Abstract
Objective
Postoperative cognitive dysfunction (POCD) is associated with adverse outcomes of cardiac surgery. This study investigated the potential of pre-operative hypersensitive C-reactive protein (Hs-CRP) as a prognostic indicator of POCD in valvular disease (VHD).
Methods
This study retrospectively analyzed 372 VHD patients admitted to the Department of Cardiac Surgery, Fujian Medical University Union Hospital from January 2024 to July 2024. POCD was evaluated by neuropsychological examination before and one month after surgery. Demographics, disease history, blood biochemical parameters, and perioperative data were collected. Patients were divided into a POCD group (N = 103) and a non-POCD group (N = 269) according to the occurrence of POCD. A logistic regression model was used to analyze the relationship between Hs-CPR and POCD in VHD patients.
Results
The 1-month incidence of POCD in VHD patients was 27.6%. There was statistical significance in age and years of education between the two groups (P = 0.047, P = 0.001). The red blood cell count in the POCD group was lower than that in the non-POCD group (P = 0.025), and the Hs-CRP and mechanical ventilation duration in the POCD group was higher than that in the non-POCD group, with statistical significance (P < 0.001). No significant differences were observed in the results of demographic characteristics and other laboratory measures. The incidence of hospitalization days, ICU stay time, acute renal insufficiency, and new cerebral infarction in the POCD group were higher than those in the non-POCD group (P < 0.001, P < 0.001, P = 0.001, P = 0.029). Univariate and multivariate analysis showed that Hs-CRP was an independent risk factor for POCD in patients undergoing surgery for VHD disease.
Conclusion
Our study shows that preoperative Hs-CRP is significantly elevated in POCD patients undergoing VHD surgery, and preoperative Hs-CRP is an independent predictor of POCD.
Keywords: valvular heart disease, VHD, Hs-CRP, postoperative cognitive complications, cognitive dysfunction, postoperative cognitive dysfunction
Introduction
Valvular heart disease (VHD) is the leading cause of cardiovascular morbidity and mortality worldwide, and its true prevalence is significantly higher than clinically reported prevalence.1 Currently, surgery is an effective means to treat VHD. Cardiopulmonary Bypass (CPB) is a means to maintain blood supply to vital organs during cardiac surgery. During CPB surgery, because the brain is particularly sensitive to ischemic injury changes, the brain tissue is prone to unpredictable damage due to adverse factors such as increased inflammatory mediators and changes in brain metabolism during the operation.2 Postoperative cognitive dysfunction (POCD) is the most common central nervous system complication after VHD surgery.3 In patients who underwent CPB surgery, serum inflammatory markers such as CRP were elevated, while S100b protein was a marker of blood-brain barrier breakdown and brain injury. In the study of Glumac S4 et al, it was found that corticosteroids can inhibit the inflammatory response and reduce CRP. S100b levels were lower in the dexamethasone group, which reduced the incidence or severity of POCD. According to recent reports, the incidence of POCD in cardiac surgery was 33.0%-83.0%,5 and the decline of compound cognitive Z score was greater at one month.6 POCD can not only lead to longer hospital stays7 and higher admission rates8 but also increase the risk of death 6–12 months after surgery by 2.1 times9 and the risk of dementia within 1 year by 7.5 times.10 Some recognized risk factors for POCD include old age, surgical procedures, induction of anesthesia, and metabolic diseases.11 Currently, predictive biomarkers for identifying POCD risk in valve surgery patients are critical for risk stratification.
Hypersensitive C-reactive protein (Hs-CRP) is a test used to determine whether there is inflammatory infection, trauma, and other abnormalities in the body. It is mainly determined through venous blood extraction and has high clinical application value for inflammation, infection, and cardiovascular diseases.12 It is worth noting that recent studies have shown that Hs-CRP is an early prognostic factor for certain diseases, including intracranial hemorrhage,13 interstitial lung disease,14 pediatric bloodstream infection,12 and traumatic stroke.15 Hs-CRP increases sharply in plasma when the body is infected or tissue damaged, binds to complement, accelerates phagocytosis,16 promotes the expression of local adhesion molecules and PAI-1, reduces endothelial NO bioavailability, changes the uptake of LDL by macrophages, causes the aggregation of complement in atherosclerotic lesions, and affects cognitive changes.15 The study of Hou Min et al17 found that the incidence of acute myocardial infarction in patients with elevated Hs-CRP was 3 times that of normal patients. As one of the inflammatory indicators, Hs-CRP was associated with the prognosis of aortic aneurysm.18 Compared with other inflammatory indicators, Hs-CRP is stronger in predicting the risk of future cardiovascular events and death.19 The increase of serum Hs-CRP level is involved in the recovery of neurological function in patients with cerebral infarction and is closely related to the rate of cognitive function decline.20,21 However, the predictive value of preoperative Hs-CRP for POCD in patients undergoing VHD surgery remains to be further explored.
Hs-CRP is a protein that rises sharply in plasma when the body is infected or tissue is damaged. It is also a relatively sensitive indicator of acute disease. The purpose of this study was to investigate the predictive value of pre-operative Hs-CRP for POCD in patients with VHD.
Material and Methods
Study Design, Setting, and Participants
This is a retrospective study, 372 patients with VHD who were treated in the Department of Cardiac Surgery at Fujian Medical University Union Hospital from January 2024 to July 2024 were selected as the study objects. The inclusion criteria for this study were: (1) at least 18 years of age; (2) Emergency surgical treatment after admission: the operation was performed under general anesthesia and with the support of extracorporeal circulation; (3) The preoperative Montreal Scoring Scale (MOCA) indicated no cognitive impairment (illiteracy > 19, primary > 22, junior high > 24). Exclusion criteria: (1) a history of severe central nervous system disease or mental illness (stroke, transient ischemic attack, ischemic attack, severe anxiety, drug or alcohol abuse) or brain surgery; (2) Taking neurological or psychotropic drugs; (3) Unable to receive a cognitive function assessment due to hearing/vision impairment; (4) Other cardiovascular diseases requiring additional surgery; (5) The patient died while in hospital. All patients were admitted to the cardiac surgical ICU and serum samples were drawn from venous blood before emergency surgery without medication. This study follows the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Medical University Union Hospital (Number: 2023KY142).
The following data were collected from the patient’s electronic medical records: (1) general baseline data (age, sex, BMI, years of education, smoking history, drinking history, etc).; (2) clinical data including medical history (hypertension, diabetes, cerebrovascular disease, coronary heart disease); (3) Biochemical indicators (serum creatinine [SCr], D-dimer, glycosylated hemoglobin [GHb], Hypersensitive C-reactive protein [Hs-CRP], triglyceride, low-density lipoprotein [LDL], high-density lipoprotein [HDL], white blood cells [WBC], neutrophil count, hemoglobin, platelet count, mechanical ventilation duration [MV], etc)., (4) Surgical data (duration of operation, duration of cardiopulmonary bypass, duration of aortic occlusion), etc.
Outcomes and Covariates
The primary outcome measure of this study was POCD, and secondary outcome measures included postoperative new arrhythmia (NOA), acute renal insufficiency, acute liver insufficiency, cerebral infarction, ICU stays, and length of hospital stays.
Relevant definitions or diagnostic criteria: (1) Acute renal insufficiency was defined as a 50% increase in SCr within 7 days or a 0.3 mg/dL (26.5 μmol/L) increase in SCr within 2 days or oliguria ≥6 hours.22 (2) Acute hepatic insufficiency is defined as postoperative ALT (0–46 IU/L) and/or AST (0–46 IU/L) exceeding normal values; Increased TBIL (2–22 umol/L) and/or DBIL (0–5.9 umol/L).23 (3) Cerebral infarction is diagnosed by doctors: as ischemic stroke, which is a disease of brain tissue necrosis or softening caused by local cerebral tissue ischemia and hypoxia due to blocked cerebral blood supply.24 (4) Diagnosis of POCD: All participants underwent neuropsychological tests before and one month after surgery, mainly using the MOCA scale to measure whether patients had POCD. According to international research on POCD, Z-values were obtained for individual tests. The Z value is calculated as Z= (postoperative score - preoperative score)/corresponding standard deviation. If the Z value is ≥1.96, the diagnosis is POCD.25
Statistical Analysis
SPSS25.0 statistical software was used for data entry and analysis. Measurement data conforming to normal distribution were represented by mean ± standard deviation (Mean ± SD). An independent sample T-test and analysis of variance were used to compare the intergroups. Measurement data that do not conform to normal distribution are represented by median and quartile. Counting data were described by frequency and component ratio, and the Chi-square test or rank sum test was used for comparison between groups. Binary logistic regression analysis was used to investigate the predictive value of Hs-CRP for POCD in patients with VHD. Univariate logistic regression analysis identified potential risk factors for developing POCD in VHD patients (P < 0.1), and multivariate logistic regression analysis identified previously significant variables as independent factors (P < 0.05). Results are expressed as odds ratios (OR) and corresponding 95% confidence intervals (CI). To evaluate the predictive effect of Hs-CPR in patients with POCD, a receiver operating characteristic (ROC) curve was also constructed and the area under the curve (AUC), sensitivity, and specificity were calculated to show the predictive value of Hs-CRP. Statistical significance was defined as P < 0.05.
Results
Study Population and Baseline Characteristics
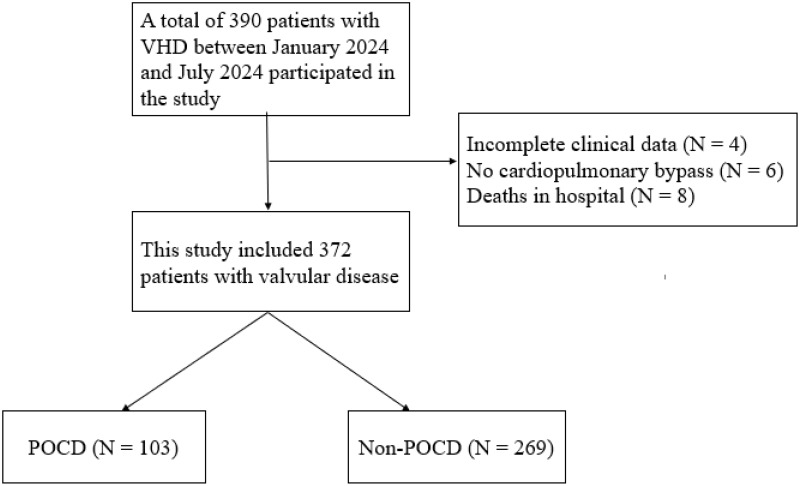
Figure 1 shows the flow chart of the study population. In this study, 372 subjects were finally included for analysis, among which the incidence of POCD in patients with VHD one month after surgery was 27.6%. Patients were divided into two groups based on whether or not they developed POCD: POCD group (n = 103) and non-POCD group (n = 269). The average age of patients in the POCD group was (57.43 ± 11.34) years old, and the average age of patients in the non-POCD group was (54.72 ± 11.84) years old, and the age difference between the two groups was statistically significant (P = 0.047). The average years of education in the POCD group was (6.46 ± 1.88) years, while the average years of education in the non-POCD group was (10.03 ± 1.75) years, and the difference was statistically significant (P = 0.001). In terms of laboratory indicators, the Hs-CRP in the POCD group was (13.32 ± 1.63) mg/L, and the RBC was (4.28 ± 0.69) ×1012/L. Hs-CRP and red blood cell count in the non-POCD group were (8.92 ± 2.96) mg/L and (4.45 ± 0.66) ×1012/L, and there were statistically significant differences in Hs-CRP and red blood cell count between the two groups (P = 0.001, P = 0.025). There were no significant differences in operation duration, cardiopulmonary bypass duration, and aortic occlusion duration between the two groups (P > 0.05). The mechanical ventilation time of the POCD group was longer than that of the non-POCD group (P < 0.001). The baseline characteristics of the two groups are shown in Table 1.
Figure 1.
Flowchart of the study population.
Table 1.
Preoperative Baseline Characteristics of Patients in the Two Groups
| Baseline Characteristics | Total | Non-POCD | POCD | P-value |
|---|---|---|---|---|
| Age (years) | 55.47 ± 11.75 | 54.72 ± 11.84 | 57.43 ± 11.34 | 0.047 |
| BMI (kg/m2) | 23.42 ± 3.60 | 23.43 ± 3.55 | 23.39 ± 3.74 | 0.937 |
| Educational level (years) | 9.04 ± 2.39 | 10.03 ± 1.75 | 6.46 ± 1.88 | 0.001 |
| Male | 204 (54.8) | 151 (40.6) | 53 (14.2) | 0.243 |
| Female | 168 (45.2) | 118 (31.7) | 50 (13.4) | |
| Smoking | 116 (31.2) | 82 (22.0) | 34 (9.1) | 0.363 |
| Drinking | 129 (34.7) | 92 (24.7) | 37 (9.9) | 0.422 |
| Hypertension | 102 (27.4) | 73 (19.6) | 29 (7.8) | 0.470 |
| Diabetes | 33 (8.9) | 26 (7.0) | 7 (1.9) | 0.257 |
| Coronary heart disease | 40 (10.8) | 31 (8.3) | 9 (2.4) | 0.283 |
| Cerebrovascular disease | 31 (8.3) | 20 (5.4) | 11 (3.0) | 0.208 |
| LVEF (%) | 62.71 ± 9.17 | 63.21 ± 8.53 | 61.40 ± 10.60 | 0.090 |
| Scr (umol/L) | 78.27 ± 30.43 | 76.84 ± 29.85 | 82 ± 31.73 | 0.144 |
| D-dimer (umol/L) | 1.39 ± 2.14 | 1.38 ± 2.06 | 1.42 ± 2.36 | 0.869 |
| GHb (%) | 5.875 ± 0.80 | 5.91 ± 0.84 | 5.77 ± 0.67 | 0.118 |
| Hs-CRP (mg/L) | 10.14 ± 3.30 | 8.92 ± 2.96 | 13.32 ± 1.63 | 0.001 |
| Glucose (mg/dl) | 5.21 ± 1.57 | 5.17 ± 1.41 | 5.32 ± 1.94 | 0.414 |
| HDL (mmol/L) | 1.11 ± 0.30 | 1.10 ± 0.30 | 1.14 ± 0.28 | 0.190 |
| LDL (mmol/L) | 2.84 ± 1.21 | 2.78 ± 0.75 | 3.00 ± 1.95 | 0.110 |
| Triglyceride (mmol/L) | 1.38 ± 1.03 | 1.41 ± 1.12 | 1.29 ± 0.72 | 0.291 |
| Serum albumin (g/dl) | 38.54 ± 4.15 | 38.78 ± 4.06 | 37.93 ± 4.35 | 0.079 |
| WBC (10^9/L) | 6.72 ± 2.52 | 6.69 ± 2.59 | 6.82 ± 2.34 | 0.645 |
| Neutrophil (10^9/L) | 4.23 ± 2.42 | 4.18 ± 2.49 | 4.36 ± 2.23 | 0.507 |
| Lymphocyte (10^9/L) | 1.83 ± 0.65 | 1.86 ± 0.65 | 1.77 ± 0.67 | 0.262 |
| PLT (10^9/L) | 205.92 ± 60.84 | 209.74 ± 58.76 | 195.94 ± 65.21 | 0.050 |
| RBC (10^12/L) | 4.40 ± 0.68 | 4.45 ± 0.66 | 4.28 ± 0.69 | 0.025 |
| Hemoglobin (g/L) | 129.55 ± 21.50 | 130.64 ± 20.66 | 126.71 ± 23.42 | 0.115 |
| MV (hours) | 26.04 ± 26.55 | 19.14 ± 14.47 | 44.07 ± 39.50 | 0.000 |
| Operation duration (Minutes) | 365.55 ± 105.19 | 364.70 ±103.36 | 367.78 ± 110.31 | 0.801 |
| Duration of CPB (Minutes) | 147.96 ± 56.64 | 144.91 ± 56.75 | 155.90 ± 55.83 | 0.094 |
| Duration of ACC (Minutes) | 91.65 ± 70.97 | 87.30 ± 43.23 | 102.97 ±114.88 | 0.057 |
Abbreviations: POCD, Postoperative cognitive dysfunction; BMI, Body Mass Index; LVEF, Left Ventricular Ejection Fraction; SCr, Serum creatinine; GHb, Glycosylated hemoglobin; Hs-CRP, Hypersensitive C-reactive protein; LDL, Low-Density Lipoprotein Cholesterin; HDL, High-Density Lipoprotein cholesterol; WBC, White Blood Cell; PLT, Blood Platelet Count; RBC, Red Blood Cell; MV, Mechanical Ventilation; CPB, Cardiopulmonary bypass; ACC, Aortic occlusion.
Postoperative Clinical Features of Two Groups of Patients
As shown in Table 2: Among the 372 VHD patients, 103 patients developed POCD. The number of days in hospital and ICU stays in the POCD group was longer than that in the non-POCD group (P < 0.001), and the incidence of acute renal insufficiency and new cerebral infarction was higher than that in the non-POCD group (P = 0.001, P = 0.029), with no statistical significance in other clinical outcomes (P > 0.05).
Table 2.
Postoperative Clinical Features of the Two Groups
| Outcomes | Total | Non-POCD | POCD | P-value |
|---|---|---|---|---|
| Length of stays (days) | 20.14 ± 10.87 | 17.15 ± 7.57 | 27.92 ± 13.92 | 0.000 |
| ICU stays (hours) | 66.63 ± 64.99 | 47.57 ± 33.62 | 116.39 ± 94.52 | 0.000 |
| Acute renal insufficiency | 48 (12.9) | 23 (6.2) | 25 (6.7) | 0.001 |
| Acute liver insufficiency | 80 (21.5) | 52 (14.0) | 28 (7.5) | 0.067 |
| NOA | 43 (11.6) | 32 (8.6) | 11(3.0) | 0.450 |
| New cerebral infarction | 26 (7.0) | 12 (3.2) | 14 (3.8) | 0.029 |
Abbreviations: POCD, Postoperative cognitive dysfunction; NOA, New Postoperative Arrhythmia.
Univariate analysis showed that several factors were significantly correlated with the risk of POCD in patients with VHD after surgery, including age (OR=1.021, 95% CI: 1.000–1.042, P = 0.048) and years of education (OR=0.322, 95% CI: 0.257–0.405, P = 0.001), Hs-CRP (OR=1.806, 95% CI: 1.594–2.046, P = 0.001), RBC (OR=0.676, 95% CI: 0.478–0.955, P = 0.026) and mechanical ventilation duration (OR=1.041, 95% CI: 1.028–1.005, P = 0.001) predicted the risk of POCD in VHD patients. The results are shown in Table 3.
Table 3.
Results of POCD Single-Factor Analysis
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age (years) | 1.021 (1.000–1.042) | 0.048 |
| Gender | 1.207 (0.766–1.903) | 0.418 |
| Educational level (years) | 0.322 (0.257–0.405) | 0.001 |
| Smoking | 0.638 (0.691–1.827) | 0.638 |
| BMI (kg/m2) | 0.997 (0.936–1.063) | 0.936 |
| Drinking | 1.079 (0.671–1.734) | 0.755 |
| Hypertension | 1.052 (0.634–1.746) | 0.844 |
| Diabetes | 0.681 (0.286–1.622) | 0.386 |
| Coronary heart disease | 0.735 (0.337–1.603) | 0.439 |
| Cerebrovascular disease | 1.489 (0.687–3.227) | 0.314 |
| LVEF (%) | 0.979 (0.956–1.003) | 0.091 |
| Scr (umol/L) | 1.005 (0.998–1.012) | 0.163 |
| HDL (mmol/L) | 1.646 (0.781–3.472) | 0.190 |
| LDL (mmol/L) | 1.158 (0.939–1.429) | 0.171 |
| D-dimer (umol/L) | 1.009 (0.909–1.120) | 0.868 |
| GHb (%) | 0.770 (0.554–1.072) | 0.122 |
| Hs-CRP (mg/L) | 1.806 (1.594–2.046) | 0.001 |
| Glucose (mg/dl) | 1.059 (0.923–1.215) | 0.415 |
| Triglyceride (mmol/L) | 0.858 (0.644–1.143) | 0.295 |
| Serum albumin (g/dl) | 0.952 (0.901–1.006) | 0.080 |
| WBC (10^9/L) | 1.021 (0.935–1.115) | 0.645 |
| Neutrophil (10^9/L) | 1.031 (0.942–1.128) | 0.507 |
| Lymphocyte (10^9/L) | 0.816 (0.572–1.164) | 0.262 |
| PLT (10^9/L) | 0.996 (0.992–1.000) | 0.051 |
| RBC (10^12/L) | 0.676 (0.478–0.955) | 0.026 |
| Hemoglobin (g/L) | 0.992 (0.981–1.002) | 0.117 |
| Operation duration (Minutes) | 1.000 (0.998–1.002) | 0.800 |
| Duration of CPB (Minutes) | 1.003 (0.999–1.007) | 0.095 |
| Duration of ACC (Minutes) | 1.003 (0.999–1.007) | 0.126 |
| MV (hours) | 1.041 (1.028–1.005) | 0.001 |
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; POCD, Postoperative cognitive dysfunction; BMI, Body Mass Index; LVEF, Left Ventricular Ejection Fraction; SCr, Serum creatinine; GHb, Glycosylated hemoglobin; Hs-CRP, Hypersensitive C-reactive protein; LDL, Low-Density Lipoprotein Cholesterin; HDL, High-Density Lipoprotein cholesterol; WBC, White Blood Cell; PLT, Blood Platelet Count; RBC, Red Blood Cell; MV, Mechanical Ventilation; CPB, Cardiopulmonary bypass; ACC, Aortic occlusion.
The results of multivariate analysis are shown in Table 4: years of education (OR=0.282, 95% CI: 0.201–0.397, P = 0.001), Hs-CRP (OR=1.809, 95% CI: 1.478–2.214, P = 0.001), duration of mechanical ventilation (OR=1.057, 95% CI: 1.034–1.080, P = 0.001) and operation duration (OR=0.995, 95% CI: 0.990–0.999, P = 0.02) were associated with an increased risk of POCD in VHD patients. The years of education in the POCD group were lower than those in the non-POCD group (P = 0.001), and the level of Hs-CRP in the POCD group was higher than that in the non-POCD group (P = 0.001). The duration of operation and MV in the POCD group was higher than in the non-POCD group. Studies have shown that Hs-CRP can predict POCD in patients with VHD after cardiac surgery.
Table 4.
Results of POCD Multi-Factor Analysis
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age (years) | 0.999 (0.960–1.040) | 0.959 |
| Educational level (years) | 0.260 (0.181–0.375) | 0.001 |
| Hs-CRP | 1.854 (1.504–2.287) | 0.001 |
| RBC (10^12/L) | 1.383 (0.646–2.962) | 0.404 |
| MV (hours) | 1.062 (1.039–1.086) | 0.001 |
| Operation duration (Minutes) | 0.995 (0.990–0.999) | 0.02 |
Note: Adjusted covariates included LEVF, Serum albumin, PLT, Duration of CPB.
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; Hs-CRP, Hypersensitive C-reactive protein; RBC, Red Blood Cell; MV, Mechanical Ventilation.
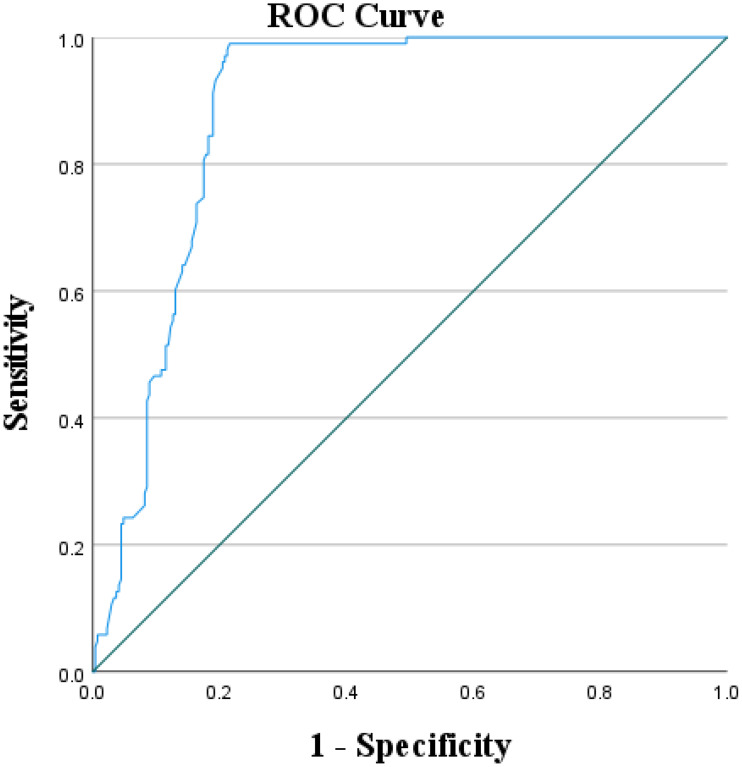
As shown in Figure 2, the ROC curve visually describes the predictive value of Hs-CRP on POCD in VHD patients. The area under the curve AUC is 0.884, and the optimal critical value of Hs-CRP is 10.44 mg/L. The sensitivity was 97.2% and the specificity was 78.4%. The results show that Hs-CRP, a non-specific marker of systemic inflammatory response in the acute phase, is one of the strongest predictors of POCD in VHD patients.
Figure 2.
ROC curve of Hs-CRP and POCD in VHD patients.
Discussion
This study is the first to evaluate the predictive value of preoperative Hs-CRP for POCD in patients with VHD. After 1 month of postoperative cognitive function assessment, it was found that the preoperative Hs-CRP of the POCD patients receiving VHD was higher than that of the non-POCD group, and the results showed that the risk of POCD occurrence was increased in the VHD patients with high Hs-CRP before surgery.
POCD is one of the most common postoperative complications of VHD, which seriously interferes with the recovery and prognosis of patients. Greaves D26 et al found that; The incidence of POCD in patients with CABG was as high as 43.0% one month after surgery, and 7% one month after transcatheter aortic valve implantation.27 In our study, the incidence of POCD 1 month after surgery in VHD patients was 27.6%, similar to previous reports. Studies have shown that the incidence of POCD after cardiac surgery is high, and the incidence of POCD may be different for different diseases.28 Our study shows that preoperative Hs-CRP is associated with cognitive decline in patients receiving VHD. In VHD patients undergoing CPB surgery, when the brain tissue is hypoxic and ischemic, inflammatory cells in the central nervous system are activated, and the activation of microglia will produce more inflammatory factors, increase the injury process, and form a vicious cycle.29 So far, the pathogenesis of POCD needs to be further explored. Early identification and perioperative risk factor management are the best ways to deal with POCD after VHD.30
In this study, the results of the multi-factor analysis showed that years of education, Hs-CRP, duration of mechanical ventilation, and duration of operation were the predictors of POCD in VHD patients (P = 0.001, P = 0.001, P = 0.001, P = 0.02). Among them, patients with more education than high school have lower rates of POCD compared to patients with less education, and in educated populations, the brain is exposed to persistently challenging mental activities that can delay the presentation of dementia by harnessing neuronal reserve and increase the efficacy of synapses to reroute around damaged areas.31 In our study, the average years of education of POCD patients were (6.46 ± 1.88) years, at the primary or middle school level, while the average years of education of patients in the non-POCD group were (10.03 ± 1.75) years, at the middle or high school level or above. The study also found that the risk of POCD was reduced by 10.0% for each additional year of schooling.32 Hs-CRP can be independently used as a predictor of POCD in VHD patients, and our study shows that Hs-CRP levels in POCD patients are higher than those in non-POCD patients. In a study of female heart surgery patients, postoperative POCD was independently associated with Hs-CRP.33 In our study, there was no statistically significant difference in gender between VHD patients. It is undeniable that mechanical ventilation (MV) is an important life-support tool, but it is associated with higher levels of brain damage, neuroinflammation, and neuronal apoptosis compared to subjects who breathe less or on their own.34 There is an association between brain damage and poorer cognitive scores in patients with prolonged mechanical ventilation. The mechanism of MV causing POCD includes dopamine-induced apoptosis, which leads to the death of nerve cells in the hippocampus of mice and increases the risk of POCD.35,36 Several studies have shown that operation time is also one of the risk factors for predicting POCD after cardiac surgery, which is similar to the results of this study.37,38 The operation itself is a serious injury to the patient, and the risk of developing POCD in patients whose operation time is more than 2 hours is 2.53 times that of those within 2 hours.39
The length of ICU stay and hospital stay in the POCD group were higher than those in the non-POCD group (P < 0.001), and the patients with acute renal insufficiency and new cerebral infarction in the POCD group were higher than those in the non-POCD group (P = 0.001, P = 0.029). A large number of studies have confirmed that POCD increases the length of hospital stay (LOS), and patients with POCD have a higher length of hospital stay than those without POCD,40 which is consistent with our study. In the study of Li Yao38 et al, 185 of 409 patients in intensive care were diagnosed with post-ICU cognitive dysfunction, which was related to the length of stay in ICU, similar to other studies, making our study more reliable.41 Acute renal insufficiency (AKI) is associated with lower scores on simple mental state tests, where soluble THF receptor 1 concentrations mediate the risk ratio of POCD,42 and some renal indicators are associated with a higher risk of dementia or cognitive decline. With the POCD combination and higher AKI than the non-POCD group in our study, renal function management may be a promising indicator for predicting or preventing dementia.43 VHD patients with POCD combined with postoperative new cerebral infarction were more likely (P = 0.029). Cerebral microembolization is an important cause of POCD in patients with CPB, and the incidence of microembolization events may be a predictor of the progression of cerebral infarction. Hypoxia caused by cerebral microembolization occurs after CPB and cerebral hypoxia is located in the CA3 region of the hippocampus.29 Hypoxia will produce a large number of free radicals, degrade the hippocampus, and lead to neuronal dysfunction. And aggravate the occurrence of POCD.44 In clinical practice, we should also pay attention to the complications of POCD after cardiac surgery, which is crucial for long-term prognosis management.
In our study, Hs-CRP, as one of the inflammatory markers, has good predictive value. Previous studies have shown that inflammation can promote the progression of atherosclerosis. Hs-CRP is an approximate serum marker of inflammation, and when Hs-CRP levels are elevated, it usually indicates the presence of infection or inflammation in the body. Preoperative examination of Hs-CRP can determine the inflammation and infection of VHD patients.45 It has been proved that the new biomarker can predict the prognosis of POCD after cardiac surgery. The study of Wiberg S et al46 [43] showed that neuron-specific enolase (NSE), tua, neurofilament light chain (NFL) and glial fibrocalcific protein (GFAP) could predict POCD after cardiac surgery, but the area under the curve was only 0.64, and biomarkers were not easily obtained in the clinic. Studies have shown that Hs-CRP is a better predictor of cardiovascular events and death risk than other inflammatory indicators.47 In the study of Li Yunwei et al48 due to the limitation of CRP sensitivity, low serum CRP level is difficult to detect, and Hs-CRP is more sensitive than standard CRP detection, which has superior clinical application value. Hs-CRP≥1mg/L can increase the nutritional risk of gastric cancer patients,49 > 5mg/L can increase the risk of death from lung cancer,50 and > 2.16 mg/L can predict all-cause death from hyperglycemia in Chinese adults for 10 years. Studies have shown that the optimal threshold value of serum Hs-CRP in the diagnosis of acute coronary syndrome (ACS) is 6.12 mg/L, with a diagnostic sensitivity of 65.44% and a specificity of 90%. However, other studies have proved that Hs-CRP≥10 mg/L may be more suitable for patients with ACS.51 The optimal critical value of Hs-CRP in our study was 10.44 mg/L, with a sensitivity of 97.2% and a specificity of 78.4%. Hs-CRP can increase the incidence of cerebral infarction. In our study, postoperative complications of new cerebral infarction in the POCD group showed statistical significance (P = 0.029). During the process of CPB, cerebral hypoxia and ischemia lead to the generation of a large number of free radicals, which leads to the degeneration of the hippocampus and leads to the occurrence of POCD. Hs-CRP is a typical downstream marker involved in immune cell chemotaxis and macrophage phagocytosis, suggesting that clinicians can improve patient outcomes by better controlling the inflammatory response.
Limitations: (1) The 1-month incidence of POCD was selected in this study, which is a short-term follow-up outcome, so a longer follow-up period is required to validate our findings; (2) This study was conducted in patients receiving VHD and therefore needed to be confirmed in other population Settings; (3) The sample size is relatively small, and larger sample sizes and multi-center studies are needed to confirm our results.
Conclusion
Hs-CRP, a highly sensitive indicator of inflammation, is significantly elevated in POCD patients undergoing VHD surgery. Preoperative Hs-CRP>10.44 mg/l was an independent risk factor for POCD in patients with VHD. Simple and feasible Hs-CRP may be an effective preoperative assessment and screening tool. To prevent POCD, patients with high Hs-CRP need early clinical intervention.
Acknowledgments
We would also like to thank the hospital for supporting the data collection of this study.
Funding Statement
China Nursing Association 2021 annual research topic (ZHKYQ202321) and Fujian Cardiovascular Disease Medical Center (2021-76).
Data Sharing Statement
The data for this study were available by contacting the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study follows the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Medical University Union Hospital (Number: 2023KY142).
Consent for Publication
Patient information was hidden in our study. The participants all signed informed consent forms at the time of the survey.
Author Contributions
Aai Zhao and Yanchun Peng should be considered joint first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–e71. doi: 10.1161/cir.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 2.Hu YN, Hsieh TH, Tsai MT, et al. Cognitive function deterioration after cardiopulmonary bypass: can intraoperative optimal cerebral regional tissue oxygen saturation predict postoperative cognitive function? J Cardiothorac Vasc Anesth. 2023;37(5):715–723. doi: 10.1053/j.jvca.2023.01.025 [DOI] [PubMed] [Google Scholar]
- 3.Oldham MA, Vachon J, Yuh D, Lee HB. Cognitive outcomes after heart valve surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2018;66(12):2327–2334. doi: 10.1111/jgs.15601 [DOI] [PubMed] [Google Scholar]
- 4.Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomized controlled trial. Eur J Anaesthesiol. 2017;34(11):776–784. doi: 10.1097/eja.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 5.Bhushan S, Li Y, Huang X, Cheng H, Gao K, Xiao Z. Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: a review article. Int J Surg. 2021;95:106163. doi: 10.1016/j.ijsu.2021.106163 [DOI] [PubMed] [Google Scholar]
- 6.Urcun YS, Altun Y, Pala AA. Early and late predictors of postoperative neurocognitive dysfunction in cardiac surgery. [A posztoperatív neurokognitív diszfunkció korai és késői prediktorai szívsebészeti beavatkozás után]. Ideggyogy Sz. 2022;75(7–08):231–240. doi: 10.18071/isz.75.0231 [DOI] [PubMed] [Google Scholar]
- 7.Oyoshi T, Maekawa K, Mitsuta Y, Hirata N. Predictors of early postoperative cognitive dysfunction in middle-aged patients undergoing cardiac surgery: a retrospective observational study. J Anesth. 2023;37(3):357–363. doi: 10.1007/s00540-023-03164-w [DOI] [PubMed] [Google Scholar]
- 8.Florido-Santiago M, Pérez-Belmonte LM, Osuna-Sánchez J, et al. Assessment of long-term cognitive dysfunction in older patients who undergo heart surgery. Neurologia. 2021. doi: 10.1016/j.nrl.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Stanley ME, Sellke FW. Neurocognitive decline in cardiac surgery patients: what do we know? J Thorac Cardiovasc Surg. 2023;166(2):543–552. doi: 10.1016/j.jtcvs.2022.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evered LA, Silbert BS, Scott DA, Maruff P, Ames D. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology. 2016;125(1):62–71. doi: 10.1097/aln.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol. 2020;130:110791. doi: 10.1016/j.exger.2019.110791 [DOI] [PubMed] [Google Scholar]
- 12.Li D, Li J, Zhao C, et al. Diagnostic value of procalcitonin, hypersensitive C-reactive protein, and neutrophil-to-lymphocyte ratio for bloodstream infections in pediatric tumor patients. Clin Chem Lab Med. 2023;61(2):366–376. doi: 10.1515/cclm-2022-0801 [DOI] [PubMed] [Google Scholar]
- 13.Peng Q, Hou J, Wang S, et al. Hypersensitive C-reactive protein-albumin ratio predicts symptomatic intracranial hemorrhage after endovascular therapy in acute ischemic stroke patients. BMC Neurol. 2021;21(1):47. doi: 10.1186/s12883-021-02066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock CJW, Bray WG, Kouranos V, et al. Serum C-reactive protein is associated with earlier mortality across different interstitial lung diseases. Respirology. 2024;29(3):228–234. doi: 10.1111/resp.14609 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Yang P, Liu HC, Sun S, Zhang JL, Kang J. The significance of the detection of serum lactate dehydrogenase, hypersensitive C-reactive protein, and N-terminal pro-brain natriuretic peptide for the evaluation of the severity and progression of pediatric patients with traumatic brain injury. Curr Neurovasc Res. 2022;19(2):219–224. doi: 10.2174/1567202619666220713110941 [DOI] [PubMed] [Google Scholar]
- 16.Cimmino G, Ragni M, Cirillo P, et al. C-reactive protein induces expression of matrix metalloproteinase-9: a possible link between inflammation and plaque rupture. Int J Cardiol. 2013;168(2):981–986. doi: 10.1016/j.ijcard.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 17.Hou M, Ren YP, Wang R, Lu LX. Early cardiopulmonary resuscitation on serum levels of myeloperoxidase, soluble ST2, and hypersensitive C-reactive protein in acute myocardial infarction patients. World J Clin Cases. 2021;9(34):10585–10594. doi: 10.12998/wjcc.v9.i34.10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köprülü D, Hassan MO, Atmaca H, Albayrak S, Işcanlı E. Inflammatory markers and aortic aneurysms: exploring the role of Hs-CRP and MHR in ascending aortic aneurysm development. Int J Gen Med. 2024;17:2899–2905. doi: 10.2147/ijgm.S465873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–1301. doi: 10.1016/s0140-6736(23)00215-5 [DOI] [PubMed] [Google Scholar]
- 20.Mantovani L, Mikus E, Tenti E, et al. Post-operative delirium and cognitive dysfunction in aged patients undergoing cardiac surgery: a randomized comparison between two blood oxygenators. Bioengineering. 2023;10(12). doi: 10.3390/bioengineering10121429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RY, Zhao DL, Yu JW, et al. Intracranial plaque characteristics on high-resolution MRI and high-sensitivity C-reactive protein levels: association and clinical relevance in acute cerebral infarction. Clin Radiol. 2023;78(5):e442–e450. doi: 10.1016/j.crad.2023.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Lameire NH, Levin A, Kellum JA, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2021;100(3):516–526. doi: 10.1016/j.kint.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Peng Y, Zhang X, et al. The blood glucose-potassium ratio at admission predicts in-hospital mortality in patients with acute type A aortic dissection. Sci Rep. 2023;13(1):15707. doi: 10.1038/s41598-023-42827-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saver JL. Proposal for a universal definition of cerebral infarction. Stroke. 2008;39(11):3110–3115. doi: 10.1161/strokeaha.108.518415 [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–289. doi: 10.1034/j.1399-6576.2001.045003275.x [DOI] [PubMed] [Google Scholar]
- 26.Greaves D, Psaltis PJ, Ross TJ, et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol. 2019;289:43–49. doi: 10.1016/j.ijcard.2019.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghezzi ES, Ross TJ, Davis D, Psaltis PJ, Loetscher T, Keage HAD. Meta-analysis of prevalence and risk factors for cognitive decline and improvement after transcatheter aortic valve implantation. Am J Cardiol. 2020;127:105–112. doi: 10.1016/j.amjcard.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbey T, Milne B, de Somer F, Kunst G. Neurologic complications after cardiopulmonary bypass - A narrative review. Perfusion. 2023;38(8):1545–1559. doi: 10.1177/02676591221119312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Deng R, Wang X, et al. Mechanisms of hypoxia in the hippocampal CA3 region in postoperative cognitive dysfunction after cardiopulmonary bypass. J Cardiothorac Surg. 2022;17(1):106. doi: 10.1186/s13019-022-01865-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin Interv Aging. 2018;13:2267–2273. doi: 10.2147/cia.S133896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J, Ma Q, Ma D. Low-dose sevoflurane attenuates cardiopulmonary bypass (CPB)- induced postoperative cognitive dysfunction (POCD) by regulating hippocampus apoptosis via PI3K/AKT pathway. Curr Neurovasc Res. 2020;17(3):232–240. doi: 10.2174/1567202617666200513085403 [DOI] [PubMed] [Google Scholar]
- 32.Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. 2017;114(7):110–117. doi: 10.3238/arztebl.2017.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogue CW, Hershey T, Dixon D, et al. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein concentrations. Anesth Analg. 2006;102(6):1602–1608. doi: 10.1213/01.Ane.0000219591.10826.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Chen C, Zhang Z, Zou Y, Peng M, Wang Y. Toll-like receptor 4 knockout ameliorates neuroinflammation due to lung-brain interaction in mechanically ventilated mice. Brain Behav Immun. 2016;56:42–55. doi: 10.1016/j.bbi.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 35.Wei W, Sun Z, He S, et al. Mechanical ventilation induces lung and brain injury through ATP production, P2Y1 receptor activation and dopamine release. Bioengineered. 2022;13(2):2346–2359. doi: 10.1080/21655979.2021.2022269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell ML, Shum DHK, Mihala G, Murfield JE, Aitken LM. Long-term cognitive impairment and delirium in intensive care: a prospective cohort study. Aust Crit Care. 2018;31(4):204–211. doi: 10.1016/j.aucc.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 37.Bendikaite R, Vimantaite R. Cognitive impairment prevalence and impact on quality of life for patients after cardiac surgery. Heart Surg Forum. 2020;23(5):E590–e594. doi: 10.1532/hsf.2819 [DOI] [PubMed] [Google Scholar]
- 38.Yao L, Li Y, Yin R, et al. Incidence and influencing factors of post-intensive care cognitive impairment. Intensive Crit Care Nurs. 2021;67:103106. doi: 10.1016/j.iccn.2021.103106 [DOI] [PubMed] [Google Scholar]
- 39.Wei W, Zhang A, Liu L, et al. Effects of subanaesthetic S-ketamine on postoperative delirium and cognitive function in elderly patients undergoing non-cardiac thoracic surgery: a protocol for a randomised, double-blinded, placebo-controlled and positive-controlled, non-inferiority trial (SKED trial). BMJ open. 2022;12(8):e061535. doi: 10.1136/bmjopen-2022-061535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Huang X, Li M, Jiang Y, Zhang H. Identification of individuals at risk for postoperative cognitive dysfunction (POCD). Ther Adv Neurol Disord. 2022;15:17562864221114356. doi: 10.1177/17562864221114356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collet MO, Egerod I, Thomsen T, et al. Risk factors for long-term cognitive impairment in ICU survivors: a multicenter, prospective cohort study. Acta Anaesthesiol Scand. 2021;65(1):92–99. doi: 10.1111/aas.13692 [DOI] [PubMed] [Google Scholar]
- 42.Bhatraju PK, Zelnick LR, Stanaway IB, et al. Acute kidney injury, systemic inflammation, and long-term cognitive function: ASSESS-AKI. Clin J Am Soc Nephrol. 2024;19(7):829–836. doi: 10.2215/cjn.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi HC, Liu Y, Tan CC, Zhang YC, Tan L, Xu W. Adult renal dysfunction and risk of dementia or cognitive decline: brain-kidney axis hypothesis based on a systematic review and meta-analysis. J Prev Alzheimers Dis. 2023;10(3):443–452. doi: 10.14283/jpad.2023.35 [DOI] [PubMed] [Google Scholar]
- 44.Liu YH, Wang DX, Li LH, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass graft surgery. Anesth Analg. 2009;109(4):1013–1022. doi: 10.1213/ane.0b013e3181aed2bb [DOI] [PubMed] [Google Scholar]
- 45.Gao Y, Wang M, Wang R, et al. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: evidence from a large national cohort study. Lipids Health Dis. 2024;23(1):66. doi: 10.1186/s12944-024-02055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiberg S, Holmgaard F, Zetterberg H, et al. Biomarkers of cerebral injury for prediction of postoperative cognitive dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36(1):125–132. doi: 10.1053/j.jvca.2021.05.016 [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Wu Q, Li H, et al. Joint high level of oxidized low-density lipoprotein and high-sensitivity c-reactive protein are associated with recurrent stroke and poor functional outcome in minor stroke or transient ischemic attack. J Am Heart Assoc. 2022;11(20):e027665. doi: 10.1161/jaha.122.027665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Zhong X, Cheng G, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 49.Mao Y, Liu J, Li J, et al. Elevation of preoperative serum hs-CRP is an independent risk factor for malnutrition in patients with gastric cancer. Front Oncol. 2023;13:1173532. doi: 10.3389/fonc.2023.1173532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller DC, Larose TL, Hodge A, et al. Circulating high sensitivity C reactive protein concentrations and risk of lung cancer: nested case-control study within lung cancer cohort consortium. BMJ. 2019;364:k4981. doi: 10.1136/bmj.k4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC study group. fragmin during instability in coronary artery disease. N Engl J Med. 2000;343(16):1139–1147. doi: 10.1056/nejm200010193431602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study were available by contacting the corresponding author upon reasonable request.