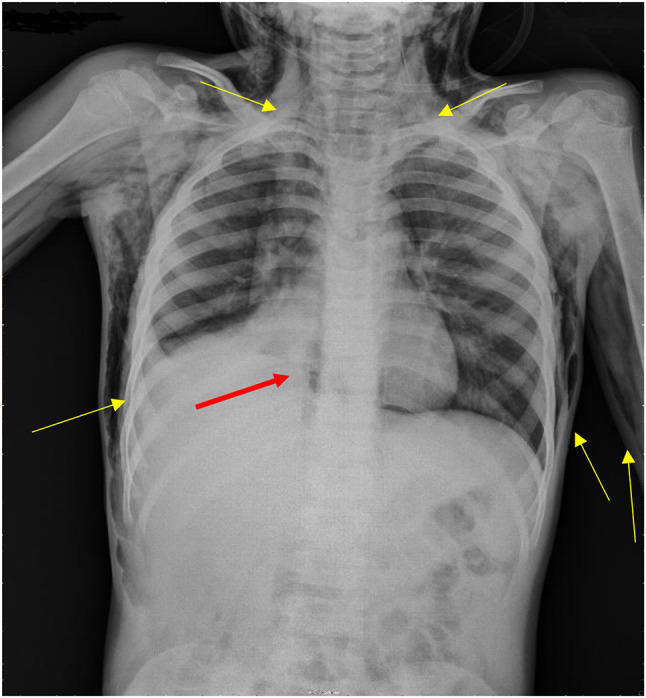
Right lower lobe atelectasis with obscuration of the right heart border (red arrow), extensive bilateral soft-tissue subcutaneous emphysema (yellow arrows)
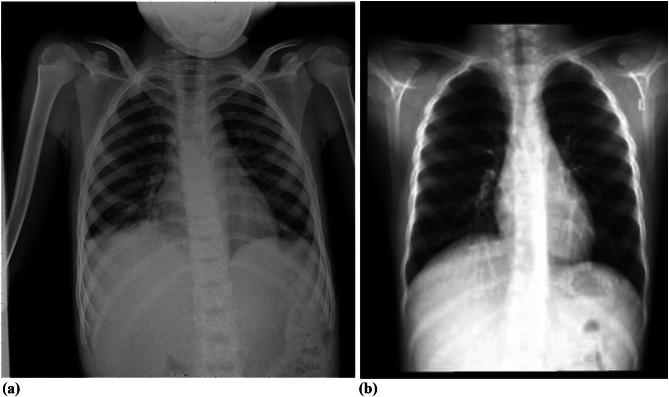
The patient was admitted to the Pediatric Intensive Care Unit (PICU) because of a severe asthma attack and SE. He received treatment that included salbutamol, ipratropium bromide nebulizers, magnesium sulfate, parenteral methylprednisolone, and intravenous antibiotics. Additionally, given poor air entry and poor response to nebulized bronchodilators, an intravenous salbutamol infusion was started. He was kept nil per os (NPO) and received intravenous fluid. The magnesium sulfate was discontinued due to hypotension, and the salbutamol infusion dose was subsequently increased. SE was treated conservatively with a high percentage of inspired oxygen therapy and analgesia for chest pain. On the 3rd day of PICU admission, swelling decreased, and oxygen levels were gradually tapered over the next three days. The salbutamol infusion was discontinued on the fourth day, and oral feeding was resumed. On the 5th day, SE was markedly reduced, and the patient was transferred to the general ward. He was discharged four days later and advised to avoid activities that could increase intrathoracic pressure, such as lifting heavy objects, using the Valsalva maneuver, blowing against resistance, and participating in competitive sports. The patient was closely monitored after discharge from the hospital until he had complete resolution of his SE (follow-up serial chest X-rays, Fig. 3).
Fig. 3.
a and b: Follow-up serial chest X-rays showed a complete resolution of subcutaneous emphysema
Discussion
Asthma exacerbation is rarely complicated by serious complications that can deteriorate the clinical condition of patients and become life-threatening, including PM, SE, and pneumothorax. This report presents the case of a male child with bronchial asthma exacerbation and atelectasis in the right lower lobe who developed extensive SE that threatened his life but was treated successfully and managed with conservative treatment.
Air leak syndrome is a group of diseases that include SE, PM, pneumopericardium (PP), and pulmonary interstitial emphysema. PM is defined by the presence of air that leakage to mediastinal space from various potential sites, including the lung, esophagus, trachea, and neck [3]. The etiology can be divided into two types: SPM and secondary (or traumatic) PM. The definition of SPM is controversial because of variations in the underlying pulmonary pathology in affected patients [4]. However, it is generally defined as air in the mediastinum that occurs without any traumatic or iatrogenic causes. SPM can be subdivided into either primary, occurring in previously healthy individuals without underlying disease, or secondary, associated with any predisposing pulmonary diseases such as asthma, viral respiratory infections, or pneumonia [1, 5].
SPM is described as a benign condition occurring in older children and young adults due to a sudden pressure change within the intrathoracic cavity [6]. Its pathogenesis is linked to the rupture of alveoli from an abrupt increase in intra-alveolar pressure, allowing air to escape into the pulmonary interstitial spaces. The air then dissects along the bronchovascular sheath. Subsequently, it enters the mediastinum (which is characterized by negative pressure compared with that in the lung parenchyma), as first reported by Macklin [4, 7]. As a result, PM and pneumothorax may occur. If the air extends to the subcutaneous tissue, SE will occur in addition to retropharyngeal and, in rare cases, retroperitoneal. It may reach the epidural space, causing pneumorrhachis [8, 9].
SE, which is the extension of air into subcutaneous tissues, tends to decompress the mediastinum and relieve any potential mediastinal circulatory disturbances [10]. During an acute asthma attack, the marked increase in intra-alveolar pressure is caused by partial or complete mechanical airway obstruction. In addition, atelectasis due to mucous or pneumonic reactions secondary to infection may play a role. Moreover, atelectasis is often associated with an area of compensatory overdistention, and it may be possible for air leakage to occur from a comparative area of compensatory alveolar ectasia [11].
The incidence of PM varies widely, likely due to varying referral patterns and diagnostic criteria. The reported incidence in previous studies ranged between 0.3% and 5% [2]. It is more commonly observed in thin, young males. Nevertheless, there are reports of SPM that have been described in children as young as five months, with no difference in incidence between sexes. Notably, patients with PM related to respiratory issues tend to be younger [1, 8, 12, 13].
Bronchial asthma is known to be associated with PM; among 20,000 patients who visit the ED with acute exacerbation of bronchial asthma, one has PM [8]. Patients with PM resulting from asthma attacks often have different clinical courses and prognoses than those with irreversible respiratory disease do [5]. Airway obstruction, atelectasis, coughing and concurrent infection may lead to SPM in asthmatic patients. The most common clinical symptom is acute substernal pleuritic chest pain, which is exacerbated during deep inspiration and may radiate to the neck, shoulders, or arms. Other symptoms include dyspnea, cough, facial swelling, lightheadedness, weakness, and SE [1, 14, 15]. SE is also a common PM-related condition [13]. Additional but less frequent PM symptoms include neck pain and pain while swallowing, epigastric or back pain, and low-grade fever. Severe cases may present with cyanosis, hemodynamic instability, and pneumopericardium [16]. Chest compression due to the accumulation of air can be life-threatening [12]. Diagnosis may be delayed because of symptom overlap between asthma exacerbation and PM. A history of Valsalva’s maneuvers, cough, vomiting, choking, athletic exertion, huffing, respiratory diseases, and asthma exacerbations is essential to identify. Interestingly, PE can present with a first attack of asthma or poorly controlled asthma [1, 8, 17]. Moreover, foreign body aspiration needs to be considered in children with asthma who show no response to initial treatment [17]. In addition, a history of trauma is essential for differentiating between SPM and secondary PM.
Physical findings of PM may include tachypnea, tachycardia, wheezing, and SE (finding of crepitus) extending to the face, neck, and upper part of the chest, which may resemble edema, as in nephrotic syndrome, allergic, or angioneurotic edema [12, 15]. Hamman’s sign, the crepitus heard with a heartbeat on chest auscultation, is a pathognomonic indicator of PM [7].
Chest X-ray (CXR), including lateral views, is the primary diagnostic tool for diagnosing PM. It shows hyperlucency along the mediastinum lines. Additionally, air can usually be identified in the soft tissue of the neck and along the cardiac silhouette in the posterior-anterior view, as well as in front of the heart in the lateral view. Lateral neck radiographs are helpful in uncertain cases. Chest computed tomography (CT) scans can reveal air in the perivascular and peribronchial sheaths, which are pathognomonic findings for SPM. Nevertheless, when indicated, CT scans can be performed to rule out other serious etiologies of nonspontaneous PM and evaluate the underlying respiratory diagnoses of PM [1, 4, 7, 8].
Thoracic ultrasound can rapidly identify PM as a cause of acute-onset chest pain. However, in the presence of subcutaneous emphysema, thoracic ultrasound may not provide accurate results due to the interference caused by the air in the subcutaneous tissues. If suspicion of pneumopericardium is present, echocardiography may be used. Electrocardiogram (ECG) and laboratory findings are nonspecific. Therefore, a more detailed patient history can help to identify the possible cause of PM and SE, and a diagnostic workup can be completed to rule out other potential causes [15]. In asthmatic patients with PM, the peak expiratory flow rate is contraindicated [2].
Conservative management is the recommended treatment option for SPM and SE. These conditions usually resolve spontaneously by treating the underlying problem. As bronchospasm resolves, it reduces alveolar distension, which decreases the pressure gradient between the alveoli and the surrounding vascular structures, ultimately decreasing airflow into the mediastinum [18]. Supportive care, including oxygen inhalation therapy, is recommended for managing SPM and SE. Because inhaling 100% oxygen leads to the removal of nitrogen from the bloodstream by the absorption of nitrogen from body cavities, such as the extrapleural space, and subsequent exhalation of nitrogen from the lungs, an increase in the gradient for gas absorption and a four-to-sixfold increase in the reduction rate of subcutaneous and mediastinal emphysema are observed. One of the complications of oxygen therapy is absorption atelectasis, which must be considered [19, 20].
In addition to oxygen therapy, other management methods include rest, analgesia, avoidance of Valsalva maneuvers, and treating the underlying disease. Hospital admission should be considered for severe or unstable cases. Dietary restrictions and antibiotics are recommended if clinically indicated. As PM may lead to airway compression and cardiac tamponade that require surgical intervention, close monitoring of patients is warranted [15, 21].
Several surgical techniques for treating SE have been reported in adult medical literature, including infraclavicular “blow holes,” subcutaneous pigtail or large-bore drains, trocar-type drains with suction, and fenestrated catheters. Although some of these interventions are effective in adult patients, it is unclear whether they can be used to treat SE in pediatric patients. Physicians unfamiliar with SE may believe that surgery is necessary. However, surgical intervention is rarely needed, as these issues usually improve when the underlying asthma is treated. In the case of compartment syndrome due to severe SE, surgical decompression may be necessary, especially for unstable patients or when hypotension is present [22].
As PM self-resolves through air resorption in mediastinal tissues, symptoms generally improve within 3–15 days without sequelae. Conservative management of SE, PM, and PP reduces the risk of infection associated with surgically invasive management. In some cases, it may be notable on imaging for up to 6 months.
Effective management should focus on stabilizing the patient’s respiratory status, treating PM and SE, and treating asthma exacerbation with bronchodilator therapy, oxygen supplementation, steroids, and mechanical ventilation if necessary. In severe cases, surgical intervention may be needed to relieve air accumulation and facilitate lung expansion.
Although PM recurrence is rare, approximately 1%, identifying patients at risk of recurrence is essential [15, 21]. The prognosis of severe asthma patients with SE largely depends on the promptness of diagnosis and intervention. However, a delay in diagnosis or inappropriate treatment may lead to complications such as tension pneumothorax or PM, which can be life-threatening.
Conclusion
Conservative management of PM and SE, particularly in cases related to asthma exacerbations, can lead to a favorable prognosis and a relatively short hospital stay, especially when diagnosis and management occur early. However, delayed diagnosis due to overlapping symptoms of asthma exacerbation and PM may negatively impact the prognosis.
Abbreviations
- SPM
Spontaneous Pneumomediastinum
- SE
Subcutaneous Emphysema
- PM
Pneumomediastinum
Author contributions
All authors including Amal H. Aljohani, Hamdi Alsufiani, Ghousia Ahmed, contributed equally to this research and read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The Institutional Review Board (IRB) of KSMC, Research Ethics Committee - gave this study ethical approval.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Noorbakhsh KA, Williams AE, Langham JJW, Wu L, Krafty RT, Furtado AD, et al. Management and outcomes of spontaneous Pneumomediastinum in Children. Pediatr Emerg Care. 2021;37(12):e1051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalumeau M, Le Clainche L, Sayeg N, Sannier N, Michel JL, Marianowski R, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31(1):67–75. [DOI] [PubMed] [Google Scholar]
- 3.Yılmaz F, Çiftçi O, Özlem M, Komut E, Altunbilek E. Subcutaneous emphysema, pneumo-orbita and pneumomediastinum following a facial trauma caused by a high-pressure car washer. Ulus Travma Acil Cerrahi Derg. 2014;20(2):147–50. [DOI] [PubMed] [Google Scholar]
- 4.Okada M, Adachi H, Shibuya Y, Ishikawa S, Hamabe Y. Diagnosis and treatment of patients with spontaneous pneumomediastinum. Respir Investig. 2014;52(1):36–40. [DOI] [PubMed] [Google Scholar]
- 5.Kobashi Y, Okimoto N, Matsushima T, Soejima R. Comparative study of mediastinal emphysema as determined by etiology. Intern Med. 2002;41(4):277–82. [DOI] [PubMed] [Google Scholar]
- 6.Stack AM, Caputo GL. Pneumomediastinum in childhood asthma. Pediatr Emerg Care. 1996;12(2):98–101. [DOI] [PubMed] [Google Scholar]
- 7.Banki F, Estrera AL, Harrison RG, Miller CC 3rd, Leake SS, Mitchell KG, et al. Pneumomediastinum: etiology and a guide to diagnosis and treatment. Am J Surg. 2013;206(6):1001–6. discussion 6. [DOI] [PubMed] [Google Scholar]
- 8.Tortajada-Girbés M, Moreno-Prat M, Ainsa-Laguna D, Mas S. Spontaneous pneumomediastinum and subcutaneous emphysema as a complication of asthma in children: case report and literature review. Ther Adv Respir Dis. 2016;10(5):402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yılmaz F, Zortuk Ö. Subcutaneous Emphysema, Pneumomediastinum and spinal epidural Emphysema as complications of violent coughing: a Case Report. Acta Biomed. 2021;92(S1):e2021141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsh MM, Orvald TO. Mediastinal and subcutaneous emphysema complicating acute bronchial asthma. Chest. 1970;57(6):580–1. [DOI] [PubMed] [Google Scholar]
- 11.Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine. 1944;23(4):281–358. [Google Scholar]
- 12.Pandey D, Jaret P, Sharma R, Sharma A, Thakur S. Subcutaneous emphysema secondary to pulmonary cavity in absence of pneumothorax or pneumomediastinum. Respir Med. 2007;101(2):363–5. [DOI] [PubMed] [Google Scholar]
- 13.Colavita L, Cuppari C, Pizzino M, Sturiale M, Mondello B, Monaco F, et al. Pneumomediastinum, subcutaneous emphysema and pneumorrhachis in asthmatic children. J Biol Regul Homeost Agents. 2016;30(2):585–8. [PubMed] [Google Scholar]
- 14.Crespo MD, Iglesias FC, Márquez dlPL, Panadero CE, Vázquez LP, editors. Spontaneous idiopathic pneumomediastinum: apropos of a case. Anales de pediatria (Barcelona, Spain: 2003); 2006. [DOI] [PubMed]
- 15.Susai CJ, Banks KC, Alcasid NJ, Velotta JB. A clinical review of spontaneous pneumomediastinum. Mediastinum. 2024;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küçükosmanoglu O, Karakoç GB, Yilmaz M, Altintas D, Kendirli SG. Pneumomediastinum and pneumopericardium: unusual and rare complications of asthma in a 4 years old girl. Allergol Immunopathol. 2001;29(1):28–30. [DOI] [PubMed] [Google Scholar]
- 17.Nimrey-Atrash N, Bentur L, Elias N. Subcutaneous emphysema and pneumomediastinum due to foreign body aspiration in children with asthma. Pediatr Pulmonol. 2012;47(1):88–90. [DOI] [PubMed] [Google Scholar]
- 18.Dattwyler R, Goldman M, Bloch K. Pneumomediastinum as a complication of asthma in teenage and young adult patients. J Allergy Clin Immunol. 1979;63(6):412–6. [DOI] [PubMed] [Google Scholar]
- 19.Grasmuk-Siegl E, Valipour A. Nitrogen Wash-Out in non-hypoxaemic patients with spontaneous pneumothorax: a narrative review. J Clin Med. 2023;12(13). [DOI] [PMC free article] [PubMed]
- 20.Butler DA, Orlowski JP. Nitrogen washout therapy for pneumothorax. Cleve Clin J Med. 1983;50(3):311–5. [DOI] [PubMed] [Google Scholar]
- 21.Giuliani S, Franklin A, Pierce J, Ford H, Grikscheit TC. Massive subcutaneous emphysema, pneumomediastinum, and pneumopericardium in children. J Pediatr Surg. 2010;45(3):647–9. [DOI] [PubMed] [Google Scholar]
- 22.Sucena M, Coelho F, Almeida T, Gouveia A, Hespanhol V. [Massive subcutaneous emphysema--management usingsubcutaneous drains]. Rev Port Pneumol. 2010;16(2):321–9. 10.1016/s0873-2159(15)30030-1 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.