Abstract
Objective:
To identify and critically evaluate models predicting insomnia treatment response in adult populations.
Methods:
Pubmed, EMBASE, and PsychInfo databases were searched from January 2000 to January 2023 to identify studies reporting the development or validation of multivariable models predicting insomnia treatment outcomes in adults. Data were extracted according to CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) guidelines and study quality was assessed using the Prediction model study Risk Of Bias Assessment Tool (PROBAST).
Results:
Eleven studies describing 53 prediction models were included and appraised. Treatment response was most frequently assessed using wake after sleep onset (n = 10; 18.9%), insomnia severity index (n = 10; 18.9%), and sleep onset latency (n = 9, 17%). Dysfunctional Beliefs About Sleep (DBAS) score was the most common predictor in final models (n = 33). R2 values ranged from 0.06 to 0.80 for models predicting continuous response and area under the curve (AUC) ranged from 0.73 to 0.87 for classification models. Only two models were internally validated, and none were externally validated. All models were rated as having a high risk of bias according to PROBAST, which was largely driven by the analysis domain.
Conclusion:
Prediction models may be a useful tool to assist clinicians in selecting the optimal treatment strategy for patients with insomnia. However, no externally validated models currently exist. These results highlight an important gap in the literature and underscore the need for the development and validation of modern, methodologically rigorous models.
Keywords: Sleep, Insomnia, Prediction, Treatment, Outcomes
1. Introduction
Insomnia is a common sleep disorder characterized by difficulty falling asleep, staying asleep, and/or early morning awakening, despite adequate opportunity for sleep. The prevalence of insomnia in the United States ranges between 10% and 40% based on definition and occurs more frequently in older people, women, and those with certain medical and psychiatric comorbidities (Crowley, 2011). In many cases, insomnia is comorbid with other conditions, including depression, anxiety, cancer, and diabetes (Morin et al., 2015). Both acute and chronic insomnia can have a significant negative impact on patient wellbeing; the primary symptoms are fatigue and diminished cognitive functioning, which can lead to worsening physical health, poor work-place performance, and worse quality of life (Kuppermann et al., 1995). Growing evidence also suggests that chronic insomnia may increase an individual’s risk of developing chronic illnesses and mood disorders, incurring significant personal and societal costs (Hertenstein et al., 2019).
Currently, there are a variety of insomnia treatments available. Pharmacological options, including benzodiazepine and non-benzodiazepine medications, are effective in treating insomnia (Sateia et al., 2017). Benzodiazepines are effective in the short-term but can lead to dependency and tolerance if used long-term (Buscemi et al., 2007). Tolerance appears to be less problematic for non-benzodiazepine options, such as dual orexin receptor antagonists and benzodiazepine receptor agonists (Sateia et al., 2017; Krystal et al., 2008; Michelson et al., 2014). Cognitive behavioral therapy for insomnia (CBT-I) is the primary non-pharmacological treatment strategy and is often preferred over medication because it does not carry any harmful side effects or potential for abuse. For those reasons, it has been recommended as a first-line treatment for chronic insomnia by the American College of Physicians (Qaseem et al., 2016). However, CBT-I is often less accessible than medication as its delivery requires trained health professionals.
While CBT-I and medications are generally effective treatment options, there is significant heterogeneity in patients’ responses and many fail to achieve remission or relapse after treatment ends. For example, one study evaluating the effect of 12 weeks of Ramelteon therapy on insomnia severity found that only 26.2% of patients responded to treatment. However, among patients with a baseline insomnia severity index (ISI) score≥10, approximately 44% responded (Uchiyama et al., 2019). In another study, 76.7% of the sample showed a therapeutic response to benzodiazepine treatment but fewer than half achieved remission (Pillai et al., 2017). Additionally, despite being well-established as an effective treatment option, studies suggest that up to 40% of patients don’t achieve remission after CBT-I (Morin et al., 2009; Castronovo et al., 2018; Edinger and Means, 2005). Treatment response is thought to be influenced by a complex interplay of individual-level factors, such as patient demographics, medical history, sleep habits, beliefs, and environment (Edinger and Means, 2005).
Given that insomnia treatment response varies widely depending on the individual, prediction models may be a promising tool to help clinicians identify which treatments are most likely to benefit a particular patient. Accurate prediction models for insomnia treatment outcomes could have significant clinical benefits, including improved patient care, reduced insomnia-related healthcare costs, and minimizing the risk of adverse events associated with inappropriate treatment selection. Technical and methodologic advancements over the past few decades have enabled the creation of sophisticated models that can analyze many variables to generate accurate predictions, and researchers have successfully developed models that predict individual treatment response in several disease areas, including cancer, depression, and obstructive sleep apnea (Lee et al., 2018; Pengo et al., 2020; Staal et al., 2021). While considerably less explored in the context of insomnia, a small number of studies have attempted to apply similar methodologies to predict insomnia treatment outcomes. To date, there have not been any articles synthesizing this type of evidence for insomnia. Thus, our primary aim was to systematically identify and appraise multivariable models that can be used to predict primary and secondary insomnia treatment outcomes in the general adult population. Our secondary aim was to provide an overview of the most common predictors used in the models.
2. Methods
2.1. Data source and search procedure
This systematic review was registered with the Prospective Register of Systematic Reviews (CRD42021291824) and conducted according to the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). A comprehensive literature search was performed using PubMed, Embase, and PsychInfo to identify primary articles reporting on the development and/or validation of models predicting treatment outcomes in insomnia that were published between January 1st, 2000 and January 1st, 2023. Supplementary Tables S1a, S1b, and S1c summarize the literature search criteria for each database, including Medical Subject Headings (MeSH) terms and keywords. Briefly, the search terms included combinations of keywords related to insomnia/sleep disorders, outcomes, prediction, and algorithms. Relevant references from identified articles were also screened for eligibility.
2.2. Eligibility criteria
The study question was defined according to the PICOTS system as presented in the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) checklist (Moons et al., 2014), see Table S2 in the supplement. To be eligible for inclusion, studies were required to meet the following criteria: 1) develop and/or validate one or more multivariable models predicting treatment outcome(s) for primary or secondary insomnia, 2) study population included adults with insomnia, 3) report a measure of the model’s predictive performance (e.g., area under the curve, R2, mean squared error), 4) treatment outcomes were assessed using a validated measure or well-defined EMR-based definition (if using EMR data), and 5) written in English. Insomnia treatments could be pharmacologic or non-pharmacologic (i.e., CBT-I). Animal studies, biomolecular studies, letters, reviews, editorials, systematic reviews, meta-analyses, case reports, case series, case-control studies and cross-sectional studies were excluded, as were studies on children with insomnia and studies that did not report a measure of model performance.
2.3. Phase I – screening of titles and abstracts
Titles and abstracts of identified studies were independently screened by two reviewers (EH and CB) for further consideration. Disagreements were discussed between reviewers and resolved by a third party (AO) if no consensus was reached. Eligible articles were 1) written in English, 2) concerned insomnia patients, and 3) reported on the prediction of treatment outcomes in a multivariable fashion. Articles that did not meet these criteria were excluded from phase II.
2.4. Phase II – review of full articles
To be considered for quality assessment, articles were required to 1) measure insomnia treatment outcomes using a validated instrument or well-defined measure based on EMR data (e.g., changes in healthcare utilization), and 2) report a measure of model performance. If an article reported more than one prediction model, each model was reviewed individually and evaluated against the inclusion criteria. Articles and models that did not meet these criteria were excluded from phase III.
2.5. Phase III – quality assessment and data extraction
The quality of models eligible for phase III was independently assessed by two reviewers (EH, YD) using the Prediction model study Risk Of Bias Assessment Tool (PROBAST) tool. Disagreements were discussed between reviewers and resolved by a third party (AO) if no consensus was reached. The PROBAST tool consists of 20 signaling questions organized into four domains: participants, predictors, outcome, and analysis (Wolff et al., 2019). Data elements were extracted into a standardized form according to CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) guidelines (Moons et al., 2014). Briefly, the data elements included descriptions of 1) data source, 2) participants, 3) outcome to be predicted, 4) candidate predictors, 5) sample size, 6) missing data, 7) model development, 8) model performance, 9) model evaluation, 10) results, and 11) discussion.
3. Results
3.1. Flow of included studies
The initial search yielded 4144 unique papers. Upon title and abstract review, 2659 articles were excluded. Of the 39 articles that underwent full text review, 25 were excluded for not reporting a measure of model performance, two for not predicting insomnia treatment outcomes, and one for not using a multivariable model, leaving 11 studies for data extraction. These 11 studies included a total of 65 unique prediction models. Out of those 65 models, five models (Blom et al., 2021; Pruiksma et al., 2020) were excluded due to not reporting performance measures and seven models (Jansson-Frojmark and Linton, 2008) were excluded for non-standard outcome measures. The remaining 53 models were reviewed (Fig. 1) (see Table 1).
Fig. 1.

Flowchart of literature search for models predicting insomnia treatment outcomes.
Table 1.
Summary of included models grouped by outcome definition.
| Author (year) | Population (data source, location) |
Treatment | Outcome definition (timing) |
N | No. with outcome/ mean response |
Predictors included in final model | Model type, performance |
|---|---|---|---|---|---|---|---|
| Insomnia Severity Index | |||||||
| Blom et al. (2021) | Adults with insomnia (4 RCTs, Sweden) | CBT-I | ISI (EOT) | 252 | Δ ISI = 5.68 | Baseline ISI, baseline SRBQ (items 14, 17, 19, 24), factor 3 (blocking thoughts), factor 8 (napping), factor 10 (checking time) | Linear regression, R = 0.21 |
| ISI <8 or Δ ISI >7 and ISI <15 (3–10yrs) | 199 | 133/199 (67%) | Baseline ISI, Δ ISI, Δ DBAS, Δ SRBQ, Δ SPAQ | Logistic regression, Tjur’s R2 = 0.37 | |||
| ISI <8 or Δ ISI >7 and ISI <15 (3–10yrs) | 199 | 133/199 (67%) | Baseline ISI, Δ ISI, baseline DBAS (items 1, 3, 5), Δ DBAS (items 5, 10), baseline SRBQ (items 4, 5, 7, 15), Δ SRBQ (items 7, 15), Δ Factor 4 (blaming poor sleep), Δ Factor 10 (checking time) | Logistic regression, Tjur’s R2 = 0.59 | |||
| Constantino et al. (2007) | Adults with insomnia referred for CBT by sleep specialist (Sleep center, USA) | CBT-I | Δ daytime interference (EOT) | 29 | Δ DI: 2.88 | Group Therapy Session Report (expectancy, affiliation, critical confrontation, expectancy*affiliation, expectancy*critical confrontation, affiliation*critical confrontation) | Linear regression, Adjusted R2 = −0.09 |
| Pruiksma et al. (2020) | Active-duty military members with insomnia on psychotropic and/or hypnotic medications for at least one month (RCT, USA) | CBT-I | Δ ISI (EOT) | 99 | NR | Baseline ISI, baseline BDI score, history of head injury, baseline TST | Linear regression, R = 0.24 |
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ ISI–P (EOT) | 47 | NR | Δ DBAS (sleep expectations, insomnia consequences), ABS (time arising from bed, avoidance of napping), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.26 |
| Δ ISI–P (6m) | 47 | NR | Δ DBAS (sleep expectation, insomnia consequences), ABS (time arising from bed, avoidance of napping), Δ HADS (total), TEPCQ, TAPQ-Participant | Linear regression, R = 0.19 | |||
| Δ ISI–C (EOT) | 47 | NR | Δ DBAS (control of sleep, insomnia consequences), ABS (avoidance of napping, arising during night awakenings), Δ HADS (anxiety subscale), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.18 | |||
| Δ ISI–C (6m) | 47 | NR | Δ DBAS (insomnia consequences subscale, sleep habits subscale), ABS (bedtime hour, arising during night awakenings), Δ HADS (depression subscale), TEPCQ, TAPQ-Global | Linear regression, R = 0.33 | |||
| Forsell et al. (2022) | Non-depressed adults with insomnia (RCT, Sweden) | CBT-I | ISI >8 or Δ ISI ≤7 (EOT) | 199 | 66/199 (33%) | ISI, MADRS-S, CORE-9, DBAS (items 4, 11, 17, 20, 24, 25, 28, 29), insomnia knowledge, GSE, SRBQ (items 7,9,25,28), SPAQ (items 1,2,3,4 and additional item), PSS-4, WAI score, TCS score, clinician ratings (activity, contact, sleep restriction and stimulus control, attitude towards sleep and CBT-I, homework, sleep medication, affected adherence, affected sleep, motivation) | Logistic regression, Accuracy = 0.67 |
| Pittsburgh Sleep Quality Index | |||||||
| Currie et al. (2002) | Adults ≤60 years with insomnia secondary to chronic pain, RCT (RCT, Canada) | CBT-I | PSQI RCI>1.96 (EOT) | 51 | 20/51 (39.2%) | Sleep self-efficacy scale | Logistic regression, Accuracy = 0.71 |
| Morgan et al. (2003) | Adults ≥30 years using hypnotics for at least the previous month and requested a refill (RCT, UK) | CBT-I | Δ PSQI (3m) | 55 | Δ PSQI: 2.8 | baseline PSQI score, IPQ (cure/control subscale) | Linear regression, R = 0.35 |
| Shi et al. (2021) | Adults with insomnia age 18–70 (Cohort study, China) | TMS | Δ PSQI (EOT) | 25 | Δ PSQI: 3.20 | baseline power envelope connectivity, age, sex | PLS regression, Cross-validated R2: Alpha band: 0.60 Beta band: 0.80 Gamma band: 0.66 Delta band: 0.46 Theta band: 0.58 |
| Δ PSQI (1m) | 25 | Δ PSQI: 5.84 | baseline power envelope connectivity, age, sex | PLS regression, Cross-validated R2: Alpha band: 0.52 Beta band: 0.62 Gamma band: 0.31 Delta band: 0.28 Theta band: 0.55 |
|||
| Sleep Efficiency | |||||||
| Jansson-Frojmark and Linton (2008) | Adults 18–65 years with insomnia (RCT, Sweden) | CBT-I | Δ SE (1 yr) | 64 | Δ SE: +13% | # of CBT sessions, baseline DBAS | Linear regression, R = 0.15 |
| Morgan et al. (2003) | Adults ≥30 years who had been taking hypnotics for at least the previous month and requested a refill (RCT, UK) | CBT-I | Δ SE (3m) | 54 | Δ SE: +11.3% | baseline SE, IPQ (cure/control subscale) | Linear regression, R = 0.54 |
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ SEa (EOT) | 47 | NR | Δ DBAS (control of sleep subscale, insomnia consequences subscale), ABS (time of arising from bed, arising during night awakenings), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.28 |
| Δ SEa (6m) | 47 | NR | Δ DBAS (insomnia consequences subscale, sleep habits subscale), ABS (bedtime hour, avoidance of napping), Δ HADS (anxiety subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.31 | |||
| Δ SEb (EOT) | 47 | NR | Δ DBAS (causal attributions subscale, sleep expectations subscale), ABS (time arising from bed, avoidance of napping), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.18 | |||
| Δ SEb (6m) | 47 | NR | Δ DBAS (insomnia consequences subscale, sleep habits subscale), ABS (time arising from bed, avoidance of napping), Δ HADS (depression subscale), TEPCQ, TAPQ-Global | Linear regression, R = 0.20 | |||
| Sleep Onset Latency | |||||||
| Currie et al. (2002) | Adults ≤60 years with insomnia secondary to chronic pain, RCT (RCT, Canada) | CBT-I | SOL ≤30 min (EOT) | 51 | 5/51 (29.4%) | BDI, smoking status, sleep medications | Logistic regression, Accuracy = 0.71 |
| Espie et al. (2001) | Adults with insomnia referred to a sleep clinic (Primary care, Scotland) | CBT-I | Δ SOL ≥50% (12m) | 109 | 45/109 (41.3%) | Insomnia severity, DBAS factor II | Logistic regression, Sensitivity = 0.56, Specificity = 0.74 |
| SOL ≤30 min (12m) | 109 | 70/109 (64.2%) | Insomnia severity, DBAS factor II | Logistic regression, Sensitivity = 0.88, Specificity = 0.35 | |||
| Δ SOL ≥50% and SOL ≤30 min (12m) | 109 | 38/109 (34.9%) | DBAS factor II | Logistic regression, Sensitivity = 0.91, Specificity = 0.21 | |||
| Inoue et al. (2015) | Adults aged 20–84 with insomnia (RCT, Japan) | Eszopiclone | SOL ≤30 min (4 wk) | 58 | 35/58 (60.3%) | Age, sex, BMI, number of comorbidities, baseline SOL | Logistic regression, AUC = 0.73 |
| Morgan et al. (2003) | Adults ≥30 years who had been taking hypnotics for at least the previous month and requested a refill (RCT, UK) | CBT-I | Δ SOL (3m) | 56 | Δ SOL: 27.7 min | baseline SOL | Linear regression, R = 0.37 |
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ SOLa (EOT) | 47 | NR | Δ DBAS (control of sleep subscale, sleep habits subscale), ABS (avoidance of napping, sleep restriction), Δ HADS (total), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.21 |
| Δ SOLa (6m) | 47 | NR | Δ DBAS (control of sleep subscale, sleep habits subscale), ABS (time arising from bed, avoidance of napping), Δ HADS (depression subscale), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.29 | |||
| Δ SOLb (EOT) | 47 | NR | Δ DBAS (control of sleep subscale, sleep expectations subscale), ABS (time of arising from bed, sleep restriction), Δ HADS (depression subscale), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.23 | |||
| Δ SOLb (6m) | 47 | NR | Δ DBAS (sleep expectations subscale, insomnia consequences subscale), ABS (bedtime hour, time of arising from bed), Δ HADS (total), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.27 | |||
| Total Sleep Time | |||||||
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ TSTa (EOT) | 47 | NR | Δ DBAS (control of sleep, insomnia consequences), ABS (time of arising from bed, sleep restriction), Δ HADS (anxiety subscale), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.26 |
| Δ TSTa (6m) | 47 | NR | Δ DBAS (insomnia consequences, sleep habits), ABS (bedtime hour, avoidance of napping), Δ HADS (total), TEPCQ, TAPQ-Participant | Linear regression, R = 0.26 | |||
| Δ TSTb (EOT) | 47 | NR | Δ DBAS (causal attributions, sleep expectations), ABS (avoidance of napping, arising during night awakenings), Δ HADS (anxiety), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.32 | |||
| Δ TSTb (6m) | 47 | NR | Δ DBAS (sleep expectations, sleep habits), ABS (time of arising from bed, arising during night awakenings), Δ HADS (total), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.32 | |||
| Wake After Sleep Onset | |||||||
| Currie et al. (2002) | Adults ≤60 years with insomnia secondary to chronic pain, RCT (RCT, Canada) | CBT-I | WASO ≤30 min (EOT) | 51 | 23/51 (45.1%) | Pain severity, smoking status | Logistic regression, Accuracy = 0.63 |
| Espie et al. (2001) | Adults with insomnia referred to a sleep clinic (Primary care, Scotland) | CBT-I | Δ WASO ≥50% (12m) | 109 | 54/109 (49.5%) | BDI score, SDQ factor IV, insomnia severity | Logistic regression, Sensitivity = 0.70, Specificity = 0.64 |
| WASO ≤30 min (12m) | 109 | 69/109 (63.3%) | Insomnia severity, STAI state | Logistic regression, Sensitivity = 0.77, Specificity = 0.46 | |||
| Δ WASO ≥50% and WASO ≤30 min (12m) | 109 | 44/109 (40.4%) | BDI score, SDQ factor IV | Logistic regression, Sensitivity = 0.87, Specificity = 0.42 | |||
| Inoue et al. (2015) | Adults aged 20–84 with insomnia, 46 sleep clinics in (RCT, Japan) | Eszopiclone | WASO ≤30 min (4 wk) | 51 | 35/51 (68.6%) | Age, sex, BMI, number of comorbidities, baseline WASO | Logistic regression, AUC = 0.87 |
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ WASOa (EOT) | 47 | NR | Δ DBAS (sleep expectations, insomnia consequences), ABS (time of arising from bed, arising during night awakenings), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.38 |
| Δ WASOa (6m) | 47 | NR | Δ DBAS (insomnia consequences, sleep habits), ABS (time of arising from bed, arising during night awakenings), Δ HADS (anxiety), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.43 | |||
| Δ WASOb (EOT) | 47 | NR | Δ DBAS (causal attributions, insomnia consequences), ABS (avoidance of napping, sleep restriction), Δ HADS (total), TEPCQ, TAPQ-Clinician | Linear regression, R = 0.29 | |||
| Δ WASOb (6m) | 47 | NR | Δ DBAS (sleep habits, sleep expectations), ABS (bedtime hour, time of arising from bed), Δ HADS (anxiety), TEPCQ, TAPQ-Global | Linear regression, R = 0.22 | |||
| Total Wake Time | |||||||
| Tremblay et al. (2009) | Adult women with chronic insomnia secondary to breast cancer (RCT, Canada) | CBT-I | Δ TWTa (EOT) | 47 | NR | Δ DBAS (control of sleep, insomnia consequences), ABS (time of arising from bed, arising during night awakenings), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.28 |
| Δ TWTa (6m) | 47 | NR | Δ DBAS (control of sleep, insomnia consequences), ABS (time of arising from bed, avoidance of napping), Δ HADS (anxiety subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.30 | |||
| Δ TWTb (EOT) | 47 | NR | Δ DBAS (causal attributions, insomnia consequences), ABS (avoidance of napping, time of arising from bed), Δ HADS (depression subscale), TEPCQ, TAPQ-Participant | Linear regression, R = 0.32 | |||
| Δ TWTb (6m) | 47 | NR | Δ DBAS (insomnia consequences, sleep habits), ABS (time of arising from bed, avoidance of napping), Δ HADS (depression subscale), TEPCQ, TAPQ-Global | Linear regression, R = 0.23 | |||
| Constantino et al. (2007) | Adults with insomnia referred for CBT by sleep specialist (Sleep center, USA) | CBT-I | Δ TWT (EOT) | 49 | Δ TWT: 28.47% | Group Therapy Session Report (expectancy, affiliation, critical confrontation, expectancy*affiliation, expectancy*critical confrontation, affiliation*critical confrontation) | Linear regression, Adjusted R2 = 0.06 |
| Sleep Quality | |||||||
| Jansson-Frojmark and Linton (2008) | Adults age 18–65 with insomnia (RCT, Sweden) | CBT-I | Δ SQ (1 yr) | 64 | Δ SQ: 1.3 | Age Δ DBAS (total), insomnia duration | Linear regression, R = 0.09 Linear regression, R = 0.31 |
| Number of Nocturnal Awakenings | |||||||
| Espie et al. (2001) | Adults with insomnia referred to a sleep clinic (Primary care, Scotland) | CBT-I | Δ nWAKE ≥50% (1yr) | 109 | 31/109 (28.4%) | BDI | Logistic regression, Sensitivity = 0.13, Specificity = 1.0 |
| Enjoyment of Sleep | |||||||
| Espie et al. (2001) | Adults with insomnia referred to a sleep clinic (Primary care, Scotland) | CBT-I | Δ Enjoy ≥50% (1yr) | 109 | 29/109 (26.6%) | PSWQ, marital status, credibility | Logistic regression, Sensitivity = 0.17, Specificity = 0.94 |
| Outcome: hypnotic-free nights/week | |||||||
| Morgan et al. (2003) | Adults ≥30 years who had been taking hypnotics for at least the previous month and requested a refill (RCT, UK) | CBT-I | Δ hypnotic-free nights/week (3m) | 57 | Δ hypnotic-free nights/week: −2.2 | HADS (anxiety), baseline # hypnotic-free nights/week | Linear regression, R = 0.22 |
Abbreviations: CBT-I = cognitive behavioral therapy for insomnia, ISI = insomnia severity index, PSQI = Pittsburgh Sleep Quality Index, WASO = wake after sleep onset, SOL = sleep onset latency, SE = sleep efficiency, TST = total sleep time, SQ = sleep quality, TWT = total wake time, RCI = reliable change index, PLS = partial least squares, NR = not reported, Δ = change, EOT = end of treatment, m = months, yr = years, wk = weeks, nWAKE = number of nocturnal awakenings, enjoy = enjoyment of sleep, DBAS = Dysfunctional Beliefs About Sleep questionnaire, HADS = Hospital Anxiety and Depression Scale, TEPCQ = Treatment Expectancies and Perceived Credibility Questionnaire, TAPQ = Therapeutic Alliance Perception Questionnaire, ABS = Adherence to Behavioral Strategies, SDQ = Sleep Disorders Questionnaire, BDI = Beck Depression Inventory, SPAQ = Sleep Practices and Attitudes Questionnaire, SRBQ = Sleep Related Behaviors Questionnaire, BMI = body mass index, DI = daytime interference, IPQ = Illness Perception Questionnaire.
from sleep diary
from polysomnogram.
3.2. Study characteristics
Three studies were based in Sweden (Blom et al., 2021; Jansson--Frojmark and Linton, 2008; Forsell et al., 2022), two in the United States (Pruiksma et al., 2020; Constantino et al., 2007), two in the United Kingdom (Espie et al., 2001; Morgan et al., 2003), two in Asia (Inoue et al., 2015; Shi et al., 2021), and two in Canada (Tremblay et al., 2009; Currie et al., 2002). In total, nine studies (Blom et al., 2021; Pruiksma et al., 2020; Jansson-Frojmark and Linton, 2008; Forsell et al., 2022; Constantino et al., 2007; Espie et al., 2001; Morgan et al., 2003; Tremblay et al., 2009; Currie et al., 2002) predicted response to CBT-I, one predicted response to eszopiclone therapy (Inoue et al., 2015), and one predicted response to transcranial magnetic stimulation (Shi et al., 2021). While all studies included adults with insomnia, three studies were restricted to specific subpopulations. Specifically, one study was limited to women with insomnia secondary to breast cancer (Tremblay et al., 2009), another required insomnia secondary to chronic pain (Currie et al., 2002), and a third required participants be using hypnotic medications (Morgan et al., 2003). The median sample size across studies was 47 participants (range 25–252). A full summary of the included models, grouped by outcome, is presented in Table 2.
Table 2.
Overview of outcome measures used in included studies.
| Measure | Definition |
|---|---|
| PSQI | 24-item self-report measure of sleep quality. Range: 0–21. Score >6 indicates poor sleep. (Buysse et al., 1989) |
| SOL | Measured by sleep diary or polysomnogram; length of time (minutes) from turning light off to falling asleep. SOL ≤30 min is normal. |
| WASO | Measured by sleep diary or polysomnogram; length of time (minutes) of wakefulness after initial onset of sleep. WASO ≤30 min is normal. |
| TST | Measured by sleep diary or polysomnogram; length of time (minutes) spent sleeping while in bed. |
| TWT | Measured by sleep diary or polysomnogram; length of time (minutes) of wakefulness during the in-bed period. |
| SE | Measured by sleep questionnaire, sleep diary, or polysomnogram; percentage of time asleep while in bed. |
| ISI | 7-item self-report measure of insomnia severity. Range: 0–28. A change in score >7 is clinically meaningful and a score ≥11 indicates insomnia. (Morin, 1993; Morin et al., 2015) |
| Sleep Quality | Item from sleep diary (“How would you rate the quality of your sleep?“), rated on a 5-point Likert scale from “very poor” to “very good”. |
| Nighttime awakenings | The discrete number of nighttime awakenings recalled by an individual. |
| Hypnotic-free nights/week | The number of nights per week that an individual did not use hypnotic medications for sleep. |
| Enjoyment of sleep | Enjoyment of sleep rated on a 5-point Likert scale from 0 (not at all) through 4 (extremely). |
Abbreviations: ISI = insomnia severity index, PSQI = Pittsburgh Sleep Quality Index, WASO = wake after sleep onset, SOL = sleep onset latency, SE = sleep efficiency, TST = total sleep time, TWT = total wake time.
3.3. Insomnia treatment response
There was substantial variation in how treatment response was assessed. Across all models, the most common methods of measuring treatment response were the insomnia severity index (Morin, 1993) (ISI) and sleep onset latency (SOL; n = 10 models, respectively). Other methods included wake after sleep onset (WASO; n = 9), sleep efficiency (n = 6), total wake time (TWT; n = 5), total sleep time (TST; n = 4), Pittsburgh Sleep Quality Index (Buysse et al., 1989) (PSQI; n = 4), sleep quality (n = 2), number of nighttime awakenings (n = 1), enjoyment of sleep (n = 1), and number of hypnotic free nights/week (n = 1), see Table 2. All metrics were sourced from patient-reported questionnaires except for SOL, WASO, and sleep efficiency, which could come from either patient-reported sleep diaries (Carney et al., 2012) or polysomnograms. In terms of timing, 51 models (Pruiksma et al., 2020; Jansson-Frojmark and Linton, 2008; Forsell et al., 2022; Constantino et al., 2007; Espie et al., 2001; Morgan et al., 2003; Inoue et al., 2015; Shi et al., 2021; Tremblay et al., 2009; Currie et al., 2002) predicted treatment response within one year of treatment completion and two models (Blom et al., 2021) predicted “long term outcome”, which consisted of patient questionnaires completed either 3- or 10-years post-treatment.
In total, 37 models predicted a continuous treatment outcome. Of those, seven models predicted post-treatment or pre/post-treatment change in ISI score (Blom et al., 2021; Pruiksma et al., 2020; Constantino et al., 2007; Tremblay et al., 2009), three predicted post-treatment PSQI score (Morgan et al., 2003; Shi et al., 2021), six predicted pre/post-treatment change in sleep efficiency (Jansson-Frojmark and Linton, 2008; Morgan et al., 2003; Tremblay et al., 2009), five predicted pre/post-treatment change in SOL (Morgan et al., 2003; Tremblay et al., 2009), four predicted pre/post-treatment change in TST (Tremblay et al., 2009), four predicted pre/post-treatment change in WASO (Tremblay et al., 2009), five predicted pre/post-treatment change in TWT (Constantino et al., 2007; Tremblay et al., 2009), one predicted pre/post-treatment change in the sleep quality (sleep diary item) (Jansson-Frojmark and Linton, 2008), and one predicted pre/post-treatment change in the number of hypnotic-free nights per week (Morgan et al., 2003).
Sixteen models predicted categorical treatment response. Three models (Blom et al., 2021; Forsell et al., 2022) defined treatment response using established ISI thresholds (post-treatment ISI score <8, which indicates insomnia remission (Morin and Espie, 2003), ISI score improvement from pretreatment >7, indicating clinically-meaningful change (Morin et al., 2011), and ISI <15, an established cutpoint differentiating mild and moderate insomnia (Morin and Espie, 2003)). One model (Currie et al., 2002) assessed treatment response using the PSQI, defining response as a reliable change index (RCI) score >1.96. Five models (Espie et al., 2001; Inoue et al., 2015; Currie et al., 2002) defined treatment response as a posttreatment SOL ≤30 min, SOL decrease of ≥50% from baseline, or a combination of both, and another five models defined treatment response using those same cutoffs but for WASO rather than SOL (Espie et al., 2001; Inoue et al., 2015; Currie et al., 2002). These cutoffs for SOL and WASO are commonly used in the literature (Morin, 1993). Two classification models defined treatment response as ≥ 50% change from baseline in enjoyment of sleep and number of nocturnal awakenings, respectively (Espie et al., 2001). Both enjoyment of sleep and number of nocturnal awakenings correspond to questions in an older version of the sleep diary (Espie, 1991).
3.4. Predictors
The median number of candidate predictors for the included models was 16 (range 3–27). In total, 54 different types of candidate predictors were included, which fell into the following general categories: patient demographics and clinical characteristics, sleep diary items, sleep questionnaires, mood questionnaires, CBT questionnaires, and other questionnaires.
While the number of candidate predictors was relatively large, the number of predictors included in the final models was much smaller (median = 5, range 1–11). Nearly all studies selected a subset of the candidate predictors for the final model using univariable associations or automated selection procedures. When considering the final models, the most frequently included variables were Dysfunctional Beliefs about Sleep (DBAS) score (n = 32), Treatment Expectancies and Perceived Credibility Questionnaire (TAPQ, n = 26), and the Hospital Depression and Anxiety Scale (HADS, n = 25), see Fig. 2. Of the 32 models using DBAS as a predictor, 3121,23,24,26,30 used the DBAS-10 (Espie et al., 2000) and one (Tremblay et al., 2009) used a 21-item short form derived from the original version (Morin et al., 1993). The most common predictors in final models, by outcome (for outcomes with >3 models), are presented in Figs. S1-S7. Nine models (Blom et al., 2021; Pruiksma et al., 2020; Forsell et al., 2022; Morgan et al., 2003) included baseline outcome measurements as predictors.
Fig. 2. Frequency of predictors in final prediction models (all).

abrAbbreviationsBMI = body mass index, EEG = electroencephalogram, SOL = sleep onset latency, WASO = wake after sleep onset, TST = total sleep time, SE = sleep efficiency, ISI = insomnia severity index, PSQI = Pittsburgh Sleep Quality Index, SSE = sleep self-efficacy scale, DBAS = Dysfunctional Beliefs About Sleep questionnaire, SRBQ = Sleep-related beliefs questionnaire, SPAQ = Sleep Problem Acceptance Questionnaire, BDI = Beck Depression Inventory, HADS = Hospital Anxiety and Depression Scale, PSWQ = Penn State Worry Questionnaire, STAI = State-Trait Anxiety Inventory, MADRS-S = Montgomery-Asberg Depression Rating Scale self-report, CORE = Clinical Outcomes in Routine Evaluation, PSS-4 = Perceived Stress Scale 4, IPQ = Illness Perception Questionnaire, GSE = General Self Efficacy scale, CBT = cognitive behavioral therapy, TEPCQ = Treatment Expectancies and Perceived Credibility Questionnaire, GTSR = Group Therapy Session Report, ABS = Adherence to Behavioral Strategies, TAPQ = Therapeutic Alliance Perception Questionnaire, WAI = Working Alliance Inventory.
3.5. Model development and performance
The context and performance of these models varied substantially. In total, 37 models (Blom et al., 2021; Pruiksma et al., 2020; Jansson--Frojmark and Linton, 2008; Constantino et al., 2007; Morgan et al., 2003; Shi et al., 2021; Tremblay et al., 2009) predicted continuous treatment outcomes using linear regression and reported R2 values ranging from 0.06 to 0.80. The remaining 16 models (Blom et al., 2021; Forsell et al., 2022; Espie et al., 2001; Inoue et al., 2015; Currie et al., 2002) predicted binary treatment outcomes using logistic regression, reporting accuracies and AUCs ranging from 0.60 to 0.71 and from 0.73 to 0.87, respectively. Eight classification models (Espie et al., 2001) reported sensitivity and specificity, which ranged from 0.13 to 0.91 and 0.21 to 1.0, respectively.
3.6. Critical appraised
A summary of the risk of bias assessments is presented in Fig. 3 and detailed assessments for each model are reported in Supplementary Table S3. The risk of bias for the participant domain was low for all 53 models. Several models (n = 29) were assessed as having high risk of bias in the predictors domain due to using variables in the models that are not available prior to treatment initiation. All models had a low risk of bias for the outcome domain. However, bias in the analysis domain was extensive and affected all 53 models. This was largely driven by a lack of validation, improper handling of missing data, and having too few events per candidate variable (EPV); notably, only two models (Shi et al., 2021) were internally validated and none were externally validated. Furthermore, only four had an EPV greater than 10, the minimum recommended to avoid overfitting (Austin and Steyerberg, 2017), and all studies that had missing data performed a complete-case analysis. Since all models had high risk of bias in the analysis domain, the overall risk of bias for all models was also considered high. Approximately half of the models (n = 29) were considered to have high applicability concerns for including variables in the models that are not available at the intended time of use.
Fig. 3.
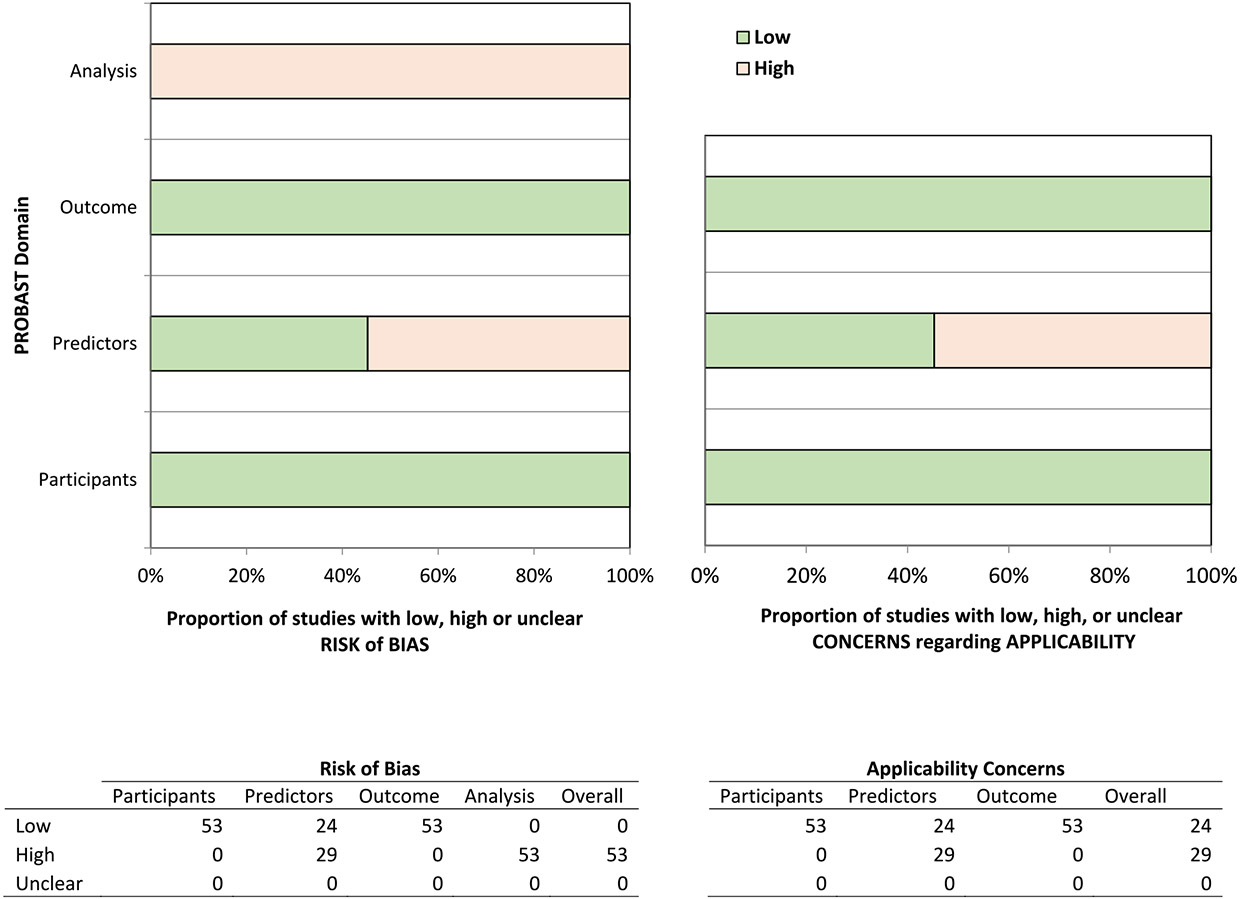
Risk of bias assessment (PROBAST) for included models.
4. Discussion
After reviewing the literature, we identified and systematically assessed 53 models predicting insomnia treatment outcomes. Of those, 49 attempted to predict patient response to CBT-I, two predicted response to eszopiclone therapy, and two predicted response to transcranial magnetic stimulation. Treatment response was assessed using sleep diaries, polysomnograms, ISI, and the PSQI, and most models predicted continuous treatment response rather than categorical. Of the sixteen classification models, thirteen defined treatment response using established cutoffs from the literature. One model had PSQI as the outcome but defined treatment response as RCI >1.96 rather than the established cutoff score of 533. While RCI can useful, it determines whether there was a statistically significant change in an individual’s scores on a particular assessment, not whether the change is clinically meaningful (Jacobson and Truax, 1991). Two classification models predicted improvement on individual questions from an old version of the sleep diary, which do not appear to have established cutoffs. While candidate predictors varied substantially, DBAS score was included in final models more than any other predictor, closely followed by TEPCQ and HADS scores. Of note, the nine models that included baseline outcome measures as predictors showed that they explained most of the variation in treatment response. To our knowledge, this is the first systematic review of models predicting insomnia treatment outcomes.
All models used either linear or logistic regression rather than machine learning algorithms. The models that showed the best performance were those that predicted response to treatments other than CBT-I; Shi et al.‘s models predicting response to TMS, which included baseline EEG connectivity, age, and sex, performed the best of those predicting continuous responses (beta band R2 = 0.62) (Shi et al., 2021). The best performing classifiers were those predicting response to eszopiclone (WASO AUC = 0.87, SOL AUC = 0.73) (Inoue et al., 2015). It’s possible that the models predicting response to TMS performed robustly because they included electrophysiological connectivity as a predictor, which may serve as a neural network biomarker. Eszopiclone prediction models may have demonstrated better performance than those predicting response to CBT-I due to the different mechanisms of action of the two treatments and predictors that were included. Eszopiclone is a sedative-hypnotic that acts on gamma-aminobutyric acid-A (GABA-A) receptors (Rösner et al., 2018). In contrast, sleep restriction, a core component of CBT-I, is thought to reduce sleep latency and time awake at night by increasing the homeostatic sleep drive (Walker et al., 2022). However, it is difficult to conclude the utility and generalizability of any of the models given that they all had high risk of bias, which was largely driven by concerns about the analysis domain.
One of the most evident findings of our review is that no externally validated models to predict insomnia treatment outcomes currently exist. Despite being described as prediction models, most studies focused on exploring factors associated with the outcome as is done when the goal is statistical inference. Indeed, our systematic review revealed that all the identified prediction models suffered from substantial methodologic pitfalls. Only two models were internally validated and zero were externally validated, raising questions about performance optimism and generalizability. Assessing a model using the same set of patients used to develop it often overestimates performance; this issue is typically addressed using either internal or external validation. Internal validation methods, such as cross-validation or bootstrapping, provide more accurate estimates of how the model would perform on new patients, while external validation using different data gives insight about how well the model might generalize (Steyerberg and Harrell, 2016). Notably, all but four models had EPVs of less than ten, further increasing the risk of overfitting (Austin and Steyerberg, 2017). None of the models predicting a binary outcome reported calibration, a measure that compares predicted risk to observed risk (Steyerberg et al., 2010).
Predicting insomnia treatment outcomes remains a complex task rife with logistical challenges. Lack of data may be the greatest barrier given that the types of data required for models predicting treatment outcomes (i.e., patient questionnaires) are typically only collected in randomized controlled trials, which are expensive and time-consuming to conduct and, as evidenced by the studies included in this review, often limited to relatively small sample sizes. Small samples are detrimental because they restrict the number of predictors that can be included in the models without overfitting and can limit generalizability. These findings illustrate the need for larger patient cohorts, an issue that could potentially be addressed through greater collaboration and the pooling of deidentified study data. Alternatively, researchers could leverage EMR and/or claims databases to obtain larger cohorts for prediction modeling, although doing so would limit the outcomes that could be investigated to items that are readily available in such databases (e.g., insomnia medication use, healthcare utilization, etc.), which may lack well-established definitions of treatment response compared to patient-reported measures. It is also unclear which outcome is best to measure insomnia treatment response. Given the considerable heterogeneity found in insomnia, even within studies using Diagnostic and Statistical Manual (DSM) or International Classification of Sleep Disorders (ICSD) criteria, it is plausible that the relationship between predictors treatment effects may be sensitive to the type of outcome measure used and patient population. Additionally, a reliable set of predictors has yet to be established. Physiologic predictors, such as those from EEGs, have been correlated with treatment response and could possibly be used to improve model accuracy (Shi et al., 2021; Krystal and Edinger, 2010). Despite these challenges, prediction models remain a promising tool in the pursuit of precision medicine and have the potential to enhance insomnia treatment through personalized treatment recommendations.
Taken together, our findings suggest that none of the currently published models are suitable for use in clinical practice; the process of trial-and-error when treating insomnia will continue, at least for the time being. That does not mean that the pursuit of accurate prediction models for insomnia treatment models should be abandoned, however. Instead, it highlights an opportunity for researchers to develop modern prediction models that follow recently established model development guidelines (Collins et al., 2015) and leverage machine learning methods that could significantly improve model performance.
This review has several limitations. First, only English language articles were included so it is likely that some models were not considered. The included studies all had a high risk of bias, mainly due to analytic methods, and thus provide inconclusive evidence regarding their predictive ability and clinical utility. Additionally, the heterogeneity of the included studies makes it difficult to directly compare model performance or generalize the prognostic value of individual predictors across study populations. Some studies only included participants of specific categories (i.e., women with breast cancer, active-duty military) and therefore do not apply to the general adult population. Finally, we were unable to conduct a meta-analysis due to heterogeneous outcome measures.
5. Conclusion
Insomnia is a common and debilitating sleep disorder that affects a significant portion of the population. While there are a variety of insomnia treatments available, their effectiveness can vary substantially depending on the individual patient. Prediction models may be a useful tool to assist clinicians in selecting the optimal treatment strategy for patients with insomnia, however, external validation studies are needed to determine the utility and generalizability of the currently published models. Additional work is also needed to determine which outcome measure is best suited for measuring treatment response and to establish a reliable set of predictors. These results highlight an important gap in the literature and underscore the need for modern, methodologically rigorous models. Future research efforts focused on developing and validating such models could enable clinicians to tailor treatment strategies to the unique needs of each patient, potentially leading to better outcomes and improved quality of life for individuals suffering from insomnia.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
CRediT authorship contribution statement
Emma Holler: Formal analysis, Writing – original draft, Writing – review & editing, Conceptualization. Yu Du: Formal analysis, Writing – review & editing. Cristina Barboi: Formal analysis, Writing – review & editing. Arthur Owora: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2023.12.017.
Conflict of interest disclosures
None to disclose.
References
- Austin PC, Steyerberg EW, 2017. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat. Methods Med. Res 26 (2), 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom K, Hentati Isacsson N, Forsell E, et al. , 2021. An investigation and replication of sleep-related cognitions, acceptance and behaviours as predictors of short- and long-term outcome in cognitive behavioural therapy for insomnia. J. Sleep Res 30 (5), e13376. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Friesen C, et al. , 2007. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J. Gen. Intern. Med 22 (9), 1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res 28 (2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, et al. , 2012. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 35 (2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo V, Galbiati A, Sforza M, et al. , 2018. Long-term clinical effect of group cognitive behavioral therapy for insomnia: a case series study. Sleep Med. 47, 54–59. [DOI] [PubMed] [Google Scholar]
- Collins GS, Reitsma JB, Altman DG, Moons KGM, 2015. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 13 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino MJ, Manber R, Ong J, Kuo TF, Huang JS, Arnow BA, 2007. Patient expectations and therapeutic alliance as predictors of outcome in group cognitive-behavioral therapy for insomnia. Behav. Sleep Med 5 (3), 210–228. [DOI] [PubMed] [Google Scholar]
- Crowley K, 2011. Sleep and sleep disorders in older adults. Neuropsychol. Rev 21 (1), 41–53. [DOI] [PubMed] [Google Scholar]
- Currie SR, Wilson KG, Curran D, 2002. Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain. J. Behav. Med 25 (2), 135–153. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK, 2005. Cognitive–behavioral therapy for primary insomnia. Clin. Psychol. Rev 25 (5), 539–558. [DOI] [PubMed] [Google Scholar]
- Espie CA, 1991. The Psychological Treatment of Insomnia. Wiley. [Google Scholar]
- Espie CA, Inglis SJ, Harvey L, Tessier S, 2000. Insomniacs’ attributions: psychometric properties of the dysfunctional beliefs and attitudes about sleep scale and the sleep disturbance questionnaire. J. Psychosom. Res 48 (2), 141–148. [DOI] [PubMed] [Google Scholar]
- Espie CA, Inglis SJ, Harvey L, 2001. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: analysis of outcome data at 12 months posttreatment. J. Consult. Clin. Psychol 69 (1), 58–66. [DOI] [PubMed] [Google Scholar]
- Forsell E, Jernelöv S, Blom K, Kaldo V, 2022. Clinically sufficient classification accuracy and key predictors of treatment failure in a randomized controlled trial of Internet-delivered Cognitive Behavior Therapy for Insomnia. Internet Interv 29, 100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein E, Feige B, Gmeiner T, et al. , 2019. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med. Rev 43, 96–105. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Kamijo A, Nagai R, 2015. Patient background factors affecting the therapeutic outcomes in response to eszopiclone in adult patients with chronic insomnia: a post hoc analysis of a double-blind phase III study in Japan. J. Clin. Sleep Med 11 (10), 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, Truax P, 1991. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol 59 (1), 12–19. [DOI] [PubMed] [Google Scholar]
- Jansson-Frojmark M, Linton SJ, 2008. The role of sleep-related beliefs to improvement in early cognitive behavioral therapy for insomnia. Cognit. Behav. Ther 37 (1), 5–13. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, 2010. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep 33 (5), 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T, 2008. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep 31 (1), 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann M, Lubeck DP, Mazonson PD, et al. , 1995. Sleep problems and their correlates in a working population. J. Gen. Intern. Med 10 (1), 25–32. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ragguett R-M, Mansur RB, et al. , 2018. Applications of machine learning algorithms to predict therapeutic outcomes in depression: a meta-analysis and systematic review. J. Affect. Disord 241, 519–532. [DOI] [PubMed] [Google Scholar]
- Michelson D, Snyder E, Paradis E, et al. , 2014. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 13 (5), 461–471. [DOI] [PubMed] [Google Scholar]
- Moons KG, de Groot JA, Bouwmeester W, et al. , 2014. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 11 (10), e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Thompson J, Dixon S, Tomeny M, Mathers N, 2003. Predicting longer-term outcomes following psychological treatment for hypnotic-dependent chronic insomnia. J. Psychosom. Res 54 (1), 21–29. [DOI] [PubMed] [Google Scholar]
- Morin CM, 1993. Insomnia: Psychological Assessment and Management. Guilford Press, New York, NY, US. [Google Scholar]
- Morin CM, Espie CA, 2003. Insomnia: A Clinician’s Guide to Assessment and Treatment. Springer Science & Business Media. [Google Scholar]
- Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S, 1993. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol. Aging 8 (3), 463–467. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Guay B, et al. , 2009. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA 301 (19), 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H, 2011. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34 (5), 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Drake CL, Harvey AG, et al. , 2015. Insomnia disorder. Nat. Rev. Dis. Prim 1, 15026. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. , 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengo MF, Soranna D, Giontella A, et al. , 2020. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta-analysis. Eur. Respir. J 55 (5), 1901945. [DOI] [PubMed] [Google Scholar]
- Pillai V, Roth T, Roehrs T, Moss K, Peterson EL, Drake CL, 2017. Effectiveness of benzodiazepine receptor agonists in the treatment of insomnia: an examination of response and remission rates. Sleep 40 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruiksma KE, Hale WJ, Mintz J, et al. , 2020. Predictors of cognitive behavioral therapy for insomnia (CBTi) outcomes in active-duty U.S. Army personnel. Behav. Ther 51 (4), 522–534. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, 2016. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med 165 (2), 125–133. [DOI] [PubMed] [Google Scholar]
- Rösner S, Englbrecht C, Wehrle R, Hajak G, Soyka M, 2018. Eszopiclone for insomnia. Cochrane Database Syst. Rev 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL, 2017. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med 13 (2), 307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Guo Y, Zhu L, et al. , 2021. Electroencephalographic connectivity predicts clinical response to repetitive transcranial magnetic stimulation in patients with insomnia disorder. Sleep Med. 88, 171–179. [DOI] [PubMed] [Google Scholar]
- Staal FCR, van der Reijd DJ, Taghavi M, Lambregts DMJ, Beets-Tan RGH, Maas M, 2021. Radiomics for the prediction of treatment outcome and survival in patients with colorectal cancer: a systematic review. Clin. Colorectal Cancer 20 (1), 52–71. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW, Harrell FE Jr., 2016. Prediction models need appropriate internal, internal-external, and external validation. J. Clin. Epidemiol 69, 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW, Vickers AJ, Cook NR, et al. , 2010. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21 (1), 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay V, Savard J, Ivers H, 2009. Predictors of the effect of cognitive behavioral therapy for chronic insomnia comorbid with breast cancer. J. Consult. Clin. Psychol 77 (4), 742–750. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Sakamoto S, Miyata K, 2019. Effect of ramelteon on insomnia severity: evaluation of patient characteristics affecting treatment response. Sleep Biol. Rhythm 17 (4), 379–388. [Google Scholar]
- Walker J, Muench A, Perlis ML, Vargas I, 2022. Cognitive behavioral therapy for insomnia (CBT-I): a primer. Klin Spec Psihol 11 (2), 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff RF, Moons KG, Riley RD, et al. , 2019. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med 170 (1), 51–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


