ABSTRACT
Musculoskeletal tuberculosis (TB) accounts for approximately 10% of all extrapulmonary TB cases in the United States and is the third most common site of extrapulmonary TB after pleural and lymphatic disease. Vertebral involvement (tuberculous spondylitis, or Pott’s disease) is the most common type of skeletal TB, accounting for about half of all cases of musculoskeletal TB. The presentation of musculoskeletal TB may be insidious over a long period and the diagnosis may be elusive and delayed, as TB may not be the initial consideration in the differential diagnosis. Concomitant pulmonary involvement may not be present, thus confusing the diagnosis even further. Early diagnosis of bone and joint disease is important to minimize the risk of deformity and enhance outcome. The introduction of newer imaging modalities, including MRI (imaging procedure of choice) and CT, has enhanced the diagnostic evaluation of patients with musculoskeletal TB and for directed biopsies of affected areas of the musculoskeletal system. Obtaining appropriate specimens for culture and other diagnostic tests is essential to establish a definitive diagnosis and recover M. tuberculosis for susceptibility testing. A total of 6 to 9 months of a rifampin-based regimen, like treatment of pulmonary TB, is recommended for the treatment of drug susceptible musculoskeletal disease. Randomized trials of tuberculous spondylitis have demonstrated that such regimens are efficacious. These data and those from the treatment of pulmonary TB have been extrapolated to form the basis of treatment regimen recommendations for other forms of musculoskeletal TB.
INTRODUCTION
Musculoskeletal tuberculosis (TB) accounts for approximately 10% of all extrapulmonary TB cases in the United States and is the third most common type of extrapulmonary TB after pleural and lymphatic disease. Vertebral involvement (tuberculous spondylitis, or Pott’s disease) is the most common type of skeletal TB, accounting for about half of all cases of musculoskeletal TB. The presentation of musculoskeletal TB may be insidious over a long period and the diagnosis may be elusive and delayed, as TB may not be the initial consideration in the differential diagnosis. The diagnosis is often confused with malignancy. Concomitant pulmonary involvement may not be present, thus confusing the diagnosis even further.
Ancient skeletal remains dating back several thousand years have preserved the history of skeletal TB. Egyptian mummies are some of the oldest specimens and demonstrate evidence of spinal TB, as well as psoas abscesses (1). PCR has confirmed that these ancient lesions are due to Mycobacterium tuberculosis and not Mycobacterium bovis as others have suggested (2). There is also evidence of skeletal TB, including vertebral disease, in pre-Columbian, New World remains (3). Molecular diagnostic studies have also confirmed these lesions as M. tuberculosis (4, 5). These findings demonstrate that TB was present in the New World prior to the arrival of Europeans, which had been disputed for some time.
Vertebral TB was described by Hippocrates in ancient Greece. Sir Percivall Pott in 1779 was the first to describe the modern presentation of the clinical aspects of vertebral TB when he described a patient that had spinal deformity with paraplegia. He even proposed drainage of adjacent paraspinal abscesses, which frequently are seen in skeletal TB, and reported improvement in symptoms after the procedure (6). It was not until the late 19th century, after the description of the tubercle bacillus in 1882 by Robert Koch, that Pott’s disease was linked to illness caused by M. tuberculosis.
EPIDEMIOLOGY
The incidence of TB in the United States declined significantly during most of the 20th century and into the 21st century. However, in the mid-1980s through the early 1990s, there was a resurgence of this ancient disease. Between 1985 and 1992 there were an additional 40,000 cases of TB in the United States that were unexpected. This resurgence of TB was due to several factors, including the human immunodeficiency virus (HIV) epidemic and a breakdown in the public health infrastructure in the United States for TB control due to decreases in funding. As cases of pulmonary TB increased, a rise in the number of extrapulmonary disease cases, including musculoskeletal TB, was seen. Enhanced efforts at TB control (including increased funding) were implemented in response to the resurgence and led to a subsequent decline in TB cases and incidence in the United States beginning in 1993. In 2015, a total of 9,557 TB cases were reported in the United States, for a TB rate of 3.8 cases per 100,000 population, which is the lowest ever reported (7). Most TB cases in the United States (approximately two-thirds) now occur among foreign-born persons, reflecting the global TB epidemic (7). The large majority of extrapulmonary TB cases, including musculoskeletal TB, diagnosed in the United States also occur among foreign-born persons.
Bone and joint TB cases have consistently accounted for 2 to 3% of all reported TB cases in the United States despite an overall decrease in total TB cases (Table 1); in some series reported from outside the United States, >6% of TB cases have been due to bone and joint disease (8). Data from the preantibiotic era demonstrated that half of those with musculoskeletal TB had evidence of coexisting pulmonary disease (9). As the number of reported TB cases and incidence of TB disease have decreased in the United States, an increasing racial disparity has been noted, with more cases occurring among minorities and the foreign-born. Reports from the United States and United Kingdom have indicated that about three-fourths of all patients with musculoskeletal TB are foreign-born (10). This is important for clinicians to remember, as multiple studies have shown that immigrants (i.e., foreign-born persons) have accounted for an increasing percentage of extrapulmonary TB cases and the diagnosis is often delayed (7, 8, 10, 11). Extrapulmonary TB is more common among patients with HIV infection, but musculoskeletal TB is not necessarily increased in HIV-seropositive patients compared to those that are HIV seronegative (12). Tumor necrosis factor alpha (TNF-α) inhibitors have been shown to greatly increase the risk of disseminated TB disease among patients with latent TB infection, and recent reports have noted that this includes the development of serious musculoskeletal infections (13).
TABLE 1.
Numbers and proportions of musculoskeletal TB cases in the United States, 1993 to 2015a
| Yr | No. of bone/joint/skeletal TB cases | % of U.S. cases due to bone/joint/skeletal TB | Total no. of TB cases reported (U.S.) |
|---|---|---|---|
| 1993 | 641 | 2.55 | 25,102 |
| 1994 | 563 | 2.33 | 24,206 |
| 1995 | 541 | 2.38 | 22,726 |
| 1996 | 559 | 2.64 | 21,210 |
| 1997 | 511 | 2.59 | 19,751 |
| 1998 | 491 | 2.69 | 18,286 |
| 1999 | 471 | 2.69 | 17,499 |
| 2000 | 457 | 2.80 | 16,308 |
| 2001 | 433 | 2.72 | 15,945 |
| 2002 | 462 | 3.07 | 15,055 |
| 2003 | 435 | 2.93 | 14,835 |
| 2004 | 435 | 3 | 14,499 |
| 2005 | 423 | 3.01 | 14,060 |
| 2006 | 421 | 3.07 | 13,728 |
| 2007 | 376 | 2.83 | 13,282 |
| 2008 | 389 | 3.02 | 12,893 |
| 2009 | 357 | 3.1 | 11,520 |
| 2010 | 357 | 3.2 | 11,159 |
| 2011 | 357 | 3.4 | 10,510 |
| 2012 | 343 | 3.45 | 9,942 |
| 2013 | 328 | 3.43 | 9,550 |
| 2014 | 313 | 3.33 | 9,406 |
| 2015 | 297 | 3.11 | 9,557 |
Data obtained from CDC, Division of TB Elimination.
In countries where TB is endemic, older children and young adults are most commonly affected with musculoskeletal TB, while in developed countries, the disease is often seen in older persons (14). Historically, musculoskeletal TB was a disease of children and adolescents, often seen developing within a few years after primary infection. In resource-limited areas, this is still often the case, whereas in the developed countries, musculoskeletal TB most commonly results from reactivation.
Osteoarticular lesions result from hematogenous spread of a primary infection. Any bone, joint, or bursa can be infected, but the spine, hip, and knee, in order of frequency, are the preferred sites of infection, representing 70 to 80% of infections (14, 15). The diagnosis of musculoskeletal TB is often delayed due to the indolent nature of the disease and low clinical suspicion in areas with a low incidence of TB disease. In a series of 194 patients from India with musculoskeletal TB, 30% of cases occurred during the second decade of life, 22% in the first decade, 18% in the third decade, and 14% in the fourth decade (16). However, in developed countries with a lower prevalence of TB, musculoskeletal TB is seen more frequently in the adult age group, especially among foreign-born persons.
Vertebral or spinal TB is the most common type of musculoskeletal TB, accounting for about 50% of cases in most series. In Los Angeles County, CA, a total of 220 cases of musculoskeletal TB were registered between 1990 and 1995; the distribution of sites was as follows: 118 (54%) had vertebral TB, 56 (26%) had joint involvement (29 cases [13%] with knee involvement, 18 [8%] with hip involvement, and 9 [4%] with wrist involvement), and 10 (4%) had soft tissue/muscle involvement (Table 2) (15). A report from India with 194 cases of musculoskeletal TB noted reported the distribution of cases as follows: 49% had vertebral disease, 34 (18%) had knee involvement, 32 (16%) had hip involvement, 15 (8%) had ankle/foot involvement, 8 (4%) had elbow involvement, 4 (2%) had hand involvement, and 3 (1%) had wrist involvement (16). Other sites in this series with two or fewer cases included the ileum, shoulder, rib, pubis, calcaneus, femur, and sacro-iliac joint. TB can also cause disease of the peripheral joints, skull, and ribs, but these are less common manifestations (17).
TABLE 2.
Anatomic sites of musculoskeletal TB reported in Los Angeles County from 1990 to 1995
| Group | Site | No. | % of group | % of total |
|---|---|---|---|---|
| Spine (n = 118) | Cervical | 6 | 5 | 3 |
| Thoracic | 45 | 38 | 21 | |
| Lumbar | 65 | 55 | 30 | |
| Sacrum | 2 | 2 | 1 | |
| Peripheral joints (n = 78) | Hip | 18 | 23 | 8 |
| Knee | 29 | 37 | 13 | |
| Ankle | 5 | 6 | 2 | |
| Foot | 7 | 9 | 3 | |
| Shoulder | 4 | 5 | 2 | |
| Elbow | 5 | 6 | 2 | |
| Wrist | 9 | 12 | 4 | |
| Finger | 1 | 1 | 0.5 | |
| Other (n = 24) | Other Bone/soft/tissue/muscle | 14 | 58 | 6 |
| 10 | 42 | 4 |
PATHOGENESIS
The common lesion in skeletal TB consists of both osteomyelitis and arthritis. Bone involvement usually is the result of hematogenous spread of M. tuberculosis (especially occurring following primary infection), but bone and joint involvement can also be due to lymphatic drainage or secondary to a contiguous focus of disease. The growth plates (metaphyses) receive the richest blood supply and are most often the initial site of infection. Tubercle bacilli invade the end arteries, causing endarteritis and bone destruction through the epiphysis. After crossing the epiphysis, bacilli can drain into the joint space, resulting in tuberculous arthritis, or form a sinus tract after being released from the destroyed bone. M. tuberculosis does not produce any cartilage-destroying enzymes as are seen in pyogenic infections. If the infection progresses without treatment, abscesses surrounding the joint or bone may develop. These are often described as being “cold” abscesses. The abscesses may rupture, forming sinus tracts which have long been associated with musculoskeletal TB. Healing of musculoskeletal TB, especially of the joints, involves the formation of fibrous scar tissue. Calcifications are also frequently seen in healed lesions, especially if an abscess, infected bursa, or paraspinous mass was involved. A calcified psoas muscle in someone with healed Pott’s disease (spinal TB) is a classic example of this. The same hematogenous spread of tubercle bacilli can also primarily infect the synovium, bursae, or tendon sheaths, although this occurs much less frequently than bone involvement.
In children, the main route of infection in skeletal TB is through hematogenous spread from a primary source. Children may also experience musculoskeletal TB from reactivation of a quiescent focus after the development of latent TB infection, as occurs not uncommonly among adults. Children historically have been most affected with musculoskeletal TB because of the increased vascularity of their bones during growth, thus making them more susceptible during the period of hematogenous dissemination (e.g., following primary infection). Large weight-bearing bones and joints are the most commonly affected. Muscles are rarely primarily infected in adults or children, but tuberculous myositis may occur secondarily from contiguous bone infection or a draining sinus tract, as is seen with psoas muscle involvement that occurs secondary to Pott’s disease (18).
After the dissemination of bacilli to the bone, a granulomatous inflammatory response ensues. Biopsies of bone samples from those with skeletal TB reveal few organisms compared with pulmonary TB. The infected area consists of abscess and granulation tissue, and histology demonstrates giant cells, epithelioid histiocytes, and a mantle of lymphocytes and plasma cells, with an outer layer of proliferating fibroblasts and granulation tissue. As the area of infection enlarges, the center becomes necrotic, resulting in an area of caseating necrosis. This caseation may progress to cause bone expansion and eventually destruction of the cortex. A pathological feature of tuberculous osteomyelitis is that there is usually no bone regeneration (sclerosis) or periosteal reaction (19).
PATHOPHYSIOLOGY
Tuberculous Spondylitis
Sir Percivall Pott described the classic presentation of vertebral TB in 1779 as destruction of two or more contiguous vertebrae and apposed end plates, commonly associated with a paraspinal mass or abscess (20). In 1936, Compere and Garrison from the University of Chicago provided classic descriptions of vertebral TB, comparing radiologic findings with pathology and autopsy findings (21). They also compared findings at autopsy of patients with tuberculous spondylitis and pyogenic infections. Their descriptions of tuberculous spondylitis noted that the anterior portion of the vertebral body is much more commonly affected than the posterior components of the vertebrae. From this site, TB may spread to adjacent intervertebral disks. More than one vertebra is usually involved because of the contiguous spread along the anterior longitudinal ligaments. Skip lesions can also occur.
Tuberculous spondylitis begins with infection of the subchondral bone that then spreads to the cortex. The cartilage resists destruction by M. tuberculosis and despite there being a rich blood supply to the vertebrae, there is no blood supply to the disk (22). The anterior portion of the vertebral body is the most affected, with sparing of the posterior components. Involvement of the posterior components (i.e., laminae, pedicles, transverse processes, and spinous processes) is rare (23). In children, the intervertebral disk is vascularized; therefore, tuberculous diskitis in children may be the result of primary infection. In adults, the disk is avascular and disk disease is due to the contiguous spread of infection from the vertebral body. The narrowing disk space visible in adults on plain radiography is more often due to collapse of the vertebral end plate than to destruction of the disk itself (24). Collapse of the anterior spinal elements results in a kyphotic deformity, giving the hunchback appearance and Gibbus deformity associated with Pott’s disease. TB of the vertebral skeleton causes lytic destruction without new bone formation (sclerosis). The infection can extend to the soft tissue, forming paraspinal abscesses. The degree of the kyphosis is proportional to the initial loss of vertebral body volume and continues until the vertebral bodies meet anteriorly or until the caseous material and granulation tissue mature into bone (25).
Vertebral TB most commonly involves the thoracic and lumbar regions of the spine. Historically, the thoracic vertebrae have been the most commonly affected area of the spine. Some reports have indicated that the thoracic spine is involved in about 50% of spinal TB cases, whereas the lumbar spine is involved in 25% of cases (26). Cervical and sacral involvement occurs less frequently. However, other more recent reports have suggested that among adults, lumbar involvement may be most common. Of 118 cases of vertebral TB reported from a series in Los Angeles, 65 patients had lumbar tuberculous spondylitis, 45 thoracic, 6 cervical, and 2 sacral. Cervical involvement is frequently associated with retropharyngeal abscess and severe neurologic defects (27).
Spinal deformity and paraplegia/quadriplegia are the most common and serious complications of TB of the spine. Rarely do they result directly from the kyphotic defect unless it is severe enough to cause subluxation of the spine. Paraplegia can be due to compression of the spinal canal by an adjacent abscess, sequestra of the vertebral body or disk, or direct dural invasion. Cervical vertebral TB is highly associated with early and severe neurologic compromise (28).
Paraspinal abscesses are quite common in vertebral TB, occurring in more than 90% of cases. The abscess may extend anteriorly to adjacent ligaments and soft tissues, or it may also extend posteriorly into the epidural space. Because the abscesses can spread beneath the ligament, distant sites may be involved. There are also reports of tuberculous paraspinal abscesses eroding into internal organs or to the body surface (29). Lumbar disease may spread into or beneath the psoas-iliac muscle, causing abscesses in the thigh. Sacral lesions have been reported to extend into the perineum.
Pyogenic abscesses differ from vertebral TB in several ways. Pyogenic abscesses destroy the disk rapidly, resulting in early disk space narrowing. Calcification of a large paraspinous abscess, which may be seen with TB, is not a prominent feature of a pyogenic abscess. In a study comparing pyogenic, tuberculous, and brucellar vertebral osteomyelitis in Spain, patients with vertebral TB were found to be more likely to have a prolonged clinical course, thoracic segment involvement, absence of fever, presence of spinal deformity, neurologic deficit, and paraspinal or epidural masses (30).
Tuberculous Osteomyelitis and Arthritis
Tuberculous osteomyelitis may extend to a joint or tenosynovium. In adults, the lesion may be single and affect any bone, including long bones, the pelvis, ribs, and skull. In children, multiple lesions in long bones dominate, but the bones of the hands and feet may be affected. Tuberculous dactylitis (involvement of the short bones of the hands or feet) is more common among children than adults. Tuberculous osteomyelitis has a predilection for the metaphysis of long bones such as the femur, tibia, and ulna. In children, TB may violate the growth plate and lesions of the epiphysis may extend into the joint space. Destruction of the epiphyseal growth plates in children can also result in shortening of the affected limb. Although uncommon, TB can also involve the ribs and skull. The skull contains little cancellous bone which is usually affected by M. tuberculosis. Disease involving the skull occurs more often in children and anecdotally may be associated with head trauma (31). In a review of 223 cases of TB involving the skull, Strauss found that only 15 had associated central nervous system disease (10 with meningitis and 5 with cerebral TB). This is thought to be due to the dura being resistant to infection with M. tuberculosis (32). TB of the ribs can occur either by hematogenous seeding of the ribs or, in some cases, due to contiguous spread in a patient with pulmonary disease. TB of the ribs (Fig. 1) is an uncommon manifestation of the disease but is the second most common cause of nontraumatic rib lesions after malignancy (33). A closed cystic form of skeletal TB can occur, especially in the long bones, and may not have associated sclerosis, osteopenia, or abscess/sinus tract formation as in other forms of skeletal TB. This form of TB is more likely to occur in children and may be misdiagnosed as a malignancy (34, 35).
FIGURE 1.
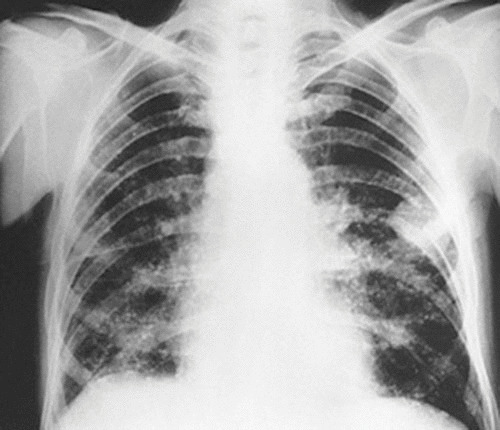
TB of the rib. Shown is a postero-anterior radiographic view of the chest of a man after 3 months of successful anti-TB chemotherapy. Note the mass in the left chest with destruction of a portion of the adjacent rib. A biopsy and culture confirmed TB. The mass resolved with continued therapy.
Tuberculous osteomyelitis is frequently complicated by tuberculous arthritis (discussed below) as well as by the development of cold abscesses that may form around the adjacent bone process and can rupture, creating draining sinus tracts (Fig. 2). Cold abscesses are composed of white blood cells, products of caseating necrosis from the tubercle, bone debris, and tubercle bacilli. Cold abscesses appear to occur commonly among HIV-infected patients.
FIGURE 2.
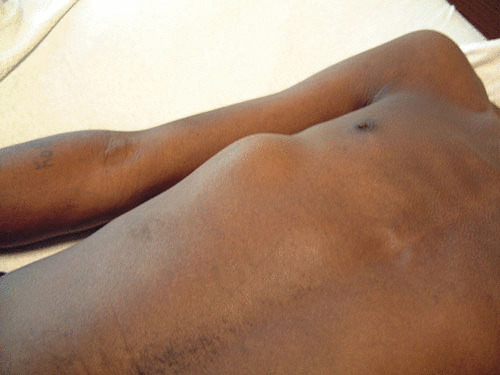
This photograph demonstrates a cold abscess of the chest wall in a patient with TB. Aspiration of this mass yielded material that was AFB smear positive, and the culture yielded M. tuberculosis.
Several reports have noted an association between mechanical factors such as trauma and the development of skeletal TB. In a Canadian study of 99 patients with skeletal TB, 30 had a history of trauma preceding their presentation and 7 had a recent history of intra-articular steroid injection. This may also explain why weight-bearing joints are most frequently involved. Trauma may be associated with skeletal TB because of resulting increased vascularity, decreased resistance, or unmasking of latent infection (15, 31).
Tuberculous Arthritis
Tuberculous arthritis most typically involves large weight-bearing joints such as the hip and the knee, although any other joint may be affected. Invasion of the joint space may be either hematogenous seeding or indirectly from lesions in epiphyseal bone (in adults) or metaphyseal bone (in children) eroding into the joint space. Contiguous spread of TB from other organs to bones may also occur. In long bones, hematogenous spread commonly affects the synovium, causing an erosive deforming arthritis that is monoarticular in about 90% of cases. Initially, the synovium develops an inflammatory reaction, followed by formation of granulation tissue, which leads to the development of a pannus. The pannus can erode the margins and surface of the joint. As the effusion develops, fibrin may precipitate, forming “rice bodies” seen in the synovial fluid, bursae, and tendon sheaths. Rice bodies are not unique to TB, as they can also be seen in rheumatoid arthritis. The infection then spreads to the epiphysis and upper metaphysis on either side of the joint through the periarticular vasculature. The granulation tissue erodes and eventually destroys the cartilage, eventually leading to demineralization of the bone and caseating necrosis. In advanced and late disease, paraosseous cold abscesses develop surrounding the joints. Spontaneous drainage of cold abscesses results in sinus tract formation.
Poncet’s Disease (Poncet’s Arthritis)
Poncet’s disease is a reactive polyarthritis usually associated with extrapulmonary TB (e.g., visceral or disseminated TB) in the absence of any evidence of mycobacterial infection of the joints. Poncet’s disease was originally described by Charcot in 1864 and Lancereaux in 1871, but Anton Poncet first gave a detailed description of this syndrome when he described polyarthritis occurring in a 15-year-old with suppurative TB of the hip in 1897 (36). Poncet’s arthritis is a reactive form of arthritis and is a separate entity from TB directly affecting joint spaces. Usually, it occurs during acute TB infection and is a polyarticular process associated with fever. Erythema nodosum may be a hallmark of disease. The pathogenesis of Poncet’s disease is unclear. As noted, joint fluid analysis does not reveal the presence of M. tuberculosis disease in the joint space, and clinical symptoms resolve with anti-TB therapy. In a recent review, extrapulmonary TB was present in half of the patients with Poncet’s disease, and only 6% of the patients presented with erythema nodosum (37).
Tuberculous Myositis
Tuberculous myositis is an uncommon manifestation of the disease and usually the result of contiguous infection, especially seen in Pott’s disease (38, 39). Tuberculous myositis can also be “primary” (i.e., a cause of pyomyositis), resulting from hematogenous spread, but this is a less common manifestation of disease and much less likely to occur than myositis due to secondary or contiguous spread (18, 40). The tuberculous lesion of primary myositis may present as a solitary nodule with epithelioid granuloma and caseating necrosis or a cystic formation containing a gelatinous material enclosed by a thick wall (41). The most common presentation of tuberculous myositis is a psoas abscess due to a complication of and contiguous spread from tuberculous spondylitis; this may extend below the inguinal ligament (38). Tuberculous myositis has been reported more frequently among HIV-infected patients (42).
CLINICAL FEATURES
The onset of musculoskeletal TB is usually an insidious process that often takes months and occasionally years from the first symptoms to the time of diagnosis. Presentation depends upon the stage of the disease, site of the disease, and presence of complications such as neurologic deficits, abscesses, or sinus tracts. Local pain and tenderness are generally the presenting symptoms, followed by impairment of function and swelling of the affected part. Regional muscle wasting and joint deformity are common findings. In some cases, a painless cold abscess (Fig. 2) may be the only presenting clinical feature for an extended period. Systemic symptoms such as fever, night sweats, and weight loss may be seen with early disease but are more likely to occur with advanced cases. About half of the cases of musculoskeletal TB have evidence of active or healed pulmonary TB (16). A single site of involvement is generally seen, but multiple locations are not uncommon. Multiple lesions occur more often in those who are immunocompromised, including persons with HIV infection. Several studies have reported that >90% of patients with musculoskeletal TB were tuberculin skin test positive (43); however, these studies were largely carried out in the pre-HIV era.
Tuberculous Spondylitis
Tuberculous spondylitis remains the most common manifestation of musculoskeletal TB. The progression of disease is usually slow and insidious, and the main symptom, back pain, is not specific. This frequently results in delayed diagnosis, resulting in diagnosis from weeks to years after the onset of symptoms. Pertuiset et al. reported a median of 4 months’ duration of symptoms prior to diagnosis, with a range of 1 week to 3 years among 103 patients with spinal TB, and noted weight loss in 48%, fever (>38°C) in 31%, and night sweats in 18% (44). As the disease advances, cold abscesses, neurologic deficits, sinus tract formation, and kyphotic deformities can develop. Cold abscesses of the paraspinal tissues or psoas muscle abscesses may be large and found to protrude under the inguinal ligament when a patient is examined for the first time. Some degree of kyphosis is frequently present. Weakness and paralysis of the lower extremities may occur early during the disease process. HIV infection has not been shown to alter the clinical presentation or course of tuberculous spondylitis (12).
On physical examination, there may be focal tenderness over the spinal processes as well as back spasm. Fluctuance, erythema, and focal warmth on exam are unusual findings, as the spinal infection typically involves the anterior column of the spine (45). Range-of-motion testing may produce severe pain, and especially with advanced disease, focal kyphosis can be seen on physician examination. Neurologic symptoms may be subtle at first and progress over time. Initially, these include numbness and tingling in the lower extremities or a subjective sense of weakness with activity. With more advanced disease there is evidence of spinal cord compression, which can result in paraplegia. Between 10 and 25% of patients in reported case series have had paraplegia (44). Patients with cervical disease are prone to developing neurologic compromise very quickly and may develop retropharyngeal abscesses (27, 46, 47). Cervical TB can also manifest as torticollis, dysphagia, hoarseness, and cranial nerve 12 palsy, depending upon which level of the cervical spine is affected (48). The degree of neurologic damage correlates with prognosis, with those presenting with complete motor loss being unlikely to recover neurologically. Extrinsic cord compression can occur due to vertebral subluxation, collapse of a vertebral body, or an extradural abscess.
Tuberculous Arthritis
Tuberculous arthritis usually occurs as a monoarthritis mostly of weight-bearing joints such as the knee, hip, or ankle. It is slowly progressive and characterized by painful, boggy swelling caused by synovial hypertrophy and effusion (Fig. 3). Eventually, ankylosis of the joint may occur. Periarticular abscesses and draining sinus tracts are late findings. Pain is such a prominent symptom that it may lead to immobility of the affected joint. Prolonged immobility may eventually lead to deformity, especially of the knee and hip. Tuberculous arthritis clinically can mimic other processes such as gout or juvenile rheumatoid arthritis, making the diagnosis confusing and delayed (49). Polyarticular tuberculous arthritis has been reported but is rare (50).
FIGURE 3.
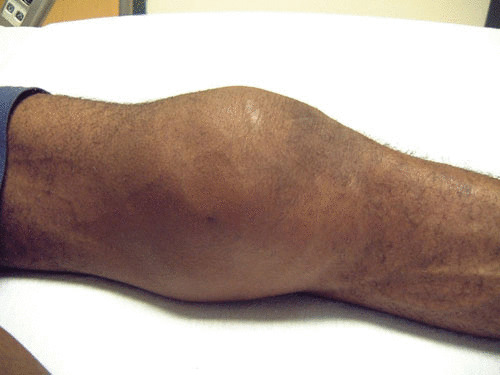
Swollen knee of a patient with tuberculous arthritis. An HIV-infected patient presented with a painful, swollen knee. He had a recent history of trauma to the knee. On examination the knee was warm and an effusion was present. Culture of the synovial fluid following arthrocentesis grew M. tuberculosis.
Tuberculous Osteomyelitis
Tuberculous osteomyelitis often occurs in conjunction with tuberculous arthritis, but it can occur as a distinct entity without joint involvement. In adults, tuberculous osteomyelitis without joint involvement usually presents as a single lesion, usually in the metaphysis of long bones (e.g., femur and humerus), although the ribs, pelvis, skull, mastoid, and mandible can be affected. In children, older adults, and immunocompromised persons, including those with HIV infection, the lesions may be multiple (51). In children, the lesions may affect the short bones of the hands and feet; tuberculous dactylitis has been reported to occur in adults but is unusual. Patients with widespread lesions may be misdiagnosed as having a malignant process. Bacterial superinfection can also mask the diagnosis and presentation, as there are reports of infection due to coexisting Staphylococcus aureus infection and TB (52, 53).
Tuberculous osteomyelitis usually manifests with pain and swelling adjacent to the bone, with eventual limitation of movement of the affected limb. Symptoms may be present for 6 to 24 months before a diagnosis is made. Fever, weight loss, and night sweats are often present. Abscesses and sinus tracts may occur, often later in the course. Tuberculous involvement of the skull may be associated with headaches and soft tissue masses. TB involving the ribs manifests with chest pain and sometimes with a “cold” chest wall mass (Fig. 2). Infection of bones of the head and neck, especially the mastoid and mandible, has been reported to result from tuberculous otitis and disease involving the oral cavity. Facial paralysis can occur secondarily to tuberculous mastoiditis (54). TB of the temporomandibular joint has also been reported as a cause of chronic temporomandibular joint pain (55). TB of the sternum can manifest as anterior chest pain (56).
Tuberculous Tenosynovitis
Tuberculous tenosynovitis is an unusual manifestation of the disease. When it occurs, it may occur in conjunction with another form of skeletal TB, such as TB of the carpal bones in the hand or, rarely, hematogenous spread to the synovium (57). Carpal tunnel syndrome is a common presentation of tuberculous synovitis (58, 59). Carpal tunnel syndrome occurs when the median nerve, going through the flexor compartment of the wrist, is compressed secondarily to thickening and edema of the tendon sheath. Diagnosis is often made late in the course due to its slow progression, indolent symptoms, and the fact that the diagnosis may be delayed because infectious causes are not considered initially (60). Presenting symptoms include swelling, followed by wrist pain, numbness and tingling of the fingers, and decreased range of motion (57). Other causes of carpal tunnel syndrome in the differential diagnosis include trauma, diabetes, amyloidosis, and sarcoidosis. Another presentation of tuberculous tenosynovitis is a ganglion formation along the volar carpal ligament that manifests with soft tissue swelling above the flexor retinaculum (61).
DIAGNOSIS
A high index of suspicion is needed for the diagnosis of musculoskeletal TB, especially given the insidious onset of symptoms and reports of a long duration between onset of symptoms and diagnosis of disease. In countries with a high burden of TB disease, musculoskeletal complaints may be attributed to TB correctly based on clinical and radiologic examination. In the developed world with a lower incidence of TB, the diagnosis may not be initially considered and the diagnosis is frequently delayed. Any bone or joint may be involved, but the spine and weight-bearing joints are the most common sites of infection. Pain is the most common complaint that leads a patient to seek medical care, and TB should be considered in the differential diagnosis of the cause of skeletal pain. Interestingly, local pain, swelling, and limitation of movement may even on occasion precede radiographic findings by up to 8 weeks (62). Cold abscesses can occur and sometimes with draining sinus tracts, but this is usually seen in advanced, untreated disease or among patients with HIV infection. The differential diagnosis of musculoskeletal TB includes other infectious causes of musculoskeletal disease (bacterial, fungal, and other mycobacterial pathogens), as well as malignancy, rheumatologic conditions, and sarcoidosis.
Imaging techniques, which include conventional radiography, computed tomography (CT), and magnetic resonance imaging (MRI), are useful in evaluation of patients with suspected musculoskeletal TB and other skeletal diseases. Use of newer techniques such as CT, MRI, and CT-guided fine-needle aspiration biopsy has revolutionized the diagnostic approach and has resulted in more accurate results and much less invasive procedures than when only plain radiography and open biopsy were available. Previously, conventional radiography had been the mainstay in the diagnosis of tuberculous arthritis and osteomyelitis. However, MRI is now accepted as the imaging modality of choice for diagnosis of tuberculous spondylitis and other types of musculoskeletal TB and can demonstrate the extent of the disease of tuberculous spondylitis and soft tissue TB (63, 64). MRI may be very helpful in providing diagnostic clues in the evaluation of spondylodiscitis, as it may easily demonstrate anterior corner destruction, the relative preservation of the intervertebral disk, multilevel involvement with or without skip lesions, and a large soft tissue abscess, as these are all arguments in favor of a tuberculous spondylitis (versus a pyogenic infection).
Since there are no pathognomonic radiographic findings, the diagnosis is usually made by tissue biopsy and/or culture data (14). Needle aspiration and biopsy can confirm the diagnosis with the findings of caseating granuloma and the presence of acid-fast bacilli (AFB). A positive culture for M. tuberculosis provides definitive evidence of tuberculous disease and allows antimicrobial susceptibility testing to be performed, which is essential for helping to prescribe optimal therapy. Fine-needle aspiration biopsy of involved bone (often CT directed) to obtain specimens for culture is useful diagnostically as well as for the draining of abscesses in certain situations (65). In addition to modern culture techniques performed on specimens obtained by biopsy of involved tissues, the use of molecular diagnostics to detect the presence of M. tuberculosis has the potential to improve the ability to diagnose skeletal and other types of musculoskeletal TB. While nucleic acid amplification tests have demonstrated high sensitivity and specificity for AFB smear-positive respiratory specimens, there are limited data on the utility of these tests for extrapulmonary TB (66). This is especially the case for the use of these molecular diagnostic tests for musculoskeletal TB. The currently commercially available and FDA-approved nucleic acid amplification tests are not approved for use in extrapulmonary TB, including musculoskeletal cases. While further data are needed on the utility of these tests in the aid of diagnosis of musculoskeletal TB, recent reports from South Africa appear promising and suggest that Xpert MTB/RIF may be a valuable diagnostic test for musculoskeletal TB in both adults and children (67, 68). Recent TB diagnostic guidelines published by the American Thoracic Society, Infectious Diseases Society of America, and CDC suggest that the quality of data for the utility of nucleic acid amplification tests performed on specimens from patients with suspected extrapulmonary TB is low: the test results are specific but may lack sensitivity (69). This suggests that a positive Xpert MTB/RIF is valuable but that a negative test does not rule out extrapulmonary TB, in this case musculoskeletal TB.
Tuberculous Spondylitis
Plain radiography is often the first imaging technique employed by clinicians when considering tuberculous spondylitis. At least 30 to 50% of the vertebra needs to be destroyed before the disease can be detected on plain radiography, making this an insensitive diagnostic tool (Fig. 4) (19, 70). Plain radiography may reveal several features indicative of tuberculous spondylitis, such as osteoporotic end plates, involvement of multiple levels, and anterior destruction leading to collapse (Table 3). Sometimes a paravertebral abscess or enlarged psoas muscle may be observed on plain radiography. Plain radiography will show calcifications in abscesses if they are present. Atypical features that may be seen on radiography include involvement of posterior elements, single vertebral involvement, and an “ivory” vertebra, which is the result of diffuse sclerosis (71).
FIGURE 4.
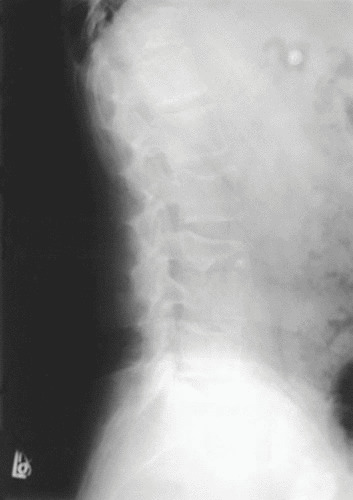
Plain film radiograph of the lumbar spine of a patient with tuberculous spondylitis demonstrating anterior end plate destruction, sclerosis, loss of disk space, and evidence of bony debris. These findings are suggestive of tuberculous spondylitis. A CT-directed biopsy was performed to obtain material for cultures, which yielded M. tuberculosis.
TABLE 3.
Radiographic characteristics of tuberculous spondylitis
| Multiple levels involved |
| Lytic destruction of anterior portion of vertebral body |
| Disk space narrowing |
| Vertebral end plate osteoporosis |
| Increased anterior wedging |
| Collapse of vertebral bodies |
| Paravertebral shadow of an abscess, sometimes with calcification |
| Enlarged psoas muscle shadow, often with calcifications |
Nuclear medicine imaging is not very useful in diagnosing tuberculous spondylitis because of its low sensitivity and is not recommended as a diagnostic imaging modality in the evaluation of patients with spondylitis. CT scanning is superior to both plain radiography and nuclear medicine imaging. CT has also proved useful in determining the extent of soft tissue infection, such as abscesses and draining sinus tracts as well as soft tissue calcifications (Fig. 5). Irregular lytic lesions, sclerosis, and disk collapse all can be demonstrated on CT. CT is also very helpful in providing guidance for percutaneous drainage and biopsy of vertebra-associated lesions and abscesses (72).
FIGURE 5.
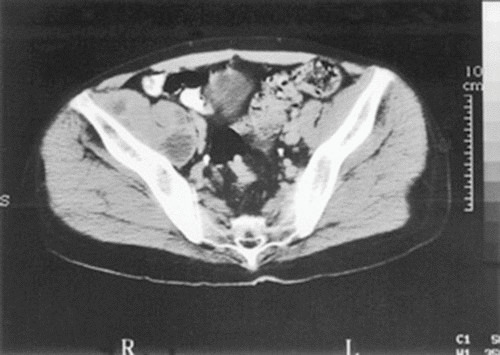
CT evaluation of patients with tuberculous spondylitis. CT demonstrated a large right psoas abscess in an HIV-infected patient with tuberculous spondylitis of the lumbar spine. A percutaneous drain was placed into the psoas abscess, and the fluid culture yielded M. tuberculosis.
As noted above, MRI is the imaging modality of choice for the evaluation of patients with suspected tuberculous spondylitis because it detects early marrow and paraspinal soft tissue changes with multiplanar capabilities and excellent soft tissue contrast resolution (19, 70). Contrast-enhanced MRI scanning is the optimal test for defining intraspinal extension, focal myelopathy, and spinal cord or nerve root compression (14). Paravertebral abscesses are often seen at multiple levels and show peripheral enhancement with central necrosis on contrast-enhanced images (20). MRI allows the entire spine to be viewed and provides high-contrast resolution. By comparing T1 and T2 weighted images, one can accurately differentiate various diseased tissues, delineate clearly vertebral bodies, and detect marrow infiltration, intervertebral disks, intraspinal contents, posterior vertebral components, meningeal involvement, and paraspinal tissues (Fig. 6). MRI best demonstrates the origin of the pathological tissue causing spinal cord compression (Fig. 6) by differentiating pus and granulation tissue from the bony, disk, or fibrotic causes of compression, thus helping to avoid unnecessary and aggressive surgical decompression (73).
FIGURE 6.
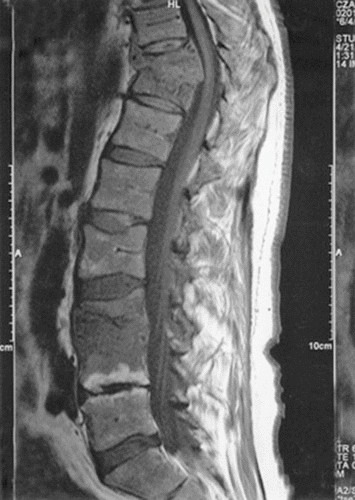
MRI of a patient with multifocal tuberculous spondylitis who has both thoracic and lumbar spine involvement which is not contiguous (i.e., skip lesions). The thoracic lesion reveals anterior collapse of adjacent vertebrae and a Gibbus formation leading to kyphosis. There is also evidence of lumbar disease in this patient.
Newer imaging techniques are helpful in distinguishing tuberculous spondylitis from other infectious etiologies and from neoplastic processes (Table 4). Two distinct patterns of vertebral TB may be seen. The first is the classic finding of spondylodiscitis, characterized by destruction of two or more contiguous vertebrae and opposed end plates, disk infection, and commonly a paraspinal mass or collection. The second pattern, increasing in frequency, is an atypical form of spondylitis without disk involvement (74). The combination of clinical findings, characteristic lesions on plain radiography, CT, and MRI, and sometimes either with a positive tuberculin skin test or gamma interferon release assay or evidence of pulmonary TB strongly suggests the diagnosis of tuberculous spondylitis. CT-directed biopsy can also be helpful by providing means for a directed biopsy to definitively make the diagnosis by obtaining material for pathological examination, culture, nucleic acid amplification testing (e.g., Xpert MTB/RIF), and susceptibility testing. It is essential to obtain appropriate specimens for mycobacterial cultures and other diagnostic tests to establish a definitive diagnosis. In spinal TB, a CT-guided biopsy often yields the diagnosis. Open spinal biopsy is generally reserved for cases of difficult diagnosis or when surgical therapy is otherwise indicated (14, 26).
TABLE 4.
Imaging features associated with tuberculous spondylitisa
| Imaging features suggestive of tuberculous spondylitis versus other infectious etiologies |
| More than one vertebra involved |
| Multicentric involvement |
| Relative sparing of the disk |
| Large paravertebral abscess |
| Paravertebral osseous debris |
| Subligamentous spread |
| Heterogenous signal intensity on MRI |
| Rim enhancement pattern on MRI |
| Imaging features suggestive of tuberculous spondylitis versus neoplastic disease |
| Paravertebral abscess |
| Paravertebral osseous debris |
| Subligamentous spread |
| Predominant distribution adjacent to end plates or vertebral corners |
Adapted from reference 71.
Tuberculous Arthritis
The classic triad of juxta-articular osteoporosis, peripheral osseous erosion, and gradual narrowing of the joint space comprises radiologic characteristics of tuberculous arthritis which were described by Phemister and Hatcher in 1933 (75). The joint space is preserved early in tuberculous arthritis, as opposed to most pyogenic infections, in which there is early destruction of cartilage due to the production of proteolytic enzymes by bacterial pathogens. The radiologic characteristics of pyogenic, tuberculous, and rheumatoid arthritis are shown in Table 5. The differential diagnosis of tuberculous arthritis includes bacterial and fungal infections of the joint as well as noninfectious processes (e.g., rheumatologic). Pyogenic (bacterial) arthritis is usually a monoarticular process as is seen with tuberculous arthritis, but the time course is usually more acute in those with a pyogenic bacterial infection than the more chronic and initially indolent course seen among patients with tuberculous arthritis. Trauma or bacteremia is frequently associated with pyogenic arthritis. Nocardia spp., Brucella spp., and Sporothrix schenckii can also cause a chronic monoarthritis resembling TB. In addition to pyogenic and other inflammatory processes, pigmented villonodular synovitis, which can cause synovial thickening and joint erosions, needs to be included in the radiographic differential diagnosis of TB arthritis (71).
TABLE 5.
Radiologic characteristics in the differential diagnosis of tuberculous arthritis
| Condition | Radiologic result | ||
|---|---|---|---|
| Osteopenia | Marginal erosions | Joint space narrowing | |
| TB | + | + | Late, mild |
| Pyogenic | +/− | + | Early, significant |
| Rheumatoid arthritis | + | + | Early, significant |
| Gout | Mild, absent | + | 0 |
Radiologic studies may provide some clues to the diagnosis as discussed above (Table 5) but cannot definitively differentiate bacterial from tuberculous arthritis. Because the process begins with synovial thickening and an effusion, joint space swelling is the first radiographic sign. Bone sequestration and dense triangular collections may be found at the edge of the joint. Marginal erosions are especially prominent in the weight-bearing joints (Fig. 7). Sclerosis is usually not seen in tuberculous arthritis except in children, in whom a layered periosteal reaction can be seen (19, 76). Eventually, severe joint destruction and fibrous ankylosis occur when the process is left untreated (Fig. 8 and 9).
FIGURE 7.
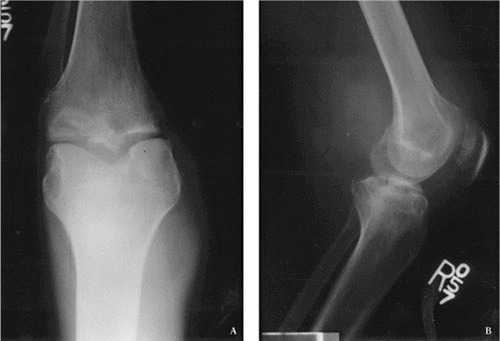
Radiographs of the knee of a patient with tuberculous arthritis. (A) Plain radiograph of the knee shown in Fig. 3. Marginal erosions are visible, along with soft tissue swelling.
FIGURE 8.
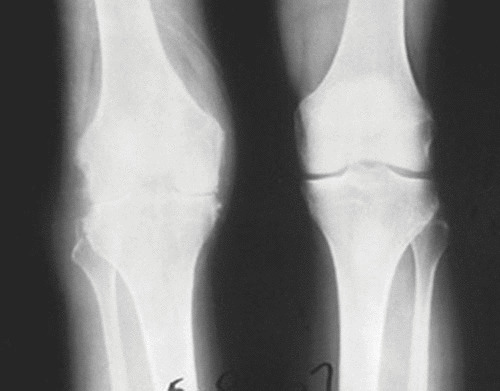
TB of the knee. The radiograph shows findings of TB (left) and the normal knee (right). Note the narrowed joint space, lytic bone destruction in the distal femur and proximal tibia, and soft tissue swelling in the abnormal knee, which had shown clinical evidence of TB for more than 10 years (but the patient had not undergone treatment).
FIGURE 9.
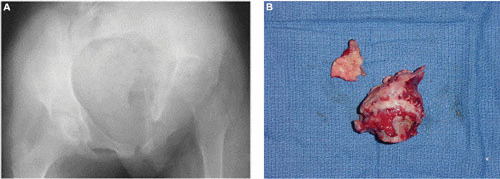
TB of the hip. (A) Plain radiograph of a 12-year-old girl who presented with an abnormal gait for several months. The left femoral head is completely destroyed. (B) Operative specimen of the destroyed femoral head.
Martini and Ouahes (43) summarized the radiologic changes of tuberculous arthritis in the following classification, which also reflects the pathogenesis of the infectious process:
Stage I: no bony lesions, localized osteoporosis
Stage II: one or more erosions or lytic lesions in the bone; discrete diminution of the joint space
Stage III: involvement and destruction of the whole joint without gross anatomic disorganization
Stage IV: gross anatomic disorganization
CT imaging of the joint may be useful for evaluating bony destruction and soft tissue swelling or abscess and any evidence of bony sequestration (Fig. 10), but in general, MRI is considered the imaging test of choice for musculoskeletal TB. MRI can detect earlier changes, especially synovial thickening and periarticular soft tissue changes, and has even been shown to demonstrate rice bodies in the joint space (77). An absence of marrow enhancement on MRI along with bony erosions is suggestive of tuberculous arthritis rather than a pyogenic process (76). MRI may reveal hemosiderin deposits in the synovium and demonstrate the erosions that occur with advanced disease and are often centrally located. As discussed below, a definitive diagnosis should be made. Pursuing arthrocentesis to obtain appropriate specimens (e.g., synovial fluid) or performing a synovial biopsy to obtain specimens for bacterial, fungal, and mycobacterial culture is crucial in establishing a definitive diagnosis and to recover the organism to make a microbiological diagnosis so that susceptibility testing can be performed. The synovial fluid is often nondiagnostic, and biopsy and culture of the synovium and periarticular bone are often necessary to make the diagnosis (14).
FIGURE 10.
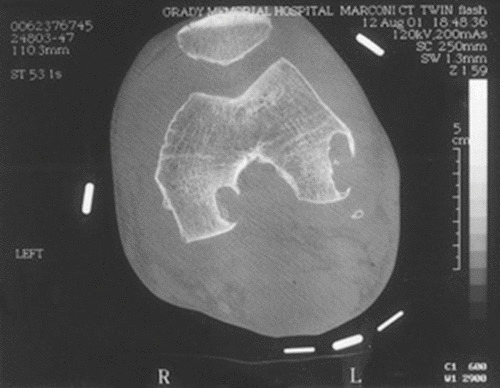
CT imaging of the knee of a patient with tuberculous arthritis, showing extensive marginal destruction as well as erosions.
Tuberculous Osteomyelitis
Radiographically, tuberculous osteomyelitis is often confused with malignancy, especially if the lesions are diffuse and lytic. Plain radiographs may show osteoporosis, lytic lesions, sclerosis, and periostitis. Sequestra may appear as spicules of increased radiodensity within the area of destruction. Cystic lesions may be seen, especially in children and young adults. The differential diagnosis of cystic bone lesions is shown in Table 6. The lesions in children are less well defined than in adults, in whom well-defined margins of sclerosis are usually present (34). Multifocal disease is an uncommon presentation and occurs primarily in children and the immunocompromised (78, 79). MRI is useful in detecting osteomyelitis early because of changes in the bone marrow. The normal marrow fat signal on T1 weighted images is replaced by low signal intensity, with corresponding high signal intensities on T2 weighted images and enhancement of T1 weighted images after gadolinium (19). Tuberculous lesions are rarely seen in the hands and feet, but tuberculous dactylitis occurring in children is a well-recognized entity. The typical radiologic appearance is a ballooned-out configuration of “spina ventosa” in which the dissolution of bone causes absorption of trabeculae and expansion of the affected digit (78).
TABLE 6.
Differential diagnosis of cystic bone lesions
| Cystic TB |
| Syphilis |
| Eosinophilic granuloma |
| Sarcoidosis |
| Cystic angiomatosis |
| Multiple myeloma |
| Neoplasm (metastatic, lymphoma, neuroblastoma) |
| Fungal infection (blastomycosis, coccidioidomycosis) |
Tuberculous Myositis
Tuberculous myositis is usually discovered secondarily to a known skeletal focus. Primary myositis can occur, especially in the immunocompromised patient, whereas secondary tuberculous myositis, which is much more common, is usually seen in conjunction with vertebral TB. The differential diagnosis of primary myositis or pyomyositis is shown in Table 7. Plain radiography may show calcifications or, in the case of a psoas abscess, an enlarged psoas shadow. CT imaging shows a well-marginated tumor-like lesion, either hypodense or isodense compared with normal muscle (Fig. 5 and 11). MRI (the imaging procedure of choice) can demonstrate a distinct mass that can be of either lower or higher density than the muscle depending on the T1 or T2 weighted image (80). CT and MRI cannot really differentiate tuberculous myositis from other causes of muscle lesions, but the diagnosis may be highly suspected in the proper clinical setting (e.g., TB at a contiguous or distant site (41). Ultimately, a definitive diagnosis needs to be based on histologic and microbiological studies. Psoas abscesses are often associated with vertebral TB, but other etiologies should be considered as well (Table 8). CT and MRI are both useful in detecting a psoas abscess. A calcified psoas muscle on plain film is pathognomonic of a tuberculous abscess secondary to vertebral disease (81).
TABLE 7.
Differential diagnosis of primary myositis
| TB |
| Actinomycosis |
| Sarcoidosis |
| Malignancy (sarcoma) |
| Bursa or tendon cyst |
| Hematoma |
| Pyogenic myositis |
| Melioidosis |
FIGURE 11.
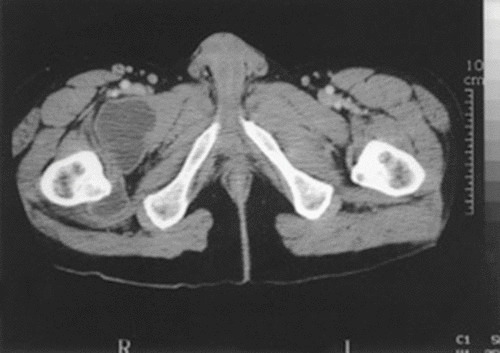
CT imaging of a psoas muscle abscess. The CT shows the lower extremities in a patient with lumbar tuberculous spondylitis who has a psoas abscess that extends into the right thigh.
TABLE 8.
Etiologies of a psoas muscle abscess
| Vertebral TB (Pott’s disease) |
| Diverticulitis |
| Appendicitis |
| Crohn’s disease |
| Postpartum infection |
| Neoplasm of spine or bowel |
| Renal calculi |
| Hematoma |
| Pyogenic infection (especially Staphylococcus aureus) |
| Actinomycosis |
Mycobacteriological and Histologic Diagnosis
Radiologic methods may suggest a diagnosis of TB, but bacteriological or histologic confirmation is required to prove the diagnosis. Every attempt should be made to establish a microbiologically confirmed diagnosis (i.e., recovery of M. tuberculosis from culture) so that an isolate is available for susceptibility testing. A presumptive diagnosis can be made by observing caseating granulomas with or without AFB on histologic examination of specimens. However, granulomas in musculoskeletal specimens do not always indicate infection with M. tuberculosis. Other diseases producing synovial granulomas include fungal diseases, infection with nontuberculous mycobacteria, sarcoidosis, traumatic fat necrosis, brucellosis, and foreign-body giant cell reactions. In developing countries where TB is highly endemic and resources, including imaging and diagnostic capabilities, are limited, the diagnosis of musculoskeletal TB is often based on the clinical and radiologic (i.e., plain film) findings.
Differentiating pyogenic, tuberculous, or inflammatory arthritis can be difficult radiographically, and ultimately a microbiological diagnosis needs to be made. For tuberculous arthritis, synovial fluid examination is the first procedure performed. As noted above, biopsy of the synovium may be required to establish a diagnosis of tuberculous arthritis. Synovial fluid in early stages of disease appears xanthochromic, and as the disease process advances the fluid becomes yellow-white and thick and gelatinous in nature because of the presence of degenerated cartilage and bony debris (82). An elevated protein over 2.5 g/dl is a uniform finding. The joint fluid glucose level is thought to be low compared to serum levels; the synovial fluid glucose is usually 40 mg/dl less than the blood glucose among patients with tuberculous arthritis. The synovial fluid white blood cell count can vary widely, with the average being in the range of 10,000 to 20,000/ml, but white blood cell counts of 50,000 to 100,000/ml (in the range of what is often reported for bacterial or “septic” arthritis) have been reported. These findings can be consistent with rheumatoid arthritis as well. For these reasons, a bacteriological and/or histologic diagnosis is imperative.
AFB staining of synovial fluid has a sensitivity that is low but is thought to be higher than that seen in examination of fluid from other serosal membranes (e.g., pleura, pericardium, and peritoneum). Synovial biopsy and culture had a higher yield, with 94% of specimens having a positive histology and yielding a positive culture for M. tuberculosis. Open biopsy and fine-needle aspiration biopsy also have a high likelihood of yielding a positive culture (>90%) but are generally not required. Mondal reported a series of 116 fine-needle aspiration biopsies in which TB was diagnosed in 38 patients; metastatic tumor was diagnosed in the remaining 78 patients. Thirty-four of the 38 patients with tuberculous arthritis had cultures positive for M. tuberculosis (89%), and 11 (39%) were smear positive for AFB. The remaining four cases of tuberculous arthritis were confirmed histologically, and the patients demonstrated improvement with anti-TB treatment (83). Masood performed fine-needle aspiration biopsy for diagnosis of bone and soft tissue lesions in 11 patients, and 64% were smear positive for AFB and 84% were culture positive; the author concluded that fine-needle biopsy is as good as open biopsy and less invasive (84). As noted, additional data are needed on the utility of molecular diagnostic techniques (e.g., nucleic acid amplification tests) for the diagnosis of tuberculous arthritis.
Debeaumont showed that the total bacterial population of an infected spine may comprise less than 1 million organisms, whereas an expectorated sputum specimen may produce up to 300,000 bacilli in 1 ml (85). The lower burden of organisms may help explain why it is sometimes difficult to recover tubercle bacilli in paraspinal or psoas abscesses and draining sinus tracts. The yield from culturing material from a sinus tract can be increased if the specimen is collected with a syringe rather than a swab (86). The low bacillary burden also implies that there is a lower chance of developing drug-resistant TB in skeletal disease than in pulmonary TB.
TREATMENT
Early diagnosis and initiation of appropriate anti-TB therapy are important, as early treatment can prevent loss of function and mobility. There is now abundant evidence to show that musculoskeletal TB, detected early, can be cured by chemotherapy without the previously expected, inevitable sequelae of deformity in the spine or ankylosis in the limb joints. Even if minor radiologic changes have occurred, full restoration of function without deformity can be confidently expected if the diagnosis is made early enough. Simple survival of the patient in a moderately disabled condition is no longer acceptable. Early diagnosis may indeed be the greatest contribution that surgery adds in modern management of musculoskeletal TB.
The basic principles that underlie the treatment of pulmonary TB also apply to extrapulmonary forms of the disease (87, 88). Regimens that are effective for the treatment of drug-susceptible pulmonary TB are appropriate for the treatment of susceptible musculoskeletal TB. Several studies have examined the treatment of bone and joint TB and have shown that 6- to 9-month (short-course) regimens containing rifampin are at least as effective as 18-month regimens that do not contain rifampin for the treatment of drug-susceptible disease (89–91). Because of the difficulties in assessing response, some experts tend to favor the 9-month duration, and in the setting of extensive orthopedic hardware, some experts extend the duration of treatment further to 12 months (88). As discussed below, no additional benefit of surgical debridement in combination with chemotherapy compared with chemotherapy alone for spinal tuberculosis has been found in clinical trials. Surgical therapy for spinal TB is recommended only in selected cases based on expert opinion, as discussed below. Concomitant use of corticosteroids in the treatment of persons with bone and joint disease is not recommended unless there is evidence of concomitant tuberculosis meningitis; such cases should be treated as is done for tuberculous meningitis (88). There are few reports of multidrug-resistant skeletal TB, but the same treatment principles as used in the treatment of pulmonary multidrug-resistant TB should apply to musculoskeletal TB.
Tuberculous Spondylitis
Historical perspective
Before the availability of effective anti-TB chemotherapy, Pott’s disease was treated with immobilization using prolonged bed rest or a body cast. In Dobson’s series of 914 patients, all received prolonged immobilization, with either a brace or a body plaster cast, and 54 underwent a spinal fusion (92). The mortality rate was 20%, and 22% were readmitted for relapses. Anti-TB chemotherapy was gradually introduced into the treatment of osteoarticular TB after the studies of Canetti et al. (93) and Debeaumont (85) demonstrated the effect of chemotherapy in osteoarticular disease. Hodgson et al. described the first series combining a surgical approach using an anterior approach for decompression and autologous bone grafting for fusion with chemotherapy and reported a high success rate (94). Konstam and Blesovsky were the first to report an ambulatory, medical approach for tuberculous spondylitis (95). They reported treatment of 207 patients with isoniazid and p-aminosalicylic acid (PAS) for at least 12 months (until there was radiographic improvement) and did not include bracing or immobilization as part of the therapy. Surgery was performed only in 27 patients who needed abscesses drained. Eighty-six percent of patients exhibited complete recovery. It was demonstrated that patients with vertebral TB could be cured with chemotherapy alone and without prolonged immobilization or a complicated surgical procedure.
It was this divergence of opinion and practice that led the British Medical Research Council to set up a series of randomized controlled clinical trials in different centers (96–103). Patients included in these research studies had evidence of active TB of the thoracic or lumbar spine. These studies were carried out in Korea, Zimbabwe (then called Rhodesia), South Africa, and Hong Kong. All patients received chemotherapy (in most studies this consisted of 18 months of isoniazid and PAS supplemented with streptomycin during the first 3 months for some patients). The methods of treatment were determined by the therapy available at that time plus locally available resources and included outpatient anti-TB therapy, chemotherapy with immobilization by bed rest or body casts, chemotherapy and conservative debridement of infected bone without fusion, and, in Hong Kong and South Africa, chemotherapy with “radical surgery.” The radical surgical therapy consisted of anterior resection, debridement of granulation tissue and nonviable bone, and autologous bone strut grafting (90, 96). Nonoperative treatment produced a favorable status in 67% of subjects at 18 months, 85% at 3 years, and 88% at 5 years. The continued improvement that became apparent with the passage of time, from 67% to 85 to 90% at 5 to 10 years, is a valid warning against panic if all does not seem well at the end of the initial period of chemotherapy. No statistically significant difference could be shown at any stage between the results in patients who underwent 6 months of inpatient care, 9 months in plaster jackets, or neither. In addition, no statistically significant advantage could be shown from the addition of streptomycin to PAS and isoniazid. It was therefore concluded that in this series of cases, from the point of view of the preservation of life and health, ambulant outpatient chemotherapy gave excellent results, as judged over a period of 5 years, in patients whose drug taking had been supervised as strictly as possible under the prevailing conditions. Debridement by open operation with or without anterior spinal fusion, in addition to chemotherapy, gave no better ultimate results than did ambulant outpatient treatment. Favorable outcome in these studies was defined as “full physical activity with clinically and radiographically quiescent disease, with no sinuses, abscesses or myelopathy with functional impairment, all without modification of the allocated regimen” (98).
Radical surgery, as performed in Hong Kong, gave similar results in the long term so far as preservation of life and health is concerned. The Hong Kong operation consisted of anterior resection, debridement of infected bone, and autologous bone grafting. At the end of the period of chemotherapy (18 months), 89% of patients had already reached a favorable status; at 3 years, 87% of patients had a favorable status; and at 5 years, 89% were considered to have a favorable status. The Hong Kong operation in conjunction with non-rifampin-based regimens (in the era before the availability of rifampin), however, produced certain distinct advantages: (i) abscesses, including mediastinal abscesses, resolved more rapidly than after conservative treatment and even after debridement; (ii) bony fusion occurred much earlier; and (iii) most importantly, kyphosis did not worsen (96). The Hong Kong radical procedure was also performed in South Africa, but unlike in the reports from Hong Kong, it did not show advantages over debridement alone. In the era before the availability of rifampin, spinal TB was best treated by a combination of appropriate chemotherapy and the Hong Kong radical operation if, and only if, adequate surgical expertise, anesthesia, and nursing facilities were available. If not, surgery was to be avoided.
Modern therapy for tuberculous spondylitis
Stimulated by the efficacy of short-course chemotherapy for pulmonary TB with isoniazid and rifampin, the British Medical Research Council Working Party started a second series of trials in Hong Kong, Korea, and South India (89–91). In Hong Kong, all patients underwent the radical operation with either 6 or 9 months of chemotherapy with isoniazid and rifampin supplemented by streptomycin for 6 months. In Korea, only ambulatory chemotherapy with a regimen of 6 or 9 months of isoniazid plus rifampin was compared with a regimen of 9 or 18 months of isoniazid plus PAS or ethambutol. In Madras (now Chennai), South India, ambulatory chemotherapy with a regimen of 6 or 9 months of isoniazid and rifampin was compared with a regimen of 6 or 9 months of isoniazid plus rifampin and the Hong Kong radical operation. After 3 years, all studies showed that >95% had favorable results for regimens containing isoniazid and rifampin (89–91). These results confirmed the efficacy of a short course of rifampin-based regimens, 6 or 9 months, for spinal TB. The resolution of sinuses and abscesses present on admission was more rapid in patients treated with isoniazid plus rifampin than in patients treated with isoniazid plus PAS or ethambutol. There was no recurrence in patients prescribed rifampin-containing regimens. The resolution of myelopathy was very good in ambulatory regimens, with 83 to 88% of patients making a full recovery. There was no clear evidence that 9 months of treatment with isoniazid plus rifampin had any advantages over 6 months of treatment. Primary resistance to isoniazid and streptomycin was a problem, particularly in the Hong Kong studies.
Thus, on the basis of the results of these more recent randomized trials carried out by the Medical Research Council Working Party on Tuberculosis of the Spine, tuberculous spondylitis of the thoracolumbar spine due to drug-susceptible organisms is best treated by a rifampin-based regimen including isoniazid and rifampin for a period of 6 to 9 months, supplemented by pyrazinamide for 2 months (and ethambutol pending susceptibility results), similar to those regimens used in the treatment of pulmonary TB (88). Among patients taking rifampin-based short-course regimens, there was no demonstrated additional benefit of surgical debridement or radical operation (resection of the spinal focus and bone grafting) in combination with chemotherapy compared with chemotherapy alone (91). In addition, myelopathy with or without functional impairment most often responds to chemotherapy. In two studies conducted in Korea, 24 of 30 patients in one study (89) and 74 of 85 patients in an earlier study (104) had complete resolution of myelopathy or complete functional recovery when treated medically.
As noted above, several trials found no additional benefit of surgical debridement in combination with chemotherapy compared with chemotherapy alone for spinal TB (89, 91, 104, 105). Therefore, the most recent TB treatment guidelines from the American Thoracic Society, CDC, and Infectious Diseases Society of America recommend that uncomplicated cases of spinal tuberculosis be managed with medical rather than surgical treatment (88). In some circumstances, however, surgery appears to be beneficial and may be indicated. Based on expert opinion, surgery can be considered in situations in which (i) there is poor response to chemotherapy with evidence of ongoing infection or ongoing deterioration, (ii) relief of cord compression is needed in patients with persistence or recurrence of neurologic deficits, or (iii) there is instability of the spine or progressive kyphosis of the spine (88).
Despite the large number of patients enrolled in the Medical Research Council trials, patients with obvious myelopathy were excluded, so there are no controlled trials evaluating the role of surgery when paresis is present. In addition, patients with tuberculous spondylitis of the cervical spine were not included in the Medical Research Council studies, in part because of the low incidence of cervical disease. In two case series which reported on a total of 46 patients with upper and lower cervical TB, all patients received anti-TB chemotherapy and surgery (106, 107). Medical therapy consisted of isoniazid plus rifampin for 12 to 15 months or isoniazid plus PAS for 15 to 21 months. Of the six patients with upper cervical disease, four recovered without sequelae. There were 40 patients with lower cervical disease; all experienced a meaningful clinical and radiologic recovery. Twelve patients with cord compression experienced full neurologic recovery. Treatment recommendations for cervical TB are based on case series (106–108). Surgical intervention of the cervical spine is often indicated because of the likely association of neurologic deficits, frequent abscesses that can cause respiratory compromise, and the relative instability of the cervical spine, although in some cases, such as those reported by Jain et al. (108), there can be recovery without surgical intervention, as was reported for about two-thirds of the patients in their series. An anterior approach is considered the treatment of choice. A laminectomy should be avoided because it is not as effective in relieving cord compression and can lead to instability of the cervical spine.
Tuberculous Arthritis and Osteomyelitis
There are no controlled trials assessing treatment of musculoskeletal TB, except for tuberculous spondylitis, which is discussed above. Based on experience from treating tuberculous spondylitis and the experience with treating other forms of extrapulmonary disease, it is recommended that treatment of drug-susceptible tuberculous arthritis and osteomyelitis be carried out using rifampin-based short-course regimens like those that are used for the treatment of pulmonary disease. Surgery is generally reserved for diagnosis and when necessary to drain an abscess that is not responding to medical therapy or to drain a large abscess to relieve pressure (109). Late treatment or inadequate treatment results in ankylosis of the affected joint by fibrosis or bony fusion. The function of the affected joint is compensated by other joints which in the long term are overused and may become painful because of degenerative arthritis. Recent technical developments have stimulated interest in indications for arthroplasty in joints with old, healed TB (110–112). Su et al. reported 16 cases of patients with a history of TB of the knee who underwent total knee replacement; 8 patients had a preoperative diagnosis of TB and had received at least 2 months of anti-TB medications, and 8 were diagnosed at the time of the operation. Five patients, four of whom did not receive anti-TB chemotherapy at the time of the operation, suffered a recurrence of disease after arthroplasty (112). There are no formal recommendations, but some experts have suggested that patients requiring total arthroplasty for quiescent TB receive perioperative chemotherapy for at least 3 weeks before and at least 6 to 9 months after surgery to minimize the risk of reactivation (113).
PROSTHETIC JOINT INFECTIONS
Prosthetic joint infections with M. tuberculosis are uncommon, but with an increasing number of procedures being done yearly and globalization, the potential to see a rise in cases exists, especially as more prosthetic joints are being placed in areas where TB endemicity is higher, such as India. Prosthetic joint TB may be a reactivation of a latent focus that reaches the joint by hematogenous spread or local reactivation. A history of TB and immunosuppressive diseases or medications appear to be risk factors. No large series exist in the literature, but based on case reports, most cases appear to be due to reactivation versus primary infection (114–116). Reactivation has occurred as late as 42 years after initial TB infection (117). In 11 cases reported by Tokumoto et al., symptoms were present from a range of 2 weeks to 2 years before diagnosis; the most common symptom was pain, and swelling and/or a draining sinus tract was the most common physical finding (116).
There are no specific treatment recommendations available, but treatments often require a combined medical and surgical approach. A series of cases of TB involving prosthetic knee arthroplasties and review of the literature showed that early infection (up to 8 weeks postoperative) in three of four patients who had no loosening of hardware or draining sinus tracts were treated with prolonged standard TB therapy and one also had concurrent debridement (114). The authors concluded that with late presentation, greater than 2 months postoperatively, a two-stage reconstruction along with standard anti-TB therapy was indicated. Late-onset presentation may be associated with osteolysis, hardware loosening, or draining sinus tracts. Of nine patients who were treated with a two-stage regimen, none had recurrent disease (114). However, none of these data involve randomized trials, and they are extrapolated from the literature and based on anecdotal experience.
CONCLUSIONS
The diagnosis of musculoskeletal TB is often delayed because of failure to consider the diagnosis. Early diagnosis of bone and joint disease is important to minimize the risk of deformity and enhance outcome. The introduction of newer imaging modalities, including MRI (imaging procedure of choice) and CT, has enhanced the diagnostic evaluation of patients with musculoskeletal TB and for directed biopsies of affected areas of the musculoskeletal system. Obtaining appropriate specimens for culture and other diagnostic tests is essential to establish a definitive diagnosis and recover M. tuberculosis for susceptibility testing. A total of 6 to 9 months of a rifampin-based regimen, like treatment of pulmonary TB, is recommended for the treatment of drug-susceptible musculoskeletal disease. Randomized trials of tuberculous spondylitis have demonstrated that such regimens are efficacious. These data and those from the treatment of pulmonary TB have been extrapolated to form the basis of treatment regimen recommendations for other forms of musculoskeletal TB. Finally, ensuring adherence to chemotherapy is essential for good outcomes; therefore, directly observed therapy is recommended for the treatment of all persons with musculoskeletal TB.
REFERENCES
- 1.Zink A, Haas CJ, Reischl U, Szeimies U, Nerlich AG. 2001. Molecular analysis of skeletal tuberculosis in an ancient Egyptian population. J Med Microbiol 50:355–366. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Crubézy E, Ludes B, Poveda JD, Clayton J, Crouau-Roy B, Montagnon D. 1998. Identification of Mycobacterium DNA in an Egyptian Pott’s disease of 5,400 years old. C R Acad Sci III 321:941–951.[PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Konomi N, Lebwohl E, Mowbray K, Tattersall I, Zhang D. 2002. Detection of mycobacterial DNA in Andean mummies. J Clin Microbiol 40:4738–4740. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donoghue HD, Spigelman M, Greenblatt CL, Lev-Maor G, Bar-Gal GK, Matheson C, Vernon K, Nerlich AG, Zink AR. 2004. Tuberculosis: from prehistory to Robert Koch, as revealed by ancient DNA. Lancet Infect Dis 4:584–592. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Salo WL, Aufderheide AC, Buikstra J, Holcomb TA. 1994. Identification of Mycobacterium tuberculosis DNA in a pre-Columbian Peruvian mummy. Proc Natl Acad Sci U S A 91:2091–2094. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang HS, Ong GB, Hodgson AR. 1964. Anterior spinal fusion: the operative approaches. Clin Orthop Relat Res 35:16–33. [PubMed] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2016. Reported Tuberculosis in the United States, 2015. CDC, Atlanta, GA. [PubMed] [Google Scholar]
- 8.Hodgson SP, Ormerod LP. 1990. Ten-year experience of bone and joint tuberculosis in Blackburn 1978–1987. J R Coll Surg Edinb 35:259–262. [PubMed] [PubMed] [Google Scholar]
- 9.Lafond EM. 1958. An analysis of adult skeletal tuberculosis. J Bone Joint Surg Am 40-A:346–364. [PubMed] [PubMed] [Google Scholar]
- 10.Talbot JC, Bismil Q, Saralaya D, Newton DA, Frizzel RM, Shaw DL. 2007. Musculoskeletal tuberculosis in Bradford—a 6-year review. Ann R Coll Surg Engl 89:405–409. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colmenero JD, Jiménez-Mejías ME, Reguera JM, Palomino-Nicás J, Ruiz-Mesa JD, Márquez-Rivas J, Lozano A, Pachón J. 2004. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis 23:477–483. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Leibert E, Schluger NW, Bonk S, Rom WN. 1996. Spinal tuberculosis in patients with human immunodeficiency virus infection: clinical presentation, therapy and outcome. Tuber Lung Dis 77:329–334. [DOI] [PubMed] [Google Scholar]
- 13.Franco-Paredes C, Díaz-Borjon A, Senger MA, Barragan L, Leonard M. 2006. The ever-expanding association between rheumatologic diseases and tuberculosis. Am J Med 119:470–477. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Ludwig B, Lazarus AA. 2007. Musculoskeletal tuberculosis. Dis Mon 53:39–45. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Davidson PT, Horowitz I. 1970. Skeletal tuberculosis. A review with patient presentations and discussion. Am J Med 48:77–84. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Agarwal RP, Mohan N, Garg RK, Bajpai SK, Verma SK, Mohindra Y. 1990. Clinicosocial aspect of osteo-articular tuberculosis. J Indian Med Assoc 88:307–309. [PubMed] [PubMed] [Google Scholar]
- 17.Evanchick CC, Davis DE, Harrington TM. 1986. Tuberculosis of peripheral joints: an often missed diagnosis. J Rheumatol 13:187–189. [PubMed] [PubMed] [Google Scholar]
- 18.Belzunegui J, Plazaola I, Uriarte E, Pego JM. 1995. Primary tuberculous muscle abscess in a patient with systemic lupus erythematosus. Br J Rheumatol 34:1177–1178. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. 2003. Imaging features of musculoskeletal tuberculosis. Eur Radiol 13:1809–1819. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Moore SL, Rafii M. 2001. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am 39:329–342. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Compere EL, Garrison M. 1936. Correlation of pathologic and roentgenologic findings in tuberculosis and pyogenic infections of the vertebrae: the fate of the intervertebral disk. Ann Surg 104:1038–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer PES. 2001. The Imaging of Tuberculosis: With Epidemiological, Pathological, and Clinical Correlation. Springer-Verlag, New York, NY. [Google Scholar]
- 23.Naim-ur-Rahman. 1980. Atypical forms of spinal tuberculosis. J Bone Joint Surg Br 62-B:162–165. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Calderone RR, Larsen JM. 1996. Overview and classification of spinal infections. Orthop Clin North Am 27:1–8. [PubMed] [PubMed] [Google Scholar]
- 25.Boachie-Adjei O, Squillante RG. 1996. Tuberculosis of the spine. Orthop Clin North Am 27:95–103. [PubMed] [PubMed] [Google Scholar]
- 26.Watts HG, Lifeso RM. 1996. Tuberculosis of bones and joints. J Bone Joint Surg Am 78:288–298. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Al Soub H. 1996. Retropharyngeal abscess associated with tuberculosis of the cervical spine. Tuber Lung Dis 77:563–565. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Wurtz R, Quader Z, Simon D, Langer B. 1993. Cervical tuberculous vertebral osteomyelitis: case report and discussion of the literature. Clin Infect Dis 16:806–808. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Burke HE. 1950. The pathogenesis of certain forms of extrapulmonary tuberculosis; spontaneous cold abscesses of the chest wall and Pott’s disease. Am Rev Tuberc 62(1-B):48–67. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J, Martos F, García de las Heras J, Pachón J. 1997. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 56:709–715. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeRoux PD, Griffin GE, Marsh HT, Winn HR. 1990. Tuberculosis of the skull—a rare condition: case report and review of the literature. Neurosurgery 26:851–855; discussion, 855–856. [PubMed] [PubMed] [Google Scholar]
- 32.Strauss DC. 1933. Tuberculosis of the flat bones of the vault of the skull. Surg Gynecol Obstet 57:384–398. [Google Scholar]
- 33.Asnis DS, Niegowska A. 1997. Tuberculosis of the rib. Clin Infect Dis 24:1018–1019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.González Herranz J, Farrington DM, Angulo Gutiérrez J, Rodriguez Ferrol P. 1997. Peripheral osteoarticular tuberculosis in children: tumor-like bone lesions. J Pediatr Orthop B 6:274–282. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Zahraa J, Johnson D, Lim-Dunham JE, Herold BC. 1996. Unusual features of osteoarticular tuberculosis in children. J Pediatr 129:597–602. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Isaacs AJ, Sturrock RD. 1974. Poncet’s disease—fact or fiction? A re-appraisal of tuberculous rheumatism. Tubercle 55:135–142. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Kroot EJ, Hazes JM, Colin EM, Dolhain RJ. 2007. Poncet’s disease: reactive arthritis accompanying tuberculosis. Two case reports and a review of the literature. Rheumatology (Oxford) 46:484–489. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Huang DY. 1990. Tuberculous muscle abscess: an unusual presentation of tuberculosis. Am J Med 88:57N–59N. [PubMed] [PubMed] [Google Scholar]
- 39.Plummer WW, Sanes S, Smith WS. 1934. Hematogenous tuberculosis of skeletal muscle; report of the case with involvement of gastrocnemius muscle. J Bone Joint Surg Am 16:631–639. [Google Scholar]
- 40.Ahmed J, Homans J. 2002. Tuberculosis pyomyositis of the soleus muscle in a fifteen-year-old boy. Pediatr Infect Dis J 21:1169–1171. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Kotoura Y, Hosono M, Tsuboyama T, Sakahara H, Konishi J. 1995. Solitary muscular involvement by tuberculosis: CT, MRI, and scintigraphic features. Comput Med Imaging Graph 19:237–240. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Lupatkin H, Bräu N, Flomenberg P, Simberkoff MS. 1992. Tuberculous abscesses in patients with AIDS. Clin Infect Dis 14:1040–1044. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Martini M, Ouahes M. 1988. Bone and joint tuberculosis: a review of 652 cases. Orthopedics 11:861–866. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Pertuiset E, Beaudreuil J, Lioté F, Horusitzky A, Kemiche F, Richette P, Clerc-Wyel D, Cerf-Payrastre I, Dorfmann H, Glowinski J, Crouzet J, Bardin T, Meyer O, Dryll A, Ziza JM, Kahn MF, Kuntz D. 1999. Spinal tuberculosis in adults. A study of 103 cases in a developed country, 1980–1994. Medicine (Baltimore) 78:309–320. [DOI] [PubMed] [Google Scholar]
- 45.McLain RF, Isada C. 2004. Spinal tuberculosis deserves a place on the radar screen. Cleve Clin J Med 71:537–539, 543–549. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.García S, Combalía A, Serra A, Segur JM, Ramón R. 1997. Unusual locations of osteoarticular tuberculosis. Arch Orthop Trauma Surg 116:321–323. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Neumann JL, Schlueter DP. 1974. Retropharyngeal abscess as the presenting feature of tuberculosis of the cervical spine. Am Rev Respir Dis 110:508–511. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Lukhele M. 1996. Tuberculosis of the cervical spine. S Afr Med J 86:553–556. [PubMed] [PubMed] [Google Scholar]
- 49.Al-Matar MJ, Cabral DA, Petty RE. 2001. Isolated tuberculous monoarthritis mimicking oligoarticular juvenile rheumatoid arthritis. J Rheumatol 28:204–206. [PubMed] [PubMed] [Google Scholar]
- 50.Valdazo JP, Perez-Ruiz F, Albarracin A, Sanchez-Nievas G, Perez-Benegas J, Gonzalez-Lanza M, Beltran J. 1990. Tuberculous arthritis. Report of a case with multiple joint involvement and periarticular tuberculous abscesses. J Rheumatol 17:399–401. [PubMed] [PubMed] [Google Scholar]
- 51.Gros T, Soriano V, Gabarre E, Tor J, Sabriá M. 1992. Multifocal tubercular osteitis in a female patient infected with the human immunodeficiency virus. Rev Clin Esp 191:35–37. (In Spanish.) [PubMed] [PubMed] [Google Scholar]
- 52.Babhulkar SS, Pande SK. 2002. Unusual manifestations of osteoarticular tuberculosis. Clin Orthop Relat Res 398:114–120. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Franco-Paredes C, Blumberg HM. 2001. Psoas muscle abscess caused by Mycobacterium tuberculosis and Staphylococcus aureus: case report and review. Am J Med Sci 321:415–417. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Chernoff WG, Parnes LS. 1992. Tuberculous mastoiditis. J Otolaryngol 21:290–292. [PubMed] [PubMed] [Google Scholar]
- 55.Wu H, Wang QZ, Jin Y. 1998. Tuberculosis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:243. [PubMed] [DOI] [PubMed] [Google Scholar]
- 56.McLellan DG, Philips KB, Corbett CE, Bronze MS. 2000. Sternal osteomyelitis caused by Mycobacterium tuberculosis: case report and review of the literature. Am J Med Sci 319:250–254. [PubMed] [DOI] [PubMed] [Google Scholar]
- 57.Jaovisidha S, Chen C, Ryu KN, Siriwongpairat P, Pekanan P, Sartoris DJ, Resnick D. 1996. Tuberculous tenosynovitis and bursitis: imaging findings in 21 cases. Radiology 201:507–513. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Klofkorn RW, Steigerwald JC. 1976. Carpal tunnel syndrome as the initial manifestation of tuberculosis. Am J Med 60:583–586. [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Rashid M, Sarwar SU, Haq EU, Islam MZ, Rizvi TA, Ahmad M, Shah K. 2006. Tuberculous tenosynovitis: a cause of carpal tunnel syndrome. J Pak Med Assoc 56:116–118. [PubMed] [PubMed] [Google Scholar]
- 60.Hoffman KL, Bergman AG, Hoffman DK, Harris DP. 1996. Tuberculous tenosynovitis of the flexor tendons of the wrist: MR imaging with pathologic correlation. Skeletal Radiol 25:186–188. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Cramer K, Seiler JG III, Milek MA. 1991. Tuberculous tenosynovitis of the wrist. Two case reports. Clin Orthop Relat Res 262:137–140. [PubMed] [PubMed] [Google Scholar]
- 62.Tuli SM. 2002. General principles of osteoarticular tuberculosis. Clin Orthop Relat Res 398:11–19. [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.De Backer AI, Mortelé KJ, Vanhoenacker FM, Parizel PM. 2006. Imaging of extraspinal musculoskeletal tuberculosis. Eur J Radiol 57:119–130. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.De Backer AI, Vanhoenacker FM, Sanghvi DA. 2009. Imaging features of extraaxial musculoskeletal tuberculosis. Indian J Radiol Imaging 19:176–186. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handa U, Garg S, Mohan H, Garg SK. 2010. Role of fine-needle aspiration cytology in tuberculosis of bone. Diagn Cytopathol 38:1–4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Pai M, Ling DI. 2008. Rapid diagnosis of extrapulmonary tuberculosis using nucleic acid amplification tests: what is the evidence? Future Microbiol 3:1–4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Held M, Laubscher M, Zar HJ, Dunn RN. 2014. GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test. Bone Joint J 96-B:1366–1369. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Held M, Laubscher M, Mears S, Dix-Peek S, Workman L, Zar H, Dunn R. 2016. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary tuberculosis in children with musculoskeletal infections. Pediatr Infect Dis J 35:1165–1168. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O’Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. 2017. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 64:111–115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raut AA, Naphade PS, Ramakantan R. 2016. Imaging spectrum of extrathoracic tuberculosis. Radiol Clin North Am 54:475–501. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Griffith JF, Kumta SM, Leung PC, Cheng JC, Chow LT, Metreweli C. 2002. Imaging of musculoskeletal tuberculosis: a new look at an old disease. Clin Orthop Relat Res 398:32–39. [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Clementsen P, Hansen M, Conrad C, Myhre O. 1988. Percutaneous drainage of tuberculous abscess of the psoas muscle. Tubercle 69:63–65. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Hoffman EB, Crosier JH, Cremin BJ. 1993. Imaging in children with spinal tuberculosis. A comparison of radiography, computed tomography and magnetic resonance imaging. J Bone Joint Surg Br 75:233–239. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.De Backer AI, Mortelé KJ, Vanschoubroeck IJ, Deeren D, Vanhoenacker FM, De Keulenaer BL, Bomans P, Kockx MM. 2005. Tuberculosis of the spine: CT and MR imaging features. JBR-BTR 88:92–97. [PubMed] [PubMed] [Google Scholar]
- 75.Phemister DB, Hatcher CH. 1933. Correlation of the pathological findings in the diagnostic of tuberculous arthritis. Am J Roentgenol Radium Ther Nucl Med 29:736–740. [Google Scholar]
- 76.Hong SH, Kim SM, Ahn JM, Chung HW, Shin MJ, Kang HS. 2001. Tuberculous versus pyogenic arthritis: MR imaging evaluation. Radiology 218:848–853. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Moore SL, Rafii M. 2003. Advanced imaging of tuberculosis arthritis. Semin Musculoskelet Radiol 7:143–153. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Morris BS, Varma R, Garg A, Awasthi M, Maheshwari M. 2002. Multifocal musculoskeletal tuberculosis in children: appearances on computed tomography. Skeletal Radiol 31:1–8. [PubMed] [DOI] [PubMed] [Google Scholar]
- 79.Tiwari A, Sud A, Mehta S, Kanojia RK, Kapoor SK. 2007. Multifocal skeletal tuberculosis presenting as multiple bone cysts. Ann Acad Med Singapore 36:1038–1039. [PubMed] [PubMed] [Google Scholar]
- 80.Abdelwahab IF, Bianchi S, Martinoli C, Klein M, Hermann G. 2006. Atypical extraspinal musculoskeletal tuberculosis in immunocompetent patients: part II, tuberculous myositis, tuberculous bursitis, and tuberculous tenosynovites. Can Assoc Radiol J 57:278–286. [PubMed] [PubMed] [Google Scholar]
- 81.Graves VB, Schreiber MH. 1973. Tuberculous psoas muscle abscess. J Can Assoc Radiol 24:268–271. [PubMed] [PubMed] [Google Scholar]
- 82.Wallace R, Cohen AS. 1976. Tuberculous arthritis: a report of two cases with review of biopsy and synovial fluid findings. Am J Med 61:277–282. [DOI] [PubMed] [Google Scholar]
- 83.Mondal A. 1994. Cytological diagnosis of vertebral tuberculosis with fine-needle aspiration biopsy. J Bone Joint Surg Am 76:181–184. [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Masood S. 1992. Diagnosis of tuberculosis of bone and soft tissue by fine-needle aspiration biopsy. Diagn Cytopathol 8:451–455. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Debeaumont A. 1966. Bacteriology of osteoarticular tuberculosis under chemotherapy. Bibl Tuberc 22:125–188. (In French.) [PubMed] [PubMed] [Google Scholar]
- 86.Mousa HA. 1998. Tuberculosis of bones and joints: diagnostic approaches. Int Orthop 22:245–246. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O’Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA, American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O’Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. 2016. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 63:e147–e195. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medical Research Council Working Party on Tuberculosis of the Spine. 1993. Controlled trial of short-course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis. Results at three years of a study in Korea. Twelfth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 75:240–248. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Medical Research Council Working Party on Tuberculosis of the Spine. 1986. A controlled trial of six-month and nine-month regimens of chemotherapy in patients undergoing radical surgery for tuberculosis of the spine in Hong Kong. Tenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Tubercle 67:243–259. [DOI] [PubMed] [Google Scholar]
- 91.Darbyshire J, Medical Research Council Working Party on Tuberculosis of the Spine. 1999. Five-year assessment of controlled trials of short-course chemotherapy regimens of 6, 9 or 18 months’ duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery. Fourteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Int Orthop 23:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dobson J. 1951. Tuberculosis of the spine; an analysis of the results of conservative treatment and of the factors influencing the prognosis. J Bone Joint Surg Br 33-B:517–531. [PubMed] [DOI] [PubMed] [Google Scholar]
- 93.Canetti G, Debeyre J, Seze SD. 1957. Sterilization of lesions in osteo-articular tuberculosis by antibacillary chemotherapy. Rev Tuberc (Paris) 21:1337–1344. (In French.) [PubMed] [Google Scholar]
- 94.Hodgson AR, Stock FE, Fang HS, Ong GB. 1960. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg 48:172–178. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Konstam PG, Blesovsky A. 1962. The ambulant treatment of spinal tuberculosis. Br J Surg 50:26–38. [DOI] [PubMed] [Google Scholar]
- 96.Medical Research Council Working Party on Tuberculosis of the Spine. 1982. A 10-year assessment of a controlled trial comparing debridement and anterior spinal fusion in the management of tuberculosis of the spine in patients on standard chemotherapy in Hong Kong. Eighth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 64:393–398. [PubMed] [DOI] [PubMed] [Google Scholar]
- 97.Medical Research Council Working Party on Tuberculosis of the Spine. 1985. A 10-year assessment of controlled trials of inpatient and outpatient treatment and of plaster-of-Paris jackets for tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan, Korea. Ninth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 67:103–110. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.Medical Research Council Working Party on Tuberculosis of the Spine. 1973. A controlled trial of ambulant out-patient treatment and in-patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy a study in Masan, Korea. First report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 55:678–697. [PubMed] [PubMed] [Google Scholar]
- 99.Medical Research Council Working Party on Tuberculosis of the Spine. 1974. A controlled trial of anterior spinal fusion and débridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in Hong Kong. Br J Surg 61:853–866. [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Medical Research Council Working Party on Tuberculosis of the Spine. 1978. A controlled trial of anterior spinal fusion and débridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in two centres in South Africa. Seventh Report of the Medical Research Council Working Party on tuberculosis of the spine. Tubercle 59:79–105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Medical Research Council Working Party on Tuberculosis of the Spine. 1973. A controlled trial of plaster-of-Paris jackets in the management of ambulant outpatient treatment of tuberculosis of the spine in children on standard chemotherapy. A study in Pusan, Korea. Second report of the Medical Research Council Working Party on Tuberculosis of the Spine. Tubercle 54:261–282. [DOI] [PubMed] [Google Scholar]
- 102.Medical Research Council Working Party on Tuberculosis of the Spine. 1976. A five-year assessment of controlled trials of in-patient and out-patient treatment and of plaster-of-Paris jackets for tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan, Korea. Fifth report of the Medical Research Council Working Party on tuberculosis of the spine. J Bone Joint Surg Br 58-B:399–411. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Medical Research Council Working Party on Tuberculosis of the Spine. 1978. Five-year assessments of controlled trials of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine. Studies in Bulawayo (Rhodesia) and in Hong Kong. Sixth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 60-B:163–177. [PubMed] [DOI] [PubMed] [Google Scholar]
- 104.Pattisson PR, Chir B. 1986. Pott’s paraplegia: an account of the treatment of 89 consecutive patients. Paraplegia 24:77–91. [PubMed] [DOI] [PubMed] [Google Scholar]
- 105.Jutte PC, Van Loenhout-Rooyackers JH. 2006. Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database Syst Rev 2006(1):CD004532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang D, Leong JC, Fang HS. 1983. Tuberculosis of the upper cervical spine. J Bone Joint Surg Br 65:47–50. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Hsu LC, Leong JC. 1984. Tuberculosis of the lower cervical spine (C2 to C7). A report on 40 cases. J Bone Joint Surg Br 66:1–5. [PubMed] [DOI] [PubMed] [Google Scholar]
- 108.Jain AK, Kumar S, Tuli SM. 1999. Tuberculosis of spine (C1 to D4). Spinal Cord 37:362–369. [PubMed] [DOI] [PubMed] [Google Scholar]
- 109.Aguirre M, Bago J, Martin N. 1989. Tuberculosis of the knee. Surgical or conservative treatment? Acta Orthop Belg 55:22–25. [PubMed] [PubMed] [Google Scholar]
- 110.Eskola A, Santavirta S, Konttinen YT, Tallroth K, Lindholm ST. 1988. Arthroplasty for old tuberculosis of the knee. J Bone Joint Surg Br 70:767–769. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Laforgia R, Murphy JC, Redfern TR. 1988. Low friction arthroplasty for old quiescent infection of the hip. J Bone Joint Surg Br 70:373–376. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.Su JY, Huang TL, Lin SY. 1996. Total knee arthroplasty in tuberculous arthritis. Clin Orthop Relat Res 323:181–187. [DOI] [PubMed] [Google Scholar]
- 113.Kramer SB, Lee SHS, Abramson SB. 2004. Nonvertebral infections of the musculoskeletal system by Mycobacterium tuberculosis, p 577–586. In Rom WN, Garay SM (ed), Tuberculosis, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 114.Harwin SF, Banerjee S, Issa K, Kapadia BH, Pivec R, Khanuja HS, Mont MA. 2013. Tubercular prosthetic knee joint infection. Orthopedics 36:e1464–e1469. [PubMed] [DOI] [PubMed] [Google Scholar]
- 115.Spinner RJ, Sexton DJ, Goldner RD, Levin LS. 1996. Periprosthetic infections due to Mycobacterium tuberculosis in patients with no prior history of tuberculosis. J Arthroplasty 11:217–222. [DOI] [PubMed] [Google Scholar]
- 116.Tokumoto JI, Follansbee SE, Jacobs RA. 1995. Prosthetic joint infection due to Mycobacterium tuberculosis: report of three cases. Clin Infect Dis 21:134–136. [PubMed] [DOI] [PubMed] [Google Scholar]
- 117.Johnson R, Barnes KL, Owen R. 1979. Reactivation of tuberculosis after total hip replacement. J Bone Joint Surg Br 61-B:148–150. [PubMed] [DOI] [PubMed] [Google Scholar]


