Abstract
The efficacy of therapeutic aerosolized amphotericin B (AMB) was studied in a steroid-immunosuppressed murine model of invasive pulmonary aspergillosis. Nebulized liposomal AMB can be a valid approach to the treatment of this infection, with subjects showing significantly improved survival relative to that of subjects given intravenous deoxycholate AMB, as well as lower lung weights and pulmonary glucosamine levels.
Pulmonary disease is the most frequent form of invasive aspergillosis (4), a fungal infection of growing incidence among immunocompromised patients (4), chiefly caused by Aspergillus fumigatus and associated with high mortality rates (10), despite a therapeutic arsenal that has been broadened with the approval of new drugs (5, 7, 15). To improve the poor results obtained with current treatment regimens, the potential usefulness of methods which may increase the concentration of amphotericin B (AMB) at the infectious focus, including its use by nebulization, has been suggested (8, 16, 19).
The efficacy of nebulized deoxycholate AMB or liposomal AMB was compared to that of conventional intravenous (i.v.) dosages of both formulations, using a murine model of invasive pulmonary aspergillosis (IPA). The potential benefits of inhalation plus i.v. administration were also explored. This experimental protocol was approved by the Ethics Committee of Vall d'Hebron Hospitals.
Female Wistar rats (180 to 200 g; Harland Iberica, Spain), fed with a low-protein diet, were immunosuppressed with 125 mg of subcutaneous cortisone acetate (Sigma Chemical Co., St. Louis, MO) per kg of body weight three times per week, from 14 days before infection to the end of the experiment (11).
On day 0, animals were intratracheally challenged with 0.3 ml of a conidial suspension prepared from a 1-week-old subculture on Sabouraud dextrose agar of a clinical isolate of A. fumigatus (AF19/68). Conidia were counted in a hemocytometer and adjusted to 8 × 106 cells/ml in sterile saline.
Intravenous treatments were administered through a central venous catheter (11). Aerosols were generated by a CR60 compressor (Medic-Aid Ltd., West Sussex, United Kingdom; flow rate, 10 liter/min) attached to a Nebulizer II Oxinova (Carburos Metálicos, Barcelona, Spain) and were led through a nose-only exposure inhalation chamber (Panlab, Spain) over a 60-min period. The total dosage of AMB administered to each animal was calculated with the following formula: the total dosage = the chamber concentration of AMB (mg/liter) × the minute volume (liter/min) × the duration of exposition (min) × the animal's weight (kg−1) (1). The minute volume is equal to the body weight0.75 (grams) × 0.00254 (6).
Animals were randomized to receive 5% dextrose i.v. (control) (n = 23), deoxycholate AMB (d-AMB) at 1 mg/kg i.v. (Fungizone; Bristol-Myers Squibb Group, Spain) (n = 25), liposomal AMB (L-AMB) at 5 mg/kg i.v. (AmBisome; Gilead Sciences, Spain) (n = 27), nebulized d-AMB (nd-AMB) at 1.13 mg/kg (n = 18), nebulized L-AMB (nL-AMB) at 2.3 mg/kg (n = 16), or nL-AMB at 2.3 mg/kg plus L-AMB at 5 mg/kg i.v. (n = 16). Treatment was administered daily, starting 24 h after infection, for a total of 10 days.
Surviving rats were sacrificed 24 h after the last dose of treatment. All animals were aseptically dissected. Lungs were weighed and homogenized in sterile distilled water, and aliquots were serially diluted and plated onto Sabouraud dextrose agar for colony counts. Lung homogenates were processed for chitin assay, as described elsewhere (9), to quantify fungal burden. In brief, homogenates were suspended in 21.4 M KOH after being washed with 0.10 M sodium dodecyl sulfate (Sigma Chemical Co., St. Louis, MO) and heated at 130°C for 1 h. After cooling, 8 ml of 75% ethanol and 0.3 ml of a Celite 545 suspension (Sigma) were added. Pellets were washed with 40% ethanol and distilled water and suspended in 0.5 ml of 0.7 M NaNO2 and 0.5 ml of 0.4 M KHSO4. Volumes of the supernatants were mixed with 1.10 M ammonium sulfamate, followed by 3-methyl-2-benzothiazolinone hydrazone hydrochloride hydrate monohydrate (Sigma) and 0.03 M FeCl3 · 6H2O. The optical density was spectrophotometrically read (UV-160A; Shimadzu Corp., Japan) at 650 nm.
Survival was analyzed using Kaplan-Meier analysis and the log rank test. Lung weight (grams), chitin content (micrograms of glucosamine/paired lungs), and numbers of CFU/gram of lung (log10 numbers of CFU/gram) were expressed as means and 95% confidence intervals of the means and compared using one-way analysis of variance and Bonferroni's test. P values of ≤0.01 were considered significant.
Results obtained from animals surviving 5 days or longer with the treatment are shown in Table 1 and Fig. 1 and 2. Results were pooled, since preliminary studies revealed that pulmonary glucosamine reaches a plateau by this time in animals left untreated (J. Gavaldà, P. López, T. Martín, X. Gomis, M. Rosal, B. Almirante, C. Pigrau, and A. Pahissa, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1682, 2000).
TABLE 1.
Therapeutic efficacy in the treatment groups
| Group | No. of rats | Value for rats surviving ≥5 days of treatmentb
|
|||
|---|---|---|---|---|---|
| No. of rats (% of total) | Wt of paired lungs (g)a | Amt of glucosamine/paired lungs (μg)a | Log10 no. of CFU/ga | ||
| Control | 23 | 11 (47.83) | 3.15 (2.63-3.68) | 176.25 (117.06-237.44) | 5.08 (4.53-5.63) |
| d-AMB at 1 mg/kg/day, i.v. | 25 | 17 (68) | 2.97 (2.61-3.33) | 148.36 (96.37-200.35) | 5.07 (4.83-5.30) |
| L-AMB at 5 mg/kg/day, i.v. | 27 | 19 (70.37) | 2.37 (2.06-2.68)* | 78.26 (31.30-125.22) | 4.99 (4.75-5.24) |
| nd-AMB at 1.13 mg/kg/day | 18 | 15 (83.33) | 2.26 (1.94-2.58)*† | 97.89 (40.18-155.60) | 4.56 (4.14-4.97) |
| nL-AMB at 2.3 mg/kg/day | 16 | 14 (87.5) | 1.87 (1.60-2.14)*† | 28.31 (5.34-51.29)*† | 4.48 (3.80-5.17) |
| nL-AMB + L-AMB, i.v. | 16 | 16 (100) | 1.82 (1.59-2.05)*† | 46.28 (10.58-81.97)* | 4.30 (3.89-4.70) |
Results are expressed as means ± 95% confidence intervals. *, P ≤ 0.01 versus value for control; †, P ≤ 0.01 versus value for d-AMB administered i.v. at 1 mg/kg/day.
Values are the results of three experiments combined.
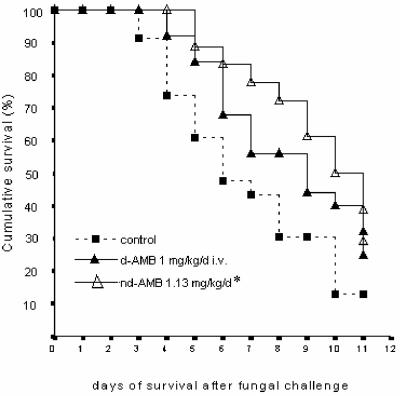
FIG. 1.
Effects of administration of nebulized d-AMB versus intravenous administration of d-AMB in steroid-immunosuppressed rats with experimental invasive aspergillosis. *, significant relative to value for control.
FIG. 2.
Effects of administration of nebulized L-AMB versus intravenous administration of L-AMB in steroid-immunosuppressed rats with experimental invasive aspergillosis. *, significant relative to value for control; †, significant relative to value for i.v. d-AMB; ‡, significant relative to value for nd-AMB.
Nebulized d-AMB improved survival (P = 0.019) and lung weight (P = 0.006) compared to those of controls, whereas the nL-AMB group showed significantly improved survival, pulmonary weights, and glucosamine levels compared to control and i.v. d-AMB groups (P ≤ 0.003); compared to the i.v. L-AMB group, the survival of animals receiving nL-AMB was prolonged, reaching levels close to significance (P = 0.066).
Aerosolization plus i.v. administration of L-AMB produced results similar to those with nL-AMB alone compared with the control. Compared to results with i.v. d-AMB, an important reduction of glucosamine was noted (P = 0.016), although the combined administration achieved the most remarkable diminution of log10 numbers of CFU (P = 0.071, versus i.v. d-AMB results). Discrepancies between CFU counts and chitin measurements have been previously reported, due to the peculiar structure of molds (2).
Pulmonary concentrations achieved with recommended doses (up to 5 mg/kg/day) of L-AMB can be considered suprainhibitory according to in vitro data, but in vivo, they might be insufficient to eradicate the fungus. Therefore, pulmonary AMB concentrations could be increased by nebulization. Experimental animal studies have shown that aerosolization leads to measurable concentrations of AMB in lungs (1, 14, 16), and levels have been reported to be higher with lipid formulations (1, 3). Nebulization minimizes the risk of adverse events by avoiding systemic exposure to the drug (16, 18), focusing the drug activity at the site of infection.
The efficacy of aerosol AMB prophylaxis against Aspergillus infection has been extensively studied. Experimental models of IPA have shown that nebulized AMB increases survival with respect to that of controls (1, 3, 18), and it has also been described as a protective factor against Aspergillus species infection in lung transplant recipients (12, 13). In contrast, few studies have investigated the efficacy of nebulized AMB for the treatment of IPA (17, 18). Nebulized L-AMB has proved to be useful for the treatment of experimental invasive aspergillosis of neutropenic rats (17). In humans, Ruffini and coworkers (E. Ruffini, S. Baldi, D. Libertucci, P. Solidoro, P. di Marzio, A. Cavallo, and M. Mancuso, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1237, 2002) have reported that nL-AMB alone resolved nine episodes of colonization, and in association with L-AMB i.v., it resolved all four cases of invasive aspergillosis.
Our work shows that aerosolization of AMB is a useful approach to the treatment of experimental IPA, with a more favorable outcome achieved with nL-AMB than with nd-AMB. This may suggest that both nd-AMB and nL-AMB reached satisfactory concentrations in the lungs, although concentrations were probably slightly higher with the liposomal formulation.
Combined administration of nebulized plus i.v. L-AMB produced results similar to those with nL-AMB alone, probably because the two methods rendered little difference in pulmonary concentrations of AMB. Being outside the scope of the study, we did not investigate whether the combination prevented the risk of extrapulmonary dissemination. Since in humans dissemination is difficult to discard, administering aerosolized plus i.v. L-AMB seems prudent.
We conclude that the use of nebulized AMB, in particular L-AMB, may be a useful approach to the treatment of experimental IPA. Further studies might be warranted.
Acknowledgments
We thank Celine Cavallo for English language assistance.
This work has been supported by the Spanish Fondo de Investigaciones Sanitarias (grants FIS 01/1412 and FIS G03/075 RESITRA).
REFERENCES
- 1.Allen, S. D., K. N. Sorensen, M. J. Nejdl, C. Durrant, and R. T. Proffit. 1994. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J. Antimicrob. Chemother. 34:1001-1013. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. J., S. de Marie, M. H. Fens, W. C. Hop, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2002. Enhanced antifungal efficacy in experimental invasive pulmonary aspergillosis by combination of AmBisome with Fungizone as assessed by several parameters of antifungal response. J. Antimicrob. Chemother. 49:813-820. [DOI] [PubMed] [Google Scholar]
- 3.Cicogna, C. E., M. H. White, E. M. Bernard, T. Ishimura, M. Sun, W. P. Tong, and D. Armstrong. 1997. Efficacy of prophylactic aerosol amphotericin B lipid complex in a rat model of pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:259-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W., C. C. Kibler, and R. A. Barnes on behalf of the British Society for Medical Mycology. 2003. British Society for Medical Mycology proposed standards of care for patients with invasive fungal infections. Lancet Infect. Dis. 3:230-240. [DOI] [PubMed] [Google Scholar]
- 6.Guyton, A. C. 1947. Measurement of respiratory volumes of laboratory animals. Am. J. Physiol. 150:70-77. [DOI] [PubMed] [Google Scholar]
- 7.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin. J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw, for the Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi, T., K. Kubo, T. Kaneki, M. Hanaoka, T. Hayano, T. Miyahara, K. Okada, K. Fujimoto, H. Yamamoto, T. Kobayashi, and M. Sekiguchi. 1998. Pharmacokinetic evaluation of amphotericin B in lung tissue: lung lymph distribution after intravenous injection and airspace distribution after aerosolization and inhalation of amphotericin B. Antimicrob. Agents Chemother. 42:1597-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, S.-J., J. Schranz, and S. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 11.Martín, M. T., J. Gavaldà, P. López, X. Gomis, J. L. Ramírez, D. Rodríguez, O. Len, Q. Jordano, I. Ruiz, M. Rosal, B. Almirante, and A. Pahissa. 2003. Efficacy of high doses of liposomal amphotericin B in the treatment of experimental aspergillosis. J. Antimicrob. Chemother. 52:1032-1034. [DOI] [PubMed] [Google Scholar]
- 12.Minari, A., R. Husni, R. K. Avery, D. L. Longworth, M. DeCamp, M. Bertin, R. Schilz, N. Smedira, M. T. Haug, A. Mehta, and S. M. Gordon. 2002. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl. Infect. Dis. 4:195-200. [DOI] [PubMed] [Google Scholar]
- 13.Monforte, V., A. Román, J. Gavaldà, C. Bravo, L. Tenorio, A. Ferrer, J. Maestre, and F. Morell. 2001. Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J. Heart Lung Transplant. 20:1274-1281. [DOI] [PubMed] [Google Scholar]
- 14.Niki, Y., E. M. Bernard, H.-J. Schmitt, W. P. Tong, F. F. Edwards, and D. Armstrong. 1990. Pharmacokinetics of aerosol amphotericin B in rats. Antimicrob. Agents Chemother. 34:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrosky-Zeichner, L., K. A. Marr, J. H. Rex, and S. H. Cohen. 2003. Amphotericin B: time for a new “gold standard.” Clin. Infect. Dis. 37:415-425. [DOI] [PubMed] [Google Scholar]
- 16.Ruijgrok, E. J., A. G. Vulto, and E. W. M. Van Etten. 2000. Aerosol delivery of amphotericin B desoxycholate (Fungizone) and liposomal amphotericin B (AmBisome): aerosol characteristics and in-vivo amphotericin B deposition in rats. J. Pharm. Pharmacol. 52:619-627. [DOI] [PubMed] [Google Scholar]
- 17.Ruijgrok, E. J., A. G. Vulto, and E. W. M. Van Etten. 2001. Efficacy of aerosolized amphotericin B deoxycholate and liposomal amphotericin B in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. J. Antimicrob. Chemother. 48:89-95. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt, H. J., E. M. Bernard, M. Häuser, and D. Armstrong. 1988. Aerosol amphotericin B is effective for prophylaxis and therapy in a rat model of pulmonary aspergillosis. Antimicrob. Agents Chemother. 32:1676-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinbach, W. J., and D. A. Stevens. 2003. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin. Infect. Dis. 37(Suppl. 3):157-187. [DOI] [PubMed] [Google Scholar]