Abstract
Purpose
Abnormal melanin synthesis causes hyperpigmentation disorders like melasma and lentigines, impacting psychological well-being. RNA interference (RNAi) uses small RNA molecules to inhibit gene expression by targeting specific mRNA, silencing genes involved in undesirable cellular functions. This study assessed INTASYL compounds, self-delivering RNAi molecules, designed to target and reduce tyrosinase gene expression to decrease pigmentation.
Methods
36 INTASYL compounds were designed to target and reduce TYR gene expression and tested in a screening assay. RXI-231, the lead compound, was tested in normal human epithelial melanocytes and the MelanoDerm™ model, a 3D reconstituted human epidermal culture. RXI-231 was evaluated for its ability to reduce tyrosinase mRNA expression, in vitro dopachrome formation, and melanin content. Penetration of fluorescently labeled INTASYL compounds through the stratum corneum into the epidermis was tested in cultured porcine skin explants using a DermaPen® microneedle device and a proprietary mixture of penetration enhancers. RXI-231 was also tested for skin irritation in the MatTek EpiDerm™ model to determine its non-irritant profile.
Results
RXI-231 significantly reduced tyrosinase mRNA expression, dopachrome formation, and melanin content in both normal human melanocytes and the MelanoDerm model. Application of INTASYL compounds every other day visibly reduced pigmentation in the 3D epidermal cultures. Penetration studies showed efficient delivery into the epidermis, overcoming the stratum corneum barrier. RXI-231 showed no irritation, with viability above 50% in the MatTek EpiDerm model, confirming its non-irritant profile.
Conclusion
RXI-231 effectively reduced tyrosinase activity and melanin synthesis, showing promise for treating hyperpigmentation disorders. Further characterization and planned human patient testing are necessary to confirm its clinical potential patient.
Keywords: hyperpigmentation, tyrosinase inhibitors, RNA interference (RNAi), melanin biosynthesis, epidermal culture models
Introduction
Hyperpigmentation disorders, such as melasma, post-inflammatory hyperpigmentation, and solar lentigines, are characterized by the overproduction of melanin, resulting in darkened skin patches.1 These conditions are not purely cosmetic; they can severely impact the quality of life and psychological well-being of affected individuals.2 Studies have shown that visible skin disorders, such as hyperpigmentation, can lead to low self-esteem, social anxiety, and depression, especially when affecting highly visible areas such as the face.3,4 The psychological toll can be profound, driving affected individuals to seek treatment for aesthetic reasons and to alleviate associated emotional distress.
Despite the availability of various treatment options, including topical agents like hydroquinone, retinoids, and chemical peels, the efficacy of current therapies is often limited. Moreover, these treatments come with a risk of adverse effects such as skin irritation, rebound hyperpigmentation, or even permanent alterations in skin color.5 Hydroquinone, for instance, remains the gold standard for hyperpigmentation treatment but can cause ochronosis, a disfiguring condition that paradoxically leads to skin darkening.6 These limitations highlight the pressing need for novel, effective, and safer therapeutic options that address both the overproduction of melanin and the patient’s psychosocial concerns.
Melanin biosynthesis is a complex process involving multiple enzymatic reactions, and tyrosinase plays a pivotal role as the key rate-limiting enzyme in this pathway. Tyrosinase is responsible for catalyzing two crucial reactions: the oxidation of L-tyrosine to L-DOPA (L-3,4-dihydroxyphenylalanine) and the subsequent oxidation of L-DOPA to dopaquinone.7 These reactions initiate the cascade of chemical transformations that ultimately lead to the production of melanin, including both eumelanin (black/brown pigment) and pheomelanin (red/yellow pigment).8
As the rate-limiting enzyme, tyrosinase controls the pace of melanin synthesis, making it a critical regulatory node in the pathway. Its activity is tightly regulated at the transcriptional, translational, and post-translational levels to ensure precise control of melanin production in response to various physiological and environmental cues, such as UV exposure.5 Given its central role, tyrosinase is a prime target for therapeutic and cosmetic interventions aimed at reducing hyperpigmentation. By inhibiting tyrosinase activity, melanin production can be effectively downregulated, providing a strategic approach to treat conditions like melasma, post-inflammatory hyperpigmentation, and other pigmentation disorders.
While existing melanin inhibitors—such as kojic acid, arbutin, ascorbic acid, and hydroquinone—target tyrosinase, they often affect other pathways as well, leading to undesirable effects such as skin irritation or unintended depigmentation in areas of normal pigmentation.9,10
In response to this unmet clinical need, we have developed INTASYL compounds targeting tyrosinase. INTASYL compounds are stable, self-delivering RNA interference (RNAi) molecules that incorporate the unique features of RNAi and antisense technology (Figure 1A). RNAi is a natural cellular process that uses small RNA molecules to silence specific genes (Figure 1B), in this case, tyrosinase (TYR).11 siRNAs are short, double-stranded RNA molecules, typically 20–25 nucleotides in length, that guide the RNA-induced silencing complex (RISC) to target mRNA molecules. The siRNA directs RISC to bind to complementary sequences in the target mRNA, leading to its cleavage and subsequent degradation, thereby preventing translation into protein.12 The INTASYL platform allows for the rapid and efficient delivery of these compounds into cells without the need for additional delivery systems, such as lipid nanoparticles or electroporation which can often cause unwanted side effects. These compounds demonstrate potent activity, stability, and delivery to a wide variety of cell types.13
Figure 1.
INTASYLs are asymmetric RNAi compounds comprised of a small duplex region (<15 base pairs) and a single-stranded phosphorothioate tail (≥ 6 nucleotides). In addition, INTASYL compounds are chemically modified with stabilizing and hydrophobic modifications (eg sterol), which confer stability, efficient cellular uptake and reduced inflammatory response (A). INTASYL functions through the RNAi pathway, a naturally occurring cellular process. Introduction of short double stranded RNA, such as INTASYL, into cells can result in association with the RNA induced silencing complex (RISC) to target and degrade complementary mRNA sequences and silence target genes (B).
INTASYL technology is already in clinical development for various indications, including oncological, dermatological, and ophthalmologic conditions (NCT06014086, NCT02079168, NCT02599064), and shows promise in providing a novel approach to managing skin pigmentation disorders.
This study introduces RXI-231, a TYR-targeting INTASYL compound designed to address the limitations of existing hyperpigmentation therapies, by providing a more targeted and efficient approach to reducing melanin production through RNA interference. By specifically silencing the TYR gene, RXI-231 offers a potentially safer and more effective alternative that minimizes off-target effects.
Materials and Methods
INTASYL Compound Synthesis
36 INTASYL sequences targeting TYR (NM_000372.5) were identified using a proprietary algorithm. RNA synthesis involved standard phosphoramidite chemistry on a Mermade 12 synthesizer (LGC Biosearch). After synthesis, oligonucleotides were cleaved from the support and deprotected using ammonia and ethanol. The crude products were purified via ion exchange chromatography and analyzed by reverse-phase HPLC (Shimadzu Prominence). Sense strands were purified through ionic precipitation, desalted, and then assessed for purity and molecular weight using HPLC and ESI-MS (Novatia).
Cell Culture and INTASYL Treatment
The human melanoma cell line, SK-MEL-5 (ATCC), was cultured in Eagle’s Minimum Essential Medium (EMEM) (ThermoFisher) supplemented with 10% Fetal Bovine Serum (FBS) (ThermoFisher). The Primary Epidermal Melanocytes (Normal), HEMn cell line (ATCC) was cultured in Medium 254 (ThermoFisher) supplemented with 10% FBS. Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. Cells were subcultured upon reaching 70–80% confluence and regularly monitored under a microscope to verify healthy growth and morphology.
For treatment of cell cultures, cells were seeded into tissue culture plates containing prediluted INTASYL compounds at final concentrations of 0–2 µM. The cells were incubated for 48 h, at which point they were collected for analysis.
3-Dimensional Tissue Construct Culture and Treatment
The MelanoDerm epidermal skin model tissues (MatTek) was used to further explore the effects of RXI-231. This reconstructed epidermal model consists of human keratinocytes and melanocytes, mimicking the structure and function of human skin, including pigmentation and barrier properties. The MelanoDerm model provides a reliable in vitro system for studying skin pigmentation. MelanoDerm tissues were handled according to the manufacturer’s protocol. Tissues were equilibrated at 37°C with 5% CO₂ for 24 hours before treatment prior to being exposed to INTASYL compounds at concentrations ranging from 0–5 µM in culture media for 14 days. Media changes were performed every other day according to the manufacturer’s protocol. Following treatment, tissues were washed with PBS and prepared for mRNA quantification, melanin content analysis, and histology.
Determination of Gene Silencing by RT-qPCR and bDNA Assay
For screening of TYR compounds and determination of mRNA reduction in 3-dimensional culture models, RT-qPCR was performed. Total RNA was isolated from duplicate or triplicate wells for each condition using the PureLink RNA Mini Kit (Thermo Fisher) according to the manufacturer’s recommendations. The isolated mRNA was quantified using an Epoch microplate spectrophotometer (BioTek), and ~75 ng of mRNA was used as an input for duplicate gene expression analysis via single-plex RT‒qPCR using the TaqMan™ RNA-to-CT 1-Step Master Mix (Thermo Fisher) and TaqMan™ probes for the specific detection of human TYR-FAM (Thermo Fisher; Hs00165976_m1) and PPIB-FAM (Thermo Fisher; Hs00168719_m1) on a StepOne RT‒qPCR machine (Applied Biosystems). Cycle thresholds (Ct) from TYR gene analytes were normalized to those of PPIB, and relative quantification (RQ; 2−ΔΔϹt) was calculated as the fold change relative to the UTC condition.
For confirmation of hit compounds, TYR mRNA expression levels were measured using the QuantiGene bDNA assay kit (ThermoFisher). Cells were harvested from triplicate wells, lysed, and incubated at room temperature for 30 minutes. The target-specific probe was added to the lysate and incubated at 55°C for 16 to 20 hours. Signal amplification steps followed with sequential 1-hour incubations at 55°C. After adding the label probe, the resulting signal was detected with a luminometer (BioTek) and normalized to a housekeeping gene.
Determination of Inhibition of Dopachrome Activity and Reduction of Melanin Content
To confirm a reduction in tyrosinase activity, a dopachrome assay was performed. Human Epidermal Melanocytes (HEMn) were seeded at a density of 1 × 10⁴ cells per well in 24-well plates one day prior to the dopachrome assay, with cells maintained in normal growth media. Compound dilutions were prepared in media and added to the cells. A chemically-matched non-targeting siRNA control (NTC) was used as a negative control. Following administration of the compounds, the cells were incubated at 37°C in 5% CO₂ for 5 to 7 days, with media changes twice weekly.
Following incubation with compounds, the cells were lysed and the lysates were transferred to microfuge tubes. The lysates were centrifuged to pellet. For the Dopachrome assay, 80 µL of the cleared lysate was combined with 20 µL of 5 mm L-Dopa (Sigma) in a 96-well plate. Tyrosinase (Sigma) was used as a positive control. Absorbance was measured at 475 nm every hour from hours 2 to 10 and normalized to protein quantity.
Following cell lysis and Dopachrome assay, the remaining pellets were used for the determination of melanin content. The melanin pellets were dissolved in 1 N NaOH containing 20% DMSO and incubated for 1 hour at 95°C. After incubation, the solution was vortexed and spun briefly to collect the dissolved melanin. The absorbance of the solution was measured at 490 nm, and the melanin content was calculated against a known standard of synthetic melanin (Sigma).
Ex vivo Topical Application
Fluorescently labeled INTASYL compounds were applied to full-thickness porcine explant skin to evaluate dermal penetration and cellular uptake. The skin was pre-treated with a DermaPen fractional microneedle device, creating 1.0 mm deep microchannels to enhance permeability. The INTASYL compounds were then applied, and the explants were placed in a Franz diffusion cell system for 24 hours at 37°C. After incubation, the skin was sectioned and analyzed using fluorescence microscopy.
Several proprietary formulations were developed and tested on porcine ear skin using an ex vivo culture model. For these experiments, 8 mm biopsy punches of skin were placed into transwell culture inserts, which were then positioned in 6-well culture dishes containing complete medium. The test articles were topically applied to the skin, and the explants were cultured for 48 hours. Following incubation, the skin samples were processed and stained for histological analysis to assess the effects of the formulations.
Histological Evaluation
After 14 days of treatment, MelanoDerm tissues were visualized using light microscopy, then fixed in 10% neutral-buffered formalin for 24 hours. The fixed tissues were dehydrated, cleared, and embedded in paraffin. Transverse sections (5 µm) were cut using a microtome and mounted on glass slides. The sections were deparaffinized, rehydrated, and stained using the Fontana-Masson method to identify melanin. Slides were then counterstained with nuclear fast red, dehydrated, cleared, and cover slipped. Melanin was visualized as dark brown or black deposits under light microscopy.
Porcine skin explants treated with fluorescently labeled INTASYL compounds were fixed in 10% neutral buffered formalin for 24–48 hours, then dehydrated through a graded series of ethanol (70%, 95%, 100%) and cleared in xylene. Samples were embedded in paraffin at 60°C, sectioned at 5 µm, and mounted on slides. Sections were stained with Hoechst dye for 2 minutes to visualize nuclei. Fluorescence microscopy was used to detect INTASYL compound distribution.
Skin Irritation Testing
The RXI-231 compound was evaluated using the MatTek EpiDerm™ skin irritation model. This model is a widely used in vitro system designed to evaluate the potential irritation effects of chemicals on human skin. This model consists of a three-dimensional, human-derived epidermal tissue, which mimics the structure and function of human skin, including the stratum corneum, viable epidermis, and dermal-epidermal junction. The EpiDerm™ model is cultured using human keratinocytes, and it responds to irritants in a manner similar to human skin, making it a reliable tool for assessing skin irritation potential. In the assay, the tissue is exposed to test compounds, and various endpoints, such as cell viability, tissue morphology, and cytokine release, are measured to evaluate irritation potential. In this assay, the construct was treated with RXI-231. After 24 hours, cell viability was assessed. Negative controls (untreated constructs) and positive controls (constructs treated with a known irritant) were included to validate the assay. A reduction in viability to less than 50% of the negative control indicates potential irritancy.
Statistical Methods
GraphPad Prism 10.3.1 was used for statistical analyses. A one-way analysis of variance (ANOVA) was conducted to determine if there were statistically significant differences between the means of the groups. Following the ANOVA, Dunnett’s multiple comparison test was performed to compare each treatment group to a single control group.
Results
Screening and Optimization
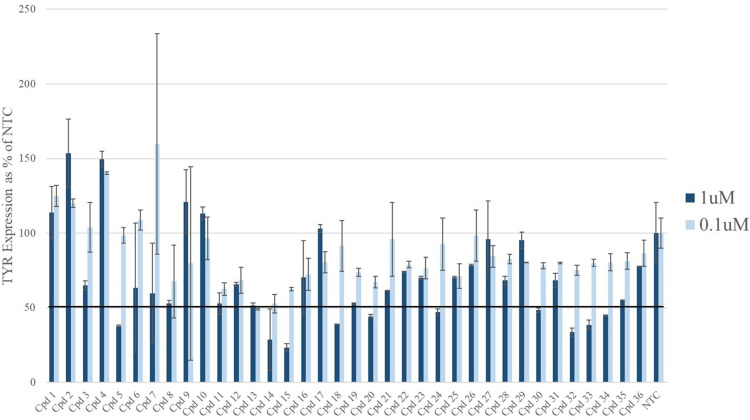
The initial screening of 36 INTASYL compounds targeting TYR was performed in SK-MEL-5 cells. Multiple compounds showed greater than 50% reduction in TYR mRNA levels compared to the non-targeting control (NTC) (Figure 2). These compounds were selected for further chemical optimization to enhance their potency and stability.
Figure 2.
For the initial compound screening, SK-MEL-5 cells were treated with 1 uM and 0.1 uM of TYR targeting INTASYL compounds 1–36 for 48 hours. mRNA levels were assessed by qPCR and plotted relative to the non-targeting control (NTC). Hits were identified as those compounds with silencing of greater than 50%. These compounds were chosen for further chemical optimization.
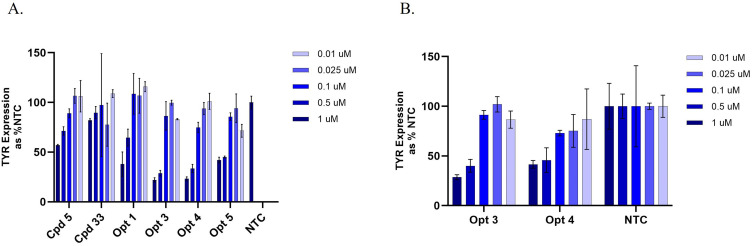
Subsequent screening of the optimized INTASYL compounds was performed using 5-point dose-response curves in SK-MEL-5 cell line and HEMn primary melanocyte cells. TYR mRNA levels were evaluated after 48 hours using a bDNA assay. The optimized compounds demonstrated improved silencing efficiency compared to the original hits (Figure 3A and B). At the 1 µM concentration, Opt 3 was able to silence TYR mRNA by approximately 70% in both SK-MEL-5 and HEMn cell types as compared to the NTC. Opt 3 was chosen for further evaluation in phenotypic assays and renamed RXI-231.
Figure 3.
Hit compounds identified in the original screen underwent further chemical optimization to enhance potency and stability. The resulting optimized compounds were screened with the original compounds for comparison. Screening was performed in SK-MEL-5 cells using 5-point dose response curves. TYR mRNA levels were assessed by bDNA assay (A). Confirmation of the two best hits from the SK-MEL-5 testing was performed in primary human melanocytes (HEMn) (B). Opt 3 was selected as the lead compound and renamed RXI-231.
In vitro Effects
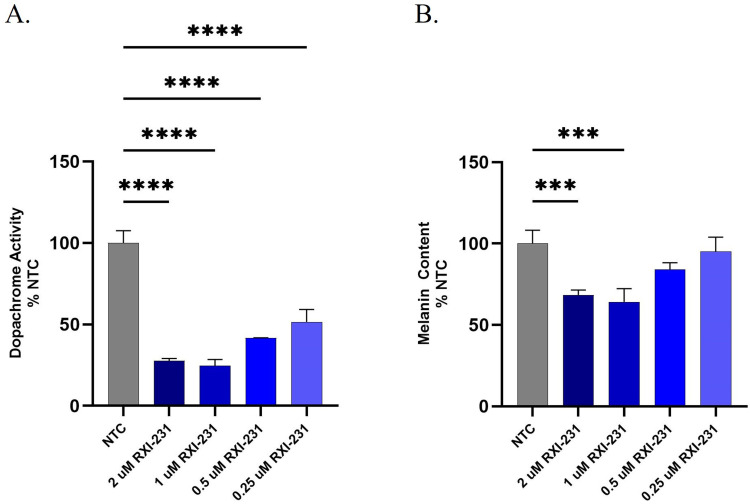
RXI-231 was evaluated for its ability to inhibit tyrosinase activity and reduce melanin synthesis in HEMn cells. Dopachrome formation, an indicator of tyrosinase activity, was reduced in a dose-dependent manner after two weekly treatments over a 14-day period (Figure 4A) resulting in a reduction of nearly 75% compared to NTC. Additionally, melanin protein content was reduced by over 40% following treatment with RXI-231 (Figure 4B). These results indicate that RXI-231 effectively inhibits melanin production at the cellular level.
Figure 4.
Tyrosinase enzyme activity in HEMn cells was evaluated by measuring formation of dopachrome (a melanin precursor) in a 3-point dose response after 14 days in culture. Cells were treated with RXI-231 on days 1 and 7 and had media replenishment twice weekly. RXI-231 showed a dose-dependent reduction of dopachrome with only 2 weekly doses of compound (A). Primary human melanocytes were also evaluated for melanin content following the 14 day transfection. Treatment with RXI-231 resulted in a 40% reduction in melanin content (B). ****p<0.0001, ***p<0.001.
In vivo Relevance
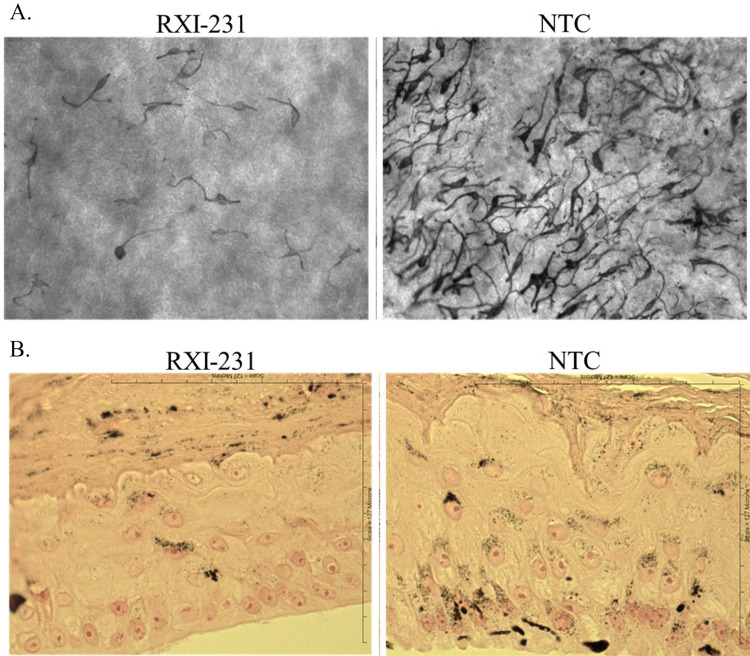
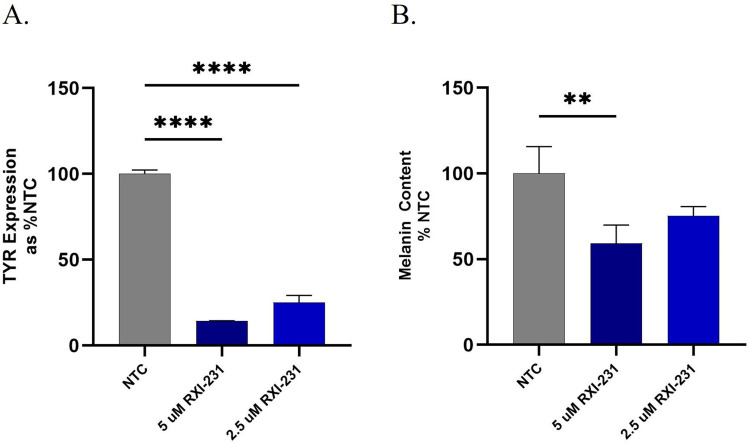
To assess the impact of RXI-231 on melanin production in a more physiologically relevant model, it was tested in the MelanoDerm models. MelanoDerm is a highly differentiated, three-dimensional tissue culture model of human epidermis that contains normal human melanocytes (NHM) and keratinocytes. NHM within MelanoDerm exhibit a dendritic morphology, are localized to the basal cell layer, and produce melanin granules which progressively populate the layers of tissue as it is cultured. Over a 4 week period, cultures become increasingly pigmented with retention of normal epithelial morphology. MelanoDerm tissues were treated with RXI-231 in culture media for 14 days. Over a 4-week period, RXI-231 treatment resulted in a visible reduction in pigmentation and a 70–80% reduction in TYR mRNA levels (Figures 5A and 6B). Additionally, melanin content in the treated tissues decreased by approximately 40% (Figure 6B). Importantly, RXI-231-treated tissues maintained their epidermal structure while exhibiting fewer pigmented melanocytes (Figure 5B). These findings highlight the potential of RXI-231 to not only reduce pigmentation effectively but also to preserve the integrity of the skin, which is crucial for developing a clinically viable treatment option.
Figure 5.
MelanoDerm tissues were treated with RXI-231 at 5uM in culture media for 14 days with media changes every other day, as per manufacturer’s protocol. After 14 days, tissues were visualized using light microscopy (20x) (A). Tissues treated with TYR targeting INTASYL have fewer melanocytes in the tissues while maintaining the structure of the epidermis (B).
Figure 6.
RXI-231-treated MelanoDerm tissues were assessed for TYR expression and melanin content. Treatment with RXI-231 results in a reduction in TYR mRNA of ~80% (A) and melanin content by ~40% (B). ****p<0.0001, **p<0.01.
Delivery Method Optimization
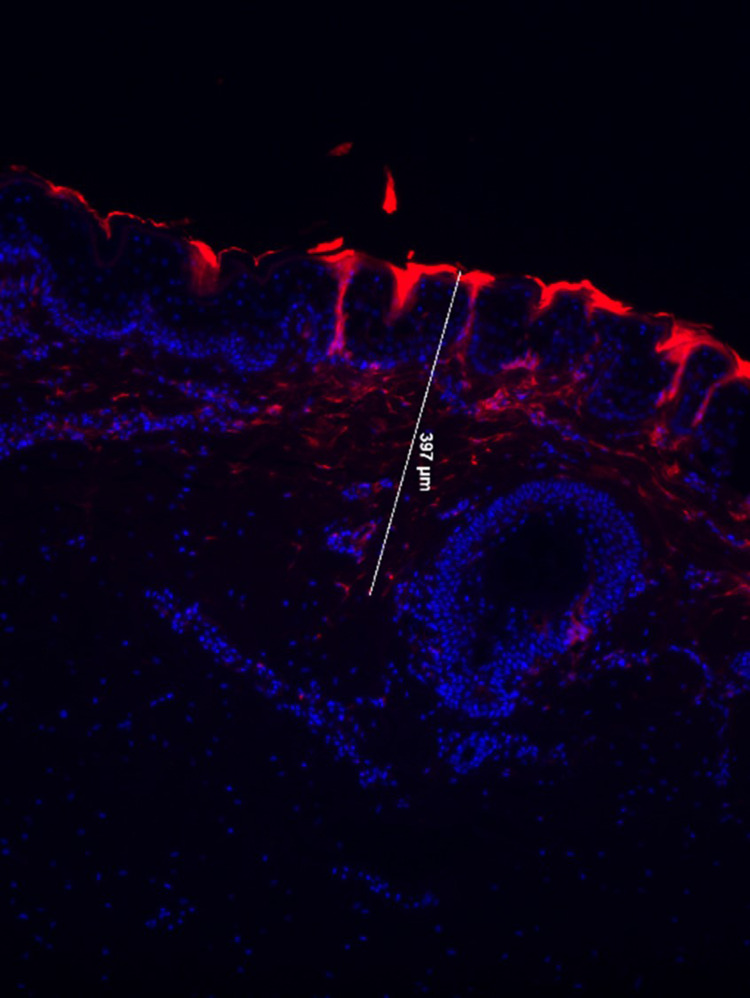
To enhance the delivery of RXI-231, we employed fractional microneedle-assisted delivery using a DermaPen device. This approach created microchannels in full-thickness porcine skin, facilitating RXI-231 penetration to a depth of 400 µm with significant cellular uptake (Figure 7). Fluorescence microscopy confirmed effective delivery through the stratum corneum and into the dermis. This method offers a promising strategy for improving the efficacy of topical treatments.
Figure 7.
Fluorescently labeled RXI-231 was applied to the surface of full thickness porcine explant skin. A DermaPen® fractional microneedle device was passed over the surface of the skin to create microchannels (1.0 mm depth) through which the compounds enter the skin. Following 24 hours on a Franz cell system, dermal penetration is detected by fluorescence microscopy (20x magnification). Dermal penetration to a depth of 400 nm was achieved with good cellular uptake observed.
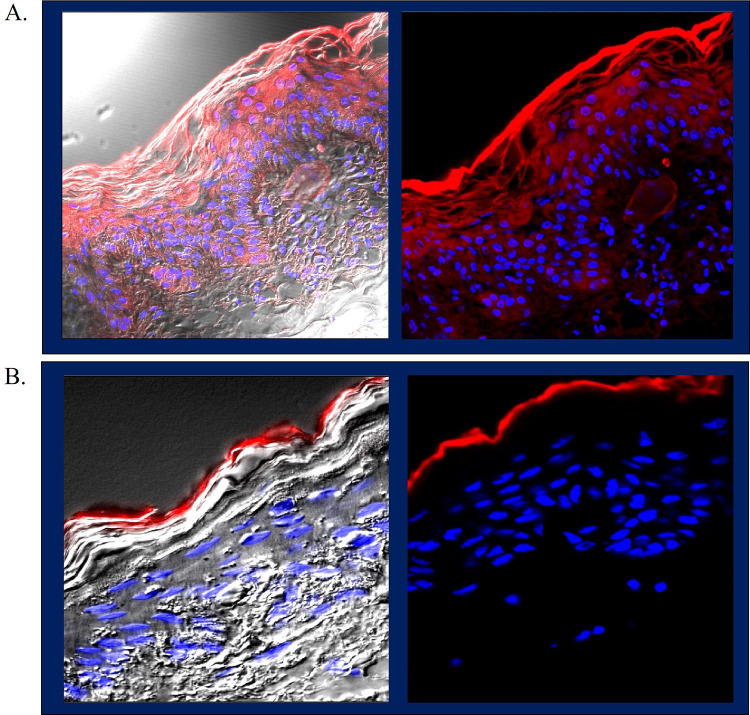
We also developed a proprietary formulation of RXI-231 that enables non-invasive epidermal delivery of our compounds. This formulations uses a proprietary mixture of penetration enhancers that can be used in cosmetic or pharmaceutical formulations. 8 mm biopsy punches of porcine ear skin were seated in transwell culture inserts and placed into 6 well culture dishes containing complete medium. After topical application of the test formulation, the skin pieces were cultured for 48 hours followed by processing and staining for histological analysis. The final formulation showed significant penetration of fluorescently labeled INTASYL through the stratum corneum, with some dermal delivery observed (Figure 8A) compared to formulation in PBS (Figure 8B). Left panels an overlay of light and fluorescence microscopy and right panels are fluorescence only. This formulation represents a less invasive alternative to microneedle delivery, potentially enhancing patient comfort and compliance.
Figure 8.
A proprietary topical formulation was made and tested on porcine ear skin using an ex vivo culture model. (A) The final formulation shows significant penetration through the stratum corneum with some dermal delivery compared to RXI-231 formulated in PBS control (B) (63x magnification).
Safety Assessment
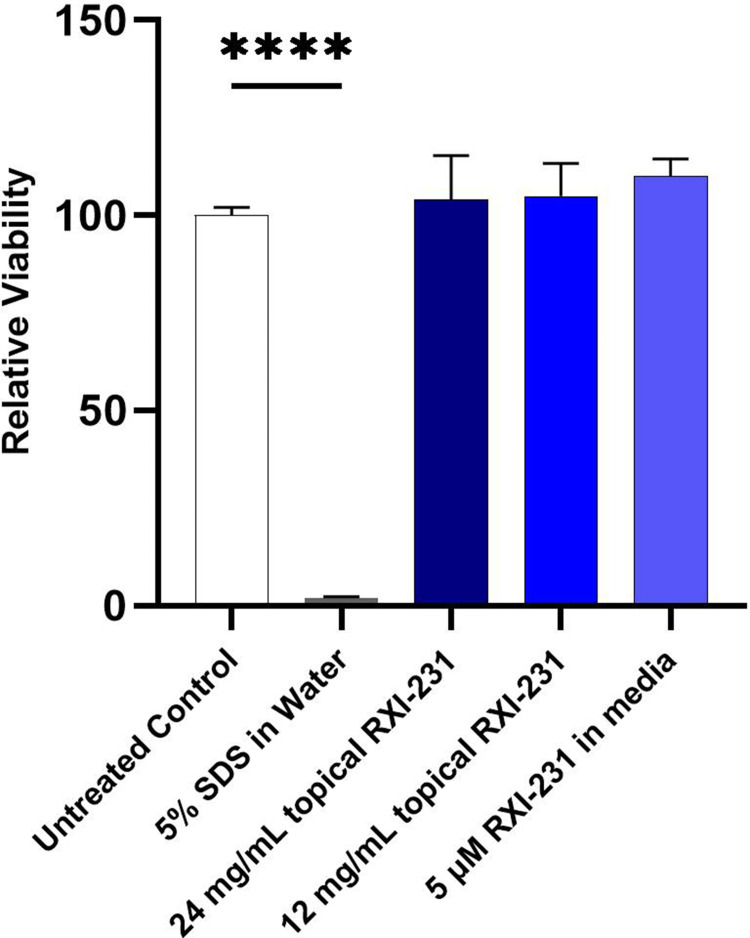
The RXI-231 formulation was assessed for safety using the MatTek EpiDerm skin irritation model. The results demonstrated no significant reduction in cell viability, indicating that RXI-231 is non-irritating and suitable for dermatological applications (Figure 9). The safety profile of RXI-231 is particularly noteworthy and suggests a lower risk of adverse effects compared to traditional treatments.
Figure 9.
The RXI-231 formulation was tested in the MatTek EpiDerm skin irritation model. Briefly, the 3 dimensional skin construct is treated with the test article and viability is assessed after 24 hours. Irritancy is predicted if the viability is reduced to lower than 50% of the viability of the negative controls. RXI-231 in formulation showed no reduction in viability.
Discussion
The efficacy and safety of RXI-231, a self-delivering RNAi compound from the INTASYL platform, designed for the treatment of hyperpigmentation disorders by silencing tyrosinase (TYR) expression has been explored in this study. RXI-231 provides a novel, targeted mechanism for reducing melanin production by directly downregulating TYR, the enzyme responsible for catalyzing key steps in melanin biosynthesis. These findings demonstrate that RXI-231 can effectively reduce tyrosinase mRNA, dopachrome formation, and overall melanin content in both 2D melanocyte cultures and 3D MelanoDerm models, providing a promising new approach to treating hyperpigmentation.
Clinical Implications and Advantages Over Current Treatments
RXI-231 offers a unique mechanism of action as a gene-silencing RNAi molecule. By directly targeting tyrosinase mRNA, RXI-231 circumvents the limitations of current tyrosinase inhibitors, which often act indirectly or exhibit off-target effects.2,6 This specificity provides a more focused therapeutic approach, reducing melanin production with potentially fewer side effects. Unlike conventional treatments, RXI-231’s silencing tyrosinase gene expression ensures that melanin production is decreased specifically at its source, minimizing off-target risks.
Additionally, RXI-231’s mechanism offers potential long-term benefits by addressing the root cause of hyperpigmentation—overproduction of tyrosinase. This reduces the risk of rebound hyperpigmentation, common with treatments like hydroquinone, and may provide longer-lasting effects post-treatment.
The findings from this study suggest that RXI-231 has the potential as a treatment for hyperpigmentation, either as a monotherapy or in combination with other treatments, such as retinoids or chemical peels, depending on the severity of the disorder. Importantly, RXI-231 may offer an alternative for patients who cannot tolerate traditional treatments due to skin sensitivity or who have experienced limited success with existing therapies.
Topical application of RXI-231 could provide a non-invasive, patient-friendly option. Our ex vivo penetration studies demonstrated effective delivery of RXI-231 through the stratum corneum, suggesting that it can be formulated for topical use without requiring additional invasive methods. The incorporation of microneedle-assisted delivery, as tested with the DermaPen®, offers another potential delivery strategy, particularly for patients with more recalcitrant pigmentation. This technique facilitated deep penetration into the skin and enhanced cellular uptake, further increasing the clinical utility of RXI-231.
As RXI-231 showed no irritancy in the MatTek EpiDerm skin irritation model, it has a potential for chronic topical use without the risk of irritation or damage to the skin barrier. The absence of significant adverse effects in the in vitro and ex vivo models highlight the potential of RXI-231 to be a safer alternative to hydroquinone and other tyrosinase inhibitors, which are associated with higher risk profiles.
Future Directions
While the results are promising, further research is needed to confirm the durability of effect, efficacy, and safety of RXI-231 in vivo, particularly in human clinical testing. The next steps would involve evaluating RXI-231 in consumer testing settings to better understand its therapeutic potential and long-term safety profile. Additionally, continued optimization of delivery methods, including the refinement of topical formulations or further exploration of microneedle-assisted delivery, will be crucial to maximize its effectiveness.
Another area of potential interest is the use of RXI-231 in combination therapies. Given the multifactorial nature of hyperpigmentation,5 combining RXI-231 with other treatments targeting different stages of melanogenesis or the skin’s inflammatory response could further enhance outcomes. Furthermore, investigating RXI-231’s role in preventing or reducing the recurrence of hyperpigmentation, a common challenge with existing treatments, would be valuable.
Study Limitations and Future Research Needs
Although the in vitro and ex vivo findings presented in this study are promising, they are not without limitations. Most of the data were generated from controlled lab models, such as 2D melanocyte cultures and the 3D MelanoDerm model, which may not fully replicate the complexities of human skin in vivo. The efficacy, safety, and long-term effects of RXI-231 need to be confirmed in clinical trials involving human patients to ensure that these results translate effectively into therapeutic benefits. Additionally, challenges such as optimizing dosing, delivery methods, and ensuring consistent results across diverse skin types will need to be addressed. The potential for unforeseen side effects or limitations in large-scale, real-world use should also be carefully monitored in future in vivo studies.
Exploration of the ability of INTASYL compounds to target and silence other genes involved in hyperpigmentation would also be of interest. TYRP1,14 MC1R,15 and KITLG16 would all be of interest in this pathway.
Conclusion
RXI-231, as a novel RNAi-based therapy, represents a promising new approach to the treatment of hyperpigmentation disorders. Its targeted mechanism of action, combined with its safety profile and the potential for non-invasive delivery, positions it as a potential alternative to current therapies that are often limited by side effects or incomplete efficacy. Further research, including clinical testing, is warranted to fully explore the therapeutic potential of RXI-231 and to integrate it into the dermatological treatment landscape. This study underscores the innovative potential of INTASYL compounds and their capacity to address unmet needs in dermatological conditions, such as hyperpigmentation, offering patients safer and more effective treatment options.
Acknowledgments
The authors would like to express our gratitude to all those who supported and contributed to this research. We appreciate the valuable feedback and suggestions from our colleagues, which greatly improved the manuscript. Additionally, we acknowledge the technical and administrative assistance received during the course of this research.
Abbreviations
Acetonitrile, CH₃CN; Controlled Pore Glass, CPG; Cycle threshold, Ct; Dimethyl Sulfoxide, DMSO; Eagle’s Minimum Essential Medium, EMEM; Electrospray Ionization Mass Spectrometry, ESI-MS; Fetal Bovine Serum, FBS; High-Performance Liquid Chromatography, HPLC; Human Epidermal Melanocytes (Normal), HEMn; Human Melanocyte Growth Supplement, HMGS; hour, h; INTASYL, self-delivering RNAi technology; L-3,4-Dihydroxyphenylalanine, L-DOPA; Microliter, µL; Micromolar, µM; Molecular Weight, MW; nanometer, nm; Phosphate Buffered Saline, PBS; Peptidylprolyl Isomerase B, PPIB; Relative Quantification, RQ; Reverse Transcription Quantitative Polymerase Chain Reaction, RT-qPCR; RNA interference, RNAi; RXI-231, a lead INTASYL compound targeting tyrosinase; SK-MEL-5, a human melanoma cell line; Sodium Bromide, NaBr; Sodium Hydroxide, NaOH; Sodium Phosphate Monobasic, NaH2PO₄; Untreated Control, UTC.
Ethical Statement
The study was approved by the institutional ethics committee of Phio Pharmaceuticals. The study was conducted according to the Declaration of Helsinki principles.
Disclosure
Ms Melissa Maxwell reports a patent 62/487,454 pending to Phio Pharmaceuticals. Dr James Cardia is a previous employee of Phio Pharmaceuticals. M Maxwell is currently employed by Phio Pharmaceuticals. The other authors (K Holton, R Looby, M Bryne, J Cardia) were employed by Phio Pharmaceuticals at the time this work was conducted. The author(s) report no conflicts of interest in this work.
The abstract of this paper was presented at the Society for Investigative Dermatology Annual Meeting, held on 11-14 May 2016 as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Journal of Investigative Dermatology, Volume 136, Issue 5, S115: https://www.jidonline.org/article/S0022-202X(16)30746-1/fulltext.
References
- 1.Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7(3):305–318. doi: 10.1007/s13555-017-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vashi NA, Kundu RV. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013;169:41–56. doi: 10.1111/bjd.12536 [DOI] [PubMed] [Google Scholar]
- 3.Jankowski M, Jasiński K, Płocka M. The aggressive face of melasma: unveiling the influence of upper lip pigmentation on social perception. Adv Dermatol Allergol. 2024;41(2):173–180. doi: 10.5114/ada.2024.138678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Wan Y, Sun Y, Gao C, Li J. Prevalence of depression in melasma: a systematic review and meta-analysis. Front Psychiatry. 2024;14:1276906. doi: 10.3389/fpsyt.2023.1276906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thawabteh AM, Jibreen A, Karaman D, Thawabteh A, Karaman R. Skin pigmentation types, causes and treatment—a review. Molecules. 2023;28(12):4839. doi: 10.3390/molecules28124839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishack S, Lipner SR. Exogenous ochronosis associated with hydroquinone: a systematic review. Int J Dermatol. 2022;61(6):675–684. doi: 10.1111/ijd.15878 [DOI] [PubMed] [Google Scholar]
- 7.Zolghadri S, Bahrami A, Hassan Khan MT, et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34(1):279–309. doi: 10.1080/14756366.2018.1545767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hearing VJ, Jiménez M. Analysis of mammalian pigmentation at the molecular level. Pigment Cell Res. 1989;2(2):75–85. doi: 10.1111/j.1600-0749.1989.tb00166.x [DOI] [PubMed] [Google Scholar]
- 9.Hollinger JC, Angra K, Halder RM. Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J Clin Aesthetic Dermatol. 2018;11(2):28–37. [PMC free article] [PubMed] [Google Scholar]
- 10.Boo YC. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants. 2021;10(7):1129. doi: 10.3390/antiox10071129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. 1998;391:806–811—. [DOI] [PubMed] [Google Scholar]
- 12.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- 13.Byrne M, Tzekov R, Wang Y, et al. Novel hydrophobically modified asymmetric RNAi compounds (sd-rxRNA) demonstrate robust efficacy in the eye. J Ocul Pharmacol Ther off J Assoc Ocul Pharmacol Ther. 2013;29(10):855–864. doi: 10.1089/jop.2013.0148 [DOI] [PubMed] [Google Scholar]
- 14.Wagatsuma T, Suzuki E, Shiotsu M, et al. Pigmentation and TYRP1 expression are mediated by zinc through the early secretory pathway-resident ZNT proteins. Commun Biol. 2023;6(1):403. doi: 10.1038/s42003-023-04640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guida S, Guida G, Goding CR. MC1R functions, expression, and implications for targeted therapy. J Invest Dermatol. 2022;142(2):293–302.e1. doi: 10.1016/j.jid.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 16.Picardo M, Cardinali G. The genetic determination of skin pigmentation: KITLG and the KITLG/c-Kit pathway as key players in the onset of human familial pigmentary diseases. J Invest Dermatol. 2011;131(6):1182–1185. doi: 10.1038/jid.2011.67 [DOI] [PubMed] [Google Scholar]