Abstract
Chemotherapy of tuberculosis caused by multiple-drug-resistant (MDR) strains is problematic because of choices limited to relatively inefficacious and toxic drugs. Some beta-lactam antibiotics are active against Mycobacterium tuberculosis in vitro. We investigated the efficacy of imipenem in a mouse model of tuberculosis and in humans with MDR tuberculosis. Mice infected with M. tuberculosis strain H37Rv were treated with isoniazid or imipenem. Residual organisms in lung and spleen and survival of imipenem-treated mice were compared to those of untreated or isoniazid-treated mice. Ten patients with MDR tuberculosis also were treated with imipenem in combination with other first- or second-line agents; elimination of M. tuberculosis from sputum samples was measured by quantitative culture. Although it was less effective than isoniazid, imipenem significantly reduced the numbers of M. tuberculosis organisms in lungs and spleens and improved survival of mice. Eight of 10 patients with numerous risk factors for poor outcomes responded to imipenem combination therapy with conversion of cultures to negative. Seven remained culture-negative off of therapy. There were two deaths, one of which was due to active tuberculosis. Organisms were eliminated from the sputa of responders at a rate of 0.35 log10 CFU/ml/week. Relapse upon withdrawal of imipenem and development of resistance to imipenem in a nonresponder suggest that imipenem exerts antimycobacterial activity in humans infected with M. tuberculosis. Imipenem had antimycobacterial activity both in a mouse model and in humans at high risk for failure of treatment for MDR tuberculosis.
Treatment of tuberculosis caused by multiple-drug-resistant (MDR) (i.e., resistant to both isoniazid [INH] and rifampin) strains of Mycobacterium tuberculosis (MDR TB) is a challenge. Second-line antituberculous agents are less efficacious and more toxic than first-line drugs (5, 13). Fluoroquinolones, which can significantly improve response rates, are an important recent addition to the therapeutic armamentarium (13, 17), but other alternatives are desperately needed.
Beta-lactams have not been regarded as useful drugs for treatment of tuberculosis because M. tuberculosis is naturally resistant to most of these antibiotics in vitro. Resistance is thought to be mediated by a class A beta-lactamase that hydrolyzes penicillins and cephalosporins (4, 6, 8). Resistance may be overcome by two means. One is inhibition of the beta-lactamase, and the other is the use of an antibiotic that is not a substrate for it. An example of the former is the use of the beta-lactam-beta-lactamase inhibitor combination amoxicillin-clavulanate, which is active in vitro (3) and has early bactericidal activity in patients with pulmonary tuberculosis (1). Anecdotally, amoxicillin-clavulanate in combination with other second-line agents has been successfully used in selected patients infected with MDR strains (11, 19). This approach has met considerable skepticism, and the role, if any, of amoxicillin-clavulanate remains unclear (18).
Carbapenems offer a second approach for overcoming beta-lactam resistance of M. tuberculosis. They are poor substrates for both class A and class C enzymes, and two carbapenems, meropenem and imipenem (IMI), are active in vitro against M. tuberculosis (15). Our experience with imipenem in mice infected with tuberculosis and in 10 patients with MDR TB indicates that further clinical evaluation of these potentially useful agents is warranted.
MATERIALS AND METHODS
Mouse model of tuberculosis.
These experiments were conducted in accordance with a protocol approved by the University of California San Francisco Committee on Animal Research. Infection was established in strain CD-1 female mice by tail vein injection of a 0.1-ml suspension of M. tuberculosis strain H37Rv in PBS plus 0.05% Tween-80 containing approximately 5 × 108 CFU/ml. The MICs of isoniazid and imipenem for this strain are 0.1 and 4 μg/ml, respectively (2). Mice were untreated or received either imipenem, 100 mg/kg of body weight, subcutaneously twice daily (b.i.d.), or isoniazid (Apothecon), 25 mg/kg, subcutaneously once daily, a standard regimen for this model (9). Imipenem serum concentrations, measured by an agar diffusion bioassay method with Escherichia coli strain ATCC 25922, achieved in uninfected mice 1 hour after single 100 mg/kg doses were a mean (± standard deviation) of 10.1 ± 3.8 μg/ml (n = 3), and the half-life of imipenem was 24 min. Antibiotic was administered on a Monday-through-Friday schedule starting 2 to 3 days after inoculation. Untreated mice were sacrificed, and spleens and lungs were harvested on days 0 (defined as the first day that antibiotics were administered), 14, and 28 of the experiment. Treated mice were sacrificed, and spleens and lungs were harvested on days 14 and 28 approximately 3 days after their last doses of antibiotic. Mice that died prior to the scheduled sacrifice time had spleens and lungs removed and stored at −20°C; these were processed and cultured at the scheduled sacrifice time. Spleens and lungs were weighed, suspended in 1 ml of 0.9% saline, and homogenized. The homogenate was serially diluted 10-fold in saline plus 0.05% Tween 80 and quantitatively cultured onto 7H10 agar. Colonies were counted after a 4-week incubation. Tissue burden of M. tuberculosis was expressed as log10 CFU per gram of tissue cultured.
Data from mice dying on or before day 14 were combined with data from those sacrificed on day 14; data from mice dying after day 14 were combined with data from those sacrificed at day 28. Analysis of variance was used to determine whether differences between or among groups achieved statistical significance, defined as a P value of <0.05. Kaplan-Meier survival curves were used to compare the treatment outcomes over time, and the log rank test was used to test differences in survival over the 28-day treatment period for statistical significance.
Human studies.
These studies were approved by the University of California San Francisco Committee on Human Research and conducted in association with the General Clinical Research Center of the University of California San Francisco at San Francisco General Hospital. To be eligible, patients had to have tuberculosis caused by a strain resistant to both INH and rifampin. Exclusion criteria were inability to provide informed consent, serious underlying medical illnesses (e.g., liver failure, renal failure, decompensated heart failure), and hypersensitivity or contraindication to beta-lactam antibiotics or aminoglycoside. Patients were admitted to the General Clinical Research Center, and baseline sputum samples were obtained for quantitative culturing. Patients then received 1 g imipenem twice daily (30 mg/kg in two divided doses for patients weighing less than 50 kg) intravenously administered as a 1- to 2-h infusion, in addition to other antituberculous agents at standard doses (12). Whenever possible, amikacin at 15 mg/kg once daily five to seven times a week (dose adjusted based on renal function) intravenously or streptomycin administered intravenously at the same dose was included in the regimen. Infusions were administered through a percutaneously inserted central venous catheter. Patients were discharged home after approximately 2 weeks, and self-administered therapy was begun under the supervision of a visiting nurse. Sputum samples were obtained at regular intervals for quantitative culturing (1, 16). Parenteral agents were discontinued at approximately 6 months into treatment (range, 4 to 9 months; see Table 1 and Results for exceptions), and therapy was completed with an oral regimen of three or more drugs to which the organism was susceptible in vitro for a total duration of therapy of 18 to 24 months.
TABLE 1.
Clinical summary of 10 cases of multiple-drug resistant tuberculosisa
| Patient | Duration of active TB prestudy | Resistances in prestudy isolates | Prestudy regimen | Study regimen (no. of mo IMI was administered) | Weeks to negative culture | Outcome (yr of follow-up off of therapy) |
|---|---|---|---|---|---|---|
| 1 | 11 yr | INH, RIF, PZA, EMB, ETH, OFX, STM | CS, CLO, EMB, PAS, STM | IMI (6), AMK, CLO, CS, EMB, PAS | 7.5 | Cured (4) |
| 2 | 1 mo | INH, RIF, PZA, EMB, STM | INH, RIF, PZA, EMB | IMI (6), AMK, OFX | 9.5 | Cured (0.5) |
| 3 | 2 yr | INH, RIF, PZA, EMB, KAN, STM, ETH, PAS | AMK, CIP, CS, PZA | IMI (6), AMK, CLO, ETH#, OFX | 1 | Cured (3) |
| 4 | 1.5 yr | INH, RIF, PZA, EMB, AMK, CAP, KAN, ETH, OFX, RBT | CS, EMB, OFX, STM | IMI (4.5), STM, CLO, CS | >18 | Failed |
| 5 | 1 mo | INH, RIF, PZA, EMB, STM, ETH | INH, RIF, PZA, EMB | IMI (8), AMK, CIP, CS | 9.5 | Cured (3) |
| 6 | 10 mo | INH, RIF, EMB, STM, RBT | PZA, EMB, CIP, STM | IMI (4), AMK, OFX, ETH, EMB | 12 | Cured (2) |
| 7 | 1.5 yr | INH, RIF, EMB, STM, ETH, RBT, OFX‡ | EMB, PZA, CAP, OFX | IMI (4), AMK, CLO, CS, OFX‡ | 12 | Cured (2) |
| IMI (9), AMK, CLO, EMB, PZA, CS | 8 | |||||
| 8 | 1 mo | INH, RIF, AMK, STM, KAN, PZA | LEVO, CAP, PZA, CS | IMI (9), CAP, PZA, CS, LEVO | 16 | Cured (2) |
| IMI (5.5), CAP, PZA, CS, LEVO | 1 | |||||
| 9 | 24 yr | INH; RIF, PZA, EMB, AMK, KAN, STM, OFX, PAS, CS | INH, CS, CIP | IMI (7.5), CAP, EMB, CLO, ETH# | >32 | Failed |
| 10 | 1 yr | INH, RIF, PZA, STM, ETH | LEVO, EMB, CLO, CS | IMI (6), AMK, EMB, LEVO, CS, CLO | — | Partial response, relapse |
Abbreviations (dose used in the study regimen): AMK, amikacin (10 to 15 mg/kg/day); CAP, capreomycin (750 to 1,000 mg every day [qd]); CLO, clofazimine (200 mg/d); CIP, ciprofloxacin (750 mg b.i.d.); CS, cycloserine (250 mg b.i.d. to three times a day [t.i.d.]); EMB, ethambutol (50 mg/kg twice a week [b.i.w.]); ETH, ethionamide (250 mg b.i.d. to t.i.d.); IMI, imipenem (1 g every 12 h); INH, isoniazid; KAN, kanamycin; LEVO, levofloxacin (500 mg qd); OFX, ofloxacin (600 to 800 mg qd); PAS, para-aminosalicylic acid; PZA, pyrazinamide (25 mg/kg/day or 50 mg/kg b.i.w.); RBT, rifabutin; RIF, rifampin; STM, streptomycin. A double dagger (‡) indicates that the isolate was originally susceptible to ofloxacin but that resistance emerged during therapy. A pound sign (#) indicates that ETH at 250 mg b.i.d. was initially included in the regimen but was discontinued after 3 months due to intolerance. The dash (—) indicates that weeks to culture negativity were not determined, but cultures were negative at endoscopy performed after 6 months of therapy.
Susceptibility studies.
Susceptibility to antibiotics other than imipenem was determined either by an agar proportion method or by a Bactec broth dilution method (Becton-Dickenson Diagnostic Instrumentation Systems, Sparks, MD) (7). Instability of imipenem, which has a half-life of only 9.6 h in aqueous medium, and the variable and slow growth of MDR isolates prevented accurate and reproducible determination of in vitro susceptibility to imipenem. To test for the emergence of resistance, the relative susceptibilities of pretreatment and posttreatment isolates were compared by using Bactec 12B broth inoculated with 103 to 104 CFU/ml of M. tuberculosis. When growth corresponding to 100 to 300 cpm was achieved, imipenem at concentrations of 4, 8, or 16 μg/ml was added. An untreated vial served as the control.
RESULTS
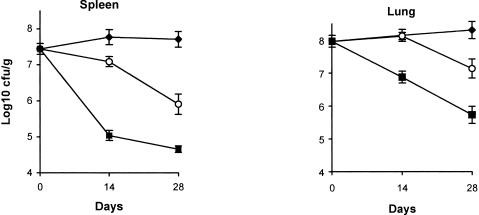
Mice treated with imipenem had significantly fewer organisms than untreated mice (Fig. 1). By day 28, imipenem-treated mice had 1.8 log10 CFU/g fewer organisms in splenic tissue (P < 0.001) and 1.2 log10 CFU/g fewer in lung tissue (P < 0.04) than untreated mice. Isoniazid was more effective than imipenem in eliminating organisms from both spleen and lung (P < 0.001 for each organ).
FIG. 1.
Mean burdens of M. tuberculosis strain H37Rv as log10 CFU/g in spleen and lung tissues. The numbers of mice are as follows: for untreated mice (black diamonds), 14, 19, and 14 on days 0, 14, and 28, respectively; for imipenem-treated mice (open circles), 22 and 15 for days 14 and 28, respectively; and for isoniazid-treated (black squares), 14 and 15 for days 14 and 28, respectively. The standard errors of the means are indicated by the bars.
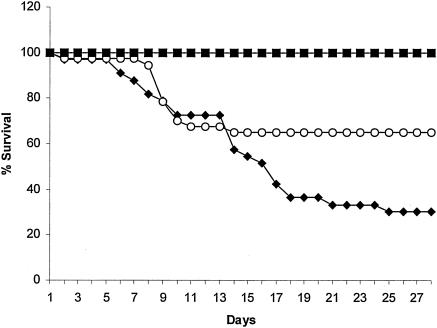
Imipenem-treated mice also had improved survival compared to controls, with a 28-day mortality rate of 35% versus 70% (Fig. 2) (P = 0.016 by log rank test). The survival benefit was delayed, however, with similar mortality rates for untreated and imipenem-treated mice during the first 2 weeks of treatment. Survival of INH-treated mice was 100% (P values of <0.001 versus imipenem).
FIG. 2.
Survival curves for untreated mice (black diamonds), imipenem-treated mice (open circles), and isoniazid-treated mice (black squares).
Ten patients, nine with smear-positive pulmonary infection and one with AIDS and gastrointestinal tuberculosis, received imipenem in combination with two or more other agents. The mean age (± standard deviation) was 45.3 ± 12.8 years (range of 26 to 61 years). Eight patients were foreign born (five Asians and three Mexican-Americans), and five were women. Eight had radiographic evidence of cavitary disease. The clinical isolates were resistant to 7 ± 2 antituberculous agents (range, 5 to 10) (Table 1); four isolates were fluoroquinolone resistant. With the exceptions that it produced rash (two patients) and occasional diarrhea, the imipenem combination regimen was well tolerated.
Eight patients (95% confidence interval, 50 to 100%) initially responded with conversion of cultures to negative. Seven patients (95% confidence interval, 35 to 100%) remained culture negative off of therapy and were considered cured. Two patients died.
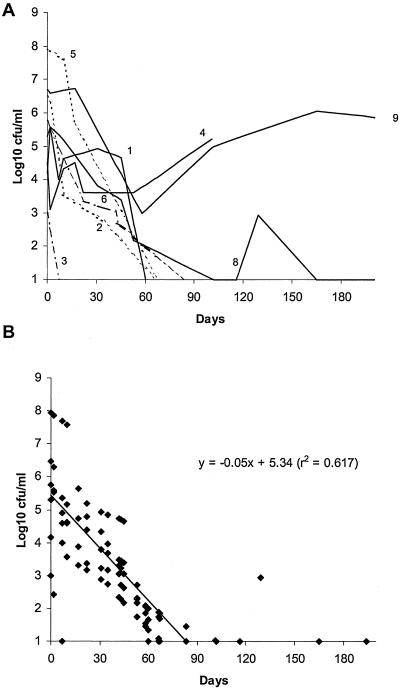
Six of the eight patients who responded had serial quantitative cultures performed. These showed a continuous and sustained reduction of mycobacterial burden in sputa averaging approximately 0.35 log10 CFU/ml/week (Fig. 3). Patients 7 and 10 had only qualitative cultures performed. Patient 7, with 40 prestudy weeks of documented positive smears and cultures, became smear and culture negative after 3 months of imipenem plus other agents. Patient 10, with smear-positive and culture-positive tuberculosis, with ulcerations and perforation of the stomach and duodenum, had healing documented by follow-up endoscopy after 6 months of imipenem combination therapy. Smears and cultures of the duodenal biopsy specimen were negative for M. tuberculosis.
FIG. 3.
Results of quantitative sputum cultures indicating M. tuberculosis burdens, expressed as log10 CFU/ml, over time in sputa of individual patients (numbers correspond to patient numbers in Table 1) (A) and as a calculation of the overall elimination rate by linear regression for those patients who responded to treatment (B). The linear regression equation is shown.
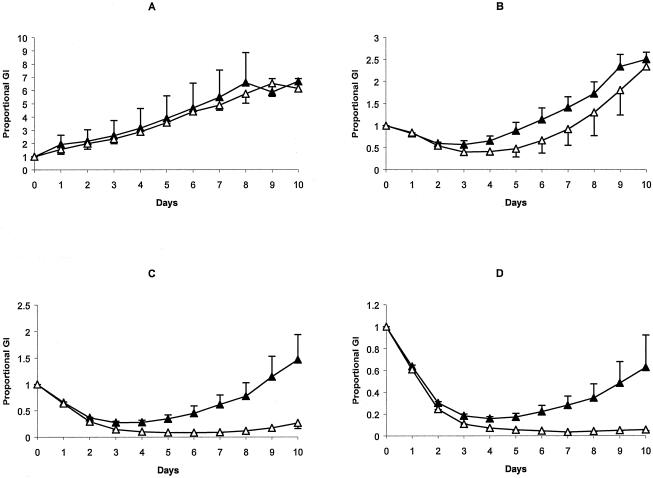
Two patients, patients 4 and 9, each of whom had an isolate that was resistant to 10 drugs, were treatment failures. In both cases, there were transient reductions in the numbers of organisms in sputa, but these eventually returned to pretreatment levels (Fig. 3A). The 18-week clinical isolate from patient 4 had acquired secondary resistance to streptomycin during treatment. This isolate also had acquired resistance to imipenem, as it was able to grow slowly in the presence of imipenem at concentrations of 8 and 16 μg/ml; growth of the prestudy isolate was inhibited at these concentrations (Fig. 4). Whether resistance was beta-lactamase mediated, was due to target alterations, or was a penetration defect was not determined.
FIG. 4.
Means and standard errors for proportional growth indices (GI) (the ratio of counts per minute on each day to counts per minute for the strain at day 0) for three separate experiments performed with starting growth indices ranging between 100 and 300. The open triangles indicate the prestudy isolate, and the closed triangles indicate the 18-week isolate for untreated cultures (A) or cultures to which imipenem at concentrations of 4 μg/ml (B), 8 μg/ml (C), or 16 μg/ml (D) had been added on day 0.
Three patients, i.e., patients 7, 8, and 10, relapsed shortly after the discontinuation of imipenem and aminoglycoside or capreomycin in the regimen. In patient 7, 3 months after discontinuing imipenem and amikacin, given for 4 months, smears and cultures were again positive, and secondary resistance to ofloxacin was documented. Imipenem and amikacin were reinstituted, with smears and cultures converting to negative after 1 and 2 months, respectively. Imipenem, amikacin, and oral agents were administered for another 9 months; patient 7 remained culture negative for the 36-month follow-up period.
Patient 8, who had a single positive culture on week 18 of imipenem therapy, received an initial course of 9 months of imipenem and capreomycin in addition to oral agents. Three months after imipenem and capreomycin were discontinued, multiple sputum smears and a single culture were again positive. Smears and cultures again converted to negative during a second 5.5-month course of imipenem and capreomycin in addition to oral medications, which were given for a total of 18 months after the last positive culture. This patient had no evidence of active tuberculosis after 2 years of follow-up off of therapy.
Patient 10 relapsed 6 months after the discontinuation of imipenem, which was stopped because of severe rash, and died of disseminated tuberculosis. Patient 2 died of cor pulmonale and chronic respiratory insufficiency 6 months after completing therapy; there was no evidence of active tuberculosis.
DISCUSSION
Proving the clinical efficacy of any agent, new or old, for treatment of tuberculosis, especially that due to MDR strains, is exceedingly difficult. The need for drug combinations in treating active tuberculosis and the lack of a standardized regimen for comparison confounds the assessment of the activity of any single agent and its contribution to the outcome. The need to control for the many variables known to affect outcome (5, 13, 17), including prior drug-exposure history, duration of disease, the number of drug resistances, prior treatment with or resistance to fluoroquinolones, and the presence of cavitary disease, further complicates study design. Thus, medical therapy of MDR tuberculosis is based almost entirely on noncomparative observational studies, case series, and case reports, despite their weaknesses and limitations.
With this in mind, the following conclusions about the efficacy of imipenem seem warranted. The murine studies indicate that imipenem as a single agent has activity against tuberculosis: it reduced the numbers of organisms in target organs and improved survival. These results are particularly encouraging given the brevity of the period of exposure of organisms to imipenem in the infected mice, because of a short half-life and lower serum concentrations in mice compared to humans.
Viewed within the context of the animal studies, even though only 10 patients were treated, the data indicate that imipenem was therapeutically active and useful. There was a strong temporal association between the institution of imipenem combination therapy and the elimination of M. tuberculosis in sputa as assayed by quantitative culture. While it can be argued that it was other agents, and not imipenem, that were responsible for this, it is likely that imipenem contributed significantly to the overall efficacy. As a group, these patients were at high risk for treatment failure: nine were smear positive at study entry, eight had cavitary disease, eight had previously received a fluoroquinolone, and four had a fluoroquinolone-resistant isolate. The prestudy isolate average was seven drug resistances, and no isolate was resistant to fewer than five drugs. Our patients were quite similar to those reported by Goble et al. (5), as were cure rates, i.e., 70% versus 59%. Cure rates (77 to 96%) higher than these have been reported previously (13, 14, 17), but the patients in those studies were infected by strains resistant to fewer drugs, typically five or fewer, and fluoroquinolones were used.
Individual responses further indicate that imipenem had antimycobacterial activity. Case 1 had been culture positive for 11 years, failing pneumonectomy and numerous courses of directly observed therapy. Compared to the most recent prestudy regimen, imipenem was the only substantive addition, although amikacin was substituted for streptomycin because of secondary resistance. In case 2, conversion of sputum cultures to negative was accomplished with a regimen of only three drugs, i.e., ofloxacin, imipenem, and amikacin. Case 4 is particularly instructive, because microbiologic failure was associated with the emergence of resistance to imipenem as well as to streptomycin. Ironically, failure associated with emergence of resistance is perhaps the best evidence of drug activity in vivo (10), with the caveat that drug potency is difficult to assess because less-active drugs still will select for resistant mutants. Three patients, i.e., patients 7, 8 and 10, after initially responding to imipenem plus amikacin or capreomycin, relapsed when these drugs were discontinued from the regimen. Two remained culture negative for at least 2 years and presumably were cured after a second course of imipenem combination therapy. The third patient, who could not be retreated because of a severe allergy to imipenem and who had AIDS, eventually died of disseminated tuberculosis.
The fact that imipenem must be given parenterally is a drawback because of increased cost associated with this mode of drug administration and added risks associated with maintaining long-term vascular access. However, with the assistance of a visiting nurse, the patients in this study learned to self-administer the drug. Because prolonged administration of an injectable aminoglycoside or capreomycin is often required in the treatment of MDR TB, imipenem and either streptomycin, amikacin, or capreomycin were administered intravenously to avoid weeks to months of painful daily intramuscular injections. Parenteral administration of drugs also can be an advantage, because there are only limited choices of parenteral agents for those who are poorly compliant, who are unable to take oral medications, or who have malabsorption.
These clinical data and the experimental animal data indicate that imipenem is likely to be clinically useful in selected patients infected with MDR organisms. It may be considered for use in cases where the choice of second-line agents is extremely limited because of resistance or intolerance. Further studies are needed to document its efficacy relative to those of other drugs and to define which companion drugs are most efficacious. Aminoglycosides in particular should be examined. An aminoglycoside or capreomycin was used in conjunction with imipenem in all 10 cases, making it impossible to separate their individual contributions. In addition, aminoglycosides enhance beta-lactam antibiotic in vitro activity against numerous bacterial species, including M. tuberculosis (unpublished data). Ideally, imipenem would be evaluated in a randomized trial employing some measure of early bactericidal activity, in which a standardized regimen is compared to a standardized regimen with imipenem. Testing a regimen intended for use in MDR tuberculosis in patients with active infections caused by drug-susceptible strains is probably not an option, given that the regimen is likely to be less effective and more toxic than the standard short-course isoniazid-rifampin regimen. Studying imipenem probably is not feasible in the United States or other industrialized countries, where tuberculosis and MDR case rates are too low. Study sites would have to be established in developing countries where both tuberculosis and multiple-drug-resistant disease are prevalent. This would be difficult and expensive, requiring commitment of significant resources for infrastructure, laboratory support, and personnel to assure adequate follow-up.
Acknowledgments
This research was supported by the U.S. Public Health Service grant AI-33702 from the National Institutes of Health (H.F.C.). This work was conducted at the General Clinical Research Center at San Francisco General Hospital (RR00083-35) with support from the National Center for Research Resources.
H.F.C. is a shareholder in Merck as part of a diversified portfolio.
REFERENCES
- 1.Chambers, H. F., T. Kocagoz, T. Sipit, J. Turner, and P. C. Hopewell. 1998. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin. Infect. Dis. 26:874-877. [DOI] [PubMed] [Google Scholar]
- 2.Chambers, H. F., D. Moreau, D. Yajko, C. Miick, C. Wagner, C. Hackbarth, S. Kocagoz, E. Rosenberg, W. K. Hadley, and H. Nikaido. 1995. Can penicillins and other β-lactam antibiotics be used to treat tuberculosis? Antimicrob. Agents Chemother. 39:2620-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cynamon, M. H., and G. S. Palmer. 1983. In vitro activity of amoxicillin in combination with clavulanic acid against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 24:429-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finch, R. 1986. Beta-lactam antibiotics and mycobacteria. J. Antimicrob. Chemother. 18:6-8. [DOI] [PubMed] [Google Scholar]
- 5.Goble, M., M. D. Iseman, L. A. Madsen, D. Waite, L. Ackerson, and C. R. Horsburgh, Jr. 1993. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N. Engl. J. Med. 328:527-532. [DOI] [PubMed] [Google Scholar]
- 6.Hackbarth, C. J., I. Unsal, and H. F. Chambers. 1997. Cloning and sequence analysis of a class A β-lactamase from Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 41:1182-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heifets, L. B. 1994. Antimycobacterial drugs. Semin. Respir. Infect. 9:84-103. [PubMed] [Google Scholar]
- 8.Kasik, J. 1965. The nature of mycobacterial penicillinase. Am. Rev. Respir. Dis. 91:117-119. [DOI] [PubMed] [Google Scholar]
- 9.Klemens, S. P., C. A. Sharpe, M. C. Rogge, and M. H. Cynamon. 1994. Activity of levofloxacin in a murine model of tuberculosis. Antimicrob. Agents Chemother. 38:1476-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall, G., J. W. S. Blacklock, C. Cameron, N. B. Capon, R. Cruickshank, et al. 1948. Streptomycin treatment of pulmonary tuberculosis: a medical research council investigation. Br. Med. J. 2:769-782. [Google Scholar]
- 11.Nadler, J. P., J. Berger, J. A. Nord, R. Cofsky, and M. Saxena. 1991. Amoxicillin-clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest 99:1025-1026. [DOI] [PubMed] [Google Scholar]
- 12.Petri, W. A. 2001. Drugs used in the chemotherapy of tuberculosis, Mycobacterium avium complex disease, and leprosy, p. 1273-1294. In J. G. Hardman and L. E. Limbard (ed.), The pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, N.Y.
- 13.Tahaoglu, K., T. Torun, T. Sevim, G. Atac, A. Kir, L. Karasulu, I. Ozmen, and N. Kapakli. 2001. The treatment of multidrug-resistant tuberculosis in Turkey. N. Engl. J. Med. 345:170-174. [DOI] [PubMed] [Google Scholar]
- 14.Telzak, E. E., K. Sepkowitz, P. Alpert, S. Mannheimer, F. Medard, W. el-Sadr, S. Blum, A. Gagliardi, N. Salomon, and G. Turett. 1995. Multidrug-resistant tuberculosis in patients without HIV infection. N. Engl. J. Med. 333:907-911. [DOI] [PubMed] [Google Scholar]
- 15.Watt, B., J. R. Edwards, A. Rayner, A. J. Grindey, and G. Harris. 1992. In vitro activity of meropenem and imipenem against mycobacteria: development of a daily antibiotic dosing schedule. Tuber. Lung Dis. 73:134-136. [DOI] [PubMed] [Google Scholar]
- 16.Yajko, D. M., C. Wagner, V. J. Tevere, T. Kocagoz, W. K. Hadley, and H. F. Chambers. 1995. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J. Clin. Microbiol. 33:1944-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yew, W. W., C. K. Chan, C. H. Chau, C. M. Tam, C. C. Leung, P. C. Wong, and J. Lee. 2000. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 117:744-751. [DOI] [PubMed] [Google Scholar]
- 18.Yew, W. W., and C. H. Chau. 1996. New antimycobacterial agents. Monaldi Arch. Chest Dis. 51:394-404. [PubMed] [Google Scholar]
- 19.Yew, W. W., C. F. Wong, J. Lee, P. C. Wong, and C. H. Chau. 1995. Do beta-lactam-beta-lactamase inhibitor combinations have a place in the treatment of multidrug-resistant pulmonary tuberculosis? Tuber. Lung Dis. 76:90-92. [DOI] [PubMed] [Google Scholar]