Abstract
Background
Co-inhibition of immune checkpoints lymphocyte-activation gene 3 (LAG-3) and PD-1 is believed to enhance cancer immunotherapy through synergistic effects. Herein, we evaluate the safety and efficacy of IBI110 (anti-LAG-3 antibody) with sintilimab (an anti-PD-1 antibody) in Chinese patients with advanced solid tumors.
Methods
In this open-label phase I study, phase Ia dose escalation of IBI110 monotherapy and phase Ib combination dose escalation of IBI110 plus sintilimab were conducted in patients with advanced solid tumors. Additionally, phase Ib combination dose expansion of IBI110 plus sintilimab and chemotherapy was conducted in previously untreated, advanced squamous non-small cell lung cancer (sqNSCLC) and HER-2 negative gastric cancer (GC). In phase Ia dose escalation, patients received IBI110 monotherapy at 0.01/0.1/0.3/1/3/10/20 mg/kg Q3W. In phase Ib dose escalation, patients received IBI110 at 0.3/0.7/1.5/3/5/8/10 mg/kg Q3W plus sintilimab 200 mg Q3W. In phase Ib combination dose expansion, patients received IBI110 at recommended phase 2 dose (RP2D) plus sintilimab 200 mg Q3W and chemotherapy. The primary endpoints were safety, tolerability and efficacy including objective response rate (ORR), disease control rate (DCR), duration of response (DoR), progression-free survival (PFS) assessed by RECIST v1.1 and overall survival (OS). The secondary endpoints included pharmacokinetics, pharmacodynamics and immunogenicity.
Results
In phase Ia dose escalation (n = 28), treatment-related adverse events (TRAEs) occurred in 67.9% patients and grade ≥ 3 TRAEs occurred in 21.4% patients. In phase Ib combination dose escalation (n = 45), TRAEs occurred in 75.6% patients and grade ≥ 3 TRAEs occurred in 22.2% patients. No dose-limiting toxicity (DLT) was observed. The most common TRAE was anemia (17.9%, including 3.6% ≥ G3) in phase Ia dose escalation of IBI110 monotherapy (n = 28), aspartate aminotransferase increased (28.9%, all G1-G2) in phase Ib dose escalation of IBI110 plus sintilimab (n = 45), anemia (70.0%, all G1-G2) in phase Ib dose expansion in sqNSCLC (n = 20), and neutrophil count decreased (64.7%, including 17.6%≥ G3) in phase Ib dose expansion in GC (n = 17). The RP2D of IBI110 was determined at 200 mg (3 mg/kg) Q3W. ORR in phase Ia/Ib dose escalation was 3.6% with IBI110 monotherapy and 14% with IBI110 plus sintilimab. In phase Ib combination dose expansion of IBI110 plus sintilimab and chemotherapy, unconfirmed and confirmed ORR in sqNSCLC (n = 20) was 80.0% (95% CI, 56.3–94.3) and 75.0% (95% CI, 50.9–91.3), respectively and in GC (n = 17) was 88.2% (95% CI, 63.6–98.5) and 70.6% (95% CI, 44.0-89.7), respectively.
Conclusions
IBI110 monotherapy and in combination with sintilimab were well-tolerated in Chinese patients with advanced solid tumors. Encouraging efficacy of IBI110 in combination with sintilimab and chemotherapies was observed in sqNSCLC and GC.
Trial registration
ClinicalTrials.gov Identifier: NCT04085185.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01651-5.
Keywords: LAG-3, PD-1, Monoclonal antibody, Non-small cell lung cancer, Gastric cancer
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death receptor 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) have emerged as promising targets for developing new drugs to treat various malignancies [1]. Patients sensitive to ICI treatments showed improved efficacy and durable response; whereas some patients have no response to ICI treatments due to primary resistance or acquired ICI resistance following an initial treatment response [2]. Significant unmet medical needs remain to overcome resistance and optimize the clinical efficacy of ICIs. Beyond PD-1/L1 and CTLA-4, other immune checkpoints also play important roles in the response to ICI treatment and have emerged as promising novel immunotherapeutic targets [3, 4].
Lymphocyte-activation gene 3 (LAG-3, also known as CD223), mainly expressed on T cells and natural killer cells, is an inhibitory receptor to control T cell functions [5]. Inhibition of LAG-3 could block the interaction between LAG-3 and its ligand major histocompatibility complex (MHC) class II to relieve inhibitory effect of LAG-3 on T cell activation and enhance anti-tumor immune response of T cells [3–5]. Expression of multiple immune checkpoints has been reported to lead to T-cell exhaustion and induce immunosuppressive effects, potentially resulting in the failure of ICI treatment [6]. The co-expression of LAG-3 and PD-1 have been frequently observed, with synergic effects on T cell inhibition and promoting cancer immune escape [7]. Therefore, inhibition of LAG-3 alone and co-inhibition of LAG-3 and PD-1 appears to be promising approaches for improving current ICI treatments [8].
A number of LAG-3 inhibitors as monoclonal antibody, bispecific antibody or soluble protein have been developed [9]. The U.S. Food and Drug Administration recently approved the combination of relatlimab (anti-LAG-3 antibody) and nivolumab (anti-PD-1 antibody) for the treatment of untreated advanced melanoma based on the results of RELATIVITY-047 study [10, 11]. Additionally, other anti-LAG-3 antibodies plus anti-PD-1 antibodies have been reported in early phase studies [12, 13]. Clinical studies of LAG-3 inhibitors were mainly conducted in Western populations with a notable lack of clinical data in Chinese patients.
IBI110 is an IgG4κ recombinant human anti-LAG-3 monoclonal antibody designed to directly bind to LAG-3 on T cells. Herein, we present the first-in-human clinical study of IBI110 in Chinese patients with advanced solid tumors to evaluate the safety, efficacy, pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of IBI110 both as monotherapy and in combination with sintilimab (anti-PD-1 antibody).
Methods
Study design
This open-label phase I study included phase Ia dose escalation of IBI110 monotherapy, phase Ib dose escalation of IBI110 in combination with sintilimab and phase Ib dose expansion of IBI110 in combination with sintilimab and chemotherapy in previously untreated patients with advanced squamous non-small cell lung cancer (sqNSCLC) or HER-2 negative patients with gastric cancer (GC). The schematic study design was presented in Supplementary Figure S1. The primary endpoints were safety, tolerability and efficacy including objective response rate (ORR), disease control rate (DCR), duration of response (DoR), progression survival (PFS) and overall survival (OS). The secondary endpoints included PK, PD and immunogenicity. The dose escalation in phase Ia was implemented with accelerated titration (0.01/0.1 mg/kg) and classic 3 + 3 design (0.03/1/3/10/20 mg/kg). The dose escalation in phase Ib was implemented with classic 3 + 3 design. Crossover from monotherapy to combination therapy was allowed at progression. Dose-limiting toxicity (DLT) criteria were detailed in Supplementary Methods. Patients received IBI110 monotherapy or in combination with sintilimab up to 24 months or until disease progression, loss to follow-up, death, intolerable toxicity, informed consent withdrawal and other reasons leading to study discontinuation (whichever occurs first).
Patients
The phase Ia and Ib escalation enrolled patients with locally advanced, recurrent, or metastatic solid tumors who failed standard therapy. The phase Ib expansion enrolled previously untreated patients with advanced sqNSCLC or HER-2 negative GC. Inclusion and exclusion criteria are detailed in Supplementary Methods.
Safety assessments
Adverse events (AEs) were coded by the Medical Dictionary for Regulation Activities (MedDRA) and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Treatment-relative AEs (TRAEs) and immune-related AEs (irAEs) were assessed by investigators. Safety assessment was performed since the sign of informed consent and up to 90 ± 7 days after the last dose or before the initiation of a new anti-tumor treatment (whichever occurs first). All AEs were followed until they recovered to baseline or grade 0–1, or the invesitgator considers that no further follow-up is required for reasonable reasons (e.g., no recovery or improvement).
Efficacy assessments
Treatment efficacy was assessed by CT or MRI by investigators according to RECIST v1.1 and iRECIST. Baseline assessment was conducted within 28 days prior to the first dose. For patients with first recorded complete response (CR) or partial response (PR), additional assessment was conducted after 4 to 6 weeks to confirm the response. Subsequently, assessments were conducted every 6 weeks ± 7 days until disease progression, initiation of new anti-tumor treatment, informed consent withdrawal and other reasons leading to study discontinuation (whichever occurs first).
Preclinical study
Preclinical methods were detailed in the Supplementary Methods including affinity measurement of IBI110 with LAG-3, binding activity of IBI110 to HEK293-hLAG-3 or human CD4+ T cells or cynomolgus monkey PBMC, protein-based blocking assay, cell-based blocking assay, LAG-3 blockade reporter assay, receptor occupancy in the Human CD4+ T Cells, and mixed leukocyte reaction.
PK and PD analysis
During phase Ia and Ib escalation, blood samples were collected for PK and PD analysis. PK parameters assessed after single and multiple IBI110 doses included maximum concentration (Cmax), time to reach maximum concentration (Tmax), area under the concentration-time curve (AUC), clearance (CL), volume of distribution (V), elimination half-life (t1/2). PK analysis was performed in phase Ia patients (including dose escalation and expansion) using non-compartmental analysis (NCA).
Statistical analysis
Patients who received at least one dose of IBI110 were included for safety and efficacy analysis. Continuous variables were described by number of cases, mean, standard deviation, median, minimum and maximum. Categorical variables were described by frequency and percentage. The 95% confidence interval (CI) for ORR and DCR was calculated using the Clopper-Pearson method. DoR, PFS and OS were analyzed using the Kaplan-Meier method, with the median time and 95% CI. Statistical analysis was performed using SAS version 9.4.
Results
Preclinical study
Preclinical pharmacological and toxicological studies were performed both in vivo and in vitro to evaluate safety and efficacy of IBI110 (Supplementary Results). In brief, IBI110 showed high affinity to human LAG-3 and cynomolgus monkey LAG-3. It disrupts MHCII/LAG-3 binding and triggers downstream signaling. IBI110 plus sintilimab showed enhanced IL-2 secretion in vitro and more favorable antitumor effect in vivo.
Patients
From December 4, 2019 to January 20, 2022, the phase Ia dose escalation of IBI110 monotherapy enrolled 28 patients and the phase Ib combination dose escalation of IBI110 plus sintilimab enrolled 45 patients (Table 1). In phase Ia escalation, patients were allocated to receive IBI110 every three weeks (Q3W) from 0.01 mg/kg (n = 1), 0.1 mg/kg (n = 1), 0.3 mg/kg (n = 3), 1 mg/kg (n = 3), 3 mg/kg (n = 6), 10 mg/kg (n = 7), to 20 mg/kg (n = 7). In phase Ib escalation part, patients were allocated to receive IBI110 Q3W from 0.3 mg/kg (n = 3), 0.7 mg/kg (n = 3), 1.5 mg/kg (n = 3), 3 mg/kg (n = 11), 5 mg/kg (n = 15), 8 mg/kg (n = 6), to 10 mg/kg (n = 4) plus sintilimab 200 mg Q3W. The CONSORT diagram is shown in Supplementary Figure S2.
Table 1.
Baseline characteristics
| Ia escalation | Ib escalation | Ib expansion | ||
|---|---|---|---|---|
| n = 28 | n = 45 | sqNSCLC, n = 20 | GC, n = 17 | |
| Gender, n (%) | ||||
| Male | 18 (64.3) | 37 (82.2) | 19 (95.0) | 13 (76.5) |
| Female | 10 (35.7) | 8 (17.8) | 1 (5.0) | 4 (23.5) |
| Age, years | ||||
| median (range) | 60.5 (35–72) | 60.0 (33–74) | 63.0 (52–74) | 61.0 (39–73) |
| ECOG PS, n (%) | ||||
| 0 | 14 (50.0) | 14 (31.1) | 4 (20.0) | 3 (17.6) |
| 1 | 14 (50.0) | 31 (68.9) | 16 (80.0) | 14 (82.4) |
| SLD, mm | ||||
| median (range) | 60.8 (10.0-179.0) | 61.3 (10.0-209.0) | 85.5 (22.8–163.0) | 42.0 (12.0-110.5) |
| Tumor types, n (%) | ||||
| Lung cancer | 12 (42.9) | 33 (73.3) | 20 (100) | 0 |
| Colorectal cancer | 3 (10.7) | 2 (4.4) | 0 | 0 |
| Gastric cancer | 2 (7.1) | 1 (2.2) | 0 | 17 (100) |
| Kidney cancer | 2 (7.1) | 0 | 0 | 0 |
| Ovarian cancer | 2 (7.1) | 1 (2.2) | 0 | 0 |
| Melanoma | 2 (7.1) | 0 | 0 | 0 |
| other | 5 (17.9) | 8 (17.8) | 0 | 0 |
| Histology, n (%) | ||||
| adeno | 14 (50.0) | 14 (31.1) | 0 | 17 (100) |
| squamous | 5 (17.9) | 21 (46.7) | 20 (100) | 0 |
| other | 9 (32.1) | 10 (22.2) | 0 | 0 |
| Tumor stage, n (%) | ||||
| IIIa | 1 (3.6) | 5 (11.1) | 0 | 0 |
| IIIb | 1 (3.6) | 7 (15.6) | 3 (15.0) | 2 (11.8) |
| IIIc | 0 | 2 (4.4) | 2 (10.0) | 0 |
| IV | 26 (92.9) | 30 (66.7) | 15 (75.0) | 15 (88.2) |
| N/A | 0 | 1 (2.2) | 0 | 0 |
*SLD: sum of the longest diameter of the target lesions
The phase Ib expansion of IBI110 plus sintilimab and chemotherapy was conducted in previously untreated patients with advanced sqNSCLC or HER-2 negative GC. As of October 25, 2022, the sqNSCLC cohort enrolled 20 patients, including 5 patients with TNM stage III (25.0%) and 15 patients with TNM stage IV (75.0%). The median treatment duration of IBI110 plus sintilimab was 53.1 weeks (range: 6.1–70.3) with 10 patients remained on treatment (50.0%). As of March 22, 2023, the GC cohort enrolled 17 patients including 2 patients with TNM stage III (11.8%) and 15 patients with TNM stage IV (88.2%). There were 7 patients with liver metastasis (41.2%). The median treatment duration of IBI110 plus sintilimab was 16.6 weeks (range: 3.1–72.1) with 3 patients remained on treatment (17.6%). The baseline characteristics of each cohorts were listed in Table 1.
PK and PD
When IBI110 was administered as monotherapy or in combination with sintilimab at relatively low dose levels ranging from 0.01 to 1.5 mg/kg, decreased clearance rates were observed with escalating doses. These non-linear PK profiles were typical characteristics of target-mediated drug disposition (TMDD). When IBI110 dose increased to ≥ 3 mg/kg (200 mg), the clearance rates became stabilized and linear PK characteristics were observed (Supplementary Figure S3). Patients in phase Ia and Ib escalation were also tested for anti-drug antibody (ADA) to evaluate immunogenicity. No clinically significant changes in immunogenicity were observed across different dose levels indicating that immunogenicity was not dose-dependent. The PD analysis of IBI110 was described in Supplementary Results and presented in Supplement Figure S4.
Safety
During phase Ia escalation of IBI110 alone and phase Ib escalation of IBI110 plus sintilimab, no DLT was observed across all dose groups. The maximum tolerated dose was not reached. Safety profiles of phase Ia and Ib escalation as well as Ib expansion were summarized in Table 2.
Table 2.
Safety overview
| n, % | Ia escalation | Ib escalation | Ib expansion | |
|---|---|---|---|---|
| n = 28 | n = 45 | sqNSCLC, n = 20 | GC, n = 17 | |
| TRAEs | 19 (67.9) | 34 (75.6) | 20 (100) | 17 (100) |
| TRAEs, Grade ≥ 3 | 6 (21.4) | 10 (22.2) | 16 (80) | 11 (64.7) |
| TRSAEs | 4 (14.3) | 5 (11.1) | 5 (25) | 7 (41.2) |
| TRAEs leading to dose interruption | 2 (7.1) | 6 (13.3) | 8 (40) | 14 (82.4) |
| TRAEs leading to treatment discontinuation | 1 (3.6) | 0 | 4 (20) | 6 (35.3) |
| TRAEs leading to death | 0 | 0 | 0 | 0 |
| irAEs | 3 (10.7) | 14 (31.1) | 14 (70) | 10 (58.8) |
| irAEs, Grade ≥ 3 | 1 (3.6) | 3 (6.7) | 4 (20) | 3 (17.6) |
In phase Ia escalation, TRAEs of any grade occurred in 19 patients (67.9%) while TRAEs of grade ≥ 3 occurred in 6 patients (21.4%). Common TRAEs were presented in Supplementary Table S1 with the most common being anemia (17.9%, including 3.6% ≥ G3). TRAEs leading to dose interruption and treatment discontinuation occurred in 2 (7.1%) and 1 (3.6%) patients, respectively. No TRAEs led to death. Immune-related adverse events (irAEs) occurred in 3 patients (10.7%) including 1 patient (3.6%) had grade ≥ 3 irAE (Supplementary Table S2). In phase Ib escalation, TRAEs of any grades occurred in 34 patients (75.6%) while TRAEs of grade ≥ 3 occurred in 10 patients (22.2%). Common TRAEs were presented in Supplementary Table S3 with the most common being aspartate aminotransferase increased (28.9%, all G1-G2). TRAEs leading to dose interruption in 6 patients (13.3%). No TRAEs led to treatment discontinuation or death. irAEs occurred in 14 patients (31.1%) including 3 patients (6.7%) had grade ≥ 3 irAE (Supplementary Table S4).
The phase Ib expansion of IBI110 plus sintilimab and chemotherapy was conducted in patients with advanced sqNSCLC or HER-2 negative GC. In sqNSCLC cohort (n = 20), TRAEs of any grade occurred in all patients while TRAEs of grade ≥ 3 occurred in 16 patients (80.0%). Common TRAEs in sqNSCLC cohort were presented in Supplementary Table S5 with the most common being anemia (70.0%, all G1-G2). TRAEs leading to dose interruption and treatment discontinuation occurred in 8 (40.0%) and 4 (20.0%) patients, respectively. No TRAEs lead to death. irAEs occurred in 14 patients (70.0%) including 4 patients (20.0%) had grade ≥ 3 irAE (Supplementary Table S6). In GC cohort (n = 17), TRAEs of any grade occurred in all patients (100%) while TRAEs of grade ≥ 3 occurred in 11 patients (64.7%). Common TRAEs in GC cohort were presented in Supplementary Table S7 with the most common being neutrophil count decreased (64.7%, including 17.6%≥ G3). TRAEs leading to dose interruption and treatment discontinuation occurred in 14 (82.4%) and 6 (35.3%) patients, respectively. No TRAEs lead to death. irAEs occurred in 10 patients (58.8%) including 3 patients (17.6%) had grade ≥ 3 irAE (Supplementary Table S8).
Efficacy
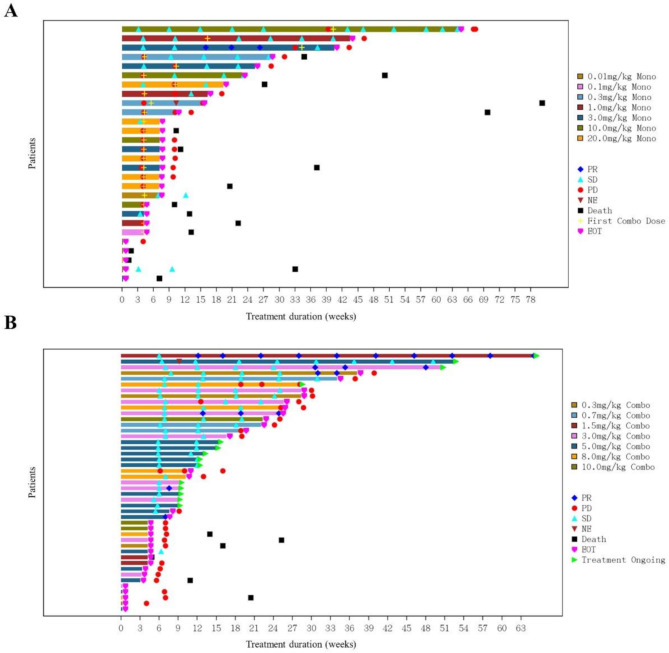
As of Jan 20, 2022, ORR and DCR of IBI110 monotherapy in phase Ia escalation (n = 28) were 3.6% and 25.0%, respectively (Fig. 1A). Only 1 patient with ovarian cancer at 3 mg/kg had PR. In phase Ib escalation, 43 of 45 patients had undergone at least 1 post-baseline tumor assessment. The ORR and DCR of IBI110 plus sintilimab were 14.0% and 67.4%, respectively (Fig. 1B). Based on the safety and efficacy profiles observed in phase Ia and Ib escalation, the RP2D was determined as IBI110 200 mg (3 mg/kg) Q3W plus sintilimab 200 mg Q3W. In phase Ib expansion, 20 patients with previously untreated, advanced sqNSCLC received RP2D of IBI110 in combination with sintilimab plus paclitaxel and carboplatin (TP) as first-line treatment; while 17 patients with previously untreated, advanced HER-2 negative GC received RP2D of IBI110 in combination with sintilimab plus capecitabine and oxaliplatin (XELOX) as first-line treatment (Table 3).
Fig. 1.
Tumor assessment of each patient in phase Ia (A) and Ib (B) dose escalation. (A) In phase Ia escalation of IBI110, the best response assessed by investigator was partial response (PR) in 1 patient and stable disease (SD) in 6 patients. After progressive disease (PD), patients may cross to combination treatment of IBI110 plus sintilimab while 8 patients had SD. (B) In phase Ib escalation of IBI110 plus sintilimab, 43 of 45 patients had undergone at least 1 post-baseline tumor assessment. The best response assessed by investigator was PR in 6 patients (4 non-small cell lung cancer, 1 small cell lung cancer and 1 endometrial cancer) and SD in 23 patients
Table 3.
Efficacy in phase Ib expansion
| Ia escalation | Ib expansion | |||
|---|---|---|---|---|
| IBI110 monotherapy (n = 28) | IBI110 plus sintilimab (n = 43)* | sqNSCLC cohort (n = 20) | GC cohort (n = 17) | |
| Confirmed Best Overall Response, n (%) | ||||
| Partial response(PR) | 1 (3.6) | 3 (7.0) | 15 (75.0) | 12 (70.6) |
| Stable disease(SD) | 6 (21.4) | 26 (60.5) | 2 (10.0) | 4 (23.5) |
| Progressive disease(PD) | 21 (75.0) | 14 (32.6) | 3 (15.0) | 1 (5.9) |
| unconfirmed ORR, % (95% CI) | 3.6% (0-10.5) | 14.0% (3.6–24.3) | 80.0% (56.3–94.3) | 88.2% (63.6–98.5) |
| confirmed ORR, % (95% CI) | 3.6% (0-10.5) | 7.0% (0-14.6) | 75.0% (50.9–91.3) | 70.6% (44.0-89.7) |
| DCR, % (95% CI) | 25.0% (9.0–41.0) | 67.4% (53.4–81.5) | 85.0% (62.1–96.8) | 94.1% (71.3–99.9) |
*43 of 45 patients in phase Ib combination dose escalation had undergone at least 1 post-baseline tumor assessment as of the cutoff date
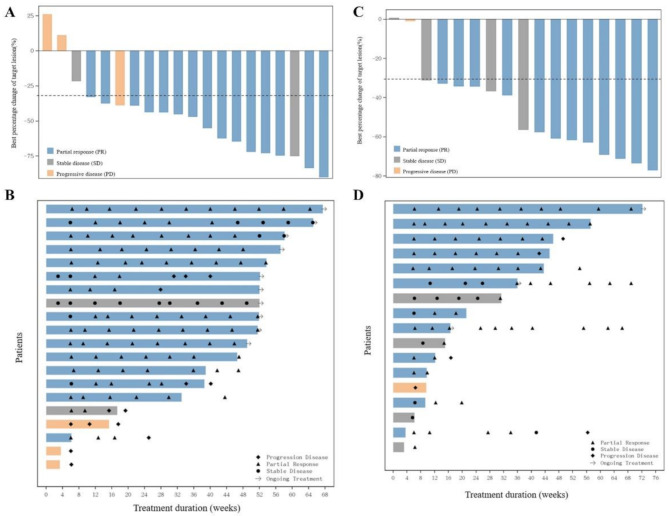
As of October 25, 2022, the unconfirmed and confirmed ORRs in sqNSCLC cohort were 80.0% (95% CI, 56.3–94.3) and 75.0% (95% CI, 50.9–91.3), respectively (Fig. 2A and B). The DCR was 85.0% (95% CI, 62.1–96.8). The median DoR was not reached with events occurring in 4 of 15 patients with confirmed objective response. The 12-month DoR rate was 73.3% (95% CI, 43.6–89.1). The median PFS was not reached with events occurring in 8 (40.0%) patients. The 12-month PFS rate was 60.0% (95% CI, 35.7–77.6). The median OS was not reached with event occurred in 3 (15%) patients. The 12-month OS rate was 85.0% (95% CI, 60.4–94.9).
Fig. 2.
Efficacy of IBI110 in combination with sintilimab and chemotherapy in sqNSCLC and GC patients. (A) Confirmed best overall response in sqNSCLC: 1 patient had increase of 11.04% in size of the target lesion and 1 patient had decrease of 38.66% in size of the target lesion, but their overall responses were PD due to the presence of new lesions. (B) Tumor assessment in patients with sqNSCLC: 1 patient had the first and second tumor assessment of iUPD, and continued treatment until the third tumor assessment of iCPD. (C) Confirmed best overall response in GC: 1 patient had decrease of 0.83% in size of the target lesion, but the overall response was PD due to the presence of new lesion. (D) Tumor assessment in patients with GC
As of March 22, 2023, the unconfirmed and confirmed ORRs in GC cohort were 88.2% (95% CI, 63.6–98.5) and 70.6% (95% CI, 44.0-89.7), respectively (Fig. 2C and D). The DCR was 94.1% (95% CI, 71.3–99.9). The median DoR was 10.6 months (95% CI, 2.5–14.4). With a median follow up of 13.1 months (95% CI, 7.1-NR [not reported]), the progression-free survival (PFS) was 12.9 months (95% CI, 3.8–15.8). With a median follow up of 15.8 months (95% CI, 13.4–16.6), the median overall survival (OS) was 15.8 months (95% CI, 8.5-NR), and the 12-months OS rate was 70.6% (95% CI, 43.1–86.6).
Discussion
In this phase Ia/Ib study, we evaluated the safety and efficacy of IBI110 in Chinese patients with advanced solid tumors. No DLT was observed in all dose groups during phase Ia dose escalation of IBI110 monotherapy and phase Ib combination dose escalation of IBI110 plus sintilimab. The RP2D of IBI110 was determined as 200 mg (3 mg/kg) Q3W in monotherapy or in combination with sintilimab 200 mg Q3W.
The PK characteristics of IBI110 were essentially the same when administered alone or in combination with sintilimab. Following intravenous infusion of IBI110 in patients with advanced tumors, the drug exhibited TMDD characteristics within the dose range of 0.01 to 1.5 mg/kg. At doses of ≥ 3 mg/kg, both Cmax and AUC increased in proportional to the dose, while the clearance rate remained constant, indicating linear PK characteristics, which also represents the potential effective dose range. These PK properties are similar to those of relatlimab, demonstrating TMDD characteristics in the low-dose group and linear PK characteristics in the high-dose group [14]. LAG3 is an immune checkpoint inhibitor that negatively regulates T cell activation and contributes to T cell exhaustion. Soluble LAG3 (sLAG3) is the soluble form of LAG3 released from the cell surface, providing negative feedback on immune activation. Baseline levels of sLAG3 have been reported to be associated with the prognosis of various cancer types [15, 16]. In our study, we measured total serum sLAG3 to assess immune activation after treatment. The observed dose-dependent increases in sLAG3 with either monotherapy or combination therapy suggest the effectiveness of IBI110 in activating immune cells. In the RELATIVITY-020 study of relatlimab [17], treatment-induced decreases in serum free sLAG3 indicate target engagement, which has a different implication compared to measuring total serum sLAG3 in our study.
During dose escalation, TRAEs of any grade occurred in 67.9% patients (including 21.4% of grades ≥ 3) with IBI110 monotherapy and in 75.6% patients (including 22.2% of grades ≥ 3) with IBI110 plus sintilimab. The incidences of TRAEs in any grade and grades ≥ 3 were comparable to clinical studies of other LAG-3 inhibitors [11–13]. In dose escalation of IBI110 monotherapy, TRAEs leading to treatment discontinuation occurred in 1 patient (3.6%) only. In combination dose escalation of IBI110 with sintilimab, no patient had TRAEs leading to treatment discontinuation which was different with other studies (0% vs. 14.6% in relatlimab plus nivolumab, 4.1% in ieramilimab [LAG525, anti-LAG-3 antibody] plus spartalizumab [anti-PD-1 antibody] and 5.6% in favezelimab [MK-4280, anti-LAG-3 antibody] plus pembrolizumab) [11–13]. The safety profiles worsened during the phase Ib combination dose expansion in sqNSCLC and GC cohorts of which 80% and 64.7% patients had grades ≥ 3 TRAEs while 20% and 35.3% patients had TRAEs leading to treatment discontinuation. The increased toxicities may be associated with the added chemotherapy for the treatments of sqNSCLC and GC treatment.
Despite similar TRAE incidences, the spectrums of adverse events were largely varied across different studies of anti-LAG-3 antibodies. In previous studies, fatigue and gastrointestinal toxicities including diarrhea and nausea were frequently observed [11–13]. Unlike western populations, no patients treated with IBI110 plus sintilimab in our study had fatigue, diarrhea and nausea, while liver toxicities featured by aspartate aminotransferase increased and alanine aminotransferase increased were frequently observed. Other common TRAEs in our study included anemia, hypothyroidism and hypertension.
The clinical efficacy of IBI110 monotherapy was relatively low with only 1 of 28 patient had PR (ORR of 3.6%). Similar results were also observed in studies of ieramilimab and favezelimab in which no patient responded to single-agent treatment (ORR of 0%) [12, 13]. Inhibiting LAG-3 alone may not provide sufficient anti-tumor activity, while co-inhibition of LAG-3 and PD-1 appears to be a feasible and rational approach. IBI110 plus sintilimab showed ORR of 14% in patients with various advanced tumors during phase Ib combination dose escalation. Comparable results were observed in another phase I study with ORR of 10.7% in ieramilimab combined with spartalizumab [12]. In previously treated, advanced microsatellite stable colorectal cancer, patients treated with favezelimab plus pembrolizumab had confirmed ORR of 6.3% [13]. A higher response rate of LAG-3 and PD-1 co-inhibition was reported in melanoma, which is known to be more sensitive to ICI treatments [18]. The recent update of RELATIVITY-047 study reported ORR of 43.1% vs. 32.6% in patients with previously untreated unresectable or metastatic melanoma receiving relatlimab plus nivolumab or nivolumab alone [19]. A recent study in mouse models of melanoma indicated that CD8+ T cells deficient in both PD-1 and LAG-3 mediate enhanced tumor clearance and long-term survival [20]. Another study in melanoma patients also revealed that the combination of relatlimab and nivolumab could enhance CD8+ T cell receptor signaling and CD8+ T cell differentiation, which contributed to boosting cytotoxicity while maintaining the exhaustion state [21].
During dose expansion, we observed confirmed ORR of 75% in sqNSCLC and 70.6% in HER-2 negative GC patients treated with IBI110 plus sintilimab and chemotherapy. In a randomized, double-blind, phase 3 trial (ORIENT-12), sintilimab plus platinum and gemcitabine (GP) as first-line treatment for advanced or metastatic sqNSCLC showed confirmed ORR of 44.7% in the sintilimab-GP group and 35.4% in the placebo-GP group [22]. Another randomized, double-blind, phase 3 trial (ORIENT-16) in patients with previously untreated, advanced gastric or gastroesophageal junction cancer (G/GEJC) reported ORR of 58.2% in sintilimab plus chemotherapy versus 48.4% in chemotherapy alone [23]. Recently, the open-label phase II study (RELATIVITY-060) in LAG-3 positive (≥ 1%) G/GEJC observed ORR of 48% with first-line nivolumab and relatlimab plus chemotherapy and 61% with nivolumab plus chemotherapy. Although no improvement on ORR was observed with nivolumab and relatlimab plus chemotherapy, numerical improvements in mPFS and mOS were observed in some subgroups of patients during exploratory biomarker analysis, including LAG-3 and PD-L1 expression [24]. Despite promising ORRs of IBI110 plus sintilimab and chemotherapy in sqNSCLC and HER-2 negative GC, the cross-trial comparison should be interpreted with caution given substantial differences in baseline characteristics, interventions and tumor types. The added value of IBI110 in different tumor types and patient subgroups warrants further clinical investigations.
Some limitations of our study should be noted. As a single-arm study, it lacks a control arm and has a limited sample size. In addition, the study does not include an analysis of biomarkers such as PD-L1 and LAG-3 expression, which might be informative to identify patients most likely to benefit from IBI110 treatment.
In conclusion, IBI110 monotherapy and in combination with sintilimab were well-tolerated during dose escalation and expansion in Chinese patients with advanced solid tumors. Encouraging efficacy of IBI110 in combination with sintilimab was observed, but further clinical studies with larger sample size and informative biomarkers are warranted.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families, as well as the research staff at all participating sites, for their contributions to this study. Medical writing assistance was provided by Han Han-Zhang, PhD and Yuxi Zhou, MSc, from Innovent Biologics (Suzhou) Co., Ltd.
Abbreviations
- LAG
3 Lymphocyte-Activation Gene 3
- sqNSCLC
Squamous Non-Small Cell Lung Cancer
- GC
Gastric Cancer
- RP2D
Recommended Phase 2 Dose
- ORR
Objective Response Rate
- DCR
Disease Control Rate
- DoR
Duration of Response
- PFS
Progression-Free Survival
- OS
Overall Survival
- DLT
Dose-Limiting Toxicity
- TRAE
Treatment-Related Adverse Event
- irAE
Immune-Related Adverse Event
- ICI
Immune Checkpoint Inhibitor
- PD-1
Programmed Cell Death Receptor 1
- PD-L1
Programmed Cell Death Ligand 1
- CTLA-4
Cytotoxic T Lymphocyte-Associated Antigen-4
- MHC
Major Histocompatibility Complex
- PK
Pharmacokinetic
- PD
Pharmacodynamic
- NR
Not reported
Author contributions
Conceptualization: CZ and NX. Investigation: CM, AX, JQ, WW, YL, TZ, CZ and NX. Supervision: CM, AX, JQ, CZ and NX. Data curation: CM, AX, JQ, WW, YL and TZ. Formal analysis: CM, AX, JQ, ZW, HN, JL, SL, LZ and YC. Writing – original draft: CM, AX and JQ. Writing – review & editing: All authors.
Funding
The study was funded by Innovent Biologics (Suzhou) Co., Ltd.
Data availability
The datasets (including de-identified individual data) generated during the current study are available from the corresponding author upon reasonable request. Requestors will need to submit a proposal to the corresponding author and sign a data access agreement to gain the data access.
Declarations
Ethical approval
The study protocol was approved by the institutional review board and ethics committee of all participating sites. This study was registered at clinicaltrials.gov (NCT04085185) and conducted following Good Clinical Practice Guidelines, the Declaration of Helsinki, and relevant local regulatory policy. Written informed consents were required before patient enrollment.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenyu Mao, Anwen Xiong and Jiong Qian contributed equally to this work.
Contributor Information
Caicun Zhou, Email: caicunzhoudr@163.com.
Nong Xu, Email: nongxu@zju.edu.cn.
References
- 1.Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- 2.Vesely MD, Zhang T, Chen L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu Rev Immunol. 2022;40:45–74. [DOI] [PubMed] [Google Scholar]
- 3.Borgeaud M, Sandoval J, Obeid M, Banna G, Michielin O, Addeo A et al. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treatment Reviews [Internet]. 2023 [cited 2023 Dec 5];120. https://www.cancertreatmentreviews.com/article/S0305-7372(23)00107-X/fulltext [DOI] [PubMed]
- 4.Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal V, Workman CJ, Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023;24:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Han Y, Zhang Y, Zhao Q, Wang H, Wei J, et al. Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms. Cell Biosci. 2023;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy—current perspectives and future directions. Cell. 2023;186:1652–69. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J Thorac Oncol. 2017;12:814–23. [DOI] [PubMed] [Google Scholar]
- 9.Maruhashi T, Sugiura D, Okazaki I-M, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik J. Nivolumab Plus Relatlimab: First Approval. Drugs. 2022;82:925–31. [DOI] [PubMed] [Google Scholar]
- 11.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schöffski P, Tan DSW, Martín M, Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer. 2022;10:e003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garralda E, Sukari A, Lakhani NJ, Patnaik A, Lou Y, Im S-A, et al. A first-in-human study of the anti-LAG-3 antibody favezelimab plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. ESMO Open. 2022;7:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Hu Z, Bathena SP, Keidel S, Miller-Moslin K, Statkevich P, et al. Model-Informed Clinical Pharmacology Profile of a Novel Fixed-Dose Combination of Nivolumab and Relatlimab in Adult and Adolescent Patients with Solid Tumors. Clin Cancer Res. 2024;30:3050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botticelli A, Zizzari IG, Scagnoli S, Pomati G, Strigari L, Cirillo A, et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. JPM. 2021;11:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Wang W, Tian J, Zhou Y, Shen Y, Wang M, et al. Clinical Significance of Soluble LAG-3 (sLAG-3) in Patients With Cervical Cancer Determined via Enzyme-Linked Immunosorbent Assay With Monoclonal Antibodies. Technol Cancer Res Treat. 2023;22:15330338231202650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ascierto PA, Lipson EJ, Dummer R, Larkin J, Long GV, Sanborn RE, et al. Nivolumab and Relatlimab in Patients With Advanced Melanoma That Had Progressed on Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy: Results From the Phase I/IIa RELATIVITY-020 Trial. JCO. 2023;41:2724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002–14. [DOI] [PubMed] [Google Scholar]
- 19.Long GV, Stephen Hodi F, Lipson EJ, Schadendorf D, Ascierto PA, Matamala L, et al. Overall Survival and Response with Nivolumab and Relatlimab in Advanced Melanoma. NEJM Evid. 2023;2:EVIDoa2200239. [DOI] [PubMed] [Google Scholar]
- 20.Andrews LP, Butler SC, Cui J, Cillo AR, Cardello C, Liu C, et al. LAG-3 and PD-1 synergize on CD8 + T cells to drive T cell exhaustion and hinder autocrine IFN-γ-dependent anti-tumor immunity. Cell. 2024;187:4355–e437222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cillo AR, Cardello C, Shan F, Karapetyan L, Kunning S, Sander C, et al. Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8 + T cells to promote antitumor immunity. Cell. 2024;187:4373–e438815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab Plus Platinum and Gemcitabine as First-Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12). J Thorac Oncol. 2021;16:1501–11. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA. 2023;330:2064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegewisch-Becker S, Mendez G, Chao J, Nemecek R, Feeney K, Van Cutsem E, et al. First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study. J Clin Oncol. 2024;42:2080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets (including de-identified individual data) generated during the current study are available from the corresponding author upon reasonable request. Requestors will need to submit a proposal to the corresponding author and sign a data access agreement to gain the data access.