ABSTRACT
Humans are exceptional among vertebrates in that their living tissue is directly exposed to the outside world. In the absence of protective scales, feathers, or fur, the skin has to be highly effective in defending the organism against the gamut of opportunistic fungi surrounding us. Most (sub)cutaneous infections enter the body by implantation through the skin barrier. On intact skin, two types of fungal expansion are noted: (A) colonization by commensals, i.e., growth enabled by conditions prevailing on the skin surface without degradation of tissue, and (B) infection by superficial pathogens that assimilate epidermal keratin and interact with the cellular immune system. In a response-damage framework, all fungi are potentially able to cause disease, as a balance between their natural predilection and the immune status of the host. For this reason, we will not attribute a fixed ecological term to each species, but rather describe them as growing in a commensal state (A) or in a pathogenic state (B).
INTRODUCTION
Humans are exceptional among vertebrates in that their living tissue is directly exposed to the outside world. In the absence of protective scales, feathers, or fur, the skin has to be highly effective in defending the organism against a gamut of opportunistic fungi surrounding us. Most (sub)cutaneous infections enter the body by implantation through the skin barrier. On intact skin, two types of fungal expansion are noted: (A) colonization by commensals, i.e., growth enabled by conditions prevailing on the skin surface without degradation of tissue, and (B) infection by superficial pathogens that assimilate epidermal keratin and interact with the cellular immune system. In a response-damage framework (1), all fungi are potentially able to cause disease, as a balance between their natural predilection and the immune status of the host. For this reason we will not attribute a fixed ecological term to each species, but rather describe them as growing in a commensal state (A) or in a pathogenic state (B).
Colonization of human skin by allochthonous (i.e., having their preferred niche outside the human host) commensals is coincidental (Fig. 1A). The conditions provided on the skin resemble some essential factors of their natural niche in the environment. The fungi have a “sloppy fitness space” (2); i.e., as long as these factors are met, the fungi are able to survive in habitats that are otherwise unsuitable. Mechanisms for transmission are lacking and thus the infection is a spillover with negative impact on fitness of the etiologic agent. A typical example is the black yeast Hortaea werneckii. The species causes tinea nigra, characterized by blackish patches on excessively salty skin of hyperhydrotic individuals acquired, for example, after a holiday at the beach (3). Coastal waters are the natural habitat of the fungus (4). It is a halophile, its ecology being determined by Hog1 homologues of the mitogen-activated protein kinase of Saccharomyces cerevisiae (5), being able to grow even in near-saturated NaCl solutions.
FIGURE 1.

Overview of basic types of fungal occurrence on human skin and modes of transmission.
Malassezia species have their natural niche on humans, living on human products, i.e., they are autochthonous commensals (Fig. 1B). Clinically, they are recognizable in several common skin disorders including seborrheic dermatitis as pityriasis versicolor (PV), a common skin disorder associated with hyper- or hypopigmentation caused by Malassezia (6–8). The lipid-dependent fungi are also part of the benign residential skin flora of most warm-blooded animals including humans (9).
Dermatophytes cause cutaneous infections. The evolutionary history of dermatophytes is determined by their ability to degrade keratin. In humans, they are highly specialized, infecting almost exclusively nails, hair, and epidermis. Three broad ecological groups can be distinguished (Fig. 1C). Ancestral species are geophilic, having a saprobic life cycle in the soil and digesting remains of vertebrate keratin such as feathers and hair. Human infection is sporadic and decreases the fungus’ fitness. Zoophilic species reside in closer association with the mammalian host, being carried in pelts and feathers, mostly without causing inflammatory reactions. Anthropophilic species are the most derived dermatophytes, naturally growing on naked human skin. The fungi have developed an intimate interplay with innate and acquired immunity.
This review focuses on the natural eukaryotic skin flora: i.e., dermatophytes and Malassezia.
DERMATOPHYTES
Biodiversity
Common dermatophytes on humans are classically identified by clinical presentation and macro- and micromorphology. Today, methods of choice to display the total biodiversity and the coherence of species in a phylogenetic system involve multilocus sequencing. Polymorphisms of the rDNA internal transcribed spacer (ITS) region were able to resolve a large number of species (10). The topology of their phylogenetic tree has repeatedly been confirmed by other molecular markers such as BT2 (11), TEF1 (12), and TEF3 (13), and thus the taxonomy of Arthrodermataceae seems to have reached stability. In all trees, Trichophyton is polyphyletic, containing clinically and ecologically widely different species. Therefore de Hoog et al. (13) confined Trichophyton and Epidermophyton to mainly anthropophilic species located in derived clusters. Zoophilic species were located at the top and around the middle of the tree and were classified in Trichophyton, Microsporum, and Nannizzia, while the highly diverse geophilic species were located in an ancestral position and were mainly classified in Arthroderma, Lophophyton, and Nannizzia (Fig. 2).
FIGURE 2.
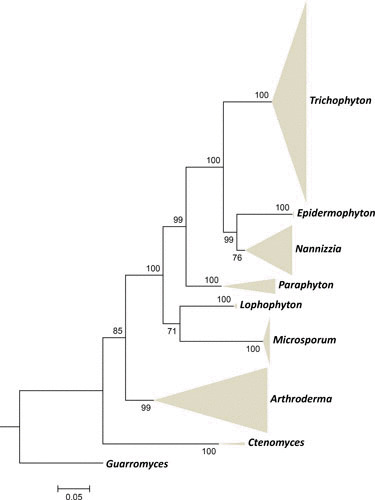
Maximum likelihood phylogenetic tree (RAxML v.8.0.0) based on ITS and partial LSU, TUB, and 60S L10 sequences of Arthrodermataceae species using GTRCAT as model, with 1,000 bootstrap replications, shown as collapsed when bootstrap values >70%. Guarromyces ceretanicus was selected as outgroup. Reprinted from de Hoog et al. (13).
Virulence Factors
Because dermatophytes are almost exclusively localized in keratinized tissues, research on mechanisms of invasion primarily focuses on secreted proteases. All dermatophytes grow well in a medium containing hard keratin as the sole source of carbon and nitrogen. The spectrum of proteases secreted by dermatophytes is similar to that of Aspergillus, but differs by multiple endoprotease members of the S8 (subtilisins), M35 (deuterolysin), and M36 (fungalysins) families (14, 15). These belong to endoprotease gene families that have expanded in the Onygenales, and to exopeptidases of the M14 family (metallocarboxypeptidases) and M28 (aminopeptidases). At neutral or alkaline pH, dermatophytes secrete two major subtilisins, Sub3 and Sub4, and two major fungalysins, Mep3 and Mep4, as endopeptidases (14, 16–19). In comparison with proteinase K and subtilisin Carlsberg, Trichophyton rubrum secretes subtilisins Sub3 and Sub4, which are more active on keratin azure than on other protein sources such as elastin, suggesting specificity toward hard keratin substrates (17). In addition, dermatophytes secrete various aminopeptidases, e.g., leucine aminopeptidases (Lap1 and Lap2) and dipeptidyl-peptidases (DppIV and DppV). These enzymes show activities similar to Aspergillus fumigatus orthologues (20). Dermatophytes were also found to secrete a carboxypeptidase of the MEROPS M14A subfamily that is homologous to the human pancreatic carboxypeptidase A (21).
In a protein medium at acidic pH, dermatophytes secrete an aspartic protease of the pepsin family (Pep1) as an endoprotease, as well as tripeptidyl peptidases of the sedolisin family, prolyl peptidases and carboxypeptidases of the S10 family, and exoproteases (14). Analysis of the repertoire of secreted exoproteases in dermatophytes suggests basic mechanisms of extracellular proteolysis similar to those known in Aspergillus species at acidic and neutral pH (22, 23).
The proteases secreted in vitro are considered as virulence factors. However, transcriptome analysis performed with RNA from guinea pigs infected by the dermatophyte Trichophyton benhamiae and proteomic analyses of proteins extracted from infected nail beds of patients with onychomycosis revealed that a particular subtilisin (Sub6), not detected in vitro, was the major protease during infection (24, 25). Additional secreted proteins were detected, including the closely related Sub7 and the dipeptidyl peptidase DppV. Surprisingly, most of the proteases secreted in vitro during keratin digestion were not detected during the establishment of an infection. The results from in vivo experiments are of interest because Sub6 and DppV in T. rubrum were previously identified and described as major dermatophyte allergens, Tri r 2 and Tri r 4, respectively (26).
Other secreted hydrolases, e.g., ceramidases and lipases, are also putatively involved in degradation of the skin barrier. Microarray analysis revealed upregulation of genes such as heat shock proteins and transporters during Arthroderma benhamiae infection in guinea pigs, but the precise role of these genes in pathogenicity remains unclear (24). Marked upregulation of genes encoding enzymes of the glyoxylate cycle (i.e., isocitrate lyase and malate synthase) focused attention because this cycle was found to be necessary for virulence in Candida albicans (27) and for persistence in macrophages in Mycobacterium tuberculosis (28). However, virulence of deletion mutants defective in malate synthase was not attenuated in guinea pigs (29). The effects of the absence of the glyoxylate pathway might be suppressed by other metabolic pathways during infection.
Clinical Manifestations Distant from Dermatophyte Infections: Asthma and Skin Dermatophytides
Dermatophyte infections can provoke secondary immune reactions such as severe asthma and eczematous skin reactions (dermatophytids) in susceptible individuals. Dermatophytids, like Trichophyton-related asthma, can be controlled with systemic antifungal therapy.
Dyshidrotic and vesicular eczema on the hands (palms and/or fingers) associated with tinea pedis and/or tinea unguium in adults (generally caused by T. rubrum and Trichophyton interdigitale) are common dermatophytids (30). Eczema on the chest, trunk, and back associated with scalp ringworm due to zoophilic and anthropophilic species is less frequent but has been reported (31–33). A set of diagnostic criteria was developed to identify a dermatophytid reaction (33, 34): (i) there is a proven dermatophytic infection in a body site other than the locus of the eczematous skin reaction; (ii) the eczema appeared after the dermatophyte infection. The fungus is not present in the site of the cutaneous eruption. From this site, culture assays remain sterile and direct mycological examination is negative; (iii) the dermatophytid symptoms only resolve after eradication of the primary focus of fungal infection; (iv) the patients are sensitized to dermatophyte antigens and show a positive skin test response to fungal extracts (termed “Trichophytin”). In contrast to knowledge on Trichophyton asthma (35–37), individual antigens involved in dermatophytids have not been identified. Proteins secreted in vivo by dermatophytes are the best candidates as antigens involved in the host immune response.
Comparative Genomics
Genomes of dermatophytes are somewhat smaller than usual in filamentous ascomycetes, ranging from 22.5 Mb for T. rubrum to 24.1 Mb for Trichophyton equinum (38). Important functions concern secondary metabolism, proteases, and LysM binding domains, i.e., motifs that bind to various types of peptidoglycan and chitin. In contrast, genes involved in sugar metabolism and plant cell wall degradation are relatively depleted. There are only a few low-GC (31 to 36%) transposable elements, representing 1.3 to 7.2% of the genome (38), with low GC. Genomes of dermatophytes are colinear among each other, with only a few inversions. The core set comprises 6,168 orthologous groups of which 308 are unique to the dermatophytes. Species-specific genes have little functional information, and, hence, different clinical predilections between species cannot be explained with genome data alone. Three orthologous groups of the glycosyltransferase family (GT54) may be involved in immunomodulation of mannan upon infection. Burmester et al. (15) analyzed the global secretome during keratin degradation by growth with human keratinocytes. Dermatophytes produce a broad repertoire of genes encoding hydrolytic enzymes, lipases for cutaneous lipid degradation, and an exceptionally large number of proteases. Proteases do not differ significantly between species, indicating that proteolytic abilities are ancestral. Dermatophytes have an unusually high degree of nonreducing polyketide synthases. Possibly this is linked to the often striking colony colors due to extracellular metabolites of the polyketide synthase pathway, such as the mycotoxin xanthomegnin, the red color of T. rubrum, which has been detected in clinical samples of epidermal material infected by T. rubrum, in contrast to noninfected controls.
Sexuality
Many geophilic and zoophilic species produce sexual phases on suitable media after confrontation of strains of opposite mating types, and, in such species, mating type distribution is balanced. Li et al. (39) matched molecular structure of the mating type locus idiomorphs (alpha and high-mobility group [HMG] domains) with in vitro mating results. Symoens et al. (40) showed balanced frequencies of + and − mating types in strains of the zoophilic species T. benhamiae. Anzawa et al. (41) showed mating of a highly competent Arthroderma simii tester strain producing a fertile F1 generation with a strain of T. rubrum, although, with a hybridization depression, only 1 of 35 ascospores was a real recombinant of the two species. On the basis of mating alone, some authors maintain rather wide species concepts uniting entities that are clinically and phenotypically different from each other (42). Another approach is a hypothesis of an atavistic ability to undergo genetic exchange via sexual reproduction/hybridization in response, e.g., the stressful conditions of a newly inhabited environment (13).
Over the phylogenetic tree, a gradual loss of sexual vigor can be observed. Kano et al. (43) confirmed that in the zoophilic species Trichophyton verrucosum idiomorphs of the mating type locus (alpha domain and HMG domain genes) exhibited only a single molecular mating type. Gräser et al. (Kosanke S, Hamann L, Gräser Y, unpublished data) noticed that this is the prevalent situation in several other zoophilic and most anthropophilic species. This suggests that anthropophilic species do not have an environmental niche where sexuality could take place, being transmitted between hosts. It may be hypothesized that clonal offshoots of interbreeding species are ecologically specialized and may be in danger of extinction upon changes in their niche on the human host (44).
Host Defense Mechanisms
Zoophilic and geophilic species of dermatophytes (e.g., T. benhamiae, Trichophyton erinacei, T. verrucosum, and Microsporum canis) cause highly inflamed lesions in humans. A dermatophyte provokes a more intense inflammatory reaction on a host to which it is not adapted than to its natural host; but on the other hand such lesions more rapidly lead to spontaneous resolution. Both innate and adaptive immunity are involved in host defense mechanisms against dermatophytes.
Innate immunity
Innate immunity in superficial dermatophytosis implies the action of keratinocytes and neutrophils. A broad spectrum of cytokines is produced by keratinocytes upon exposure to a dermatophyte (45), including interleukin-8 (IL-8), a potent chemoattractant for neutrophils that can kill dermatophytes, and the proinflammatory tumor necrosis factor α (TNF-α) (46). Production is higher in zoophilic than in anthropophilic dermatophytes (45, 47), which is consistent with the clinical features by the respective dermatophyte groups. Keratinocytes also secrete a wide variety of antimicrobial peptides with antifungal properties. Human β-defensin (48), cathelicidin LL-37 (49), psoriasin (50), and disulfide-reduced psoriasin (51) were proven to be either fungistatic or fungicidal in vitro against T. rubrum. Disulfide-reduced psoriasin was isolated from psoriatic lesions and confers resistance to fungal infections (51).
Zoonotic dermatophyte infections tend to be more inflammatory than those caused by anthropophilic species (52). Rare cases of deep dermatophytosis have been described in HIV and immunosuppressed patients (53, 54), but also in immunocompetent people, mainly from North African families with consanguinity (55). These patients were found to bear homozygous mutations in the gene coding for a caspase recruitment domain containing protein (CARD9). A stop codon mutation (Q289*) was detected in 15 patients from seven Algerian and Tunisian families (55). Two missense mutations, R101C and R101L, were detected in two Moroccan siblings and a Brazilian patient, respectively (55, 56). The mutation Q289* was also detected in a patient of Egyptian origin with extensive skin and nail dermatophytosis (57). CARD9 is an adaptor protein in the signaling pathway downstream from lectin receptors, such as dectin 1 and dectin 2 involved in the recognition of pathogenic fungi, and plays an important role in the innate immune response against fungal pathogens. The gene is expressed mainly in myeloid cells and is involved in the stimulation of proinflammatory responses. CARD9-deficient cells show low levels of IL-6 production after stimulation with zymosan, an agonist of dectin 1 (55).
Adaptive immunity
Zoophilic and geophilic dermatophytes induce a delayed type hypersensitivity (DTH) cell-mediated response, which usually results in recovery and subsequent protection against reinfection. The response is characterized by elevated levels of the key cytokines IL-12 and gamma interferon (IFN-γ), which trigger T-helper 1 (Th1) cells for the activation of macrophages as effector cells. The overexpression of transforming growth factor β, IL-1β, and IL-6 mRNA during infection of T. benhamiae in a mouse model also suggests a role of the Th17 pathway in the establishment of immunity with recruitment of polymorphonuclear neutrophils (58). In contrast, anthropophilic species (e.g., T. rubrum, T. interdigitale, and Trichophyton tonsurans) tend to be associated with less inflammatory, but more chronic and persistent infections. These infections are correlated with poor specific DTH, elevated specific IgE and IgG4, and IgE-mediated immediate hypersensitivity, with the production of Th2 cytokines by mononuclear leukocytes (37, 59). While a cell-mediated Th1 response to dermatophytes is effective in eradicating the infection, Th2-mediated immediate hypersensitivity responses are not protective.
MALASSEZIA
Biodiversity
Malassezia species are among the most prominent microorganisms growing on human skin, where they form a significant part of the skin microbiome (60) thus corroborating older culture-based studies (61 and references therein). Because Malassezia spp. are extremely fastidious in culture, culture-based studies are potentially biased for the fast-growing and more robust species including M. furfur, M. sympodialis, and allies and against slower and fastidious species such as M. restricta, M. globosa, or M. obtusa. As such, in this review, we will focus on more recent DNA-based studies and point out specific differences where relevant.
It has long been known that all Malassezia species are lipophilic, and it has recently been shown that all species, including M. pachydermatis, are actually fully lipid dependent and lack a fatty acid synthase (9). They require long-chain fatty acid (e.g., C12 or longer) supplementation for growth (62). Within the Fungi, the genus Malassezia occupies a rather isolated phylogenetic position. Recent multigene phylogenetic studies placed the genus in its own class, Malasseziomycetes in the phylum Basidiomycota, subphylum Ustilaginomycotina, that otherwise mainly comprises plant pathogens (63). Taxonomically, the genus has been significantly enlarged during the last decades and currently contains 15 species (Fig. 3) (64–66). For a long time, only two species were recognized, namely the lipid-dependent and human-associated M. furfur and the then considered non-lipid-dependent and animal-associated M. pachydermatis. Major improvements in our understanding of the diversity and phylogenetic relationships of the Malassezia species resulted from molecular phylogenetic studies, especially those initiated by Guého et al. (67). The initial studies used the D1/D2 domains of the large subunit ribosomal DNA (LSU rDNA) and were later followed by the addition of the ITS (61, 68 and references therein). Improved phylogenetic insights based on these rDNA domains presently identify 14 species (66, 69). A recent phylogenomic study resulted in a fully resolved, well-supported phylogenetic tree in which four subclades were apparent (Fig. 4; 9). The most basal lineage is formed by M. slooffiae and M. cuniculi (clade C), with two larger terminal sister clades, namely clade A with M. japonica, M. yamatoensis, M. obtusa, and M. furfur, and clade B with two subclades, namely B1 with M. pachydermatis, M. nana, M. caprae, M. dermatis, and M. sympodialis, and B2 with the most common human skin commensal organisms, M. restricta and M. globosa (9). This core-eukaryotic gene tree, in general, agrees with a previous four-gene tree (70) and an amplified fragment length polymorphism (AFLP)-based tree (71).
FIGURE 3.

Malassezia species tree was constructed using concatenated sequences of 164 core eukaryotic genes that are present in all Malassezia, U. maydis, and S. cerevisiae genomes. Sequences were aligned using MUSCLE and the phylogeny constructed using a maximum likelihood (ML) approach by RAxML. RAxML was run using “–f a –m PROTGAMMAJTT” with 400 bootstraps.
FIGURE 4.
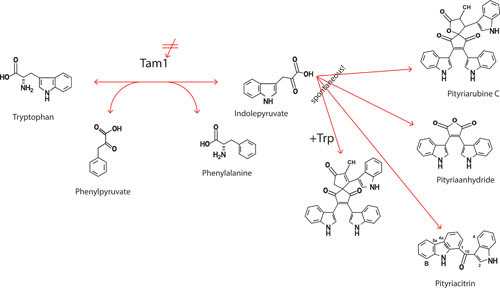
Synthesis of indole-derived pigments by one enzymatic step (TAM1) and possible intervention by TAM inhibitors.
Occurrence on Healthy Human Skin
Malassezia species have been considered commensal skin-inhabiting fungi that may be implicated in skin diseases (72). Their ubiquitous presence on humans has implied early maternal transmission, which is supported by a combination of culture and molecular studies (73). Recent advances in human mycobiome studies revealed that Malassezia species represent a commonly occurring group of fungi on and in the human body. When compared to bacteria, the abundance of fungi was generally lower, but M. globosa and M. restricta were more abundant near the ears and forehead (60). Malassezia species predominated on 11 body and arm sites of healthy individuals, namely the antecubital crease, back, external auditory canal, glabella, hypothenar palm, inguinal crease, manubrium, nare, occiput, retroauricular crease, and volar forearm. Eleven species were found to occur on various body sites (74), and a reanalysis of available metagenomics data from 12 individuals revealed that at least 12 Malassezia species can occur on skin. In terms of both frequency and abundance, in all individuals, M. restricta and M. globosa were the nondisputed numbers 1 and 2, with M. sympodialis ranking third (9).
Topological differences were observed. On the back, M. globosa was found to be most abundant on most individuals, with the exception of a single individual where M. sympodialis was dominant. The latter occurs in almost all cases as the second most abundant species on the back. In the antecubital or alar crease, M. restricta was most abundant in all subjects. Two of these species, and in many cases all three, were present in all individuals and also at most body sites sampled, but differences between individuals are observed. M. slooffiae occurred as a rather abundant species on one individual, whereas M. obtusa, M. pachydermatis, M. caprae, and M. equina were much less abundant. The occurrence of these latter species seems largely dependent on the individual. Interestingly, individuals showed the same spectrum of species present over a 3-month period (60). The factors that determine the presence of the rarer species are unknown. Many more individuals from different ethnic and geographic origins, together with large-scale assessment of their skin conditions and other associated metadata, probably have to be investigated to understand any causal relationships. Furthermore, data as yet are primarily in Caucasians of Western descent and location, and more data will be required to understand the breadth of Malassezia diversity in other ethnicities and from people residing in different climates and geographies.
Occurrence in Human Skin Disorders
Malassezia spp. were found to be the most abundantly occurring fungi on both healthy and diseased skin with M. restricta, M. globosa, M. sympodialis, and M. furfur occurring on both and M. slooffiae only on nonlesional skin (75). The number of sequence reads of M. restricta and M. globosa differed significantly between both types of skin, with M. restricta being more dominant at lesional skin and M. globosa on nonlesional skin. No differences were observed in colonization of both skin types for M. furfur, M. sympodialis, or M. slooffiae (75). A French study that compared dandruff versus nondandruff scalp reported that the presence of dandruff correlated with a disequilibrium between M. restricta and Staphylococcus epidermidis or Propionibacterium acnes (63, 76). With the use of ITS and D1/D2 of LSU rDNA, it was found that M. restricta was the major fungus on both types of scalp with 97% of sequence reads from healthy scalp and 84% from scalp with dandruff (76). The difference in healthy versus dandruff scalp may also be due to intrinsic host factors separate from fungal metabolism, termed “individual susceptibility” (77).
M. globosa is a diverse species, the species most highly correlated with human disease and containing multiple genotypes (78). Four IGS1 genotypes have been described, and it has been suggested that one of those may be related to atopic dermatitis (79). The species is known from healthy skin, but also from lesions of PV and seborrheic dermatitis (SD) patients and animal skin (64, 80). Skin mycobiome studies revealed that M. globosa occurs on the back, occiput, and inguinal crease. The species may co-occur with M. restricta in SD/dandruff and atopic dermatitis patients (81). The abundance of M. globosa was significantly higher on SD than on healthy skin (75).
The second most important species on healthy and diseased skin, particularly of the head, including scalp, neck, face, and ears, is M. restricta (61). This species is known from the skin of healthy subjects, SD, dandruff, and atopic dermatitis patients (6, 81). It occurs abundantly on both SD and healthy skin (75), being isolated from approximately 52% of SD patients compared to 64% in healthy humans (82).
M. sympodialis (83) is known from healthy human skin, in particular, the back and chest, but also from human PV, atopic dermatitis, skin, auditory tract, and various animals (64, 84). A recent Polish study using a culturing approach found that it was the most abundant Malassezia species on skin (85), but these results differ from many other studies that found M. restricta or M. globosa as the most common skin inhabitants. The discrepancy may have been caused by more ready isolation of M. sympodialis than of M. globosa and M. restricta.
Less commonly isolated species from human skin are M. caprae, previously known from healthy skin of goats and horses (86), and M. dermatis, known from lesions of atopic dermatitis and healthy skin (61). M. furfur has been reported from deep-seated infections, blood, urine, and vagina, and from septicemia in neonates that receive lipid supplementation via catheters (87, 88). Eight AFLP subtypes have been reported (68) that, however, did not fully coincide with subtypes based on sequences of the D1/D2 domains of the LSU rDNA. It has been suggested that isolates of AFLP genotype 4 may be more frequently involved in deep-seated infections, but the numbers that have been reported are low, hampering statistical analysis (71).
M. japonica is known from the skin of healthy Japanese women, but also from skin of atopic dermatitis patients (79). M. obtusa is known from the human groin, dermatitis skin, the nasal vestibule, but also from animals, such as goat, horse, and dog, and from canine otitis (89). M. yamatoensis is known from lesions of seborrheic and atopic dermatitis patients, but also from healthy human skin (64). It has also been suggested that several Malassezia species may be involved in skin carcinogenesis because of the production of aryl-hydrocarbon receptor ligands (90).
Occurrence Outside Human Skin
The mycobiome of the nasal vestibule of healthy humans and those suffering from allergic rhinitis is dominated by Malassezia species and comprises at least eight species with M. restricta as the most abundant and with M. globosa ranking as number 2. Further species observed were M. cuniculi, M. dermatis, M. obtusa, M. pachydermatis, M. slooffiae, and M. sympodialis (89). Malassezia species were found to be present with 100% colonization (91) and may also occur in the human gut, where five species were demonstrated using pyrosequencing of rDNA ITS (92). However, this approach is very sensitive and cannot discriminate between live resident flora and dead transient DNA. Using a PCR approach, M. restricta, M. globosa, and M. pachydermatis were found in stool of various individuals from different geographic locations, i.e., Amazonia, Polynesia, India, and Senegal (93). Another condition that has attracted interest is the prevalence of fungi in cystic fibrosis patients. Malassezia species, including M. globosa and M. restricta, were present in all sputum samples investigated, but with 10- to 50-fold lower levels than the more abundantly present Candida species (94). Finally, Malassezia species have been demonstrated to occur in the oral cavity where they have been suggested to be part of the basal oral mycobiome (95, 96).
A study using pyrosequencing of part of the small subunit ribosomal DNA (SSU rDNA) of sputum from asthma and nonatopic humans suggested that M. pachydermatis occurred commonly in asthmatic patients but was absent from healthy humans. A similar trend was seen for M. furfur, but the number of reads was much lower than that for M. pachydermatis (97). M. pachydermatis was also found in the nasal vestibules of healthy humans as well as those with allergic rhinitis (89).
Importantly, M. pachydermatis may cause catheter-related septicemia in neonates who receive lipid supplementation (98), and it also may cause sepsis in immune-suppressed adults. Domestic pet animals, especially dogs, have been identified as a source for transmission to neonates (99).
Occurrence Outside Humans
Many animals, such as cats, dogs, but also birds, horses, goats, cows, and pigs, may be infected by Malassezia species (65). Malassezia sequences have also been demonstrated from diverse terrestrial and marine ecosystems (100). Diverse M. pachydermatis isolates populate animal skin, including seven D1/D2 LSU rDNA genotypes that are known to exhibit some host specificity (101). Sequevar a originated from humans, sequevar b occurred in various animals, sequevar c came from rhinoceros, and cats and dogs were colonized by two and three genotypes, respectively.
M. equina was reported from healthy skin of a horse (anus) and cow in Spain (86), and M. nana from the otitis externa of cats, but also from cows in Brazil with or without otitis externa (102). M. pachydermatis is especially known from the external ear canal of many warm-blooded animals, especially dogs and cats, but also from bears, fox, rhinoceros, California sea lion, and many more (103 and references therein).
M. globosa is known from animal skin as well as from ears of healthy and diseased bovines and, for the latter, a link with nematodes has been suggested (104). DNA of the species was detected in Malenchus nematodes in a European forest soil (105), and also in Antarctic soils where a connection with nematodes has been further postulated (106).
Biochemistry
Of the 15 currently recognized Malassezia species, M. globosa is thought to play a key role in the pathogenesis of PV (80). However, PV characteristics such as pigmentation alterations, fluorescence of PV lesions, or minimal signs of inflammation despite high fungal load (107) cannot be explained just by occurrence of M. globosa, which is also the predominant species on healthy skin. Instead, it was shown that, with tryptophan as a nitrogen source, M. furfur produces a number of indole pigments and alkaloids, which might be related to the clinical appearance of PV (Table 1) (108). Despite its complex biochemistry, the pathway does not represent secondary metabolism, as is the case with most of the mycotoxins. In the related and completely sequenced organism Ustilago maydis, it was shown that tryptophan and keto acids are converted to indole-3-pyruvic acid (IPyA) and the corresponding amino acids involving a tryptophan aminotransferase (Tam1; UM01804, XM_752858) as the initial and only enzymatic step. The indole pigments and alkaloids are then formed spontaneously from IPyA (109; Fig. 1). Tam1 of M. furfur (MfTam1) was shown to be differentially expressed under pigment-producing and nonproducing conditions (110, 111). The mftam1 gene (1,431 bp) was cloned by a reversed PCR strategy (112). MfTam1 (molecular weight [MW] 52,500; JX 453496, EC 2.6.1.27) is specific for transamination of l-tryptophan with Km values for the substrate and α-ketoglutarate in the low millimolar range (∼7 mM). Its activity is highly dependent on pyridoxal phosphate (PLP) and compounds interfering with PLP, such as cycloserine (CS) and aminooxyacetate (AOA), inhibit the MfTam1 reaction. Mayser and Rieche (113) showed that a 200 mM CS solution topically applied for 5 days on PV lesions reversed the hyperpigmentation, and found with AOA tests the same effect as for CS on PV. However, AOA was more potent than CS (P. Mayser, unpublished data). Interestingly, comparison with the genome of M. globosa showed that the gene is also present in this species (MGL_2601, XM_001730167) (111). However, MgTam (MW ∼35,500) shows a weaker activity in vitro than MfTam (Mayser, unpublished data). For CS and AOA inhibition of MgTam1 is expected, because the sequence is similar to the one of M. furfur and the inhibitors target PLP-dependent enzymes.
TABLE 1.
Selected Trp-derived indole compounds and their potential relationship to clinical phenomena in PV
| Isolated compound | Pharmacological property | Phenomenon in PV |
|---|---|---|
| Pityriacitrin | UV protection | Lack of sunburn in depigmented areas of PV alba |
| Fluorescent metabolites (e.g., pityrialactone) | Fluorescence | Fluorescence of lesions in PV |
| Pityriarubins | Inhibition of oxidative burst in granulocytes | Lack of inflammation in lesions of PV |
| Malassezin | Induction of apoptosis in human melanocytes | Depigmentation in PV alba |
| AHRa-agonists (e.g., malassezin, pityriazepin, indolo[3,2-b]carbazole) | Activation of cytochrome, activation of AHR | Immunomodulation, effects on cell differentiation and homeostasis, depigmentation? desquamation? |
| Diversity of pigmented compounds | Pigmentation | Brick-red to brownish pigmentation of lesions in PV |
AHR, aryl hydrocarbon receptor.
PV is very common in tropical climates and is associated with sweating and hyperhidrosis (114). The tryptophan concentration in sweat, however, is very low (∼70 μM). It was shown that glycine metabolism of M. furfur results in Malassezia growth (115). However, metabolism of tryptophan is responsible for pigment production. When glycine and tryptophan are present, the fungi first grow and then, after a growth plateau is reached, start producing pigments. These data indicate that M. furfur preferentially metabolizes glycine. When the glycine concentration decreases, the metabolism switches to deamination of tryptophan with pigment formation as a by-product. In light of the fact that glycine is highly abundant in sweat (∼2 to 4 mM) (116), it was hypothesized that severe sweating changes the local amino acid concentrations, resulting in an enrichment of the poorly water-soluble tryptophan on the skin. Thus, M. furfur (and probably M. globosa) might convert Trp to IP in a situation that lacks other nitrogen sources. The subsequently spontaneously generated indole pigments could be responsible for the clinical course of this common skin disease. TAM 1 inhibitors might represent a new approach in the therapy of this widespread disease.
Malassezia biology is also implicated in the common skin disorders dandruff and SD (9). These disorders are thought to be downstream of Malassezia lipid metabolism. In brief, Malassezia lacks the enzymes necessary to generate required fatty acids (no fatty acid synthase) or to assimilate unsaturated fatty acids (lacking a Δ9-desaturase). These deficiencies are compensated by the secretion of a number of hydrolytic enzymes that break down human sebaceous lipids. The Malassezia cells then assimilate the saturated fatty acids for survival (117), and leave behind irritating fatty acids that have been shown to induce dandruff-like desquamation in susceptible individuals (77).
Comparative Genomics
Twenty-four Malassezia isolates have been fully sequenced (Table 2), including all 14 currently accepted species and multiple isolates of the most common inhabitants of human skin (M. globosa, M. restricta, and M. sympodialis) and the most common human pathogenic species, M. furfur (9). The Malassezia genomes were compared with widely divergent fungal genomes, which identified features defining Malassezia. A large set of common fungal genes has been lost, corresponding to the compactness of the genome and adaptation to the skin environment. Malassezia genomics shows the genus has a propensity for gene turnover and highlights the importance of gathering nutrients from a sparse environment via secretion of large families of lipases, phospholipases, aspartyl proteases, and other peptidases. Recently, multiple genomes of M. sympodialis were generated via long read sequencing, and the genomes annotated via proteogenomics. This has significantly increased the reliability of the M. sympodialis genome annotation (118).
TABLE 2.
Malassezia genome statisticsa
| Cluster | Strain | Genome (Mbp) | N50 (kbp) | Chromosome no. (PFGE) | Mitochondrial genome |
|---|---|---|---|---|---|
| Human skin | M. globosa 7966 | 8.9 | 724 | 9b | 34689 |
| M. globosa 7990 | 8.9 | 415 | 9 | 38672 | |
| M. globosa 7874 | 8.9 | 398 | 9 | 34808 | |
| M. restricta 7877 | 7.2 | 403 | 10 | 38499 | |
| M. restricta 8742 | 7.3 | 667 | 10 | 40172 | |
| M. sympodialis 42132 | 7.5 | 54 | 8a | 38623 | |
| M. sympodialis 44340 | 7.5 | 60 | 8 | 38776 | |
| M. sympodialis 96806 | 7.4 | 45 | 8 | 38538 | |
| M. dermatis 9169 | 7.5 | 189 | NDc | NDc | |
| Animal skin | M. caprae 10434 | 7.6 | 110 | NDc | NDc |
| M. equina 9969 | 7.7 | 372 | NDc | NDc | |
| M. nana 9557 | 7.6 | 492 | NDc | NDc | |
| M. pachydermatis 1879 | 8.2 | 957 | 5d | 28337 | |
| slooffiae-like | M. slooffiae 7956 | 8.3 | 16 | 9b | 40575 |
| M. cuniculi 11721 | 7.5 | 522 | NDc | NDc | |
| furfur-like | M. japonica 9431 | 8.3 | 66 | NDc | 28009 |
| M. yamatoensis 9725 | 8.1 | 1,448 | NDc | 41233 | |
| M. obtusa 7876 | 7.7 | 23 | NDc | 60147 | |
| M. furfur 1878 | 13.5 | 15 | 10-11 | 48161 | |
| M. furfur 4172 | 14 | 16 | 10-11 | 48279 | |
| M. furfur 7019 | 13.4 | 16 | 8 | 49305 | |
| M. furfur 7710 | 14.8 | 15 | 7 | 47901 | |
| M. furfur JPLK13 | 8.5 | 1,649,308 | 7 | NDc | |
| M. furfur JPLK23 | 7.6 | 15 | 7b | 48933 | |
| M. furfur 7982 | 7.7 | 21 | 7 | 47903 |
Genomic data genebank accession: BioProject ID: PRJNA286710.
Confirmed via PacBio sequencing.
ND, neither pulsed-field gel electrophoresis (PFGE) nor PacBio sequence, only Illumina sequence available, mitochondrial genome not assembled.
PFGE of pachydermatis 7925.
Several important facets of Malassezia warrant consideration. Malassezia produces a set of secreted hydrolases similar to C. albicans, a human skin pathogen, but is phylogenetically more closely related to the plant pathogen U. maydis. Ustilago produces a different set of secreted hydrolases, adapted for life on plants. The set of secreted enzymes utilized by each genus is likely adaptation to the host niche and may be involved in pathogenicity (9, 72, 119). Malassezia species are all missing multiple enzymes required for robust lipid metabolism, including fatty acid synthase, Δ9-desaturase, and Δ2,3-enoyl-CoA isomerase. These gene losses likely explain their lipid dependence. Genuswide variation in these families also likely supports each species’ niche specificity. Malassezia species have multiple gene duplications into several families. These genes have undergone lineage-specific duplications since divergence from Ustilago (9, 119). Malassezia species all encode proteins similar to the M. sympodialis allergens, supporting a role in skin and inhalational allergy. Malassezia genomes all have genes associated with mating, although mating has not been observed. They are likely among the first recorded fungi with a pseudobipolar mating phenotype (83, 120, 121).
Malassezia species have been shown to have gained and lost many specific gene families during their complex evolution. Of particular note is the likely gain of a single gene, conserved in all Malassezia but absent in any other sequenced basidiomycetes, suggesting a gene transfer at the very root of the Malassezia. The gene is likely functional, as it was found in M. globosa to be both transcribed and its transcription regulated. A second important potentially horizontally transferred gene family was located at the root of clades B1/B2 and was shown to be related to a catalase, which could adaptively protect Malassezia from a group of their own secreted proteins that generate hydrogen peroxide (9). Another example of Malassezia receiving genes via horizontal gene transfer is acquisition of a flavohemoglobin A from the Corynebacterium of Actinobacteria, potentially adding nitric oxide resistance into Malassezia (118).
Malassezia genomics also has revealed that they are likely to mate (9, 119). Fungal mating may be involved in pathogenesis, by increasing genetic diversity (122, 123). The mating loci suggest bipolar or pseudobipolar mating may be present in all Malassezia species. Studies using sequencing technology to build larger contiguous regions will be needed to define the mating systems in Malassezia, and further gene deletion or model systems to study any role in pathogenesis. Now that transformation and genetic engineering of Malassezia have been shown to be possible (124, 125), it is likely that the roles of these and other genes and pathways will be clarified.
Host Defense Mechanisms
As residents of the skin of all animals, and by definition then all human beings, Malassezia represents a unique opportunity to study the interaction of commensal microorganisms with the human immune system. As such, Malassezia has been shown to directly modulate cytokine expression by isolated human peripheral blood mononuclear cells (126). This report indicates that the Malassezia species most commonly found on human skin (M. globosa, M. restricta) downregulate expression of IL-6 or IL-1β, while the less common M. sympodialis does not. It was also indicated that the ability to modulate immune cells may be related to lipid metabolism, because extraction of Malassezia lipids via chloroform:methanol abrogated this effect. Malassezia lipids are further implicated in their relationship with the immune system in that the overall hydrophobicity of isolates correlates with their ability to stimulate secretion of proinflammatory cytokines from isolated human keratinocytes. An additional report further implicates a direct interaction with human monocyte-derived dendritic cells (127), where human NK cells activated monocyte-derived dendritic cells and induced an even stronger activation when Malassezia was present. Upregulation of CD83 and CD86 indicates that NK cells mature DCs and improve their costimulatory capacity.
M. sympodialis is able to directly elicit a human immune response, shown by the presence of human antibodies that cross-react with a group of Malassezia-secreted proteins termed “allergens” (128). In this series of reports, it was shown that individuals with specific forms of atopic dermatitis had serum IgG that could react with protein lysates from M. sympodialis and that subjects with head and neck atopic dermatitis had higher levels of anti-Malassezia IgG (129, 130). The allergens were identified, cloned, and sequenced, and later shown to be present and likely secreted in all Malassezia species (9). The Malassezia allergens are contained and secreted in nanovesicles, and the secretion and activity are modulated by skin pH (72).
REFERENCES
- 1.Casadevall A, Pirofski LA. 2015. What is a host? Incorporating the microbiota into the damage-response framework. Infect Immun 83:2–7 10.1128/IAI.02627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agosta SJ, Janz N, Brooks DR. 2010. How specialists can be generalists: resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia Curitiba 27:151–162 10.1590/S1984-46702010000200001. [DOI] [Google Scholar]
- 3.Bonifaz A, Badali H, de Hoog GS, Cruz M, Araiza J, Cruz MA, Fierro L, Ponce RM. 2008. Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Stud Mycol 61:77–82 10.3114/sim.2008.61.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunde-Cimermana N, Zalarb P, de Hoog S, Plemenitaš A. 2000. Hypersaline waters in salterns - natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240. [DOI] [PubMed] [Google Scholar]
- 5.Kejžar A, Cibic M, Grøtli M, Plemenitaš A, Lenassi M. 2015. The unique characteristics of HOG pathway MAPKs in the extremely halotolerant Hortaea werneckii. FEMS Microbiol Lett 362:fnv046 10.1093/femsle/fnv046. [DOI] [PubMed] [Google Scholar]
- 6.Batra R, Boekhout T, Guého E, Cabañes FJ, Dawson TL Jr, Gupta AK. 2005. Malassezia Baillon, emerging clinical yeasts. FEMS Yeast Res 5:1101–1113 10.1016/j.femsyr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. 2004. Skin diseases associated with Malassezia species. J Am Acad Dermatol 51:785–798 10.1016/j.jaad.2003.12.034. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. 2012. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev 25:106–141 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, Zhao H, Li C, Rajapakse MP, Wong WC, Xu J, Saunders CW, Reeder NL, Reilman RA, Scheynius A, Sun S, Billmyre BR, Li W, Averette AF, Mieczkowski P, Heitman J, Theelen B, Schröder MS, De Sessions PF, Butler G, Maurer-Stroh S, Boekhout T, Nagarajan N, Dawson TL Jr. 2015. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet 11:e1005614 10.1371/journal.pgen.1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gräser Y, de Hoog GS, Kuijpers AFA. 2000. Recent advances in the molecular taxonomy of dermatophytes. In Kushwaha RKS, Guarro J (ed), Biology of Dermatophytes and other Keratinophilic Fungi. Rev Iberoam Micol 17:17–21. [Google Scholar]
- 11.Rezaei-Matehkolaei A, Mirhendi H, Makimura K, de Hoog GS, Satoh K, Najafzadeh MJ, Shidfar MR. 2014. Nucleotide sequence analysis of beta tubulin gene in a wide range of dermatophytes. Med Mycol 52:674–688 10.1093/mmy/myu033. [DOI] [PubMed] [Google Scholar]
- 12.Mirhendi H, Makimura K, de Hoog GS, Rezaei-Matehkolaei A, Najafzadeh MJ, Umeda Y, Ahmadi B. 2015. Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol 53:215–224 10.1093/mmy/myu088. [DOI] [PubMed] [Google Scholar]
- 13.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y. 2017. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182:5–31 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriranganadane D, Waridel P, Salamin K, Feuermann M, Mignon B, Staib P, Neuhaus JM, Quadroni M, Monod M. 2011. Identification of novel secreted proteases during extracellular proteolysis by dermatophytes at acidic pH. Proteomics 11:4422–4433 10.1002/pmic.201100234. [DOI] [PubMed] [Google Scholar]
- 15.Burmester A, Shelest E, Glöckner G, Heddergott C, Schindler S, Staib P, Heidel A, Felder M, Petzold A, Szafranski K, Feuermann M, Pedruzzi I, Priebe S, Groth M, Winkler R, Li W, Kniemeyer O, Schroeckh V, Hertweck C, Hube B, White TC, Platzer M, Guthke R, Heitman J, Wöstemeyer J, Zipfel PF, Monod M, Brakhage AA. 2011. Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol 12:R7 10.1186/gb-2011-12-1-r7. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jousson O, Léchenne B, Bontems O, Capoccia S, Mignon B, Barblan J, Quadroni M, Monod M. 2004. Multiplication of an ancestral gene encoding secreted fungalysin preceded species differentiation in the dermatophytes Trichophyton and Microsporum. Microbiology 150:301–310 10.1099/mic.0.26690-0. [DOI] [PubMed] [Google Scholar]
- 17.Jousson O, Léchenne B, Bontems O, Mignon B, Reichard U, Barblan J, Quadroni M, Monod M. 2004. Secreted subtilisin gene family in Trichophyton rubrum. Gene 339:79–88 10.1016/j.gene.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Giddey K, Favre B, Quadroni M, Monod M. 2007. Closely related dermatophyte species produce different patterns of secreted proteins. FEMS Microbiol Lett 267:95–101 10.1111/j.1574-6968.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaugg C, Monod M, Weber J, Harshman K, Pradervand S, Thomas J, Bueno M, Giddey K, Staib P. 2009. Gene expression profiling in the human pathogenic dermatophyte Trichophyton rubrum during growth on proteins. Eukaryot Cell 8:241–250 10.1128/EC.00208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monod M, Léchenne B, Jousson O, Grand D, Zaugg C, Stöcklin R, Grouzmann E. 2005. Aminopeptidases and dipeptidyl-peptidases secreted by the dermatophyte Trichophyton rubrum. Microbiology 151:145–155 10.1099/mic.0.27484-0. [DOI] [PubMed] [Google Scholar]
- 21.Zaugg C, Jousson O, Léchenne B, Staib P, Monod M. 2008. Trichophyton rubrum secreted and membrane-associated carboxypeptidases. Int J Med Microbiol 298:669–682 10.1016/j.ijmm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Byun T, Kofod L, Blinkovsky A. 2001. Synergistic action of an X-prolyl dipeptidyl aminopeptidase and a non-specific aminopeptidase in protein hydrolysis. J Agric Food Chem 49:2061–2063 10.1021/jf001091m. [DOI] [PubMed] [Google Scholar]
- 23.Sriranganadane D, Waridel P, Salamin K, Reichard U, Grouzmann E, Neuhaus JM, Quadroni M, Monod M. 2010. Aspergillus protein degradation pathways with different secreted protease sets at neutral and acidic pH. J Proteome Res 9:3511–3519 10.1021/pr901202z. [DOI] [PubMed] [Google Scholar]
- 24.Staib P, Zaugg C, Mignon B, Weber J, Grumbt M, Pradervand S, Harshman K, Monod M. 2010. Differential gene expression in the pathogenic dermatophyte Arthroderma benhamiae in vitro versus during infection. Microbiology 156:884–895 10.1099/mic.0.033464-0. [DOI] [PubMed] [Google Scholar]
- 25.Méhul B, Gu Z, Jomard A, Laffet G, Feuilhade M, Monod M. 2016. Sub6 (Tri r 2), an onychomycosis marker revealed by proteomics analysis of Trichophyton rubrum secreted proteins in patient nail samples. J Invest Dermatol 136:331–333 10.1038/JID.2015.367. [DOI] [PubMed] [Google Scholar]
- 26.Woodfolk JA, Wheatley LM, Piyasena RV, Benjamin DC, Platts-Mills TA. 1998. Trichophyton antigens associated with IgE antibodies and delayed type hypersensitivity. Sequence homology to two families of serine proteinases. J Biol Chem 273:29489–29496 10.1074/jbc.273.45.29489. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 28.McKinney JD, Höner zu Bentrup K, Muñoz-Elías EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735–738 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 29.Grumbt M, Defaweux V, Mignon B, Monod M, Burmester A, Wöstemeyer J, Staib P. 2011. Targeted gene deletion and in vivo analysis of putative virulence gene function in the pathogenic dermatophyte Arthroderma benhamiae. Eukaryot Cell 10:842–853 10.1128/EC.00273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veien NK, Hattel T, Laurberg G. 1994. Plantar Trichophyton rubrum infections may cause dermatophytids on the hands. Acta Derm Venereol 74:403–404. [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.Gianni C, Betti R, Crosti C. 1996. Psoriasiform id reaction in tinea corporis. Mycoses 39:307–308 10.1111/j.1439-0507.1996.tb00144.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Liu ZH, Shen H, Xu AE. 2011. Severe kerion with dermatophytid reaction presenting with diffuse erythema and pustules. Mycoses 54:e650–e652 10.1111/j.1439-0507.2010.01973.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheng N, Rucker Wright D, Cohen BA. 2011. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics 128:e453–e457 10.1542/peds.2010-2757. [DOI] [PubMed] [Google Scholar]
- 34.Ilkit M, Durdu M, Karakaş M. 2012. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol 38:191–202 10.3109/1040841X.2011.645520. [DOI] [PubMed] [Google Scholar]
- 35.Slunt JB, Taketomi EA, Woodfolk JA, Hayden ML, Platts-Mills TA. 1996. The immune response to Trichophyton tonsurans: distinct T cell cytokine profiles to a single protein among subjects with immediate and delayed hypersensitivity. J Immunol 157:5192–5197. [PubMed] [PubMed] [Google Scholar]
- 36.Woodfolk JA, Platts-Mills TA. 2001. Diversity of the human allergen-specific T cell repertoire associated with distinct skin test reactions: delayed-type hypersensitivity-associated major epitopes induce Th1- and Th2-dominated responses. J Immunol 167:5412–5419 10.4049/jimmunol.167.9.5412. [DOI] [PubMed] [Google Scholar]
- 37.Woodfolk JA. 2005. Allergy and dermatophytes. Clin Microbiol Rev 18:30–43 10.1128/CMR.18.1.30-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez DA, Oliver BG, Gräser Y, Goldberg JM, Li W, Martinez-Rossi NM, Monod M, Shelest E, Barton RC, Birch E, Brakhage AA, Chen Z, Gurr SJ, Heiman D, Heitman J, Kosti I, Rossi A, Saif S, Samalova M, Saunders CW, Shea T, Summerbell RC, Xu J, Young S, Zeng Q, Birren BW, Cuomo CA, White TC. 2012. Comparative genome analysis of Trichophyton rubrum and related dermatophytes reveals candidate genes involved in infection. MBio 3:e00259-12 10.1128/mBio.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Metin B, White TC, Heitman J. 2010. Organization and evolutionary trajectory of the mating type (MAT) locus in dermatophyte and dimorphic fungal pathogens. Eukaryot Cell 9:46–58 10.1128/EC.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Symoens F, Jousson O, Packeu A, Fratti M, Staib P, Mignon B, Monod M. 2013. The dermatophyte species Arthroderma benhamiae: intraspecies variability and mating behaviour. J Med Microbiol 62:377–385 10.1099/jmm.0.053223-0. [DOI] [PubMed] [Google Scholar]
- 41.Anzawa K, Kawasaki M, Mochizuki T, Ishizaki H. 2010. Successful mating of Trichophyton rubrum with Arthroderma simii. Med Mycol 48:629–634 10.3109/13693780903437884. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki M. 2011. Verification of a taxonomy of dermatophytes based on mating results and phylogenetic analyses. Med Mycol J 52:291–295 10.3314/mmj.52.291. [DOI] [PubMed] [Google Scholar]
- 43.Kano R, Yoshida E, Yaguchi T, Hubka V, Anzawa K, Mochizuki T, Hasegawa A, Kamata H. 2014. Mating type gene (MAT1-2) of Trichophyton verrucosum. Mycopathologia 177:87–90 10.1007/s11046-013-9722-4. [DOI] [PubMed] [Google Scholar]
- 44.Gräser Y, De Hoog S, Summerbell RC. 2006. Dermatophytes: recognizing species of clonal fungi. Med Mycol 44:199–209 10.1080/13693780600606810. [DOI] [PubMed] [Google Scholar]
- 45.Shiraki Y, Ishibashi Y, Hiruma M, Nishikawa A, Ikeda S. 2006. Cytokine secretion profiles of human keratinocytes during Trichophyton tonsurans and Arthroderma benhamiae infections. J Med Microbiol 55:1175–1185 10.1099/jmm.0.46632-0. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Kano R, Hasegawa A, Watanabe S. 2002. Interleukin-8 and tumor necrosis factor alpha production in human epidermal keratinocytes induced by Trichophyton mentagrophytes. Clin Diagn Lab Immunol 9:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tani K, Adachi M, Nakamura Y, Kano R, Makimura K, Hasegawa A, Kanda N, Watanabe S. 2007. The effect of dermatophytes on cytokine production by human keratinocytes. Arch Dermatol Res 299:381–387 10.1007/s00403-007-0780-7. [DOI] [PubMed] [Google Scholar]
- 48.Jensen JM, Pfeiffer S, Akaki T, Schröder JM, Kleine M, Neumann C, Proksch E, Brasch J. 2007. Barrier function, epidermal differentiation, and human beta-defensin 2 expression in tinea corporis. J Invest Dermatol 127:1720–1727 10.1038/sj.jid.5700788. [DOI] [PubMed] [Google Scholar]
- 49.López-García B, Lee PH, Gallo RL. 2006. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J Antimicrob Chemother 57:877–882 10.1093/jac/dkl078. [DOI] [PubMed] [Google Scholar]
- 50.Fritz P, Beck-Jendroschek V, Brasch J. 2012. Inhibition of dermatophytes by the antimicrobial peptides human β-defensin-2, ribonuclease 7 and psoriasin. Med Mycol 50:579–584 10.3109/13693786.2012.660203. [DOI] [PubMed] [Google Scholar]
- 51.Hein KZ, Takahashi H, Tsumori T, Yasui Y, Nanjoh Y, Toga T, Wu Z, Grötzinger J, Jung S, Wehkamp J, Schroeder BO, Schroeder JM, Morita E. 2015. Disulphide-reduced psoriasin is a human apoptosis-inducing broad-spectrum fungicide. Proc Natl Acad Sci USA 112:13039–13044 10.1073/pnas.1511197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nenoff P, Uhrlaß S, Krüger C, Erhard M, Hipler U-C, Seyfarth F, Herrmann J, Wetzig T, Schroedl W, Gräser Y. 2014. Trichophyton species of Arthroderma benhamiae - a new infectious agent in dermatology. J Dtsch Dermatol Ges 12:571–581. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.da Silva BC, Paula CR, Auler ME, Ruiz LS, Dos Santos JI, Yoshioka MC, Fabris A, Castro LG, Duarte AJ, Gambale W. 2014. Dermatophytosis and immunovirological status of HIV-infected and AIDS patients from Sao Paulo city, Brazil. Mycoses 57:371–376. [DOI] [PubMed] [Google Scholar]
- 54.Wu LC, Sun PL, Chang YT. 2013. Extensive deep dermatophytosis cause by Trichophyton rubrum in a patient with liver cirrhosis and chronic renal failure. Mycopathologia 176:457–462 10.1007/s11046-013-9696-2. [DOI] [PubMed] [Google Scholar]
- 55.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Boudghene Stambouli O, Guellil B, Jacobs F, Goffard JC, Schepers K, del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux ME, Boudia M, Abel L, Lortholary O, Casanova JL, Picard C, Grimbacher B, Puel A. 2013. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 369:1704–1714 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grumach AS, de Queiroz-Telles F, Migaud M, Lanternier F, Filho NR, Palma SM, Constantino-Silva RN, Casanova JL, Puel A. 2015. A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J Clin Immunol 35:486–490 10.1007/s10875-015-0170-4. [DOI] [PubMed] [Google Scholar]
- 57.Jachiet M, Lanternier F, Rybojad M, Bagot M, Ibrahim L, Casanova JL, Puel A, Bouaziz JD. 2015. Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Dermatol 151:192–194 10.1001/jamadermatol.2014.2154. [DOI] [PubMed] [Google Scholar]
- 58.Cambier L, Weatherspoon A, Defaweux V, Bagut ET, Heinen MP, Antoine N, Mignon B. 2014. Assessment of the cutaneous immune response during Arthroderma benhamiae and A. vanbreuseghemii infection using an experimental mouse model. Br J Dermatol 170:625–633 10.1111/bjd.12673. [DOI] [PubMed] [Google Scholar]
- 59.Mignon B, Tabart J, Baldo A, Mathy A, Losson B, Vermout S. 2008. Immunization and dermatophytes. Curr Opin Infect Dis 21:134–140 10.1097/QCO.0b013e3282f55de6. [DOI] [PubMed] [Google Scholar]
- 60.Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program, Kong HH, Segre JA. 2014. Biogeography and individuality shape function in the human skin metagenome. Nature 514:59–64 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boekhout T, Mayser P, Guého-Kellermann E, Velegraki A (ed). 2010. Malassezia and the Skin. Springer, Berlin. 10.1007/978-3-642-03616-3 [DOI] [Google Scholar]
- 62.Porro MN, Passi S, Caprill F, Nazzaro P, Morpurgo G. 1976. Growth requirements and lipid metabolism of Pityrosporum orbiculare. J Invest Dermatol 66:178–182 10.1111/1523-1747.ep12481919. [DOI] [PubMed] [Google Scholar]
- 63.Wang QM, Theelen B, Groenewald M, Bai FY, Boekhout T. 2014. Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia 33:41–47 10.3767/003158514X682313. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guého-Kellermann E, Boekhout T, Begerow D. 2010. Biodiversity, phylogeny and ultrastructure, p 17–63. In Boekhout T, Mayser P, Guého-Kellermann E, Velegraki A (ed), Malassezia and the Skin. Springer Verlag, Berlin. [Google Scholar]
- 65.Cabañes FJ. 2014. Malassezia yeasts: how many species infect humans and animals? PLoS Pathog 10:e1003892 10.1371/journal.ppat.1003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cabañes FJ, Vega S, Castellá G. 2011. Malassezia cuniculi sp. nov., a novel yeast species isolated from rabbit skin. Med Mycol 49:40–48 10.3109/13693786.2010.493562. [DOI] [PubMed] [Google Scholar]
- 67.Guého E, Midgley G, Guillot J. 1996. The genus Malassezia with description of four new species. Antonie van Leeuwenhoek 69:337–355 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 68.Gupta AK, Boekhout T, Theelen B, Summerbell R, Batra R. 2004. Identification and typing of Malassezia species by amplified fragment length polymorphism and sequence analyses of the internal transcribed spacer and large-subunit regions of ribosomal DNA. J Clin Microbiol 42:4253–4260 10.1128/JCM.42.9.4253-4260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guého-Kellermann E, Batra R, Boekhout T. 2011. Malassezia Baillon (1889), p 1807–1836. In Kurtzman C, Fell JW, Boekhout T (ed), The Yeasts, a Taxonomic Study, 5th ed. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 70.Castellá G, Coutinho SDA, Cabañes FJ. 2014. Phylogenetic relationships of Malassezia species based on multilocus sequence analysis. Med Mycol 52:99–105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Theelen B, Silvestri M, Guého E, van Belkum A, Boekhout T. 2001. Identification and typing of Malassezia yeasts using amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD) and denaturing gradient gel electrophoresis (DGGE). FEMS Yeast Res 1:79–86 10.1111/j.1567-1364.2001.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 72.Saunders CW, Scheynius A, Heitman J. 2012. Malassezia fungi are specialized to live on skin and associated with dandruff, eczema, and other skin diseases. PLoS Pathog 8:e1002701 10.1371/journal.ppat.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagata R, Nagano H, Ogishima D, Nakamura Y, Hiruma M, Sugita T. 2012. Transmission of the major skin microbiota, Malassezia, from mother to neonate. Pediatr Int 54:350–355 10.1111/j.1442-200X.2012.03563.x. [DOI] [PubMed] [Google Scholar]
- 74.Findley K, Grice EA. 2014. The skin microbiome: a focus on pathogens and their association with skin disease. PLoS Pathog 10:e1004436 10.1371/journal.ppat.1004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka A, Cho O, Saito M, Tsuboi R, Kurakado S, Sugita T. 2014. Molecular characterization of the skin fungal microbiota in patients with seborrheic dermatitis. J Clin Exp Dermatol Res 5:239 10.4172/2155-9554.1000239. [DOI] [Google Scholar]
- 76.Clavaud C, Jourdain R, Bar-Hen A, Tichit M, Bouchier C, Pouradier F, El Rawadi C, Guillot J, Ménard-Szczebara F, Breton L, Latgé JP, Mouyna I. 2013. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One 8:e58203 10.1371/journal.pone.0058203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeAngelis YM, Gemmer CM, Kaczvinsky JR, Kenneally DC, Schwartz JR, Dawson TL Jr. 2005. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J Investig Dermatol Symp Proc 10:295–297 10.1111/j.1087-0024.2005.10119.x. [DOI] [PubMed] [Google Scholar]
- 78.Gemmer CM, DeAngelis YM, Theelen B, Boekhout T, Dawson TL Jr. 2002. Fast, noninvasive method for molecular detection and differentiation of Malassezia yeast species on human skin and application of the method to dandruff microbiology. J Clin Microbiol 40:3350–3357 10.1128/JCM.40.9.3350-3357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. 2003. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol 41:4695–4699 10.1128/JCM.41.10.4695-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crespo Erchiga V, Ojeda Martos A, Vera Casaño A, Crespo Erchiga A, Sanchez Fajardo F. 2000. Malassezia globosa as the causative agent of pityriasis versicolor. Br J Dermatol 143:799–803 10.1046/j.1365-2133.2000.03779.x. [DOI] [PubMed] [Google Scholar]
- 81.Sugita T, Tajima M, Tsubuku H, Tsuboi R, Nishikawa A. 2006. Quantitative analysis of cutaneous malassezia in atopic dermatitis patients using real-time PCR. Microbiol Immunol 50:549–552 10.1111/j.1348-0421.2006.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 82.Oh BH, Song YC, Lee YW, Choe YB, Ahn KJ. 2009. Comparison of nested PCR and RFLP for identification and classification of Malassezia yeasts from healthy human skin. Ann Dermatol 21:352–357 10.5021/ad.2009.21.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gioti A, Nystedt B, Li W, Xu J, Andersson A, Averette AF, Münch K, Wang X, Kappauf C, Kingsbury JM, Kraak B, Walker LA, Johansson HJ, Holm T, Lehtiö J, Stajich JE, Mieczkowski P, Kahmann R, Kennell JC, Cardenas ME, Lundeberg J, Saunders CW, Boekhout T, Dawson TL, Munro CA, de Groot PWJ, Butler G, Heitman J, Scheynius A. 2013. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. MBio 4:e00572-12 10.1128/mBio.00572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simmons RB. 1990. A new species of Malassezia. Mycol Res 94:1146–1149 10.1016/S0953-7562(09)81349-X. [DOI] [Google Scholar]
- 85.Jagielski T, Rup E, Ziółkowska A, Roeske K, Macura AB, Bielecki J. 2014. Distribution of Malassezia species on the skin of patients with atopic dermatitis, psoriasis, and healthy volunteers assessed by conventional and molecular identification methods. BMC Dermatol 14:3 10.1186/1471-5945-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabañes FJ, Theelen B, Castellá G, Boekhout T. 2007. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res 7:1064–1076 10.1111/j.1567-1364.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 87.Boekhout T, Bosboom RW. 1994. Karyotyping of Malassezia yeasts: taxonomic and epidemiological implications. Syst Appl Microbiol 17:146–153 10.1016/S0723-2020(11)80043-3. [DOI] [Google Scholar]
- 88.Iatta R, Cafarchia C, Cuna T, Montagna O, Laforgia N, Gentile O, Rizzo A, Boekhout T, Otranto D, Montagna MT. 2014. Bloodstream infections by Malassezia and Candida species in critical care patients. Med Mycol 52:264–269 10.1093/mmy/myt004. [DOI] [PubMed] [Google Scholar]
- 89.Jung WH, Croll D, Cho JH, Kim YR, Lee YW. 2015. Analysis of the nasal vestibule mycobiome in patients with allergic rhinitis. Mycoses 58:167–172 10.1111/myc.12296. [DOI] [PubMed] [Google Scholar]
- 90.Velegraki A, Cafarchia C, Gaitanis G, Iatta R, Boekhout T. 2015. Malassezia infections in humans and animals: pathophysiology, detection, and treatment. PLoS Pathog 11:e1004523 10.1371/journal.ppat.1004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cleland EJ, Bassiouni A, Boase S, Dowd S, Vreugde S, Wormald PJ. 2014. The fungal microbiome in chronic rhinosinusitis: richness, diversity, postoperative changes and patient outcomes. Int Forum Allergy Rhinol 4:259–265 (Erratum, 5:92.) 10.1002/alr.21297. [DOI] [PubMed] [Google Scholar]
- 92.Suhr MJ, Banjara N, Hallen-Adams HE. 2016. Sequence-based methods for detecting and evaluating the human gut mycobiome. Lett Appl Microbiol 62:209–215 10.1111/lam.12539. [DOI] [PubMed] [Google Scholar]
- 93.Gouba N, Raoult D, Drancourt M. 2014. Eukaryote culturomics of the gut reveals new species. PLoS One 9:e106994 10.1371/journal.pone.0106994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, Filkins LM, O’Toole GA, Moulton LA, Ashare A, Sogin ML, Hogan DA. 2014. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2:40 10.1186/2049-2618-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI, Strausbaugh LD. 2014. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One 9:e90899 10.1371/journal.pone.0090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu H, Dongari-Bagtzoglou A. 2015. Shaping the oral mycobiota: interactions of opportunistic fungi with oral bacteria and the host. Curr Opin Microbiol 26:65–70 10.1016/j.mib.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Woerden HC, Gregory C, Brown R, Marchesi JR, Hoogendoorn B, Matthews IP. 2013. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis 13:69 10.1186/1471-2334-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chryssanthou E, Broberger U, Petrini B. 2001. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr 90:323–327 10.1080/080352501300067712. [DOI] [PubMed] [Google Scholar]
- 99.Chang HJ, Miller HL, Watkins N, Arduino MJ, Ashford DA, Midgley G, Aguero SM, Pinto-Powell R, von Reyn CF, Edwards W, McNeil MM, Jarvis WR, Pruitt R. 1998. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med 338:706–711 10.1056/NEJM199803123381102. [DOI] [PubMed] [Google Scholar]
- 100.Amend A. 2014. From dandruff to deep-sea vents: malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog 10:e1004277 10.1371/journal.ppat.1004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guillot J, Guého E, Chévrier G, Chermette R. 1997. Epidemiological analysis of Malassezia pachydermatis isolates by partial sequencing of the large subunit ribosomal RNA. Res Vet Sci 62:22–25 10.1016/S0034-5288(97)90174-0. [DOI] [PubMed] [Google Scholar]
- 102.Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, Yamaguchi H, Hasegawa A. 2004. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol 54:623–627 10.1099/ijs.0.02776-0. [DOI] [PubMed] [Google Scholar]
- 103.Sugita T, Boekhout T, Velegraki A, Guillot J, Hadina S, Cabañes FJ. 2010. Epidemiology of Malassezia-related skin infections, p 65–119. In Boekhout T, Mayser P, Guého-Kellermann E, Velegraki A (ed), Malassezia and the Skin. Springer, Berlin. 10.1007/978-3-642-03616-3_3 [DOI] [Google Scholar]
- 104.Duarte ER, Resende JC, Rosa CA, Hamdan JS. 2001. Prevalence of yeasts and mycelial fungi in bovine parasitic otitis in the State of Minas Gerais, Brazil. J Vet Med B Infect Dis Vet Public Health 48:631–635 10.1046/j.1439-0450.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- 105.Renker C, Alphei J, Buscot F. 2003. Soil nematodes associated with the mammal pathogenic fungal genus Malassezia (Basidiomycota: Ustilaginomycetes) in Central European forests. Biol Fertil Soils 37:70–72. 10.1007/s00374-002-0556-3 [DOI] [Google Scholar]
- 106.Fell JW, Scorzetti G, Connell L, Craig S. 2006. Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with <5% soil moisture. Soil Biol Biochem 38:3107–3119 10.1016/j.soilbio.2006.01.014. [DOI] [Google Scholar]
- 107.Mayser P, Gaitanis G. 2010. Physiology and biochemistry, p 121–137. In Boekhout T, Guého E, Mayser P, Velegraki A (ed), Malassezia and the Skin. Springer, Berlin. 10.1007/978-3-642-03616-3_4 [DOI] [Google Scholar]
- 108.Mexia N, Gaitanis G, Velegraki A, Soshilov A, Denison MS, Magiatis P. 2015. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch Biochem Biophys 571:16–20 10.1016/j.abb.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuther K, Mayser P, Hettwer U, Wu W, Spiteller P, Kindler BL, Karlovsky P, Basse CW, Schirawski J. 2008. The tryptophan aminotransferase Tam1 catalyses the single biosynthetic step for tryptophan-dependent pigment synthesis in Ustilago maydis. Mol Microbiol 68:152–172 10.1111/j.1365-2958.2008.06144.x. [DOI] [PubMed] [Google Scholar]
- 110.Hort W, Lang S, Brunke S, Mayser P, Hube B. 2009. Analysis of differentially expressed genes associated with tryptophan-dependent pigment synthesis in M. furfur by cDNA subtraction technology. Med Mycol 47:248–258 10.1080/13693780802238842. [DOI] [PubMed] [Google Scholar]
- 111.Lang SK, Hort W, Mayser P. 2011. Differentially expressed genes associated with tryptophan-dependent pigment synthesis in Malassezia furfur--a comparison with the recently published genome of Malassezia globosa. Mycoses 54:e69–e83 10.1111/j.1439-0507.2009.01848.x. [DOI] [PubMed] [Google Scholar]
- 112.Preuss J, Hort W, Lang S, Netsch A, Rahlfs S, Lochnit G, Jortzik E, Becker K, Mayser PA. 2013. Characterization of tryptophan aminotransferase 1 of Malassezia furfur, the key enzyme in the production of indolic compounds by M. furfur. Exp Dermatol 22:736–741 10.1111/exd.12260. [DOI] [PubMed] [Google Scholar]
- 113.Mayser P, Rieche I. 2009. Rapid reversal of hyperpigmentation in pityriasis versicolor upon short-term topical cycloserine application. Mycoses 52:541–543 10.1111/j.1439-0507.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 114.Reed WB, Pidgeon J, Becker SW. 1961. Patients with spinal cord injury. Clinical cutaneous studies. Arch Dermatol 83:379–385 10.1001/archderm.1961.01580090029002. [DOI] [PubMed] [Google Scholar]
- 115.Barchmann T, Hort W, Krämer HJ, Mayser P. 2011. Glycine as a regulator of tryptophan-dependent pigment synthesis in Malassezia furfur. Mycoses 54:17–22 10.1111/j.1439-0507.2009.01758.x. [DOI] [PubMed] [Google Scholar]
- 116.Liappis N, Kelderbacher SD, Kesseler K, Bantzer P. 1979. Quantitative study of free amino acids in human eccrine sweat excreted from the forearms of healthy trained and untrained men during exercise. Eur J Appl Physiol Occup Physiol 42:227–234 10.1007/BF00423292. [DOI] [PubMed] [Google Scholar]
- 117.Ro BI, Dawson TL. 2005. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J Investig Dermatol Symp Proc 10:194–197 10.1111/j.1087-0024.2005.10104.x. [DOI] [PubMed] [Google Scholar]
- 118.Wisecaver JH, Alexander WG, King SB, Hittinger CT, Rokas A. 2016. Dynamic evolution of nitric oxide detoxifying flavohemoglobins, a family of single-protein metabolic modules in bacteria and eukaryotes. Mol Biol Evol 33:1979–1987 10.1093/molbev/msw073. [DOI] [PubMed] [Google Scholar]
- 119.Zhu Y, et al. 2017. Proteogenomics produces comprehensive and highly accurate protein-coding gene annotation in a complete genome assembly of Malassezia sympodialis. Nucleic Acids Res 45:2629–2643. 10.1093/nar/gkx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, Kronstad JW, Deangelis YM, Reeder NL, Johnstone KR, Leland M, Fieno AM, Begley WM, Sun Y, Lacey MP, Chaudhary T, Keough T, Chu L, Sears R, Yuan B, Dawson TL Jr. 2007. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci USA 104:18730–18735 10.1073/pnas.0706756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ianiri G, Averette AF, Kingsbury JM, Heitman J, Idnurm A. 2016. Gene function analysis in the ubiquitous human commensal and pathogen Malassezia genus. MBio 7:e01853-16 10.1128/mBio.01853-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Celis AM, Vos AM, Triana S, Medina CA, Escobar N, Restrepo S, Wösten HA, de Cock H. 2017. Highly efficient transformation system for Malassezia furfur and Malassezia pachydermatis using Agrobacterium tumefaciens-mediated transformation. J Microbiol Methods 134:1–6 10.1016/j.mimet.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Coelho MA, Sampaio JP, Gonçalves P. 2010. A deviation from the bipolar-tetrapolar mating paradigm in an early diverged basidiomycete. PLoS Genet 6:e1001052 10.1371/journal.pgen.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nielsen K, Heitman J. 2007. Sex and virulence of human pathogenic fungi, p 143–173. In Dunlap JC (ed), Advances in Genetics. Fungal Genomics. Academic Press, Cambridge, MA 10.1016/S0065-2660(06)57004-X [DOI] [PubMed] [Google Scholar]
- 125.Heitman J, Carter DA, Dyer PS, Soll DR. 2014. Sexual reproduction of human fungal pathogens. Cold Spring Harb Perspect Med 4:a019281 10.1101/cshperspect.a019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kesavan S, Holland KT, Ingham E. 2000. The effects of lipid extraction on the immunomodulatory activity of Malassezia species in vitro. Med Mycol 38:239–247 10.1080/mmy.38.3.239.247. [DOI] [PubMed] [Google Scholar]
- 127.Buentke E, D’Amato M, Scheynius A. 2004. Malassezia enhances natural killer cell-induced dendritic cell maturation. Scand J Immunol 59:511–516 10.1111/j.0300-9475.2004.01416.x. [DOI] [PubMed] [Google Scholar]
- 128.Johansson C, Tengvall Linder M, Aalberse RC, Scheynius A. 2004. Elevated levels of IgG and IgG4 to Malassezia allergens in atopic eczema patients with IgE reactivity to Malassezia. Int Arch Allergy Immunol 135:93–100 10.1159/000080651. [DOI] [PubMed] [Google Scholar]
- 129.Scheynius A, Johansson C, Buentke E, Zargari A, Linder MT. 2002. Atopic eczema/dermatitis syndrome and Malassezia. Int Arch Allergy Immunol 127:161–169 10.1159/000053860. [DOI] [PubMed] [Google Scholar]
- 130.Kato H, Sugita T, Ishibashi Y, Nishikawa A. 2006. Detection and quantification of specific IgE antibodies against eight Malassezia species in sera of patients with atopic dermatitis by using an enzyme-linked immunosorbent assay. Microbiol Immunol 50:851–856 10.1111/j.1348-0421.2006.tb03860.x. [DOI] [PubMed] [Google Scholar]


