Abstract
The tripartite motif 5α protein (TRIM5α) is one of several factors expressed by mammalian cells that inhibit retrovirus replication. Human TRIM5α (huTRIM5α) inhibits infection by N-tropic murine leukemia virus (N-MLV) but is inactive against human immunodeficiency virus type 1 (HIV-1). However, we show that replacement of a small segment in the carboxy-terminal B30.2/SPRY domain of huTRIM5α with its rhesus macaque counterpart (rhTRIM5α) endows it with the ability to potently inhibit HIV-1 infection. The B30.2/SPRY domain and an additional domain in huTRIM5α, comprising the amino-terminal RING and B-box components of the TRIM motif, are required for N-MLV restriction activity, while the intervening coiled-coil domain is necessary and sufficient for huTRIM5α multimerization. Truncated huTRIM5α proteins that lack either or both the N-terminal RING/B-Box or the C-terminal B30.2/SPRY domain form heteromultimers with full-length huTRIM5α and are dominant inhibitors of its N-MLV restricting activity, suggesting that homomultimerization of intact huTRIM5α monomers is necessary for N-MLV restriction. However, localization in large cytoplasmic bodies is not required for inhibition of N-MLV by huTRIM5α or for inhibition of HIV-1 by chimeric or rhTRIM5α.
Several intrinsic immune mechanisms exist in mammalian cells that inhibit the replication of viruses (2, 9). Among these are APOBEC3G and related proteins (26) and zinc-finger antiviral protein (8), which modify and/or destabilize viral nucleic acids (8, 11, 19, 32). Products of the tripartite motif 5 (TRIM5) gene in primates and the Friend virus susceptibility factor 1 (Fv1) gene in mice constitute a class of restriction factors that inhibit retrovirus infection by targeting incoming capsids and preventing the establishment of a provirus in the target cell (1, 28).
Naturally occurring variation in TRIM5 and Fv1 sequence impacts the spectrum of retroviruses that are inhibited. For example, two principal Fv1 alleles exist in laboratory mice (Fv1n and Fv1b) that inhibit infection by B-tropic murine leukemia virus (B-MLV) and N-tropic MLV (N-MLV), respectively (16, 17, 22, 24). The differential sensitivity of N-MLV and B-MLV to Fv1n and Fv1b is determined by a single amino acid in the MLV capsid (15), whereas the differential specificity of Fv1n and Fv1b is governed by sequence variation at three positions within the C-terminal domain of the protein (3, 4). The reciprocal specificity of inhibition of MLV strains by Fv1 variants is the basis of the compelling (albeit unproven) hypothesis that Fv1-based restriction involves direct recognition of the incoming MLV capsid by the Fv1 protein (10, 27).
Like Fv1 variants, TRIM5 proteins from different primate species exhibit noticeable variation in the specificity with which they inhibit retrovirus infection (13, 14, 23, 28, 30). For example, human immunodeficiency virus type 1 (HIV-1) is strongly blocked by rhesus monkey and African green monkey (AGM) TRIM5α (rhTRIM5α and AGM TRIM5α) but not by human TRIM5α (huTRIM5α). Conversely SIVMAC is blocked to at least some extent by AGM TRIM5α but is largely resistant to huTRIM5α and rhTRIM5α. Several TRIM5α variants can exhibit a remarkable breadth in the specificity with which they block retrovirus infection. For example, several primate TRIM5α proteins block infection by both equine infectious anemia virus and MLV, whose capsids share little sequence homology and AGM TRIM5α blocks infection by most retroviruses that have been tested, the exceptions being lentiviruses that are naturally found in AGMs and, paradoxically, B-MLV (13, 14, 30). What TRIM5α domains are responsible for specific recognition of widely divergent incoming retroviral capsids is largely unclear. Like many other TRIM proteins, TRIM5 takes various forms as a consequence of alternative mRNA splicing, but all TRIM5 proteins share a common amino-terminal TRIM motif, comprising RING, B-box, and coiled-coil domains (25). Conversely, each spliced variant encodes a unique carboxy-terminal domain. In humans, rhesus macaques, and African green monkeys, the α splice-variant of TRIM5, which encodes a B30.2/SPRY C-terminal domain, has been shown to exhibit restriction activity against widely divergent retroviruses (13, 14, 23, 28, 30). Conversely the γ splice-variant of rhTRIM5, which lacks the B30.2/SPRY domain, is inactive against HIV-1. Mutations in the amino-terminal RING domain of rhTRIM5α also reduce HIV-1 restriction activity (28). Thus, both RING and B30.2/SPRY domains of rhTRIM5α are required for full inhibition of HIV-1 infection, but at present it is not clear precisely what function each domain provides in inhibiting retrovirus infection, or whether inhibition of other retroviruses by other TRIM5α variants exhibits similar requirements for each TRIM5α domain. A naturally occurring TRIM5 protein that lacks a B30.2/SPRY domain occurs in owl monkeys due to insertion of a CypA pseudogene insertion in the TRIM5 locus. Because this unusual protein displays the HIV-1 capsid specific recognition properties of CypA (18), coupled with the infection inhibiting properties of TRIM5α (28), the B30.2/SPRY domain is an excellent candidate for conferring specific recognition of incoming retrovirus capsids.
In the present study, we determined which domains of huTRIM5α are required for N-MLV restriction and are responsible for the inability of huTRIM5α to block HIV-1 infection. These experiments also provide evidence that TRIM5α homomultimerization is required for inhibition of N-MLV by huTRIM5α but that formation of large cytoplasmic bodies is not essential for inhibition of N-MLV by huTRIM5α or for inhibition of HIV-1 by rhTRIM5α.
MATERIALS AND METHODS
Chimeric huTRIM5α/rhTRIM5α proteins.
The TRIM5α proteins used in the present study were derived from HeLa and FRhK4 cell cDNAs, respectively, and have been described previously (13). The coding sequences of each of these proteins were amplified by PCR with a forward primer that incorporated XhoI site 5′ to the translation initiating codon and a reverse primer that introduced a NotI site in place of a translation terminating codon. These amplicons were inserted into an LNCX2-derived vector modified so as to introduce a hemagglutinin (HA) tag at the C terminus of the expressed protein. All huTRIM5α-based chimeras and truncation mutants were generated in this background so that each protein was appended with a C-terminal HA tag. For the first set of chimeras (Fig. 1B), we took advantage of a BglII restriction site underlying amino acid D234 in huTRIM5α, as well as a BamHI site underlying I372 in huTRIM5α (I376 in rhTRIM5α) coding sequence. In addition, a BglII site underlying D236 in rhTRIM5α was introduced by PCR-based mutagenesis. Using these sites, we reciprocally exchanged 3′ (BglII-NotI) fragments of huTRIM5α and rhTRIM5α sequence to generate the hu(rh237-497) and rh(hu235-493) chimeras. Similarly, exchange of BamHI-NotI or BglII-BamHI fragments was used to generate the rh(hu372-493) and hu(rh237-376) chimeras.
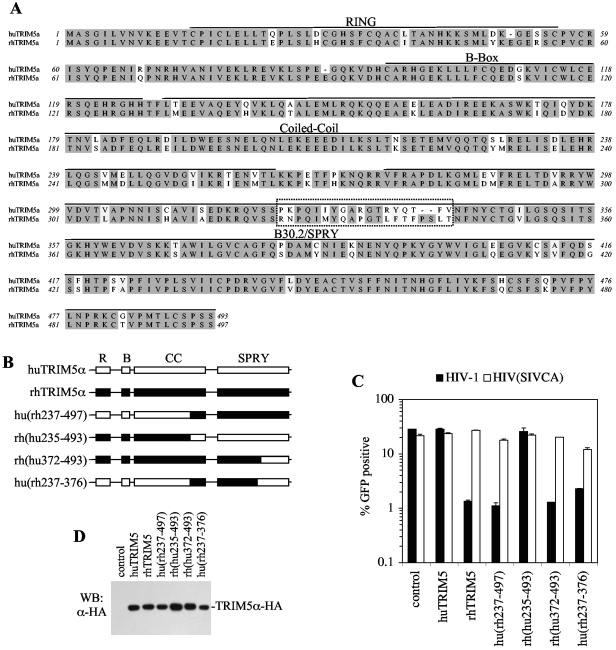
FIG. 1.
The huTRIM5α and rhTRIM5α B30.2/SPRY domain contains determinants of restriction specificity. (A) Alignment of human and rhesus TRIM5α amino acid sequences with key domains and a hypervariable segment within the B30.2/SPRY domain indicated. (B) Schematic representation of chimeras generated between human and rhesus TRIM5α, with the RING (R), B-box (B), coiled-coil (CC), and B30.2/SPRY (SPRY) domains indicated (see text for details). (C) Infection of unmodified MDTF cells (control) or MDTF cells stably expressing the indicated TRIM5α protein with wild-type HIV-1 (▪) or with HIV(SIVCA) (□). For each virus-TRIM5α combination, the percentage of infected (GFP-positive) cells as determined by fluorescence-activated cell sorting (FACS) analysis is plotted. (D) Western blot analysis of lysates of MDTF cells expressing the indicated wild-type and chimeric TRIM5α proteins using an α-HA antibody.
For the next set of chimeras (Fig. 2A), silent mutagenesis was used to introduce a KpnI site underlying amino acids G333-T334 in huTRIM5α (G335-T336 in rhTRIM5α). These were then used to generate the hu(rh325-344), hu(rh325-335), and hu(rh336-344) chimeras. This was achieved by using chimeric PCR primers incorporating the respective huTRIM5α and rhTRIM5α sequences and KpnI sites at their 5′ ends, followed by insertion of amplicons into huTRIM5α using BglII, KpnI, and BamHI sites. The hu(rh237-335) and hu(rh336-376) chimeras were generated by exchange of BglII-KpnI and KpnI-BamHI fragments of huTRIM5α with their rhTRIM5α equivalents.
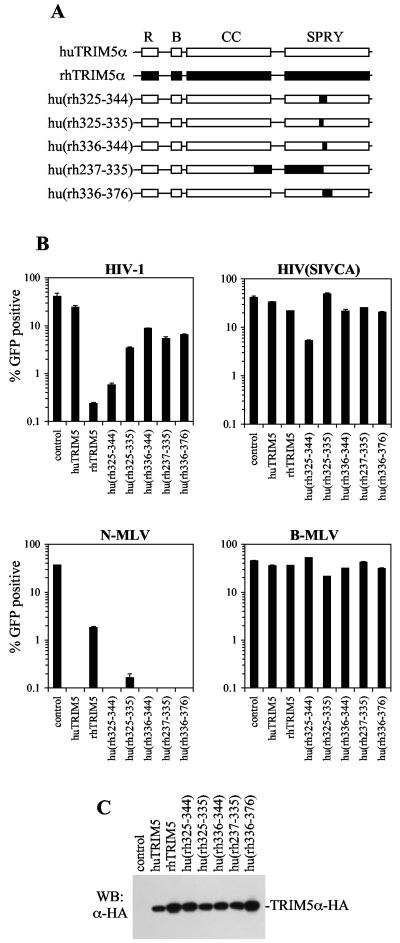
FIG. 2.
Mapping of a motif in huTRIM5α/rhTRIM5α important for restriction specificity. (A) Schematic representation of chimeras generated between huTRIM5α/rhTRIM5α, with the RING (R), B-box (B), coiled-coil (CC), and B30.2/SPRY (SPRY) domains indicated. (B) Infection of unmodified MDTF cells (control) or single-cell clones of MDTF cells expressing the indicated TRIM5α proteins with HIV-1 (upper left panel), HIV(SIVCA) (upper right panel), N-MLV (lower left panel), or B-MLV (lower right panel). For each virus-TRIM5α combination, the percentage of infected (GFP-positive) cells as deter-mined by FACS analysis is plotted. (C) Western blot analysis of lysates of MDTF cells expressing the indicated wild-type and chimeric TRIM5α proteins using an α-HA antibody.
Truncated forms of huTRIM5α.
To generate the truncated forms of huTRIM5α, forward PCR primers were used that introduced an XhoI site and a translation initiating codon appended to sequences beginning at S61 for BCCSPRY, T128 for CCSPRY, and V294 for SPRY. These forward primers were used together with the reverse NotI primer described above. Reverse primers introducing a NotI site were directed to sequences terminating at H127 or D293 and were used in conjunction with the appropriate forward primers described above to generate the RB, RBCC, and CC truncation mutants. Each PCR product was inserted as an XhoI-NotI fragment into LNCX2/HA for expression in mammalian cells with an appended carboxy-terminal HA epitope tag or into pVP16/HA for expression in yeast fused to a VP16 activation domain.
Retroviral vectors.
In most cases, HIV-1, HIV(SIVCA), N-MLV, and B-MLV vectors that carried a green fluorescent protein (GFP)-reporter gene were generated by using combinations of three expression vectors. In each case, the retroviral Gag-Pol was encoded on a separate expression plasmid from the packaged viral genome which expressed GFP. Details of the Gag-Pol and vector expression plasmids have been described previously (6, 12). Vectors were pseudotyped with vesicular stomatitis virus G glycoprotein (VSV-G) to enable the efficient entry into the mammalian cell lines used in these experiments and were made by transient transfection of 293T cells, as described previously (6, 12). Doses of vectors applied to cells were normalized by reverse transcriptase assay (Cavidi-Tech) or by titration on nonrestricting Mus dunni tail fibroblast (MDTF) cells.
Measurement of TRIM5-based retrovirus restriction activity.
293T cells were transfected with LNCX2-based retroviral vectors expressing the various TRIM5α-derived proteins, along with plasmids expressing MLV Gag-Pol and VSV-G expression plasmids. These vectors were used to transduce cell lines from humans (HeLa) or mice (MDTF). The transduced cells were selected in 1 mg of G418/ml for 7 to 10 days and then either used as a pool or, alternatively, single-cell clones were isolated by limiting dilution. To test sensitivity to retrovirus infection, target cells were seeded in 24-well plates at 2 × 104 cells per well and inoculated with GFP-reporter vector stocks in the presence of 5 μg of Polybrene/ml. The virus dose was selected so as to infect 20 to 50% of unmodified cells. GFP-positive cells were enumerated 48 h later by using a FACSCalibur instrument (Becton Dickinson).
Yeast two-hybrid assays.
A bait plasmid expressing a GAL4-huTRIM5α fusion protein was generated by insertion of huTRIM5α coding sequences into pGBKT7 (Clontech). Yeast Y190 cells were transformed with pGBKT7/huTRIM5α and pVP16/HA(5) derivatives expressing the various huTRIM5α truncation mutants. Interactions were measured by using a β-galactosidase reporter assay as previously described (5, 20).
Coprecipitation assays.
LNCX2-based plasmids expressing the HA-tagged truncated proteins were cotransfected in 293T cells with a plasmid expressing full-length huTRIM5α fused to glutathione S-transferase (GST) at its N terminus or a plasmid expressing unfused GST as a control. After 48 h the cells were lysed in buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 5% glycerol, 1% Triton X-100, and a protease inhibitor mixture [Roche]), and clarified lysates were incubated with glutathione-Sepharose beads for 4 to 5 h at 4°C. The beads were washed three times with buffer containing 0.1% Triton X-100. Aliquots of the unfractionated cell lysates and glutathione-bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with an α-HA monoclonal antibody (Covance) and an α-mouse immunoglobulin G conjugated to peroxidase (Chemicon).
Microscopy.
MDTF or HeLa cells transduced with LNCX2-based vectors expressing wild-type, truncated, or chimeric TRIM5α proteins were plated on 35-mm glass-bottom dishes coated with collagen (MatTek). The cells were fixed with paraformaldehyde and stained with an α-HA monoclonal antibody (Covance), followed by the addition of α-mouse immunoglobulin G conjugated to AlexaFluor 594 (Molecular Probes). Images were collected and deconvolved with a Deltavision microscope and software (Applied Precision).
RESULTS
Replacement of a small segment of huTRIM5α in the B30.2/SPRY domain confers potent anti-HIV-1 activity.
Of the TRIM5α variants in primates characterized thus far, several have broad antiretroviral activity, and only the human protein lacks the ability to inhibit HIV-1. We adopted a gain-of-function approach to determine which sequences in huTRIM5α are responsible for its inability to inhibit HIV-1. Like other members of the tripartite motif family of proteins, TRIM5α contains RING, B-box, and coiled-coil domains (25). In addition, TRIM5α contains a carboxy-terminal B30.2/SPRY domain. The rhesus monkey and human TRIM5α proteins share 87% amino acid identity (28), and many of the differences cluster in the B30.2/SPRY domain (Fig. 1A). In assays where TRIM5α is overexpressed by using retroviral vectors, both proteins restrict N-MLV, but only rhTRIM5α inhibits HIV-1 (13, 28, 30). Therefore, we generated a series of chimeras of human and rhesus TRIM5α proteins (see Fig. 1B). The chimeric proteins were appended with a C-terminal epitope tag from influenza virus HA and were stably expressed by using retrovirus vectors in MDTF cells. As a control, the parental wild-type huTRIM5α and rhTRIM5α proteins, also containing a C-terminal HA tag, were stably expressed in MDTF cells. The addition of an HA tag to the C terminus of TRIM5α did not affect the efficiency with which the proteins inhibited infection (28; data not shown). Cells expressing the intact and chimeric TRIM5α proteins were then infected with a GFP-expressing HIV-1-based vector. The vector was packaged by using either an intact HIV-1 Gag-Pol expression plasmid or an otherwise identical Gag-Pol expression plasmid encoding a capsid domain from SIVMAC, termed HIV(SIVCA), which is not sensitive to rhTRIM5α (13, 28).
As can be seen in Fig. 1C, a chimera that contained the C-terminal half (residues 237 to 497) from rhTRIM5α and the N-terminal half of huTRIM5α, termed hu(rh237-497), conferred resistance to infection by the HIV-1 vector to the same extent as did the intact rhTRIM5α protein. Neither protein affected the infectivity of the HIV(SIVCA) vector. Conversely, the reciprocal rh(hu235-493) chimera, like huTRIM5α and unlike rhTRIM5α, did not confer resistance to HIV-1 infection (Fig. 1C). Western blot analysis showed that each of these proteins was expressed at similar levels (Fig. 1D), and minor variations did not correlate with restriction activity. Thus, the C-terminal half of the rhTRIM5α contains determinants required for restriction of HIV-1 infection that are lacking in huTRIM5α. Therefore, further huTRIM5α-based chimeras containing smaller portions of the C-terminal half of the rhTRIM5α protein were generated (Fig. 1B). Cells expressing the rh(hu372-493) chimera were as susceptible to HIV-1 vectors as cells expressing huTRIM5α, whereas cells expressing the hu(rh237-376) chimera behaved similarly to cells expressing rhTRIM5α in that they were highly resistant to HIV-1 but susceptible to HIV(SIVCA) (Fig. 1C). All of the aforementioned chimeras conferred resistance to N-MLV but not B-MLV (data not shown), as do both the intact rhesus and human TRIM5α proteins. In contrast, two reciprocal chimeras, hu(rh376-497) and rh(hu235-372), although efficiently expressed, did not confer resistance to any retrovirus tested, including N-MLV. Because we cannot be certain that hu(rh376-497) and rh(hu235-372) were inactive for irrelevant reasons, such as misfolding, they were considered uninformative (data not shown). Nonetheless, these results show that huTRIM5α amino acids 235 to 372 harbor the defect that confers inactivity against HIV-1 infection, which can be rescued by replacement with their rhTRIM5α (amino acid 237 to 376) equivalents.
To determine more precisely the amino acids within this region responsible for the differential ability of huTRIM5α and rhTRIM5α to inhibit HIV-1, we generated further chimeras (see Fig. 2A). In contrast to the chimeras shown in Fig. 1, some of these additional chimeras exhibited intermediate HIV-1 restriction activity, and in several cases it was difficult to distinguish whether the cells that became infected did so because of partial restriction activity or because there exists a small fraction of the transduced pool of MDTF cells that did not express the chimeric TRIM5α proteins. Indeed, immunofluorescence analysis with an α-HA antibody revealed that a few cells in the TRIM5α-expressing cell pools expressed undetectable levels of TRIM5α, and these were preferentially infected by the HIV-1 vector (data not shown). Therefore, to ensure that any differences in the apparent degree of restriction activity among TRIM5α chimeras was not due to variable frequencies of cells that did not express the chimeras, single-cell clones of MDTF cells expressing each chimera were isolated and tested. The results from these experiments are shown in Fig. 2. MDTF clones expressing intact huTRIM5α and rhTRIM5α were also derived for control purposes and, as expected, clones in which 100% of cells expressed the rhTRIM5α proteins were more resistant to HIV-1 infection than were rhTRIM5α-expressing pools (compare Fig. 1C and Fig. 2B). For each chimera, at least two representative single-cell clones were used, and similar levels of chimeric TRIM5α expression in each of these clones were verified by Western blot analysis (Fig. 2C). Each pair of clones behave similarly, and for simplicity results from one clone of each pair is shown in Fig. 2B.
Within the 237- to 376-amino-acid region of rhTRIM5α, residues that were different to the huTRIM5α cluster between amino acids 325 and 344. This coincides with a position in the TRIM5α protein where the AGM TRIM5α carries a 20-amino-acid insertion relative to huTRIM5α. We reasoned that this apparently hypervariable segment of TRIM5α was a likely candidate for the determinant of the inability of huTRIM5α to inhibit HIV-1 and therefore replaced a short 18-amino-acid stretch in huTRIM5α with the corresponding rhTRIM5α sequence to generate the hu(rh325-344) chimera (Fig. 2A). The level of resistance to HIV-1 induced by hu(rh325-344) was almost 100-fold and comparable to that observed in single cell clones expressing rhTRIM5α (Fig. 2B). Thus, rhTRIM5α amino acids 325 to 344 contain determinants that are able to confer on huTRIM5α the ability to potently inhibit HIV-1 infection.
We also tested the degree to which chimeras induced resistance to N-MLV because this property differed between huTRIM5α, which restricted N-MLV by >200-fold, and rhTRIM5α, which restricted N-MLV by ∼20-fold (Fig. 2B). Interestingly, the hu(rh325-344) chimera inhibited N-MLV infection as potently as huTRIM5α and more potently than rhTRIM5α, suggesting that residues outside the hypervariable segment contribute to the potency with which huTRIM5α restricts N-MLV. In addition, clones expressing the hu(rh325-344) chimera exhibited a modest degree of resistance to infection by the HIV(SIVCA) vector (Fig. 2B). These cells were equivalently susceptible to B-MLV, demonstrating that the observed differences in N-MLV, HIV-1, and HIV(SIVCA) sensitivity were not due to a generalized loss of susceptibility to retroviral infection in the cell clones expressing this chimera (Fig. 2B). This finding indicates that, at least when overexpressed, chimeric TRIM5α proteins can also inhibit retroviruses that are not targeted by either parental TRIM5α variant. Thus, the specificity with which incoming retroviral capsids are recognized is likely determined in a combinatorial manner by more than one sequence motif in TRIM5α.
Within the 323-340/325-344 hypervariable segment of huTRIM5α/rhTRIM5α, 11 of 18 residues are different and 2 residues are inserted in rhTRIM5α compared to huTRIM5α (Fig. 1A). To determine whether the entire rhTRIM5α hypervariable segment was required for HIV-1 inhibition, we generated additional huTRIM5α-based chimeras containing the N-terminal (residues 325 to 335) or C-terminal (residues 336 to 344) halves of the rhTRIM5α segment (Fig. 2A). The hu(rh325-335) chimera indeed induced resistance to HIV-1, but the degree to which this occurred (12-fold resistance) was less than that induced by hu(rh325-344) or rhTRIM5α. The hu(rh336-344) chimera also induced modest resistance to HIV-1 (four- to fivefold). Both of these chimeras strongly restricted N-MLV but did not affect B-MLV infection (Fig. 2B). Two further chimeras were generated that contained larger segments of rhTRIM5α in addition to the N-terminal or C-terminal halves of the hypervariable segment, namely, hu(rh237-335) and hu(rh336-376) (Fig. 2A). Both chimeras conferred levels of resistance to HIV-1 comparable to those encoding only the respective halves of the hypervariable segment (Fig. 2B), suggesting differences between huTRIM5α and rhTRIM5α outside the 325 to 344 segment do not contribute in a major way to HIV-1 specific restriction. Again, there were only minor variations in the expression level of each of the chimeras that did not correlate with restriction activity (Fig. 2C). Therefore, transfer of the entire 325 to 344 segment from rhTRIM5α to huTRIM5α was both necessary and sufficient to attain the levels of anti-HIV-1 activity induced using the wild-type rhTRIM5α protein.
huTRIM5α domains required for anti-N-MLV activity.
Although sequence differences in the B30.2/SPRY domain account for the inability of huTRIM5α to restrict HIV-1, huTRIM5α is clearly able to confer resistance to other retroviruses such as N-MLV and equine infectious anemia virus (13, 14, 23, 30). Therefore, we next sought to determine which TRIM5α domains are required for N-MLV restriction activity. We generated a series of truncation mutants of huTRIM5α, removing entire domains but retaining the HA tag at the C terminus of each protein. For simplicity, the mutants are named according to which of the four domains, namely, RING (R), B-box (B), coiled-coil (CC), and SPRY, were retained in the truncation mutant (Fig. 3A). These mutants were stably expressed in MDTF cells, and their restriction activity was tested against N-MLV, which is highly sensitive to full-length huTRIM5α, and B-MLV, which is resistant (13, 14, 23, 30).
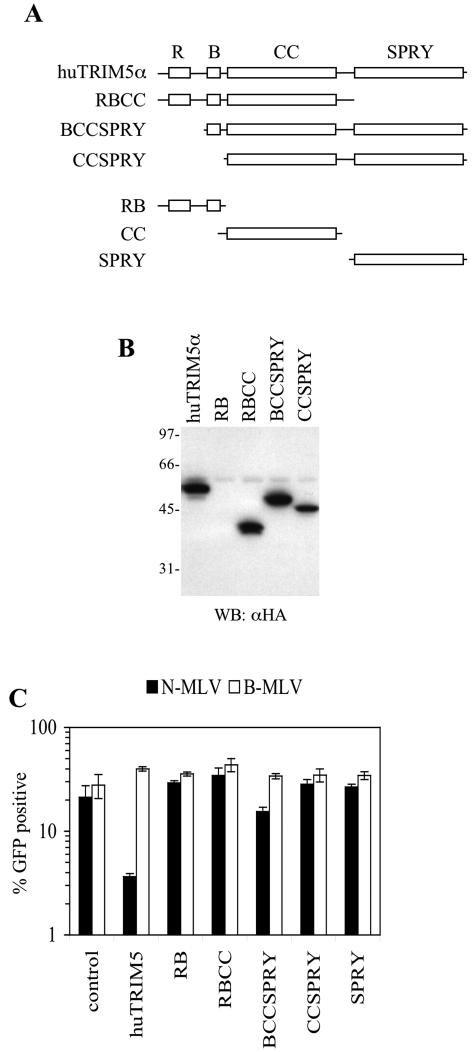
FIG. 3.
TRIM5 domains required for retrovirus restriction. (A) Schematic representation of truncation mutants of huTRIM5α (see the text for details). (B) Expression of TRIM5α truncation mutants in MDTF cells. Lysates from each pool of transduced cells expressing the indicated HA-tagged truncation mutant were analyzed by Western blotting by using an α-HA monoclonal antibody. (C) Restriction activity of huTRIM5α truncation mutants upon expression in MDTF cells. Unmodified MDTF cells (control) or MDTF cells stably expressing the protein indicated were infected with N-MLV (▪) or B-MLV (□). The percentage of infected (GFP-positive) cells as determined by FACS analysis is plotted.
The truncated RBCC, BCCSPRY, and CCSPRY proteins were expressed as efficiently as full-length huTRIM5α (Fig. 3B). Indeed, the BCCSPRY mutant, which lacks the RING domain did exhibit a weak but significant (twofold) inhibitory activity against N-MLV (Fig. 3C). Conversely, the CCSPRY mutant, which lacks both the RING and the B-box domains mutant was devoid of restriction activity (Fig. 3C), as was the RBCC mutant, which lacks the SPRY domain. (Fig. 3C). Of the remaining mutants, the RB protein was expressed at very low levels and was detectable only after overexposure of the Western blot, and expression of the isolated SPRY protein was undetectable (data not shown). Not surprisingly, therefore, these truncated proteins did not exhibit any N-MLV restriction activity. The CC protein was not tested in this assay because, based on the fact that it lacks two domains (RB and SPRY) that appeared necessary for activity necessary, (Fig. 3C) it was not expected to be active.
Truncated huTRIM5α proteins that multimerize are dominant inhibitors of restriction activity.
It has been previously shown that expression of rhTRIM5γ, a form of TRIM5 that lacks the SPRY domain and anti-HIV-1 activity, relieves the block to HIV-1 infection when expressed in rhesus cells but that RING domain mutants lack this activity (28). We sought to determine whether the huTRIM5α truncation mutants depicted in Fig. 3A could exhibit dominant-negative activity when expressed in HeLa cells. N-MLV is strongly restricted in HeLa cells, and this phenotype is accentuated by transduction with a retrovirus vector expressing huTRIM5α (Fig. 4A). However, expression of either the RBCC or the CCSPRY proteins completely abolished restriction, and HeLa cells expressing these truncated proteins were approximately equivalently sensitive to N-MLV and B-MLV (Fig. 4A). The BCCSPRY protein was also efficiently expressed in HeLa cells (Fig. 4B) and also significantly enhanced N-MLV titers therein. However, it did not completely abolish restriction (Fig. 4A), probably because it has weak restricting activity itself (Fig. 3C). Notably, the CC protein, which encodes only the TRIM5 coiled-coil domain also suppressed restriction activity in HeLa cells. Incomplete suppression by the CC protein may be due to the fact that it is expressed at very low levels compared to the RBCC and CCSPRY proteins (Fig. 4B). Thus, each truncation mutant that retained the coiled-coil domain of huTRIM5α acted as a dominant inhibitor of the activity of the full-length protein endogenously expressed by HeLa cells.
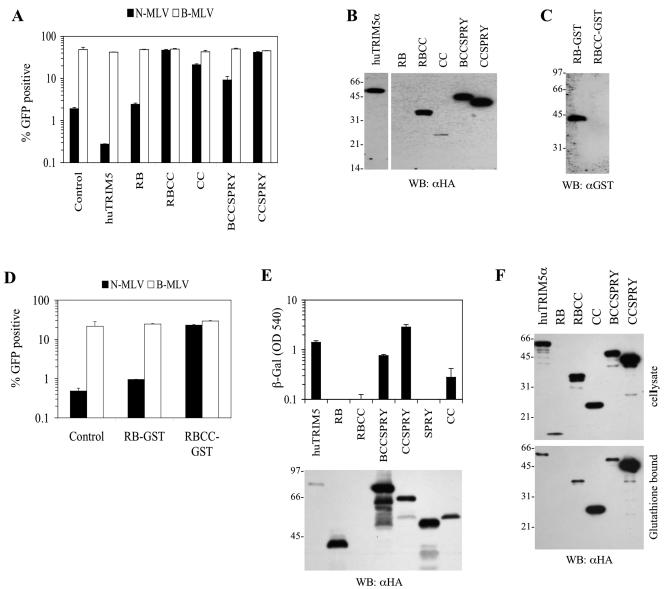
FIG. 4.
TRIM5α domains required for dominant-negative activity and multimerization. (A) Inhibition of restriction by huTRIM5α truncation mutants expressed in HeLa cells. Unmodified HeLa cells (control) or HeLa cells stably expressing full-length or truncated huTRIM5α proteins as indicated were infected with N-MLV (▪) or B-MLV (□). (B) Expression of huTRIM5α truncation mutants in HeLa cells. Lysates from each cell line used in A were analyzed by Western blotting with an α-HA monoclonal antibody. The full-length huTRIM5α was run on the same gel as the mutants, was exposed for the same time, and has been repositioned in the figure for clarity. (C) Expression of the truncated TRIM5α-GST fusion proteins. Lysates from each pool of cells stably expressing the indicated GST-fused huTRIM5α truncation mutant were analyzed by Western blotting with an α-GST monoclonal antibody. (D) Inhibition of restriction activity by GST-fused huTRIM5α truncation mutants in HeLa cells. Unmodified HeLa cells (control) or cells stably expressing RB-GST or RBCC-GST fusion proteins as indicated were infected with N-MLV (▪) or B-MLV (□). (E) Yeast two-hybrid analysis of TRIM5α multimerization. β-Galactosidase reporter levels in yeast expressing GAL4-TRIM5α and the indicated TRIM5α protein fused to VP16 and an HA epitope tag are plotted. Lysates of the yeast were analyzed by Western blotting with an α-HA antibody. (F) Coprecipitation analysis of TRIM5α multimerization in mammalian cells. 293T cells were transiently cotransfected with a GST-TRIM5α fusion protein expression plasmid along with plasmids expressing the indicated HA-tagged TRIM5α truncation mutants. Samples of clarified cell lysates and glutathione-bound proteins were analyzed by Western blotting with an antibody to HA.
As was the case in MDTF cells, neither the RB protein nor the SPRY protein were well expressed in HeLa cells, and neither suppressed N-MLV restriction therein (Fig. 4A and B and data not shown). Nevertheless, we could not exclude the possibility that these proteins could also have dominant-negative activity if it were possible to overexpress them. Consequently, these proteins were fused to the N terminus of GST in an attempt to generate fusion proteins with improved expression levels. This strategy was successful in the case of RB-GST fusion protein, which was expressed in transduced HeLa cells at high levels (Fig. 4C). However, RB-GST overexpression did not relieve restriction (Fig. 4D). As a control, an RBCC-GST fusion was also generated and restored N-MLV infectivity as efficiently as the original, HA-tagged RBCC protein (Fig. 4D). In fact, this protein was quite a potent inhibitor of endogenous TRIM5α restriction activity because we were unable to detect RBCC-GST protein expression (Fig. 4C). It should be noted that the GST antibody is less efficient than the HA antibody (data not shown); thus, the expression levels of HA versus GST fusion proteins cannot be meaningfully compared. Nonetheless, the experiments in Fig. 4A to D demonstrate that expression of the huTRIM5α coiled-coil domain in the context of a truncated protein is necessary and sufficient to inhibit the activity of the full-length protein.
Coiled-coil domains often promote protein multimerization and, like many TRIM proteins, TRIM5α has been reported to homomultimerize (25). Thus, it seemed possible that the dominant-negative activity of the truncation mutants was due to their ability to multimerize with the full-length protein. To test this, the truncation mutants depicted in Fig. 3A were also tested for their ability to multimerize with the full-length protein in a yeast two-hybrid assay. In this assay, huTRIM5α exhibits quite strong homomultimerization activity (Fig. 4E). All of the truncated proteins retaining the coiled-coil domain, namely, BCCSPRY, CCSPRY, and CC, bound to full-length huTRIM5α in the yeast two-hybrid assay. The exception was the RBCC truncation mutant which, surprisingly, was not expressed in yeast cells (Fig. 4E). Proteins that lacked the coil-coil domain, namely, RB and SPRY, failed to multimerize with full-length huTRIM5α even though they were efficiently expressed in yeast cells (Fig. 4E).
To confirm these findings in mammalian cells, each HA-tagged TRIM5α truncation mutant was transiently expressed in 293T cells together with a GST-huTRIM5α fusion protein or, as a control, an unfused GST protein. Protein complexes were then precipitated by using glutathione-Sepharose and analyzed by Western blotting with an α-HA antibody. Full-length huTRIM5α and all of the truncation mutants retaining the coiled-coil domain coprecipitated with GST-huTRIM5α (Fig. 4F). The expression of the RB protein was easily detected in transiently transfected 293T cells, albeit at lower levels than the other truncation mutants, but coimmunoprecipitation with GST-huTRIM5α was not detected even after overexposure of the Western blot (Fig. 4F and data not shown). The CC protein appeared to interact at least as strongly with GST-huTRIM5α as did any other truncation mutant or full-length TRIM5α, based on their relative expression levels (Fig. 4F). Overall, the ability of each huTRIM5α truncation mutant to multimerize with the full-length protein in mammalian cells correlated with their ability to do so in the yeast two-hybrid assays and with their ability to inhibit the activity of endogenous huTRIM5α in HeLa cells. Thus, the coiled-coil domain is necessary and sufficient for huTRIM5α multimerization and for dominant inhibition of the restriction activity of the full-length huTRIM5α protein.
Subcellular localization of restricting and nonrestricting, intact and truncated TRIM5α proteins.
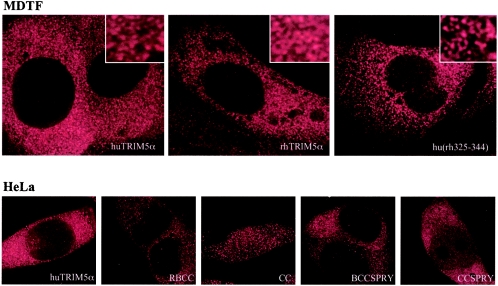
Previously, TRIM5 has been reported to concentrate in large and discrete structures in the cytoplasm, termed cytoplasmic bodies (25). To examine whether this is a property of TRIM5α that is gained or lost in active and inactive TRIM5α truncation mutants and chimeras, we analyzed their localization when stably expressed in MDTF and/or HeLa cells by immunofluorescence with the α-HA antibody. In MDTF cell clones that strongly restrict N-MLV or HIV-1 infection (for example, see Fig. 2), huTRIM5α and rhTRIM5α did not accumulate in large cytoplasmic bodies but were distributed throughout the cell cytoplasm (Fig. 5). High-resolution deconvolution imaging revealed that both huTRIM5α and rhTRIM5α were present in small puncta and, upon close examination, TRIM5α seemed to form a three-dimensional irregular net-like structure with α-HA reactive linkages between the puncta (Fig. 5, upper-panel insets).
FIG. 5.
Subcellular localization of TRIM5α variants, chimeras and deletion mutants. MDTF or HeLa cells, as indicated, were transduced with LNCX2-based retrovirus vectors expressing the indicated TRIM5-derived proteins bearing a C-terminal HA epitope tag. Cells were fixed and stained as described in the text, and the images shown are deconvolved single optical sections.
It is likely that the differences in localization between this and previously published data using transiently transfected GFP-TRIM5 forms (25) are due to lower expression levels of the tagged TRIM5α proteins in our study. Indeed, we were able to reproduce the previous findings of large cytoplasmic bodies in 293T cells transiently transfected with huTRIM5α-HA and rhTRIM5α-HA expression plasmids (data not shown). Thus, distributions observed in Fig. 5 should be interpreted with caution in the absence of a definitive phenotype for endogenously expressed TRIM5α because, even in the context of the stable cell lines shown in Fig. 5, TRIM5α is overexpressed. Nonetheless, the active and inactive TRIM5α chimeras and mutants did not appear to differ significantly in their subcellular distribution (Fig. 5 and data not shown). The exceptions to this was the CC and, to a lesser extent, the CC-SPRY truncation mutants that were observed in the nucleus and in the cytoplasm, presumably because they are small enough to permit passive diffusion through nuclear pores.
DISCUSSION
These studies indicate that separable domains of huTRIM5αcontribute distinct functions to inhibit retrovirus infection. The B30.2/SPRY domain appears at least partly responsible for determining the specificity with which incoming capsids are recognized and inhibited, and within this domain we mapped a determinant that is present in rhTRIM5α but not in huTRIM5α that is required for restriction of HIV-1. This determinant was a hypervariable segment comprising residues 325 to 344 of the rhTRIM5α protein. It is probably not coincidental that the AGM TRIM5α protein, which has remarkably broad restriction specificity, contains a 20-amino-acid insertion within this hypervariable segment (13, 14, 30). Attempts to further dissect the differences between huTRIM5α and rhTRIM5α residues that are responsible for their differential ability to inhibit HIV-1 infection indicated that multiple residues within the hypervariable segment contributed to restriction specificity. Indeed, independent transfer of either of two short and nonoverlapping portions of rhTRIM5α hypervariable segment (residues 325 to 335 or 336 to 344) into the huTRIM5α context resulted in proteins with at least some ability to inhibit HIV-1 infection. Nonetheless, both portions were required to give a chimeric TRIM5α protein that restricted HIV-1 with similar potency to that of the intact rhTRIM5α protein. While this study was in review, Yap et al. reported that a single amino acid change (R332P) within the hypervariable segment of huTRIM5α was sufficient to confer anti-HIV-1 activity (31). However, this change was apparently not sufficient to recapitulate the entire activity of the intact rhTRIM5α protein, a finding consistent with our conclusion that at least two elements within the hypervariable segment were required to give a huTRIM5α protein that inhibited HIV-1 with a potency similar to that of rhTRIM5α. Moreover, the hypervariable segment contained in residues 325 to 344 is unlikely to be the sole determinant of the specificity with which retrovirues are inhibited in general. This conclusion is based on the fact that the hu(rh325-344) chimeric protein partly restricted an HIV-1 engineered to carry an SIVMAC capsid, a property exhibited by neither huTRIM5α nor rhTRIM5α. In addition, the hu(rh325-344) protein restricted N-MLV as potently as huTRIM5α and more potently than rhTRIM5α. Thus, restriction specificity is likely governed in a combinatorial manner by more than one determinant within TRIM5α, including but not limited to the hypervariable segment within the B30.2/SPRY domain identified herein as an important specificity determinant. This combinatorial contribution of sequences in the C-terminal portion of TRIM5α is reminiscent of previous findings with Fv1, where new MLV restriction specificities could be obtained by mixing determinants unique to the Fv1n and Fv1b proteins (3).
The N-terminal TRIM domains from rhesus and human TRIM5α were fully competent to mediate restriction of N-MLV or HIV-1 when fused to a B30.2/SPRY domain of the appropriate specificity. Within the TRIM motif, the presence of the RING domain of huTRIM5α strongly enhanced restriction of N-MLV but was not absolutely required, a result that is similar to previous finding using RING domain point mutants of rhTRIM5α and HIV-1 as the target retrovirus (28). These results suggest that there exists broad similarity in the mechanism by which HIV-1 and N-MLV are restricted by TRIM5α proteins. Further truncation of the B-box domain of huTRIM5α completely inactivated the N-MLV restricting properties of the protein. The partial requirement for the RING domain in HIV-1 restriction is also evident independently of whether the restriction specificity was conferred by a B30.2/SPRY domain or a CypA domain (D. Perez-Caballero and T. Hatziioannou, unpublished observations). This suggests that the RING domain is unlikely to contribute to capsid recognition, and rather provides some other function, perhaps recruitment of additional factors or ubiquitin ligation activity (29), during restriction.
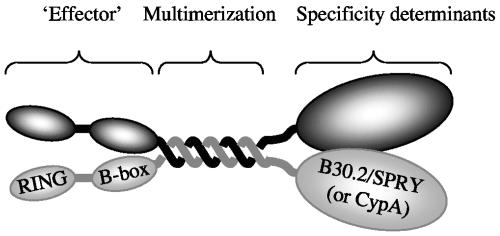
Overall, therefore, these studies suggest that TRIM5-based restriction factors can be considered as being composed of distinct functional domains, shown schematically in Fig. 6. These domains consist of (i) a C-terminal B30.2/SPRY or CypA domain that determines the specificity with which incoming capsids are recognized, (ii) a central coiled-coil domain required for TRIM5 multimerization, and (iii) an N-terminal “effector” domain comprising the RING and B-box domains that appears to be required in a general manner for restriction. huTRIM5α proteins lacking either the effector or specificity determining domains exhibited potent dominant inhibitory activity on the endogenous full-length protein in HeLa cells. In fact, the coiled-coil domain that was necessary and sufficient for multimerization was also necessary and sufficient to mediate dominant inhibition of full-length huTRIM5α. This suggests that the active form of TRIM5α that mediates restriction is a multimeric complex. In principle, therefore, TRIM5α-based restriction factors could recognize incoming capsids in a polyvalent manner. Indeed, observations based on saturation of the owl monkey TRIM-CypA protein with particles derived from a panel of HIV-1 Gag mutants suggest that polyvalent interactions between incoming capsids and restriction factors occur (7). However, although multimerizing properties of TRIM5α likely drive the formation of cytoplasmic bodies or aggregates at high expression levels (25), immunofluorescent localization of TRIM5α in strongly restricting cells indicated that formation of large cytoplasmic bodies is unlikely to be an integral part of the mechanism by which TRIM5 inhibits retrovirus infection.
FIG. 6.
Summary of the organization of TRIM5 based restriction factors indicating domains either demonstrated or implied, based on genetic evidence, to be responsible for the specificity with which incoming retrovirus capsids are recognized and for effecting restriction. Although this schematic drawing represents TRIM5 as a dimer, it is possible that TRIM5 forms higher-order multimers.
Owl monkey TRIM-CypA inhibition of HIV-1 is the only restriction axis where binding of the restriction factor a viral capsid protein has been clearly demonstrated (21). However, given that the B30.2/SPRY domain determines the specificity with which incoming capsids are recognized by huTRIM5α, it seems very likely that this TRIM5α domain binds directly to the incoming retroviral capsid. If the B30.2/SPRY domain is solely responsible for capsid binding, then recognition and restriction of retrovirus infection involves discrete domains of TRIM5α. Further details of the mechanism by which TRIM5-based restriction factors block retrovirus infection remain to be resolved. In particular, it will be interesting to determine how the amino-terminal domains of TRIM5-based restriction factors function to alter the fate of incoming retroviral capsids.
Acknowledgments
We thank Greg Towers for reagents and helpful discussions.
This study was supported by a grant from the National Institutes of Health R01AI050111 and R01AI64003 and by a postdoctoral fellowship from the American Foundation for AIDS research to T.H. P.D.B. is an Elizabeth Glaser scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 2.Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109-1115. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, K. N., M. Bock, G. Towers, and J. P. Stoye. 2001. Identification of the regions of Fv1 necessary for murine leukemia virus restriction. J. Virol. 75:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogerd, H. P., R. A. Fridell, W. S. Blair, and B. R. Cullen. 1993. Genetic evidence that the Tat proteins of human immunodeficiency virus types 1 and 2 can multimerize in the eukaryotic cell nucleus. J. Virol. 67:5030-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forshey, B. M., J. Shi, and C. Aiken. 2005. Structural requirements for recognition of the human immunodeficiency virus type 1 core during host restriction in owl monkey cells. J. Virol. 79:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, G., X. Guo, and S. P. Goff. 2002. Inhibition of retroviral RNA production by ZAP a CCCH-type zinc finger protein. Science 297:1703-1706. [DOI] [PubMed] [Google Scholar]
- 9.Goff, S. P. 2004. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 38:61-85. [DOI] [PubMed] [Google Scholar]
- 10.Goff, S. P. 1996. Operating under a Gag order: a block against incoming virus by the Fv1 gene. Cell 86:691-693. [DOI] [PubMed] [Google Scholar]
- 11.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 12.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc. Natl. Acad. Sci. USA 101:10774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keckesova, Z., L. M. Ylinen, and G. J. Towers. 2004. The human and African green monkey TRIM5α genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. USA 101:10780-10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225:300-305. [DOI] [PubMed] [Google Scholar]
- 16.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45:163-169. [PubMed] [Google Scholar]
- 17.Lilly, F. 1967. Susceptibility to two strains of Friend leukemia virus in mice. Science 155:461-462. [DOI] [PubMed] [Google Scholar]
- 18.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 19.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defense by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 21.Nisole, S., C. Lynch, J. P. Stoye, and M. W. Yap. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA 101:13324-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odaka, T., and T. Yamamoto. 1965. Inheritance of susceptibility to Friend mouse leukemia virus. 11. Spleen foci method applied to test the susceptibility of crossbred progeny between a sensitive and a resistant strain. Jpn J. Exp. Med. 35:311-314. [PubMed] [Google Scholar]
- 23.Perron, M. J., M. Stremlau, B. Song, W. Ulm, R. C. Mulligan, and J. Sodroski. 2004. TRIM5α mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. USA 101:11827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pincus, T., J. W. Hartley, and W. P. Rowe. 1971. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J. Exp. Med. 133:1219-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reymond, A., G. Meroni, A. Fantozzi, G. Merla, S. Cairo, L. Luzi, D. Riganelli, E. Zanaria, S. Messali, S. Cainarca, A. Guffanti, S. Minucci, P. G. Pelicci, and A. Ballabio. 2001. The tripartite motif family identifies cell compartments. EMBO J. 20:2140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 27.Stoye, J. P. 1998. Fv1, the mouse retrovirus resistance gene. Rev. Sci. Technol. 17:269-277. [DOI] [PubMed] [Google Scholar]
- 28.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 29.Xu, L., L. Yang, P. K. Moitra, K. Hashimoto, P. Rallabhandi, S. Kaul, G. Meroni, J. P. Jensen, A. M. Weissman, and P. D'Arpa. 2003. BTBD1 and BTBD2 colocalize to cytoplasmic bodies with the RBCC/tripartite motif protein, TRIM5δ. Exp. Cell Res. 288:84-93. [DOI] [PubMed] [Google Scholar]
- 30.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 101:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap, M. W., S. Nisole, and J. P. Stoye. 2005. A single amino acid change in the SPRY domain of human Trim5α leads to HIV-1 restriction. Curr. Biol. 15:73-78. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]