Abstract
Heart failure (HF) is a complex syndrome and a leading cause of mortality worldwide. While current medical treatment is based on known pathophysiology and is effective for many patients, the underlying cellular mechanisms are poorly understood. Energy deficiency is a characteristic of HF, marked by complex alterations in metabolism. Within the tricarboxylic acid cycle, anaplerosis emerges as an essential metabolic process responsible for replenishing lost intermediates, thereby playing a crucial role in sustaining energy metabolism and consequently cardiac function. Alterations in cardiac anaplerosis are commonly observed in HF, demonstrating potential for therapeutic intervention. This review discusses recent advances in understanding the anaplerotic adaptations that occur in HF. We also explore therapeutics that can directly modulate anaplerosis or are likely to confer cardioprotective effects through anaplerosis, which could potentially be implemented to rescue the failing heart.
Keywords: Anaplerosis, Mitochondria, Cardiac metabolism, Heart failure, Cardiovascular disease
Graphical Abstract
Graphical Abstract.
1. Introduction
Heart failure (HF) results from an inability of the heart to pump and/or fill with blood to meet the demands of the body.1 The clinical syndrome of HF is characterized by structural and/or functional abnormalities corroborated by elevated levels of natriuretic peptides and objective evidence of pulmonary or systemic congestion.2 Considered a global epidemic, HF currently affects an estimated 64.3 million individuals.3 As such, it has become a priority to reduce the economic and social burden of this condition.
The heart is an energetically demanding organ that relies heavily on adenosine triphosphate (ATP) production to support its contractile function.4 To meet its demands, cardiomyocytes rely on multiple energy sources, including fatty acids (FAs), glucose, lactate, amino acids, and ketone bodies, which are ultimately converted into acetyl coenzyme A (acetyl-CoA) prior to entry into the tricarboxylic acid (TCA) cycle (Figure 1).4–8 The TCA cycle is a critical process in the mitochondrial production of ATP through the oxidation of carbon energy substrates. Once acetyl-CoA enters the TCA cycle, it is combined with oxaloacetate (OAA) to form citrate, followed by a series of reactions that produce reducing equivalents which are used by the electron transport chain (ETC) to produce ATP. During this process, two carboxyl groups are lost as CO2, balancing the two carbon units added by the incorporation of acetyl-CoA into the TCA cycle.
Figure 1.
Overview of metabolism in healthy cardiomyocytes. Under aerobic conditions, FAs, glucose, lactate, ketone bodies, and BCAAs are converted to acetyl-CoA which enters the TCA cycle and permits the generation of reducing equivalents NADH and FADH2 which transfer electrons to the mitochondrial ETC for the production of ATP. AcAc, acetoacetate; BHB, β-hydroxybutyrate; BCKA, branched chain keto acids; IMS, intermembrane space; MM, mitochondrial matrix. Figure created using Biorender.
Kornberg first coined the term ‘anaplerosis’ to describe the replenishment of TCA cycle intermediates in bacteria.9 This crucial process replaces carbon that has been removed from the TCA cycle, allowing for the continued production of reducing equivalents. In contrast, cataplerosis refers to the removal of intermediates from the TCA cycle for biosynthetic purposes, such as the production of amino acids or glucose. Perturbations in anaplerosis can lead to the depletion of TCA cycle intermediates which can severely impact cellular ATP production with consequences for organs with high energetic demands, including the heart. Current medical treatment of HF is primarily focused on symptom reduction and curative therapy has not yet been established. Improved understanding of the underlying molecular pathology of HF, including how altered mitochondrial energy metabolism and perturbed anaplerosis play a role in disease pathogenesis, could help identify novel defined therapeutic targets.
2. Cardiac anaplerosis: substrates and metabolic pathways
The more specific phrase ‘cardiac anaplerosis’ was conceived to describe the replenishment of TCA cycle intermediates in the heart.10 The earliest studies of cardiac anaplerosis confirmed its importance wherein isolated perfused rat hearts exhibited decreased myocardial function when perfusate lacked anaplerotic substrates.11,12 Further work in the same model and also in vivo in swine hearts confirmed the observation that a lack of anaplerotic substrates resulted in contractile dysfunction.13–19 However, studies investigating cardiac anaplerosis in humans are limited.
The reactions of the TCA cycle allow for a total recovery of cycle intermediates, as the two carbons lost as CO2 are matched by two carbons entering the TCA cycle from acetyl-CoA (Figure 2). However, a portion of TCA cycle intermediates are released from mitochondria to participate in other metabolic or biosynthetic pathways.20 This process, known as cataplerosis, depletes TCA intermediates, necessitating their replenishment to sustain catabolic function and meet the heart’s energy demands.21,22 To maintain metabolic homeostasis, anaplerosis plays a vital role in counterbalancing this loss, restoring essential intermediates to the TCA cycle, and ensuring continuous energy production.
Figure 2.
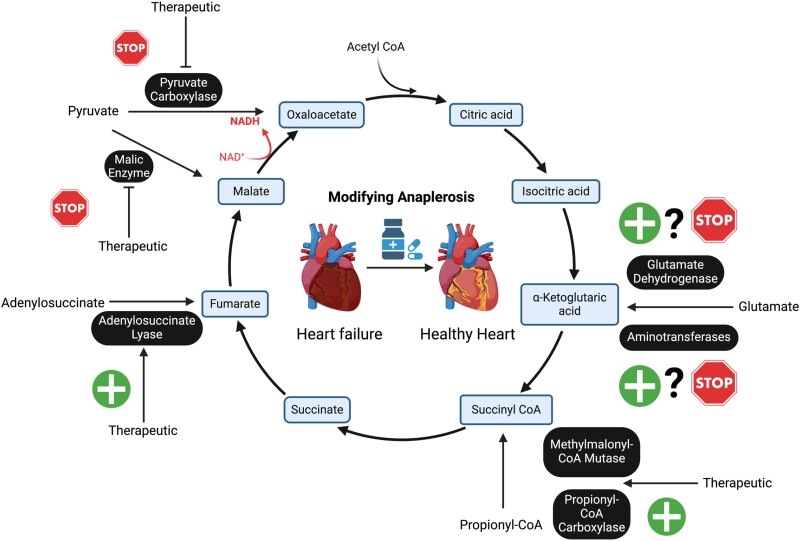
Anaplerotic and cataplerotic pathways with entry points, anaplerotic enzymes, and the molecules directly or indirectly involved in anaplerosis in the TCA cycle. Double-headed arrows represent reversible reactions involved in anaplerosis and cataplerosis. Single-headed arrows indicating non-reversible anaplerotic reactions. Blue single-headed arrows indicate cataplerotic efflux of TCA cycle intermediates. PC, pyruvate carboxylase; ME, malic enzyme; AT, aminotransferases; GLS, glutaminase; GDH, glutamate dehydrogenase; PCC, propionyl-CoA carboxylase; ADSL, adenylosuccinate lyase. Figure created in Biorender.
There are five anaplerotic entry points into the TCA cycle including malate, OAA, α-KG, succinyl-CoA, and fumarate.10 Direct anaplerotic substrates include pyruvate, aspartate, adenylosuccinate, propionyl-CoA, and glutamate. Indirect anaplerotic substrates are short- or odd-chain FAs (OCFAs) [i.e. propionate (C3), C15, and C17] and C5-ketone bodies which are all precursors of propionyl-CoA. In addition, glucose, FAs, lactate, and amino acids [including glutamine and branched chain amino acids (BCAAs)] also contribute to cardiac anaplerosis (Figure 2).23
3. Anaplerosis in the healthy heart
Pyruvate is the end-product of glycolysis in the cytosol, after which it is transported into the mitochondrial matrix to participate in the TCA cycle. While the majority of pyruvate is destined for acetyl-CoA production for the TCA cycle, some of this pyruvate can be used for anaplerosis through three different pathways. Pyruvate can undergo reversible nicotinamide adenine dinucleotide phosphate (NADP+)-dependent pyruvate carboxylation reactions, catalyzed by malic enzyme (ME) to produce malate. Alternatively, pyruvate can undergo irreversible pyruvate carboxylation, catalyzed by pyruvate carboxylase (PC), to produce OAA. Studies have shown that ME plays a more prominent role in anaplerosis compared to PC.13,24 OAA may also be replenished through a transamination reaction catalyzed by aspartate aminotransferase (AST). Lastly, the reverse reaction of lactate dehydrogenase catalyzes the conversion of lactate to pyruvate and thus indirectly contributes to anaplerosis through subsequent PC- and ME-catalyzed reactions.
Mitochondrial OAA levels are influenced by the malate–aspartate shuttle which links cytosolic glycolysis with the TCA cycle for transport of decreased nicotinamide adenine dinucleotide (NADH) molecules in cataplerotic metabolism.25–27 The malate–aspartate shuttle allows for cataplerosis and export of TCA cycle intermediates such as α-KG and malate, facilitated by the malate-α-KG antiporter (SLC25A11). In the cytosol, α-KG and aspartate undergo transamination by AST to form glutamate and OAA, respectively, and OAA is then converted back to malate by cytosolic malate dehydrogenase. The malate is re-imported into the mitochondria, completing the shuttle. This exchange coordinates anaplerotic and cataplerotic fluxes, allowing the TCA cycle intermediates to participate in biosynthetic capacity, redox balance, and energy production while maintaining TCA cycle homeostasis.
Glutamine is another anaplerotic precursor that enters the mitochondrial matrix to be processed via glutaminolysis.28,29 First, glutamine is deaminated into glutamate in a rate-limiting reaction catalyzed by glutaminase (GLS). Then, glutamate is converted into α-KG by two different reversible reactions: one catalyzed by aminotransferases, and the other by glutamate dehydrogenase (GDH).10,30 Succinyl-CoA represents an entry point into the TCA cycle for several molecules including OCFAs, cholesterol, propionate, and the BCAAs (particularly valine and isoleucine) which converge into propionyl-CoA, then succinyl-CoA.31 Fumarate replenishment occurs through the guanine triphosphate-hydrolyzing reaction of the purine nucleotide cycle, and the deamination of aspartate.32
Branched chain amino transferase 2 (BCAT2) also contributes to anaplerosis, converting branched chain ketoacids (BCKAs) to BCAAs, and glutamate to α-KG. However, BCAT2 can also function in the opposite direction, converting α-KG to glutamate in the first step of BCAA oxidation. The directionality of BCAT2 depends largely on the levels of BCKAs and BCAAs, but this enzyme primarily functions in the cataplerotic direction as BCAA levels usually predominate.33
4. Alterations in cardiac anaplerosis in HF
Recognizing the critical role of anaplerosis in the healthy heart, it is important to consider how these pathways may be altered in HF. Diminished levels of TCA cycle intermediates contribute to the reported defects of the TCA cycle in HF, and alterations in cardiac anaplerosis are associated with the development or worsening severity of HF in animals and humans.31,34–36 Multiple studies have suggested that abnormalities of cardiac anaplerosis exist across varying HF stages and in varying pathological states associated with HF including hypertension, ischaemia, toxin-mediated cardiomyopathy, and hypertrophy.22,37,38
Anaplerosis enables the heart to balance carbon between ATP generation and the replenishment of TCA cycle intermediates as needed. Disruptions in anaplerosis can destabilize cellular energy balance, redox homeostasis, and the availability of precursors necessary for biosynthetic processes.39–41 In HF, protein synthesis is upregulated, often in conjunction with by decreased amino acid degradation, which increases the demand for anaplerotic inputs like pyruvate to provide biosynthetic precursors. Furthermore, diminished anaplerosis can exacerbate oxidative stress by lowering decreased NADP (NADPH), NADH, and flavin adenine dinucleotide (FADH2) levels, leading to redox imbalance, increased electron leakage, and the generation of ROS.40,41
5. Pyruvate anaplerosis in HF
Cardiac hypertrophy is a physiological adaptation, but can also precede the onset of HF with decreased ejection fraction (HFrEF) which develops as a consequence of pressure overload.42 Hypertrophy initially helps to maintain cardiac function but eventually becomes maladaptive leading to decompensated hypertrophy and HF.42,43 Myocardial hypertrophy is commonly associated with increased rates of glycolysis, decreased mitochondrial oxidative metabolism, and altered anaplerosis.44–49 In response to diminished glucose oxidation, there is increased conversion of pyruvate to lactate and increased anaplerotic pyruvate flux bypassing pyruvate dehydrogenase (PDH), at the expense of energy efficiency (see Supplementary material online, Table S1).22,37,50,51 These changes are driven by the required upregulation of biosynthetic pathways to support protein synthesis in hypertrophy.52
Sorokina et al. induced hypertrophy in rat hearts by subjecting them to 10 weeks of transaortic constriction (TAC) and assessed cardiac metabolism using dynamic 13C-nuclear magnetic resonance (NMR). The isolated hearts were perfused with 13C-palmitate and glucose.22 The study detected diminished FA oxidation alongside increased glycolysis, without significant changes in glucose oxidation via PDH.22 Flux measurements indicated an enhanced anaplerotic flow into the TCA cycle, accompanied by increased ME mRNA expression, suggesting that this anaplerotic pathway compensates for the diminished contribution of glycolysis-derived pyruvate. The energetic state was monitored using 31P-NMR spectroscopy to assess phosphocreatine and ATP content. Despite the activation of anaplerosis and the preservation of TCA cycle flux, cardiac ATP generation in hypertrophy was inefficient, as evidenced by diminished ATP levels compared to control rat hearts.22
Pound et al.50 later validated these findings in TAC-subjected isolated rat hearts perfused with 13C-labeled palmitate or glucose. They detected an increase in anaplerotic flux through cytosolic ME, and increased levels of ME protein in TAC-treated rat hearts compared to sham-operated animals.50 Aside from increased anaplerotic flux via ME, levels of myocardial triacylglycerol were decreased in TAC-treated rats compared to sham-operated animals, suggesting lower rates of lipogenesis. This was reversed with PDH activation which competitively decreased anaplerosis, resulting in a significant increase in triacylglycerol content and enhanced cardiac contractility. Overall, this suggests that anaplerosis of pyruvate by ME could be depleting NADPH, a biosynthetic factor and electron donor required for lipogenesis and protection against oxidative stress.
Lahey et al.51 studied TAC-induced hypertrophic rat hearts and exposed them to an adenovirus carrying miRNA targeting cytoplasmic rat ME1 which inhibits the enzyme. Next, the hearts were isolated, perfused with 13C-labelled palmitate, glucose, and lactate and divided into three groups, TAC, sham, and miRNA-ME1. To measure cardiac anaplerosis, the authors used high-resolution 13C-NMR spectroscopy and applied isotopomer analysis. Increased anaplerosis was seen in the TAC group but was lowered in the miRNA-ME1 group. Moreover, inhibiting ME1 restored increased NADPH levels and as a result increased levels of antioxidant glutathione.51 Overall these findings indicate that failing hearts rely on anaplerosis at the expense of decreasing the rate of biosynthesis. Supporting these animal observations, quantitative polymerase chain reaction analysis confirmed increased expression of ME1 in failing human myocardium.51
Turer et al.37 subjected mice to severe TAC (sTAC) for 6–8 weeks, to quickly transition the animals from compensated hypertrophy to decompensated hypertrophy and severe HF. To study cardiac substrate preference, the authors used a perfusate containing 13C-labelled glucose, ethylacetoacetate, and enriched FAs and performed 13C flux measurements using liquid chromatography-mass spectrometry (LC-MS). Cardiac anaplerosis was assessed using 13C-NMR spectroscopy. Surprisingly, substrate utilization studies showed that glucose was the only energy substrate that showed significantly increased contribution to acetyl-CoA in overt HF, suggestive of increased glucose oxidation. This reveals an important difference between the models of sTAC and TAC-induced hypertrophy, wherein a decrease in glucose oxidation is observed.44–49 Furthermore, in sTAC-treated animals, succinyl-CoA anaplerosis was elevated, while pyruvate anaplerosis remained unchanged, underscoring another key difference between the sTAC and TAC models of HF. However, Turer et al. did not identify the specific anaplerotic substrate responsible for replenishing succinyl-CoA, leaving it unclear whether this metabolic alteration has a beneficial or detrimental effect on cardiac function.
Other groups have studied pyruvate anaplerosis by genetically modulating FA oxidation in animal models of HF. Deletion of acetyl-CoA carboxylase 2 (ACC2) increases the entry of long-chain FAs into the mitochondria, thereby preserving substrate utilization and FA oxidation in the heart. Kolwicz et al.53 performed a cardiac-specific Cre-induced deletion of ACC2 in mice and subjected them to 8 weeks of TAC. The isolated mouse hearts were then perfused with 13C-labeled glucose and FAs, and ventricular tissue metabolites were analysed using LC-MS and gas chromatography-mass spectrometry (GC-MS). In addition, myocardial energetics were assessed via 31P-NMR spectroscopy. This study identified increased glycolysis and increased anaplerosis as a response to TAC, representing a metabolic signature of cardiac hypertrophy in mice.53 However, in TAC-treated mice with a cardiac-specific deletion of ACC2, substrate utilization did not differ from the profile observed in sham controls. Moreover, these mice had preserved FA oxidation, and no increases in glycolysis or anaplerosis.53 This genetic modification was also associated with decreased cardiac fibrosis and hypertrophy and preserved systolic function. These data suggest that inhibiting anaplerosis leads to improved metabolic and cardiac function.
More recently, Choi et al.54 created a tamoxifen-inducible cardiac-specific knockout of ACC2 in 12- to 14-week-old mice and infused these animals with angiotensin-II. The mice hearts were then isolated and perfused with 13C-labelled glucose and 13C-labelled FAs.13C-NMR spectroscopy and isotopomer analysis were used to measure and quantify substrate utilization and anaplerosis. Deletion of ACC2 sustained FA oxidation, enhanced glycolytic-oxidative metabolism, and decreased anaplerosis, leading to decreased cardiac hypertrophy and fibrosis while preserving diastolic function in TAC-treated mice.54 Overall, these studies suggest that modifying FA oxidation reduces anaplerosis and protects cardiac function, and that anaplerosis may not always be beneficial.
Anaplerotic changes are not unique to surgically induced cardiac hypertrophy, as similar findings have been found in alternative models. In rat hearts with triiodothyronine-induced cardiac hypertrophy, Atherton et al.55 performed an in vivo evaluation of TCA cycle metabolites using 2-13C pyruvate and magnetic resonance spectroscopy. A unique pattern was identified in which the incorporation of label into [1-13C]citrate, [5-13C]glutamate, and [1-13C]acetylcarnitine was significantly decreased in hypertrophied rat hearts compared to controls. A significant lowering in PDH flux, diminished glucose oxidation, and increased anaplerosis through PC were evident in this model of cardiac hypertrophy. Interestingly, maintaining PDH activity with dichloroacetic acid treatment decreased hypertrophy but did not significantly impact anaplerosis through PC or alter cardiac output.55 This illustrates that metabolic abnormalities can persist despite augmenting glucose oxidation. Therefore, inhibiting anaplerosis could be a potential alternative strategy to enhance cardiac output.
In summary, the evidence suggests that an activation of pyruvate anaplerosis plays a significant role in driving pathological cardiac hypertrophy by diverting glucose metabolism away from efficient energy production and depleting NADPH reserves, which are crucial for antioxidant defense. This shift in metabolism not only reduces the heart’s energetic efficiency but may also exacerbate oxidative stress and promote detrimental remodelling of the heart muscle, ultimately impairing cardiac contractility and function. It is also clear that PC activity in the heart is minimal, indicating that ME is the predominant enzyme mediating pyruvate anaplerosis in HF.13,24 Moreover, while pyruvate is a major source for anaplerosis via ME and PC, aspartate can contribute via transamination reactions that generate OAA. However, aspartate remains relatively understudied in the context of HF. Investigating how aspartate metabolism impacts cardiac function could yield new insights, particularly in terms of its contributions to energy homeostasis and redox balance. Based on the current evidence, the inhibition of ME may represent a multifaceted therapeutic strategy to not only improve cardiac energy efficiency but also to reduce cardiac hypertrophy, protect cardiomyocytes from oxidative stress, and thereby improve heart function.
6. Targeting pyruvate anaplerosis in HF
Pyruvate is a significant source of acetyl-CoA fuelling the TCA cycle, as well as a substrate for cardiac anaplerosis. If the failing heart is energetically inefficient, modifying pyruvate anaplerosis represents a potential therapeutic option. Many animal and human studies have tested various interventions to modify pyruvate anaplerosis in HF.
Bøgh et al.56 performed pulmonary valve suturing in porcine hearts to induce volume overload and eventually right-sided HF. A combination of magnetic resonance imaging and hyperpolarized [1-13C] pyruvate magnetic resonance spectroscopy were used to assess cardiac contractility, hypertrophy and metabolism. Consistent with the findings of Pound et al.,50 administering dichloroacetate, a PDH kinase inhibitor which increases PDH flux, improved pyruvate oxidation and contractile function and decreased hypertrophy.56 These results demonstrate that recoupling glycolysis to mitochondrial metabolism can reduce inefficient pyruvate anaplerosis, contributing to enhanced energy efficiency and cardiac performance.
Some studies have attempted to stimulate pyruvate metabolism in HF by altering the mitochondrial pyruvate carrier (MPC), a transporter composed of two subunits (MPC1 and MPC2) that is responsible for the transport of pyruvate across the mitochondrial membrane.57 Mice with a MPC2 deletion spontaneously developed cardiac dilation and contractile dysfunction at 6 weeks.58 Targeted metabolomics analysis of MPC2-deficient mice hearts revealed an accumulation of pyruvate, unaltered lactate levels, a decrease in acetyl-CoA, and an accumulation of fumarate, malate, and OAA suggesting that anaplerosis is unaffected by MPC2 deletion. This study also found decreased protein levels and mRNA expression of both MPC1 and MPC2 in heart tissue samples from HF patients undergoing left ventricular assist device (LVAD) implantation. This finding is consistent with the observation that myocardial recovery in HF patients after LVAD implantation is associated with increased myocardial expression of MPC.59 These findings were further validated in adult mice with Cre-induced cardiac-specific deletion of MPC1, which led to cardiac hypertrophy and HF.59 The impact of MPC downregulation was further analysed using 13C-glucose tracing and MS in H9c2 cells with an inducible shRNA-mediated knockdown of MPC1.59 An increase in extracellular and intracellular 13C-lactate was observed as well as an overall decrease in labelled citrate, malate, and fumarate, suggesting a reduction in mitochondrial pyruvate oxidation and anaplerosis. Increased lactate export was also supported by increased protein expression of monocarboxylate transporter 4 (MCT4) in hypertrophied H9c2 cells. The overexpression of MPC and inhibition of MCT4 attenuated the drug-induced hypertrophy in cultured cardiomyocytes.59 Overall, the evidence on the impact of modifying MPC on pyruvate anaplerosis is limited and somewhat conflicting. McCommis et al.58 suggest that anaplerosis remains partially preserved despite diminished pyruvate oxidation. In contrast, findings from Cluntun et al.59 indicate that decreased pyruvate oxidation is accompanied by a decline in pyruvate-driven anaplerosis. The implications of these pre-clinical findings for optimal cardiac energy metabolism and the role of pyruvate anaplerosis remain unclear.
When investigating the therapeutic potential of pyruvate anaplerosis in human HF it is crucial to consider the type and stage of HF that this intervention aims to target. For example, considering that increased pyruvate anaplerosis is associated with cardiac hypertrophy in animal models of HF, it may be beneficial to stimulate pyruvate decarboxylation through the activation of PDH.50,51 However, this approach may not be reasonable in later stages of HF because studies in patients with end-stage systolic HF or ischaemic cardiomyopathy have reported increased PDH expression.60,61 To further complicate this, findings in end-stage non-ischaemic dilated cardiomyopathy (DCM) reveal that PDH subunit levels remain largely unchanged.36 Instead, PDH activity is notably enhanced in DCM, primarily due to the decreased expression of PDH kinase 4, an inhibitor of PDH.
The majority of clinical studies to-date have explored supplementation with pyruvate in HF, exploiting pyruvate’s role both as a source of acetyl-CoA for the TCA cycle and as an antioxidant. Early studies attempted to elevate physiological pyruvate levels in HF patients to elicit cardioprotection.23 Hasenfuss and colleagues showed marked improvements in cardiac function following administration of pyruvate to patients with HF, without inducing adverse side effects.62,63 Years later, this finding was validated in a study that showed improved systolic and diastolic function in HF patients who received intracoronary infusions of pyruvate.64 Although the utilization of pyruvate as an energetic fuel is necessary for enhancement of cardiac function, it is not entirely clear if cardioprotection with pyruvate supplementation was conferred due to increases in pyruvate oxidation, increased anaplerosis, or its antioxidant properties.65–68 Furthermore, at least for humans, limited data exist regarding the optimal dose of pyruvate needed to rescue the failing heart. Herman et al.62 observed that two 15-min intracoronary doses of 3 or 6 mmol/L significantly improved cardiac stroke-volume indices and decreased pulmonary capillary wedge pressure.
In conclusion, research on pyruvate as a therapeutic option for HF has shown promising potential, especially regarding its roles in energy metabolism, anaplerosis, and antioxidant effects. Although studies indicate that enhancing pyruvate metabolism could be cardioprotective, the precise mechanisms, whether through anaplerosis, oxidative benefits, or other pathways, remain unclear. Pyruvate’s cardioprotective effects likely extend beyond anaplerosis, suggesting intricate interactions with oxidative stress reduction, mitochondrial efficiency, and the broader metabolic adaptations of failing cardiomyocytes. These pathways, including pyruvate’s role in modulating redox balance and NADPH availability, offer crucial insights into how metabolic dysfunction contributes to HF progression. Despite the promising results, it remains unclear whether pyruvate exerts its protective effects predominantly through energy replenishment, antioxidant capacity, or a combination of pathways that affect overall cardiac function. Addressing this gap requires a deeper understanding of how pyruvate metabolism intersects with broader metabolic networks, including the underexplored roles of metabolites such as aspartate and OAA. Therefore, further pre-clinical and clinical studies are necessary—not only to elucidate these mechanisms but also to identify the most effective methods of delivering pyruvate therapeutically, optimizing its benefits for patients with HF. By unravelling these connections, we can potentially harness pyruvate’s role in both stabilizing cardiac function and mitigating the metabolic disturbances associated with HF.69
7. Glutamine anaplerosis in HF
Being the most prevalent amino acid in the bloodstream, glutamine serves as a major carbon source for replenishing TCA cycle intermediates through mitochondrial glutaminolysis.70 Ultimately, glutamine is converted to glutamate and then α-KG (Figure 2). Multiple studies highlight the cardioprotective effects of glutamine supplementation, but the precise mechanism of action is unknown.71–74 Fewer studies have been conducted to study glutamine anaplerosis in HF, although current knowledge creates a foundation for further investigation and potential clinical translation.
Lauzier et al.75 perfused ex vivo rat hearts with 13C-labeled carbohydrates and oleate in the control group, and restricted pyruvate anaplerosis by removing insulin and pyruvate in the experimental group. Using GC-MS, this study showed that the addition of physiologically relevant levels of glutamine significantly improved cardiac function in ex vivo rat hearts under conditions of restricted pyruvate anaplerosis. In agreement with previous work, the isotopic labelling patterns of TCA cycle intermediates derived from 5-13C glutamine suggest that anaplerotic mechanisms may not be the reason for the cardioprotective effects of glutamine. Instead, glutamine activates the hexosamine biosynthetic pathway and increases the recruitment of FA transporter CD36, leading to a rewiring of cardiac metabolism. More specifically, Lauzier et al. suggest that glutamine shifts cardiac substrate utilization from carbohydrates to FAs and promotes FA oxidation.10,75,76
However, more recently, Watanabe et al. treated rat neonatal cardiomyocytes with H2O2 to create an in vitro model of oxidative stress-induced HF.70 With LC-MS analysis they showed decreased intracellular levels of glutamine, glutamate and α-KG in H2O2-treated cells. Stable isotope tracing with [U-13C]-glutamine and GC-MS were used to assess glutamine anaplerosis. They found increased relative amounts of α-KG, glutamate, glutamine, fumarate, and malate derived from 13C-glutamine, indicating increased anaplerotic flux of glutamine. Supporting this observation, the enzymatic activity of GLS increased over time following H2O2 treatment. However, ideally, CO2 production should be measured to validate the anaplerosis results. Lastly, the authors also tested the impact of the GLS inhibitor compound ‘968’ and dimethyl α-ketoglutarate (DMKG), a membrane-permeable ester of α-KG. Inhibition of GLS decreased cardiac cell viability, ATP production, and glutathione in rat neonatal cardiomyocytes exposed to H2O2 which was reversed with DMKG-treatment. Overall, Watanabe et al.70 suggest that modulation of glutamine anaplerosis could prevent metabolic remodelling, improve energetic status and ameliorate the oxidative stress associated with HF.
Another group showcased the cardioprotective effects of α-KG supplementation in pressure overload-induced HF.77 In TAC-subjected mice, α-KG supplementation decreased myocardial hypertrophy and fibrosis and improved cardiac function and LV strain.77 These data aligned with in vitro work where myocardial hypertrophy and injury were induced by angiotensin-II.77 Neonatal rat ventricular myocytes treated with α-KG in the presence of angiotensin-II maintained their mitochondrial membrane potential, displayed enhanced myocardial mitophagy, and showed decreased levels of hypertrophy, injury, apoptosis, and oxidative damage.77
Conversely, Yoshikawa et al.29 saw increases in glutamine anaplerosis in angiotensin-II treated hypertrophic murine hearts, rat neonatal cardiomyocytes, and rat neonatal cardiac fibroblasts. Contrary to their previous in vitro findings, this study suggested that glutamine anaplerosis is a reason for pathological cardiac hypertrophy and fibrosis, and that its inhibition could be therapeutic in HF.29,70
Recently, Li et al. found that α-KG has regenerative effects in the heart. More specifically, an accumulation of α-KG in cardiomyocytes activates KDM5 demethylases, leading to the demethylation of H3K4me3 histone protein, and a reprogramming of cells to a less mature, more proliferative state. This process promotes heart regeneration by enhancing cellular de-differentiation and tissue repair following injury.78
In summary, recent data underscore the ambiguity of glutamine anaplerosis in HF. Although glutamine serves as a crucial source of carbon for replenishing TCA cycle intermediates, its anaplerotic effects and therapeutic potential remain poorly understood. Some studies suggest that glutamine supplementation could protect from HF, but there is conflicting evidence for benefit in the context of cardiac hypertrophy.29,70,75 These discrepancies raise important questions about the differential regulation of glutamine metabolism in normal vs. failing hearts and how these variations influence both energy efficiency and the progression of hypertrophic remodelling. Understanding these distinctions could be key to determining whether glutamine acts as a metabolic buffer or exacerbates maladaptive remodelling under certain conditions. Therefore, additional pre-clinical investigation of glutamine anaplerosis is warranted, especially in cellular and animal models of HF.
8. The role of glutamine anaplerosis in dilated cardiomyopathy with ataxia syndrome fibroblasts
The dilated cardiomyopathy with ataxia syndrome (DCMA) is a rare mitochondrial disease that is associated with DCM and early mortality due to intractable HF.79–81 Deficiency of DNAJC19, a poorly characterized and understudied mitochondrial protein, underlies DCMA.81–83 Previous work revealed mitochondrial fragmentation in DCMA patient-derived fibroblasts and in cardiomyocytes differentiated from patient-derived induced pluripotent stem cells.84,85 Mitochondrial diseases such as DCMA are commonly associated with alterations in oxidative phosphorylation and glutamine metabolism.86 Recent work in DCMA fibroblasts has demonstrated that DCMA is a disorder of mitochondrial glutamine metabolism with suspected defects in glutamine anaplerosis.87 King et al.87 demonstrated normal TCA cycle functioning in fibroblasts from patients with both mild and severe cardiac dysfunction. However, DCMA fibroblasts illustrated a greater reliance on glutamine metabolism concurrent with increased glutamate secretion and diminished glutamine recycling. Given the heart’s reliance on mitochondrial metabolism for ATP production, altered glutamine metabolism could impact cardiac function by disrupting the balance between energy demand and supply in cardiomyocytes. A greater dependence on glutamine metabolism may indicate an adaptive response to mitochondrial dysfunction, but it could also contribute to metabolic inefficiency and redox imbalance, exacerbating HF in DCMA patients. Future research should focus on how these metabolic shifts in glutamine anaplerosis directly affect cardiomyocyte function, remodelling, and progression toward HF.
9. Propionyl-CoA anaplerosis in HF
Propionyl-CoA carboxylation is an important source of anaplerotic succinyl-CoA for the heart. There are several molecules that provide substrates for this reaction including BCAAs, short-chain FAs (i.e. propionate), OCFAs (particularly C15 and C17) and C5-ketone bodies. Propionyl-CoA carboxylase (PCC) and methylmalonic CoA mutase (MCM) play a role in replenishing succinyl-CoA and deficiency of PCC or MCM are associated with diminished anaplerosis.88,89 Abnormalities in propionyl-CoA anaplerosis may contribute to cardiomyopathy and HF by disrupting the replenishment of succinyl-CoA and causing a build-up of propionic acid.88–91 He et al.92 subjected ex vivo rat hearts to ischaemia by stopping the perfusate flow and measured the metabolite profile using GC-MS. Hearts exposed to prolonged ischaemia (30 min) exhibited an accumulation of propionyl-CoA and lowered cardiac levels of succinyl-CoA. Takada et al.31 have shown that mice with coronary artery ligation (CAL)-induced ischaemic chronic HF exhibit lowered cardiac levels of succinyl-CoA, compared to sham controls. Flam et al.36 employed plasma and cardiac tissue metabolomics to analyse 87 explanted human hearts, comparing 39 patients with end-stage HF to 48 non-failing donor hearts. Failing human cardiac tissue had a diminished relative abundance of propionyl-CoA. In summary, propionyl-CoA originates from various sources that all contribute to replenishing succinyl-CoA. This process appears to be essential for normal cardiac function but is poorly understood in the context of cardiac pathology and HF.
10. Branched chain amino acid anaplerosis in HF
BCAAs play a key role in cardiac metabolism. Aside from providing acetyl-CoA for mitochondrial oxidative metabolism, they may also be an important anaplerotic source of propionyl-CoA and succinyl-CoA (Figure 2). Cardiac BCAAs are reversibly transaminated into their respective BCKAs by mitochondrial branched-chain amino-transaminase (BCATm). Subsequently, BCAA dehydrogenase (BCKDH) converts BCKAs to either acetyl-CoA or succinyl-CoA. Protein phosphatase 2Cm (PP2Cm) activates BCKDH through dephosphorylation and mitochondrial branched-chain α-keto acid dehydrogenase kinase (BCKDK) inactivates it via phosphorylation.
Decreased BCAA oxidation occurs in HF, usually resulting in the accumulation of BCAAs and BCKAs.93–95 Elevated levels of cardiac BCAAs have been noted in metabolomic studies of CAL- and TAC-treated murine hearts. In conducting metabolomic profiling of both CAL- and TAC-treated murine hearts, previous groups observed accumulation of cardiac BCAAs, suggesting impairments in BCAA oxidation.94,95 In agreement with these findings, a transcriptomic and metabolomic analysis by Sun et al.93 identified a downregulation of genes responsible for BCAA catabolism and accumulation of BCKAs in TAC-treated mice. Their group validated the animal findings by observing the same gene expression findings in human myocardial samples from humans with dilated cardiomyopathy.93
Despite evidence of defective BCAA catabolism in HF, Murashige et al.96 proposed that BCAA oxidation is unchanged in failing human myocardium and enhanced in a CAL-treated murine model of HF. However, this study did not actually directly measure BCAA oxidation, but rather assessed enrichment of 13C-labelled TCA cycle intermediates originating from 13C-BCAAs. These levels of enrichment are primarily dependent on the levels of the TCA cycle intermediates, as opposed to the actual rates of BCAA oxidation. Therefore, differences in anaplerosis or cataplerosis in HF could provide an alternative explanation for these results. Most studies continue to show suppressed BCAA oxidation in HF.97 Yang et al.97 subjected 12-week-old mice to TAC and provided them with either a BCAA-containing diet or a BCAA-free diet for 4 days. After 1–2 weeks of TAC, they measured histone propionylation by assessing the enrichment of H3K23Pr through chromatin immunoprecipitation and ChIP-Seq. This study found that diminished BCAA oxidation in TAC-subjected mice may lead to lower propionyl-CoA production, indicating that diminished propionyl-CoA anaplerosis could result from decreased BCAA oxidation. Moreover, diminished BCAA oxidation slowed the progression of cardiac hypertrophy. However, the impact of removing dietary BCAAs (potentially decreasing anaplerosis) on cardiac metabolism and energy status was not measured. Overall, pre-clinical studies suggest impaired BCAA oxidation which may lead to decreased anaplerosis. However, direct evidence for this effect has not been demonstrated.
Findings in human HF studies align with those from animal HF models, both demonstrating impaired BCAA metabolism and potential reduction of BCAA anaplerosis.34,36,98,99 Diakos et al.99 also showed diminished levels of BCAAs in failing hearts compared to non-failing hearts in humans. A down regulation of genes associated with BCAA metabolism and an accumulation of BCKAs has been shown in myocardial samples from humans with DCM.93 Uddin et al.98 used NMR spectroscopy to validate this finding, showing a significant elevation of BCAAs concomitant with a downregulation of BCAA catabolic enzymes in LV tissue of patients with DCM. Flam et al.36 have also shown that BCAAs and BCKAs levels are elevated in failing human cardiac tissue along with decreased gene and protein expression of BCAA catabolism enzymes. Most recently, Hahn et al.34 performed a metabolomics analysis of human myocardium samples from patients with HFpEF and HFrEF. This study identified a significant elevation of myocardial BCAAs in HFpEF myocardium and a similar but non-significant trend in HFrEF compared to non-HF controls. In addition, a lowering in in BCAA-derived catabolites were found both in HFrEF and HFpEF samples, further confirming defects in BCAA catabolism. Overall, these human studies suggest impairments of BCAA metabolism in HF. As a result, anaplerosis from BCAA metabolism could be decreased. However, this was not directly assessed in these studies.
Apart from reducing anaplerosis, decreased BCAA catabolism could also lead to the preservation of TCA cycle intermediates by reducing the flux of α-KG to glutamate (a cataplerotic process) catalyzed by BCAT2. Increased extracellular BCAAs and decreased expression of BCKDH could promote cataplerosis. However, pre-clinical studies indicate that BCAT2 expression is downregulated in the failing heart, suggesting a reduction in cataplerosis.93,98,100
In summary, the accumulation of BCAAs and/or downregulation of BCAA-metabolizing enzymes is a common finding in HF. Impaired BCAA oxidation may result in lowered anaplerosis or increased cataplerosis, disrupting the balance of intermediates within the TCA cycle. As such, the interplay between anaplerosis and cataplerosis of BCAAs in the TCA cycle should be further studied using dual stable isotope labelled BCAAs and measurement of CO2 production.101 The data illustrating impairments of BCAA oxidation in HF are convincing and suggest a limitation in propionyl-CoA and succinyl-CoA anaplerosis, which are critical for maintaining the function of the TCA cycle. Given the relatively small contribution of BCAAs to ATP production in healthy and failing hearts, an anaplerotic deficiency due to BCAA oxidation defects may not significantly impact overall myocardial energy metabolism.102–105 While BCAAs are not a major source of ATP, their role in anaplerosis and cataplerosis could still have important implications for metabolic flexibility and efficiency in the failing heart. Enhancing BCAA oxidation might alleviate anaplerotic stress, as has been observed in other metabolic diseases.106–108 However, further studies are needed to determine whether restoring BCAA metabolism can significantly improve cardiac function or mitigate progression of HF.
11. Targeting BCAA anaplerosis in HF
Several groups have studied the therapeutic effects of BCAAs in HF through dietary and pharmacological interventions. Tanada et al. fed Dahl salt-sensitive rats a high-salt diet and supplemented their drinking water with BCAAs starting at 11 weeks of age, a point when cardiac hypertrophy was present but cardiac function remained preserved. In rats with HF and cardiac cachexia, the addition of BCAAs in drinking water preserved cardiac metabolism and function and prolonged survival.109 More recently, a study demonstrated promising cardioprotective effects of essential amino acids in HFrEF.100 Ragni et al. subjected adult wild-type and PP2Cm knockout mice to TAC for 4 weeks and provided a saturated fatty acid-essential amino acid (SFA-EAA) diet either before or after TAC surgery. Providing a SFA-EAA diet before TAC surgery preserved cardiac function and prevented cardiac enlargement and fibrosis. Similarly, providing a SFA-EAA diet after TAC surgery prevented the progression of HF. LC-MS metabolomics showed that the SFA-EAA diet lowered the accumulation of BCAA catabolites. Gene expression data showed that SFA-EAA reversed the decreased expression of BCAA oxidation genes and decreased protein expression of PP2Cm. The cardioprotective effects of stimulating BCAA catabolism was confirmed, as the SFA-EAA diet did not provide cardioprotection in PP2Cm-null mice, a model characterized by impaired BCAA oxidation.
Despite the promising results of dietary BCAAs, it is not entirely clear if the strategy is exclusively beneficial. Clinical and basic science studies suggest that increasing serum BCAAs and an accumulation of their metabolites (BCKAs) may exacerbate HF.98,110–117 One explanation for this negative effect is decreased insulin-stimulated glucose oxidation in the heart in response to an accumulation of BCKAs.98 Considering that HF is associated with BCAA oxidation defects, dietary supplementation with BCAAs may lead to further accumulation of BCAA metabolites and potentially intensify HF severity.
Precise pharmacological modulation of BCAA oxidation may be an alternative therapeutic strategy. Stimulating BCATm, may reduce BCAA levels and reduce mammalian target of rapamycin (mTOR) signalling and cardiac hypertrophy.98,118 However, this stimulation would also lead to elevated levels of the BCKAs, reducing insulin-mediated glucose oxidation which may aggravate the maladaptive glycolysis-glucose oxidation mismatch and increased pyruvate anaplerosis seen in HF.98,117,118 Conversely, inhibition of BCATm may improve insulin-mediated glucose oxidation in HF and reduce pyruvate anaplerosis, while decreasing the loss of α-KG. However, the secondary accumulation of BCAAs would likely increase cardiac hypertrophy via activation of the mTOR signalling pathway.98 Modifying BCATm is therefore a nuanced therapeutic strategy for HF. It is plausible that modulating BCATm will alter anaplerosis in the failing heart, but this remains to be investigated.
BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid) is an inhibitor of BCKDK and has demonstrated positive effects in animal models of HF.93,96,98,119 BT2 has also been shown to increase gene expression of enzymes responsible for glucose and FA oxidation which would provide the failing heart with additional fuel.6,93,98 Sun et al.93 tested the impact of BT2 on wild-type and PP2Cm knockout mice subjected to TAC for 8 weeks. Treatment with BT2 increased BCKDK activity, decreased plasma BCKA levels, and preserved cardiac function. In a proof-of-concept study, Chen et al.119 demonstrated the therapeutic effectiveness of BT2 in 6- to 8-week-old mice subjected to TAC for 2 weeks. Administration of BT2 for 6 weeks after TAC decreased cardiac levels of BCAAs and BCKAs and prevented deterioration of systolic function. Uddin et al.98 subjected 10-week-old mice to TAC and administered BT2 starting 1-week post-surgery for a duration of 3 weeks. The production of 14CO2 released by metabolism of U-14C valine/leucine/isoleucine was measured to quantify BCAA oxidation. Isolated working hearts from TAC-subjected mice showed increased BCAA oxidation, decreased accumulation of BCAAs in hearts in response to BT2 and improved systolic function. Voronova et al.120 validated the findings of these animal studies in silico by studying a quantitative systemics pharmacology model simulating BCAA metabolism. This study showed that BT2 decreased BCAA levels in cardiac tissues and improved heart function in HF models, leading to a 12% improvement in left ventricular ejection fraction.
Murashige et al.96 recently conducted a metabolomics analysis after infusing U-13C-labelled BCAAs into BT2-treated CAL/TAC-treated mice. In response to BT2 treatment, TAC-treated mice were protected from HF, with increased vascular relaxation and decreased blood pressure.96 Cardiac-specific, skeletal muscle-specific and whole body-knockouts of BCKDK prevented the cardioprotective effects typically induced by BT2.96 However, BCAA oxidation measurements by Murashige et al. were indirect and only illustrated the enrichment of TCA cycle intermediates as opposed to BCAA oxidation rates. While this is informative, TCA cycle flux must be measured to truly assess BCAA oxidation.
PP2Cm is a protein that can stimulate BCAA oxidation, and thereby potentially stimulate anaplerosis, through the dephosphorylation and activation of BCKDH.118 Deficiency of PP2Cm was shown to render TAC-treated mice more susceptible to contractile dysfunction.93 Stimulating PP2Cm is a potential therapeutic approach for HF, though it remains relatively unexplored.
In summary, modifying BCAA metabolism may represent a viable potential therapeutic strategy for HF. Pharmacological interventions targeting BCATm and PP2Cm may be more precise in targeting BCAA anaplerosis compared to dietary interventions.101 Enhancing BCAA oxidation in the heart is a strategy that could potentially increase anaplerosis or cataplerosis and warrants further study. Although this strategy may theoretically support anaplerosis, excessive accumulation of metabolites such as BCKAs poses a risk of exacerbating metabolic dysfunction, particularly in glucose oxidation. Further research is required to clarify whether increasing BCAA oxidation can enhance anaplerosis or cataplerosis without promoting maladaptive metabolic consequences, such as cardiac hypertrophy through mTOR signalling. This underscores the importance of a more refined understanding of BCAA metabolism in HF, particularly through direct measurements of TCA cycle flux and BCAA oxidation rates, to optimize therapeutic strategies
12. OCFA anaplerosis in HF
Altered FA metabolism is a characteristic of HF but may be increased or decreased depending on the specific type of HF. FAs serve as the primary source of energy for cardiomyocytes and they can be categorized into three main types based on their length: short-chain fatty acids (SCFAs, <6-carbons), medium-chain fatty acids (6–12 carbons) and long-chain fatty acids (LCFAs, >12 carbons). FAs can also be further classified based on whether they contain an odd or even number of carbon atoms. OCFAs, such as propionate (C3), heptadecanoate (C17), and pentadecanoate (C15), are important sources for anaplerosis. Medium-length OCFAs (C17 and C15) are metabolized into acetyl-CoA and propionyl-CoA in the final spiral of β-oxidation. Therefore, medium and long OCFAs are also a source of gluconeogenic OAA. Conversely, short OCFAs (propionate) are direct precursors of propionyl-CoA, an anaplerotic source of succinyl-CoA.
Evidence from animal and human studies demonstrate increased short chain fatty acid (SCFA) metabolism in HF.92,105,121–123 Lewandowski et al.122 subjected pig hearts to 5 h of CAL, after which the in vivo hearts were perfused with [2-¹³C] butyrate and [2-¹³C] glucose. NMR spectroscopy data indicated that SCFAs contributed more significantly to acetyl-CoA production, suggesting increased and preferential oxidation of SCFAs in pig hearts subjected to CAL. Carley et al.121 subjected rat hearts to 14 weeks of TAC, after which hearts were isolated and perfused with either 4-13C-labelled ketone (D3-hydroxybutyrate) or 4-13C-labelled butyrate in the presence of glucose and palmitate. NMR spectroscopy, along with mRNA and protein expression data, indicated that failing hearts preferentially use ketones and exhibit increased butyrate oxidation. He et al.92 perfused isolated rat hearts using the Langendorff model, followed by exposure to varying durations of stop-flow global ischaemia (0, 5, 15, and 30 min). The metabolic profiles were analysed using LC-MS to understand the relationship between ischaemia and metabolism. Cardiac ischaemia led to a build-up of propionyl-CoA and a decrease in methylmalonyl-CoA levels, suggesting decreased propionate anaplerosis.
Murashige et al.105 used metabolomics to quantify nutrient uptake and release in failing and non-failing human hearts by measuring the arteriovenous gradient of 277 metabolites in 110 patients. This study suggested that SCFAs, particularly acetate, are actively taken up by the heart in both failing and non-failing conditions.105 The uptake of SCFAs was found to be directly proportional to their circulating levels, suggesting that substrate availability drives consumption without requiring regulation. However, a recent study by Kirschner et al.123 found that patients with HF had significantly lower plasma concentrations of key SCFAs, including propionate, butyrate, and isovalerate, compared to healthy controls. decreased SCFA levels were associated with poorer muscle quality, higher emotional distress, and impaired cognitive function in the HF group.
In summary, discrepancies exist between animal models of HF and human HF studies regarding OCFA metabolism. Overall, most studies suggest increased SCFA utilization. However, findings could be related to the decreased microbial diversity seen in HF patients, leading to decreased propionate production through fermentation and lower absorption.124 Lower levels of propionate utilization may contribute to decreased energy metabolism in HF by reducing succinyl-CoA replenishment. Furthermore, the reliance on SCFA metabolism in failing hearts may reflect an adaptive mechanism to maintain ATP production under metabolic stress. However, whether this shift compensates for impaired anaplerotic pathways or exacerbates metabolic dysfunction remains to be clarified. Propionate anaplerosis and its role in HF remain poorly understood, and additional research is needed to clarify its contribution to TCA cycle replenishment and overall cardiac metabolism.
13. Targeting OCFA anaplerosis in HF
Propionate has been investigated as a potential HF therapeutic in pre-clinical studies. In rat hearts perfused with acetoacetate, cardiac function was rescued by adding propionate and lactate.14 Dietary supplementation with propionate has cardioprotective effects in animal models of HF.125–129 Bartolomaeus et al.126 explored the impact of propionate on cardiovascular damage induced by hypertension in mouse models. This study involved administering propionate to wild-type and ApoE-knockout mice, with atherosclerosis and hypertension induced by infusion with Ang-II. Propionate significantly decreased cardiac hypertrophy, fibrosis, vascular dysfunction, and atherosclerosis. Additionally, propionate was found to moderately decrease blood pressure and lower the risk of arrhythmia. Tang et al.128 explored the influence of gut microbiota and SCFAs, including propionate, on cardiac healing after CAL-induced myocardial infarction in mice. By depleting gut microbiota with antibiotics, they observed worsened cardiac repair, increased mortality, and decreased immune cell response. Supplementation with SCFAs, including propionate, partially reversed these effects by enhancing survival and promoting immune cell infiltration in the heart. Kaye et al.127 used wild-type mice, germ-free mice, and SCFA receptor knockout strains to study the effects of a low-fibre diet and SCFA supplementation on hypertension and cardiac remodelling. Mice were fed either a low-fibre or high-fibre diet while hypertension was induced using Ang-II. Propionate supplementation in mice fed a low-fibre diet significantly decreased blood pressure, cardiac hypertrophy, and fibrosis, even in the presence of a hypertensive stimulus. Zhou et al. investigated the effects of propionate on ventricular arrhythmias and cardiac electrophysiological remodelling using a rat CAL model. Male Sprague-Dawley rats were divided into four groups: a sham control group, a sham group treated with propionate, a CAL control group, and a CAL group treated with propionate. Propionate was administered in drinking water for 7 days before myocardial infarction induction. The study revealed that propionate significantly enhanced heart rate variability, decreased ventricular arrhythmias, and improved cardiac electrical stability in CAL-treated rats, primarily through promoting parasympathetic activity via the gut-brain axis. Deng et al.125 employed a myocardial ischaemia-reperfusion (I/R) injury model in male mice, inducing ischaemia via CAL followed by reperfusion. Ang-II was administered during reperfusion to worsen cardiac injury, allowing the investigation of propionate’s protective effects under these conditions. Mice were pre-treated with sodium propionate in their drinking water for 15 days prior to I/R induction. The study demonstrated that propionate decreased myocardial infarct size, lowered oxidative stress, and enhanced cardiac function, even with Ang-II present. Overall, studies in animal models of HF indicate that propionate supplementation could serve as a nonpharmacological approach for managing HF. However, it remains unclear whether enhanced propionate anaplerosis directly contributes to its cardioprotective effects.
Since administration of propionate in humans is hindered by its toxicity, researchers have employed propionyl-L-carnitine (PLC) as an alternative to increasing propionyl-CoA levels and enhancing anaplerosis.130,131 Early studies highlighted the therapeutic potential of PLC in acetoacetate perfused rat hearts.15 In pre-clinical HF studies, PLC has shown cardioprotective effects such as improving exercise tolerance and cardiac function.132–134 PLC has also been tested in HF clinical trials, showing improved exercise tolerance and cardiac function.135–138 Zamani et al. are currently recruiting HFpEF patients to study the effects of PLC-treated perfusate on exercise tolerance and oxidative phosphorylation in skeletal muscle (NCT04913805).
Triheptanoin (Dojolvi®) is a source of medium (C7) OCFAs and is an approved therapeutic for human FA oxidation disorders.139 Triheptanoin is catabolized into glycerol and three heptanoate molecules, the latter of which is a source of acetyl-CoA and anaplerotic molecules including propionyl-CoA and C5-ketone bodies (β-ketopentanoate and β-hydroxypentanoate).140 Triheptanoin enhances anaplerosis by providing propionyl-CoA for PCC-catalyzed carboxylation into succinyl-CoA. The therapeutic efficacy of triheptanoin has been demonstrated in animal and human studies.141–145 Studies of patients with long chain fatty acid oxidation disorders and HF have demonstrated that treatment with triheptanoin may be more effective compared to supplementation with even-chain FAs.146,147 Clinical studies demonstrate that plasma levels of long (C15 and C17) OCFAs are inversely associated with cardiovascular disease.148 Humans with low levels of pentadecanoic acid (C15) are more prone to developing cardiovascular disease including HF.149–153
C5 ketones are exported from the liver as a byproduct of OCFA metabolism.154 They are metabolized into acetyl-CoA and propionyl-CoA by cardiomyocytes, thereby making them an important anaplerotic substrate.155 In both animals and humans, HF, especially in its advanced stages, is often associated with elevated levels of plasma ketones, concurrent with increased ketone oxidation. However, the precise role of C5 ketones in HF is unknown and understudied. The effectiveness of triheptanoin may be in part due to its effect of providing anaplerotic C5 ketones.156
In summary, studies suggest that altered OCFA metabolism is a potential therapeutic target for HF. Considering their distinct role in cardiac metabolism, the utilization and oxidation of OCFAs and C5 ketones should be further studied in HF. Quantifying the metabolism of C5 ketones in earlier stages of HF and identifying their potential anaplerotic role in end-stage HF are important research goals. While studies highlight the cardioprotective effects of propionate supplementation in animal models, the precise mechanisms, whether through direct anaplerosis or indirect metabolic modulation, remain unclear. In particular, the contribution of propionyl-CoA to succinyl-CoA formation via OCFA metabolism requires further exploration to determine its full therapeutic potential in HF. PLC and triheptanoin hold therapeutic potential for HF patients, but their effectiveness across different HF types and stages remains to be fully elucidated.
14. Adenylosuccinate
The anaplerosis of adenylosuccinate is a part of the purine nucleotide cycle, which functions to maintain a balance of adenine nucleotides in the heart. A deficiency of ATP in the failing heart leads to an imbalance of flux through the purine nucleotide synthesis pathways (de novo synthesis, degradation, and salvage). Increased purine nucleotide degradation is a compensatory response to increased ATP demand or chronic ischaemia in HF.157 This degradation depletes cardiac adenine nucleotides which is associated with mechanical dysfunction of the heart.158–161 Lopez et al.159 subjected male rats to TAC-induced pressure overload, resulting in cardiac hypertrophy and decompensated HF. This study found that elevated adenosine monophosphate deaminase activity and depletion of adenine nucleotides were associated with mechanical dysfunction. The increase in purine nucleotide degradation observed in HF could also lead to reductions of adenylosuccinate anaplerosis, further contributing to energy deficiency. However, the activity of adenylosuccinate lyase and anaplerosis of adenylosuccinate were not measured. Pre-clinical investigation of this pathway could identify novel therapeutic targets. For example, adenylosuccinic acid, an orphan drug previously investigated in Duchenne muscular dystrophy, may have potential to improve adenylosuccinate anaplerosis in HF.162
15. Conclusions
Alterations in cardiac energy metabolism are a hallmark of HF with specific defects identified in TCA cycle intermediates and anaplerosis. However, the relationship between HF and cardiac anaplerosis remains complex and poorly understood. While the molecular pathways governing anaplerosis are known, what remains elusive is a functional understanding of how these metabolic shifts contribute to the overall energy deficit in HF. Collectively these studies show that there is a complex interplay between metabolic function and HF. Despite this, multiple lines of evidence suggest that metabolism can contribute to the progression of HF or potentially its rescue. Understanding these complex metabolic-physiology dynamics will require a comprehensive understanding of metabolism in the context of cardiac function.
Furthermore, the heterogeneity of HF pathophysiology underscores the need for tailored metabolic interventions. For example, understanding how defects in anaplerosis differ between HFpEF and HFrEF could inform more precise therapeutic strategies. Similarly, the role of specific metabolic substrates, like OCFAs or BCAA-derived metabolites, must be further explored to determine whether they offer cardioprotective benefits or contribute to maladaptive remodelling.
To achieve meaningful progress, additional pre-clinical, translational, and clinical studies must integrate accurate measurements of cardiac TCA cycle flux, intermediates, and metabolic outcomes in the context of myocardial function. Only through this integrative approach will we be able to identify novel, metabolism-based therapeutic targets that are both effective and specific to the underlying cause and stage of HF.
Supplementary Material
Acknowledgements
S.C.G. acknowledges funding from the Heart and Stroke Foundation. I.A.L. and G.D.L. acknowledge funding from the Canadian Institutes of Health Research.
Contributor Information
Karm A Alhasan, Department of Cardiac Sciences and Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1; Department of Pediatrics and Alberta Children’s Hospital Research Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 1N4; Department of Biochemistry & Molecular Biology, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1.
Melissa A King, Department of Cardiac Sciences and Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1; Alberta Centre for Advanced Diagnostics, Department of Biological Sciences, University of Calgary, Calgary, AB, Canada T2N 1N4.
Badal S B Pattar, Department of Cardiac Sciences and Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1.
Ian A Lewis, Alberta Centre for Advanced Diagnostics, Department of Biological Sciences, University of Calgary, Calgary, AB, Canada T2N 1N4.
Gary D Lopaschuk, Cardiovascular Research Centre, University of Alberta, Edmonton, AB, Canada T6G 2S2.
Steven C Greenway, Department of Cardiac Sciences and Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1; Department of Pediatrics and Alberta Children’s Hospital Research Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 1N4; Department of Biochemistry & Molecular Biology, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada T2N 4N1.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Author contributions
K.A.A., M.A.K., and B.S.B.P. wrote the manuscript. S.C.G. and G.D.L. reviewed the draft manuscript. All authors have approved the final version of the manuscript.
References
- 1. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol 2012;21:365–371. [DOI] [PubMed] [Google Scholar]
- 2. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Michael Felker G, Filippatos G, Fiuzat M, Fonarow GC, Gomez-Mesa J, Heidenreich P, Imamura T, Jankowska EA, Januzzi J, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, Seferović P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–380. [DOI] [PubMed] [Google Scholar]
- 3. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272–3287. [DOI] [PubMed] [Google Scholar]
- 4. Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 2013;113:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fragasso G. Deranged cardiac metabolism and the pathogenesis of heart failure. Card Fail Rev 2016;2:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res 2021;128:1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng SM, Neubauer S, Rider OJ. Myocardial metabolism in heart failure. Curr Heart Fail Rep 2023;20:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, Bi X. Integrated control of fatty acid metabolism in heart failure. Metabolites 2023;13:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kornberg HL. Anaplerotic sequences and their role in metabolism. Essays Biochem 1966;2:1–31. [Google Scholar]
- 10. Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res 2011;90:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson JR, Krebs HA. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J 1961;80:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmerman AN, Meijler FL, Hulsmann WC. The inhibitory effect of acetoacetate on myocardial contraction. Lancet 1962;2:757–758. [DOI] [PubMed] [Google Scholar]
- 13. Russell RR, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol Heart Circ Physiol 1991;261:H1756–H1762. [DOI] [PubMed] [Google Scholar]
- 14. Russell RR, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest 1991;87:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell RR, Mommessin JI, Taegtmeyer H. Propionyl-L-carnitine-mediated improvement in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol 1995;268:H441–H447. [DOI] [PubMed] [Google Scholar]
- 16. Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol 2000;279:H2390–H2398. [DOI] [PubMed] [Google Scholar]
- 17. Panchal AR, Comte B, Huang H, Dudar B, Roth B, Chandler M, Des Rosiers C, Brunengraber H, Stanley WC. Acute hibernation decreases myocardial pyruvate carboxylation and citrate release. Am J Physiol Heart Circ Physiol 2001;281:H1613–H1620. [DOI] [PubMed] [Google Scholar]
- 18. Olson AK, Hyyti OM, Cohen GA, Ning X-H, Sadilek M, Isern N, Portman MA. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol 2008;295:H2315–H2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russell RR III, Taegtmeyer H. Coenzyme A sequestration in rat hearts oxidizing ketone bodies. J Clin Invest 1992;89:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inigo M, Deja S, Burgess SC. Ins and outs of the TCA cycle: the central role of anaplerosis. Annu Rev Nutr 2021;41:19–47. [DOI] [PubMed] [Google Scholar]
- 21. Wang ZV, Hill JA. Protein quality control and metabolism: bidirectional control in the heart. Cell Metab 2015;21:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 2007;115:2033–2041. [DOI] [PubMed] [Google Scholar]
- 23. Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis 2006;29:327–331. [DOI] [PubMed] [Google Scholar]
- 24. Sundqvist KE, Hiltunen JK, Hassinen IE. Pyruvate carboxylation in the rat heart. Role of biotin-dependent enzymes. Biochem J 1989;257:913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peuhkurinen KJ, Nuutinen EM, Pietiläinen EP, Hiltunen JK, Hassinen IE. Role of pyruvate carboxylation in the energy-linked regulation of pool sizes of tricarboxylic acid-cycle intermediates in the myocardium. Biochem J 1982;208:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garland PB, Randle PJ. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J 1964;93:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiltunen JK, Hassinen IE. Energy-linked regulation of glucose and pyruvate oxidation in isolated perfused rat heart. Role of pyruvate dehydrogenase. Biochim Biophys Acta 1976;440:377–390. [DOI] [PubMed] [Google Scholar]
- 28. Bowtell JL, Bruce M. Glutamine: an anaplerotic precursor. Nutrition 2002;18:222–224. [DOI] [PubMed] [Google Scholar]
- 29. Yoshikawa S, Nagao M, Toh R, Shinohara M, Iino T, Irino Y, Nishimori M, Tanaka H, Satomi-Kobayashi S, Ishida T, Hirata K-I. Inhibition of glutaminase 1-mediated glutaminolysis improves pathological cardiac remodeling. Am J Physiol Heart Circ Physiol 2022;322:H749–H761. [DOI] [PubMed] [Google Scholar]
- 30. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takada S, Maekawa S, Furihata T, Kakutani N, Setoyama D, Ueda K, Nambu H, Hagiwara H, Handa H, Fumoto Y, Hata S, Masunaga T, Fukushima A, Yokota T, Kang D, Kinugawa S, Sabe H. Succinyl-CoA-based energy metabolism dysfunction in chronic heart failure. Proc Natl Acad Sci U S A 2022;119:e2203628119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lowenstein J. The purine nucleotide cycle revised. Int J Sports Med 1990;11:S37–S46. [DOI] [PubMed] [Google Scholar]
- 33. Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab 2004;286:E64–E76. [DOI] [PubMed] [Google Scholar]
- 34. Hahn VS, Petucci C, Kim M-S, Bedi KC, Wang H, Mishra S, Koleini N, Yoo EJ, Margulies KB, Arany Z, Kelly DP, Kass DA, Sharma K. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 2023;147:1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shibayama J, Yuzyuk TN, Cox J, Makaju A, Miller M, Lichter J, Li H, Leavy JD, Franklin S, Zaitsev AV. Metabolic remodeling in moderate synchronous versus dyssynchronous pacing-induced heart failure: integrated metabolomics and proteomics study. PLoS One 2015;10:e0118974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flam E, Jang C, Murashige D, Yang Y, Morley MP, Jung S, Kantner DS, Pepper H, Bedi KC, Brandimarto J, Prosser BL, Cappola T, Snyder NW, Rabinowitz JD, Margulies KB, Arany Z. Integrated landscape of cardiac metabolism in end-stage human nonischemic dilated cardiomyopathy. Nat Cardiovasc Res 2022;1:817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turer A, Altamirano F, Schiattarella GG, May H, Gillette TG, Malloy CR, Merritt ME. Remodeling of substrate consumption in the murine sTAC model of heart failure. J Mol Cell Cardiol 2019;134:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peuhkurinen KJ, Takala TE, Nuutinen EM, Hassinen IE. Tricarboxylic acid cycle metabolites during ischemia in isolated perfused rat heart. Am J Physiol Heart Circ Physiol 1983;244:H281–H288. [DOI] [PubMed] [Google Scholar]
- 39. Gudbjarnason S, Telerman M, Bing RJ. Protein metabolism in cardiac hypertrophy and heart failure. Am J Physiol Legacy Content 1964;206:294–298. [DOI] [PubMed] [Google Scholar]
- 40. Mongirdienė A, Skrodenis L, Varoneckaitė L, Mierkytė G, Gerulis J. Reactive oxygen Species induced pathways in heart failure pathogenesis and potential therapeutic strategies. Biomedicines 2022;10:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moris D, Spartalis M, Spartalis E, Karachaliou G-S, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med 2017;5:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 43. Tham YK, Bernardo BC, Ooi JYY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 2015;89:1401–1438. [DOI] [PubMed] [Google Scholar]
- 44. Allard MF, Schönekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 1994;267:H742–H750. [DOI] [PubMed] [Google Scholar]
- 45. Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol 1970;218:153–159. [DOI] [PubMed] [Google Scholar]
- 46. Kagaya Y, Kanno Y, Takeyama D, Ishide N, Maruyama Y, Takahashi T, Ido T, Takishima T. Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation 1990;81:1353–1361. [DOI] [PubMed] [Google Scholar]
- 47. Nuutila P, Mäki M, Laine H, Knuuti MJ, Ruotsalainen U, Luotolahti M, Haaparanta M, Solin O, Jula A, Koivisto VA. Insulin action on heart and skeletal muscle glucose uptake in essential hypertension. J Clin Invest 1995;96:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 1988;11:416–426. [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, Merkle H, Uğurbil K, From AH, Bache RJ. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation 1995;92:1274–1283. [DOI] [PubMed] [Google Scholar]
- 50. Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 2009;104:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED. Enhanced redox state and efficiency of glucose oxidation with miR based suppression of maladaptive NADPH-dependent malic enzyme 1 expression in hypertrophied hearts. Circ Res 2018;122:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Liao H, Wang Y, Zhou J, Wang F, Xie Y, Zhao K, Gao W. KLK11 promotes the activation of mTOR and protein synthesis to facilitate cardiac hypertrophy. BMC Cardiovasc Disord 2021;21:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kolwicz SC, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 2012;111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Choi YS, de Mattos ABM, Shao D, Li T, Nabben M, Kim M, Wang W, Tian R, Kolwicz SC. Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. J Mol Cell Cardiol 2016;100:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation 2011;123:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bøgh N, Hansen ESS, Omann C, Lindhardt J, Nielsen PM, Stephenson RS, Laustsen C, Hjortdal VE, Agger P. Increasing carbohydrate oxidation improves contractile reserves and prevents hypertrophy in porcine right heart failure. Sci Rep 2020;10:8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zangari J, Petrelli F, Maillot B, Martinou J-C. The multifaceted pyruvate metabolism: role of the mitochondrial pyruvate carrier. Biomolecules 2020;10:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCommis KS, Kovacs A, Weinheimer CJ, Shew TM, Koves TR, Ilkayeva OR, Kamm DR, Pyles KD, King MT, Veech RL, DeBosch BJ, Muoio DM, Gross RW, Finck BN. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat Metab 2020;2:1232–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Krokidi AT, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab 2021;33:629–648.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sheeran FL, Angerosa J, Liaw NY, Cheung MM, Pepe S. Adaptations in protein expression and regulated activity of pyruvate dehydrogenase multienzyme complex in human systolic heart failure. Oxid Med Cell Longev 2019;2019:4532592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lopez-Vazquez P, Fernandez-Caggiano M, Barge-Caballero E, Barge-Caballero G, Couto-Mallon D, Grille-Cancela Z, Blanco-Canosa P, Paniagua-Martin MJ, Enriquez-Vazquez D, Vazquez-Rodriguez JM, Domenech N, Crespo-Leiro MG. Reduced mitochondrial pyruvate carrier expression in hearts with heart failure and reduced ejection fraction patients: ischemic vs. Non-ischemic origin. Front Cardiovasc Med 2024;11:1349417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hermann HP, Pieske B, Schwarzmüller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 1999;353:1321–1323. [DOI] [PubMed] [Google Scholar]
- 63. Hasenfuss G, Maier LS, Hermann H-P, Lüers C, Hünlich M, Zeitz O, Janssen PML, Pieske B. Influence of pyruvate on contractile performance and Ca(2+) cycling in isolated failing human myocardium. Circulation 2002;105:194–199. [DOI] [PubMed] [Google Scholar]
- 64. Hermann H-P, Arp J, Pieske B, Kögler H, Baron S, Janssen PML, Hasenfuss G. Improved systolic and diastolic myocardial function with intracoronary pyruvate in patients with congestive heart failure. Eur J Heart Fail 2004;6:213–218. [DOI] [PubMed] [Google Scholar]
- 65. Mallet RT, Sun J. Antioxidant properties of myocardial fuels. Mol Cell Biochem 2003;253:103–111. [DOI] [PubMed] [Google Scholar]
- 66. Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med (Maywood) 2005;230:435–443. [DOI] [PubMed] [Google Scholar]
- 67. Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of beta-adrenergic inotropism in stunned myocardium. J Mol Cell Cardiol 1999;31:1863–1872. [DOI] [PubMed] [Google Scholar]
- 68. Mallet RT, Squires JE, Bhatia S, Sun J. Pyruvate restores contractile function and antioxidant defenses of hydrogen peroxide-challenged myocardium. J Mol Cell Cardiol 2002;34:1173–1184. [DOI] [PubMed] [Google Scholar]
- 69. Mallet RT, Olivencia-Yurvati AH, Bünger R. Pyruvate enhancement of cardiac performance: cellular mechanisms and clinical application. Exp Biol Med (Maywood) 2018;243:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Watanabe K, Nagao M, Toh R, Irino Y, Shinohara M, Iino T, Yoshikawa S, Tanaka H, Satomi-Kobayashi S, Ishida T, Hirata K-I. Critical role of glutamine metabolism in cardiomyocytes under oxidative stress. Biochem Biophys Res Commun 2021;534:687–693. [DOI] [PubMed] [Google Scholar]
- 71. Badole SL, Jangam GB, Chaudhari SM, Ghule AE, Zanwar AA. L-glutamine supplementation prevents the development of experimental diabetic cardiomyopathy in streptozotocin-nicotinamide induced diabetic rats. PLoS One 2014;9:e92697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang H, Cui Y-C, Li K, Yang B-Q, Liu X-P, Zhang D, Li H, Wu A-L, Tang Y. Glutamine protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions through inhibition of the transforming growth factor-β1-Smad3 pathway. Arch Biochem Biophys 2016;596:43–50. [DOI] [PubMed] [Google Scholar]
- 73. Fricovsky ES, Suarez J, Ihm S-H, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 2012;303:R689–R699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y. The essential role of glutamine metabolism in diabetic cardiomyopathy: a review. Medicine (Baltimore) 2023;102:e36299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard M-E, Labarthe F, Dolinsky VW, Dyck JRB, Allen BG, Chatham JC, Des Rosiers C. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 2013;55:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Comte B, Vincent G, Bouchard B, Benderdour M, Des Rosiers C. Reverse flux through cardiac NADP(+)-isocitrate dehydrogenase under normoxia and ischemia. Am J Physiol Heart Circ Physiol 2002;283:H1505–H1514. [DOI] [PubMed] [Google Scholar]
- 77. An D, Zeng Q, Zhang P, Ma Z, Zhang H, Liu Z, Li J, Ren H, Xu D. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol 2021;46:102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li X, Wu F, Günther S, Looso M, Kuenne C, Zhang T, Wiesnet M, Klatt S, Zukunft S, Fleming I, Poschet G, Wietelmann A, Atzberger A, Potente M, Yuan X, Braun T. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023;622:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Machiraju P, Degtiarev V, Patel D, Hazari H, Lowry RB, Bedard T, Sinasac D, Brundler M-A, Greenway SC, Khan A. Phenotype and pathology of the dilated cardiomyopathy with ataxia syndrome in children. J Inherit Metab Dis 2022;45:366–376. [DOI] [PubMed] [Google Scholar]
- 80. Sparkes R, Patton D, Bernier F. Cardiac features of a novel autosomal recessive dilated cardiomyopathic syndrome due to defective importation of mitochondrial protein. Cardiol Young 2007;17:215–217. [DOI] [PubMed] [Google Scholar]
- 81. Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive barth syndrome-like condition. J Med Genet 2006;43:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Janz A, Walz K, Cirnu A, Surjanto J, Urlaub D, Leskien M, Kohlhaas M, Nickel A, Brand T, Nose N, Wörsdörfer P, Wagner N, Higuchi T, Maack C, Dudek J, Lorenz K, Klopocki E, Ergün S, Duff HJ, Gerull B. Mutations in DNAJC19 cause altered mitochondrial structure and increased mitochondrial respiration in human iPSC-derived cardiomyocytes. Mol Metab 2024;79:101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Richter-Dennerlein R, Korwitz A, Haag M, Tatsuta T, Dargazanli S, Baker M, Decker T, Lamkemeyer T, Rugarli EI, Langer T. DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate cardiolipin remodeling. Cell Metab 2014;20:158–171. [DOI] [PubMed] [Google Scholar]
- 84. Machiraju P, Wang X, Sabouny R, Huang J, Zhao T, Iqbal F, King M, Prasher D, Lodha A, Jimenez-Tellez N, Ravandi A, Argiropoulos B, Sinasac D, Khan A, Shutt TE, Greenway SC. SS-31 peptide reverses the mitochondrial fragmentation present in fibroblasts from patients with DCMA, a mitochondrial cardiomyopathy. Front Cardiovasc Med 2019;6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rohani L, Machiraju P, Sabouny R, Meng G, Liu S, Zhao T, Iqbal F, Wang X, Ravandi A, Wu JC, Khan A, Shutt T, Rancourt D, Greenway SC. Reversible mitochondrial fragmentation in iPSC-derived cardiomyocytes from children with DCMA, a mitochondrial cardiomyopathy. Can J Cardiol 2020;36:554–563. [DOI] [PubMed] [Google Scholar]
- 86. Bornstein R, Mulholland MT, Sedensky M, Morgan P, Johnson SC. Glutamine metabolism in diseases associated with mitochondrial dysfunction. Mol Cell Neurosci 2023;126:103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. King MA, Heger K, Khan A, Sinasac D, Huttlin EL, Greenway SC, Lewis IA. Altered glutamine metabolism of cultured fibroblasts predicts severity of cardiac dysfunction in the dilated cardiomyopathy with ataxia syndrome (DCMA), a mitochondrial cardiomyopathy. bioRxiv 334938 10.1101/2020.10.11.334938, 11 October 2020, preprint: not peer reviewed. [DOI]
- 88. Forny P, Bonilla X, Lamparter D, Shao W, Plessl T, Frei C, Bingisser A, Goetze S, van Drogen A, Harshman K, Pedrioli PGA, Howald C, Poms M, Traversi F, Bürer C, Cherkaoui S, Morscher RJ, Simmons L, Forny M, Xenarios I, Aebersold R, Zamboni N, Rätsch G, Dermitzakis ET, Wollscheid B, Baumgartner MR, Froese DS. Integrated multi-omics reveals anaplerotic rewiring in methylmalonyl-CoA mutase deficiency. Nat Metab 2023;5:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stanescu S, Belanger-Quintana A, Fernandez-Felix BM, Ruiz-Sala P, del Valle M, Garcia F, Arrieta F, Martinez-Pardo M. Interorgan amino acid interchange in propionic acidemia: the missing key to understanding its physiopathology. Amino Acids 2022;54:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Riemersma M, Hazebroek MR, Helderman-van den Enden ATJM, Salomons GS, Ferdinandusse S, Brouwers MCGJ, van der Ploeg L, Heymans S, Glatz JFC, van den Wijngaard A, Krapels IPC, Bierau J, Brunner HG. Propionic acidemia as a cause of adult-onset dilated cardiomyopathy. Eur J Hum Genet 2017;25:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fragaki K, Cano A, Benoist J-F, Rigal O, Chaussenot A, Rouzier C, Bannwarth S, Caruba C, Chabrol B, Paquis-Flucklinger V. Fatal heart failure associated with CoQ10 and multiple OXPHOS deficiency in a child with propionic acidemia. Mitochondrion 2011;11:533–536. [DOI] [PubMed] [Google Scholar]
- 92. He W, Berthiaume JM, Previs S, Kasumov T, Zhang G-F. Ischemia promotes acyl-coas dephosphorylation and propionyl-coa accumulation. Metabolomics 2023;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn J-Y, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui W-J, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016;133:2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 2014;7:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K, Gao E, Cheng H, Tao L. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 2016;311:H1160–H1169. [DOI] [PubMed] [Google Scholar]
- 96. Murashige D, Jung JW, Neinast MD, Levin MG, Chu Q, Lambert JP, Garbincius JF, Kim B, Hoshino A, Marti-Pamies I, McDaid KS, Shewale SV, Flam E, Yang S, Roberts E, Li L, Morley MP, Bedi KC, Hyman MC, Frankel DS, Margulies KB, Assoian RK, Elrod JW, Jang C, Rabinowitz JD, Arany Z. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab 2022;34:1749–1764.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang Z, He M, Austin J, Sayed D, Abdellatif M. Reducing branched-chain amino acids improves cardiac stress response in mice by decreasing histone H3K23 propionylation. J Clin Invest 2023;133:e169399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, Karwi QG, Altamimi T, Wishart DS, Dyck JRB, Ussher JR, Oudit GY, Lopaschuk GD. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol 2019;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY, Drakos SG. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart. JACC Basic Transl Sci 2016;1:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ragni M, Greco CM, Felicetta A, Ren SV, Kunderfranco P, Ruocco C, Carullo P, Larcher V, Tedesco L, Severi I, Giordano A, Cinti S, Valerio A, Sun H, Wang Y, Gao C, Condorelli G, Nisoli E. Dietary essential amino acids for the treatment of heart failure with reduced ejection fraction. Cardiovasc Res 2023;119:982–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McGarrah RW, White PJ. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol 2023;20:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG, Rabinowitz JD, Arany Z. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab 2019;29:417–429.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab 2018;315:E1046–E1052. [DOI] [PubMed] [Google Scholar]
- 104. Walejko JM, Christopher BA, Crown SB, Zhang G-F, Pickar-Oliver A, Yoneshiro T, Foster MW, Page S, van Vliet S, Ilkayeva O, Muehlbauer MJ, Carson MW, Brozinick JT, Hammond CD, Gimeno RE, Moseley MA, Kajimura S, Gersbach CA, Newgard CB, White PJ, McGarrah RW. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat Commun 2021;12:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC, Sun Q, Yu C, Hegde M, Li J, Wynn RM, Chuang DT, Hutson S, Lee B. Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet 2011;20:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tso S-C, Qi X, Gui W-J, Chuang JL, Morlock LK, Wallace AL, Ahmed K, Laxman S, Campeau PM, Lee BH, Hutson SM, Tu BP, Williams NS, Tambar UK, Wynn RM, Chuang DT. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain α-ketoacid dehydrogenase kinase. Proc Natl Acad Sci U S A 2013;110:9728–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vanweert F, Schrauwen P, Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes 2022;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tanada Y, Shioi T, Kato T, Kawamoto A, Okuda J, Kimura T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci 2015;137:20–27. [DOI] [PubMed] [Google Scholar]
- 110. Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J Mol Cell Cardiol 1973;5:139–147. [DOI] [PubMed] [Google Scholar]
- 111. Du X, Li Y, Wang Y, You H, Hui P, Zheng Y, Du J. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci 2018;209:167–172. [DOI] [PubMed] [Google Scholar]
- 112. Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martínez-González MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB, Rexrode KM. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fan Y, Li Y, Chen Y, Zhao Y-J, Liu L-W, Li J, Wang S-L, Alolga RN, Yin Y, Wang X-M, Zhao D-S, Shen J-H, Meng F-Q, Zhou X, Xu H, He G-P, Lai M-D, Li P, Zhu W, Qi L-W. Comprehensive metabolomic characterization of coronary artery diseases. J Am Coll Cardiol 2016;68:1281–1293. [DOI] [PubMed] [Google Scholar]
- 114. Lim L-L, Lau ESH, Fung E, Lee H-M, Ma RCW, Tam CHT, Wong WKK, Ng ACW, Chow E, Luk AOY, Jenkins A, Chan JCN, Kong APS. Circulating branched-chain amino acids and incident heart failure in type 2 diabetes: the Hong Kong diabetes register. Diabetes Metab Res Rev 2020;36:e3253. [DOI] [PubMed] [Google Scholar]
- 115. Guo X, Huang C, Lian K, Wang S, Zhao H, Yan F, Zhang X, Zhang J, Xie H, An R, Tao L. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem Biophys Res Commun 2016;480:106–113. [DOI] [PubMed] [Google Scholar]
- 116. Narita K, Amiya E. Is branched-chain amino acid nutritional supplementation beneficial or detrimental in heart failure? World J Cardiol 2021;13:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Uddin GM, Karwi QG, Pherwani S, Gopal K, Wagg CS, Biswas D, Atnasious M, Wu Y, Wu G, Zhang L, Ho KL, Pulinilkunnil T, Ussher JR, Lopaschuk GD. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metabolism 2021;124:154871. [DOI] [PubMed] [Google Scholar]
- 118. Karwi QG, Lopaschuk GD. Branched-chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther 2023;37:413–420. [DOI] [PubMed] [Google Scholar]
- 119. Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, Chuang DT, Wang Y, Sun H. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc 2019;8:e011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Voronova V, Sokolov V, Morias Y, Boezelman MJ, Wågberg M, Henricsson M, Hansson K, Goltsov A, Peskov K, Sundqvist M. Evaluation of therapeutic strategies targeting BCAA catabolism using a systems pharmacology model. Front Pharmacol 2022;13:993422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Carley AN, Maurya SK, Fasano M, Wang Y, Selzman CH, Drakos SG, Lewandowski ED. Short-chain fatty acids outpace ketone oxidation in the failing heart. Circulation 2021;143:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lewandowski ED, Kudej RK, White LT, O’Donnell JM, Vatner SF. Mitochondrial preference for short chain fatty acid oxidation during coronary artery constriction. Circulation 2002;105:367–372. [DOI] [PubMed] [Google Scholar]
- 123. Kirschner SK, Deutz NEP, Rijnaarts I, Smit TJ, Larsen DJ, Engelen MPKJ. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. JPEN J Parenter Enteral Nutr 2022;46:660–670. [DOI] [PubMed] [Google Scholar]
- 124. Jin L, Shi X, Yang J, Zhao Y, Xue L, Xu L, Cai J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 2021;12:346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Deng F, Zhang L-Q, Wu H, Chen Y, Yu W-Q, Han R-H, Han Y, Zhang X-Q, Sun Q-S, Lin Z-B, Wang Y, Liu Y-P, Chen J-Y, Liu K-X, Hu J-J. Propionate alleviates myocardial ischemia-reperfusion injury aggravated by angiotensin II dependent on caveolin-1/ACE2 axis through GPR41. Int J Biol Sci 2022;18:858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt K-U, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, Horlock D, Vijay A, Giam B, Vinh A, Johnson C, Fiedler A, Donner D, Snelson M, Coughlan MT, Phillips S, Du X-J, El-Osta A, Drummond G, Lambert GW, Spector TD, Valdes AM, Mackay CR, Marques FZ. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 2020;141:1393–1403. [DOI] [PubMed] [Google Scholar]
- 128. Tang TWH, Chen H-C, Chen C-Y, Yen CYT, Lin C-J, Prajnamitra RP, Chen L-L, Ruan S-C, Lin J-H, Lin P-J, Lu H-H, Kuo C-W, Chang CM, Hall AD, Vivas EI, Shui J-W, Chen P, Hacker TA, Rey FE, Kamp TJ, Hsieh PCH. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019;139:647–659. [DOI] [PubMed] [Google Scholar]
- 129. Zhou M, Li D, Xie K, Xu L, Kong B, Wang X, Tang Y, Liu Y, Huang H. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct 2021;12:12580–12593. [DOI] [PubMed] [Google Scholar]
- 130. Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci 2004;1033:79–91. [DOI] [PubMed] [Google Scholar]
- 131. Mingorance C, Rodriguez-Rodriguez R, Justo ML, Herrera MD, de Sotomayor MA. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr Rev 2011;69:279–290. [DOI] [PubMed] [Google Scholar]
- 132. Koh SG, Brenner DA, Korzick DH, Tickerhoof MM, Apstein CS, Saupe KW. Exercise intolerance during post-MI heart failure in rats: prevention with supplemental dietary propionyl-L-carnitine. Cardiovasc Drugs Ther 2003;17:7–14. [DOI] [PubMed] [Google Scholar]
- 133. Leasure JE, Kordenat K. Effect of propionyl-L-carnitine on experimental myocardial infarction in dogs. Cardiovasc Drugs Ther 1991;5:85–95. [DOI] [PubMed] [Google Scholar]
- 134. Sethi R, Wang X, Ferrari R, Dhalla NS. Improvement of cardiac function and beta-adrenergic signal transduction by propionyl L-carnitine in congestive heart failure due to myocardial infarction. Coron Artery Dis 2004;15:65–71. [DOI] [PubMed] [Google Scholar]
- 135. Anand I, Chandrashekhan Y, De Giuli F, Pasini E, Mazzoletti A, Confortini R, Ferrari R. Acute and chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc Drugs Ther 1998;12:291–299. [DOI] [PubMed] [Google Scholar]
- 136. Caponnetto S, Canale C, Masperone MA, Terracchini V, Valentini G, Brunelli C. Efficacy of L-propionylcarnitine treatment in patients with left ventricular dysfunction. Eur Heart J 1994;15:1267–1273. [DOI] [PubMed] [Google Scholar]
- 137. Anand IS, Francis G, Maseri A, Milazzotto F, Pepine CJ, Yusuf S, Beaufils P, Ferrari R, Poole-Wilson PA, Remme WJ, Schlepper M. Study on propionyl-L-carnitine in chronic heart failure. Eur Heart J 1999;20:70–76. [DOI] [PubMed] [Google Scholar]
- 138. Mancini M, Rengo F, Lingetti M, Sorrentino GP, Nolfe G. Controlled study on the therapeutic efficacy of propionyl-L-carnitine in patients with congestive heart failure. Arzneimittelforschung 1992;42:1101–1104. [PubMed] [Google Scholar]
- 139. Shirley M. Triheptanoin: first approval. Drugs 2020;80:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wehbe Z, Tucci S. Therapeutic potential of triheptanoin in metabolic and neurodegenerative diseases. J Inherit Metab Dis 2020;43:385–391. [DOI] [PubMed] [Google Scholar]
- 141. Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Triheptanoin alleviates ventricular hypertrophy and improves myocardial glucose oxidation in rats with pressure overload. J Card Fail 2015;21:906–915. [DOI] [PubMed] [Google Scholar]
- 142. Okere IC, McElfresh TA, Brunengraber DZ, Martini W, Sterk JP, Huang H, Chandler MP, Brunengraber H, Stanley WC. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J Appl Physiol 2006;100:76–82. [DOI] [PubMed] [Google Scholar]
- 143. Gillingham MB, Heitner SB, Martin J, Rose S, Goldstein A, El-Gharbawy AH, Deward S, Lasarev MR, Pollaro J, DeLany JP, Burchill LJ, Goodpaster B, Shoemaker J, Matern D, Harding CO, Vockley J. Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial. J Inherit Metab Dis 2017;40:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Vockley J, Charrow J, Ganesh J, Eswara M, Diaz GA, McCracken E, Conway R, Enns GM, Starr J, Wang R, Abdenur JE, Sanchez-de-Toledo J, Marsden DL. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol Genet Metab 2016;119:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vockley J, Burton B, Berry G, Longo N, Phillips J, Sanchez-Valle A, Chapman K, Tanpaiboon P, Grunewald S, Murphy E, Lu X, Cataldo J. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: results from an open-label, long-term extension study. J Inherit Metab Dis 2021;44:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Labarthe F, Gélinas R, Des Rosiers C. Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc Drugs Ther 2008;22:97–106. [DOI] [PubMed] [Google Scholar]
- 147. Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest 2002;110:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty Acids12. Adv Nutr 2016;7:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Khaw K-T, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med 2012;9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Trieu K, Bhat S, Dai Z, Leander K, Gigante B, Qian F, Korat AVA, Sun Q, Pan X-F, Laguzzi F, Cederholm T, de Faire U, Hellénius M-L, Wu JHY, Risérus U, Marklund M. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: a cohort study, systematic review, and meta-analysis. PLoS Med 2021;18:e1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Djousse L, Biggs ML, Matthan NR, Ix JH, Fitzpatrick AL, King I, Lemaitre RN, McKnight B, Kizer JR, Lichtenstein AH, Mukamal KJ, Siscovick DS. Serum individual non-esterified fatty acids and risk of heart failure in older adults. Cardiology 2021;146:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liang J, Zhou Q, Kwame Amakye W, Su Y, Zhang Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: a systematic review and meta analysis of prospective studies. Crit Rev Food Sci Nutr 2018;58:1122–1130. [DOI] [PubMed] [Google Scholar]
- 153. Venn-Watson S, Schork NJ. Pentadecanoic acid (C15:0), an essential fatty acid, shares clinically relevant cell-based activities with leading longevity-enhancing compounds. Nutrients 2023;15:4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lehninger principles of biochemistry - NLM Catalog - NCBI https://www.ncbi.nlm.nih.gov/nlmcatalog/101303262.
- 155. Schenkl C, Heyne E, Doenst T, Schulze PC, Nguyen TD. Targeting mitochondrial metabolism to save the failing heart. Life 2023;13:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Roe CR, Brunengraber H. Anaplerotic treatment of long-chain fat oxidation disorders with triheptanoin: review of 15 years experience. Mol Genet Metab 2015;116:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lopez-Schenk R, Collins NL, Schenk NA, Beard DA. Integrated functions of cardiac energetics, mechanics, and purine nucleotide metabolism. Compr Physiol 2023;14:5345–5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bashore TM, Magorien DJ, Letterio J, Shaffer P, Unverferth DV. Histologic and biochemical correlates of left ventricular chamber dynamics in man. J Am Coll Cardiol 1987;9:734–742. [DOI] [PubMed] [Google Scholar]
- 159. Lopez R, Marzban B, Gao X, Lauinger E, Van den Bergh F, Whitesall SE, Converso-Baran K, Burant CF, Michele DE, Beard DA. Impaired myocardial energetics causes mechanical dysfunction in decompensated failing hearts. Function (Oxf) 2020;1:zqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Starling RC, Hammer DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem 1998;180:171–177. [PubMed] [Google Scholar]
- 161. Unverferth DV, Magorien RD, Kolibash AJ, Lewis RP, Lykens M, Altschuld R, Baba N, Leier CV. Biochemical and histologic correlates of ventricular end-diastolic pressure. Int J Cardiol 1981;1:133–142. [DOI] [PubMed] [Google Scholar]
- 162. Rybalka E, Kourakis S, Bonsett CA, Moghadaszadeh B, Beggs AH, Timpani CA. Adenylosuccinic acid: an orphan drug with untapped potential. Pharmaceuticals 2023;16:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.