Abstract
Background
Pharmacological treatment is a cornerstone of chronic obstructive pulmonary disease (COPD) management, with general practitioners providing the most care. However, the lack of data on prescribing trends in initial pharmacotherapy in primary care hinders the understanding of how scientific and technical developments impact patient care and may also perpetuate suboptimal practices. Hence, this study aims to analyze trends in the initial pharmacological treatment of newly diagnosed COPD patients in Dutch primary care from 2010 to 2021.
Methods
A repeated cross-sectional study was conducted via the PHARMO GP Database. Data were extracted from the electronic health records of individuals managed by general practitioners in the Netherlands within the PHARMO Data Network. Individuals aged ≥ 40 years at diagnosis with an International Classification of Primary Care code for COPD (R95) were included. Initial pharmacological treatment was identified based on the first prescription issued within 90 days postdiagnosis. The annual proportions of individuals receiving a specific treatment among those diagnosed were calculated and directly standardized by age and sex according to the 2021 Dutch population structure. Trend analysis was performed via joinpoint regression.
Results
A total of 54,628 COPD patients were included (median [IQR] age: 65 [57–73]; 53.7% male), with 36.4% not receiving respiratory medication within 90 days of diagnosis, and 4.2% on other treatments. Trend analysis revealed that LAMA monotherapy increased from 13.4% in 2010 to 15.1% in 2015 and then declined to 11.0% by 2021. Moreover, LABA-ICS decreased from 17.6% to 8.5% between 2010 and 2018, after which it plateaued. In contrast, LABA-LAMA sharply increased, from 0.6% in 2010 to 9.6% in 2021. LABA monotherapy increased from 2.6% in 2010 to 5.7% in 2021. Triple therapy has remained constant. For reliever-only therapies, SABA increased from 8.5% in 2010 to 14.3% in 2018 and then stabilized, whereas SAMA and SABA-SAMA remained low throughout.
Conclusions
Shifts in initial pharmacological COPD treatment from 2010 to 2021 likely reflect the introduction of new inhalers and updated management strategies. However, a significant proportion of patients remain without GP prescriptions, which warrants further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03073-w.
Keywords: Chronic obstructive pulmonary disease, Primary health care, Drug therapy
Background
Chronic obstructive pulmonary disease (COPD) ranks third in global mortality and is projected to become the fourth leading cause of disability by 2050 [1, 2]. In many healthcare systems, such as the Dutch model, most COPD patients are diagnosed and managed by general practitioners (GPs), with severe cases typically referred to specialists. Pharmacotherapy is a cornerstone of COPD management and is aimed at reducing symptoms, improving lung function, and decreasing the risk and frequency of exacerbations [3, 4]. Real-world evidence indicates frequent delays in initiating inhaled therapy and persistent overuse of inhaled corticosteroids (ICSs) across different settings [5–7]. However, prescribing trends in primary care have not yet been sufficiently studied. This undermines the potential to optimize COPD management and limits our understanding of how scientific and technical developments impact patient care [8, 9]. Ultimately, this lack of knowledge could perpetuate suboptimal treatment practices and adversely affect patient outcomes [10]. Hence, the aim of this study was to analyze trends in the initial pharmacological treatment of newly diagnosed COPD patients in Dutch primary care from 2010 to 2021.
Methods
Study design and participants
This study is reported in accordance with the REporting of studies Conducted using Observational Routinely Collected Health Data (RECORD) statement [11].
A population-based, repeated cross-sectional study was conducted using data from the PHARMO GP database between January 1, 2010, and December 31, 2021 [12]. The database contains data extracted from the electronic health records of over 800 practices in the Netherlands [12, 13].
Participants were eligible if they had a COPD diagnosis (International Classification of Primary Care, ICPC-1 code R95) assigned by a GP, with at least 90 days of follow-up in PHARMO both before and after diagnosis and were aged ≥ 40 years at the time of diagnosis. Individuals could have a concomitant chronic bronchitis (R91.01) diagnosis, but those diagnosed with other respiratory conditions, such as bronchiectasis or asthma, were excluded. The diagnosis date was the first recorded entry of a COPD code during the study period.
Pharmacological treatments were identified using the Anatomical Therapeutic Chemical (ATC) [14] 2nd level R03 code from prescriptions issued by GPs. A complete list of the ATC codes for which there were prescriptions is provided in the supplementary material (eTable 1). The initial pharmacological treatment for COPD was based on the first prescription within 90 days of diagnosis and could include both inhaled and systemic medications (e.g., methylxanthines, phosphodiesterase-4 inhibitors). Participants were grouped based on their initial pharmacological treatment into reliever-only therapy [short-acting beta-agonists (SABA), short-acting muscarinic antagonists (SAMA), or SAMA-SABA combinations] or maintenance therapy [long-acting beta-agonists (LABA), long-acting muscarinic antagonists (LAMA) monotherapy, LABA-LAMA combinations, and ICS-containing therapies (LABA-ICS and LABA-LAMA-ICS)]. Fixed and open combinations of inhaler therapies were included. Patients prescribed maintenance therapies could also receive relievers, and those in both groups could be prescribed systemic medications. Patients outside these categories were labeled “other,” while those without respiratory medications were labeled “no prescription.” Non-respiratory medications prescribed during the 90 days before and after diagnosis were retrieved and categorized as cardiac (B01, C01-C03, C07-C09), metabolic (C10, A10), psychotropic (N05, N06), or gastrointestinal (A02) agents, which serve as proxies for comorbidities at diagnosis.
Statistical analyses
The annual proportion of individuals receiving a specific treatment was calculated by dividing the number of patients prescribed that treatment in a given year by the total number of individuals diagnosed within the same year [15]. Direct standardization by age (defined in 5-year intervals: 40–44 years, 45–49 years, and up to ≥95 years) and sex was conducted using data from the 2021 Dutch population structure provided by CBS – Statistics Netherlands [16] to enable comparisons across calendar years. Standard errors were calculated assuming a Poisson distribution, and 95% confidence intervals (CIs) were derived from a Gamma distribution following the method of Fay and Feuer [17, 18]. Data preprocessing, analysis, and visualisation were conducted using R statistical software (version 4.2.2). Temporal trends were assessed via joinpoint regression analysis with the National Cancer Institute's joinpoint software (version 5.0.2) [19]. Proportions were log-transformed for trend analysis, with a positive constant (0.5) added when counts were zero. The results are reported as the annual percentage change (APC) with corresponding 95% CIs, which are estimated via the empirical quantile method [20]. The APC represents the predicted change within a segment, meaning that the estimated proportion from one year to the next equals the predicted percentage for that year multiplied by the APC, added to the predicted proportion from the previous year. The maximum number of joinpoints depends on the number of data points; in this case, there were 12 data points, allowing for a maximum of two joinpoints [21]. The weighted Bayesian information criterion was used for model selection.
Secondary analyses
Coincidence tests were conducted with crude proportions (i.e., unstandardized) to determine whether trends were consistent across age groups (40–64, 65–79, and ≥ 80 years) and between sexes [22]. Standard errors were computed via the standard formula for a given sample proportion, whereas 95% CIs were calculated with the Wilson interval method [23]. The significance threshold (P-value < 0.0056) was adjusted by applying the Bonferroni correction for pairwise comparisons. Additionally, for individuals who did not receive any prescriptions within 90 days of diagnosis, we assessed: (a) how many had a prescription in the 90 days before diagnosis, (b) how many received a prescription between 91 and 180 days after diagnosis, and (c) among those without a prescription between 91 and 180 days, how many received one between 181 and 365 days after diagnosis.
Results
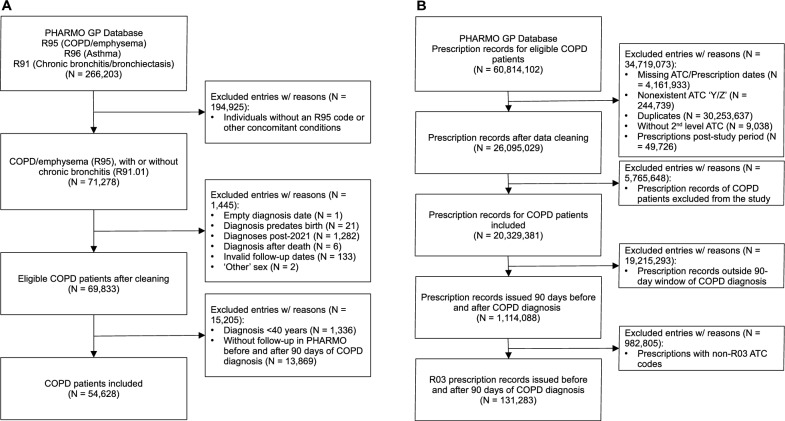
The study included data from 54,628 COPD patients (Fig. 1), with a median age of 65 years [interquartile range (IQR): 57–73], of whom 29,316 (53.7%) were male. The number of new diagnoses per year is available in the supplemental file (eTable 2).
Fig. 1.
Flowchart illustrating inclusion and exclusion criteria for adults newly diagnosed with COPD (A) and medications prescribed by general practitioners (B) in Dutch primary care from the PHARMO GP database
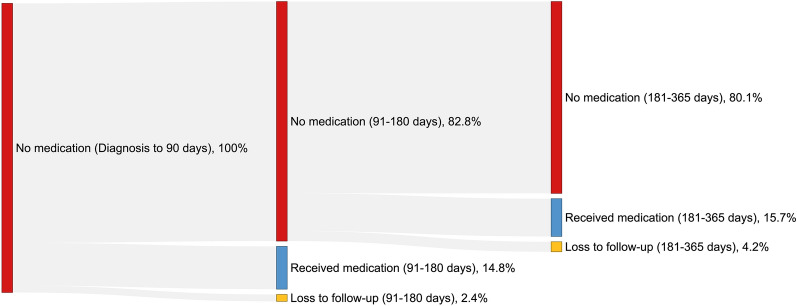
A total of 34,768 patients (63.6%) received respiratory medication within 90 days post-diagnosis, whereas 19,860 patients (36.4%) did not. Secondary analysis showed that among those who did not receive respiratory medication within 90 days post-diagnosis, only 3,254 (16.4%) had such a prescription in the 90 days before diagnosis. Additionally, 16,453 (82.5%) did not receive any respiratory medication within 180 days post-diagnosis, and of these, 13,177 (80.1%) remained without prescriptions up to one year after diagnosis (Fig. 2).
Fig. 2.
Sankey diagram showing newly diagnosed COPD patients without medication in the first 90 days, those who received at least one respiratory medication within 180 days, and those who received their first medication between 181 and 365 days after diagnosis
LAMA monotherapy was the most prescribed maintenance therapy (9,426; 17.3%), followed by LABA-ICS (7,508; 13.7%), LABA-LAMA-ICS (2,402; 4.4%), LABA-LAMA (2,324; 4.3%), and LABA monotherapy (2,080; 3.8%) (Table 1). SABA was the most prescribed among reliever-only therapies (6,041; 11.1%), followed by SAMA (2,225; 4.1% ) and SABA-SAMA (459; 0.8%). Additionally, 2303 individuals (4.2%) received "other" therapies, mainly ICS monotherapy.
Table 1.
Numbers and proportions of individuals with chronic obstructive pulmonary disease prescribed initial pharmacotherapy per treatment group within 90 days postdiagnosis in Dutch primary care, overall and stratified by age and sex, from 2010–2021
| Total (N = 54,628) |
Females (N = 25,312) |
Males (N = 29,316) |
|||||
|---|---|---|---|---|---|---|---|
| 40–64 years (N = 13,024) |
65–79 years (N = 9544) |
≥ 80 years (N = 2744) |
40–64 years (N = 13,222) |
65–79 years (N = 12,780) |
≥ 80 years (N = 3314) |
||
| No prescriptions | 19,860 (36.4) | 4720 (36.2) | 3144 (32.9) | 790 (28.8) | 5467 (41.3) | 4662 (36.5) | 1077 (32.5) |
| SABA# | 6041 (11.1) | 1860 (14.3) | 1017 (10.7) | 267 (9.7) | 1528 (11.6) | 1081 (8.5) | 288 (8.7) |
| SAMA# | 2225 (4.1) | 441 (3.4) | 458 (4.8) | 216 (7.9) | 359 (2.7) | 566 (4.4) | 185 (5.6) |
| SABA-SAMA# | 459 (0.8) | 75 (0.6) | 88 (0.9) | 42 (1.5) | 85 (0.6) | 119 (0.9) | 50 (1.5) |
| SABA-SAMA | 457 (0.8) | 75 (0.6) | 88 (0.9) | 42 (1.5) | 85 (0.6) | 117 (0.9) | 50 (1.5) |
| SABA-SAMA, Xanthines | 2 (0.0) | – | – | – | – | 2 (0.0) | – |
| LABA | 2080 (3.8) | 464 (3.6) | 413 (4.3) | 133 (4.8) | 429 (3.2) | 488 (3.8) | 153 (4.6) |
| LABA | 1879 (3.4) | 417 (3.2) | 366 (3.8) | 117 (4.3) | 392 (3.0) | 450 (3.5) | 137 (4.1) |
| LABA, SABA | 122 (0.2) | 33 (0.3) | 23 (0.2) | 9 (0.3) | 27 (0.2) | 21 (0.2) | 9 (0.3) |
| LABA, SABA Xanthines | 1 (0.0) | 1 (0.0) | – | – | – | – | – |
| LABA, SAMA | 66 (0.1) | 10 (0.1) | 22 (0.2) | 7 (0.3) | 9 (0.1) | 13 (0.1) | 5 (0.2) |
| LABA, SABA-SAMA | 12 (0.0) | 3 (0.0) | 2 (0.0) | - | 1 (0.0) | 4 (0.0) | 2 (0.1) |
| LAMA | 9426 (17.3) | 1896 (14.6) | 1809 (19.0) | 517 (18.8) | 2023 (15.3) | 2528 (19.8) | 653 (19.7) |
| LAMA | 8709 (15.9) | 1725 (13.2) | 1665 (17.4) | 482 (17.6) | 1859 (14.1) | 2375 (18.6) | 603 (18.2) |
| LAMA, SABA | 580 (1.1) | 151 (1.2) | 109 (1.1) | 27 (1.0) | 142 (1.1) | 118 (0.9) | 33 (1.0) |
| LAMA, SAMA | 101 (0.2) | 14 (0.1) | 24 (0.3) | 6 (0.2) | 18 (0.1) | 27 (0.2) | 12 (0.4) |
| LAMA, SABA-SAMA | 34 (0.1) | 6 (0.0) | 11 (0.1) | 2 (0.1) | 3 (0.0) | 7 (0.1) | 5 (0.2) |
| LAMA, Xanthines | 2 (0.0) | – | – | – | 1 (0.0) | 1 (0.0) | – |
| LABA-LAMA | 2324 (4.3) | 417 (3.2) | 509 (5.3) | 104 (3.8) | 495 (3.7) | 631 (4.9) | 168 (5.1) |
| LABA-LAMA | 2043 (3.7) | 351 (2.7) | 445 (4.7) | 89 (3.2) | 435 (3.3) | 572 (4.5) | 151 (4.6) |
| LABA-LAMA, SABA | 201 (0.4) | 51 (0.4) | 36 (0.4) | 12 (0.4) | 46 (0.3) | 45 (0.4) | 11 (0.3) |
| LABA-LAMA, SABA, Xanthines | 1 (0.0) | – | – | – | 1 (0.0) | – | – |
| LABA-LAMA, SAMA | 26 (0.0) | 7 (0.1) | 9 (0.1) | – | 5 (0.0) | 3 (0.0) | 2 (0.1) |
| LABA-LAMA, SAMA, Xanthines | 1 (0.0) | – | 1 (0.0) | – | – | – | – |
| LABA-LAMA, SABA-SAMA | 51 (0.1) | 8 (0.1) | 18 (0.2) | 3 (0.1) | 7 (0.1) | 11 (0.1) | 4 (0.1) |
| LABA-LAMA, Xanthines | 1 (0.0) | – | – | – | 1 (0.0) | – | – |
| LABA-ICS | 7508 (13.7) | 1940 (14.9) | 1278 (13.4) | 439 (16.0) | 1801 (13.6) | 1580 (12.4) | 470 (14.2) |
| LABA-ICS | 6370 (11.7) | 1598 (12.0) | 1088 (11.0) | 366 (13.0) | 1551 (11.0) | 1374 (10.0) | 393 (11.0) |
| LABA-ICS, SABA | 786 (1.4) | 274 (2.1) | 120 (1.3) | 37 (1.3) | 201 (1.5) | 121 (0.9) | 33 (1.0) |
| LABA-ICS, SABA, Xanthines | 3 (0.0) | 1 (0.0) | - | - | 1 (0.0) | - | - |
| LABA-ICS, SAMA | 249 (0.5) | 50 (0.4) | 48 (0.5) | 29 (1.1) | 33 (0.2) | 60 (0.5) | 29 (0.9) |
| LABA-ICS, SAMA, Xanthines | 5 (0.0) | - | 1 (0.0) | 1 (0.0) | 1 (0.0) | 1 (0.0) | 1 (0.0) |
| LABA-ICS, SABA-SAMA | 92 (0.2) | 17 (0.1) | 19 (0.2) | 6 (0.2) | 14 (0.1) | 22 (0.2) | 14 (0.4) |
| LABA-ICS, SABA-SAMA, Xanthines | 1 (0.0) | - | – | – | – | 1 (0.0) | – |
| LABA-ICS, Xanthines | 2 (0.0) | 1 (0.0) | – | – | – | 1 (0.0) | – |
| LABA-LAMA-ICS | 2402 (4.4) | 557 (4.3) | 435 (4.6) | 94 (3.4) | 499 (3.8) | 675 (5.3) | 142 (4.3) |
| LABA-LAMA-ICS | 1986 (3.6) | 452 (3.5) | 353 (3.7) | 75 (2.7) | 386 (2.9) | 586 (4.6) | 134 (4.0) |
| LABA-LAMA-ICS, SABA | 330 (0.6) | 85 (0.7) | 64 (0.7) | 14 (0.5) | 95 (0.7) | 65 (0.5) | 7 (0.2) |
| LABA-LAMA-ICS, SAMA | 38 (0.1) | 9 (0.1) | 9 (0.1) | 2 (0.1) | 7 (0.1) | 11 (0.1) | – |
| LABA-LAMA-ICS, SAMA, Xanthines | 1 (0.0) | – | – | – | – | 1 (0.0) | – |
| LABA-LAMA-ICS, SABA-SAMA | 38 (0.1) | 9 (0.1) | 8 (0.1) | 2 (0.1) | 10 (0.1) | 8 (0.1) | 1 (0.0) |
| LABA-LAMA-ICS, SABA-SAMA, Xanthines | 2 (0.0) | 1 (0.0) | – | – | 1 (0.0) | – | – |
| LABA-LAMA-ICS, Xanthines | 7 (0.0) | 1 (0.0) | 1 (0.0) | 1 (0.0) | – | 4 (0.0) | – |
| Other | 2303 (4.2) | 654 (5.0) | 393 (4.1) | 142 (5.2) | 536 (4.1) | 450 (3.5) | 127 (3.9) |
| ICS | 1158 (2.1) | 324 (2.5) | 191 (2.0) | 64 (2.3) | 264 (2.0) | 248 (1.9) | 67 (2.0) |
| ICS, SABA | 472 (0.9) | 165 (1.3) | 74 (0.8) | 26 (0.9) | 137 (1.0) | 53 (0.4) | 17 (0.5) |
| ICS, LAMA | 160 (0.3) | 38 (0.3) | 26 (0.3) | 10 (0.4) | 24 (0.2) | 49 (0.4) | 13 (0.4) |
| ICS, SAMA | 87 (0.2) | 18 (0.1) | 19 (0.2) | 10 (0.4) | 14 (0.1) | 18 (0.1) | 8 (0.2) |
| Other | 426 (0.8) | 109 (0.8) | 83 (0.9) | 32 (1.2) | 97 (0.7) | 82 (0.6) | 22 (0.7) |
The data are presented as numbers and percentages, N (%).LABA (long-acting beta-agonists), LAMA (long-acting muscarinic antagonists), ICS (inhaled corticosteroids), SABA (short-acting beta-agonists), SAMA (short-acting muscarinic antagonists). #Reliever-only therapies
A total of 39,157 patients were receiving non-respiratory medications at the time of COPD diagnosis (see Additional file 1, eTable 3). The most commonly prescribed agents were cardiac and blood medications (29,227, 74.6%), particularly antithrombotic agents (17,939; 45.8%), renin-angiotensin system agents (15,810; 40.4%), beta-blockers (14,034; 35.8%), and diuretics (10,389; 26.5%). Additionally, 19,742 (50.4%) were prescribed medications for acid-related disorders, 17,742 (45.3%) lipid-modifying agents, 14,060 (35.9%) psychotropic agents, and 5,528 (14.1%) diabetes medications.
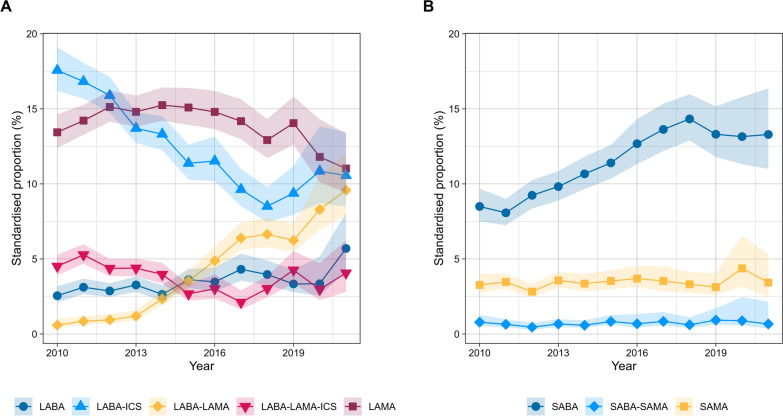
LABA monotherapy as initial therapy increased steadily from 2.6% in 2010 to 5.7% in 2021 (4.1%, 95% CI: 0.6, 7.3) (Fig. 3; see Additional file 1, eFigure 1). LAMA monotherapy increased from 13.4% in 2010 to a peak of 15.1% in 2015 (2.2%, 95% CI 0.1, 6.9) before decreasing annually to 11.0% in 2021 (−4.7%, 95% CI −10.5, −2.7) (eFigure 2). LABA-LAMA significantly increased from 0.6% in 2010 to 4.9% in 2016 (47.6%, 95% CI 39.5, 63.6) and continued to rise more gradually, reaching 9.6% by 2021 (12.1%, 95% CI 3.2, 18.7) (eFigure 3). LABA-ICS decreased sharply from 17.6% in 2010 to 8.5% in 2018 (-8.5%, 95% CI −11.9, −7.1), with no significant changes observed up to 2021 (6.5%, 95% CI −5.5, 20.8) (eFigure 4). Triple therapy remained stable throughout the study period, with proportions of 4.5% in 2010 and 4.1% in 2021 (−2.3%, 95% CI −11.6, 1.8) (eFigure 5).
Fig. 3.
Standardized proportions of initial therapy for people newly diagnosed with chronic obstructive pulmonary disease in Dutch primary care (2010–2021) from the PHARMO data network. A shows trends in maintenance therapies, and B shows trends in reliever-only therapies. SAMA (short-acting muscarinic antagonist), SABA (short-acting beta-agonist), SABA-SAMA (combination of SABA and SAMA), LABA (long-acting beta-agonist), LAMA (long-acting muscarinic antagonist), LABA-LAMA (combination of LABA and LAMA), LABA-ICS (combination of LABA and inhaled corticosteroids), and LABA-LAMA-ICS (combination of LABA, LAMA, and ICS). Shaded areas represent 95% confidence intervals
SABA as reliever-only therapy increased from 8.5% in 2010 to 14.3% in 2018 (7.6%, 95% CI 6.6, 9.0). It then plateaued between 2018 and 2021, reaching 13.3% in 2021 (−3.7%, 95% CI −11.9, 0.8) (eFigure 6). SAMA and SABA-SAMA remained consistently low and stable throughout the period, with SAMA at 3.3% in 2010 and 3.4% in 2021 (0.7%, 95% CI −1.5, 2.6) and SABA-SAMA at 0.8% in 2010 and 0.7% in 2021 (−2.6%, 95% CI −3.3, 8.4) (eFigure 7–8). A detailed breakdown of the annual proportions and corresponding 95% CIs for the entire cohort, as well as by sex and age, can be found in the supplementary material (see Additional file 1, eTable 4–12).
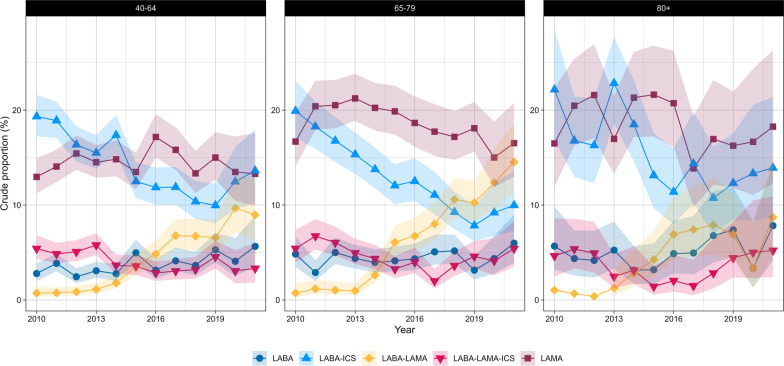
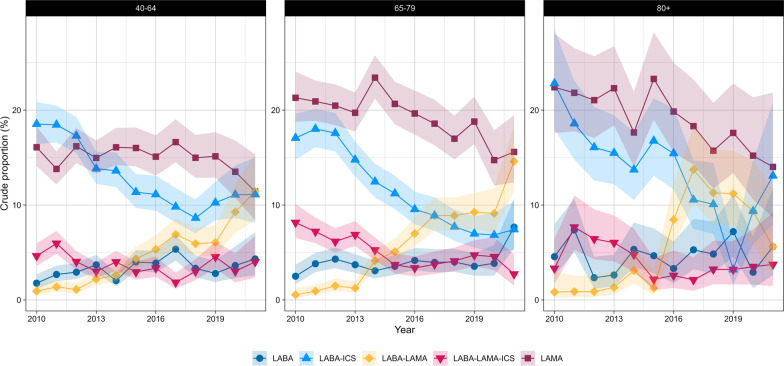
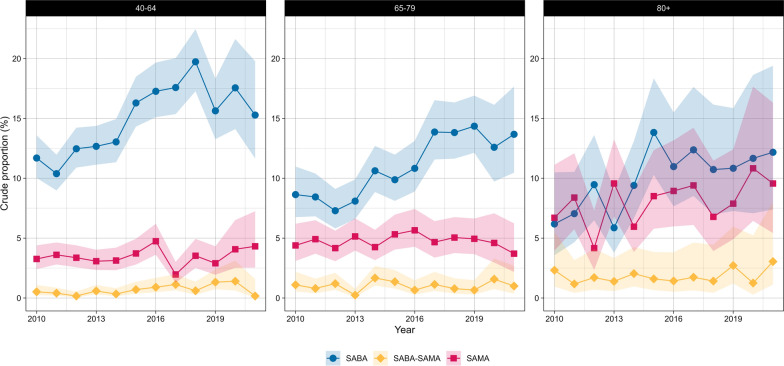
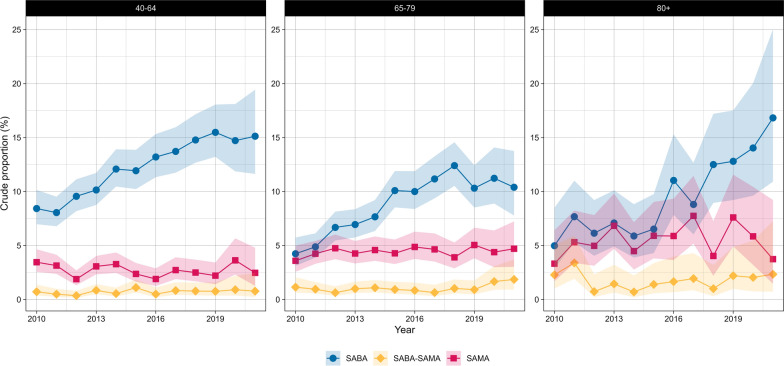
For LABA-ICS and LABA-LAMA, differences in trends were observed only between sexes (eTable 10). In contrast, LAMA, LABA-LAMA-ICS, and SABA-SAMA varied with age but not with sex. However, SABA and SAMA differed across age groups and between sexes (Figs. 4, 5, 6 and 7).
Fig. 4.
Crude proportions of maintenance therapies in Dutch primary care (2010–2021) from the PHARMO data network for females by age group. LABA (long-acting beta-agonist), LAMA (long-acting muscarinic antagonist), LABA-LAMA (combination of LABA and LAMA), LABA-ICS (combination of LABA and inhaled corticosteroids), LABA-LAMA-ICS (combination of LABA, LAMA, and ICS). Shaded areas represent 95% confidence intervals
Fig. 5.
Crude proportions of maintenance therapies in Dutch primary care (2010–2021) from the PHARMO data network for males by age group. LABA (long-acting beta-agonist), LAMA (long-acting muscarinic antagonist), LABA-LAMA (combination of LABA and LAMA), LABA-ICS (combination of LABA and inhaled corticosteroids), LABA-LAMA-ICS (combination of LABA, LAMA, and ICS). Shaded areas represent 95% confidence intervals
Fig. 6.
Crude proportions of reliever-only therapies in Dutch primary care (2010–2021) from the PHARMO data network for females by age group. SAMA (short-acting muscarinic antagonist), SABA (short-acting beta-agonist), SABA-SAMA (combination of SABA and SAMA). Shaded areas represent 95% confidence intervals
Fig. 7.
Crude proportions of reliever-only therapies in Dutch primary care (2010–2021) from the PHARMO data network for males by age group. SAMA (short-acting muscarinic antagonist), SABA (short-acting beta-agonist), SABA-SAMA (combination of SABA and SAMA). Shaded areas represent 95% confidence intervals
Discussion
This study reported significant changes in the initial pharmacological treatment of newly diagnosed COPD patients in Dutch primary care, likely driven by updated management strategies and the introduction of new fixed-dose combination inhalers [4, 24].
LAMA monotherapy and LABA-ICS were the leading maintenance therapies; however, their use as initial treatments has declined, while LABA-LAMA has grown significantly. SABA remained the preferred choice for reliever-only therapy throughout the observation period, despite limited evidence of its faster onset of action or superior bronchodilation [25]. Among long-acting monotherapies, LAMAs were more frequently prescribed as initial therapy than LABAs, likely because they more effectively reduce exacerbation risk despite no definitive evidence favoring either class for symptom relief [25–27].
The proportion of patients receiving LAMA monotherapy increased between 2010 and 2015 as new long-acting anticholinergic drugs became available, which may have expanded treatment options and influenced prescribing patterns [4]. However, from 2015 onward, LAMA monotherapy began to decline, following an already ongoing decrease in LABA-ICS. Moreover, the proportion of patients prescribed LABA-LAMA therapy increased significantly. These changes may reflect the evolving scientific landscape over the past decade. In the early 2010s, the importance of the rational use of ICSs became increasingly recognized, emphasizing careful patient selection and weighing the risks of their use [28–30]. Simultaneously, between 2013 and 2015, fixed-dose LABA-LAMA combinations were introduced [4]. Subsequent studies demonstrated that LABA-LAMA therapies were more effective than LABA-ICS in reducing exacerbation risk [31, 32] and were even superior to monotherapies [26]. These developments may have led to a shift toward increased prescribing of LABA-LAMA [33]. Nevertheless, LABA-ICS remained one of the most commonly prescribed initial therapies at the end of the decade. Future studies should monitor its use, as triple therapy is now preferred when ICS is indicated [34]. Patients currently on LABA-ICS should be reviewed to confirm either a reduction in exacerbations or a documented positive response to ICS [34].
Triple therapy was prescribed as early as 2010, despite not being recommended as a first-line therapy at that time [35]. Instead, it was indicated as maintenance therapy for COPD patients with frequent exacerbations who were not adequately controlled with either LABA-ICS or LABA-LAMA [35–37]. These findings are consistent with previous reports indicating that triple therapy was often prescribed as initial therapy for COPD patients, contrary to GOLD recommendations [38–40].
Approximately 36% of newly diagnosed COPD patients did not receive a prescription for respiratory medication within 90 days post-diagnosis, and most remained without one for up to a year. Previous data from Germany and the United Kingdom have similarly indicated that a substantial proportion of patients lack prescriptions following diagnosis [5, 6]. Delays in initiating inhaled therapy are worrisome, as they can increase the risk of exacerbations and further decrease lung function, which affects patients' quality of life and results in increased, yet preventable, healthcare costs [41, 42]. Prescribing practices are influenced by both national and international guidelines, which may explain some of these findings. For example, the Dutch NHG guidelines recommend that milder patients use SABA or SAMA “if necessary,” meaning some may not receive any medication at all [43]. Still, it is important to further explore why some patients remain without a prescription. Qualitative research with GPs could provide insight into the factors driving their decision-making and help bridge the gap between guideline recommendations and real-world implementation. Furthermore, a smaller proportion of patients received treatments that are entirely discouraged as COPD therapy, primarily ICS monotherapy [28]. This is unexpected because ICS monotherapy is a standard treatment for asthma, and patients with asthma were excluded from this study, suggesting either potential inaccuracies in patient diagnostic coding or suboptimal clinical practices.
Differences in trends across age groups and sexes may suggest the existence of prescribing biases [25]. A recent study highlighted sex-specific differences among new users of inhaled pharmacotherapies for obstructive airway diseases, indicating differential treatment that warrants further investigation [44]. However, the present analysis did not account for the distribution of the population across the GOLD groups, for example. The observed differences could be attributed to patient characteristics, such as symptom burden and exacerbation history, rather than inherent prescriber preferences on the basis of sex or age.
This study also revealed that a significant proportion of patients are already being treated for comorbidities at the time of diagnosis. The appropriate management and monitoring of comorbidities are essential because they can lower adherence to COPD treatment, exacerbate symptoms, and affect patient prognosis [45–48]. Furthermore, these findings suggest that comorbidities may share risk factors with COPD, indicating that they are not merely consequences of the disease but part of a broader health context affecting these patients [49]. Comorbidities are also likely to develop earlier in the presence of subclinical lung function impairment [50].
This study has strengths and limitations that require acknowledgment. The main strengths are as follows: (i) the use of PHARMO data, which are representative of the general Dutch population in terms of demographics and diagnoses and provide more comprehensive medication records than national statistics do [13]; and (ii) this is one of the first studies to assess temporal trends in pharmacological treatment within primary care for newly diagnosed COPD patients. Nevertheless, it cannot be ruled out that some individuals assumed to be without prescriptions may, in fact, be receiving treatment, as information on specialist co-management is lacking and some may have obtained prescriptions from GP practices outside the PHARMO catchment area. Furthermore, ICPC codes rely on recording by general practitioners, and since lung function data was not available for all individuals, the diagnosis of COPD could not be confirmed.
Conclusion
Significant shifts in initial pharmacological treatment for newly diagnosed COPD patients were observed between 2010 and 2021, possibly due to the introduction of new inhaler therapies and updated management strategies. Approximately 36% of patients remained without GP prescriptions for COPD after 90 days of diagnosis, while 4% were on ICS monotherapy or other treatments, highlighting the potential for improved COPD management in primary care.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
G.R. and B.N.B. contributed to data curation. G.R. conducted the formal analysis and wrote the original draft. J.A., Q.D., B.N.B., A.M., F.M.E.F., and M.A.S. contributed to methodology and writing-review & editing. A.M., F.M.E.F., and M.A.S. were responsible for conceptualization and supervision. F.M.E.F. and M.A.S. handled funding acquisition.
Funding
This work received financial support from AstraZeneca, Chiesi, TEVA, and Boehringer Ingelheim through the University of Aveiro/CIRO + B.V. (BI/ESSUA/9841/2023; BI/ESSUA/9878/2021). It was also supported by FCT-Fundação para a Ciência e Tecnologia, I.P. under project references UIDB/04501/2020 (DOI: https://doi.org/10.54499/UIDB/04501/2020) and UIDP/04501/2020 (DOI: https://doi.org/10.54499/UIDP/04501/2020).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but can be obtained from the PHARMO Institute upon reasonable request and with approval from the Compliance Committee of Stichting Informatievoorziening voor Zorg en Onderzoek (STIZON).
Declarations
Ethics approval and consent to participate
This study is exempt from requiring informed consent under the European Union General Data Protection Regulation (EU-GDPR), which permits the use of secondary data for scientific research when appropriate safeguards are in place. Stichting Informatievoorziening voor Zorg en Onderzoek (STIZON) is responsible for the collection, processing, and deidentification of data in accordance with the Personal Data Protection Act under Dutch legislation, allowing its anonymous use by PHARMO and/or affiliated universities or projects for research purposes.
Consent for publication
Not applicable.
Competing interests
G.R., J.A., Q.D., and A.M. declare that they have no competing interests. B.N.B. is an employee of the PHARMO Institute for Drug Outcomes Research, an independent institute conducting financially supported studies for the government, healthcare authorities, and pharmaceutical companies. F.M.E.F. has received research grants from AstraZeneca; consultancy fees from Merck Sharp & Dohme; and speaker fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Chiesi, and Novartis. All these are outside the scope of the current study. M.A.S. has received research grants from the Netherlands Lung Foundation and Stichting Astma Bestrijding, as well as consultancy fees from AstraZeneca and Boehringer Ingelheim outside the scope of the current study. All research grants and consultancy fees were paid to Ciro.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global burden of chronic respiratory diseases and risk factors, 1990–2019: an update from the Global Burden of Disease Study 2019. EClinicalMedicine. 2023;59:101936. [DOI] [PMC free article] [PubMed]
- 2.Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2204–56. [DOI] [PMC free article] [PubMed]
- 3.Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpin DMG, Dickens AP, Skinner D, Murray R, Singh M, Hickman K, et al. Identification of key opportunities for optimising the management of high-risk COPD patients in the UK using the CONQUEST quality standards: an observational longitudinal study. Lancet Reg Health Eur. 2023;29: 100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhl R, Wilke T, Picker N, Schmidt O, Hechtner M, Kondla A, et al. Real-world treatment of patients newly diagnosed with chronic obstructive pulmonary disease: a retrospective german claims data analysis. Int J Chron Obstruct Pulmon Dis. 2022;17:2355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson K, Ekberg-Jansson A, Stridsman C, Hanno M, Vanfleteren L. Adherence to treatment recommendations for chronic obstructive pulmonary disease—results from the swedish national airway register. Int J Chron Obstruct Pulmon Dis. 2021;16:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013;1(10):e29-30. [DOI] [PubMed] [Google Scholar]
- 9.Gershon AS, Lindenauer PK, Wilson KC, Rose L, Walkey AJ, Sadatsafavi M, et al. Informing healthcare decisions with observational research assessing causal effect. An official American Thoracic Society research statement. Am J Respir Crit Care Med. 2021;203(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpin DMG. Clinical management of COPD in the real world: can studies reveal errors in management and pathways to improve patient care? Pragmat Obs Res. 2023;14:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10): e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuiper JG, Bakker M, Penning-van Beest FJA, Herings RMC. Existing data sources for clinical epidemiology: the PHARMO database network. Clin Epidemiol. 2020;12:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overbeek JA, Swart KMA, Houben E, Penning-van Beest FJA, Herings RMC. Completeness and representativeness of the PHARMO general practitioner (GP) data: a comparison with national statistics. Clin Epidemiol. 2023;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ATC/DDD Index: Norwegian Institute of Public Health; 2024. https://atcddd.fhi.no/atc_ddd_index/. Accessed 23 Jul 2024.
- 15.Rasmussen L, Wettermark B, Steinke D, Pottegård A. Core concepts in pharmacoepidemiology: measures of drug utilization based on individual-level drug dispensing data. Pharmacoepidemiol Drug Saf. 2022;31(10):1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics Netherlands (CBS). Population Pyramid: Statistics Netherlands (CBS); 2024. https://www.cbs.nl/en-gb/visualisations/dashboard-population/population-pyramid.
- 17.Vollset SE. Confidence intervals for a binomial proportion. Stat Med. 1993;12(9):809–24. [DOI] [PubMed] [Google Scholar]
- 18.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. [DOI] [PubMed] [Google Scholar]
- 19.Joinpoint Regression Program: National Cancer Institute; 2024. https://surveillance.cancer.gov/joinpoint/. Accessed 23 Jul 2024.
- 20.Kim H-J, Luo J, Chen H-S, Green D, Buckman D, Byrne J, et al. Improved confidence interval for average annual percent change in trend analysis. Stat Med. 2017;36(19):3059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Number of Joinpoints - Joinpoint Regression Program: National Cancer Institute; 2024. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/number-of-joinpoints. Accessed 23 Jul 2024.
- 22.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–14. [DOI] [PubMed] [Google Scholar]
- 23.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–12. [Google Scholar]
- 24.Terry PD, Dhand R. Inhalation therapy for stable copd: 20 years of GOLD reports. Adv Ther. 2020;37(5):1812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung D. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD 2024 Report. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2024 2024.
- 26.Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12(12):Cd012620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koarai A, Sugiura H, Yamada M, Ichikawa T, Fujino N, Kawayama T, et al. Treatment with LABA versus LAMA for stable COPD: a systematic review and meta-analysis. BMC Pulm Med. 2020;20(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quint JK, Ariel A, Barnes PJ. Rational use of inhaled corticosteroids for the treatment of COPD. NPJ Prim Care Respir Med. 2023;33(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antón E. How and when to use inhaled corticosteroids in chronic obstructive pulmonary disease? Expert Rev Respir Med. 2013;7(2 Suppl):25–32. [DOI] [PubMed] [Google Scholar]
- 31.Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34. [DOI] [PubMed] [Google Scholar]
- 32.Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2(2):Cd012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi S, Wright A, Hartgers-Gubbels ES, Hechtner M, Clark B, Wright C, et al. Costs and clinical consequences of compliance with COPD GOLD recommendations or national guidelines compared with current clinical practice in Belgium, Germany, Sweden, and the United States. Int J Chron Obstruct Pulmon Dis. 2022;17:2149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Global Initiative for Chronic Obstructive Lung D. Global strategy for prevention, diagnosis and management of COPD: 2025 Report. 2024.
- 35.Gruffydd-Jones K. GOLD guidelines 2011: what are the implications for primary care? Prim Care Respir J. 2012;21(4):437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. [DOI] [PubMed] [Google Scholar]
- 37.Yawn BP, Mintz ML, Doherty DE. GOLD in practice: chronic obstructive pulmonary disease treatment and management in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2021;16:289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Lim J, Stemkowski S, Kaila S, Renda A, Shaikh A. Initiation of triple therapy maintenance treatment among patients with COPD. Am J Manag Care. 2020;26(4):e106–12. [DOI] [PubMed] [Google Scholar]
- 39.Simeone JC, Luthra R, Kaila S, Pan X, Bhagnani TD, Liu J, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt SP, Blauer-Peterson C, Buysman EK, Bengtson LGS, Paine Iii SR. Trends and characteristics of global initiative for chronic obstructive lung disease guidelines-discordant prescribing of triple therapy among patients with COPD. Chronic Obstr Pulm Dis. 2022;9(2):135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh D, Litewka D, Páramo R, Rendon A, Sayiner A, Tanni SE, et al. Delaying disease progression in COPD with early initiation of dual bronchodilator or triple inhaled pharmacotherapy (DEPICT): a predictive modelling approach. Adv Ther. 2023;40(10):4282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogart M, Glassberg MB, Reinsch T, Stanford RH. Impact of prompt versus delayed initiation of triple therapy post COPD exacerbation in a US-managed care setting. Respir Med. 2018;145:138–44. [DOI] [PubMed] [Google Scholar]
- 43.de Jong J, Bouma M. Nieuwe ontwikkelingen in de herziene NHG-Standaard COPD. Huisarts en wetenschap 2021;64:65–66.
- 44.Amegadzie JE, Gamble JM, Farrell J, Gao Z. Gender differences in inhaled pharmacotherapy utilization in patients with obstructive airway diseases (OADs): a population-based study. Int J Chron Obstruct Pulmon Dis. 2020;15:2355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skajaa N, Laugesen K, Horváth-Puhó E, Sørensen HT. Comorbidities and mortality among patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2023;10(1):e001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61. [DOI] [PubMed] [Google Scholar]
- 48.Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabbri LM, Celli BR, Agustí A, Criner GJ, Dransfield MT, Divo M, et al. COPD and multimorbidity: recognising and addressing a syndemic occurrence. Lancet Respir Med. 2023;11(11):1020–34. [DOI] [PubMed] [Google Scholar]
- 50.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but can be obtained from the PHARMO Institute upon reasonable request and with approval from the Compliance Committee of Stichting Informatievoorziening voor Zorg en Onderzoek (STIZON).