Abstract
Plasmid DNA vaccines elicit potent and protective immune responses in numerous small-animal models of infectious diseases. However, their immunogenicity in primates appears less potent. Here we investigate a novel approach that optimizes regulatory elements in the plasmid backbone to improve the immunogenicity of DNA vaccines. Among various regions analyzed, we found that the addition of a regulatory sequence from the R region of the long terminal repeat from human T-cell leukemia virus type 1 (HTLV-1) to the cytomegalovirus (CMV) enhancer/promoter increased transgene expression 5- to 10-fold and improved cellular immune responses to human immunodeficiency virus type 1 (HIV-1) antigens. In cynomolgus monkeys, DNA vaccines containing the CMV enhancer/promoter with the HTLV-1 R region (CMV/R) induced markedly higher cellular immune responses to HIV-1 Env from clades A, B, and C and to HIV-1 Gag-Pol-Nef compared with the parental DNA vaccines. These data demonstrate that optimization of specific regulatory elements can substantially improve the immunogenicity of DNA vaccines encoding multiple antigens in small animals and in nonhuman primates. This strategy could therefore be explored as a potential method to enhance DNA vaccine immunogenicity in humans.
Plasmid DNA vaccines have shown promise as a novel vaccination modality based on their simplicity and versatility (31, 32, 36). In particular, DNA vaccines can elicit potent and protective cellular and humoral immune responses in a variety of small-animal models (10). However, they have proven substantially less immunogenic in nonhuman primate studies and in clinical trials to date (8, 19, 33).
Several approaches have been explored to improve the immunogenicity of DNA vaccines. Our laboratories and others have demonstrated that the addition of plasmids expressing cytokines and immunomodulatory molecules can substantially augment DNA vaccine-elicited immune responses in both mice and nonhuman primates (3, 4, 15, 16, 21, 34, 37). However, the practical requirements of manufacturing and establishing the safety of the plasmid cytokines prior to the initiation of clinical trials may prove a limitation of this strategy (7, 26). Other approaches involve the addition of polymer adjuvants (29) and the use of in vivo electroporation techniques (24, 35). These strategies have similarly proven effective in animal models, but their practical utility in clinical trials has yet to be demonstrated.
In this study, we investigate a novel strategy involving optimization of regulatory elements in the backbone of the plasmid DNA vaccine. DNA vaccines often utilize a cytomegalovirus (CMV) enhancer, promoter, and intron to drive high-level expression of a transgene in mammalian cells (32, 38). Here, we explore the effects of adding the regulatory R region from the 5′ long terminal repeat (LTR) of human T-cell leukemia virus type 1 (HTLV-1), which acts as a transcriptional and posttranscriptional enhancer (30). We find that these CMV/R DNA vaccines elicit substantially higher human immunodeficiency virus type 1 (HIV-1)-specific cellular immune responses compared with the analogous parental DNA vaccines in both mice and cynomolgus monkeys. Optimization of regulatory elements thus represents a simple and effective strategy to augment the immunogenicity of DNA vaccines in primates.
MATERIALS AND METHODS
Plasmid construction.
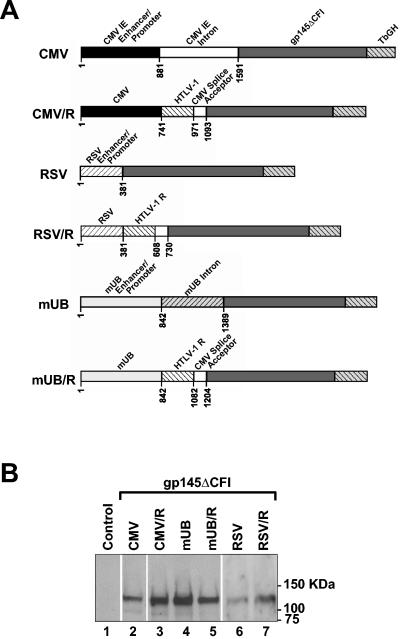
The parental 1012 DNA vaccine plasmid contains the human CMV immediate early (IE) enhancer, promoter, and intron. To construct the CMV/R regulatory element, a SacII/HpaI fragment of the 1012 plasmid containing the majority of the CMV IE intron was replaced with a 227-bp EcoRV/HpaI fragment of the HTLV-1 R region (28). The resulting CMV/R plasmid thus contains the human CMV IE enhancer/promoter, followed by the HTLV-1 R region and a 123-bp fragment of CMV IE 3′ intron. The splice donor in the R region and the splice acceptor in the CMV IE 3′ intron serve as the pair of splicing signals. The RSV/R and mUB/R plasmids were similarly constructed by replacing the CMV enhancer/promoter region of the CMV/R plasmid with a 381-bp AflIII/HindIII fragment of the Rous sarcoma virus (RSV) enhancer/promoter or an 842-bp SpeI/EcoRV fragment of the mouse ubiquitin B (mUB) enhancer/promoter, respectively. The mUB enhancer/promoter has been described previously (40).
In vitro expression studies.
Murine fibroblast 3T3 cells were transfected with 0.5 μg or parental 1012 (CMV), CMV/R, RSV, RSV/R, mUB, mUB/R DNA vaccines expressing HIV-1 Env gp145 ΔCFI (9) in six-well plates using calcium phosphate; 24 h after transfection, cells were harvested and lysed in 50 mM HEPES, 150 mM NaCl, 1% NP-40 with protease inhibitors and 10 μg total protein was electrophoresed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and gp145 expression was assessed by Western blot analysis. A 1:5,000 dilution of human HIV immunoglobulin G (IgG) was utilized as the primary antibody, and a 1:5,000 dilution of horseradish peroxidase-conjugated goat anti-human IgG was utilized as the secondary antibody. The blots were developed with the ECL Western blot developing system (Amersham Biosciences, Piscataway, NJ).
Animals and immunizations.
Six- to 8-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) were immunized intramuscularly with 50 μg HIV-1 DNA vaccines expressing either clade B Env gp145ΔCFI (9) or a Gag-Pol-Nef fusion protein (13) in 100 μl sterile saline divided between the right and left quadriceps muscles. Adult cynomolgus monkeys (Bioqual, Rockville, MD) were immunized intramuscularly with 8 mg of a multivalent HIV-1 DNA vaccine in 1 ml sterile saline delivered by Biojector inoculation into the right and left quadriceps muscles. This multiclade, multigene DNA vaccine has been previously described (17) and includes a mixture of plasmids expressing HIV-1 Env gp145ΔCFI from clades A, B, and C and a clade B Gag-Pol-Nef fusion protein (four plasmids in a 1:1:1:3 ratio) or these antigens delivered on separate plasmids as individual genes (six plasmids in a 1:1:1:1:1:1 ratio). All murine and primate studies were approved by our Institutional Animal Care and Use Committees.
ICS assays.
CD4+ and CD8+ T-lymphocyte responses were evaluated by intracellular cytokine staining (ICS) for gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) as previously described (17). Briefly, splenocytes from immunized mice were harvested and incubated with pools of 15-amino-acid peptides overlapping by 11 amino acids (2.5 μg/ml each) covering the entire HIV-1 Env protein, followed by treatment with 10 μg/ml brefeldin A (Sigma, St. Louis, MO). Cells were then fixed, permeabilized, and stained using rat anti-mouse CD3, CD4, CD8, IFN-γ, and TNF-α monoclonal antibodies (BD Pharmingen, San Diego, CA). The IFN-γ- and TNF-α positive cells in the CD4+ and CD8+ cell populations were analyzed with the program FlowJo (Tree Star, Ashland, OR).
ELISPOT assays.
ELISPOT assays were utilized to assess IFN-γ production by murine splenocytes or monkey peripheral blood mononuclear cells (PBMC) as previously described (4, 5). For cell depletion studies, murine splenocytes were incubated with magnetic microbeads coated with anti-CD4 (L3T4) or anti-CD8 (Ly-2) monoclonal antibodies (Miltenyi Biotec, Auburn, CA), and separation using MiniMACS columns was performed according to the manufacturer's instructions. Cell depletions were approximately 95 to 98% efficient. IFN-γ secretion was then measured in response to overlapping 15-amino-acid peptides spanning the entire Env, Gag, Pol, or Nef protein.
Statistical analyses.
Immunologic data are presented as means with standard errors. Statistical analyses were performed with GraphPad Prism version 4.01 (GraphPad Software, Inc., 2004). Comparisons of mean cellular immune responses between groups of animals were performed by two-tailed nonparametric Mann-Whitney tests. In all cases, P values of less than 0.05 were considered significant.
RESULTS
Optimization of expression from plasmid DNA vaccines in vitro.
To develop improved plasmid DNA expression vectors, alternative regulatory elements were constructed and assessed for their ability to drive in vitro expression of a modified HIV-1 Env protein, gp145ΔCFI (9). The regulatory R sequence from the 5′ LTR of HTLV-1 (12, 30) was incorporated between the CMV enhancer/promoter and splicing acceptor site in the parental 1012 plasmid backbone to construct CMV/R DNA vaccines (Fig. 1A). We also incorporated this R element downstream from the Rous sarcoma virus and mouse mUB enhancer/promoter elements to create, respectively, RSV/R and mUB/R DNA vaccines.
FIG. 1.
In vitro expression of HIV-1 Env gp145 ΔCFI from various DNA expression vectors. (A). Schematic diagram of the alternative cellular and viral regulatory elements in eukaryotic expression plasmids. (B). Western blot analysis of 3T3 cells transfected with empty parental 1012 (CMV) (lane 1) or those expressing HIV Env gp145 ΔCFI with the CMV (lane 2), CMV/R (lane 3), mUB (lane 4), mUB/R (lane 5), RSV (lane 6), or RSV/R (lane 7).
To assess antigen expression from plasmids containing these transcriptional regulatory elements, we transfected 3T3 cells with these expression vectors and measured gp145ΔCFI expression by Western blots. The expression of gp145ΔCFI from the CMV/R plasmid was 5- to 10-fold higher than expression from the parental 1012 plasmid (Fig. 1B, lanes 2 versus 3). Thus, addition of the HTLV-1 R element substantially increased antigen expression driven by the CMV promoter. Baseline expression from the mUB plasmid was higher than from the 1012 plasmid but was not further enhanced by addition of the R element (Fig. 1B, lanes 4 versus 5), suggesting that the effects of adding the R element were promoter dependent. An increase in expression was observed in the RSV/R compared to the RSV plasmid (Fig. 1B, lanes 6 versus 7). Expression from RSV plasmids is routinely lower than from the 1012 plasmid (23).
Immunogenicity of improved expression vectors in mice.
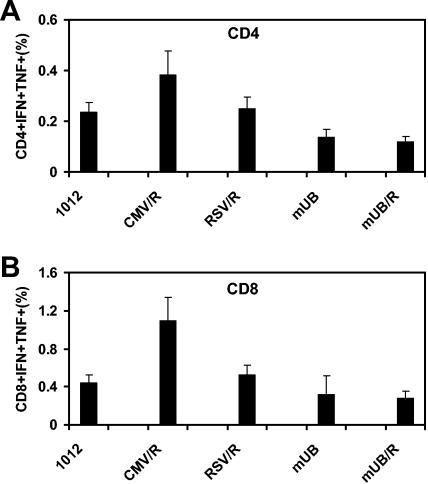
We reasoned that enhanced antigen expression may translate to improved immunogenicity of these novel DNA vaccines in vivo. To explore this possibility, we immunized BALB/c mice (n = 5/group) with 50 μg of the parental 1012 DNA vaccine or the CMV/R, RSV/R, mUB, or mUB/R DNA vaccines expressing HIV-1 Env gp145ΔCFI (9). Mice were immunized three times at weeks 0, 2, and 6. On day 10 following the final immunization, splenocytes were assessed for Env-specific cellular immune responses by IFN-γ and TNF-α intracellular cytokine staining assays. The CMV/R DNA vaccine elicited approximately twofold higher CD4+ (P = 0.15) and CD8+ (P = 0.043) T lymphocyte responses compared with the parental 1012 DNA vaccine expressing the same antigen (Fig. 2). In contrast, the RSV/R, mUB, and mUB/R DNA vaccines did not elicit enhanced CD8+ immune responses, suggesting that the HTLV-1 R element selectively improved immunogenicity in the context of the CMV promoter.
FIG. 2.
Immunogenicity of plasmid DNA vaccines expressing HIV-1 Env gp145ΔCFI in mice. Mice (n = 5/group) were immunized with 50 μg of the parental 1012 DNA vaccine or the CMV/R, RSV/R, mUB, or mUB/R DNA vaccines expressing HIV-1 Env gp145 ΔCFI at weeks 0, 2, and 6. After the third immunization, splenocytes were assessed for Env-specific cellular immune responses by IFN-γ and TNF-α ICS assays. (A) % CD3+CD4+ IFN-γ/TNF-α+ and (B) % CD3+CD8+ IFN-γ/TNF-α+ splenocytes are shown.
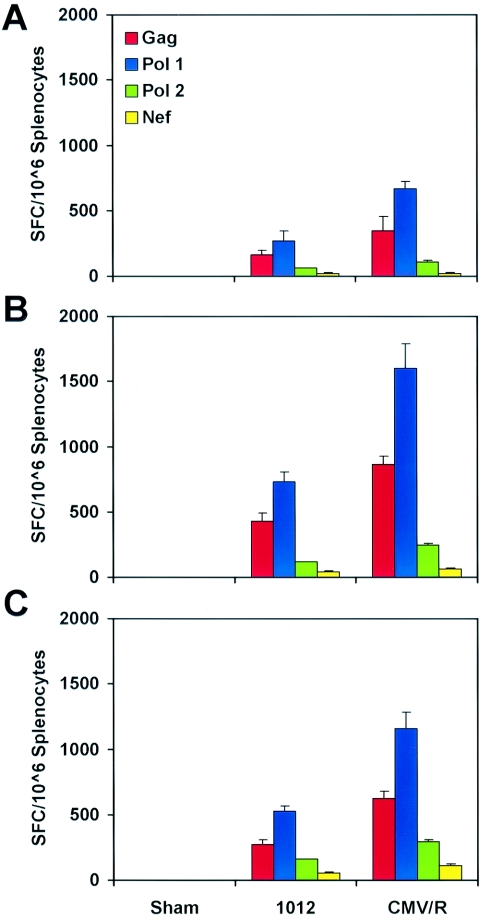
We next compared the immunogenicity of the parental 1012 DNA vaccine and the CMV/R DNA vaccine expressing other antigens. We immunized mice (n = 8/group) with sham plasmids or with these DNA vaccines expressing the HIV-1 Gag-Pol-Nef fusion protein (13). Mice were immunized twice at weeks 0 and 6, and cellular immune responses were assessed by IFN-γ ELISPOT assays using splenocytes harvested 3 weeks after the initial or boost immunization. Consistent with the prior experiment, we observed approximately twofold higher Gag- (P = 0.038) and Pol-specific (P = 0.020) responses elicited by the CMV/R DNA vaccine compared to the parental 1012 DNA vaccine following the initial immunization (Fig. 3A). Nef-specific responses elicited by both plasmids remained low. Following the boost immunization, responses elicited by the CMV/R DNA vaccine remained approximately twofold higher than responses elicited by the parental DNA vaccine using both unfractionated splenocytes (Fig. 3B) and CD8-depleted splenocytes (Fig. 3C).
FIG. 3.
Immunogenicity of CMV/R plasmid DNA vaccines expressing HIV-1 Gag-Pol-Nef in mice. Mice (n = 8/group) were immunized with 50 μg of the parental 1012 DNA vaccine or the CMV/R DNA vaccine expressing HIV-1 Gag-Pol-Nef at weeks 0 and 6. Splenocytes were assessed for Gag-, Pol-, and Nef-specific cellular immune responses by IFN-γ ELISPOT assays 3 weeks after the (A) initial and (B) week 6 boost immunizations. (C) Splenocytes depleted of CD8+ T lymphocytes were also assessed in similar ELISPOT assays following the boost immunization.
Immunogenicity of CMV/R DNA vaccines in cynomolgus monkeys.
A number of strategies have improved the immunogenicity of DNA vaccines in inbred strains of mice, but few have been assessed in outbred nonhuman primates (4, 24, 29). We therefore compared the immunogenicity of the parental 1012 DNA vaccine with CMV/R DNA vaccine expressing multiple HIV-1 antigens in cynomolgus monkeys. We immunized two groups of adult cynomolgus monkeys (n = 6/group) with four-plasmid mixtures of 1012 or CMV/R DNA vaccines expressing HIV-1 Env gp145 ΔCFI from clades A, B, and C and the Gag-Pol-Nef fusion protein from clade B in a 1:1:1:3 ratio. This multiclade, multivalent DNA vaccine has been previously described (17) and is currently being evaluated in clinical trials.
A third group of monkeys was included to investigate whether separating the Gag-Pol-Nef fusion protein into separate genes encoded on separate plasmids would further increase immune responses to these antigens. This third group of monkeys received a six-plasmid mixture of CMV/R DNA vaccines encoding HIV-1 Env gp145 from clades A, B, and C and separate Gag, Pol, and Nef proteins from clade B in a 1:1:1:1:1:1 ratio. All monkeys received three immunizations of 8 mg total DNA vaccine at weeks 0, 4, and 8.
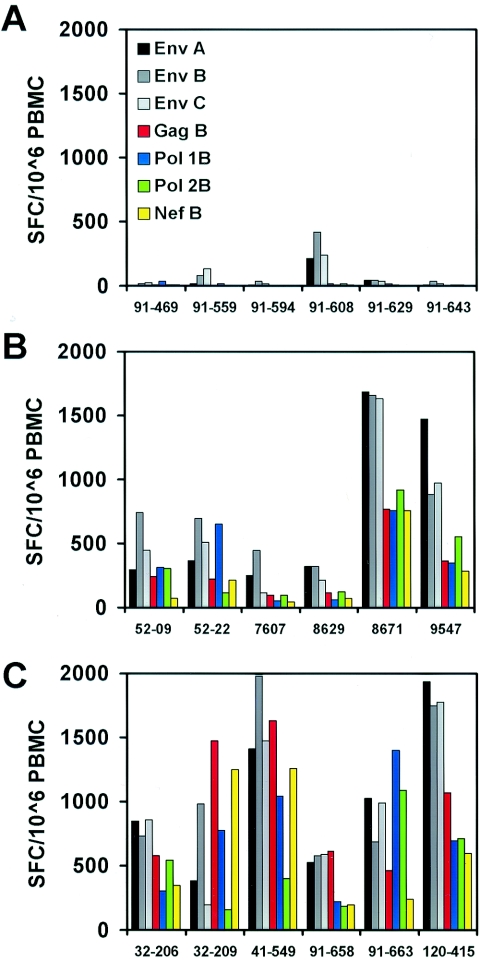
We first compared cellular immune responses against Env clade A, Env clade B, Env clade C, and Gag, Pol, and Nef from clade B in monkeys that received the four-plasmid mixtures under the control of CMV (1012) or CMV/R regulatory elements. Monkeys immunized with the parental 1012 DNA vaccines developed low and sporadic IFN-γ ELISPOT responses to Env 2 weeks following the second immunization at week 6, and no clear responses above background were detected to Gag, Pol, and Nef (Fig. 4A). In contrast, monkeys immunized with the analogous CMV/R DNA vaccines exhibited significantly higher responses to all antigens (Fig. 4B). Compared to the parental 1012 DNA vaccines, the CMV/R DNA vaccines elicited >10-fold higher ELISPOT responses to Gag (P = 0.0022), Pol (P = 0.0043), and Nef (P = 0.041) and 7- to 9-fold higher responses to Env clade A (P = 0.026), B (P = 0.0087), and C (P = 0.030) at this time point. These results demonstrate that the CMV/R DNA vaccines were markedly more immunogenic than the parental 1012 DNA vaccines for multiple HIV-1 antigens in nonhuman primates.
FIG. 4.
Immunogenicity of multivalent CMV/R DNA vaccines expressing HIV-1 antigens in cynomolgus monkeys. Cynomolgus monkeys (n = 6/group) were immunized with the four-plasmid multivalent (A) 1012 DNA vaccines or (B) CMV/R DNA vaccines expressing HIV-1 Env gp145 ΔCFI from clades A, B, and C and a Gag-Pol-Nef fusion protein. (C) A third group of monkeys was immunized with a six-plasmid multivalent CMV/R DNA vaccine expressing HIV-1 Env gp145 ΔCFI from clades A, B, and C and separate gag, pol, and nef genes. All monkeys received 8 mg total DNA at weeks 0, 4, and 8. IFN-γ ELISPOT responses were assessed 2 weeks after the second immunization at week 6.
We also observed that separating the Gag-Pol-Nef fusion protein into individual genes encoded on different plasmids further improved these responses. In particular, monkeys that received the 6-plasmid mixture of CMV/R DNA vaccines developed fourfold higher responses to Gag (P = 0.0022), a trend towards twofold higher responses to Pol (P = 0.19), and fourfold higher responses to Nef (P = 0.049) (Fig. 4C), compared to animals that received the four-plasmid mixture of CMV/R DNA vaccines that included the Gag-Pol-Nef fusion protein (Fig. 4B). As expected, Env-specific responses between these two groups of monkeys that received the four-plasmid and six-plasmid mixtures of CMV/R DNA vaccines were comparable (P = 0.48), since these groups received the same Env plasmids.
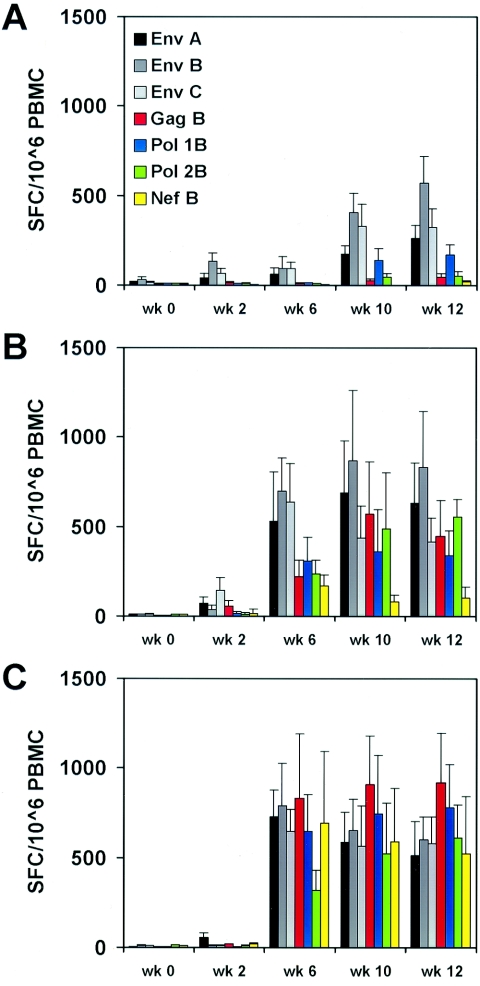
The evolution of mean IFN-γ ELISPOT responses in these groups of monkeys was evaluated at weeks 0, 2, 6, 10, and 12. Following the third DNA immunization at week 8, responses increased in all groups of monkeys (Fig. 5). At week 10, the parental 1012 DNA vaccines elicited Env- and Pol-specific responses in the majority of animals, although Gag- and Nef-specific responses remained low (Fig. 5A). In contrast, the CMV/R DNA vaccines elicited potent and broad responses to all antigens (Fig. 5B and C). At week 10, the four-plasmid CMV/R DNA vaccines (Fig. 5B) elicited >10-fold higher ELISPOT responses to Gag (P = 0.0022) and Nef (P = 0.0022), fourfold higher ELISPOT responses to Pol (P = 0.043), and trends toward 1.5- to 4-fold higher responses to Env clades A, B, and C (Fig. 5B), compared with the four-plasmid parental 1012 DNA vaccines (Fig. 5A). Gag-, Pol- and Nef-specific responses remained highest in the animals that received the six-plasmid CMV/R DNA vaccines with these genes encoded on separate plasmids (Fig. 5C). These studies confirm that the CMV/R DNA vaccines elicited substantially higher-magnitude and broader cellular immune responses to multiple antigens compared with the parental 1012 DNA vaccines. Thus, including the HTLV-1 R element and separating the gag, pol, and nef genes significantly enhanced the immunogenicity of HIV-1 DNA vaccines in nonhuman primates.
FIG. 5.
Mean responses to multivalent CMV/R DNA vaccines expressing HIV-1 antigens in cynomolgus monkeys. (A to C) Cynomolgus monkeys were vaccinated as described in the legend to Fig. 4. Mean and standard errors of IFN-γ ELISPOT responses in these groups of animals were assessed at weeks 0, 2, 6, 10, and 12 following immunization.
DISCUSSION
The relatively low immunogenicity of DNA vaccines in clinical trials to date has led to the development of various strategies to improve the immunogenicity of these vaccines. Approaches that have proven effective in nonhuman primates include the use of plasmid cytokines, polymer adjuvants, and in vivo electroporation techniques (4, 24, 29). A disadvantage of these approaches is that they require additional materials or devices that add manufacturing, regulatory, and practical complexities that may limit their large-scale clinical application. In this study, we investigated the potential utility of a strategy aimed at optimizing regulatory elements in the plasmid DNA vaccine. We demonstrated that the addition of the HTLV-1 R element to a CMV expression cassette markedly increased DNA vaccine immunogenicity in both mice and cynomolgus monkeys. Importantly, such a modification does not involve any additional components or devices and thus does not increase vaccine complexity. Optimizing immunogenicity by engineering novel regulatory elements into the plasmid backbone therefore represents a simple, practical, and effective approach that could be advanced into clinical trials.
A previous study demonstrated that adding the HTLV-1 R element downstream of an SV40 early promoter increased transgene expression by approximately 10- to 40-fold in vitro (30). The present study extends this observation by demonstrating that the R element also potentiates expression from a strong CMV promoter/enhancer. More importantly, we showed that this enhanced expression translates into significantly augmented immune responses in both rodents and nonhuman primates. We speculate that the beneficial effects of the R region likely involve recruiting key cellular transcription factors that enhance transgene transcription (12, 39), although the precise mechanism of action remains to be determined. The R region may also function as an internal ribosome entry site, suggesting that it may act at a posttranscriptional step as well (2). Alternatively, it is possible that the combination of CMV enhancer and HTLV-1 R regions stimulate optimal expression in professional antigen-presenting cells, which would best facilitate the antigen-specific T-cell response. Interestingly, the RSV/R and mUB/R DNA vaccines exhibited comparable transgene expression in vitro compared with CMV/R DNA vaccines (Fig. 1) but nevertheless failed to result in improved immunogenicity (Fig. 2). Thus, enhanced expression in vitro is not the sole determinant of enhanced immunogenicity in vivo.
In both mice and cynomolgus monkeys, CMV/R DNA vaccines expressing HIV-1 antigens elicited higher cellular immune responses than the parental 1012 DNA vaccines expressing the same antigens. However, the magnitude of the observed effects differed substantially between the two species. While the CMV/R DNA vaccines elicited only twofold higher responses in mice (Fig. 3), the CMV/R DNA vaccines elicited >10-fold higher cellular immune responses to Gag, Pol, and Nef and seven- to ninefold higher responses to Env after two immunizations in cynomolgus monkeys (Fig. 4 and 5). We suspect that this difference may reflect the lower baseline immunogenicity of the parental 1012 DNA vaccines in nonhuman primates and that the beneficial effects of the R element may appear more striking in limiting situations. Consistent with this observation, the R element had the greatest effect at enhancing the weakest responses elicited by the parental 1012 DNA vaccine against Gag and Nef. However, Env- and Pol-specific cellular immune responses were also significantly higher when induced by CMV/R DNA vaccines compared with the parental 1012 DNA vaccines.
We also observed that the six-plasmid mixture of CMV/R DNA vaccines that included Gag, Pol, and Nef on separate plasmids elicited significantly higher cellular immune responses to these antigens compared to the four-plasmid mixture of CMV/R DNA vaccines that included the Gag-Pol-Nef fusion protein. These effects are particularly notable since the separate gag, pol, and nef plasmids were each utilized at one-third the dose of the plasmid encoding the Gag-Pol-Nef fusion protein. We speculate that this may reflect enhanced translation or mRNA stability of the shorter genes compared with the fusion gene, which might potentially affect antigen processing and presentation.
A limitation of the current study is that we were not able to assess the protective efficacy afforded by the CMV/R DNA vaccines. Since HIV-1 does not infect cynomolgus monkeys, we could not perform viral challenges in these animals. However, accumulating data have confirmed the importance of cellular immune responses in controlling HIV-1 replication in humans and simian immunodeficiency virus replication in rhesus monkeys (14, 22, 27). Moreover, vaccines aimed at eliciting virus-specific cellular immune responses have afforded partial control of simian-human immunodeficiency virus and simian immunodeficiency virus challenges in rhesus monkeys (1, 4, 11, 18, 20, 25, 29). Thus, we believe that the markedly increased magnitude and breadth of HIV-1-specific cellular immune responses afforded by the CMV/R DNA vaccines in nonhuman primates in the present study will likely prove beneficial in the development of second-generation DNA vaccines for HIV-1 and other pathogens. In particular, incorporating the HTLV-1 R element and utilizing separate genes in place of fusion genes represent simple and practical strategies to improve DNA vaccines. These strategies could be advanced readily into clinical trials.
Acknowledgments
We thank Srinivas Rao, Vi Dang, Kristin Beaudry, Faye Yu, Kristi Martin, Darci Gorgone, and Michelle Lifton for generous advice, assistance, and reagents.
We acknowledge support from the NIH Vaccine Research Center (G.J.N. and N.L.L.) and NIH grant AI-58727 (D.H.B.).
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S.P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-K. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of clinical AIDS in rhesus monkeys by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Attal, J., M. C. Theron, F. Taboit, M. Cajero-Juarez, G. Kann, P. Bolifraud, and L. M. Houdebine. 1996. The RU5 (′R') region from human leukaemia viruses (HTLV-1) contains an internal ribosome entry site (IRES)-like sequence. FEBS Lett. 392:220-224. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M.-E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875-1882. [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., D. M. Truitt, and N. L. Letvin. 2004. Expression kinetics of the interleukin-2/immunoglobulin (IL-2/Ig) plasmid cytokine adjuvant. Vaccine 22:3092-3097. [DOI] [PubMed] [Google Scholar]
- 8.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 11.Egan, M. A., W. A. Charini, M. J. Kuroda, G. Voss, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. M. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 13.Huang, Y., W. P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, J. J., N. N. Trivedi, L. K. Nottingham, L. Morrison, A. Tsai, Y. Hu, S. Mahalingam, K. Dang, L. Ahn, N. K. Doyle, D. M. Wilson, M. A. Chattergoon, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 28:1089-1103. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. J., J. S. Yang, T. C. VanCott, D. J. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2000. Modulation of antigen-specific humoral responses in rhesus macaques by using cytokine cDNAs as DNA vaccine adjuvants. J. Virol. 74:3427-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong, W. P., Y. Huang, Z. Y. Yang, B. K. Chakrabarti, Z. Moodie, and G. J. Nabel. 2003. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J. Virol. 77:12764-12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 19.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 20.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D.H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS Vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore, A. C., W. P. Kong, B. K. Chakrabarti, and G. J. Nabel. 2002. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 76:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 23.Norman, J. A., P. Hobart, M. Manthorpe, P. Felgner, and C. Wheeler. 1997. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine 15:801-803. [DOI] [PubMed] [Google Scholar]
- 24.Otten, G., M. Schaefer, B. Doe, H. Liu, I. Srivastava, J. zur Megede, D. O'Hagan, J. Donnelly, G. Widera, D. Rabussay, M. G. Lewis, S. Barnett, and J. B. Ulmer. 2004. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 22:2489-2493. [DOI] [PubMed] [Google Scholar]
- 25.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker, S. E., D. Monteith, H. Horton, R. Hof, P. Hernandez, A. Vilalta, J. Hartikka, P. Hobart, C. E. Bentley, A. Chang, R. Hedstrom, W. O. Rogers, S. Kumar, S. L. Hoffman, and J. A. Norman. 2001. Safety of a GM-CSF adjuvant-plasmid DNA malaria vaccine. Gene Ther. 8:1011-1023. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 28.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA. 80:3618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 30.Takebe, Y., M. Seiki, J. Fujisawa, P. Hoy, K. Yokota, K. Arai, M. Yoshida, and N. Arai. 1988. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 8:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang, D. C., M. Devit, and S. A. Johnson. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 32.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, L. A. Hawe, K. R. Leander, D. Martinez, H. C. Perry, J. W. Shiver, D. L. Montgomery, and M. A. Liu. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 33.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, D. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, W. R., K. J. Ishii, R. C. Hedstrom, M. Sedegah, M. Ichino, K. Barnhart, D. M. Klinman, and S. L. Hoffman. 1998. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J. Immunol. 161:2325-2332. [PubMed] [Google Scholar]
- 35.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 36.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 37.Xiang, Z., and H. C. J. Ertl. 1995. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 2:129-135. [DOI] [PubMed] [Google Scholar]
- 38.Xu, L., A. Sanchez, Z. Yang, S. R. Zaki, E. G. Nabel, S. T. Nichol, and G. J. Nabel. 1998. Immunization for Ebola virus infection. Nat. Med. 4:37-42. [DOI] [PubMed] [Google Scholar]
- 39.Xu, X., S. H. Kang, O. Heidenreich, D. A. Brown, and M. I. Nerenberg. 1996. Sequence requirements of ATF2 and CREB binding to the human T-cell leukemia virus type 1 LTR R region. Virology 218:362-371. [DOI] [PubMed] [Google Scholar]
- 40.Yew, N. S., M. Przybylska, R. J. Ziegler, D. Liu, and S. H. Cheng. 2001. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol. Ther. 4:75-82. [DOI] [PubMed] [Google Scholar]