ABSTRACT
Genetically engineered bacteria have the potential to diagnose and treat a wide range of diseases linked to the gastrointestinal tract, or gut. Such engineered microbes will be less expensive and invasive than current diagnostics and more effective and safe than current therapeutics. Recent advances in synthetic biology have dramatically improved the reliability with which bacteria can be engineered with the sensors, genetic circuits, and output (actuator) genes necessary for diagnostic and therapeutic functions. However, to deploy such bacteria in vivo, researchers must identify appropriate gut-adapted strains and consider performance metrics such as sensor detection thresholds, circuit computation speed, growth rate effects, and the evolutionary stability of engineered genetic systems. Other recent reviews have focused on engineering bacteria to target cancer or genetically modifying the endogenous gut microbiota in situ. Here, we develop a standard approach for engineering “smart probiotics,” which both diagnose and treat disease, as well as “diagnostic gut bacteria” and “drug factory probiotics,” which perform only the former and latter function, respectively. We focus on the use of cutting-edge synthetic biology tools, gut-specific design considerations, and current and future engineering challenges.
INTRODUCTION AND BACKGROUND
Genetically engineered bacteria have the potential to diagnose and treat a wide range of diseases linked to the gastrointestinal tract, or gut. Such engineered microbes will be less expensive and invasive than current diagnostics and more effective and safe than current therapeutics. Recent advances in synthetic biology have dramatically improved the reliability with which bacteria can be engineered with the sensors, genetic circuits, and output (actuator) genes necessary for diagnostic and therapeutic functions. However, to deploy such bacteria in vivo, researchers must identify appropriate gut-adapted strains and consider performance metrics such as sensor detection thresholds, circuit computation speed, growth rate effects, and the evolutionary stability of engineered genetic systems. Other recent reviews have focused on engineering bacteria to target cancer (1, 2) or genetically modifying the endogenous gut microbiota in situ (3, 4). Here, we develop a standard approach for engineering “smart probiotics,” which both diagnose and treat disease, as well as “diagnostic gut bacteria” and “drug factory probiotics,” which perform only the former and latter function, respectively. We focus on the use of cutting-edge synthetic biology tools, gut-specific design considerations, and current and future engineering challenges.
Natural Probiotics
Natural probiotics are evolved organisms that are isolated, cultured, and consumed for a health benefit (5). Many bacterial strains have been developed as natural probiotics, most prominently those of the Lactobacillus and Bifidobacterium genera (6). These microbes have been used to treat Clostridium infection (7); inflammatory diseases such as obesity, diabetes, and inflammatory bowel disease (8); and neurological conditions such as anxiety, depression, and autism spectrum disorder (6), among other pathologies. Natural probiotics are known to exert beneficial effects by directly signaling with the human host through chemical or physical means or by altering the composition and metabolism of the gut microbiota (9). However, like all bacteria, natural probiotics have evolved complex gene regulatory networks that activate or silence therapeutic pathways in response to poorly understood intracellular and extracellular signals. Furthermore, the genetic and molecular mechanisms by which natural probiotics evolved to act are poorly understood (10). As a result, it has proven difficult to improve the efficacy of probiotic therapies, and natural probiotics have thus far yielded mixed success (11).
Smart Probiotics
An exciting alternative is to genetically engineer “smart probiotics”: bacteria that sense the levels of one or more gut biomarkers, compute whether the profile of those biomarkers is diagnostic of disease, and respond by delivering a precise dose of one or more appropriate therapeutics (4, 12–14) (Fig. 1).
FIGURE 1.

The three classes of engineered gut bacteria. Smart probiotics sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and respond by delivering a precise dose of one or more appropriate therapeutics at the diseased tissue. Diagnostic gut bacteria sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and produce a reporter which can be externally measured by a clinician. Drug factory probiotics constitutively produce a therapeutic within the body.
Smart probiotics offer benefits over traditional nonliving therapeutics (i.e., chemical or biologically produced [biologic] drugs) and natural probiotics. First, both smart and natural probiotics can deliver drugs locally to diseased tissues, resulting in increased efficacy and decreased side effects relative to systemic administration of traditional therapeutics. Second, bacteria produce drugs on-site, eliminating the need for expensive purification steps involved in chemical drug synthesis or fermenter-based biologic production. Third, due to the use of well-characterized engineered gene-regulatory networks, rather than poorly characterized evolved versions, therapeutic pathways can be activated far more reliably in vivo with smart as opposed to natural probiotics. Fourth, the genetic pathways resulting in the production of the therapeutic compounds are well-defined in smart probiotics. This feature is unlike natural probiotics, which may produce undefined mixtures of bioactive compounds whose effects are difficult to disentangle. Fifth, in stark contrast to their natural counterparts which are simply taken “as-is” from nature, smart probiotics are developed using iterative cycles of design, construction, testing, and learning, which enables continual performance improvements (Fig. 2). Finally, future advances in our understanding of the biology of gut-linked diseases will result in continued improvements in smart probiotic designs and expand the number of diseases that can be treated.
FIGURE 2.

A comparison of the development process for natural and engineered probiotics. (A) Natural probiotics are isolated and then tested for efficacy without an ability to methodically improve their capabilities. (B) In contrast, engineered probiotics undergo a design-build-test-learn cycle which allows for continual probiotic improvement and knowledge gain with each iteration.
The foundation for smart probiotics was laid at the turn of the current century when Remaut and colleagues genetically modified the natural probiotic Lactococcus lactis to constitutively secrete the human anti-inflammatory cytokine protein interleukin-10 (IL-10) (Fig. 3A). We classify this IL-10-secreting L. lactis strain, and any bacterium engineered to constitutively produce a therapeutic within the body, as a “drug factory probiotic.” Because drug factory probiotics perform only the “back end” delivery function, we consider them a simpler subclass of smart probiotics (Fig. 1). As the authors intended, oral administration of the IL-10-secreting L. lactis reduces colon inflammation (colitis) by 50% in mice (15) (Fig. 3B). Furthermore, 10,000-fold lower IL-10 concentrations are required to achieve a therapeutic effect when delivered in the gastrointestinal tract by L. lactis compared to intraperitoneal administration (15). This increase in efficacy decreases the potential for unwanted immunosuppressive side effects. These benefits are clear examples of several of the advantages of smart probiotics.
FIGURE 3.

Examples of current engineered gut bacteria. (A) A drug factory probiotic was made by engineering L. lactis to constitutively produce IL-10. IL-10 is secreted by the bacteria and then bound by the IL-10 receptor in the gut, resulting in downregulation of host inflammation. (B) DSS was used to induce inflammation in mice, and disease pathology was measured with histological scores. Treatment with the IL-10 drug factory probiotic was found to decrease symptoms by 50% compared to untreated mice or mice administered the natural probiotic L. lactis. (C) A diagnostic gut bacterium was created by engineering E. coli Nissle to sense thiosulfate, which is produced in the gut during inflammation. The thiosulfate is sensed by the ttrSR TCS, which activates expression of the fluorescent protein sfGFP. (D) sfGFP fluorescence of bacteria isolated from the feces and distal and proximal colon was measured and found to be significantly increased in mice experiencing inflammation in each location. Panel adapted from Mol Syst Biol (55) with permission of the publisher. (E) E. coli NGF-1 was modified to express the ATC sensor TetR, which controlled expression of the cro TF. The cro TF is one component of the lambda phage cro/cI toggle switch. ATC altered the start of the switch to become cro-dominant, and therefore produce LacZ protein, which produces a blue pigment. (F) The ATC-sensing diagnostic gut bacteria were administered to mice which were administered ATC via drinking water. Temporary administration of ATC was found to activate LacZ production, and the memory was retained for up to 1 week. Panel adapted from Proceedings of the National Academy of Sciences (56) with permission of the publisher. (G) The native RhaR rhamnose sensor in B. thetaiotaomicron was used to control expression of the Int12 recombinase, which permanently inverts a barcode segment of DNA in the genome. (H) The rhamnose diagnostic gut bacteria switched the state of the barcode as detected via qPCR when administered rhamnose in drinking water. However, even without administration of rhamnose, the sensor toggled states, due to either leaky recombination or residual rhamnose in the plant-based chow. Panel reprinted from Cell Systems (51) with permission of the publisher.
The Remaut group later iterated on the IL-10-secreting L. lactis strain several times to improve its performance. One concern is uncontrolled growth of engineered bacteria within the gut. This may be detrimental to the health of the host or result in unintended escape into the environment and corresponding spread of synthetic DNA into natural populations. To overcome this problem, the authors introduced a genomic mutation resulting in thymidine auxotrophy, making their drug factory probiotic dependent on thymidine added to laboratory growth media for survival. Indeed, this biocontainment strategy greatly decreases survival in the porcine gut (16). Additionally, a phase I clinical trial for treatment of Crohn’s disease revealed that this second-generation IL-10-secreting L. lactis does not generate systemic or long-term side effects and that the biocontainment strategy functions as intended in humans (17). However, a subsequent phase IIA trial failed to demonstrate statistically significant beneficial effects in humans. Unfortunately, the reasons for this failure are unknown (18). Nonetheless, to improve efficacy, the strain has since been re-engineered to secrete single-chain antibodies that bind tumor necrosis factor alpha (TNFα) (i.e., anti-TNFα nanobodies), preventing its ability to stimulate inflammation (19). Clinical trials using the anti-TNFα nanobody-secreting L. lactis to treat inflammatory bowel disease are now being pursued (20; https://www.dna.com/Technologies/ActoBiotics).
Undoubtedly inspired by the L. lactis work, other groups have since engineered drug factory probiotics to treat colitis (17, 19, 21–28), diabetes (29–32), obesity (33), and numerous pathogen infections (34–38). These efforts are all based on traditional genetic engineering approaches wherein a heterologous genetic pathway is simply introduced into a gut-relevant bacterial host. On the other hand, exciting advances are being made by incorporating modern synthetic biology technologies into smart probiotic designs.
Synthetic Biology
Synthetic biology is a new engineering discipline wherein living organisms are genetically programmed to carry out unnatural behaviors, with applications in medicine, agriculture, energy, manufacturing, and fundamental biology research, among other enterprises (14, 39–41). In contrast to traditional genetic engineering approaches, synthetic biologists program cellular behaviors using the “sense-compute-respond” paradigm adapted from electrical engineering (42) (Fig. 4). Here, organisms are engineered to express genetically encoded sensors, such as signal transduction pathways, that detect specific chemical or physical inputs inside or outside the cell. Sensors convert chemical or physical inputs into biological outputs such as gene transcription. These biological outputs, in turn, serve as inputs into engineered genetic circuits, to which the sensors are linked (43, 44). Genetic circuits are networks of interacting regulatory molecules, such as transcription factors (TFs) and their target promoters, that perform computations such as multi-input logic or memory. Finally, the circuits control the activity of actuator genes, such as metabolic pathways or secreted proteins, which program the cell to change its own state or the state of its environment. Importantly, sensors, circuits, and actuators (i.e., devices) are designed to be modular, such that they function in the same manner in different genetic, organismal, or environmental contexts. This modularity enables a large number of new designs to be rapidly imagined, constructed, and tested, while increasing predictability and improving performance.
FIGURE 4.
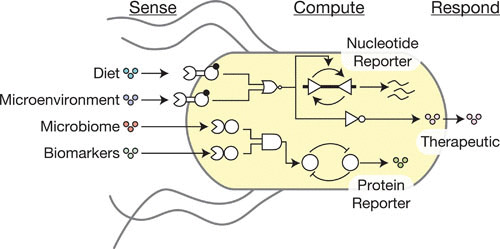
An outline of the types of sense, compute, and respond behavior an engineered gut bacterium can exhibit. Chemicals from a variety of sources within the gut may be of interest to a smart bacterium, including the host diet, compounds produced locally by the host in the bacterium’s microenvironment, signals from other components of the microbiome, and general biomarkers of host disease. Computation is performed with a variety of logic gates and memory elements. The bacterium actuates a response with a therapeutic molecule in the case of a smart probiotic, or by producing a nucleotide or protein-based reporter for a diagnostic gut bacterium.
Genetically Encoded Sensors
Bacteria have evolved thousands of genetically encoded sensors, many of which can be repurposed to diagnose disease. These fall into two major categories: one-component systems (OCSs) and two-component systems (TCSs).
OCSs, cytoplasmic TFs that directly interact with and are allosterically modulated by chemical and physical inputs, are the largest class of bacterial sensors (45) and have been used for gut-relevant sensing applications. In in vitro work, synthetic biologists have utilized OCSs that respond to acyl-homoserine lactone quorum-sensing autoinducers to program Escherichia coli to monitor communication between pathogenic Pseudomonas aeruginosa on solid agar (46) and in biofilms (47). In an ex vivo study, NorR, an OCS activated by nitric oxide, was used to detect the presence of inflammatory signaling in an epithelial cell coculture of intestinal explants (48). Finally, various orally administered gut-adapted bacteria have been used to sense dietary lactose (49), xylan (50), and rhamnose (51), as well as fucose produced by the gut microbiota (52) in the mouse gastrointestinal tract. Though they will surely play a major role in future diagnostic efforts, OCSs are limited by the fact that they cannot sense disease biomarkers that are not transported across the membrane or do not induce secondary chemical or physical changes inside the cell.
TCSs are the largest family of signal transduction pathways in biology (53, 54) and the primary means by which bacteria sense extracellular stimuli. TCSs are composed of a membrane-bound or cytoplasmic sensor histidine kinase (SK) and a cytoplasmic response regulator protein. In the presence of a specific stimulus, the SK phosphorylates the response regulator, which then binds DNA and activates or represses transcription from one or more output promoters.
In recent work, we hypothesized that thiosulfate (S2O32–) could serve as a novel biomarker of colitis and set out to engineer a gut-adapted bacterium to sense it in mouse models. However, no genetically encoded thiosulfate sensor had previously been reported. Therefore, we developed a combined computational and experimental approach to discover the first known biological thiosulfate sensor, a TCS from the marine bacterium Shewanella halifaxensis (55). We expressed this TCS, which we named ThsSR, in the natural probiotic E. coli Nissle 1917 (also known as Nissle), and encoded the reporter gene superfolder green fluorescent protein (sfgfp) as the output (Fig. 3C). We then orally administered the thiosulfate-sensing Nissle strain to control mice and mice administered the colitis-inducing compound dextran sodium sulfate (DSS) and performed flow cytometry on bacteria sampled from the colon and feces 6 hours later. As hypothesized, we observed that ThsSR is activated in mice with, but not without, colitis (Fig. 3D). Furthermore, the greater the extent of inflammation, the greater the ThsSR activity, suggesting that thiosulfate may be a novel colitis biomarker (55).
Importantly, our flow cytometry method enables identification and analysis of sensor strains without eliminating the native gut bacteria via broad-spectrum antibiotic treatment or germ-free methods. This feature enables minimally invasive measurements of biomarkers in native gut pathways and could generate more physiologically relevant results that are more likely to translate to clinical conditions. We are now investigating whether thiosulfate is a colitis biomarker in humans and whether our engineered bacterium could be advanced as a clinical diagnostic.
Our thiosulfate-sensing Nissle strain is an early example of a “diagnostic gut bacterium.” This class of engineered gut bacteria utilize sensors, or sensors linked to genetic circuits (discussed below), to diagnose a gut-linked disease via expression of a reporter gene (Fig. 2).
Genetic Circuits
Genetic circuits can be inserted between sensors and reporter genes to increase the quality, accuracy, or duration of diagnostic gut bacterial readouts. In a pioneering proof-of-principle study, Silver and coworkers used the E. coli tetracycline repressor (TetR) to express the phage λ transcriptional repressor cro, which in turn flipped the cI/cro transcriptional toggle switch from a cI- to a cro-dominant state in the presence of the tetracycline derivative anhydrotetracycline (aTc) (Fig. 3E). In this design, the cI-repressible promoter Pr is used to drive expression of the reporter gene lacZ, which is detected via production of a blue pigment indicator in X-gal agar plate growth media. First, the group transformed the mouse gut-derived E. coli strain NGF-1 with this sensor-circuit device. The device was integrated into the genome of NGF-1 to enable long-term evolutionary stability. Then they pretreated mice with the antibiotic streptomycin to clear the native gut flora, and orally administered the aTc-sensing NGF-1 strain to the mice, enabling long-term colonization (i.e., persistent growth in the gut without repeated oral administration). Once colonized, the engineered NGF-1 strain reports exposure to aTc supplied in drinking water by flipping to the cro-dominant state, which is detected by plating the fecal matter on X-gal-containing media (56). Impressively, the aTc sensor device remains activated for over 1 week after exposure to aTc due to the stability of the λ genetic switch (Fig. 3F).
Tetrathionate (S4O62–), the oxidative dimer of thiosulfate, has also been implicated as a colitis biomarker (57, 58). Recently, the Silver group modified their aTc-sensing gut bacterium to function as a tetrathionate sensor by replacing tetR with the tetrathionate-sensing Salmonella enterica serovar Typhimurium TCS ttrSR (59, 60). The tetrathionate-sensing NGF-1 was orally administered to mice pretreated with streptomycin, resulting in stable levels of engineered bacteria in the feces after 6 months. Impressively, DNA sequencing and functional screening of fecal isolates showed that the tetrathionate sensor device had not lost the ability to respond to tetrathionate. For diagnosis, the strain was then administered to control mice and the Salmonella infection and IL-10 mouse models of colitis. A significant portion of the sensor bacteria switched to the activated cro-dominant state in the colitis compared to control mice. However, activation only occurred in an average of 1% and 5% of bacteria for the Salmonella and IL-10 models, respectively, compared to 100% activation of the sensor in vitro, with some diseased mice not giving rise to any activated colonies. This limitation demonstrates the need for further design-build-test-learn cycles to improve the performance of these diagnostic gut bacteria.
Additionally, though thiosulfate and tetrathionate are both anaerobic electron acceptors for bacterial respiration, tetrathionate is preferred and may be rapidly consumed by the gut microbiota. This fact may have contributed to the low tetrathionate signal observed by Silver and coworkers. Indeed, we did not observe colitis-dependent activation of a different tetrathionate-sensing TCS in DSS-treated mice with a native microbiota (55). Taken together, these results suggest that tetrathionate may not be an ideal colitis biomarker.
Recombinases that invert promoter-containing DNA segments (61) or engineered barcode sequences (62) are an alternative circuit design for long-term memory in diagnostic gut bacteria. In a recent study by Lu, Voigt, and coworkers, a recombinase was used to invert a genomic DNA barcode in the human commensal Bacteroides thetaiotaomicron to remember exposure to diet-derived rhamnose in the mouse gut (51) (Fig. 3D). However, appreciable background recombination was observed in this study, highlighting the need for future engineering cycles to improve performance. Ultimately, though, all of these early studies clearly demonstrate the exciting potential of diagnostic gut bacteria, particularly as more biomarkers are identified and bacterial sensors developed.
Genetically Encoded Therapeutic Actuators
Drug factory probiotic design efforts have generated over 25 genetically encoded therapeutic actuators targeting diverse diseases (Table 1). A major class is bacterially secreted human proteins that augment human signaling networks. These include IL-10 (15) and anti-TNFα nanobodies (19), which treat colitis, and glycolipoprotein-1 (63), which treats diabetes. A second important class is bacterial proteins that augment human signaling networks. Examples of this class include the Toll-like receptor 2 stimulator LcrV protein from Yersinia pseudotuberculosis (25) and the superoxide dismutase SodA protein from L. lactis (22), both of which decrease symptoms of colitis. A final class is genetic pathways that produce therapeutic metabolites. For example, expression of At1g78690, an N-acyltransferase, led to production of N-acylphosphatidylethanolamines (NAPEs), which reduced food intake and obesity in mice (30). In another example, expression of the Propionibacterium acnes linoleic acid isomerase coPAI led to production of conjugated linoleic acid, which altered fatty acid composition and decreased body fat (33). The synthetic biology approach described above will enable integration of these and other therapeutic actuators into smart probiotics to increase their therapeutic efficacy and decrease associated side effects.
TABLE 1.
Examples of engineered gut bacteriaa
| Type | Disease targeted | Compound sensed | Therapeutic produced | Chassis | In vivo | Reference(s) |
|---|---|---|---|---|---|---|
| Drug factory probiotic | Autoimmune diseases | Ovalbumin | L. lactis | X | 67 | |
| Cholera | Chimeric lipopolysaccharide | E. coli E56b | X | 37 | ||
| Cholera | CAI-1 | E. coli Nissle 1917 | X | 38 | ||
| Celiac | DQ8 gliadin epitope | L. lactis | X | 68 | ||
| Colitis | IL-10 | L. lactis | X | 15, 17 | ||
| Colitis | Trefoil factors | L. lactis | X | 21 | ||
| Colitis | DNA for TGF-β1 | E. coli BM2710 | X | 64 | ||
| Colitis | SOD | L. plantarum | X | 22 | ||
| Colitis | SOD | L. lactis | X | 22 | ||
| Colitis | Manganese-dependent catalase | L. casei | X | 23 | ||
| Colitis | SOD | L. gasseri | X | 24 | ||
| Colitis | LcrV | L. lactis | X | 25 | ||
| Colitis | Anti-TNFα nanobodies | L. lactis | X | 19 | ||
| Colitis | SOD | L. casei | X | 26 | ||
| Colitis | SOD | L. casei | X | 27 | ||
| Colitis | CAT | L. casei | X | 27 | ||
| Colitis | Elafin | L. casei | X | 28 | ||
| Colitis | Elafin | L. lactis | X | 28 | ||
| Diabetes | IL-10 | L. lactis | X | 29 | ||
| Diabetes | NAPEs | E. coli Nissle 1917 | X | 30 | ||
| Diabetes | Glutamic acid decarboxylase | L. lactis | X | 31 | ||
| Diabetes | IL-10 | L. lactis | X | 31 | ||
| Diabetes | GLP-1 | L. gasseri | X | 32 | ||
| E. coli | Lactoferrin | L. casei | X | 34 | ||
| E. coli | Chimeric lipopolysaccharide | E. coli E56b | X | 35 | ||
| Listeria monocytogenes | Listeria adhesion protein | L. paracasei | X | 36 | ||
| Obesity | Conjugated linoleic acid | L. paracasei | X | 33 | ||
| Diagnostic gut bacteria | Citrobacter rodentium | Fucose | E. coli BW25113 | X | 52 | |
| Colitis | NO | E. coli | 48 | |||
| Colitis | Tetrathionate | E. coli NGF-1 | X | 60 | ||
| Colitis | Tetrathionate | E. coli Nissle 1917 | X | 55 | ||
| Colitis | Thiosulfate | E. coli Nissle 1917 | X | 55 | ||
| Diet sensor | Lactose | Streptococcus thermophilus | X | 49 | ||
| Diet sensor | ATC | E. coli NGF-1 | X | 56 | ||
| Diet sensor | Arabinogalactan | B. thetaiotaomicron | X | 51 | ||
| Diet sensor | IPTG | B. thetaiotaomicron | X | 51 | ||
| Diet sensor | Rhamnose | B. thetaiotaomicron | X | 51 | ||
| Smart probiotic | Colitis | Xylan | IL-2 | B. ovatus | 156 | |
| Colitis | Xylan | Keratinocyte growth factor-2 | B. ovatus | X | 50 | |
| Colitis | Xylan | TGF-β1 | B. ovatus | X | 157 | |
| Diabetes | Nisin | Insulin | L. lactis | 158 | ||
| Diabetes | Glucose | GLP-1 | E. coli Nissle 1917 | 63 | ||
| Diabetes | Glucose | PDX-1 | E. coli Nissle 1917 | 63 | ||
| Pseudomonas aeruginosa | 3OC12HSL | E7 lysis | E. coli | 47 | ||
| P. aeruginosa | 3OC12HSL | Bacteriocin | E. coli MG1655 | 46 |
Abbreviations: CAT, catalase; GLP-1, glucagon-like peptide 1; IPTG, isopropyl-β-d-thiogalactopyranoside; NAPE, N-acylphosphatidylethanolamine; PDX-1, pancreatic and duodenal homeobox gene 1; SOD, superoxide dismutase; TGF, transforming growth factor
ENGINEERING SMART PROBIOTICS
When setting out to construct a smart probiotic, one must make a series of basic design decisions. These include selecting a chassis organism and gut disease biomarkers, identifying the computations that need to be performed, and determining the appropriate therapeutic response. A toolkit of chassis organisms and genetic parts has been developed to accomplish these goals. Furthermore, synthetic biology techniques are available for developing new chassis and parts when current options are insufficient. Finally, deploying smart probiotics within the complex and dynamic gut environment presents additional challenges that require further design considerations.
Chassis Selection
The criteria for selecting smart probiotic chassis partially diverge from those used when selecting natural probiotics. Similar to natural probiotics, one must consider the ability of the chassis to survive transit through the gastrointestinal tract, colonize particular macroscopic or microscopic geographical locations within the gut, and reach desired densities therein. On the other hand, the amenability of the chassis to DNA transformation, the availability of tools to modify its genome, and the repertoire of sensors, genetic circuits, and actuators compatible with it are also essential to consider.
Previous studies have established a number of “off-the-shelf” chassis with different properties. Multiple strains of the Gram-negative facultative anaerobe E. coli, the traditional workhorse of synthetic biology, have been deployed in mice for different purposes. These include laboratory strains (35, 37, 52, 64), Nissle (34, 38, 55), and NGF-1 (56, 60, 65). The Gram-negative anaerobe B. thetaiotaomicron has recently been developed as a new smart probiotic chassis (51). This bacterium belongs to the phylum Bacteroidetes, which is one of the two dominant phyla in human stool (66). Gram-positive facultative anaerobe alternatives include Lactobacillus casei (23, 26–28, 34), Lactobacillus gasseri (24, 32), Lactobacillus paracasei (33, 36), Lactobacillus plantarum (22), and L. lactis (15, 17, 19, 22–28, 31, 32, 34, 67–69). Many of these lactic acid bacteria, along with Bacteroides xylanisolvens, a relative of B. thetaiotaomicron, have Generally Recognized As Safe recognition by the U.S. Food and Drug Administration (70), facilitating their use in humans.
Colonization
The ability of an organism to colonize a specific biogeographical gut location may be necessary for effective diagnosis or treatment of disease. For example, when targeting a patient with ulcerative colitis, which impacts the large intestine, it may be beneficial for the organism to colonize the large intestine (71). On the other hand, if treating the related Crohn’s disease, wherein pathology extends from the mouth to anus (71), colonization of the small intestine may also improve the therapeutic effects. Members of the Bacteroidaceae family, which are found in higher numbers in the colon, may be better suited for the former disease, whereas members of the Lactobacillaceae family, which are found in both the small intestine and colon, may be better suited for the latter (72). Furthermore, specific genetic determinants within a species can result in different colonization capabilities (72), which opens the door for engineering chassis bacteria with specific colonization patterns.
Alternatively, when engineering diagnostic gut bacteria, it may be beneficial to have bacteria that do not colonize the gut. For example, our thiosulfate-sensing Nissle strain (55) survives passage through the gut but does not appear to colonize or reach high numbers when the native gut microbiota is intact (73). These features result in minimal perturbations to the native gut microbiota relative to other strains which may outcompete native flora for specific niches and thereby alter gut microbiota or host physiology. For example, alterations to gut microbiota can lead to the highly undesirable side effects of inflammation (74) or opportunistic pathogen infection (75).
Genetic Tractability
The capacity to perform genetic modifications that yield desired outcomes is fundamental for smart probiotic design but varies widely between gut bacterial chassis. Genetic tractability requires tools for reliable DNA transformation, control of gene expression, and posttranslational control of protein processes such as secretion. A wide array of sensors, circuit components, and actuators are also needed and will be covered in depth in later sections.
E. coli has an unrivaled genetic toolbox, which we will use to exemplify the parts required to engineer smart probiotics. DNA transformation can be accomplished via chemical or electrical disruption of the membrane, phage-mediated transduction, or conjugation from another bacterium. To persist, the transferred DNA requires self-replicating elements (76) or genomic integration (77). Selectable markers such as antibiotic resistance genes are important for obtaining and maintaining transformants in the laboratory but do not appear to be required for retention of plasmid DNAs in short-term in vivo experiments (55, 73). The transcription rate of sensor, circuit, or actuator mRNAs can be precisely controlled using well-characterized constitutive promoter libraries (78, 79) or chemically inducible promoters (80, 81). Termination of transcription is important to prevent unwanted cross-talk between different transcribed elements on a contiguous piece of engineered DNA. Previous work has generated large libraries of well-characterized transcription terminators, many of them very efficient (82, 83). The mRNA translation rate is commonly used to control the protein expression level. The translation rate of a target gene can easily be tuned in E. coli by computational design of custom ribosome binding site (RBS) sequences via the RBS calculator (84–86) or through the use of the more modular “bicistronic design” RBSs developed by Endy, Arkin, and coworkers (79). Translated E. coli proteins can be functionalized with tags that facilitate their secretion from the cell (87) or rapid degradation (88). These E. coli parts have primarily been characterized in laboratory strains. However, recent work demonstrated that a complex circuit could be transferred from a laboratory strain to a native gut isolate while maintaining complete functionality (56) despite possible differences in individual part performance between strains.
When using a chassis other than E. coli, many of the aforementioned tools will be unavailable or poorly developed. Thus, techniques to rapidly generate reliable new genetic tools in non-E. coli chassis are needed. This goal was recently demonstrated by Lu, Voigt, and colleagues in B. thetaiotaomicron (51). To transform this less tractable organism, a conjugative plasmid containing the genetic construct was assembled in E. coli and subsequently transferred to B. thetaiotaomicron via conjugation. Once transformed, the plasmid expressed an integrase (89) that catalyzed its integration into a fixed site in the genome. The authors then designed a library of synthetic promoters resulting in a 20-fold range of reporter gene expression levels by inserting a 26-bp DNA sequence in different regions of the native housekeeping sigma factor’s promoter, PBT1311. They also developed three carbohydrate-inducible promoters by taking advantage of natively expressed proteins and a synthetic IPTG-inducible promoter from E. coli. To vary translation strength, they modified the native RBS of the 50S ribosomal protein gene rpiL* by targeting conserved positions, resulting in a 1,000-fold range of gene expression levels.
Several parts have been developed for Lactobacilli as well (90). These include nisin- (91), peptide pheromone SppIP- (92), IPTG-, and xylose- (93) inducible promoters and constitutive promoter libraries (94). A range of replicative plasmids (90) also exist, as do single-stranded DNA recombineering techniques to generate point mutations in chromosomal DNA (95, 96). However, the current lack of a consensus organism in this genus will require many of these parts to be recharacterized in the specific species selected as a chassis.
Sensor Design
The simplest way to engineer a smart probiotic sensor entails expressing nonnative output genes (e.g., reporters, genetic circuit components) from endogenous chemical or physical input-responsive promoters with no additional engineering of the chassis (49, 51, 52). Though convenient, this approach can produce false negatives or positives due to the poorly understood and complex gene regulatory networks that control the activity of endogenous promoters. This method can be improved upon by knocking out the sensor genes that control the promoter of interest and expressing codon-altered versions of their open reading frames under nonnative promoters, RBSs, and so on. This process, termed genetic refactoring (97–100), can break the key connections in the native control networks, making sensor performance more reliable. However, sensor proteins may still be subject to posttranslational regulation, which can interfere with their function. An even more reliable approach is to port sensors from unrelated organisms into the chassis of interest (55, 101, 102) and optimize their genetic encoding and expression levels for optimal sensor performance (103).
Sensor portability
In many instances, sensors of targeted disease biomarkers have not evolved in the chassis of interest. Thus, sensors often need to be ported between organisms. The successful transfer of LacI (51, 93) and TetR homologs (104, 105) between evolutionarily distant organisms demonstrates the portability of OCSs. OCS portability is improved by the fact that a single protein performs both the sensing and transcriptional control functions.
This feature stands in contrast to TCSs, which have different sensing and effecting proteins, resulting in the need to perform a two-dimensional gene expression optimization (55, 102, 103). SKs are often membrane bound, which could compromise TCS functionality when transported between organisms with drastically different membrane structures. Additionally, SKs often require poorly characterized accessory sensor proteins that allosterically modulate the SK in the presence of the input. If known, such accessory proteins must also be expressed and optimized in the new chassis. If unknown, the TCS will fail to function if the chassis lacks them. Thus, despite their benefits in sensing diverse extracellular signals, TCSs are sometimes more difficult to engineer than OCSs.
Sensor performance characteristics
Most synthetic biological sensors have been characterized in bench experiments where the growth media, growth phase, density, and other factors that influence the function of synthetic genetic systems are controlled. However, chemical, microbial, and host biological aspects of the gut are poorly understood, spatially heterogeneous, and vary over time in a single individual or between individuals. Gut environment variations impact the growth and physiology of engineered gut bacteria and may therefore introduce variability in sensor performance.
One way to overcome sensor variability is to increase the dynamic range (i.e., the ratio of sensor output in the maximally versus minimally active states) (106). A larger dynamic range allows for more distinguishable bits of information to pass through a sensor, resulting in more accurate measurements of the input. Sensor dynamic range can be increased by optimizing the expression level of sensor proteins or the sequence of sensor output promoters to eliminate cryptic constitutive transcriptional start sites that increase leakiness (103). Additionally, TCSs utilize phosphorylation/dephosphorylation cycles that make them more robust than OCSs to fluctuations in the expression levels of sensor proteins (107). This benefit may make TCSs more reliable sensors than OCSs in the gut.
The threshold sensitivity of a sensor to its input is an important and often overlooked factor that is fundamental to gut performance. Clinically relevant biomarker levels are likely to fall within specific ranges, and sensors must match these ranges to successfully diagnose a patient. Few studies have examined general methods of tuning the threshold sensitivities of sensors (108), and much work remains to address this problem. Additionally, many biological sensors are sensitive to only a small range of input concentrations and do not respond outside of that range, which makes them ill suited for quantifying a wide range of biomarker levels. Recent techniques enable the expansion of the range over which a sensor is sensitive, making it capable of measuring a broad range of input concentrations (109).
Sensor outlook
Currently, only a tiny fraction of evolved bacterial sensors have been characterized. Furthermore, it is likely that off-the-shelf sensors will not be available for new disease biomarkers identified in forthcoming gut metabolomic or similar studies. To develop new sensors, new OCS and TCS sensor genes can be easily identified via bioinformatic analyses (45, 54). However, their inputs can seldom be predicted, and there are few efficient methods for discovering them experimentally. In some cases, sensor domain homology can be utilized to predict the class of inputs (110, 111), or the local genomic context can be analyzed to identify nearby metabolic clusters which may be linked to the sense compound (55). Alternatively, the transcriptome response of a natural gut microbiome can be probed for sensors that are upregulated in a diseased state, which often indicates activation of the sensor (112). This may allow identification of sensors for a particular disease without prior knowledge of the compound that is being detected. These sensors can then be refactored and characterized in a defined environment to determine the specific disease biomarker sensed. Future advances in synthetic biology methods which allow many OCSs and TCSs to be screened for responses to inputs in high throughput should greatly increase the number of available sensors (Fig. 5A).
FIGURE 5.

The different components used to construct an engineered gut bacterium. The pros and cons of each component are listed in green and red text, respectively. (A) Sensors can be selected from OCSs or TCSs. (B) Logic circuits can be constructed using TFs, CRISPR/Cas repressors, RNA-based sRNA transcriptional activators, or serine recombinases. (C) Genetic memory can take the form of a toggle switch or recombined DNA. (D) The state of a circuit can be assayed using colorimetric, luminescent, fluorescent, or nucleotide reporters.
Genetic Logic Circuits
Genetic logic circuits can be used to increase the accuracy of smart probiotic diagnoses. For example, dietary nitrate is quickly consumed by the microbiota in the proximal gut (113), whereas nitrate produced due to inflammatory diseases can be found within the colon (114). Therefore, if nitrate was detected at the same time as a large intestine biomarker, then colitis would be likely, and it may be appropriate to secrete an anti-inflammatory compound. However, if nitrate was sensed alongside a small intestine biomarker, it would likely be of dietary origin rather than due to inflammation and should therefore be ignored. To execute such higher-order sensing, smart probiotics require multiple sensors and genetic logic circuits capable of integrating the biological inputs from those sensors simultaneously.
Parts to implement logic
TFs are proteins that bind DNA operator sites to activate or repress transcription from promoters. TFs are the primary means by which synthetic biologists engineer logic. Several TF-based part families have been engineered and used to construct a remarkable range of genetic logic circuits.
Recently, Voigt and colleagues engineered a library of over 10 orthogonal homologs of the TetR transcriptional repressor (104). Here, orthogonality refers to the fact that each TetR homolog represses only its target promoter, and not the promoter of any other TetR homolog in the library. Each orthogonal TetR/promoter pair comprises a simple transcriptional “inverter” circuit wherein high expression of a TetR homolog from an upstream (input) promoter results in low transcription from the corresponding repressible (output) promoter (104). In follow-up work, the group used the library to construct a series of NOR logic circuits, which are active only in the absence of two different TetR homologs. The group then wrote a new in silico program for automated genetic logic circuit design (named Cello, for cellular logic). Cello was used to computationally design the DNA sequences encoding 52 different genetic logic circuits, many that perform complicated operations typically associated with electronic systems. Initially, 94% of their genetic circuits failed due to undefined interactions between neighboring promoters and the genes they transcribed (115). To overcome this challenge, the group standardized the 15 bp upstream of the promoter with a random DNA sequence to remove variable effects of the upstream sequence. They also placed a ribozyme between the promoter and RBS which cleaves off any mRNA nucleotides encoded by the promoter to prevent the promoter from influencing translation initiation from the RBS (116). When these genetic parts were added alongside restrictions on the physical ordering of genetic parts along a DNA sequence to further reduce context-dependent performance changes, the success rate increased to a remarkable 71% (115). The vast majority of logical operations that one may want to engineer into an E. coli smart probiotic could now be implemented using this approach.
The CRISPR (clustered regularly interspaced short palindromic repeats) interference (CRISPRi) TF-design technology offers several benefits for genetic logic circuit design. In CRISPRi, a nuclease-deficient Streptococcus pyogenes Cas9 protein (dCas9) binds to an engineered guide RNA, which drives it to bind and repress virtually any promoter in a sequence-specific manner (117). Voigt and coworkers also engineered an orthogonal library of transcriptional inverters using CRISPRi. In particular, they designed a series of synthetic promoters with divergent spacer regions between the -35 and -10 elements, and cognate small guide RNAs (sgRNAs) that target each promoter sequence specifically. These inverters were then combined to create NOR (output is on only when no inputs are present), OR (output is on when any of multiple inputs are present), and AND (output is on only when all inputs are present) logic using one, two, and four interacting gRNAs, respectively (118). Unlike the TetR work, where the operator sites of most repressor homologs had to be discovered via arduous high-throughput protein/DNA binding array methods, these CRISPRi logic gates could be designed largely using Watson-Crick base-pairing rules. Indeed, dozens or hundreds of CRISPRi circuits can be easily engineered due to this feature (119). CRISPRi circuits are also promising because each sgRNA is on the order of 60 bp, resulting in a small and therefore inexpensive, simple to assemble, and possibly more evolutionarily stable DNA footprint. However, each sgRNA competes for a single pool of Cas9, creating a point of possible retroactivity and thus circuit failure (14). Retroactivity occurs when the activity of downstream components of a circuit alters the performance of upstream components in an unexpected fashion (120). Lastly, sgRNA-regulated promoters, when compared to traditional TF-regulated promoters, are sensitive over a broader range of regulator concentrations, resulting in a less digital-like response. This broad sensitivity can result in propagation of variability or stochastic noise through the genetic circuit, which can also cause circuits to fail (121).
RNA molecules can also be used to engineer transcriptional logic circuits. This class of designs was originally demonstrated by Arkin and coworkers, who utilized the copy number control mechanism of the pT181 plasmid to engineer an antisense RNA that promotes the formation of a transcriptional terminator on a target RNA, preventing downstream transcription. Orthogonal regulatory RNAs were created and used to construct a NOR gate and a three-step cascade (122). Lucks and coworkers built upon this work by inverting the effect of the antisense RNA through the addition of an anti-anti-terminator RNA sequence and creating the first sRNA transcriptional activators (123), which have been employed to create a two-input AND gate (123) and a three-step cascade (124). The ability to computationally generate novel regulatory RNAs (124), combined with their small size, facilitates the creation of circuits containing a large number of nodes. However, this has yet to be demonstrated and is possibly hampered by the low dynamic range frequently observed with RNA-based regulation.
Finally, serine recombinases, proteins that bind DNA at specific sequences and invert or excise intervening DNA (125), have been used to orient transcriptional control elements on DNA to control the flow of RNA polymerase. These have enabled circuits that can count to 3 (126), form a large number of two-input Boolean logic gates (61, 127), and create state machines that remember both the presence and order of input signals (128). However, it can be difficult to balance the expression levels of all circuit components, particularly when reversible serine recombinases are used (129).
Computation speed
The gut environment changes rapidly in space and time, and noncolonizing bacteria pass through the mouse gastrointestinal tract in several hours (55, 65, 73, 130). Moreover, colonizing bacteria face temporal changes in response to diet, exercise, and biogeographic position. Current transcriptional circuits exhibit dynamics that are likely to be too slow for many diagnostic and therapeutic applications. In particular, changes in stable protein concentrations are limited by the rate of dilution due to cell growth, and it may take many doublings to dilute or produce enough protein to traverse the sensitive region of a downstream node in a logic circuit (131). Circuits containing four (115) and nine TFs (132) have both been shown to take 5 hours to compute a final state. Recombinase circuits are even slower; a single inversion step has been measured to take 2 (62), 3 (127), and 6 hours (132), whereas two-part gates were found to take 8 (62) and 9 hours (127) to fully respond. A single CRISPRi inverter has been shown to have a 10- to 90-minute delay and take 4 to 5 hours to switch between on and off states when controlling a stable protein (117, 118). Furthermore, genetic circuits transiently produce incorrect outputs (115), which could lead to improper diagnoses and therapeutic responses by smart probiotics.
The response times of transcriptional logic circuits can be accelerated by adding degradation tags to TF components (133). However, this approach can generate a new problem where TF components compete for a finite supply of degradation machinery (134) and therefore create a potential point of retroactivity. This problem could be overcome by engineered orthogonal degradation systems (135), though this work is still at early stages. Unlike proteins, RNA is naturally labile, and RNA-based circuits should therefore compute solutions more quickly. In vivo rates of RNA circuits have not been measured, but in vitro work suggests they could be as low as 5 minutes for each computational step (136).
Robustness to environmental variability
A smart probiotic will experience a highly variable environment as its traverses the host from the mouth through the gut, but its circuits must function consistently to diagnose and treat disease reliably (137, 138). Growth rate is likely to change depending on factors such as gut location and variations in diet. These growth rate changes can dramatically alter circuit performance, particularly with stable proteins whose steady-state levels are linked to protein dilution. This challenge can be addressed by integrating feedback mechanisms such as negative autoregulation into computational nodes to compensate for growth effects (139, 140). Fluctuations in circuit performance appear particularly problematic for recombinase-based circuits because most implement irreversible computations. Temporary environments during administration or transit through the gut could prematurely result in permanent computation, giving false results. This may be addressable with reversible recombination (129), but this technique has not been explored in detail.
Long-term functionality
In some cases, it may be beneficial for smart probiotic circuits to function for long periods of time in the gut. To accomplish this goal, colonizing smart probiotics need to compete with the native microbiota while maintaining the ability to compute new treatments in response to alterations in disease state. If an engineered genetic circuit decreases the growth rate of the smart probiotic chassis, it could cripple the ability of the engineered organism to compete with the native gut microbiota. Such toxicity can occur with virtually all engineered genetic systems and has been reported in CRISPRi (118), TetR homolog (104, 115), and recombinase (62) circuits. Additionally, irreversible recombinase computation is particularly ill suited for long-term computation since it can only compute a response once and cannot respond to a changing environment. The only long-term study of computational circuit persistence showed that the genomic λ-derived toggle switch constructed using protein-based TFs (Fig. 3F) is capable of surviving for 6 months in an antibiotic pretreated mouse gut without losing functionality, highlighting the stability of this design strategy (60).
Logic circuit outlook
Given current technologies, we believe that the most promising method for engineering genetic logic into smart probiotics is to use TetR-based TFs (Fig. 5B). Benefits include the availability of a large library of parts and the ability to design complex circuits in an automated fashion. Additionally, there are no significant drawbacks such as the toxicity associated with CRISPRi circuits since nontoxic TetR homologs have been identified. Furthermore, TetR-based circuits are reversible (albeit slow), unlike most recombinase-based systems.
An interesting unexplored alternative is to replicate the computation observed in posttranslational networks such as phosphorelays (141). Bacteria have been shown to contain multistep histidine kinase phosphorelays which can integrate signals from different sensors with AND logic (142). The protein components of these phosphorelays have evolved in a highly modular nature (53), suggesting that they may be generally engineerable (143, 144). Such a network would provide fast posttranslational computation that may also be robust to changes in protein levels resulting from the complex gut environment (107).
Memory Circuits
For diagnostic gut bacteria to report the state of diseases within the gut after passage in feces, they require the ability to remember previous exposure to disease biomarkers. The two current approaches used to form memory in the gut are TF-based toggle switches (56, 60) and recombinases (51) (Fig. 5C).
Toggle switches
In a foundational paper in the field of synthetic biology, Collins and colleagues engineered a genetic toggle switch composed of two mutually repressing TFs, each of which prevented the other from being transcribed. They demonstrated that this circuit can remember exposure to different environmental signals even after the signals are removed (145, 146). This work was inspired by the λ cI/cro toggle switch that controls the bacteriophage’s decision to stay in a lysogenic state or to switch to a lytic state after transduction of its host (147). The previously discussed aTc-sensing (Fig. 3E) (56) and tetrathionate-sensing (60) diagnostic gut bacteria utilized the λ toggle switch “as-is” to implement memory within the gut.
Recombinases
Recent work has focused on the use of recombinases as a form of memory (51, 62, 129), in addition to their capability as logic circuit components. Recombinases have been used to record the presence of lactose (148) and rhamnose (51) within the gut. However, in both cases large amounts of background activation of the system were observed, limiting the time window in which there were identifiable differences between treatment and control mice (Fig. 3H) (51, 148). This may result from the fact that recording of the memory is irreversible, causing low levels of noisy recombination that accumulate false-positive outcomes over time. To address the irreversibility of recombination, Endy and colleagues expressed an excisionase, which reverses the recombination event, restoring DNA to its original sequence. After exhaustive tuning of expression levels, they created a strain which was able to toggle its memory between exposure to two different inducers for over seven cycles of induction (129).
Memory circuits outlook
The toggle switch has demonstrated clear superiority in its ability to accurately record a memory for a long period of time in the gut. However, if future diagnostic gut bacteria require memory of many different signals, the large number of available recombinase modules (62) may make them an attractive alternative. Additionally, future diagnosis may seek to report many different levels of exposure compared to the binary nature of the previously discussed memory elements. An interesting alternative is the use of retrons, which perform 6-bp modifications of DNA sequences at a much lower rate than serine recombinases. This has enabled continuous integration of both signal amplitude and duration over many days at a population level (149) and may be useful for diagnosis of diseases with highly dynamic biomarkers.
Genetic Actuators: Reporters
A range of reporter genes allow for measurement of sensor or genetic circuit outputs (Fig. 5D).
Colorimetric reporters
Colorimetric reporters have a long history of use in biotechnology, most prominently through the enzymatic activity of the lacZ gene on X-gal, producing a blue pigment (150), which has been employed in diagnostic gut bacteria (Fig. 3E) (56). Colorimetric assays are an attractive reporter system since they are low cost and do not require special instrumentation. When applied in a clinical setting, fecal coloration could enable simple in-home readouts of the state of engineered bacteria exiting the gut, but this approach may be limited by high background fecal pigmentation.
Luminescent reporters
Luminescent reporter genes, such as bacterial luciferase, typically yield increased sensitivity over colorimetric reporters due to very low background luminescence in fecal samples (151). Luminescence requires the addition of a luciferin substrate or its metabolic production by the bacteria themselves. Numerous luciferases are available, and each has different brightness, luciferin requirements, and output wavelengths. All luciferases require a luminometer instrument for measurement, making them less convenient than colorimetric reporters. However, high signal-to-background makes luminescent reporters desirable for clinical diagnostic gut bacteria.
Fluorescent reporters
Fluorescent measurement of bacteria from the gut allows single cell data to be collected from bacterial populations via flow cytometry (55, 65) or microscopy (52). Such analyses can be valuable in the research setting to detect more complex population-level changes such as bimodality, which are likely to occur in the complex gut environment. Single cell measurements can also be critical for troubleshooting engineered circuit performance (115). However, fluorescent reporter genes are less suited to clinical applications due to large expensive instruments and processing requirements, including a postgut aerobic incubation to allow for the oxygen-dependent maturation of the fluorophores (55).
Nucleic acid reporters
Direct sequencing of nucleic acid changes resulting from engineered bacterial recombination (51) may be the most promising diagnostic modality. A unique challenge of characterization of bacteria in the gut is the extreme cost of a single replicate compared to test tube experiments. Sequencing of barcoded DNA allows for measurement of entire libraries of bacteria in a single heterogeneous culture (152). This approach could be applied to single mice to test thousands of candidate synthetic probiotic sensors in parallel. Similarly, it would allow for the clinical use of many different probiotic diagnostics, enabling multiplexing of diagnostic gut bacteria. This approach has higher technical and infrastructure costs than other reporter methods, but these are likely to decrease in the future, making this a very promising avenue of investigation.
EXPERIMENTAL VALIDATION
Testing engineered gut bacteria in animal models or human trials is slow and expensive. Thus, it is prudent to validate and debug smart probiotic prototypes in a series of increasingly complex in vitro environments before final in vivo environments.
Defined Media
Defined media are useful for testing well-defined hypotheses on the performance of synthetic sensing, computing, or responding components. Questions about specific components may include what compounds a sensor binds or what levels of actuator can be produced. Broader system-wide questions include the following: Does expression of the synthetic system affect growth rates of the chassis (i.e., toxicity)? How do the expression levels of system proteins affect probiotic performance? and How does the growth rate of the chassis affect circuit performance? The high throughput and low cost of defined media allow for in-depth interrogation of these parameters. In addition, their relevance to more complex models may be improved by the development of standard defined media which replicate the gut environment through the inclusion of more complex carbohydrate and protein sources, as well as the use of anaerobic chambers.
Bioreactors
The effect of the microbiome can be incorporated by using in vitro bioreactors which utilize defined media seeded with murine or human microbiome samples (137, 153). The presence of a microbiome allows for testing of interactions between the synthetic probiotic constituents or by-products of the microbiota. This involves sensing by-products of the microbiome or affecting the constituents of the microbiome. Additionally, it provides a competitive environment where basic stability questions of the synthetic constructs can be preliminarily investigated. There exists a range of microbiome bioreactors which may be suited for different chassis organisms. It will be important to standardize the use of particular models and verify that they reflect in vivo performance for specific chassis organisms by comparing parameters such as growth rate and protein production levels.
Mouse Models
As with other therapeutics, mouse models are the most effective method for testing the performance of synthetic probiotics at diagnosing and treating disease. Probiotic bacteria can be integrated into existing murine disease models to demonstrate either sensing of relevant compounds (55, 60) or alleviation of disease markers (15). In addition to testing direct interaction of the synthetic system with the host, this can demonstrate the effect of the complex gut environment on the synthetic system (51, 55, 56, 60). Additionally, it can allow for investigation of differential effects due to different environments spatially throughout the gut.
A range of microbiome models are used in mouse studies, including native, antibiotic-treated, gnotobiotic, and exogenous microbiomes. Many strains of bacteria, including most E. coli used in engineered gut bacteria, have difficulty competing against the native microbiota, so antibiotic treatment is used to facilitate colonization with antibiotic-resistant engineered gut bacteria. Likewise, gnotobiotic mice, which are raised in germfree conditions and do not contain a gut microbiome, are easily colonized. However, large-scale perturbations of the gut environment may confound the study of gut-related diseases. For example, the commonly used colitis model, DSS treatment of mice, has been observed to be ineffective when conducted in the absence of a microbiome (154). Gnotobiotic mice can also be colonized with an exogenous microbiome either in the form of a defined consortium (155) or transplanted from human microbiome samples. The benefits and drawbacks of each of these models in testing engineered gut bacteria are largely unknown, making the best course of action replicating the methods traditionally used in the disease model which is being studied to allow comparison of the treatment effectiveness to more traditional therapeutic methods. Finally, pigs, whose gastrointestinal tract closely resembles that of humans, have been used to test the performance and biosafety of engineered probiotics prior to proceeding to human trials (16).
OUTLOOK
The application of tools developed by synthetic biologists to engineered gut bacteria will result in a step change in their efficacy and complexity. The ad hoc genetic implementations of drug factory probiotics over the past 2 decades will be improved through the use of well-characterized, high-performance expression systems in a wide variety of chassis organisms. This will enable higher expression of therapeutic compounds in more gut-relevant probiotic organisms, increasing their therapeutic efficacy.
Initial diagnostic gut bacteria (55, 60) will be improved upon by discovering more disease-relevant sensors and by studying their localized activation in vivo. New sensors can be generated using bioinformatic mining of microbiome data sets coupled with high-throughput library screening in mouse models using nucleotide-based reporters. Study of the biogeographic activation of disease-relevant sensors will determine the specifics of disease activity. In addition to their clinical applications, diagnostic gut bacteria will serve as scientific tools to locally measure specific metabolites within gut models.
These advances in engineering gut bacteria will enable the creation of the first smart probiotics capable of sensing and treating human disease within the gut. The engineering design-build-test cycle will allow for continual integration of knowledge gained from the study of host-microbiome interactions, natural probiotics, and synthetic biology to iteratively build upon existing smart probiotics. This will result in the bacteria being capable of sensing multiple disease states and localized environmental signals, computing a desired course of treatment, and administering this treatment from within the human gut. Localized diagnosis and treatment will make smart bacteria more effective and safer therapeutics.
DEFINITIONS
Actuator: A molecule which changes the bacterial cell state or the state of its environment, such as metabolic enzymes or secreted signaling proteins.
Biological output: A bacterially produced molecule such as an RNA, transcription factor, or therapeutic molecule.
Cellular memory: The ability of a cell to maintain a response to a sensed input after removal of that input.
Chassis: A bacterial species used to host a synthetic DNA to create an engineered gut bacterium.
Chemical or physical inputs: The signals that an engineered bacterium detects to compute an appropriate response. Examples include traditional inducers, disease biomarkers, and quorum-signaling molecules.
Colonization: The ability of bacterial species to survive in an environment for an extended period of time without repeated oral administration.
Device: A biological system composed of one or more components which performs a specific well-defined task with inputs and outputs; this can include sensors, genetic circuits, or actuators.
Diagnostic gut bacteria: Bacteria that sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and produce a reporter which can be externally measured by a clinician.
Drug factory probiotic: Bacteria engineered to constitutively produce a therapeutic molecule within the body.
Genetic circuit: A network of interacting regulatory molecules, such as transcription factors and their target promoters, that perform computations such as multi-input logic or memory to convert a chemical or physical input into a biological output.
One-component system (OCS): Cytoplasmic transcription factors that directly interact with and are allosterically modulated by chemical and physical inputs.
Retroactivity: The effect of downstream circuit component activity on the performance of upstream circuit components.
Sensor: A genetically encoded molecule, often an RNA or protein, that converts a chemical or physical input into a change in a biological signal such as kinase activity or transcription rate.
Smart probiotic: Bacteria that sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and respond by delivering a precise dose of one or more appropriate therapeutics at the diseased tissue.
Two-component system (TCS): A bacterial sensing network composed of a membrane-bound or cytoplasmic sensor histidine kinase which senses a chemical or physical input and regulates a cytoplasmic response regulator that controls promoter activity.
ACKNOWLEDGMENTS
B.L. is supported by the Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. Smart probiotic research in the Tabor laboratory is supported by the Office of Naval Research Young Investigator (N00014-14-1-0487) and NSF CAREER (1553317) programs and the Welch Foundation (C-1856).
Contributor Information
Brian P. Landry, Department of Bioengineering, Rice University, Houston, TX 77030
Jeffrey J. Tabor, Department of Bioengineering, Rice University, Houston, TX 77030 Department of Biosciences, Rice University, Houston, TX 77030.
Robert Allen Britton, Baylor College of Medicine, Houston, TX 77030.
Patrice D. Cani, Université catholique de Louvain, Louvain Drug Research Institute, Brussels 1200, Belgium
REFERENCES
- 1.Hosseinidoust Z, Mostaghaci B, Yasa O, Park B-W, Singh AV, Sitti M. 2016. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev 106(Pt A):27–44 10.1016/j.addr.2016.09.007 [PubMed]. [DOI] [PubMed] [Google Scholar]
- 2.Chien T, Doshi A, Danino T. 2017. Advances in bacteria cancer therapies using synthetic biology. Curr Opin Syst Biol 5:1–8 10.1016/j.coisb.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheth RU, Cabral V, Chen SP, Wang HH. 2016. Manipulating bacterial communities by in situ microbiome engineering. Trends Genet 32:189–200 10.1016/j.tig.2016.01.005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimee M, Citorik RJ, Lu TK. 2016. Microbiome therapeutics: advances and challenges. Adv Drug Deliv Rev 105(Pt A):44–54 10.1016/j.addr.2016.04.032. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2006. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. Food and Agriculture Organization of the UN, London, Ontario, Canada. http://www.fao.org/food/food-safety-quality/a-z-index/probiotics/en/
- 6.Wang H, Lee I-S, Braun C, Enck P. 2016. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil 22:589–605 10.5056/jnm16018. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HH, Cho Y-S. 2016. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc 49:257–265 10.5946/ce.2015.117. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron PA, Kleerebezem M, Brummer R-J, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM. 2017. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117:93–107 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. 2012. Probiotic mechanisms of action. Ann Nutr Metab 61:160–174 10.1159/000342079. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Sarkar A, Mandal S. 2016. Bifidobacteria: insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res 192:159–171 10.1016/j.micres.2016.07.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Rogers NJ, Mousa SA. 2012. The shortcomings of clinical trials assessing the efficacy of probiotics in irritable bowel syndrome. J Altern Complement Med 18:112–119 10.1089/acm.2011.0015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Holmes E, Kinross J, Gibson GR, Burcelin R, Jia W, Pettersson S, Nicholson JK. 2012. Therapeutic modulation of microbiota-host metabolic interactions. Sci Transl Med 4:137rv6 10.1126/scitranslmed.3004244. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Claesen J, Fischbach MA. 2015. Synthetic microbes as drug delivery systems. ACS Synth Biol 4:358–364 10.1021/sb500258b. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brophy JAN, Voigt CA. 2014. Principles of genetic circuit design. Nat Methods 11:508–520 10.1038/nmeth.2926. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352–1355. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. 2003. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol 21:785–789 10.1038/nbt840. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJH, Neirynck S, Peppelenbosch MP, Steidler L. 2006. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol 4:754–759 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Bermúdez-Humarán LG, Aubry C, Motta J-PP, Deraison C, Steidler L, Vergnolle N, Chatel J-MM, Langella P. 2013. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol 16:278–283 10.1016/j.mib.2013.06.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E, Cuvelier C, Rottiers P. 2010. Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol 3:49–56 10.1038/mi.2009.116. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Intrexon Corporation. 2016. ActoBiotics® platform: a novel class of oral biotherapeutics. https://www.dna.com/.
- 21.Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. 2004. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 127:502–513 10.1053/j.gastro.2004.05.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van Swam II, Kleerebezem M, Salvador-Cartier C, Hisbergues M, Bueno L, Theodorou V, Fioramonti J. 2006. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis 12:1044–1052 10.1097/01.mib.0000235101.09231.9e. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Rochat T, Bermúdez-Humarán L, Gratadoux J-J, Fourage C, Hoebler C, Corthier G, Langella P. 2007. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact 6:22 10.1186/1475-2859-6-22. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll IM, Andrus JM, Bruno-Bárcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. 2007. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 293:G729–G738 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 25.Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, Simonet M, Daniel C. 2007. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology 133:862–874 10.1053/j.gastro.2007.06.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefèvre F, Gratadoux JJ, Honvo-Hueto E, Chilmonczyk S, Blugeon S, Corthier G, Langella P, Bermúdez-Humarán LG. 2010. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol 144:35–41 10.1016/j.ijfoodmicro.2010.03.037. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc JG, del Carmen S, Miyoshi A, Azevedo V, Sesma F, Langella P, Bermúdez-Humarán LG, Watterlot L, Perdigon G, de Moreno de LeBlanc A. 2011. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J Biotechnol 151:287–293 10.1016/j.jbiotec.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Motta J-P, Bermúdez-Humarán LG, Deraison C, Martin L, Rolland C, Rousset P, Boue J, Dietrich G, Chapman K, Kharrat P, Vinel J-P, Alric L, Mas E, Sallenave J-M, Langella P, Vergnolle N. 2012. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 4:158ra144 10.1126/scitranslmed.3004212. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, Galleri L, Spagnuolo I, Steidler L, Van Huynegem K, Demetter P, Wasserfall C, Atkinson MA, Dotta F, Rottiers P, Gysemans C, Mathieu C. 2012. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122:1717–1725 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D, McGuinness OP, Niswender KD, Davies SS. 2014. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest 124:3391–3406 10.1172/JCI72517. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, Van Huynegem K, Steidler L, Caluwaerts S, Demetter P, Wasserfall CH, Atkinson MA, Dotta F, Rottiers P, Van Belle TL, Mathieu C. 2014. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 63:2876–2887 10.2337/db13-1236. [DOI] [PubMed] [Google Scholar]
- 32.Duan FF, Liu JH, March JC. 2015. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 64:1794–1803 10.2337/db14-0635. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosberg-Cody E, Stanton C, O’Mahony L, Wall R, Shanahan F, Quigley EM, Fitzgerald GF, Ross RP. 2011. Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology 157:609–615 10.1099/mic.0.043406-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Chen H-L, Lai Y-W, Chen C-S, Chu T-W, Lin W, Yen C-C, Lin M-F, Tu M-Y, Chen C-M. 2010. Probiotic Lactobacillus casei expressing human lactoferrin elevates antibacterial activity in the gastrointestinal tract. Biometals 23:543–554 10.1007/s10534-010-9298-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Paton AW, Jennings MP, Morona R, Wang H, Focareta A, Roddam LF, Paton JC. 2005. Recombinant probiotics for treatment and prevention of enterotoxigenic Escherichia coli diarrhea. Gastroenterology 128:1219–1228 10.1053/j.gastro.2005.01.050. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Koo OK, Amalaradjou MAR, Bhunia AK. 2012. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS One 7:e29277 10.1371/journal.pone.0029277. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Focareta A, Paton JC, Morona R, Cook J, Paton AW. 2006. A recombinant probiotic for treatment and prevention of cholera. Gastroenterology 130:1688–1695 10.1053/j.gastro.2006.02.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Duan F, March JC. 2010. Engineered bacterial communication prevents Vibrio cholerae virulence in an infant mouse model. Proc Natl Acad Sci USA 107:11260–11264 10.1073/pnas.1001294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordley RM, Bugaj LJ, Lim WA. 2016. Modular engineering of cellular signaling proteins and networks. Curr Opin Struct Biol 39:106–114 10.1016/j.sbi.2016.06.012. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA. 2016. Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol 14:135–149 10.1038/nrmicro.2015.24. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobrin A, Saxena P, Fussenegger M. 2016. Synthetic biology: applying biological circuits beyond novel therapies. Integr Biol 8:409–430 10.1039/C5IB00263J. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Tabor JJ, Groban ES, Voigt CA. 2009. Performance characteristics for sensors and circuits used to program E. coli, p 401–439. In Lee SY (ed), Systems Biology and Biotechnology of Escherichia coli. Springer, Dordrecht, The Netherlands. 10.1007/978-1-4020-9394-4_19 [DOI] [Google Scholar]
- 43.Olson EJ, Hartsough LA, Landry BP, Shroff R, Tabor JJ. 2014. Characterizing bacterial gene circuit dynamics with optically programmed gene expression signals. Nat Methods 11:449–455 10.1038/nmeth.2884. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Castillo-Hair SM, Igoshin OA, Tabor JJ. 2015. How to train your microbe: methods for dynamically characterizing gene networks. Curr Opin Microbiol 24:113–123 10.1016/j.mib.2015.01.008. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrich LE, Koonin EV, Zhulin IB. 2005. One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 13:52–56 10.1016/j.tim.2004.12.006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S, Bram EE, Weiss R. 2013. Genetically programmable pathogen sense and destroy. ACS Synth Biol 2:715–723 10.1021/sb4000417. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Saeidi N, Wong CK, Lo T-MT-M, Nguyen HX, Ling H, Leong SSJ, Poh CL, Chang MW. 2011. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol 7:521 10.1038/msb.2011.55. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Archer EJ, Robinson AB, Süel GM. 2012. Engineered E. coli that detect and respond to gut inflammation through nitric oxide sensing. ACS Synth Biol 1:451–457 10.1021/sb3000595. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Drouault S, Anba J, Corthier G. 2002. Streptococcus thermophilus is able to produce a β-galactosidase active during its transit in the digestive tract of germ-free mice. Appl Environ Microbiol 68:938–941 10.1128/AEM.68.2.938-941.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamady ZZR, Scott N, Farrar MD, Lodge JPA, Holland KT, Whitehead T, Carding SR. 2010. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut 59:461–469 10.1136/gut.2008.176131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Mimee M, Tucker AC, Voigt CA, Lu TK. 2015. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst 1:62–71 10.1016/j.cels.2015.06.001. (Erratum, https://doi.org/10.1016/J.CELS.2016.03.007.) [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, Chervonsky AV. 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514:638–641 10.1038/nature13823. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao R, Stock AM. 2009. Biological insights from structures of two-component proteins. Annu Rev Microbiol 63:133–154 10.1146/annurev.micro.091208.073214. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galperin MY. 2010. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol 13:150–159 10.1016/j.mib.2010.01.005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daeffler KN-M, Galley JD, Sheth RU, Ortiz-Velez LC, Bibb CO, Shroyer NF, Britton RA, Tabor JJ. 2017. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol Syst Biol 13:923 10.15252/msb.20167416. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotula JW, Kerns SJ, Shaket LA, Siraj L, Collins JJ, Way JC, Silver PA. 2014. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc Natl Acad Sci USA 111:4838–4843 10.1073/pnas.1321321111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Bäumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429 10.1038/nature09415. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winter SE, Lopez CA, Bäumler AJ. 2013. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep 14:319–327 10.1038/embor.2013.27. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hensel M, Hinsley AP, Nikolaus T, Sawers G, Berks BC. 1999. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol Microbiol 32:275–287 10.1046/j.1365-2958.1999.01345.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, Kotula JW, Gerber GK, Way JC, Silver PA. 2017. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol 35:653–658 10.1038/nbt.3879. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siuti P, Yazbek J, Lu TK. 2013. Synthetic circuits integrating logic and memory in living cells. Nat Biotechnol 31:448–452 10.1038/nbt.2510. [PubMed] [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Nielsen AAK, Fernandez-Rodriguez J, McClune CJ, Laub MT, Lu TK, Voigt CA. 2014. Permanent genetic memory with >1-byte capacity. Nat Methods 11:1261–1266 10.1038/nmeth.3147. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan F, Curtis KL, March JC. 2008. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol 74:7437–7438 10.1128/AEM.01019-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castagliuolo I, Beggiao E, Brun P, Barzon L, Goussard S, Manganelli R, Grillot-Courvalin C, Palù G. 2005. Engineered E. coli delivers therapeutic genes to the colonic mucosa. Gene Ther 12:1070–1078 10.1038/sj.gt.3302493. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Myhrvold C, Kotula JW, Hicks WM, Conway NJ, Silver PA. 2015. A distributed cell division counter reveals growth dynamics in the gut microbiota. Nat Commun 6:10039 10.1038/ncomms10039. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huttenhower C, et al, Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214 10.1038/nature11234. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huibregtse IL, Snoeck V, de Creus A, Braat H, De Jong EC, Van Deventer SJH, Rottiers P. 2007. Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin. Gastroenterology 133:517–528 10.1053/j.gastro.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 68.Huibregtse IL, Marietta EV, Rashtak S, Koning F, Rottiers P, David CS, van Deventer SJH, Murray JA. 2009. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J Immunol 183:2390–2396 10.4049/jimmunol.0802891. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caluwaerts S, Vandenbroucke K, Steidler L, Neirynck S, Vanhoenacker P, Corveleyn S, Watkins B, Sonis S, Coulie B, Rottiers P. 2010. AG013, a mouth rinse formulation of Lactococcus lactis secreting human trefoil factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol 46:564–570 10.1016/j.oraloncology.2010.04.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 70.Pontes DS, de Azevedo MSP, Chatel J-M, Langella P, Azevedo V, Miyoshi A. 2011. Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems. Protein Expr Purif 79:165–175 10.1016/j.pep.2011.06.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Conrad K, Roggenbuck D, Laass MW. 2014. Diagnosis and classification of ulcerative colitis. Autoimmun Rev 13:463–466 10.1016/j.autrev.2014.01.028. [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Donaldson GP, Lee SM, Mazmanian SK. 2016. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14:20–32 10.1038/nrmicro3552. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schultz M, Watzl S, Oelschlaeger TA, Rath HC, Göttl C, Lehn N, Schölmerich J, Linde HJ. 2005. Green fluorescent protein for detection of the probiotic microorganism Escherichia coli strain Nissle 1917 (EcN) in vivo. J Microbiol Methods 61:389–398 10.1016/j.mimet.2005.01.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Spees AM, Wangdi T, Lopez CA, Kingsbury DD, Xavier MN, Winter SE, Tsolis RM, Bäumler AJ. 2013. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. MBio 4:e00430-13 10.1128/mBio.00430-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamada N, Chen GY, Inohara N, Núñez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690 10.1038/ni.2608. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shetty RP, Endy D, Knight TF Jr. 2008. Engineering BioBrick vectors from BioBrick parts. J Biol Eng 2:5 10.1186/1754-1611-2-5. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.St-Pierre F, Cui L, Priest DG, Endy D, Dodd IB, Shearwin KE. 2013. One-step cloning and chromosomal integration of DNA. ACS Synth Biol 2:537–541 10.1021/sb400021j. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Kelly JR, Rubin AJ, Davis JH, Ajo-Franklin CM, Cumbers J, Czar MJ, de Mora K, Glieberman AL, Monie DD, Endy D. 2009. Measuring the activity of BioBrick promoters using an in vivo reference standard. J Biol Eng 3:4 10.1186/1754-1611-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mutalik VK, Guimaraes JC, Cambray G, Lam C, Christoffersen MJ, Mai Q-A, Tran AB, Paull M, Keasling JD, Arkin AP, Endy D. 2013. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat Methods 10:354–360 10.1038/nmeth.2404. [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Lutz R, Bujard H. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25:1203–1210 10.1093/nar/25.6.1203. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cox RS III, Surette MG, Elowitz MB. 2007. Programming gene expression with combinatorial promoters. Mol Syst Biol 3:145 10.1038/msb4100187. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y-J, Liu P, Nielsen AAK, Brophy JAN, Clancy K, Peterson T, Voigt CA. 2013. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Methods 10:659–664 10.1038/nmeth.2515. [DOI] [PubMed] [Google Scholar]
- 83.Cambray G, Guimaraes JC, Mutalik VK, Lam C, Mai Q-A, Thimmaiah T, Carothers JM, Arkin AP, Endy D. 2013. Measurement and modeling of intrinsic transcription terminators. Nucleic Acids Res 41:5139–5148 10.1093/nar/gkt163. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salis HM, Mirsky EA, Voigt CA. 2009. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950 10.1038/nbt.1568. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Espah Borujeni A, Channarasappa AS, Salis HM. 2014. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res 42:2646–2659 10.1093/nar/gkt1139. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farasat I, Kushwaha M, Collens J, Easterbrook M, Guido M, Salis HM. 2014. Efficient search, mapping, and optimization of multi-protein genetic systems in diverse bacteria. Mol Syst Biol 10:731 10.15252/msb.20134955. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoon SH, Kim SK, Kim JF. 2010. Secretory production of recombinant proteins in Escherichia coli. Recent Pat Biotechnol 4:23–29 10.2174/187220810790069550. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Andersen JB, Sternberg C, Poulsen LK, Bjørn SP, Givskov M, Molin S. 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 64:2240–2246. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, Shoemaker NB, Wang GR, Salyers AA. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J Bacteriol 182:3559–3571 10.1128/JB.182.12.3559-3571.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bosma EF, Forster J, Nielsen AT. 2017. Lactobacilli and pediococci as versatile cell factories: evaluation of strain properties and genetic tools. Biotechnol Adv 35:419–442 10.1016/j.biotechadv.2017.04.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717. [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Karlskås IL, Maudal K, Axelsson L, Rud I, Eijsink VGH, Mathiesen G. 2014. Heterologous protein secretion in lactobacilli with modified pSIP vectors. PLoS One 9:e91125 10.1371/journal.pone.0091125. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heiss S, Hörmann A, Tauer C, Sonnleitner M, Egger E, Grabherr R, Heinl S. 2016. Evaluation of novel inducible promoter/repressor systems for recombinant protein expression in Lactobacillus plantarum. Microb Cell Fact 15:50 10.1186/s12934-016-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rud I, Jensen PR, Naterstad K, Axelsson L. 2006. A synthetic promoter library for constitutive gene expression in Lactobacillus plantarum. Microbiology 152:1011–1019 10.1099/mic.0.28599-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.van Pijkeren JP, Britton RA. 2014. Precision genome engineering in lactic acid bacteria. Microb Cell Fact 13(Suppl 1):S10 10.1186/1475-2859-13-S1-S10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oh JH, van Pijkeren JP. 2014. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res 42:e131 10.1093/nar/gku623. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan LY, Kosuri S, Endy D. 2005. Refactoring bacteriophage T7. Mol Syst Biol 1:2005.0018 10.1038/msb4100025. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Temme K, Zhao D, Voigt CA. 2012. Refactoring the nitrogen fixation gene cluster from Klebsiella oxytoca. Proc Natl Acad Sci USA 109:7085–7090 10.1073/pnas.1120788109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou H, Vonk B, Roubos JA, Bovenberg RAL, Voigt CA. 2015. Algorithmic co-optimization of genetic constructs and growth conditions: application to 6-ACA, a potential nylon-6 precursor. Nucleic Acids Res 43:10560–10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burén S, Young EM, Sweeny EA, Lopez-Torrejón G, Veldhuizen M, Voigt CA, Rubio LM. 2017. Formation of nitrogenase NifDK tetramers in the mitochondria of Saccharomyces cerevisiae. ACS Synth Biol 6:1043–1055 10.1021/acssynbio.6b00371. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tabor JJ, Levskaya A, Voigt CA. 2011. Multichromatic control of gene expression in Escherichia coli. J Mol Biol 405:315–324 10.1016/j.jmb.2010.10.038. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramakrishnan P, Tabor JJ. 2016. Repurposing synechocystis PCC6803 UirS-UirR as a UV-violet/green photoreversible transcriptional regulatory tool in E. coli. ACS Synth Biol 5:733–740 10.1021/acssynbio.6b00068. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Schmidl SR, Sheth RU, Wu A, Tabor JJ. 2014. Refactoring and optimization of light-switchable Escherichia coli two-component systems. ACS Synth Biol 3:820–831 10.1021/sb500273n. [PubMed] [DOI] [PubMed] [Google Scholar]
- 104.Stanton BC, Nielsen AAK, Tamsir A, Clancy K, Peterson T, Voigt CA. 2014. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat Chem Biol 10:99–105 10.1038/nchembio.1411. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stanton BC, Siciliano V, Ghodasara A, Wroblewska L, Clancy K, Trefzer AC, Chesnut JD, Weiss R, Voigt CA. 2014. Systematic transfer of prokaryotic sensors and circuits to mammalian cells. ACS Synth Biol 3:880–891 10.1021/sb5002856. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beal J. 2015. Signal-to-noise ratio measures efficacy of biological computing devices and circuits. Front Bioeng Biotechnol 3:93 10.3389/fbioe.2015.00093. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinar G, Milo R, Martínez MR, Alon U. 2007. Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci USA 104:19931–19935 10.1073/pnas.0706792104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubens JR, Selvaggio G, Lu TK. 2016. Synthetic mixed-signal computation in living cells. Nat Commun 7:11658 10.1038/ncomms11658. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daniel R, Rubens JR, Sarpeshkar R, Lu TK. 2013. Synthetic analog computation in living cells. Nature 497:619–623 10.1038/nature12148. [PubMed] [DOI] [PubMed] [Google Scholar]
- 110.Rockwell NC, Martin SS, Lagarias JC. 2012. Red/green cyanobacteriochromes: sensors of color and power. Biochemistry 51:9667–9677 10.1021/bi3013565. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Rockwell NC, Martin SS, Lagarias JC. 2016. Identification of cyanobacteriochromes detecting far-red light. Biochemistry 55:3907–3919 10.1021/acs.biochem.6b00299. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.Hermsen R, Erickson DW, Hwa T. 2011. Speed, sensitivity, and bistability in auto-activating signaling circuits. PLOS Comput Biol 7:e1002265 10.1371/journal.pcbi.1002265. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lundberg JO, Govoni M. 2004. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37:395–400 10.1016/j.freeradbiomed.2004.04.027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. 2013. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nielsen AAK, Der BS, Shin J, Vaidyanathan P, Paralanov V, Strychalski EA, Ross D, Densmore D, Voigt CA. 2016. Genetic circuit design automation. Science 352:aac7341. [PubMed] [DOI] [PubMed] [Google Scholar]
- 116.Lou C, Stanton B, Chen Y-J, Munsky B, Voigt CA. 2012. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. Nat Biotechnol 30:1137–1142 10.1038/nbt.2401. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183 10.1016/j.cell.2013.02.022. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nielsen AA, Voigt CA. 2014. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol Syst Biol 10:763 10.15252/msb.20145735. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gander MW, Vrana JD, Voje WE, Carothers JM, Klavins E. 2017. Digital logic circuits in yeast with CRISPR-dCas9 NOR gates. Nat Commun 8:15459 10.1038/ncomms15459. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Vecchio D. 2015. Modularity, context-dependence, and insulation in engineered biological circuits. Trends Biotechnol 33:111–119 10.1016/j.tibtech.2014.11.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Bradley RW, Buck M, Wang B. 2016. Recognizing and engineering digital-like logic gates and switches in gene regulatory networks. Curr Opin Microbiol 33:74–82 10.1016/j.mib.2016.07.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 122.Lucks JB, Qi L, Mutalik VK, Wang D, Arkin AP. 2011. Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc Natl Acad Sci USA 108:8617–8622 10.1073/pnas.1015741108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chappell J, Takahashi MK, Lucks JB. 2015. Creating small transcription activating RNAs. Nat Chem Biol 11:214–220 10.1038/nchembio.1737. [PubMed] [DOI] [PubMed] [Google Scholar]
- 124.Westbrook AM, Lucks JB. 2017. Achieving large dynamic range control of gene expression with a compact RNA transcription-translation regulator. Nucleic Acids Res 45:5614–5624 10.1093/nar/gkx215. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Argos P, et al. 1986. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J 5:433–440. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedland AE, Lu TK, Wang X, Shi D, Church G, Collins JJ. 2009. Synthetic gene networks that count. Science 324:1199–1202. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonnet J, Yin P, Ortiz ME, Subsoontorn P, Endy D. 2013. Amplifying genetic logic gates. Science 340:599–603. [PubMed] [DOI] [PubMed] [Google Scholar]
- 128.Roquet N, Soleimany AP, Ferris AC, Aaronson S, Lu TK. 2016. Synthetic recombinase-based state machines in living cells. Science 353:aad8559. [PubMed] [DOI] [PubMed] [Google Scholar]
- 129.Bonnet J, Subsoontorn P, Endy D. 2012. Rewritable digital data storage in live cells via engineered control of recombination directionality. Proc Natl Acad Sci USA 109:8884–8889 10.1073/pnas.1202344109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Daniel C, Poiret S, Dennin V, Boutillier D, Pot B. 2013. Bioluminescence imaging study of spatial and temporal persistence of Lactobacillus plantarum and Lactococcus lactis in living mice. Appl Environ Microbiol 79:1086–1094 10.1128/AEM.03221-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alon U. 2006. An Introduction to Systems Biology: Design Principles of Biological Circuits. CRC Press, Boca Raton, FL. [Google Scholar]
- 132.Moon TS, Lou C, Tamsir A, Stanton BC, Voigt CA. 2012. Genetic programs constructed from layered logic gates in single cells. Nature 491:249–253 10.1038/nature11516. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prindle A, Selimkhanov J, Li H, Razinkov I, Tsimring LS, Hasty J. 2014. Rapid and tunable post-translational coupling of genetic circuits. Nature 508:387–391 10.1038/nature13238. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cookson NA, Mather WH, Danino T, Mondragón-Palomino O, Williams RJ, Tsimring LS, Hasty J. 2011. Queueing up for enzymatic processing: correlated signaling through coupled degradation. Mol Syst Biol 7:561 10.1038/msb.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cameron DE, Collins JJ. 2014. Tunable protein degradation in bacteria. Nat Biotechnol 32:1276–1281 10.1038/nbt.3053. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi MK, Chappell J, Hayes CA, Sun ZZ, Kim J, Singhal V, Spring KJ, Al-Khabouri S, Fall CP, Noireaux V, Murray RM, Lucks JB. 2015. Rapidly characterizing the fast dynamics of RNA genetic circuitry with cell-free transcription-translation (TX-TL) systems. ACS Synth Biol 4:503–515 10.1021/sb400206c. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moser F, Broers NJ, Hartmans S, Tamsir A, Kerkman R, Roubos JA, Bovenberg R, Voigt CA. 2012. Genetic circuit performance under conditions relevant for industrial bioreactors. ACS Synth Biol 1:555–564 10.1021/sb3000832. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tropini C, Earle KA, Huang KC, Sonnenburg JL. 2017. The gut microbiome: connecting spatial organization to function. Cell Host Microbe 21:433–442 10.1016/j.chom.2017.03.010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klumpp S, Hwa T. 2014. Bacterial growth: global effects on gene expression, growth feedback and proteome partition. Curr Opin Biotechnol 28:96–102 10.1016/j.copbio.2014.01.001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shopera T, He L, Oyetunde T, Tang YJ, Moon TS. 2017. Decoupling resource-coupled gene expression in living cells. ACS Synth Biol 6:1596–1604 10.1021/acssynbio.7b00119. [PubMed] [DOI] [PubMed] [Google Scholar]
- 141.Salvado B, Vilaprinyo E, Sorribas A, Alves R. 2015. A survey of HK, HPt, and RR domains and their organization in two-component systems and phosphorelay proteins of organisms with fully sequenced genomes. PeerJ 3:e1183 10.7717/peerj.1183. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Long T, Tu KC, Wang Y, Mehta P, Ong NP, Bassler BL, Wingreen NS. 2009. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol 7:e1000068 10.1371/journal.pbio.1000068. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walthers D, Tran VK, Kenney LJ. 2003. Interdomain linkers of homologous response regulators determine their mechanism of action. J Bacteriol 185:317–324 10.1128/JB.185.1.317-324.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakajima M, Ferri S, Rögner M, Sode K. 2016. Construction of a miniaturized chromatic acclimation sensor from cyanobacteria with reversed response to a light signal. Sci Rep 6:37595 10.1038/srep37595. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gardner TS, Cantor CR, Collins JJ. 2000. Construction of a genetic toggle switch in Escherichia coli. Nature 403:339–342 10.1038/35002131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi H, Kaern M, Araki M, Chung K, Gardner TS, Cantor CR, Collins JJ. 2004. Programmable cells: interfacing natural and engineered gene networks. Proc Natl Acad Sci USA 101:8414–8419 10.1073/pnas.0402940101. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ptashne M. 2004. A Genetic Switch Phage Lambda Revisited. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 148.Junjua M, Galia W, Gaci N, Uriot O, Genay M, Bachmann H, Kleerebezem M, Dary A, Roussel Y. 2014. Development of the recombinase-based in vivo expression technology in Streptococcus thermophilus and validation using the lactose operon promoter. J Appl Microbiol 116:620–631 10.1111/jam.12376. [DOI] [PubMed] [Google Scholar]
- 149.Farzadfard F, Lu TK. 2014. Genomically encoded analog memory with precise in vivo DNA writing in living cell populations. Science 346:1256272. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Horwitz JP, Chua J, Curby RJ, Tomson AJ, Darooge MA, Fisher BE, Mauricio J, Klundt I. 1964. Substrates for cytochemical demonstration of enzyme activity. I. Some substituted 3-indolyl-β-D-glycopyranosides. J Med Chem 7:574–575 10.1021/jm00334a044. [PubMed] [DOI] [PubMed] [Google Scholar]
- 151.Corthier G, Delorme C, Ehrlich SD, Renault P. 1998. Use of luciferase genes as biosensors to study bacterial physiology in the digestive tract. Appl Environ Microbiol 64:2721–2722. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kosuri S, Goodman DB, Cambray G, Mutalik VK, Gao Y, Arkin AP, Endy D, Church GM. 2013. Composability of regulatory sequences controlling transcription and translation in Escherichia coli. Proc Natl Acad Sci USA 110:14024–14029 10.1073/pnas.1301301110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Auchtung JM, Robinson CD, Farrell K, Britton RA. 2016. Minibioreactor arrays (MBRAs) as a tool for studying C. difficile physiology in the presence of a complex community. Methods Mol Biol 1476:235–258 10.1007/978-1-4939-6361-4_18. [PubMed] [DOI] [PubMed] [Google Scholar]
- 154.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. 2014. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104:Unit 15.25. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bucci V, Tzen B, Li N, Simmons M, Tanoue T, Bogart E, Deng L, Yeliseyev V, Delaney ML, Liu Q, Olle B, Stein RR, Honda K, Bry L, Gerber GK. 2016. MDSINE: Microbial Dynamical Systems INference Engine for microbiome time-series analyses. Genome Biol 17:121 10.1186/s13059-016-0980-6. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Farrar MD, Whitehead TR, Lan J, Dilger P, Thorpe R, Holland KT, Carding SR. 2005. Engineering of the gut commensal bacterium Bacteroides ovatus to produce and secrete biologically active murine interleukin-2 in response to xylan. J Appl Microbiol 98:1191–1197 10.1111/j.1365-2672.2005.02565.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 157.Hamady ZZR, Scott N, Farrar MD, Wadhwa M, Dilger P, Whitehead TR, Thorpe R, Holland KT, Lodge JPA, Carding SR. 2011. Treatment of colitis with a commensal gut bacterium engineered to secrete human TGF-β1 under the control of dietary xylan 1. Inflamm Bowel Dis 17:1925–1935 10.1002/ibd.21565. [PubMed] [DOI] [PubMed] [Google Scholar]
- 158.Ng DTW, Sarkar CA. 2011. Nisin-inducible secretion of a biologically active single-chain insulin analog by Lactococcus lactis NZ9000. Biotechnol Bioeng 108:1987–1996 10.1002/bit.23130. [PubMed] [DOI] [PubMed] [Google Scholar]


