Abstract
Purpose
Tanzania is the fifth country with the highest sickle cell disease (SCD) prevalence globally. Although hydroxyurea (HU) is available, only 25% of persons with SCD are reported to use it in Tanzania. Perceived disease threat is associated with medication usage in patients with chronic diseases. We assessed the factors associated with caregivers’ perceived threat of SCD complications and its relationship with HU use among children with SCD in Dar-es-Salaam.
Methods
We conducted a cross-sectional hospital-based study from May to August 2023. We enrolled 374 caregivers of health-insured children with SCD from 4 public SCD clinics. We adapted the modified original and revised Champion’s Health Belief Model Scales to derive perceived threat scores. We used Mann-Whitney and Kruskal-Wallis tests to compare the outcomes across sociodemographic characteristics and regression analysis for factors associated with perceived SCD threat.
Results
The median score (InterQuartile Range) for perceived threat of SCD complications was 559 (175, 598). Sixty-one percent of caregivers had a high SCD perceived threat. The caregivers of under-five children had 141 lower median SCD threat scores than those of children aged 13–17 years, p-value < 0.001. Participants from Regional Referral Hospitals (RRH) had lower median threat scores compared to participants attending Muhimbili National Hospital (MNH), 177 for Amana RRH, 325 Temeke RRH, 585 MNH Mloganzila, and 557 MNH Upanga, p-value <0.001. Children of caregivers with high perceived SCD threat were 3.4 times more likely to use HU compared to those with low SCD threat perception (Incidence Rate Ratio 3.4, 95% CI: 2.7–4.5).
Conclusion
The perceived threat of SCD predicts the likelihood of SCD patients using HU in Dar-es-Salaam, Tanzania. We recommend health education to caregivers aiming to improve their SCD threat perception and thus improve the use of HU among children with SCD in similar settings.
Keywords: Sickle cell disease, hydroxyurea, perceived threat
Introduction
Sickle cell disease (SCD) is the most common life-threatening monogenic disorder in the world, caused by a single base-pair point mutation in the β-globin gene.1,2 Over 300,000 individuals with SCD are born annually, and 75% of these reside in sub-Saharan Africa (SSA).2–5 Tanzania is among the five countries with the highest incidence of SCD globally.6–8 Clinical manifestations may be acute or chronic, may be physical or psychosocial, and vary from mild painful episodes to fatal complications such as severe anemia and stroke. Without comprehensive care, SCD is associated with high morbidity and mortality.1,7,9 SCD accounts for 7% of deaths in children under five years old.7,10 Hydroxyurea (HU) is a disease-modifying drug that reduces the risk of progression to SCD complications and improves the quality of life and survival of SCD patients.11–15 However, the use of this medicine in SSA is still low.7,16–18
Perceived threat is a function of perceived disease susceptibility and perceived disease severity.19 For patients with chronic diseases, perceived susceptibility refers to the belief that the person may personally develop complications related to the disease.19,20 Perceived disease severity refers to the person’s perception of the negative consequences and seriousness of the disease.19–21 When assessing the severity of the disease, individuals consider both medical consequences, such as disability and death, as well as social consequences, such as relationships with others.19,20,22,23 It has been shown that, for a person to accept the use of medicine and preventive behaviors, the perceived threat of the disease should be high.21,23–25
Patients who perceive the threat of SCD to be high have higher adherence to medication prescribed for their illness compared to patients reporting a lower perceived threat.26,27 Understanding patients’ and caregivers’ perceptions of the illness can give important information about how to intervene to modify patient’s and caregivers’ disease perceptions and knowledge of SCD, which might contribute to increased medication usage and, consequently, improved health over time.26,27 This study aimed to assess caregivers’ perceived threat of SCD and its association with the use of HU among their children with SCD.
Methods and Materials
Study Design
We conducted a cross-sectional hospital-based study employing quantitative methods at four public SCD clinics in Dar es Salaam region from 1st of May 2023 to 31st August 2023.
Study Setting
There are five public SCD clinics in Dar es Salaam region. HU was available at four of these facilities at the time of the study: Temeke and Amana Regional Referral Hospitals (RRH), Muhimbili National Hospital (MNH) Upanga and MNH Mloganzila. The SCD clinics at these facilities are clinical sites for the Sickle Pan-African Research Consortium (SPARCO)-Tanzania project. SPARCO-Tanzania has one of the largest databases of patients with SCD in the world and is part of the SickleInAfrica Consortium.28 All patients enrolled in this study were derived from the SPARCO-Tanzania database and had a confirmed diagnosis of SCD using haemoglobin electrophoresis, isoelectric focusing or high-performance liquid chromatography.
Sample Size Estimation
We used the formula for calculation of sample size for finite population. The proportion of caregivers with high perceived threat of SCD was unknown, hence we used 50% prevalence (P). We used a Z score of 1.96 and standard error of 0.05.
n = n0 / 1 + [(n0 – 1) / N], where n0 = sample size = z2 (1-P)/e2, N = Population of health-insured patients at study sites, 1829. Therefore, the minimum sample size required to attain a power of 80% was 318. To ensure the power of the study, we enrolled a total of 374 caregivers.
Study Participants and Sampling Criteria
We screened 466 caregivers and enrolled 374 in the study. Ninety-two caregivers were excluded because their children either lacked health insurance coverage, were above 17 years old, or did not have a confirmed SCD diagnosis. We recruited consecutively 374 caregivers of health-insured SCD children under 18 years of age who attended the Temeke RRH, Amana RRH, MNH-Upanga, and MNH-Mloganzila hospitals. The caregivers were screened before enrolment by asking about the age of the patients and health insurance status. The study population was selected because over 80% of individuals with SCD in Tanzania are children, as reported in the SPARCO-Tanzania database.17 Hence, the caregiver’s perceptions directly affect the care of a significant number of patients with SCD in this setting. Additionally, the National Health Insurance Funds (NHIF) and private insurance schemes cover the entire cost of HU. Therefore HU cost and unavailability were not barriers to HU use among children with SCD attending these facilities. From the SPARCO database, most of the insured patients with SCD have NHIF subscriptions.17
Data Collection Tools
We adapted standardized questionnaires that were validated in previous studies,29–35 (Supplementary data). We translated the tools into Kiswahili and adapted them to local circumstances. The original validated Revised Champion’s Health Belief Model Scale (RCHBMS) and Champion’s Health Belief Model Scale (CHBMS) questionnaires are available online.19 RCHBMS has validity of Cronbach’s alpha ranging from 0.7 to 0.9, and Cronbach’s alpha for CHBMS ranging from 0.6 to 0.9.
We calculated the reliability of the adapted tools after data collection by assessing the internal consistency of the scales using Cronbach’s alpha. We adapted the 3-question RCHBMS for assessment of perceived susceptibility of experiencing SCD complications. The adapted scale had a reliability of Cronbach’s alpha of 0.9, and an inter-item correlation of 0.8–0.9. The questions captured the caregiver’s perceived possibility, feelings, and risk of SCD child getting SCD-related complications (Table S1). The scores for perceived susceptibility to SCD complications varied between 3 and 15.
We adapted the 10-question CHBMS for assessment of perceived severity of SCD complications. The adapted scale had a reliability of Cronbach’s alpha of 0.9, and inter-item correlation of 0.7–0.9. The scale captured a range of caregivers’ perceived negative consequences that SCD may have on one’s medical and social life (Table S1). The scores for perceived severity of SCD complications varied between 10 and 50.
We used the scores obtained from perceived susceptibility and severity of SCD complications to calculate scores for perceived threat using the formula derived by Charles Abraham and Paschal Sheeran: Threat = Susceptibility + (Susceptibility x Severity).19
Procedures
We trained four research assistants in preparation for data collection in four study sites. The research assistants used the adapted standard questionnaires to collect data. We explained the aim of the study and provided written information forms to all participants. All participants signed informed consent forms before participating in the study.
Ethical Approval
Ethical approval was obtained from the institutional review boards at Muhimbili University of Health and Allied Sciences (MUHAS-REC-04-2023-1640) and the National Institution for Medical Research (NIMR). Signed written informed consent were obtained before participant enrollment into the study. The participation of caregivers was performed in accordance with the Declaration of Helsinki.
Demographic Data and Other Participant Characteristics
Socio-demographic characteristics included the caregiver’s age, sex, education level, occupation, caregiver-patient relationship, age of the child with SCD, child’s education level, type of health insurance coverage (public or private), and the hospital where they attended. Other participant characteristics included using HU among the children with SCD they cared for.
Statistical Analyses
We performed all analyses using the Statistical Package for Social Scientists (SPSS v29.0.1.0). Outcomes included the perceived threat of SCD among caregivers of health-insured children with SCD, perceived susceptibility of getting SCD complications, perceived severity of SCD complications, and HU use among health-insured children with SCD. We summarized sociodemographic characteristics as frequencies and proportions. The scores for outcomes (perceived susceptibility, severity, and threat of SCD) were not normally distributed, and thus, dispersion was better described by median and Interquartile range (IQR). We compared the medians of the outcomes with caregivers’ sociodemographic characteristics using the Mann-Whitney U test for binary categories and the Kruskal Wallis test for variables with more than two categories. We compared scores of perceived susceptibility, perceived severity, perceived threat, and HU use by using the Mann-Whitney U test. We used quantile regression for the analysis of sociodemographic factors associated with the perceived threat of SCD. From the health belief model, sociodemographic variables affect threat perception. Therefore, we included all variables in both univariate and multivariate analyses. We assessed the association between caregivers’ perceived threat of SCD complications and hydroxyurea use among their children with SCD using modified Poisson regression. The differences in outcomes with p-value of < 0.05 were considered statistically significant. From the threat perception formula, the maximum score of a perceived threat of SCD, a participant would get was 765. A cut-off score of 382.5 (50%) or less was considered a low threat perception. We noted a significant correlation between caregivers’ occupation and education level indicating collinearity with a p-value of less than 0.001. This rendered it inappropriate to include both variables in the multivariate analysis. Consequently, we incorporated education level while excluding occupation from the analysis.
Results
Baseline Characteristics
We enrolled 374 caregivers of health-insured children with SCD, of which 56 (15.0%) were recruited from Amana RRH, 82 (21.9%) from Temeke RRH, 18 (4.8%) from MNH Mloganzila, and 218 (58.3%) from MNH Upanga. The number of participants enrolled from each hospital mirrored the percentage of health-insured children with SCD attending these hospitals, according to the SPARCO-Tanzania database. Female caregivers comprised 85.6% of all participants and the majority (72.0%) were biological mothers of the children with SCD. Most caregivers (59.6%) were under the age of 40 years and had completed either primary or secondary education (84.5%). Over 57% were involved in petty businesses, whereas 24.3% were housewives, 3.7% were peasants, and 14.5% had formal employment. More than half of the participants (58.6%) had children with SCD aged between 5 and 12 years, whereas 19.8% had under-five children with SCD and 21.6% had teens with SCD (Table 1). The median (IQR) age of the children they cared for was 9 (5,12) years. The majority of the children, 98.9%, had health insurance through the government-run National Health Insurance Fund (NHIF), and the remaining were insured by other health insurance strategies that are privately owned.
Table 1.
Participants’ Baseline Characteristics, Perceived Susceptibility, Perceived Severity, and Perceived Threat Scores
| Variable | Frequency, n (%) | Median Susceptibility score (IQR) | P - value | Median Severity score (IQR) | P - value | Median Threat score (IQR) | P - value | |
|---|---|---|---|---|---|---|---|---|
| Caregiver’s age (years) | 20–29 | 65 (17.4) | 12 (8, 14) | 0.232 | 39 (27, 45) | 0.715 | 560 (168, 588) | 0.944 |
| 30–39 | 158 (42.2) | 13 (6, 14) | 39 (26, 44) | 554 (144, 599) | ||||
| 40–49 | 123 (32.9) | 13 (8, 15) | 39 (29, 42) | 559 (264, 600) | ||||
| ≥ 50 | 28 (7.5) | 13 (7,14) | 38 (24, 42) | 560 (175, 585) | ||||
| Caregiver’s Sex | Male | 54 (14.4) | 14 (12, 15) | 0.009 | 38 (29, 40) | 0.379 | 555 (150, 600) | 0.612 |
| Female | 320 (85.6) | 13 (7, 14) | 39 (26, 44) | 555 (350, 559) | ||||
| Caregiver’s Education level | No formal education | 8 (2.1) | 12 (7, 14) | 0.407 | 31 (21, 40) | 0.478 | 387 (179, 582) | 0.611 |
| Primary | 165 (44.1) | 13 (8, 14) | 39 (26, 44) | 561 (188, 598) | ||||
| Secondary | 151 (40.4) | 13 (7, 14) | 39 (26, 44) | 546 (165, 588) | ||||
| Higher education | 50 (13.4) | 13 (5, 15) | 38 (26, 43) | 552 (149, 600) | ||||
| Caregiver’s occupation | Housewives | 91 (24.3) | 13 (9, 15) | 0.485 | 39 (28, 44) | 0.346 | 564 (279, 588) | 0.419 |
| Peasants | 14 (3.7) | 13 (9, 14) | 37 (27, 44) | 528 (284, 616) | ||||
| Petty traders | 215 (57.5) | 13 (7, 14) | 39 (26, 44) | 555 (175, 598) | ||||
| Formal employment | 54 (14.5) | 13 (5, 15) | 38 (18, 42) | 554 (104, 599) | ||||
| Caregiver – patient relationship | Father | 46 (12.3) | 13 (7, 14) | 0.229 | 39 (26, 44) | 0.576 | 555 (158, 598) | 1.000 |
| Mother | 269 (71.9) | 13 (10, 15) | 38 (29, 41) | 559 (312, 586) | ||||
| Others | 59 (15.8) | 13 (8, 14) | 38 (25, 43) | 560 (175, 588) | ||||
| SCD child’s age group (years) | < 5 | 74 (19.8) | 11 (5, 14) | 0.007 | 35 (25, 42) | 0.048 | 314 (119, 585) | 0.011 |
| 5–12 | 219 (58.6) | 13 (7, 14) | 39 (26, 44) | 560 (184, 598) | ||||
| 13–17 | 81 (21.6) | 13 (12, 14) | 40 (36, 44) | 572 (372, 602) | ||||
| Hospital | Amana RRH | 56 (15.0) | 7 (3, 11) | < 0.001 | 26 (19, 34) | < 0.001 | 178 (81, 369) | < 0.001 |
| Temeke RRH | 82 (21.9) | 11 (6, 13) | 35 (22, 42) | 325 (140, 574) | ||||
| MNH Mloganzila | 18 (4.8) | 14 (12, 15) | 40 (36, 45) | 585 (360, 615) | ||||
| MNH Upanga | 218 (58.3) | 13 (12, 15) | 39 (36, 42) | 557 (492, 574) | ||||
Perceived Susceptibility to SCD Complications
The median score (IQR) for perceived susceptibility to getting SCD complications was 13 (7,14). On the other hand, 45.0% of caregivers did not perceive that their SCD children were at risk of getting SCD complications (Table S1). Significant differences in median perceived susceptibility scores were seen among caregivers attending different hospitals, and the age of their children with SCD. Caregivers of children with SCD above 5 years had higher scores than caregivers of children below 5 years of age (p=0.007). Further, caregivers who attended SCD clinics at MNH Mloganzila and MNH Upanga had higher scores than those attending SCD clinics at Amana RRH and Temeke RRH, 13 (12,15), 13 (12, 15), 7 (3,11) and 10.5 (6, 13), respectively (p <0.001). Participants aged 40 years and above had higher susceptibility scores than those younger than 40 years. However, this was not statistically significant (p-value 0.232). There were no significant differences in perceived susceptibility of SCD complications scores among participants with different education levels, occupations, and their relationship with the SCD child (Table 1).
Perceived Severity of SCD Complications
The median (IQR) score for perceived severity of SCD complications was 39 (26, 44). The majority of participants (69.0%) perceived that they would be financially affected if their children got SCD complications. However, half of the patients (51.0%) did not perceive that SCD could have long-lasting complications, and 44.0% did not perceive that SCD can lead to fatal or extremely serious complications such as stroke (Table S1). There were significant differences in perceived severity scores among caregivers with SCD children of different age groups and the attending hospitals; Caregivers with SCD children aged below five years had lower severity scores compared to those with children between 5 and 12 years, and caregivers of teens with SCD, 11 (5,14), 13 (12, 14) and 13 (12, 14), respectively (p= 0.048). Participants attending Amana RRH had the lowest scores, followed by participants at Temeke RRH, MNH Upanga, and MNH Mloganzila, 26 (19, 34), 35 (22, 42), 39 (36, 42) and 40 (36, 45), respectively (p < 0.001). Participants with no formal education had the lowest perceived severity scores of SCD, however the difference in median scores was insignificant. There were no significant differences in the perceived severity of SCD scores among participants with different age groups, occupations, and their relationship with the child with SCD (Table 1).
Perceived Threat and Predictors of Perceived Threat
The median (IQR) score for the perceived threat of SCD was 559 (176, 598). Sixty-one percent (229/374) scored above 382.5 and therefore categorized as having a high threat perception of SCD.
Statistically significant differences in threat scores were observed with the age of the children and the hospitals where the children attended. Caregivers of children with SCD aged below five years of age had a median (IQR) score of 314 (119, 585), whereas those with children aged 5 to 12 years scored 560 (184, 598) and those with children aged 12 to 17 scored 572 (372, 602) (p= 0.011). Participants attending Amana RRH had the lowest SCD threat perception scores followed by participants from Temeke RRH, MNH Mloganzila and MNH Upanga, 178 (81, 369), 325 (140, 574), 585 (360, 615) and 557 (492, 574), respectively (p < 0.001). Participants with no formal education had the least perceived threat of SCD. However, this difference in median scores was not significant. There were no significant differences in the perceived threat of SCD scores among participants with different age groups, occupations, and their relationship with the SCD child (Table 1).
Caregivers of SCD children aged below five years were found to have 260 points lower scores than caregivers of SCD children aged 13 to 17 years in univariate quantile regression analysis (Crude β coefficient −260, 95% CI −359 – −161, P-value <0.001), and had 141 points lower threat scores in multivariate analysis (adjusted β coefficient −141, 95% CI −204 – −79, P value <0.001). In univariate analysis, participants from Temeke RRH and Amana RRH had 265 and 410 lower threat scores, respectively, compared to participants from MNH Upanga (Temeke; Crude β coefficient −265, 95% CI −308 – −222, P-value <0.001. Amana; Crude β coefficient −410, 95% CI −459 – −361, P-value <0.001.), whereas in multivariate analysis participants from Temeke RRH had 224 lower threat scores and those from Amana had 388 lower threat scores compared to participants attending MNH Upanga (adjusted β coefficient −225, 95% CI −272 – −177, P value <0.001 for Temeke RRH, and adjusted β coefficient −388, 95% CI −442 – −334, P value <0.001 for Amana RRH) (Table 2).
Table 2.
Caregivers’ Scores of Perceived Threat of SCD Complications and Associated Factors
| Variable | Median threat scores (IQR) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| cβ | 95% CI | aβ | 95% CI | |||
| Caregiver’s sex | Male | 555 (150, 600) | 5 | −124 – 134 | −2 | −101 - 96 |
| Female | 555 (350, 559) | Ref | ||||
| Caregiver’s age | 20 −29 | 560 (168, 588) | 0 | −196 – 196 | 50 | −36 - 136 |
| 30–39 | 554 (144, 599) | −8 | −186 – 170 | 26 | −51 - 103 | |
| 40–49 | 559 (264, 600) | −1 | −182 – 181 | 29 | −49 - 106 | |
| ≥ 50 | 560 (175, 585) | Ref | ||||
| Caregiver’s level of education | No formal education | 387 (179, 582) | 22 | −308 – 352 | 3 | −139 – 144 |
| Primary | 561 (188, 598) | 9 | −131 – 149 | 18 | −43 – 79 | |
| Secondary | 546 (165, 588) | −6 | −147 – 135 | 16 | −44 – 75 | |
| Higher education | 552 (149, 600) | Ref | ||||
| Caregiver – patient relationship | Mother | 555 (158, 598) | −5 | −131 – 121 | 8 | −50 – 65 |
| Father | 559 (312, 586) | −1 | −173 – 171 | 32 | −76 – 140 | |
| Others | 560 (175, 588) | Ref | ||||
| SCD child’s age group (years) | < 5 | 314 (119, 585) | −260 | −359 – −161 | −141 | −204 – −79 |
| 5–12 | 560 (184, 598) | −12 | −92 – 68 | −24 | −73 – 25 | |
| 13–17 | 572 (372, 602) | Ref | ||||
| Hospital | Temeke RRH | 325 (140, 574) | −265 | −308 – −222 | −225 | −272 – −177 |
| Amana RRH | 178 (81, 369) | −410 | −459 – −361 | −388 | −442 – −334 | |
| MNH Mloganzila | 585 (360, 615) | - | −111 – 51 | −26 | −114 – 63 | |
| MNH Upanga | 557 (492, 574) | Ref | ||||
Note: The factors were adjusted for all other variables shown in the table.
Abbreviations: cβ, crude beta coefficient; aβ, adjusted beta coefficient; Ref, Reference category; CI, Confidence interval.
Caregiver’s Perceived Susceptibility, Severity, and Threat of SCD Scores versus the Use of Hydroxyurea Among SCD Children
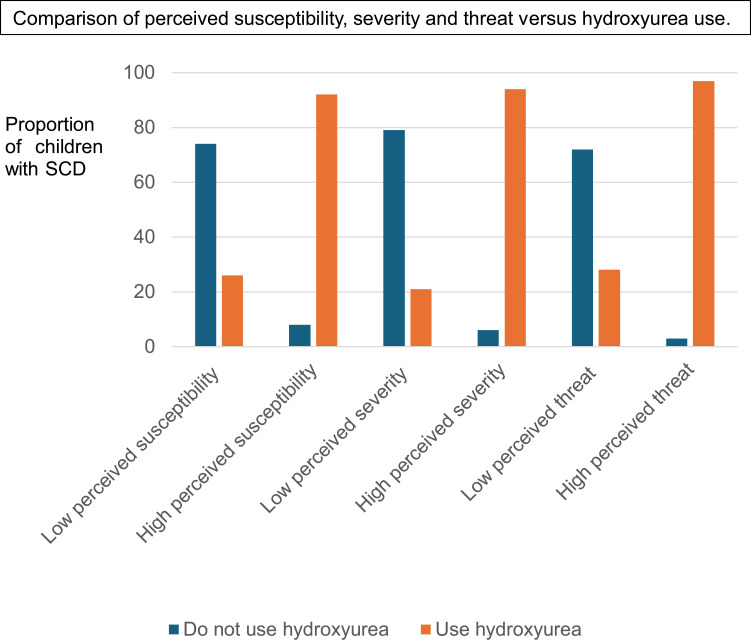
A total of 263 (70.0%) caregivers reported that their SCD children were using HU. Hospital-wise, only 27% (15/56) of caregivers from Amana RRH reported the use of HU among their SCD children, whereas 55% (45/82) of caregivers at Temeke RRH, 86% (187/218) at MNH Upanga and 89% (16/18) at MNH Mloganzila reported the use of HU among their SCD children. Nearly all (222/229, 96.9%) caregivers with higher SCD threat perception had children with SCD who were using HU compared to 28% (41/145) of caregivers with low SCD threat perception (Figure 1).
Figure 1.
Comparison of caregiver’s scores for perceived susceptibility of sickle cell disease (SCD) complications, perceived severity, and threat perceptions versus the use of hydroxyurea (HU) among children with SCD attending public SCD clinics in Dar es Salaam region. The blue bars represents proportion of children with SCD who do not use HU whereas the Orange bars represents proportion of children using HU. Caregivers with high perceived susceptibility, severity and threat of SCD complications reported higher proportions of children using HU (92%, 94% and 97% respectively) than those with low perceived susceptibility, severity and threat of SCD complications (26%, 21%, 28% respectively). The differences in scores among the caregivers of HU users and non-users were statistically significant with a p-value of < 0.001.
The median (IQR) perceived susceptibility of SCD score among caregivers whose SCD children use HU was 14 (12,15), whereas for caregivers of SCD children who do not use HU was 6 (3, 8). The median (IQR) perceived severity of SCD score among caregivers whose children with SCD use HU was 41 (38, 45), whereas for caregivers of children with SCD who do not use HU was 24 (19, 28). The median (IQR) perceived threat of SCD score among caregivers whose children with SCD use HU was 574 (546, 611), whereas for caregivers of children with SCD who do not use HU was 140 (81, 216) (Figure 1). The differences in susceptibility, severity, and threat perception scores among the caregivers of HU users and non-users were statistically significant, with a p-value < 0.001. The incidence rate ratio (IRR) of using HU was 3.4, with a 95% confidence interval of 2.7–4.5 and a p-value <0.001. There was no difference between unadjusted and age-adjusted IRR.
Discussion
Low usage of hydroxyurea is a major obstacle to optimized care for SCD in Sub-Saharan Africa. Here, we show for the first time in Tanzania that perceived susceptibility, severity, and threat of SCD complications among caregivers predicted hydroxyurea usage among their children with SCD who have unrestricted access to hydroxyurea through health insurance. Furthermore, we show that caring for children with SCD aged below 5 years of age and being attended at lower-level healthcare facilities were associated with low SCD threat perception. These findings have implications for public health measures aimed at speeding up the usage of hydroxyurea among children with SCD in Sub-Saharan Africa.
The perceived threat of illness is influenced by a person’s socio-demographics and economic status.19 However, there is a paucity of literature on SCD threat perception. In our study, the proportion of caregivers with high SCD threat perception was 61%. The age of the SCD child was found to influence the caregiver’s perception of the threat of SCD. Caregivers of under-fives with SCD had the lowest perceived threat scores. This is similar to other studies where it has been noted that the longer the duration of chronic illness, the higher the perception of the threat of the illness.24 All children with SCD enrolled in this study had the HbSS genotype, which presents the most severe clinical symptoms compared to other genotypes (HbS/β-thalassemia and HbSC).7 As patients with SCD age, the likelihood of experiencing more complications, such as major organ damage, increases.7,36 Therefore, caregivers of older children may have experienced more negative consequences of the disease and understand the disease better than caregivers of children with SCD under five years old.
In our study, caregivers who attended at National hospitals (MNH Upanga and Mloganzila), the top referral hospitals in the country, had higher threat perception than caregivers who attended the regional referral hospitals (Amana and Temeke RRH). Even though medical specialists were available in all four hospitals, the differences in threat perceptions among caregivers may be caused by the difference in qualifications among other healthcare providers involved in the care of patients with SCD, such as medical doctors and nurses, and the SCD education provided in these hospitals. Unlike Amana and Temeke RRH, MNH is a super-specialized teaching hospital, and its SCD clinics are run by hematologists, hematology trainees, and nurses with vast experience in providing health education to patients and caregivers of SCD individuals. It was noted that SCD education was often provided by nurses in the patients’ waiting area before patients and their caregivers were attended to by doctors. Additionally, healthcare providers in regional referral hospitals in Dar es Salaam were found to have inadequate knowledge of SCD and its management in 2021.37 Health education has been found to improve caregivers’ and patients’ perceptions of diseases.38 Further, MNH is the National referral hospital, therefore the majority of SCD patients who attended clinics at MNH were likely to have been referred from other regional hospitals due to having acquired major SCD complications at one point. Experience of disease complications acts as a reminder of how the patients are susceptible to complications and associated effects of the disease to patients and caregivers.19
We also found that the majority (222/229) of caregivers who perceived the threat of SCD as higher reported using HU among their children. In contrast, majority (104/145) of caregivers with lower threat scores reported their children not using HU. We found that children of caregivers with high SCD threat perception were 3.4 times more likely to use HU than those of caregivers with low SCD threat perception (IRR 3.4, 95% CI 2.7–4.5, p-value <0.001). A higher perceived threat of SCD complications indicates a greater perceived need for treatment, hence the higher rate of hydroxyurea use. This is similar to other studies that have shown that high disease threat perception is associated with preventive behaviours to either avoid the acquisition of the disease or use prescribed medications to prevent getting complications related to the disease.29,39–42 Over 50% of participants had inadequate knowledge of SCD. This was shown by caregivers who did not perceive that a child with SCD is at risk of acquiring severe SCD complications, SCD having long-lasting complications, or SCD being associated with fatal complications. Inadequate SCD knowledge and negative perceptions towards SCD, such as believing that patients with SCD have shorter lives, and therefore, no need to treat or invest in them, have been mentioned in several studies as barriers to HU use.7,18,43 To increase the uptake of medication among patients with chronic diseases such as SCD, it is advised to provide adequate education to both patients and caregivers about the disease and the role of the prescribed medication in the management of the disease. Adequate SCD knowledge is associated with correct SCD threat perception, improved medication usage for SCD management, and overall improved quality of life among SCD patients.26,27,44,45
Although there was no statistically significant difference in threat scores among caregivers with different levels of education, we observed that caregivers with at least a primary-level education had higher perceived threat scores (median scores above 540) than caregivers who did not have formal education (median 387). Other studies have shown that education levels influence the threat perception of diseases.24 The lack of association between caregivers’ education level and SCD threat perception in our study may be caused by the exclusion of caregivers of uninsured children with SCD. Health insurance subscription is influenced by education level and socioeconomic status.46 Therefore, the exclusion of caregivers of uninsured children with SCD possibly led to the exclusion of a significant number of caregivers with low or no formal education and low SCD threat perception. We excluded these participants to control other factors that may limit access to HU among study participants. In this study, most caregivers were women (85.6%). Biological mothers constituted 72% of caregivers of children with SCD. We observed that mothers had consistently higher scores of perceived susceptibility, perceived severity, and perceived threat of SCD complications than fathers of children with SCD. However, the difference was not statistically significant. Women had a better understanding and correct perception of SCD because, most often, they are the primary caregivers of children and individuals with illnesses as has been observed in other studies.47,48
Study Limitations
A strength of this study was that we enrolled caregivers of health-insured children with SCD who attended public SCD clinics in Dar es Salaam where hydroxyurea was available, and therefore, we managed to control major factors that limit access to hydroxyurea such as high cost and unavailability of HU. This allowed us to study the effects of the perceived threat of SCD disease on HU usage in the absence of these major known HU use restrictions in SSA. Another key strength of this study was that we enrolled a large sample size and compared various caregiver subgroups across different levels of healthcare facilities. On the other hand, we did not enquire about the presence of comorbidities unrelated to SCD and the number of people in households with chronic illnesses, which could further explain the level of disease threat perception among caregivers with SCD children and potentially be another predictor of HU use. Therefore, the results are applicable to patients with access to HU such as health-insured patients, able to afford medication expenses or receive HU free of charge. However, it is important to note that the predictors could differ among other groups of patients with SCD who do not share the same access to HU.
Conclusion
In conclusion, the age of the SCD child and the hospital where the child attends have shown a strong association with caregivers’ perception of susceptibility, perceived severity, and perceived threat of SCD complications, and these perceptions are associated with the use of HU among children with SCD attending SCD clinics in Dar es Salaam, Tanzania. We recommend the provision of health education on SCD to caregivers to improve their knowledge and promote an accurate understanding of the potential adverse consequences of SCD, and at the same time promote other aims such as access to affordable health insurance and sufficient availability of HU and thus improve the use of hydroxyurea among children with SCD in similar settings in Sub-Saharan Africa. Further studies to compare different approaches to education intervention, such as using peer-patient educators or community health workers assigned to families with low perceived threat of SCD and those not using HU should be prioritized.
Acknowledgment
We acknowledge the SCD patients and their families who were willing to participate in this study. Without their participation, this study would not have been complete. We wish them good health and free of SCD-related complications. The draft of this manuscript is also available on Research Square as a preprint.
This paper has been uploaded to ResearchGate and Research Square as a preprint: http://dx.doi.org/10.21203/rs.3.rs-4350150/v1
Funding Statement
1) The corresponding author, MA is sponsored by a project titled “Strengthening Doctoral Education for Health in Tanzania (DOCEHTA)”, project number 69940, funded by The Norwegian Programme for Capacity Development in Higher Education and Research for Development (NORAD). 2) MA, EB, MY, and JM are supported by the Sickle Pan-African Research Consortium (SPARCO)-Tanzania, U01HL156853, funded by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institute of Health.
Data Sharing Statement
Data collection tools are attached in this manuscript as supplementary data. The dataset used during the current study are available from the corresponding author on reasonable request.
Author contributions
All authors made a significant contribution in conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising and critically reviewing the article; approved the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Inusa BPD, Hsu LL, Kohli N, et al. Sickle cell disease—genetics, pathophysiology, clinical presentation and treatment. Int J Neonatal Screen. 2019;5(2):20. doi: 10.3390/ijns5020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawuki J, Musa TH, Obore N, Papabathini SS. Sickle cell disease in East African countries: prevalence, complications and management. J Adv Med Med Res. 2019;1–9. doi: 10.9734/jammr/2019/v30i830220 [DOI] [Google Scholar]
- 3.Makani J, Cox SE, Soka D, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minja I, Wilson E, Machibya F, et al. Dental caries in children with sickle cell disease and its association with the use of hydroxyurea and penicillin prophylaxis in Dar es Salaam. Pediatric Health Med Ther. 2024;15:121–128. doi: 10.2147/phmt.s443139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrose EE, Makani J, Chami N, Masoza T. High birth prevalence of sickle cell disease in Northwestern. Pediatr Blood Cancer. 2018;65(1):1–17. doi: 10.1002/pbc.26735.High [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ally M, Balandya E. Current challenges and new approaches to implementing optimal management of sickle cell disease in sub-Saharan Africa. Semin Hematol. 2023;60(4):192–199. doi: 10.1053/j.seminhematol.2023.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makani J, Meda E, Rwezaula S, et al. Sickle cell anaemia in East Africa: preliminary results from a cohort study. Blood. 2006;108(11):3802. doi: 10.1182/blood.V108.11.3802.3802 [DOI] [Google Scholar]
- 9.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):1–22. doi: 10.1038/nrdp.2018.10 [DOI] [PubMed] [Google Scholar]
- 10.Makani J, Soka D, Rwezaula S, et al. Health policy for sickle cell disease in Africa: experience from Tanzania on interventions to reduce under-five mortality. Trop Med Int Health. 2015;20(2):184–187. doi: 10.1111/tmi.12428.Health [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in Sub-Saharan Africa. N Engl J Med. 2019;380(2):121–131. doi: 10.1056/nejmoa1813598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akingbola TS, Tayo B, Saraf SL, et al. Low fixed dose hydroxyurea for the treatment of adults with sickle cell disease in Nigeria. Blood. 2017;130(Suppl_1):981. doi: 10.1182/blood.V130.Suppl_1.981.981 [DOI] [Google Scholar]
- 13.Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;2017(4). doi: 10.1002/14651858.CD002202.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa E, Tibalinda P, Sterzi E, et al.. Making hydroxyurea affordable for sickle cell disease in Tanzania is essential (HASTE): how to meet major health needs at a reasonable cost. Am J Hematol. 2021;96(1). doi: 10.1002/ajh.26007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGann PT, Williams TN, Olupot-Olupot P, et al. Realizing effectiveness across continents with hydroxyurea: enrollment and baseline characteristics of the multicenter REACH study in Sub-Saharan Africa. Am J Hematol. 2018;93(4):537–545. doi: 10.1002/ajh.25034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrose EE, Kidenya BR, Charles M, et al. Outcomes of hydroxyurea accessed via various means and barriers affecting its usage among children with sickle cell anaemia in North-Western Tanzania. Journal of Blood Medicine. 2023;14:37–47. doi: 10.2147/JBM.S380901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandonga D, Sangeda RZ, Masamu U, et al.. Development of the sickle Pan-African research consortium registry in Tanzania : opportunity to harness data science for sickle cell disease. Front. Hematol. 2023:1–8. doi: 10.3389/frhem.2023.1040720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okocha EC, Gyamfi J, Ryan N, et al. Barriers to therapeutic use of hydroxyurea for sickle cell disease in Nigeria: a cross-sectional survey. Front Genet. 2022;12:1–7. doi: 10.3389/fgene.2021.765958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham C, Sheeran P. The health belief model. In: Cambridge Handbook of Psychology, Health and Medicine. 2014:97–102. doi: 10.1017/CBO9780511543579.022 [DOI] [Google Scholar]
- 20.Skinner CS, Tiro J, Champion VL, Skinner CS. The health belief model. In: Health behavior and health education. 2015:21. [Google Scholar]
- 21.Anuar H, Shah SA, Gafor H, Mahmood MI, Ghazi HF. Usage of health belief model (HBM) in health behavior: a systematic review. Malaysian J Med Health Sci. 2020;16:2636–9346. [Google Scholar]
- 22.Carpenter CJ. A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Commun. 2010;25(8):661–669. doi: 10.1080/10410236.2010.521906 [DOI] [PubMed] [Google Scholar]
- 23.Jose R, Narendran M, Bindu A, Beevi N, M L, Benny PV. Public perception and preparedness for the pandemic COVID 19: a health belief model approach. Clin Epidemiol Glob Health. 2021;9. doi: 10.1016/j.cegh.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melkamu L, Berhe R, Handebo S. Does patients’ perception affect self-care practices? The perspective of health belief model. Diabetes Metab Syndr Obes. 2021;14:2145–2154. doi: 10.2147/DMSO.S306752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz CL, Tchume-Johnson T, Schapira MM, Bellamy S, Smith-Whitley K, Ellison A. Adherence to prompt fever evaluation in children with sickle cell disease and the health belief model. Pediatr Blood Cancer. 2015;62(11):1968–1973. doi: 10.1002/pbc.25634 [DOI] [PubMed] [Google Scholar]
- 26.Asnani MR, Barton-Gooden A, Grindley M, Knight-Madden J. Disease knowledge, illness perceptions, and quality of life in adolescents with sickle cell disease: is there a link?. Glob Pediatr Health. 2017;4. doi: 10.1177/2333794X17739194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asnani MR, Quimby KR, Bennett NR, Francis DK. Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications. Cochrane Database Syst Rev. 2016;2016(10). doi: 10.1002/14651858.CD011175.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makani J, Sangeda RZ, Nnodu OE, Nembaware V, Osei Okoto A, Paintsil V. SickleInAfrica. Lancet Haematol. 2020;7(2):98–99. doi: 10.1016/S2352-3026(20)30006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreira CB, Dahinten VS, Howard AF, Fernandes AFC. The revised champion’s health belief model scale: predictive validity among Brazilian women. SAGE Open Nurs. 2020;6. doi: 10.1177/2377960820940551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kueh MTW, Rahim FF, Rashid A. Development and validation of the health belief model questionnaire to promote smoking cessation for nasopharyngeal cancer prevention: a cross-sectional study. BMJ Open. 2022;12(9):e057552. doi: 10.1136/bmjopen-2021-057552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa MF. Health belief model for coronavirus infection risk determinants. Rev Saude Publica. 2020;54:1–11. doi: 10.11606/S1518-8787.2020054002494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champion VL. Instrument development for health belief model constructs. ANS Adv Nurs Sci. 1984;6(3):73–85. doi: 10.1097/00012272-198404000-00011 [DOI] [PubMed] [Google Scholar]
- 33.Parsa P, Mohd Nasir MT, Hejar AR, Nor Afiah MZ. Reliability and validity of champion’s health belief model scale for breast cancer screening among Malaysian women. Singapore Med J. 2008;49(11):897. [PubMed] [Google Scholar]
- 34.Mohamed NC, Moey SF, Lim BC. Validity and reliability of health belief model questionnaire for promoting breast self-examination and screening mammogram for early cancer detection. Asian Pac J Cancer Prev. 2019;20(9):2865–2873. doi: 10.31557/APJCP.2019.20.9.2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huaman MA, Kamimura-Nishimura KI, Kanamori M, Siu A, Lescano AG. Validation of a susceptibility, benefits, and barrier scale for mammography screening among Peruvian women: a cross-sectional study. BMC Women's Health. 2011;11(1):54. doi: 10.1186/1472-6874-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makani J, Ofori-Acquah SF, Nnodu O, Wonkam A, Ohene-Frempong K. Sickle cell disease: new opportunities and challenges in Africa. Sci World J. 2013;2013. doi: 10.1155/2013/193252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonathan A, Tutuba H, Lloyd W, et al. Healthcare workers’ knowledge and resource availability for care of sickle cell disease in dar es Salaam, Tanzania. Front Genet. 2022;12:1–10. doi: 10.3389/fgene.2021.773207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azadi NA, Ziapour A, Lebni JY, Irandoost SF, Abbas J, Chaboksavar F. The effect of education based on health belief model on promoting preventive behaviors of hypertensive disease in staff of the Iran University of medical sciences. Arch Public Health. 2021;79(1). doi: 10.1186/s13690-021-00594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vacondio M, Priolo G, Dickert S, Worry BN. Perceived threat and media communication as predictors of self-protective behaviors during the COVID-19 outbreak in Europe. Front Psychol. 2021;12. doi: 10.3389/fpsyg.2021.577992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ristvedt S, Trinkaus K, Waters E, James A. Threat sensitivity is associated with the healthcare source used most often: doctor’s office, emergency room, or none at all. Heliyon. 2019;5(5):e01685. doi: 10.1016/j.heliyon.2019.e01685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinley CJ, Ruppel EK. Exploring how perceived threat and self-efficacy contribute to college students’ use and perceptions of online mental health resources. Comput Human Behav. 2014;34:101–109. doi: 10.1016/j.chb.2014.01.038 [DOI] [Google Scholar]
- 42.Chen MF, Wang RH, Schneider JK, et al. Using the health belief model to understand caregiver factors influencing childhood influenza vaccinations. J Community Health Nurs. 2011;28(1):29–40. doi: 10.1080/07370016.2011.539087 [DOI] [PubMed] [Google Scholar]
- 43.Kilonzi M, Mlyuka HJ, Felician FF, et al. Barriers and facilitators of use of hydroxyurea among children with sickle cell disease: experiences of stakeholders in Tanzania. Hemato. 2021;2(4):713–726. doi: 10.3390/hemato2040048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houwing ME, Grohssteiner R, Teuben SAMC, et al. Health literacy, self-efficacy and knowledge of sickle cell disease among caregivers. Blood. 2019;134(Supplement_1):4841. doi: 10.1182/blood-2019-129888 [DOI] [Google Scholar]
- 45.Ezenwosu O, Chukwu B, Ndu I, et al. Effect of health education on knowledge and awareness of sickle cell disease among adolescents. Sahel Med J. 2021;24(1):43–47. doi: 10.4103/smj.smj_9_20 [DOI] [Google Scholar]
- 46.Maina JM, Kithuka P, Tororei S. Perceptions and uptake of health insurance for maternal care in rural Kenya: a cross sectional study. Pan Afr Med J. 2016;23. doi: 10.11604/pamj.2016.23.125.8936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rico-Blázquez M, Quesada-Cubo V, Polentinos-Castro E, et al. Health-related quality of life in caregivers of community-dwelling individuals with disabilities or chronic conditions. A gender-differentiated analysis in a cross-sectional study. BMC Nurs. 2022;21(1). doi: 10.1186/s12912-022-00845-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson KE, Engel C, Fietz H. The chronicity of home-making: women caregivers in disabling spaces. Space and Cult. 2023;26(3):468–482. doi: 10.1177/12063312231181534 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collection tools are attached in this manuscript as supplementary data. The dataset used during the current study are available from the corresponding author on reasonable request.