Abstract
The Rous sarcoma virus (RSV) Gag polyprotein undergoes transient nuclear trafficking as an intrinsic part of the virus assembly pathway. Nuclear export of Gag is crucial for the efficient production of viral particles and is accomplished through the action of a leptomycin B (LMB)-dependent nuclear export signal (NES) in the p10 domain (L. Z. Scheifele, R. A. Garbitt, J. D. Rhoads, and L. J. Parent, Proc. Natl. Acad. Sci. USA 99:3944-3949, 2002). We have now mapped the nuclear export activity to the C-terminal portion of the p10 sequence and identified the four hydrophobic amino acids within this region that comprise a leucine-rich NES. Alteration of these hydrophobic residues resulted in the accumulation of Gag proteins within the nucleus and a budding defect greater than that obtained with LMB treatment of cells expressing the wild-type Gag protein (Scheifele et al., Proc. Natl. Acad. Sci. USA 99:3944-3949, 2002). In addition, export of Gag from the nucleus was found to be a rate-limiting step in virus-like particle production. Consistent with a role for the NES sequence in viral replication, this cluster of hydrophobic residues in p10 is conserved across a wide range of avian retroviruses. Furthermore, naturally occurring substitutions within this region in related viruses maintained nuclear export activity and remained sensitive to the activity of LMB. Using gain-of-function approaches, we found that the hydrophobic motif in p10 was sufficient to promote the nuclear export of a heterologous protein and was positionally independent within the Gag polyprotein. Finally, the export pathway was further defined by the ability of specific nucleoporin inhibitors to prevent the egress of Gag from the nucleus, thereby identifying additional cellular mediators of RSV replication.
The Gag polyprotein coordinates the assembly of retroviral particles by serving as the precursor to the structural components of the virion, by selecting the RNA genome for encapsidation into the assembling particle, and, for some retroviruses, by directing the incorporation of the envelope glycoproteins. Gag proteins of Rous sarcoma virus (RSV) are initially synthesized on cytosolic ribosomes and then traffic through the cell nucleus (49). Within the cytoplasm, retroviral assembly intermediates can be isolated that are comprised of Gag protein multimers upon an RNA scaffold (31, 37, 50, 55). These Gag-RNA complexes are then targeted specifically to the plasma membrane, which serves as the site for higher-order virus assembly. Approximately 1,500 Gag proteins associate at the plasma membrane, where they can be seen as electron-dense aggregates driving the formation of a spherical bud. Following release, the immature virion is processed by the viral protease, cleaving the RSV Gag protein into the structural proteins matrix (MA), capsid, and nucleocapsid (NC), the enzyme protease (PR), and the peptides p2a, p2b, p10, and SP.
Coordination of retroviral assembly is directed by three functional domains within the Gag polyprotein: the membrane-binding domain mediates the selective targeting to and stable binding of the plasma membrane, the interaction domains facilitate multimerization of Gag proteins and RNA binding, and the late domain recruits host cell machinery to separate the emerging virion from the membrane (44). However, the precise compartment within the cell where each step of the assembly process occurs remains unclear. Moreover, the specific subcellular targeting signals within Gag have been only partially defined. For example, although several nuclear localization signals (NLSs) have been identified within Gag polyproteins, their roles in retroviral replication are uncertain. NLSs have been identified in the Gag proteins of the Schizosaccharomyces pombe Tf1 element (10) and the HeT-A and TART retrotransposons of Drosophila (48). Certain Gag proteins localize partially to the nucleus under steady-state conditions, including the Gag proteins of the human and equine foamy viruses (30, 62) and Moloney murine leukemia virus (38), although an NLS has not been identified within the Moloney murine leukemia virus Gag protein. Although controversial, nuclear import signals have been found in the MA region of the human immunodeficiency virus type 1 (HIV-1) Gag polyprotein (3), which might function during early steps of infection (14, 19, 20, 22).
The RSV Gag protein undergoes nuclear trafficking during viral assembly, and we are interested in defining the viral and cellular determinants of nuclear transport. We identified a noncanonical NLS within the MA domain, which is not comprised of a concise basic motif; rather, the N-terminal 88 amino acids are sufficient for nuclear accumulation (49). Nuclear localization is transient, since the Gag protein localizes exclusively to the cytoplasm under steady-state conditions. Trafficking of Gag through the nucleus is inhibited by treatment of cells with leptomycin B (LMB), a specific inhibitor of the Crm1 nuclear export pathway. Drug treatment results in retention of Gag proteins within the nucleus and consequently a significant decrease in the release of virus-like particles (VLPs), suggesting the importance of the nuclear transport step for productive retroviral replication (49).
Crm1 is a member of the importin-β superfamily of soluble nuclear transport receptors, and it mediates nuclear export through the recognition of hydrophobic nuclear export signals (NESs) within protein cargoes. Although these signals are often comprised of several closely spaced leucine residues, other hydrophobic amino acids, such as methionine, isoleucine, valine, phenylalanine and tryptophan may substitute for leucine in the recognition motif (reviewed in (21, 23, 32). Examination of the RSV Gag protein sequence for leucine-rich regions suggested the presence of a potential NES within the p10 region. Gag proteins containing a large deletion that included this sequence mimicked the phenotype of Gag proteins treated with LMB and accumulated in the nucleus (49).
The function of the p10 domain in RSV replication remains poorly defined. The sequence is enriched in proline residues and is likely a major structural determinant for the polyprotein. This structural role is reflected in the ability of the last 25 amino acids of the p10 domain to affect the morphology of VLPs assembled in vitro. Gag proteins containing deletions of p10 assemble into tubular structures in vitro (5); however, when the last 25 amino acids of the p10 domain are restored, the particles assume a spherical shape and a density resembling those of authentic RSV virions (24). In addition, p10 appears to be an important determinant of infectivity; virus particles bearing temperature-sensitive mutations in the p10 sequence are processed and assembled normally, but fail to establish infection at nonpermissive temperatures (15). Whether the effects of p10 mutations reflect solely a structural alteration that is manifested during the late stages of particle assembly or a more fundamental interruption of an early step in infection remains to be determined. Because we identified the C-terminal region of p10 as crucial for nuclear export of the Gag protein, we sought to identify the specific residues within p10 that are required for nucleus-cytosol exchange. Further characterization of the NES will allow a more detailed analysis of the role of the p10 domain in the RSV replication cycle.
MATERIALS AND METHODS
Plasmids.
Plasmids pGag-GFP, Δp10.31.Gag-GFP, Δp10.52.Gag-GFP, and ΔQM1.Gag-GFP were described previously (4, 26). pGag.FL-GFP, which restores the last seven codons of the NC domain to pGag-GFP, was created by PCR amplification of pSV.Myr0 (26) with primers PARE199 5′-(TCAGTATAGGGGCCCCGAGACGGCAGGTGGCTCA) and PARE179 5′-(CACTGATCGGATCCAGGTCAGGCTCCTGGGCCGG), digestion with ScaI and ApaI, and ligation into plasmid pMA.p2.p10-GFP (49). pGagΔPR was created by deletion of the green fluorescent protein (GFP) gene from pGag-GFP with ApaI and NotI, digestion with Klenow, and religation. Mutations were introduced into pGag.ΔPR by SstI-BspEI fragment exchange with pGag-GFP plasmids encoding the desired mutants. Mutations of pGag-GFP sequence were created using the QuickChange site-directed mutagenesis protocol according to the manufacturer's specifications (Stratagene) with the following oligonucleotides (only the sense strand is denoted), with diagnostic restriction enzyme sites underlined: L219A (PstI) (GGTCAGGCTCCTGGGCCTGCAGCGACTGACTGGGCAAGG); W222A (BssHII) (CCGGCCCTGACTGACGCGGCGCGCGTCAGGGAGGAGCTT); V225A (XhoI) (GCCCTGACTGACTGGGCAAGGGCTCGAGAGGAGCTTGCG); L229A (mutation of ScaI site) (AGGGTCAGGGAGGAGGCTGCGAGGCACTGGTCCGCCCGTG); L180,184,V187A (NarI and SstII) (GCTGCTCTCCGGCGCCCGCCGCGGAAGGATACGCACCACTCCCCAC); V225I, L229V, or L229V (mutation of ScaI site, BamHI) (CTTGGCAAGGRTCCGGGAGGAGGTTGCGAGCACTGGTCCGC); W222L,V225L (BssHII) (GCCCTGACTGACTTGGCGCGCCTCAGGGAGGAGCTTGCG); V225L (BssHII) (CTGACTGACTGGGCGCGCAGGGAGGAGCTTGCG); and V225I (BssHII) (CTGACTGACTGGGCGCGCATCAGGGAGGAGCTTGCG). Plasmids MA.NES.Gag-GFP and MA.NES/L219A.Gag.GFP were constructed using PCR with primers Pare285 5′-(CGTTTAAGCGAACTAGTAGCTCCTGGGCCGGCCCT) and Pare286 5′-(CGTTTAAGCTACTAGTGCCGGACCAGTACTCGCAAGCTC), followed by insertion of the DNA products into pΔMA6.Gag-GFP (39), which was digested with SpeI and treated with calf intestinal alkaline phosphatase (Promega). The pW222A.MA/L219A.Gag-GFP plasmid was constructed using QuickChange mutagenesis and the W222A oligonucleotide listed above. pFibrillarin-GFP was a kind gift from Mark Olson (University of Mississippi Medical Center) (13). The p10 NES motif (amino acids 219 to 234) was inserted between fibrillarin and GFP using PCR amplification of pGag-GFP with these primers: 5′-TGG CTA CCG GGT ACC CTG ACT GAC TGG GCA AGG and 5′-ACG GTA CGC GGA TCC AGG AGT ACT CGC AAG CTC CTC. The Fibrillarin.L219A-GFP variant was made using PCR amplification of pL219A.Gag-GFP with the same antisense primer and the following sense primer: 5′-TGG CTA CCG GGT GCG ACT GAC TGG GCA AGG GTC. PCR products were subcloned into pFibrillarin.GFP using Asp718I and BamHI.
Plasmids NP214-DsRed, NP98-DsRed, and NP214-DsRed were created by PCR amplification of codons 1864 to 2090 of Nup214, codons 2 to 494 of Nup98, and codons 894 to 1475 of Nup153 from plasmids ΔCAN (kindly provided by Tom Hope, University of Illinois at Chicago), NP98, and HA-NP153 (63) (kindly provided by Barbara Felber, National Cancer Institute, Frederick, Md.). PCR was used to introduce a Kozak consensus sequence and the tripeptide MAS (63) before each nucleosporin coding sequence, and amplified products were introduced into the pDsRed.N1vector (Clontech) between the XhoI and SstII sites. All mutations were confirmed using automated DNA sequencing.
Cell lines and confocal microscopy.
All experiments were carried out in the chemically transformed quail fibroblast line QT6, maintained as previously described (7, 35). Plasmid DNA was introduced by transient transfection using the calcium phosphate method, and subcellular localization of GFP fusion proteins was examined 16 to 24 h later by washing cells in Tris-buffered saline and viewing cells using either a Zeiss laser-scanning microscope (LSM10) or an inverted Leica TCS SP2 AOBS microscope at an excitation wavelength of 488 nm. Coexpression of DsRed constructs was performed by transfecting equal amounts (1 μg each) of DNA, followed by imaging with both argon (488 nm) and He-Ne lasers (643 nm).
Virus-like particle assembly and pulse-chase analysis.
Radioimmunoprecipitation assays were performed as described previously (46, 59) with polyclonal antisera against RSV (7, 45). Budding efficiency was determined by PhosphorImager analysis (Molecular Dynamics), quantitating the amount of either wild-type or mutant Gag-GFP or GagΔPR in the medium divided by the total amount of expressed protein in the cell lysate and culture medium. Wild-type values for each construct were normalized to 100% release, and P values were determined using a Student's t test. Pulse-chase analysis was performed by labeling cells for 15 min with [35S]methionine and cysteine >1,000 Ci/mmol; NEN), washing extensively in cold medium, and lysing cells every 15 min thereafter for 2.5 h. The percent release was calculated by dividing the amount of protein in the medium at each time point by the amount of protein synthesized (lysate plus medium) at the initial time point.
Retroviral sequence analysis.
Accession numbers of published avian retroviral sequences obtained from GenBank were as follows: NC_001407, AAQ55054, AAA46299, A48613, TVFVMI, P03323, P06444, CAA68260, P06936, P06937, AAA42377, P03326, and CAC28508. Sequence alignment was performed based upon progressive pairwise alignments using MultAlin software (6).
RESULTS
Following synthesis in the cytoplasm, the Gag polyprotein of RSV undergoes transient nuclear localization. Export of Gag from the nucleus depends upon the Crm1 export pathway, since treatment of Gag-expressing cells with LMB significantly reduces the production of VLPs and leads to an accumulation of Gag proteins in the nucleus (49). However, LMB is not an ideal tool for studying the role of Gag nuclear trafficking in the viral life cycle, because cellular toxicity limits the duration of drug treatment. Thus, the role of Gag nuclear transport in virus replication may be better studied either by disruption of the nuclear targeting signals within Gag or by inhibition of other cellular nuclear transport pathways. We have therefore set out to more precisely define the NES in Gag and to elucidate additional host cell factors that are involved in the nuclear export of Gag.
Definition of the hydrophobic residues comprising the p10 NES.
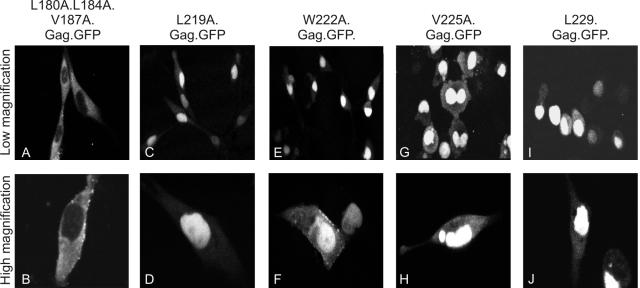
We previously identified a complex NLS in the first 88 residues of MA, and this sequence is sufficient to direct the import of the heterologous reporter protein GFP into the nucleus. As well, we determined that the NES of Gag appears to reside within the p10 domain, since deletions within the second half of the p10 sequence prevent the efficient export of Gag from the nucleus (Δp10.31 and Δp10.52) (49). However, it remained possible that these large deletions might reflect structural alterations within the Gag protein that prevent the association of an authentic NES elsewhere in Gag with host cell export machinery. To define the individual residues involved in the export of Gag from the nucleus, hydrophobic amino acids within p10 were replaced by alanine residues in the context of the Gag-GFP protein (Fig. 1). We predicted that hydrophobic residues in the first half of p10 would be dispensable for nuclear export, since a deletion encompassing the beginning of the p10 domain did not interfere with Gag localization (49). To test this idea, three codons within this upstream region (Leu180, Leu184, and Val187) were changed to encode alanine residues. The triple mutation (L180A L184A V187A) did not prevent Gag nucleocytoplasmic trafficking, and the protein was localized throughout the cytoplasm and at the plasma membrane (Fig. 2A and B).
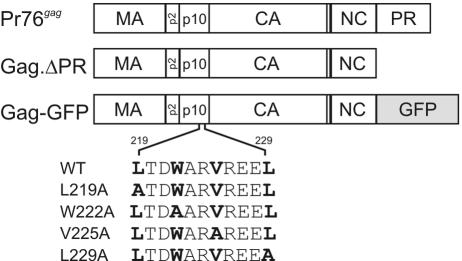
FIG. 1.
Mutants of the RSV Gag polyprotein. The wild-type Gag protein, Pr76gag, is depicted at the top, with the protease-deleted protein (Gag.ΔPR) shown below. Gag-GFP has the gene encoding GFP substituted for the last seven codons of NC and all of the PR sequence (26). The potential NES sequence encompassing residues 219 through 229 of the p10 region is highlighted. Substitution L219A, W222A, V225A, and L229A mutants are depicted below the wild-type sequence. CA, capsid.
FIG. 2.
Identification of hydrophobic residues comprising the RSV Gag NES. The subcellular localization of mutant Gag-GFP fusion proteins was determined using confocal microscopy following transient transfection of QT6 cells. The amino acid substitutions for each mutant are shown above each panel. Top panels represent lower magnification images of Gag-GFP-expressing cells, while lower panels are higher-magnification images of adjacent fields.
We next focused on the downstream hydrophobic cluster in p10 and changed each hydrophobic residue individually to alanine. Mutations in codons at position 219 or 229 (Leu), 222 (Trp), or 225 (Val) led to a dramatic redistribution of the Gag polyprotein into the nucleus (Fig. 2C to J). Each of the four individual mutations prevented the export of Gag from the nucleus, thereby mimicking the phenotype of Gag proteins containing internal p10 deletions, Δp10.31.Gag-GFP and Δp10.52.Gag-GFP (49). Western blot analysis revealed that each of the hydrophobic residue-to-alanine substitution mutants was expressed at levels equivalent to that of the wild-type Gag protein, indicating that the proteins were not unstable (data not shown). These single-amino-acid substitutions, in contrast to the large deletion mutants, likely represent a direct crippling of the NES, because the changes specifically target hydrophobic residues that comprise the putative Crm1-dependent NES. This assertion is further supported by the small proportion of cells in which the Gag-GFP proteins were visualized at the plasma membrane (as seen clearly in Fig. 2F), indicating that any proteins that can escape from or bypass the nucleus adopt a conformation similar enough to the native fold that they are competent for plasma membrane targeting.
Alanine substitutions in the p10 NES inhibit VLP release.
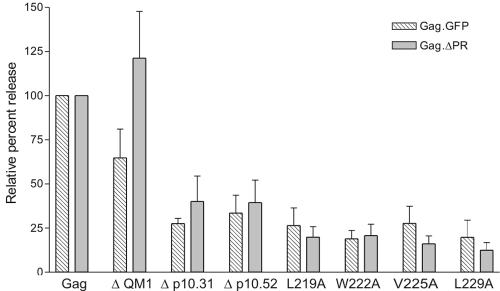
Treatment of Gag-expressing cells with LMB not only sequesters Gag proteins within the nucleus but consequently results in a significant reduction in the amount of VLPs released (49). However, the LMB experiments are limited by the toxicity of the drug in cultured cells. To determine whether deletion or mutation of the Gag NES coding sequence would have an effect on VLP release similar to that of LMB treatment, wild-type or mutant Gag proteins were expressed in QT6 cells and radioimmunoprecipitated from cell lysates and media using a polyclonal anti-RSV antibody (45). The amount of Gag protein released into the medium relative to the total intracellular and extracellular Gag protein was expressed as a percentage of wild type, which was normalized to 100%. Budding assays were performed using the Gag-GFP constructs to allow correlation with confocal microscopy data and also with the Gag.ΔPR derivatives to eliminate any potential effects of the GFP sequence on particle release (Fig. 3). Expression of Gag-GFP and Gag.ΔPR resulted in release of 24.6% ± 11.7% and 29.2% ± 7.2%, respectively, of the synthesized protein into the medium over a 2.5-h labeling period. Mutant ΔQM1 (26), which has a deletion encompassing the end of p2 and the beginning of p10 (Fig. 1, residues 176 to 200), displayed modestly reduced VLP release in the context of Gag-GFP (64.8% ± 16.3% of wild types); in contrast, budding efficiency was somewhat enhanced in the context of the Gag.ΔPR protein (121.2% ± 26.5% of WT). However, neither value is statistically significant compared to wild-type particle production (P = 0.0971 and 0.4681, respectively). This discrepancy might reflect the ability of the mutant protein to assume different conformations in the two contexts. In contrast, deletions that include the NES in p10 produced a significant decrease in the production of VLPs in the context of both Gag-GFP and Gag.ΔPR (P < 0.0001 for Δp10.31.Gag-GFP; P < 0.0090 for Δp10.31.Gag.ΔPR; P = 0.0028 for Δp10.52.Gag-GFP; and P = 0.0052 for Δp10.52.Gag.ΔPR). Thus, the budding defect in each case is consistent with the localization of the mutant Gag-GFP protein to the nucleus. The assembly block for the p10 NES deletion mutants was more substantial than we previously observed with LMB treatment of Gag-GFP-expressing cells (e.g., compare the relative particle release of 27.5% ± 3.0% for Δp10.31.Gag-GFP and 33.5% ± 10.1% for Δp10.52.Gag-GFP to the relative release of 50.8% ± 5.9% for LMB treatment [49]). Similarly, substitution of alanine for each hydrophobic residue in the NES produced a significant decrease in particle production (ranging from 18.9% to 27.7%, with P values ranging from <0.0001 to 0.0037 for Gag-GFP constructs), indicating that alteration of each critical residue individually has an effect of the same magnitude as deletion of the entire NES.
FIG. 3.
Reduced VLP assembly for NES mutant Gag proteins. Transfected cells were labeled for 2.5 h, lysed, and immunoprecipitated with polyclonal antiserum against RSV. The amount of Gag protein released into the culture medium was calculated by phosphorimager analysis following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and particle release was calculated as the amount of labeled Gag protein in the medium as a percentage of the total labeled Gag protein in the medium plus cell lysates. Percent release for each mutant was normalized to the wild-type Gag-GFP or Gag.ΔPR proteins, as appropriate, which were each assigned a value of 100%. Each bar represents the average of at least three independent experiments.
Kinetics of VLP release for NES mutant Gag proteins.
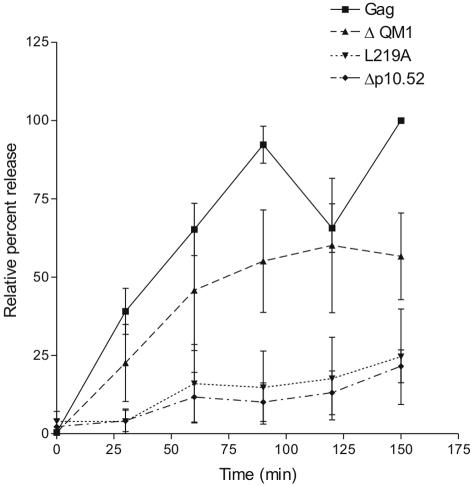
Despite the marked reduction in VLP release, Gag proteins containing NES mutations were still capable of budding, suggesting either (i) the block to nuclear export is incomplete, (ii) Gag proteins can escape the nucleus through a non-Crm1-mediated pathway, or (iii) a population of Gag proteins bypasses the nuclear compartment, traveling instead directly to the plasma membrane following synthesis. To distinguish among these possibilities, we studied the kinetics of particle release for the NES mutants using pulse-chase analysis. We reasoned that if NES-mutant Gag proteins bypassed the nucleus or utilized a non-Crm1-dependent export pathway, they would be released from the cell with wild-type kinetics; in contrast, any Gag proteins that transit through the nucleus but exit slowly (still using the crippled p10 NES) would display a severe reduction in the rate of VLP release as nuclear export became the rate-limiting step in the assembly process. Budding was measured as the ratio of Gag proteins released into the medium at each time point compared to the amount of Gag synthesized during the pulse-labeling period, with each value expressed as a percentage of wild-type (i.e., Gag.ΔPR) VLP release at the final time point (2.5 h). The Gag.ΔPR protein was rapidly released from the cell, with half-maximal release of the protein after ∼30 min (Fig. 4). Particle production peaked at 1.5 h, and budding levels reached a plateau with approximately 40% of the total protein being released from the cell. Mutant ΔQM1.Gag.ΔPR, which deletes portions of the p2 and p10 domains (excluding the p10 NES), was released with kinetics that were only slightly delayed compared to those of the wild-type protein, consistent with the preservation of functional nuclear export activity. In contrast, the two NES mutants, Δp10.52.Gag.ΔPR and L219A.Gag.ΔPR, released far fewer virus particles, and the rate of release was markedly decreased. There was no detectable subpopulation of VLPs that was released with rapid kinetics, although the half-lives of individual Gag molecules could not be assessed. These results support the hypothesis that there is a single population of Gag proteins that is released from the cell subsequent to trafficking through the nucleus.
FIG. 4.
Kinetics of NES mutant Gag protein release. Transfected QT6 cells were pulse-labeled for 15 min and then chased with cold medium. Medium samples were collected every 15 min, and the amount of Gag protein present in the medium was expressed relative to the amount of Gag.ΔPR protein synthesized during the 15-min pulse. The amount of wild-type Gag protein released after 2.5 h was standardized to 100% for each assay. Curves represent the average of four to five independent experiments, with standard deviations denoted.
Conservation of NES sequence and function among infectious avian retroviruses.
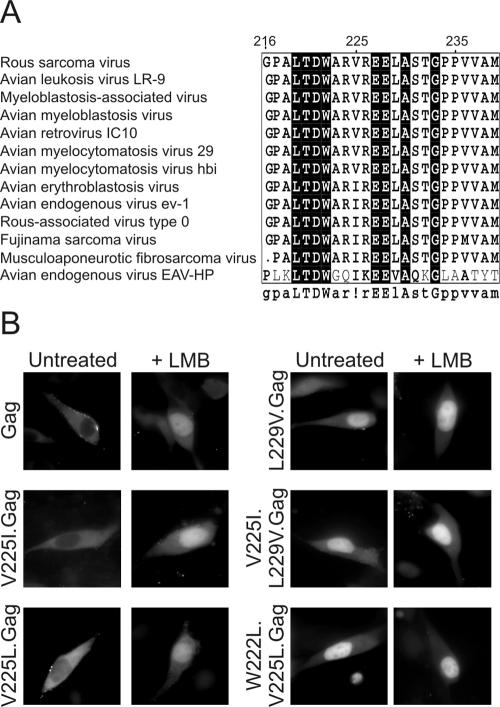
If the export signal of the Gag polyprotein is required during the life cycle of RSV, we postulated that the NES sequence might be conserved throughout evolution across a wide variety of related avian retroviruses. Avian sarcoma and leukosis viruses are stable within the genome of a broad range of avian species, revealing an ancient infection and subsequent vertical transmission of the endogenous provirus. When sequenced, these endogenous retroviruses revealed a remarkable conservation of the gag coding sequence. Indeed, highly conserved regions within Gag correspond to the membrane-binding domain, the late domain, and the NES (12). Based on the conservation of the p10 NES sequence among endogenous avian sarcoma and leukosis viruses, we sought to determine whether the same signal was preserved throughout a diverse group of exogenous avian retroviruses and whether polymorphisms in this region would preserve nuclear export function.
Examination of gag genes from the family of alpharetroviruses revealed that the C-terminal p10 region (amino acids 216 to 240) is highly conserved (Fig. 5A), although the gag genes from the avian spleen necrosis virus, lymphoproliferative disease virus, and avian leukemia virus e26 showed no overall homology throughout the gag region and are not depicted. Of the remaining viruses, 6 of the 13 depicted contain a C-terminal p10 sequence that is identical to that found within the RSV gag gene. Several viruses contained substitution of isoleucine at position 225 for the valine present in RSV. Because isoleucine is a hydrophobic residue that could potentially serve within a classical leucine-rich NES, these sequences might possibly retain nuclear export activity. We tested the ability of this sequence to serve as an NES by converting the valine codon at position 225 in RSV Gag to an isoleucine. We found that the V225I Gag mutant was able to be exported from the nucleus and was localized predominantly to the cytoplasm (Fig. 5B). As well, export of V225I.Gag-GFP from the nucleus was inhibited by treatment of cells with LMB, indicating that the new export signal remained Crm1 dependent. Therefore, the putative NES motifs present in all infectious avian retroviral p10 sequences examined are in fact functional nuclear export signals.
FIG. 5.
Conservation of NES function across avian retroviral Gag proteins. (A) Sequence alignment of the C-terminal portion of the p10 domain of a collection of alpharetroviruses using MultAlin analysis. The consensus sequence shown underneath the box represents residues of >70% sequence identity, with residues having 100% identity depicted in capital letters. (B) Subcellular localization of wild-type and mutant Gag-GFP proteins expressed in quail fibroblasts in the absence (left panels) or presence (right panels) of 18 nM LMB.
Sequence analysis also revealed a surprising absence of leucine at positions 222 and 225, considering that classical Crm1-dependent signals are leucine rich. Although we demonstrated that replacement of valine with alanine at position 225 in RSV Gag disrupts nuclear export (Fig. 2, panels G and H), we asked whether substitution of other hydrophobic residues at this position would preserve nuclear export activity. Conversion of valine to isoleucine (V225I.Gag) or valine to leucine (V225L) did not abrogate NES function or LMB sensitivity (Fig. 5B). Thus, Val225, while critical for NES function, is tolerant of conversion to other hydrophobic amino acids that can serve within leucine-rich export sequences.
Because substitution of leucine for Val225 was tolerated, we asked whether conversion of both Trp222 and Val225 to leucines, creating an NES comprised entirely of leucine residues, would be functional. Although we expected that the leucine-containing NES would mediate export, the W222L, V225L.Gag protein was unexpectedly trapped in the nucleus (Fig. 5B). Taken together, these results indicate that residues Trp222 and Val225 are sensitive to substitutions that were predicted to preserve NES function when altered in combination. This finding suggests a crucial role for these residues in maintaining the overall conformation of the Gag polyprotein or in maintaining the local structural integrity of the NES motif and is consistent with the conservation of Trp at position 222 in all avian sequences examined (Fig. 5A).
Although nearly all of the Gag sequences examined contained valine or isoleucine at position 225 as the only polymorphism within the region homologous to the RSV p10 NES, the avian endogenous retrovirus EAV-HP had substitutions of Val225 to isoleucine as well as Leu229 to valine. Despite the lack of overall conservation between the EAV-HP Gag sequence and the other avian viruses examined, it remained possible that the conservative hydrophobic substitutions at positions 225 and 229 would retain nuclear export function. To test this idea, Leu229 of RSV Gag was changed to valine (L229V.Gag), either individually or in combination with substitution of isoleucine for Val225 (V225I, L229V.Gag). Substitution of Leu-to-Val at position 229, either alone or in combination with Val-to-Ile at 225, abolished export activity (Fig. 5B, L229V.Gag and V225I, L229V.Gag). Treatment with LMB did not further enhance nuclear localization, suggesting that these combinations of hydrophobic residues do not constitute a functional NES in the context of RSV Gag. It is possible that the Gag protein derived from the endogenous retrovirus EAV-HP does not undergo nucleocytoplasmic trafficking. However, we cannot rule out the possibility that this sequence promotes nuclear export in the context of the full-length EAV-HP Gag protein or that a signal elsewhere in the protein is utilized to promote export.
The NES in p10 is positionally independent and transferable.
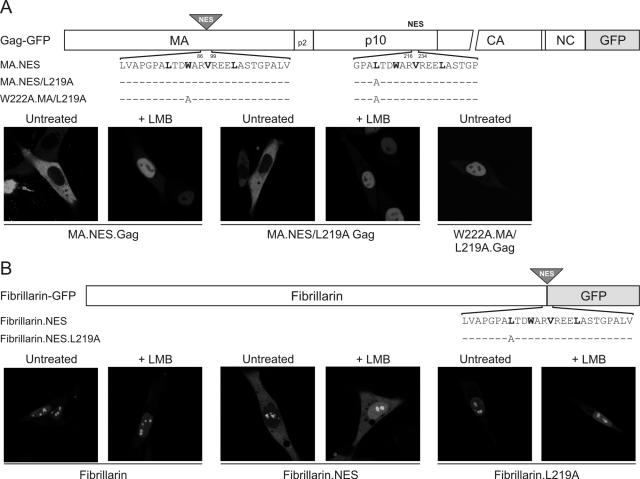
Although our mutational studies identified four hydrophobic residues within p10 that are necessary for export activity, we asked whether this sequence was sufficient to direct nuclear export. To this end, we tested whether the p10 hydrophobic motif would function in an ectopic location within the Gag protein itself. We introduced residues 216 to 234 of p10 into a portion of the MA domain that is otherwise dispensable for RSV replication (39), replacing residues 86 to 99 of MA with the p10 sequence (MA.NES.Gag-GFP) (Fig. 6A). This protein localized to the cytoplasm and plasma membrane under steady-state conditions. Importantly, treatment of MA.NES.Gag-GFP with LMB resulted in nuclear trapping of the protein, indicating that introduction of the second NES did not perturb the NLS in MA.
FIG. 6.
Mapping of a positionally independent and transferrable NES in Gag p10. (A) The position of the ectopic p10 NES sequence inserted into Gag-GFP is indicated by a shaded triangle, and the domains of Gag are indicated as MA, p2, p10, CA (capsid), NC, and PR (not drawn to scale). Residues 86 to 99 of MA were replaced with 216 to 234 of the p10 domain (plus three extra residues inserted with the restriction site introduced for cloning), and the amino acid sequences of the p10 NES and L219A and W222A mutants are shown below the diagram. Critical hydrophobic residues are indicated in bold type, and identical residues are represented as dashes. The subcellular localization of the MA.NES.Gag-GFP protein and NES variants was determined using confocal microscopy following transfection of QT6 cells that were either untreated (left panels) or incubated with 18 nM LMB (right panels). (B) Schematic diagram of fibrillarin-GFP showing the position of the inserted NES motif and the amino acid sequences of the p10 NES and the L219A mutant. QT6 cells expressing the indicated fibrillarin-derived GFP fusion proteins were untreated or treated with LMB and examined by confocal microscopy.
Using a gain-of-function approach, we asked whether the ectopic p10-derived sequence could restore nuclear export activity to a Gag protein with a crippled native NES. To this end, the MA.NES mutant was combined with the L219A knockout of the endogenous NES in p10. The resulting protein, MA.NES/L219A.Gag-GFP, was localized to the cytoplasm (Fig. 6A), indicating that the extra NES in MA rescued the L219A.Gag mutant from nuclear sequestration. Moreover, MA.NES/L219A.Gag-GFP was sensitive to LMB treatment, so the nuclear import signal in MA remained functional. Finally, to ascertain whether the p10 motif inserted into MA remained dependent on hydrophobic residues, the W222A substitution in the ectopic MA NES was combined with the L219A mutant in the native NES (Fig. 6A). As expected, the double NES knockout (W222A.MA/L219A.Gag-GFP) was confined to the nucleus in both LMB-treated and untreated cells. Importantly, this set of experiments demonstrated that the p10 NES is both positionally independent within Gag and sufficient to mediate export.
To investigate whether the p10 NES would function in a heterologous protein, residues 216 to 234 were introduced into fibrillarin-GFP (13) (Fig. 6B). Fibrillarin is normally localized in the nucleus and within nucleoli by virtue of complex nuclear and nucleolar localization signals (51). Addition of the p10 NES relocated fibrillarin to the cytoplasm and reduced the concentration in nucleoli (Fibrillarin.NES-GFP). Furthermore, treatment with LMB greatly increased the nucleoplasmic and nucleolar pools of Fibrillarin.NES-GFP, indicating that the inserted p10 NES continued to operate through the CRM1 pathway (compare the two center panels in Fig. 6B). Substitution of L219A in the inserted p10 sequence abrogated the export of fibrillarin, and localization was insensitive to LMB, as anticipated (Fibrillarin.L219A-GFP). Taken together, these results indicate that residues 216 to 234 of p10 constitute an independent and transferable motif that is sufficient for nuclear export in a heterologous system.
Identification of nuclear pore proteins that influence Gag export.
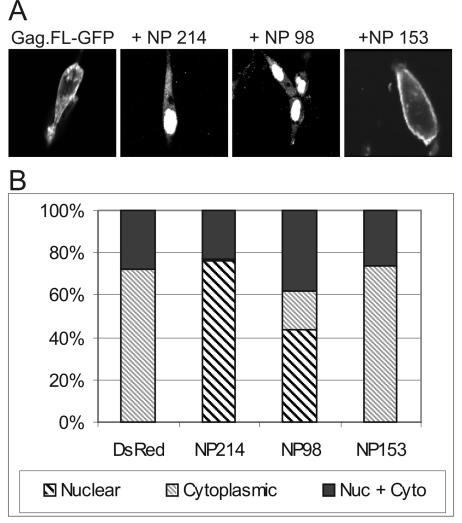
The discovery of this novel nuclear transport step in the life cycle of RSV allowed us to search for a new set of host proteins that interact with the Gag precursor to facilitate the assembly process. Crm1 is a soluble export receptor which associates with export cargo in the nucleus in complex with the small GTPase Ran. Crm1 serves as an adaptor, mediating the docking to and transport through the nuclear pore (18, 34, 53, 61). To date, many cofactors for Crm1-dependent export have been suggested. We focused on three nucleoporin proteins, which are components of the nuclear pore complex (NPC). Nup98 and Nup214 play an important role in nuclear export of the HIV Rev protein, which also interacts with Crm1, while Nup153 has a minor role in Rev export (63). Overexpression of nucleoporin cargo binding motifs, known as NP domains or FG repeat regions, allows them to act as competitive inhibitors of soluble nuclear factors that interact with nucleoporin proteins, thereby interfering with nuclear transport. To determine whether the RSV Gag protein utilized constituents of the NPC for export, the FG repeat regions on Nup98, Nup214, and Nup153 were introduced into the pDsRed.N1 vector (Clontech) and cotransfected with the pGag.FL-GFP plasmid (Fig. 7A). Fusion of DsRed allowed identification of single cells that were successfully transfected with both the NP domain and Gag-GFP using different excitation wavelengths during confocal microscopy. Coexpression of the NP domain of Nup98 or Nup214 led to a dramatic redistribution of the Gag protein into the nucleus, with a similar but more robust phenotype, as is seen with LMB treatment. In contrast, no alteration in Gag localization was seen upon expression of the NP domain of Nup153, suggesting that this nucleoporin might not be a significant mediator of Gag nuclear export. To quantitate these effects, cells expressing either NP-DsRed or DsRed alone were scored for localization of Gag.FL-GFP to either exclusively nuclear, exclusively cytosolic, or both nuclear and cytosolic (Fig. 7B). As shown in Fig. 7B, the wild-type Gag protein is cytoplasmic in 72.7% ± 5.9% of the cells, both nuclear and cytoplasmic in 27.4% ± 5.8% of cells, and not exclusively nuclear in any cells. Upon expression of NP214-DsRed or NP98-DsRed, localization of Gag is dramatically altered, with 76.4% ± 1.5% and 43.3% ± 3.8% of the cells (respectively) showing predominant localization to the nucleus. In contrast, expression of NP153-DsRed did not change Gag localization (Fig. 7A). These results suggest that in addition to Crm1, Nup214 and Nup98 are important cofactors involved in the export of the RSV Gag protein from the nucleus.
FIG. 7.
Inhibition of Gag nuclear export by dominant-negative mutants of nuclear pore proteins. (A) Cells were cotransfected with Gag.FL-GFP and either pDsRed.N1 or the DsRed constructs expressing the isolated NP domains from Nup98, Nup214, or Nup153 (63). Expression of the dominant-negative forms of Nup98 and Nup214 relocalize Gag to the cytoplasm, while the NP domain of Nup153 or the DsRed protein alone do not alter the steady-state distribution of Gag. (B) Cells expressing both Gag-GFP.FL and the DsRed protein either unconjugated or conjugated to the isolated NP domains were scored for the distribution of Gag-GFP to either the cytoplasm exclusively (thin hatched bars), both the nucleus and cytosol (black bars), or a predominant accumulation within the nucleus (thick hatched bars). Bars represent at least 200 cells from three independent experiments.
DISCUSSION
We previously reported that the RSV Gag polyprotein traffics through the nucleus of infected cells (49). Following synthesis in the cytoplasm, Gag is imported into the nucleus, likely through the use of a nuclear import signal within the MA domain. Deletions within the p10 region prevent nuclear export, leading to the accumulation of Gag in the nucleus, suggesting that the presence of an intact p10 domain is necessary for nuclear egress. However, our previous experiments did not discriminate between two possibilities: (i) whether the p10 domain is sufficient to confer export activity to Gag or (ii) whether the p10 deletions interfered with Gag export indirectly, perhaps by perturbing the overall structure of the protein and masking an export signal elsewhere in the Gag protein. In this report, the data demonstrate that specific hydrophobic residues within the p10 sequence are critical for nuclear export, and residues 216 to 234 constitute a positionally independent and transferable NES.
The high degree of conservation of p10 NES sequences among a wide range of avian retroviruses suggests that export of Gag through this pathway might be critical for the production of infectious virions. The exogenous avian retroviruses examined had only a single hydrophobic substitution, isoleucine for valine at position 225, which was functional when tested in RSV. The substitutions of hydrophobic residues that failed to confer export activity in the context of RSV Gag either were not naturally occurring or were derived from an endogenous avian retrovirus. Interestingly, substitution of leucine for native hydrophobic residues W222 and V225 was not tolerated in RSV Gag, indicating that other characteristics of the amino acids at these positions are important, perhaps for protein structure. These results raise the possibility that the C-terminal portion of the p10 domain has dual functions: one in the nuclear export of Gag, which is intrinsic to the virus assembly pathway, and the other in maintaining the proper conformation of Gag, as suggested in an in vitro assembly system (4).
The results of these studies have led us to consider the implications of the interaction of Gag with the Crm1 pathway, which is a fundamental nuclear transport system used for the export of RNA-binding proteins and specific subclasses of RNA. Crm1 is the export receptor for rRNA, 5S RNA, U snRNA, and possibly some cellular mRNAs (reviewed in reference 9). The mechanism by which Crm1 serves to export cellular RNAs is likely through its interaction with NES-containing adaptor proteins, including PHAX, which mediates transport of U snRNAs (40), and NMD3, which exports the 60S ribosomal subunit (54, 57). The adaptor for the 40S ribosomal subunit is not yet known, although Yrb2 is a candidate for this role (36). Adaptor proteins that have been proposed to mediate export of some specific mRNAs via the Crm1 pathway include the HuR-pp32-APRIL complex (2), the human nuclear export family member NXF3 (60), a splice variant of Staufen2 (33), and possibly TRA-1 in Caenorhabditis elegans, although this association has yet to be confirmed (27).
The first ligand of the Crm1 receptor identified was the HIV-1 Rev protein, which serves as an adaptor for the export of the unspliced viral RNA genome and singly spiced viral mRNA that would otherwise be restricted from leaving the nucleus (18, 53). Other retroviruses, including human T-cell leukemia virus, feline immunodeficiency virus, and equine infectious anemia virus, also possess Rev-related adaptor proteins that function through the Crm1 export pathway (42, 43). Many other viruses utilize Crm1-mediated nuclear export to enhance virus replication, often by encoding adaptor proteins that facilitate the cytoplasmic localization of genomic and subgenomic RNAs. For example, influenza virus encodes M1 and NES/NS2 proteins that facilitate export of viral ribonucleoprotein complexes from the nucleus in an LMB-sensitive manner (reviewed in reference 8). A Crm1-dependent posttranscriptional regulatory element in the genome of Woodchuck hepatitis virus is involved in export of intronless viral mRNAs, although the putative cellular factor required for its activity has not yet been found (47). For herpes simplex virus, it appears that viral transcripts are exported via redundant systems, some of which utilize the Crm1 export factor, with others relying on alternative pathways (52).
The use of the Crm1 pathway for the export of viral mRNAs and ribonucleoprotein complexes in other viral systems raises the possibility that a similar mechanism may be at work in the life cycle of RSV. The retroviral Gag protein specifically recognizes the unspliced viral RNA genome and selects it for packaging into assembling virions. We have postulated that the RSV Gag protein serves as a Crm1 adaptor protein, perhaps to facilitate export of the genomic RNA for packaging into assembling virions. Rather than serving simply as a substrate for Crm1-dependent export, Gag might serve to bridge the association of the genomic viral RNA between the export receptor and components of the NPC, including Nup98 and Nup214; further experimentation will be needed to test this idea.
We remain mindful that the Crm1-dependent NES in Gag might play other roles in RSV replication or viral pathogenesis that do not directly involve viral RNA transport. As an example of an alternative role for CRM1 in herpesvirus replication, the LMP1 protein encoded by Epstein-Barr virus possesses a Crm1-dependent NES that facilitates export of specific transcription factors to augment cellular transformation, rather than serving as a Crm1 adaptor to enhance the export of viral transcripts (41). Thus, it will be essential to analyze NES mutants of RSV to further elucidate potential roles of the Crm1-dependent export of Gag in the virus life cycle.
Although we found that residues 216 to 234 in p10 function as an LMB-sensitive NES, it should be mentioned that the spacing of hydrophobic residues in this motif is different from the spacing of leucine residues in typical NESs, like that of HIV-1 Rev. However, there are several Crm1-dependent NESs with atypical spacing and composition, including the NESs of the equine infectious anemia virus and feline immunodeficiency virus Rev-like proteins (42). An analysis of all experimentally verified leucine-rich NESs revealed that two-thirds of the NESs diverged from the consensus of L-x(2,3)-LIVFM-x(2,3)-L-x[L1] and 15% did not conform to either of the two most conserved spacing patterns (29). Not only do many LMB-dependent NESs deviate from the consensus motif, but they also vary with respect to their binding affinity for the Crm1 export receptor (1, 23); indeed, NESs are thought to require a relatively weak interaction with Crm1 to ensure proper dissociation from the receptor and translocation through the nuclear pore (17, 28). Even though the NES in RSV Gag differs from the consensus sequence, the homologous Gag protein of v-ErbA was reported to bind directly to Crm1 in vitro. Importantly, the single amino acid variation in the v-ErbA Gag p10 NES (V to I) (11) still functions in a Crm1-dependent manner in RSV Gag (V225I, Fig. 5B). Further experimentation will be needed to determine whether the isolated p10 NES binds directly to Crm1 and the affinity of the interaction as well as whether RSV Gag-Crm1 complexes can be detected in vivo.
Importantly, we have now identified additional constituents of the cellular pathway required for nucleocytoplasmic trafficking activity of the RSV Gag protein. Our data support the involvement of Nup214 and Nup98 in Gag nuclear export, since expression of the dominant-negative form of each of these NPC constituents blocked the reaccumulation of Gag in the cytoplasm. Because these same nucleoporins are integral to the function of the HIV-1 Rev protein (63) and because Rev is also Crm1 dependent, it appears that both RSV Gag and HIV-1 Rev utilize the same soluble export receptor to make contacts with identical components of the NPC. Indeed, several viruses employ direct or indirect interactions with Nup98 or Nup214 to facilitate import of their viral genomes into the nucleus, including adenovirus and HIV-1 (16, 25, 56), and vesicular stomatitis virus engages Nup98 to manipulate host gene expression (58). We anticipate that there are additional NPC components and nuclear transport factors that associate with RSV Gag during virus replication. Future experiments using more-informative genetic systems and in vitro nuclear transport assays will be needed to further define the full spectrum of interactions that Gag makes both directly and indirectly with nucleoporins and cellular trafficking pathways.
Acknowledgments
We are indebted to Rebecca Craven, John Wills, and Tom Hope for sharing reagents and for insightful discussions, Barbara Felber and Mark Olson for providing plasmids, and Scott Kenney for critical review of the manuscript. We appreciate the assistance of the Penn State Core Facility with microscopy, oligonucleotide synthesis, and DNA sequencing.
This work was supported by a grant from the National Institutes of Health to L.J.P (R01 CA76534) and a National Science Foundation predoctoral fellowship to L.Z.S. L.J.P. was supported as an MDRFA Scholar at Penn State College of Medicine. This project was funded in part by a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
REFERENCES
- 1.Askjaer, P., A. Bachi, M. Wilm, F. R. Bischoff, D. L. Weeks, V. Ogniewski, M. Ohno, C. Niehrs, J. Kjems, I. W. Mattaj, and M. Fornerod. 1999. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Mol. Cell. Biol. 19:6276-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan, C. M., I. E. Gallouzi, and J. A. Steitz. 2000. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 151:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I., N. Sharova, T. L. McDonald, T. Pushkarskaya, W. G. Tarpley, and M. Stevenson. 1993. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 90:6125-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan, E. M., and J. W. Wills. 2000. Repositioning basic residues in the M domain of the Rous sarcoma virus Gag protein. J. Virol. 74:11222-11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, S., and V. M. Vogt. 1997. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J. Virol. 71:4425-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craven, R. C., A. E. Leure-duPree, R. A. Weldon, Jr., and J. W. Wills. 1995. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J. Virol. 69:4213-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cros, J. F., and P. Palese. 2003. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 95:3-12. [DOI] [PubMed] [Google Scholar]
- 9.Cullen, B. R. 2003. Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 28:419-424. [DOI] [PubMed] [Google Scholar]
- 10.Dang, V. D., and H. L. Levin. 2000. Nuclear import of the retrotransposon Tf1 is governed by a nuclear localization signal that possesses a unique requirement for the FXFG nuclear pore factor Nup124p. Mol. Cell. Biol. 20:7798-7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong, L. J., G. M. Bonamy, E. N. Fink, and L. A. Allison. 2004. Nuclear export of the oncoprotein v-ErbA is mediated by acquisition of a viral nuclear export sequence. J. Biol. Chem. 279:15356-15367. [DOI] [PubMed] [Google Scholar]
- 12.Dimcheff, D. E., S. V. Drovetski, M. Krishnan, and D. P. Mindell. 2000. Cospeciation and horizontal transmission of avian sarcoma and leukosis virus gag genes in galliform birds. J. Virol. 74:3984-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dundr, M., T. Misteli, and M. O. Olson. 2000. The dynamics of postmitotic reassembly of the nucleolus. J. Cell Biol. 150:433-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont, S., N. Sharova, C. DeHoratius, C.-M. A. Virbasius, X. Zhu, A. G. Bukrinskaya, M. Stevenson, and M. R. Green. 1999. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature (London) 402:681-685. [DOI] [PubMed] [Google Scholar]
- 15.Dupraz, P., and P. F. Spahr. 1993. Analysis of deletions and thermosensitive mutations in Rous sarcoma virus gag protein p10. J. Virol. 67:3826-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebina, H., J. Aoki, S. Hatta, T. Yoshida, and Y. Koyanagi. 2004. Role of Nup98 in nuclear entry of human immunodeficiency virus type 1 cDNA. Microbes Infect. 6:715-724. [DOI] [PubMed] [Google Scholar]
- 17.Engelsma, D., R. Bernad, J. Calafat, and M. Fornerod. 2004. Supraphysiological nuclear export signals bind CRM1 independently of RanGTP and arrest at Nup358. EMBO J. 23:3643-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornerod, M., M. Ohno, M. Yoshida, and I. M. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export receptors. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 19.Fouchier, R. A. M., B. E. Meyer, J. H. M. Simon, U. Fischer, and M. H. Malim. 1997. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16:4531-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed, E. O., G. Englund, and M. A. Martin. 1995. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J. Virol. 69:3949-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 22.Haffar, O. K., S. Popov, L. Dubrovsky, I. Agostini, H. Tang, T. Pushkarsky, S. G. Nadler, and M. Bukrinsky. 2000. Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J. Mol. Biol. 299:359-368. [DOI] [PubMed] [Google Scholar]
- 23.Heger, P., J. Lohmaier, G. Schneider, K. Schweimer, and R. H. Stauber. 2001. Qualitative highly divergent nuclear export signals can regulate export by the competition for transport cofactors in vivo. Traffic 2:544-555. [DOI] [PubMed] [Google Scholar]
- 24.Joshi, S. M., and V. M. Vogt. 2000. Role of the Rous sarcoma virus p10 domain in shape determination of gag virus-like particles assembled in vitro and within Escherichia coli. J. Virol. 74:10260-10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiss, A., L. Li, T. Gettemeier, and L. K. Venkatesh. 2003. Functional analysis of the interaction of the human immunodeficiency virus type 1 Rev nuclear export signal with its cofactors. Virology 314:591-600. [DOI] [PubMed] [Google Scholar]
- 26.Krishna, N. K., S. Campbell, V. M. Vogt, and J. W. Wills. 1998. Genetic determinants of Rous sarcoma virus particle size. J. Virol. 72:564-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuersten, S., S. P. Segal, J. Verheyden, S. M. LaMartina, and E. B. Goodwin. 2004. NXF-2, REF-1, and REF-2 affect the choice of nuclear export pathway for tra-2 mRNA in C. elegans. Mol. Cell 14:599-610. [DOI] [PubMed] [Google Scholar]
- 28.Kutay, U., and S. Guttinger. 2005. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 15:121-124. [DOI] [PubMed] [Google Scholar]
- 29.la Cour, T., L. Kiemer, A. Molgaard, R. Gupta, K. Skriver, and S. Brunak. 2004. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. 17:527-536. [DOI] [PubMed] [Google Scholar]
- 30.Lecellier, C. H., M. Neves, M. L. Giron, J. Tobaly-Tapiero, and A. Saib. 2002. Further characterization of equine foamy virus reveals unusual features among the foamy viruses. J. Virol. 76:7220-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, E. G., A. Yeo, B. Kraemer, M. Wickens, and M. L. Linial. 1999. The gag domains required for avian retroviral RNA encapsidation determined by using two independent assays. J. Virol. 73:6282-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594, table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miki, T., and Y. Yoneda. 2004. Alternative splicing of Staufen2 creates the nuclear export signal for CRM1 (exportin 1). J. Biol. Chem. 279:47473-47479. [DOI] [PubMed] [Google Scholar]
- 34.Moroianu, J. 1998. Distinct nuclear import and export pathways mediated by members of the karyopherin beta family. J. Cell Biochem. 70:231-239. [PubMed] [Google Scholar]
- 35.Moscovici, C., M. G. Moscovici, H. Jimenez, M. M. C. Lai, M. J. Hayman, and P. K. Vogt. 1977. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell 11:95-103. [DOI] [PubMed] [Google Scholar]
- 36.Moy, T. I., and P. A. Silver. 1999. Nuclear export of the small ribosomal subunit requires the ran-GTPase cycle and certain nucleoporins. Genes Dev. 13:2118-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muriaux, D., J. Mirro, D. Harvin, and A. Rein. 2001. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. USA 98:5246-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash, M. A., M. K. Meyer, G. L. Decker, and R. B. Arlinghaus. 1993. A subset of Pr65gag is nucleus associated in murine leukemia virus-infected cells. J. Virol. 67:1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelle, T. D., and J. W. Wills. 1996. A large region within the Rous sarcoma virus matrix protein is dispensable for budding and infectivity. J. Virol. 70:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno, M., A. Segref, A. Bachi, M. Wilm, and I. W. Mattaj. 2000. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 101:187-198. [DOI] [PubMed] [Google Scholar]
- 41.Ohtani, N., P. Brennan, S. Gaubatz, E. Sanij, P. Hertzog, E. Wolvetang, J. Ghysdael, M. Rowe, and E. Hara. 2003. Epstein-Barr virus LMP1 blocks p16INK4a-RB pathway by promoting nuclear export of E2F4/5. J. Cell Biol. 162:173-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otero, G. C., M. E. Harris, J. E. Donello, and T. J. Hope. 1998. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J. Virol. 72:7593-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmeri, D., and M. H. Malim. 1996. The human T-cell leukemia virus type 1 posttranscriptional trans-activator Rex contains a nuclear export signal. J. Virol. 70:6442-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parent, L. J., R. P. Bennett, R. C. Craven, T. D. Nelle, N. K. Krishna, J. B. Bowzard, C. B. Wilson, B. A. Puffer, R. C. Montelaro, and J. W. Wills. 1995. Positionally independent and exchangeable late budding functions of Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 69:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parent, L. J., T. M. Cairns, J. A. Albert, C. B. Wilson, J. W. Wills, and R. C. Craven. 2000. RNA dimerization defect in a Rous sarcoma virus matrix mutant. J. Virol. 74:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parent, L. J., C. B. Wilson, M. D. Resh, and J. W. Wills. 1996. Evidence for a second function of the MA sequence in the Rous sarcoma virus Gag protein. J. Virol. 70:1016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popa, I., M. E. Harris, J. E. Donello, and T. J. Hope. 2002. CRM1-dependent function of a cis-acting RNA export element. Mol. Cell. Biol. 22:2057-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rashkova, S., A. Athanasiadis, and M. L. Pardue. 2003. Intracellular targeting of Gag proteins of the Drosophila telomeric retrotransposons. J. Virol. 77:6376-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheifele, L. Z., R. A. Garbitt, J. D. Rhoads, and L. J. Parent. 2002. Nuclear entry and CRM1-dependent nuclear export of the Rous sarcoma virus Gag polyprotein. Proc. Natl. Acad. Sci. USA 99:3944-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon, J. H., E. A. Carpenter, R. A. Fouchier, and M. H. Malim. 1999. Vif and the p55(Gag) polyprotein of human immunodeficiency virus type 1 are present in colocalizing membrane-free cytoplasmic complexes. J. Virol. 73:2667-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snaar, S., K. Wiesmeijer, A. G. Jochemsen, H. J. Tanke, and R. W. Dirks. 2000. Mutational analysis of fibrillarin and its mobility in living human cells. J. Cell Biol. 151:653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 54.Thomas, F., and U. Kutay. 2003. Biogenesis and nuclear export of ribosomal subunits in higher eukaryotes depend on the CRM1 export pathway. J. Cell Sci. 116:2409-2419. [DOI] [PubMed] [Google Scholar]
- 55.Tritel, M., and M. D. Resh. 2000. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J. Virol. 74:5845-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trotman, L. C., N. Mosberger, M. Fornerod, R. P. Stidwill, and U. F. Greber. 2001. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 3:1092-1100. [DOI] [PubMed] [Google Scholar]
- 57.Trotta, C. R., E. Lund, L. Kahan, A. W. Johnson, and J. E. Dahlberg. 2003. Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates. EMBO J. 22:2841-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 59.Weldon, R. A., C. R. Erdie, M. G. Oliver, and J. W. Wills. 1990. Incorporation of chimeric Gag protein into retroviral particles. J. Virol. 64:4169-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, J., H. P. Bogerd, P. J. Wang, D. C. Page, and B. R. Cullen. 2001. Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol. Cell 8:397-406. [DOI] [PubMed] [Google Scholar]
- 61.Yoneda, Y., M. Hieda, E. Nagoshi, and Y. Miyamoto. 1999. Nucleocytoplasmic protein transport and recycling of Ran. Cell Struct. Funct. 24:425-433. [DOI] [PubMed] [Google Scholar]
- 62.Yu, S. F., K. Edelmann, R. K. Strong, A. Moebes, A. Rethwilm, and M. L. Linial. 1996. The carboxyl terminus of the human foamy virus Gag protein contains separable nucleic acid binding and nuclear transport domains. J. Virol. 70:8255-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zolotukhin, A. S., and B. K. Felber. 1999. Nucleoporins Nup98 and Nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev. J. Virol. 73:120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]