ABSTRACT
There is a clear association between the gastrointestinal (GI) microbiome and the development of chronic noncommunicable diseases, providing a rationale for the development of strategies that target the GI microbiota to improve human health. In this article, we discuss the potential of supplementing the human diet with nondigestible fermentable carbohydrates (NDFCs) to modulate the composition, structure, diversity, and metabolic potential of the GI microbiome in an attempt to prevent or treat human disease. The current concepts by which NDFCs can be administered to humans, including prebiotics, fermentable dietary fibers, and microbiota-accessible carbohydrates, as well as the mechanisms by which these carbohydrates exert their health benefits, are discussed. Epidemiological research presents compelling evidence for the health effects of NDFCs, with clinical studies providing further support for some of these benefits. However, rigorously designed human intervention studies with well-established clinical markers and microbial endpoints are still essential to establish (i) the clinical efficiency of specific NDFCs, (ii) the causal role of the GI microbiota in these effects, (iii) the underlying mechanisms involved, and (iv) the degree by which inter-individual differences between GI microbiomes influence these effects. Such studies would provide the mechanistic understanding needed for a systematic application of NDFCs to improve human health via GI microbiota modulation while also allowing the personalization of these dietary strategies.
The GI MICROBIOME AND CHRONIC NONCOMMUNICABLE DISEASES
Vertebrates have evolved with dense microbial populations in their gastrointestinal (GI) tract (referred to as the GI microbiome) that contribute to performance and health of the host (1). Although symbiotic in nature, animal experiments have established that the GI microbiota plays a causative role in the development of chronic noncommunicable diseases (CNCDs) such as obesity, diabetes, cardiovascular disease, colon cancer, autism, autoimmune diseases, allergies, and other atopic diseases including asthma (Fig. 1) (2). CNCDs are often associated with microbial dysbiosis, which is typically characterized by a reduced diversity, a bloom of facultative taxa (such as enterobacteria), and a lower output of beneficial metabolites (3). These associations provide a clear rationale for the development of strategies that modulate GI microbiome structure and function for the prevention of CNCDs (4).
FIGURE 1.
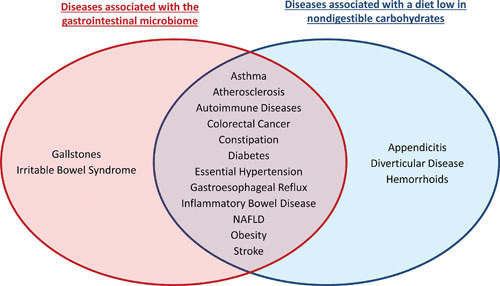
CNCDs that are associated with the GI microbiome and diets low in NDC. An industrialized lifestyle is associated with an increased prevalence of multiple CNCDs (15). Most of these diseases have now clearly been associated with the GI microbiome (pathology in animal models is dramatically different under germfree conditions, and the GI microbiome displays a dysbiosis in humans suffering from the disease). The Venn diagram designates CNCDs that are associated with the GI microbiome (2, 221, 247, 248) and a diet low in NDCs (13, 107, 244). NAFLD, nonalcoholic fatty liver disease.
Various strategies have been developed for the introduction of live microbes to modulate the GI ecosystem, through either probiotics, live biotherapeutics, or fecal transplants. These approaches have generated tremendous interest (5). However, diet has also been shown to readily alter GI microbiome structure and function (6–8), making it a particularly promising modifiable lifestyle factor of interest for the treatment of CNCDs. In addition, dietary supplements that employ nondigestible carbohydrates (NDCs) have been developed for several decades as prebiotics, targeted to support growth of beneficial GI microbiota in an attempt to improve health (9).
Interestingly, the incidence of most microbiota-associated CNCDs has substantially increased in recent decades in industrialized countries (10, 11), suggesting that practices associated with industrialized lifestyles predispose to disease. Although the exact factors that drive disease development are unknown and likely complex, the lack of NDCs is one contributing factor (12, 13). Humans likely evolved consuming more than 100 grams of NDCs daily, and nonindustrialized communities still exist today that have intakes that parallel those of our ancestors (14). In the 1970s, Denis Burkitt and colleagues compared industrialized (United States and United Kingdom) and nonindustrialized (rural Africa) populations and found strong epidemiological links between urbanization and a reduction of dietary NDC intake (13, 15). These lifestyle shifts were associated with increased prevalence of CNCDs (13, 15). Recent research demonstrated that a diet low in NDCs that are accessible to the GI microbiota (i.e., nondigestible fermentable carbohydrates [NDFCs]) resulted in reduced production of fermentation end products that provide important physiological and immunological functions to the host (16, 17). Moreover, a diet low in NDFCs has been implicated in the depletion of microbiome diversity observed in industrialized societies (18–22) and the loss of bacterial species that rely on them for growth (23). Therefore, a possible explanation for the rise in CNCDs is that by shifting away from a diet in which our human-microbiota interrelationship evolved, we have essentially disrupted this symbiosis, ultimately reducing or removing the evolutionary routed benefits provided by the microbes (12).
The connections described above provide a rationale for the application of NDFCs to modulate the composition and/or function of the GI microbiome to benefit host health. In this article, we discuss (i) the concepts by which the GI microbiome can be modulated through the intake of NDCs that are fermentable (or accessible to the microbes), (ii) the effects of these strategies on GI microbiota, (iii) the mechanisms by which these strategies promote health, and (iv) the future research needed to optimize these strategies for their use in the field of human nutrition. We will focus on strategies that allow the systematic increase of NDFCs in the human adult diet by means of supplements such as prebiotics, fermentable dietary fiber, and microbiota-accessible carbohydrates (MACs), and not through whole-food sources such as fruits and vegetables. Supplemental NDFCs could provide a promising avenue for targeted modulation of the GI microbiota once they are understood at a mechanistic level (24). They could also provide an applicable strategy for increasing NDFC consumption within the context of an industrialized lifestyle, since current whole-food-focused strategies encouraged by nutritional organizations have shown little success in increasing NDFC intake (25, 26). Furthermore, even though NDFCs such as human milk oligosaccharides are important for infant growth and development and have tremendous potential to be included in infant formula (27), we will focus this article on the application of NDFCs in weaned children and adults.
MODULATION OF THE GI MICROBIOME THROUGH NDFCs: THREE CONCEPTS
Prebiotics
Although the potential to modulate the human GI microbiome through NDFCs had been recognized decades earlier (especially in Japan), the term “prebiotics” was coined by Gibson and Roberfroid in 1995. A prebiotic was initially defined as a “nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon, and thus attempt to improve host health” (9). The definition has been adjusted various times (28), with the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus panel proposing the most recent definition: “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (29). Currently, the most commonly used definition is “a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” (30). Although most definitions do not restrict prebiotics to carbohydrates, currently only lactulose, inulin-type fructans, and trans-galactooligosaccharides are considered prebiotics (30). Polydextrose, glucooligosaccharides, lactosucrose, soybean oligosaccharides, and xylooligosaccharides have been further proposed as “candidate prebiotics” (30). However, the criteria that these carbohydrates would have to fulfill to move beyond the status of mere candidates have not been clearly established (28).
To date, most definitions, including the recent definition brought forward by the ISAPP consensus panel (29), require that prebiotics have to be “specific” for or “selective” toward health-promoting taxonomic groups. Conceptually, the idea is to shift the GI microbial community toward a more “healthy” state. This concept is derived from early findings from culture-based and later probe- and primer-based studies that found bifidobacteria and lactobacilli (putatively health-promoting organisms) to be selectively stimulated through prebiotics such as inulin and galactooligosaccharides (31). However, even though there is a strong rationale to specifically target the beneficial components and functional attributes of the GI microbiome, the prebiotic concept as it currently stands has been repeatedly criticized for being poorly defined (28, 32–34) and/or scientifically outdated (28, 35, 36). Some scientists have suggested revisions to the concept (36), while others consider it completely obsolete (5, 35). Criticism is primarily focused on the concept of selectivity and the question of how to identify beneficial microbiota that should be targeted (28, 36).
First, based on the most current scientific understanding, it is too simplistic to categorize GI microbes as either “good” or “bad.” Microbes can possess both beneficial and detrimental traits, strains of one species can differ widely in their attributes, and their role is highly context-dependent (e.g., host genetic predisposition, host physiology, GI microbial ecology, diet, etc.). Second, it has been argued that the specific targets should go beyond that of Bifidobacterium and Lactobacillus species, because many bacterial genera (Akkermansia, Eubacterium, and Fecalibacterium) have been linked to health benefits (37–40), including taxa previously considered to be detrimental, such as Clostridia and Bacteroides (41, 42). Third, given that the GI microbiota functions as a complex community, it may be imperative to support community characteristics such as diversity, stability, and ecosystem functionality (e.g., short-chain fatty acid [SCFA] output), which have all been positively correlated with health (43–45). To this end, the formation of SCFAs does not rely on selective fermentation. Moreover, although some strains of bifidobacteria and lactobacilli have been reported to produce butyrate and propionate (46), which are the SCFAs with the most evidence for health effects (17), from the metabolism of amino acids, the amounts produced (<150 μM) are less than 1% of what bacteria produce from the fermentation of carbohydrates (46, 47). Bifidobacteria and lactobacilli lack the biosynthetic pathways to produce butyrate and propionate from the fermentation of carbohydrates (48–51) and therefore cannot be the target of prebiotics that aim at boosting these SCFAs. Fourth, human studies using next-generation sequencing have shown that the response of the GI microbiome to NDFCs that are currently regarded as prebiotics, such as inulin, is not as selective as previously believed (52), while NDFCs that were considered to be broadly fermented result in restricted shifts of the GI microbiome (53–55). In this respect, it is important to consider that no carbohydrate is fermented solely by one or two species (partially because bacterial traits are shared between bacteria through horizontal gene transfer [56]), and no carbohydrate is broadly fermented, especially not under the competitive conditions within the GI tract (57). So the recent proposition by the ISAPP consensus panel that selectivity “could extend to several microbial groups, just not all” (29), would essentially mean that any carbohydrate would qualify as a prebiotic.
The notion that carbohydrates currently accepted as prebiotics are not utilized differently by the GI microbiota when compared to “regular” dietary fibers has recently been demonstrated using in vitro fecal fermentations of inulin (a well-described prebiotic) and pectin (not considered a prebiotic) (58). Both carbohydrates induced multiple substrate-specific compositional shifts. The fermentation of inulin resulted in the increased abundance of five taxa, while the fermentation of pectin resulted in the enrichment of seven different taxa. Nevertheless, both carbohydrates resulted in comparable amounts of SCFAs (58). This illustrates that both inulin and pectin can lead to a specific enrichment of different bacterial species among the GI microbiome, which would allow targeted modulation of GI microbiota. In spite of this, the rationale for why only one of them is considered a prebiotic is not obvious from these findings, especially considering that selectivity, according to the ISAPP consensus panel, “could extend to several microbial groups” (29). What constitutes a specific fermentation therefore remains ill-defined.
In this respect, it is important to point out that specificity of a prebiotic has, to date, been exclusively established only by the determination of compositional shifts. However, species that utilize a prebiotic might produce metabolites without becoming enriched. For example, Bacteroides numbers often decrease after the administration of prebiotics even if they are able to utilize them (59, 60), likely since their growth is negatively affected through a reduction in pH that results from the production of SCFAs (58, 61, 62). To truly establish whether a prebiotic is selectively fermented would require the use of techniques such as stable isotope probing (63) that allow for the identification of all microbes that are able to utilize the substrate within a complex microbial community, including those that do not become enriched. Finally, there are often substantially different inter-individual responses within the GI microbiome toward a prebiotic, yet this variation has not been considered in the concept at all (36).
Overall, we agree with recently published statements that the prebiotic concept is ill defined and based on outdated scientific views (5, 28, 32–35). To address these inconsistencies, Bindels and colleagues proposed updating the definition of a prebiotic to “a non-digestible compound that, through its metabolization by microorganisms in the GI tract, modulates composition and/or activity of the GI microbiota, thus conferring a beneficial physiological effect on the host” (28). By removing the requirement of specificity, this definition embraces the complexity of host-microbe metabolic interactions and focuses on ecological and functional features of the microbiota that are more likely to be relevant for host physiology, such as the production of SCFAs. Still, consensus on the definition of a prebiotic has not been reached (32), and some scientists prefer to abandon the term altogether (35). However, it is the opinion of the authors that the term remains helpful, as it has become well known in the scientific community and is recognized by regulators, industry, consumers, and health care professionals. Therefore, the prebiotic concept could remain valuable after the inconsistencies in the definition have been resolved.
Fermentable Dietary Fiber
Based on current definitions, most prebiotic carbohydrates are dietary fibers, but not all dietary fibers are considered to be prebiotics (64, 65). Nonetheless, a clear-cut distinction between prebiotics and nonprebiotic dietary fiber is not possible (33), and it is increasingly recognized that the fermentation of dietary fiber by the colonic microbiota contributes to human health.
The term “dietary fiber” was coined in 1953 by Eben Hipsley to describe the nondigestible components of the plant cell wall (66). Later, in the mid-1970s, Trowell and colleagues refined the definition to “remnants of plant cells resistant to hydrolysis by the alimentary enzymes of man, the group of substances that remain in the ileum but are partially hydrolyzed by bacteria in the colon” (67). Since then, the definition has undergone several revisions. The most widely used definition was put forth in 2009 by the Codex Alimentarius Commission, a joint principal branch of the Food and Agriculture Organization and the World Health Organization, which defines dietary fiber as “carbohydrate polymers with ten or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the human small intestine and belong to the following categories:
Edible carbohydrate polymers naturally occurring in the food as consumed,
Carbohydrate polymers which have been obtained from raw materials by physical, enzymatic, or chemical means and which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities, and
Synthetic carbohydrate polymers which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities” (68).
The decision to consider carbohydrates with three to nine monomeric units (oligosaccharides) as dietary fiber was left to individual country’s authorities (26). Recently, the Food and Drug Administration in the United States updated its food labeling regulations to model the Codex definition by requiring that “isolated” and “synthetic” NDCs added to foods must first demonstrate a beneficial physiological effect in humans prior to being permitted as a dietary fiber on a food label (69).
Dietary fibers can be found in plants, bacteria, and fungi and can be chemically synthesized (Fig. 2) (70, 71). Plant-derived nonstarch polysaccharides display a substantial variety of chemical structures due to the diverse functional roles that they play in plants (71). Dietary fibers also include starches that are resistant to human digestion (resistant starches [RSs]), which are divided into five subtypes based on the mechanism responsible for their inaccessibility to host digestion (72). Given the heterogenic chemical structures found in dietary fibers, their utilization within the GI tract often requires a diverse array of enzymes distributed among various microbial members. The extent by which different fiber types are utilized or fermented by the GI microbiota is therefore structure-dependent and relies on the metabolic capabilities of an individual’s microbiome, which ultimately determines the bacterial metabolites produced from the fermentation (24).
FIGURE 2.

Categories of NDCs. NDCs are a heterogeneous group of compounds that display diverse chemical structures, which is the basis for their categorization alongside their origin (24, 70, 71). Nondigestible oligosaccharides are NDCs composed of three to nine monosaccharides and are from either plant or animal origin, as well as chemically synthesized.
The chemical structure of a dietary fiber also determines other important physicochemical properties such as solubility and viscosity (73), which influence its accessibility to microbes. Most linear fibers form crystalline structures (such as cellulose), which significantly reduces their solubility in water. Meanwhile, dietary fibers with charged moieties or structural irregularities in the sugar backbone and side chains (such as β-glucan) tend to increase in solubility, which is often correlated with fermentability (74). However, one common exception is RSs, which are typically insoluble in water yet highly fermentable (33). Furthermore, most soluble dietary fibers tend to also be viscous in water (33), which relates to their ability to render a solution gelatinous via the absorption of water (74). Solubility and viscosity are important characteristics that influence the functionality of a dietary fiber, but for the purpose of modulating the GI microbiota, fermentability is of particular relevance.
“Dietary fiber” is a widely accepted and useful term to describe NDCs that influence health benefits. Most consumers recognize the term, because it is a required part of the nutrition facts label of processed food. However, to describe NDFCs that are intended to modulate the GI microbiome, the term has obvious limitations. Various mechanisms (e.g., reduced absorption through viscosity, bile acid binding, stool bulking, etc.) have been identified by which dietary fiber can benefit human health completely independently of their effects on the GI microbiome (73). In addition, as described above, not all dietary fibers are fermented by the microbiota. The definition of a prebiotic proposed by Bindels and colleagues would encompass dietary fibers for which there is evidence that they improve health via the GI microbiota (28), but as described above, there is no agreement on the definition of a prebiotic (32). What is clear is that the portion of dietary fiber that is fermentable or “accessible” by an individual’s microbiota is what determines its ability to modulate the GI microbiome. This notion is central to the concept of microbiota-accessible carbohydrates (MACs).
MACs
To address the limitations of the concepts discussed above, especially as they relate to the inter-individual differences of the human GI microbiome, Sonnenburg and Sonnenburg introduced the concept of MACs (16). MACs can be divided into dietary MACs (NDFCs such as prebiotics and dietary fiber) and host-derived MACs (such as mucosal glycans) (16). Determining whether an NDFC is considered a MAC is not entirely dependent on the NDFC’s physicochemical characteristics, because an individual’s GI microbiota must also have the enzymatic capacity to metabolize it (75, 76). For example, cellulose does not qualify as a MAC for humans, because the capacity of the human GI microbiota to ferment cellulose is extremely low (77), while it would qualify as a MAC for other host species (e.g., hindgut and foregut fermenters). On the other hand, RS type 3 would be considered a MAC for most individuals, as it is readily metabolized by their GI microbiota. However, individuals who lack the keystone species Ruminococcus bromii do not have the enzymatic capacity to metabolize RS type 3, and thus for these individuals, it would not be considered a MAC (75). The concept of MACs is therefore particularly applicable to efforts aimed at personalizing human nutrition, which could customize dietary recommendations toward the goal of incorporating specific NDFCs that are known to be accessible to an individual’s GI microbiota.
The concept of dietary MACs is essentially equivalent to that of fermentable dietary fiber, with the additional criterion regarding the individuality of the GI microbiota in their capacity to utilize certain NDFCs. Both concepts include the same types of NDFCs (16), and according to Bindels and colleagues, these carbohydrates could be considered prebiotic if they exert a health benefit via the GI microbiota (28). Because there is currently no unifying concept that describes NDFCs that target the GI microbiota for health purposes, we will continue to refer to these carbohydrates as NDFCs for the purpose of this review.
HOW DO NDFCs MODULATE GI MICROBIOTA COMPOSITION AND FUNCTION?
Modulation of GI Microbiota Composition and Diversity
Consumption of NDFCs has the potential to improve human health by changing both the composition (structure and diversity) and the function (metabolism) of the microbial communities that reside in the GI tract. Dietary administration of NDFCs alters the nutritional niches in the GI tract by providing substrates for microbial growth. Thus, in general, species that are able to utilize these substrates can expand their populations (78). For example, administration of RS has been shown to enrich specific bacterial groups (Bifidobacterium adolescentis, R. bromii, and Eubacterium rectale) in a subset of individuals (53–55). The taxa shown to be enriched differ between RS type 2 (which appears to be similar to RS type 3 [75]) and RS type 4 (55), indicating that shifts are dependent on the carbohydrate’s chemical structure. Accordingly, the consumption of galactooligosaccharides mainly induces Bifidobacterium species that possess the enzymatic machinery to efficiently utilize this substrate (60). In addition to its enzymatic capacity, the ability of a microbe to “adhere” to a substrate and to tolerate the environmental conditions generated from fermentation (e.g., low pH) determines whether a microbe can become enriched. Although RS is utilized by many members of the human GI microbiota (57, 79, 80), the species that become enriched under competition (B. adolescentis, R. bromii, and E. rectale) have been shown to directly adhere to this substrate in the human GI tract (80).
Furthermore, the degradation of many complex NDFCs does require a different species with complementary enzymatic repertoires for their degradation, which establishes syntrophic interrelationships within the GI microbiota (81–83). Primary fermenters directly degrade NDFCs, leading to the release of partial breakdown products and the production of metabolites that can benefit themselves, as well as be beneficial or inhibitory (e.g., through acidity) to other taxa (84). Secondary fermenters benefit through cross-feeding on these partial carbohydrate breakdown products and the metabolic end products released by primary fermenters (82, 85). Through coculture experiments, R. bromii has been identified as a primary fermenter of RS types 2 and 3, whereupon RS degradation-reducing sugars are released that support the growth of secondary fermenters that are not able to degrade RS directly, including B. adolescentis, E. rectale, and Bacteroides thetaiotaomicron (75). Another cross-feeding relationship exists for hydrogen-consuming species such as Blautia hydrogenotrophica, which flourish in the mouse GI tract only if in bi-association with B. thetaiotaomicron by utilizing the hydrogen generated as an end product of fermentation by the latter (86). Removal of hydrogen maintains the redox balance (NAD+/NADH ratio) in the GI tract, providing conditions favorable for fermentation, and can result in the formation of methane by methanogens (such as Methanobrevibacter smithii) and acetate by acetogens (such as B. hydrogenotrophica), which is further converted to butyrate by other taxa, including Roseburia intestinalis (87–89). Lactate is another metabolic end product of NDFC fermentation that can also be converted to butyrate through cross-feeding between lactate-producing species and lactate-utilizing butyrogenic species (83, 90).
While the processes described above lead to the enrichment of GI microbes through the fermentation of NDFCs, with different species being either directly or indirectly stimulated, there are taxa that simultaneously become inhibited through the metabolites produced. For instance, Bacteroides species often decrease in number after the administration of NDFCs (37, 59, 60), even though this group of bacteria is well equipped to utilize these substrates. These inhibitory effects are due to Bacteroides having a low tolerance for acidic conditions generated from the SCFAs produced during the fermentation of NDFCs (58, 62).
The impact of NDFCs on the GI microbiome composition displays several consistent characteristics that are important for therapeutic applications. First, the magnitude of the induced changes can be substantial, with specific species becoming enriched to constitute more than 30% of the fecal microbiota (54, 55, 60), thus providing a potential strategy for the enrichment of minority members of the GI microbiome to become dominant members. However, these changes are only maintained as long as the substrate is consumed. Once the substrate is no longer available, resilience of the microbiota results in a return to the original state. Second, the microbial response to NDFCs is highly individualized, with some individuals showing substrate-specific shifts, while others do not respond at all (55, 60). The reason for this individuality is not yet understood. Individuals might lack keystone species (75) or contain strains with varying enzymatic capacity for the substrate (91). Third, although individualized, compositional changes observed after administration of NDFCs remain restricted to certain groups of microbes. This is true for classic prebiotics such as inulin and galactooligosaccharides that are supposed to be selectively fermented (60, 92), but it also applies to substrates that were assumed to be broadly utilized, such as RSs and pectin (54, 55, 58). The reason for these observations stems from the highly competitive conditions within the human GI tract, which allow for only certain microbes to benefit directly from the NDFCs (87). Although central to the original prebiotic concept (30), whether such specific shifts are related to health outcomes still remains to be established.
Most CNCDs are associated with a dysbiosis that displays decreases in bacterial diversity and/or genetic richness of the GI microbiome (3, 43, 44). Although it is difficult to prove whether these patterns are the cause or an effect of disease, community ecology theory postulates high diversity as a beneficial trait that is attributed to stability and functionality of the ecosystem (45, 93). Several independent research groups have consistently shown that individuals from nonindustrialized populations in various parts of the world have greater GI microbiome diversity when compared to individuals from industrialized regions (94). This increased diversity is reflected not only in the number of bacterial species (or operational taxonomic units) (18–22), but also in the abundance of genetic functions encoded in the GI microbiome (20). While there are many possible factors that could cause reduced GI microbial diversity through industrialization (e.g., sanitization and antibiotic use), dietary diversity is considered a key mediator (16, 95). Research using humanized mice (mice that have been colonized with a human GI microbiota) demonstrated that a maternal diet low in MACs induced significant depletions in the GI microbiota diversity of the offspring within only a few generations and that this depletion was irreversible even after the reintroduction of MACs (23). Enrichment of the maternal diet with MACs maintained the GI microbiota diversity in the offspring over multiple generations (23). Moreover, the transition of non-human primates from wild to semicaptive to captive conditions led to a reduction in bacterial operational taxonomic unit richness. An in-depth analysis of the chloroplast sequences found within the 16S sequences obtained from fecal samples suggested that this depletion was in part driven by a reduction in the diversity of dietary plant content, and particularly by a reduction in the consumption of NDFCs (96).
Overall, there is convincing evidence that a depletion of NDFCs in the diet results in a reduction of microbial diversity within the human GI microbiome, thus providing a rationale for targeting microbial diversity through dietary modulations. An enrichment of both the amount and structural diversity of NDFCs could in theory enhance microbial diversity and gene richness by generating niche opportunities (24). Cross-sectional assessments of long-term dietary intake in overweight humans have shown that long-term consumption of fruit and vegetables, and therefore NDFCs, is associated with higher GI microbial gene richness and diversity (44, 97). Conversely, short-term dietary intervention studies have produced conflicting results (37, 98, 99). Studies supplementing fiber-rich whole foods have been shown to increase GI microbial diversity (37, 93), while most short-term feeding trials with purified NDFCs (55, 60, 98, 100) or even whole-plant-based diets (6) had no effect.
In summary, although clear associations exist between the consumption of NDFCs, GI microbial diversity, and improved metabolic and inflammatory markers of CNCDs, rigorously controlled human intervention studies with NDFCs that assess well-defined clinical and microbial endpoints are needed to determine (i) if diversity can be enhanced by NDFCs and (ii) to what extent this constitutes a microbial-dependent mechanism by which NDFCs can improve human health.
Impact of NDFCs on GI Microbiome Function
The provision of NDFCs not only impacts microbiota composition as described above, but also changes the profile of microbial-derived metabolites within the GI tract (101). The fermentation of NDFCs results in the production of beneficial metabolites (e.g., SCFAs) and microbial gases (H2, CO2, CH4), with SCFAs being the main focus of recent research (17, 101). Acetate, propionate, and butyrate are the dominant SCFAs (at >95%) (102). A significant portion of acetate (around 24%) can support the production of butyrate through microbial cross-feeding (103). The total amount and proportion of individual SCFAs produced is dependent on the type of NDFC (104), as well as the individual microbiota (105), further reinforcing that the response of human GI microbiota to NDFCs is individualized.
Fermentation of NDFCs within the GI tract leads to various systemic effects on the host, including an influence on energy homeostasis and metabolism (17). A majority of the SCFAs produced are rapidly absorbed in the GI tract, with only around 5 to 10% being excreted in the feces (101). Upon absorption, the majority of butyrate is metabolized by the colonocytes and serves as their major form of energy (106). Propionate reaches the liver via portal circulation, where it is primarily utilized for hepatic gluconeogenesis (GNG). Acetate, on the other hand, reaches peripheral circulation at extensively higher concentrations than the other SCFAs, where it is metabolized by peripheral tissues for energy, in addition to being utilized by the liver for lipogenesis (101–103). NDCs provide the host with 0 to 2.5 kcal/g (with digestible carbohydrates providing 4 kcal/g), dependent on their level of fermentability by the GI microbiota (107).
A low intake of NDFCs leads not only to a reduction in SCFAs, but also to shifts in GI microbiota metabolism toward the utilization of less favorable nutrients, particularly dietary and endogenously supplied proteins (108). For instance, moving the diet of human volunteers from a weight-maintenance diet to a high-protein, low-carbohydrate diet not only significantly reduced the production of total SCFAs and butyrate (109), but also led to an increase in potentially detrimental metabolites derived from the fermentation of amino acids, including branched chain fatty acids, ammonia, amines, N-nitroso compounds, phenolic compounds including p-Cresol, and sulfides (Fig. 3) (110, 111). These metabolites are thought to directly contribute to the development of CNCDs, particularly colon cancer (111). In addition, depletion of NDFCs within the diet subsequently causes the GI microbiota to shift their glycan-foraging behavior toward utilizing host-derived substrates such as mucins by upregulating the expression of bacterial genes necessary for the metabolization of mucosal glycoproteins (112). This shift toward the fermentation of mucosal glycans leads to a significant depletion of the epithelial mucus layer (113), which can cause GI inflammation and increases the host’s susceptibility to pathogen invasion (114).
FIGURE 3.

Mechanisms by which the metabolism of NDFCs by the GI microbiota modulates host health. NDFCs are fermented by the GI microbiota to SCFAs, which upon absorption into enterocytes can activate intestinal GNG, leading to improved satiety and glucose homeostasis. SCFAs can further stimulate enteroendocrine L-cells to secrete PYY, GLP-1, and GLP-2. Both PYY and GLP-1 act as satiety hormones, while GLP-1 also promotes glucose tolerance. Meanwhile, the secretion of GLP-2 enhances intestinal barrier function by upregulating the expression of tight junction proteins. SCFAs further enhance the intestinal barrier by stimulating mucin secretion from goblet cells, which aids in reducing the translocation of LPS through the intestinal epithelium, consequently reducing inflammation. Additionally, SCFAs exert immunomodulatory effects by regulating the production of antimicrobial peptides, the expansion of regulatory T-cells, and myeloid cell function to inhibit inflammation. Moreover, SCFAs signal to organs distant from the GI tract, such as white adipose tissue, where they may act on adipocytes promoting the secretion of leptin, another anorectic hormone. Furthermore, the presence of NDFCs inhibits the production of potentially detrimental metabolites from the fermentation of dietary proteins through lowering intestinal pH. AMP, antimicrobial peptides, BCFAs, branched-chain fatty acids; CVD, cardiovascular disease; GLP, glucagon-like peptide; GNG, gluconeogenesis; LPS, lipopolysaccharides; PYY, peptide tyrosine tyrosine; SCFAs, short-chain fatty acids; T2D, type 2 diabetes; Tregs, regulatory T-cells.
In summary, the metabolic effect of NDFCs is central to their importance in human nutrition and their effects when used as supplements (115) in the form of prebiotics or fermentable dietary fiber (65). Although the fermentation of NDFCs is considered beneficial, this subject is not without its controversies. Individuals with obesity tend to have increased fecal SCFAs when compared to their lean counterparts (116), and SCFAs might contribute to weight gain by providing energy. In addition, butyrate has a controversial role in the induction of colon cancer, because it has been shown to fuel the hyperproliferation of colon epithelial cells in a mouse model of the disease (117, 118). However, epidemiological studies consistently report a negative association between dietary fiber consumption, which would increase SCFAs, and both obesity (119) and colon cancer (120, 121). Furthermore, as discussed below, the majority of the physiological effects associated with SCFAs are considered beneficial.
PHYSIOLOGICAL EFFECTS OF NDFCs ON THE HOST
Two primary signaling mechanisms have been described by which SCFAs influence the biological responses of the host. First, SCFAs can impose epigenetic regulation through direct inhibition of histone deacetylase activity and expression (122). Histone deacetylase inhibition has been indicated as a central mechanism by which SCFAs modulate the immune system and inhibit the development of colon cancer (122). Second, SCFAs can bind to G-protein-coupled receptors (GPRs), with the primary receptors activated by SCFAs being GPR41, GPR43, and GPR109A (122). GPR41 and GPR43 are coexpressed locally on colonic enteroendocrine L-cells (46, 47), as well as systemically expressed in white adipose tissue, skeletal muscle, and the liver (46–48). GPR109A (which is also commonly referred to as niacin receptor 1 since niacin is its primary ligand [123]) has been shown to be expressed on ileal and colonic enterocytes, adipocytes, and immune cells (123–125). Agonists for GPR41 can be ranked in the following order based on potency: propionate greater than or equal to butyrate, butyrate greater than acetate; however, these SCFAs exhibit similar potencies for GPR43 (126, 127). Butyrate, on the other hand, is the only SCFA known to bind to GPR109A. Overall, SCFAs are a primary microbiota-dependent mechanism by which NDFCs modulate host health (17) through the regulation of satiety, glucose and lipid metabolism, as well as systemic inflammation (Fig. 3).
Regulation of Satiety
The regulation of the balance between hunger and satiety, which ultimately impacts energy intake, is highly complex and is influenced by multiple physiological, psychological, and environmental factors (128). SCFAs act as physiological regulators of satiety by primarily functioning as signaling molecules through the enhanced production of key anorectic hormones such as peptide tyrosine tyrosine (PYY) and glucagon-like peptide-1 (GLP-1) (Fig. 3) (47). By means of activating GPR41 and GPR43, SCFAs induce the release of both PYY and GLP-1 from colonic enteroendocrine L-cells into systemic circulation (129, 130). PYY has been shown to promote satiety by acting on the arcuate nucleus within the hypothalamus to suppress neuropeptide Y neurons and activate pro-opiomelanocortin neurons while also delaying gastric emptying (131, 132). GLP-1 similarly influences the hypothalamus by binding to the GLP-1 receptor (133, 134) while also inhibiting gastric emptying and the secretion of gastric acid (135, 136). Furthermore, the SCFAs acetate and propionate have been shown to act on white adipose tissue to stimulate the production of leptin, another anorectic hormone involved in the regulation of satiety (Fig. 3) (137, 138).
SCFAs can also regulate the interplay between hunger and satiety independent of anorectic hormones. Acetate has been shown to induce satiety through directly eliciting hypothalamic appetite suppression (139). Furthermore, propionate and butyrate promote an upregulation of intestinal GNG gene expression, which positively influences energy homeostasis and promotes satiety by portal vein glucose sensors (140, 141). Besides influencing energy intake, SCFAs may also affect energy expenditure. In rodents, SCFA supplementation promoted an increased rate of oxygen consumption while also enhancing mitochondrial function, adaptive thermogenesis, and fat oxidation (142, 143). However, the extent of this influence and its relevance to humans is not currently known.
Glucose and Lipid Metabolism
Obesity, cardiovascular disease, type 2 diabetes, and other CNCDs are associated with an altered glucose and lipid metabolism (144, 145). Human intervention studies have shown that increased consumption of NDFCs can improve glucose and lipid metabolism (146–148), thereby providing a mechanism by which they reduce the risk of developing cardiovascular disease, type 2 diabetes, and other CNCDs (149, 150). As discussed above, activation of enteroendocrine L-cells by SCFAs stimulates the release of GLP-1, which directly acts on pancreatic β-cells to promote insulin and inhibit glucagon secretion (133, 151, 152). GLP-1 is also known to improve β-cell responsiveness to glucose, even in glucose-resistant β-cells (153, 154). Additionally, SCFAs themselves, specifically propionate, have recently been shown to act directly on β-cells to stimulate insulin secretion, independent of GLP-1 (155). This would subsequently increase the uptake of glucose by skeletal muscle and adipose tissues while also decreasing hepatic-associated GNG (156). Further, SCFAs have been shown to inhibit hepatic-associated GNG via upregulation of intestinal GNG (140). This reduction in hepatic GNG is critical, because enhanced hepatic production of glucose is linked to insulin resistance and the development of some CNCDs (157, 158).
SCFAs may also directly act on the liver to further influence hepatic glucose and lipid metabolism, which may in part be mediated by GPR41 and GPR43 signaling (127). Dietary administration of SCFAs decreased lipid accumulation within the liver in mice by means of increased lipid utilization (159). This occurred primarily by downregulation of peroxisome-proliferator-activated receptor-γ expression and activity, which stimulated AMP-activated protein kinase-associated fatty acid oxidation within the liver. This SCFA-induced increase in fat oxidation was also observed in adipose tissue (159). SCFAs may play a further role in lipid metabolism by acting on adipose tissue through both intracellular and extracellular mechanisms. The acute administration of acetate and propionate in humans led to a significant reduction in serum free fatty acid levels (160). This may be in part due to a GPR43-dependent decrease in the intracellular lipolytic activity of adipocytes (161). In addition to this, propionate may also have extracellular lipolytic properties by enhancing the activity of adipose tissue lipoprotein lipase (162). Collectively, the mechanistic involvement of SCFAs in modulating glucose and lipid metabolism has been well established in animal and in vitro models, although further research is needed to clarify if these findings translate to humans.
Systemic Inflammation
Most CNCDs are characterized by a state of systemic, low-grade inflammation, which contributes to disease progression (144, 145, 163). Although the findings are to some degree variable, recent research indicates that NDFCs have anti-inflammatory effects (164, 165), primarily through SCFA-dependent mechanisms involving intestinal barrier function and regulation of immune cell responses (17).
Barrier function and endotoxemia
Diminished GI barrier function enhances the translocation of lipopolysaccharide (LPS) and other microbial-derived proinflammatory molecules across the GI epithelial layer. LPS interacts with LPS-binding protein and CD14 to stimulate Toll-like receptor-4 and subsequently promotes a proinflammatory response, including a systemic increase in acute-phase proteins and proinflammatory cytokines (166). Elevated plasma LPS in humans (endotoxemia) is positively correlated with percent body fat, excessive energy intake, metabolic inflammation, insulin resistance, and dyslipidemia (37, 167–169). In mice, endotoxemia has been demonstrated to cause obesity and insulin resistance without influencing food intake (170). Considering this, promoting GI mucosal barrier function could constitute a strategy to address CNCD-associated metabolic abnormalities.
SCFAs have been shown to enhance GI barrier function through upregulating the expression of tight junction proteins, which occurs through at least two mechanisms. First, NDFC-induced SCFA production increases GLP-2 secretion by enteroendocrine L-cells (171, 172), which leads to an upregulation in the expression of tight junction proteins (zonula occludens-1 and occludin) and, concomitantly, a reduction in LPS and systemic inflammation (Fig. 3) (173). Recently, Kelly and colleagues illustrated another mechanism by which SCFA metabolism (mainly butyrate) within intestinal epithelial cells creates a state of hypoxia, which enhances intestinal barrier integrity (174), likely through enhanced tight junction protein expression (175).
Other mechanisms by which SCFAs have been shown to improve GI barrier function include butyrate-driven upregulation of epithelial cell proliferation and differentiation (176), enhanced production of antimicrobial peptides including secretory immunoglobulin A (IgA) and intestinal alkaline phosphatase (177), stimulation of goblet cells to secrete mucus that fortifies the GI mucus layer (178, 179), and GPR109A-dependent regulation of enterocyte-derived interleukin-18, which is essential to maintain mucosal homeostasis and ultimately GI barrier function (180, 181).
Immunoregulation
Subpopulations of immunosuppressive regulatory T-cells (Tregs) play an essential role in both the maintenance of immunotolerance and, ultimately, the prevention of many CNCDs (182, 183). Microbiota-derived SCFAs, particularly butyrate and propionate, have been demonstrated to encourage immunotolerance through expansion and differentiation of Tregs (182). This process has been shown to occur through GPR43-dependent signaling (184), as well as through the epigenetic regulation of the Foxp3 promoter through inhibition of histone deacetylase (185, 186). This systemic immunoregulatory effect is thought to be determined largely by the immunologic context. For example, in a state of infection, SCFAs enhance the differentiation of proinflammatory T-helper cell subsets (i.e., Th1 and Th17 cells) instead of Tregs (187). This suggests that SCFA production from the fermentation of NDFCs may play an intricate role in host immune responses to infection beyond lowering intestinal pH, which inhibits colonization by pathogens (9).
The immunoregulatory effect of SCFAs is, however, not solely reliant upon an expansion of Tregs and can occur in organ systems throughout the body (e.g., lungs, skin) (188, 189). SCFAs have also been shown to regulate immune cells of the myeloid lineage (Fig. 3) through facilitating the polarization of macrophages toward an M2 phenotype involved in downregulating an inflammatory response (190), specifically that of GI macrophages to LPS (191). Moreover, SCFAs modulate neutrophil activity through suppressing their migratory behavior (192) while shifting their microbial killing phenotype toward increased phagocytic activity with diminished proinflammatory cytokine production (193, 194). The activity of dendritic cells has similarly been shown to be influenced by SCFAs, primarily through enhancing their ability to induce the differentiation of Tregs (180) and to promote the production of IgA by plasma cells (195). Furthermore, SCFAs act directly on β-cells to enhance the production of IgA, both by acting as an energy source and by upregulating the expression of genes necessary for plasma cell differentiation (196). SCFAs can also act on nonimmune cells to stimulate the release of antimicrobial peptides. Within the pancreas, SCFAs act on pancreatic β-cells in a GPR-dependent manner to enhance the secretion of cathelicidin-related antimicrobial peptide, which ultimately induces Treg cell expansion and protects against the development of autoimmune diabetes (197). Although additional research is needed, these animal and in vitro models elegantly illustrate the central mechanisms by which microbiota-derived SCFAs reduce inflammation.
EVIDENCE FOR MICROBIOTA-MEDIATED HEALTH EFFECTS OF NDFCs
There is substantial evidence from well-designed animal studies that NDFCs, or the metabolic products that result from their fermentation (e.g., SCFAs), have beneficial effects and that these effects are in part due to the GI microbiota. Work originating from Patrice Cani and Nathalie Delzenne’s research groups has repeatedly demonstrated, although primarily through associations and not causal assessments, that the GI microbiota is implicated in the ability of NDFCs to improve obesity-associated low-grade inflammation and insulin resistance (40, 171–173, 198). Research employing fecal microbiota transplantation methodology supports these findings and further suggests a causative link, in that upon transferring the GI microbiota from mice fed an NDFC (resistant maltodextrin) to antibiotic-treated db/db mice (a model of type 2 diabetes), the improved glucose homeostatic phenotype was likewise transferred (199).
Work with GPR41 and GPR43 knockout (KO) mice, or their specific receptor antagonists, provides additional evidence that the NDFC-associated metabolic health effects described above are mediated by the GI microbiota and specifically through the production of SCFAs. For instance, the ability of SCFAs to stimulate insulin secretion through increased GLP-1 release (200, 201), promote satiety through enhanced PYY secretion (202), reduce systemic free fatty acid levels (161), or regulate blood pressure (203) is lost in GPR41 and/or GPR43 KO mice. Moreover, the combination of GPR41 KO mice with a model of allergic asthma showed that the production of SCFAs was essential for NDFCs to protect against allergic airway inflammation, which occurred by impairing the ability of lung dendritic cells to promote allergen-reactive Th2 responses (188). A similarly designed study compared the effect of guar gum, a viscous NDFC, in wild-type, GPR43 KO, and GPR109A KO mice treated with dextran sulfate sodium, which induces colitis. The study showed that signaling through GPR43 and GPR109A was essential for the NDFC to protect against the development of colitis (204).
Although these animal studies do illustrate a clear causative link between NDFCs, host health, and GI microbiota composition and function (e.g., SCFAs), knowledge of the exact role of the GI microbiota in the health effects of NDFCs remains incomplete. One experimental approach by which this could be resolved is through the comparison of the physiological effects of NDFCs in conventionalized and germfree animals (28). However, germfree animals are not without limitations since there are important physiological features that differ from their conventional counterparts, including an immune system that is not fully developed (205, 206). Furthermore, the pathological progression of CNCD-like symptoms in several animal models is often vastly different under germfree conditions, preventing direct comparison between germfree and conventional animals (207, 208). To address these limitations, one could alternatively test if the phenotypic effects of NDFCs are able to be transferred through cohousing or fecal microbial transfers. This is based on the premise that if the beneficial effect of the NDFC is caused by a shift in the microbiome, then the same beneficial effect should be seen in the recipient animal (28).
In humans, most microbiota-associated CNCDs are also linked to a low intake of dietary NDFCs, with a substantial degree of overlap between the two (Fig. 1). This suggests that NDFCs may prevent CNCDs through modulation of the GI microbiome (209). Epidemiological studies have established convincing associations between dietary fiber intake and health (149, 150, 210), and although findings from human intervention studies are less consistent (210), health claims have been approved for dietary fibers (cancer and cardiovascular disease) (211) and prebiotics (constipation) (212). Moreover, multiple human intervention studies have been conducted that assess the effect of NDFCs on well-defined clinical outcomes while also characterizing the microbiome for compositional and functional signatures that correlate with these health outcomes (37, 52, 98, 213–216). For instance, treatment with the prebiotic inulin led to an increase in both Bifidobacterium and Faecalibacterium prausnitzii, which was inversely correlated with serum LPS levels, indicating that the prebiotic enhanced GI barrier function through modulating the GI microbiota (52). Furthermore, systematic meta-analyses have shown that prebiotic supplementation is capable of restoring bowel function (217), while also reversing multiple metabolic abnormalities associated with CNCDs, including reducing fasting insulin, triglycerides, and low-density lipoprotein cholesterol levels (146, 148, 218, 219). Supplementation with lupin kernel fiber, a viscous NDFC, also led to a significant reduction in low-density lipoprotein cholesterol levels, and this response was inversely correlated with the fecal excretion of SCFAs (220). Although these studies do detect microbial signatures that closely correlate with clinical outcomes of NDFC supplementation, they do not provide direct evidence for a causative role of the GI microbiota (28, 221).
Still, some studies have been conducted that do indicate a causative link between the GI microbiota and NDFCs. Work from Fredrik Bäckhed’s group paired a human study with a humanized germfree mouse model to demonstrate that improved glucose metabolism due to whole-grain barley intake was dependent on the presence of Prevotella copri within the participant’s GI tract (222). Furthermore, by providing SCFAs directly through colonic infusions, studies have demonstrated that SCFAs do promote a systematic increase in PYY and GLP-1, while also benefiting markers of inflammation (223, 224).
Clinical research that establishes clear connections between the health benefits of NDFCs and the GI microbiota, as well as clear evidence for the role of the GI microbiota in the health effects of NDFCs, including the underlying mechanisms involved, is altogether limited. As described above, NDFCs can exert health benefits through microbiota-independent mechanisms such as a reduction in nutrient absorption through viscosity or binding of bile acids and cholesterol (73). This clearly illustrates a need for future studies that pair rigorously designed human studies with animal models, because this would constitute a unique tool to assess causation in humans (209, 222).
FUTURE DIRECTIONS
Assessing the Clinical Efficacy of NDFCs and the Role of the GI Microbiome
Until now, most of the clinical evaluation of NDFCs has occurred through one-sided studies that solely assess either the GI microbiome or the host while completely overlooking the other (59, 225). Clinical research is needed that assesses the effect of NDFCs in rigorously designed randomized controlled trials with relevant clinical endpoints and a parallel characterization of the role of the GI microbiota in these effects. Doing so would require close collaborations between nutritionally and microbiologically focused research groups to facilitate truly interdisciplinary research.
Despite clear evidence of the benefits of NDFCs from epidemiological studies, results from human intervention studies remain inconsistent (210). These inconsistencies could stem from a variety of reasons. Most of the dietary fiber assessed in epidemiological studies is derived from whole foods (fruits, vegetables, and whole grains), in which the NDFCs are consumed intact within a food matrix that also includes components such as phytochemicals and bioactive lipids (226). These bioactive compounds are likely to act synergistically within the food matrix (227, 228), and once purified to be used as a supplement, the health effects of the NDFC might be lost or reduced (229). However, purified fibers and prebiotics, as well as their fermentation products (SCFAs), have been repeatedly shown to be beneficial in mouse models (209). The variability in human intervention studies could arise from both the highly inter-individualized nature of the human GI microbiome (53, 55, 60) and the variability in the host’s metabolic response to NDFCs (39, 222). These two factors are inherently higher in humans, because mouse colonies are often composed of inbred mice housed in a highly standardized environment and are fed homogeneous diets. Given that the GI microbiotas of human subjects differ in the degree by which they are able to utilize specific NDFCs (75, 76), inter-individual variation is likely to be more pronounced in studies using a single purified substrate instead of a mixture of substrates or a whole food containing multiple fiber chemistries. In this respect, studies should characterize the chemical structure of the NDFCs to establish structure-function relationships between NDFC-chemistry and GI microbiome gene content, because this could be used to personalize approaches (24).
What is needed are human intervention studies with clinical endpoints that compare single NDFCs and their mixtures to those effects observed from whole foods, while also including a multi-omics approach for the analysis of the GI microbiome. Metagenomic analyses can identify specific shifts in GI microbiome composition and structure (e.g., diversity) that correlate with health outcomes. Such shifts, if they exist, would suggest that health benefits are due to selective changes of the GI microbiota in accordance with the original prebiotic concept (30). However, such studies should also include predictive modeling to determine how inter-individual differences in microbiome composition and functional capacity impact clinical outcomes. These studies could provide an explanation for the high variation observed in intervention studies with NDFCs and would establish a basis for personalized NDFC applications. Pairing this approach with a metabolomic analysis can further identify the compounds that are associated with health outcomes, which could range from microbial metabolites that originate from the fermentation of NDFCs to phytochemicals and their metabolic derivatives. Research has shown that phytochemicals present in fruits, vegetables, whole grains, and many fiber extracts are also metabolically transformed by the GI microbiota and absorbed by the host, correlating with health benefits (230).
Considering that the human-microbiota symbiosis evolved with a supply of NDCs beyond 100 g/day, future human intervention studies should clearly be performed with more physiologically relevant doses (14). Supplementation of 10 to 15 grams in intervention studies ensures that participants meet current dietary fiber recommendations of around 30 g/day (26), but this intake is still far below that of our ancestors (12, 14), potentially hindering the opportunity to detect evolutionary routed interrelationships between NDFCs, the GI microbiota, and health. Although experiments in nonindustrialized populations are clearly confounded by the possibility that specific fiber-degrading bacteria have been lost (16, 23), human studies that used NDC doses greater than 50 g/day did detect health benefits through the assessment of CNCD markers. For instance, by switching African Americans over to a more traditional South African diet that consisted of 55 g/day of NDCs, markers of colon cancer were improved in only 2 weeks (231). Furthermore, following a 2-week dietary intervention that resembled an ancestral diet, with around 143 g/d of NDCs provided as green leafy vegetables, fruit, and nuts, a 25% reduction in total cholesterol was observed, which is a response comparable to cholesterol-lowering medications (232, 233). These studies clearly provide a rationale for the use of higher doses of fiber in clinical research.
The approach described in this paragraph would ultimately help to identify putative mechanisms by which NDFCs exert their health effects, which would allow the formation of hypotheses that would inform the design of clinical studies and assist in the development of dietary strategies and targeted applications of NDFCs to improve human health.
Elucidating the Exact Mechanisms
Although well-conducted human studies with clinical endpoints would be sufficient to establish efficiency of NDFCs and obtain health claims for dietary fibers or prebiotics (28, 234), it is important to point out that such research would establish only correlations and not causation. Correlations can be misleading, because directionality cannot be established, especially since host parameters altered through NDFCs (e.g., inflammation, metabolic outcomes) can in themselves have an effect on the GI microbiota (235, 236). In addition, intake of RS has clearly established that the health effects of an NDFC can be completely independent of the GI microbiota, even though clear correlations between diet-induced shifts in the microbiome and host markers exist (237).
In this respect, it is important to consider that human studies have unavoidable limitations when it comes to establishing mechanisms, because the health effects of NDFCs can be completely microbiota-independent. For example, NDCs increase fecal bulk and decrease colonic transit time (238), which in turn influences GI microbiota composition (239). Ingestion of viscous NDCs may also increase the viscosity of digesta, interrupting the rate of nutrient absorption and ultimately promoting an improvement in clinical markers, especially postprandial glycemic response (240, 241). Furthermore, NDCs sequester compounds such as sterols, bile acids, and carcinogens (242–244), inhibiting their absorbance and enhancing their excretion. Modulation of the bile acid pool alone may have systemic implications for glucose and lipid metabolism, as well as systemic inflammation through action on the farnesoid X receptor and the GPR TGR5 (245). These microbiota-independent mechanisms can be difficult to distinguish from microbiota-dependent mechanisms, especially because they still might lead to strong correlations between diet-induced shifts in microbiome composition and host markers (237).
Elucidation of the role of the GI microbiota in the health effects of NDFCs and the underlying mechanisms will require innovative experimental approaches such as utilizing animal studies in parallel with human intervention studies to directly test the role of the GI microbiota in health outcomes (209, 222). Although establishing the contribution of the GI microbiota in the health outcomes of an NDFC would in theory be required to establish its prebiotic action, such studies would be extremely difficult and costly, and probably an unrealistic demand for the purpose of defining if an NDFC qualifies as a prebiotic. Therefore, despite the limitations discussed above, the establishment of correlations between the microbiome features and health benefits of an NDFC should probably be considered sufficient from a practical standpoint to establish which NDFCs constitute prebiotics (28). Establishing the causal role of the GI microbiota and the underlying mechanisms would remain essential information for the development of improved nutritional strategies. Only an in-depth mechanistic understanding will allow for the selection of NDFCs, or mixtures thereof, to systematically target specific features of the GI microbiome (i.e., specific taxa, diversity, metabolites) with the goal of correcting both immunometabolic abnormalities and dysbiotic features that underlie CNCDs.
CONCLUSION
The human diet has clearly changed over the last millennia, resulting in diminished potential to support our GI microbial community with growth substrates due to the dramatic reduction in the intake of NDFCs to a mere fraction of what was present in the diet of our ancestors (14). Evidence points to this “fiber gap” as being one prominent driving force behind the increased prevalence of CNCDs (13, 26). Although increased consumption of NDFC-rich whole foods such as fruits, vegetables, and whole grains is preferable from a nutritional perspective, efforts to increase their consumption have been ineffective to date, because dietary fiber levels remain low despite substantial efforts from nutritional agencies (25). Humans resist long-term changes of their dietary habits (246), illustrating a need for NDFC sources that can readily enrich the standard western diet (12). A broad array of NDFCs, which have vast potential to enhance the food supply, already exist on the market; however, product development and consumer research, in combination with clinical research, is needed to determine practical and cost-effective means by which these NDFCs can be incorporated into the food supply (12).
We know that there is a high degree of inter-individual variation in GI microbial response to NDFCs (53, 55, 60), and we have the tool set available to determine to what degree this individuality affects health outcomes in clinical studies (39). This essential knowledge would ultimately support the development of a framework by which interventions with NDFCs could be personalized. This ensures tremendous potential for growth within this field, promising exciting new developments as the focus of human nutrition shifts toward targeted nourishment of our symbiotic microbial communities as a way of preventing and treating CNCDs through supplementation with NDFCs.
Contributor Information
Edward C. Deehan, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2E1
Rebbeca M. Duar, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2E1
Anissa M. Armet, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2E1
Maria Elisa Perez-Muñoz, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2E1.
Mingliang Jin, Department of Microbiology and Immunology, Northwestern Polytechnical University, Xi’an, Shaanxi, China 710065.
Jens Walter, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada T6G 2E1; Department of Biological Sciences, University of Alberta, Edmonton, Alberta, Canada T6G 2E1.
Robert Allen Britton, Baylor College of Medicine, Houston, TX 77030.
Patrice D. Cani, Université catholique de Louvain, Louvain Drug Research Institute, Brussels 1200, Belgium
REFERENCES
- 1.Walter J, Ley R. 2011. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol 65:411–429 10.1146/annurev-micro-090110-102830. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Schroeder BO, Bäckhed F. 2016. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22:1079–1089 10.1038/nm.4185. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Walker AW, Lawley TD. 2013. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res 69:75–86 10.1016/j.phrs.2012.09.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Brahe LK, Astrup A, Larsen LH. 2016. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv Nutr 7:90–101 10.3945/an.115.010587. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olle B. 2013. Medicines from microbiota. Nat Biotechnol 31:309–315 10.1038/nbt.2548. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563 10.1038/nature12820. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108 10.1126/science.1208344. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD. 2016. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65:63–72 10.1136/gutjnl-2014-308209. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Bach J-F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347:911–920 10.1056/NEJMra020100. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Bickler SW, DeMaio A. 2008. Western diseases: current concepts and implications for pediatric surgery research and practice. Pediatr Surg Int 24:251–255 10.1007/s00383-007-2095-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Deehan EC, Walter J. 2016. The fiber gap and the disappearing gut microbiome: implications for human nutrition. Trends Endocrinol Metab 27:239–242 10.1016/j.tem.2016.03.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Burkitt DP, Walker ARP, Painter NS. 1974. Dietary fiber and disease. JAMA 229:1068–1074 10.1001/jama.1974.03230460018013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Eaton SB, Eaton SB III, Konner MJ. 1997. Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. Eur J Clin Nutr 51:207–216 10.1038/sj.ejcn.1600389. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Burkitt DP. 1973. Some diseases characteristic of modern Western civilization. BMJ 1:274–278 10.1136/bmj.1.5848.274. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenburg ED, Sonnenburg JL. 2014. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20:779–786 10.1016/j.cmet.2014.07.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. 2016. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345 10.1016/j.cell.2016.05.041. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren AM, Siba PM, Greenhill AR, Walter J. 2015. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Reports 11:527–538 10.1016/j.celrep.2015.03.049. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN. 2014. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 5:3654 10.1038/ncomms4654. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya-Alarcón Ó, Lander O, McDonald J, Cox M, Walter J, Oh PL, Ruiz JF, Rodriguez S, Shen N, Song SJ, Metcalf J, Knight R, Dantas G, Dominguez-Bello MG. 2015. The microbiome of uncontacted Amerindians. Sci Adv 1:e1500183 10.1126/sciadv.1500183. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107:14691–14696 10.1073/pnas.1005963107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. 2016. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529:212–215 10.1038/nature16504. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaker BR, Tuncil YE. 2014. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 426:3838–3850 10.1016/j.jmb.2014.07.028. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.King DE, Mainous AG III, Lambourne CA. 2012. Trends in dietary fiber intake in the United States, 1999-2008. J Acad Nutr Diet 112:642–648 10.1016/j.jand.2012.01.019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Jones JM. 2014. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J 13:34 10.1186/1475-2891-13-34. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill DR, Newburg DS. 2015. Clinical applications of bioactive milk components. Nutr Rev 73:463–476 10.1093/nutrit/nuv009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bindels LB, Delzenne NM, Cani PD, Walter J. 2015. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12:303–310 10.1038/nrgastro.2015.47. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. 2017. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502 10.1038/nrgastro.2017.75. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy EF, Saulnier D, Loh G, Macfarlane S, Delzenne N, Ringel Y, Kozianowski G, Dickmann R, Lenoir-Wijnkoop I, Walker C, Buddington R. 2010. Dietary prebiotics: current status and new definition. Food Sci Tech Bull Funct Foods 7:1–19 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 31.Roberfroid M. 2007. Prebiotics: the concept revisited. J Nutr 137(Suppl 2):830S–837S. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Katsnelson A. 2016. Core concept: prebiotics gain prominence but remain poorly defined. Proc Natl Acad Sci USA 113:14168–14169 10.1073/pnas.1618366113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verspreet J, Damen B, Broekaert WF, Verbeke K, Delcour JA, Courtin CM. 2016. A critical look at prebiotics within the dietary fiber concept. Annu Rev Food Sci Technol 7:167–190 10.1146/annurev-food-081315-032749. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Suez J, Elinav E. 2017. The path towards microbiome-based metabolite treatment. Nat Microbiol 2:17075 10.1038/nmicrobiol.2017.75. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Shanahan F. 2015. Fiber man meets microbial man. Am J Clin Nutr 101:1–2 10.3945/ajcn.114.101550. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Louis P, Flint HJ, Michel C. 2016. How to manipulate the microbiota: prebiotics. Adv Exp Med Biol 902:119–142 10.1007/978-3-319-31248-4_9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, Haub MD, Walter J. 2013. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 7:269–280 10.1038/ismej.2012.104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quévrain E, Maubert M-A, Michon C, Chain F, Marquant R, Tailhades J, Miquel S, Carlier L, Bermúdez-Humarán LG, Pigneur B, Lequin O, Kharrat P, Thomas G, Rainteau D, Aubry C, Breyner N, Afonso C, Lavielle S, Grill JP, Chassaing G, Chatel J-M, Trugnan G, Xavier R, Langella P, Sokol H, Seksik P. 2016. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65:415–425 10.1136/gutjnl-2014-307649. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. 2015. Personalized nutrition by prediction of glycemic responses. Cell 163:1079–1094 10.1016/j.cell.2015.11.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110:9066–9071 10.1073/pnas.1219451110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236 10.1038/nature12331. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332:974–977 10.1126/science.1206095. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Chatelier E, et al, MetaHIT Consortium. 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546 10.1038/nature12506. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto J-M, Renault P, Doré J, Zucker JD, Clément K, Ehrlich SD, ANR MicroObes Consortium. 2013. Dietary intervention impact on gut microbial gene richness. Nature 500:585–588 10.1038/nature12480. [PubMed] [DOI] [PubMed] [Google Scholar]
- 45.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230 10.1038/nature11550. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. 2017. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 16:79 10.1186/s12934-017-0691-z. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canfora EE, Jocken JW, Blaak EE. 2015. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11:577–591 10.1038/nrendo.2015.128. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Zheng J, Ruan L, Sun M, Gänzle M. 2015. A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl Environ Microbiol 81:7233–7243 10.1128/AEM.02116-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makras L, Falony G, Van der Meulen R, De Vuyst L. 2006. Letter to the editor. J Appl Microbiol 100:1388–1389 10.1111/j.1365-2672.2006.02943.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Klijn A, Mercenier A, Arigoni F. 2005. Lessons from the genomes of bifidobacteria. FEMS Microbiol Rev 29:491–509 10.1016/j.fmrre.2005.04.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Flint HJ, Duncan SH, Scott KP, Louis P. 2015. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 74:13–22 10.1017/S0029665114001463. [PubMed] [DOI] [PubMed] [Google Scholar]
- 52.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PGB, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen J-P, Delzenne NM. 2013. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62:1112–1121 10.1136/gutjnl-2012-303304. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. 2016. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 4:33 10.1186/s40168-016-0178-x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5:220–230 10.1038/ismej.2010.118. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. 2010. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 5:e15046 10.1371/journal.pone.0015046. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. 2013. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11:497–504 10.1038/nrmicro3050. [PubMed] [DOI] [PubMed] [Google Scholar]
- 57.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131 10.1038/nrmicro1817. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Chung WSF, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. 2016. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol 14:3 10.1186/s12915-015-0224-3. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawicki CM, Livingston KA, Obin M, Roberts SB, Chung M, McKeown NM. 2017. Dietary fiber and the human gut microbiota: application of evidence mapping methodology. Nutrients 9:125 10.3390/nu9020125. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis LMG, Martínez I, Walter J, Goin C, Hutkins RW. 2011. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One 6:e25200 10.1371/journal.pone.0025200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker AW, Duncan SH, McWilliam Leitch EC, Child MW, Flint HJ. 2005. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol 71:3692–3700 10.1128/AEM.71.7.3692-3700.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan SH, Louis P, Thomson JM, Flint HJ. 2009. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol 11:2112–2122 10.1111/j.1462-2920.2009.01931.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.Tannock GW, Lawley B, Munro K, Sims IM, Lee J, Butts CA, Roy N. 2014. RNA-stable-isotope probing shows utilization of carbon from inulin by specific bacterial populations in the rat large bowel. Appl Environ Microbiol 80:2240–2247 10.1128/AEM.03799-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holscher HD. 2017. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184 10.1080/19490976.2017.1290756. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slavin J. 2013. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5:1417–1435 10.3390/nu5041417. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hipsley EH. 1953. Dietary “fibre” and pregnancy toxaemia. BMJ 2:420–422 10.1136/bmj.2.4833.420. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trowell HC. 1974. Editorial: definitions of dietary fibre. Lancet 1:503 10.1016/S0140-6736(74)92802-5. [DOI] [PubMed] [Google Scholar]
- 68.Joint FAO/WHO Food Standards Programme. 2010. Secretariat of the CODEX Alimentarius Commission: CODEX Alimentarius (CODEX) guidelines on nutrition labeling CAC/GL 2-1985 as last amended 2010. FAO, Rome, Italy. [Google Scholar]
- 69.Food and Drug Administration. 2016. Food Labeling: Revision of the Nutrition and Supplement Facts Labels. Report no. RIN 0910-AF22. FDA, College Park, MD. [PubMed] [Google Scholar]
- 70.Fuller S, Beck E, Salman H, Tapsell L. 2016. New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr 71:1–12 10.1007/s11130-016-0529-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Tungland BC, Meyer D. 2002. Nondigestible oligo- and polysaccharides (dietary fiber): their physiology and role in human health and food. Compr Rev Food Sci Food Saf 1:90–109 10.1111/j.1541-4337.2002.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 72.Raigond P, Ezekiel R, Raigond B. 2015. Resistant starch in food: a review. J Sci Food Agric 95:1968–1978 10.1002/jsfa.6966. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Mudgil D, Barak S. 2013. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: a review. Int J Biol Macromol 61:1–6 10.1016/j.ijbiomac.2013.06.044. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Guillona F, Champ M. 2000. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int 33:233–245 10.1016/S0963-9969(00)00038-7. [DOI] [Google Scholar]
- 75.Ze X, Duncan SH, Louis P, Flint HJ. 2012. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 6:1535–1543 10.1038/ismej.2012.4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hehemann J-H, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. 2010. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464:908–912 10.1038/nature08937. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Chassard C, Delmas E, Robert C, Bernalier-Donadille A. 2010. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol Ecol 74:205–213 10.1111/j.1574-6941.2010.00941.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Walter J. 2015. Murine gut microbiota-diet trumps genes. Cell Host Microbe 17:3–5 10.1016/j.chom.2014.12.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 79.Kovatcheva-Datchary P, Egert M, Maathuis A, Rajilić-Stojanović M, de Graaf AA, Smidt H, de Vos WM, Venema K. 2009. Linking phylogenetic identities of bacteria to starch fermentation in an in vitro model of the large intestine by RNA-based stable isotope probing. Environ Microbiol 11:914–926 10.1111/j.1462-2920.2008.01815.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Leitch ECM, Walker AW, Duncan SH, Holtrop G, Flint HJ. 2007. Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol 9:667–679 10.1111/j.1462-2920.2006.01186.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 81.Rakoff-Nahoum S, Coyne MJ, Comstock LE. 2014. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol 24:40–49 10.1016/j.cub.2013.10.077. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306 10.4161/gmic.19897. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. 2006. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 72:3593–3599 10.1128/AEM.72.5.3593-3599.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coyte KZ, Schluter J, Foster KR. 2015. The ecology of the microbiome: networks, competition, and stability. Science 350:663–666 10.1126/science.aad2602. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI. 2010. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem 285:22082–22090 10.1074/jbc.M110.117713. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischbach MA, Sonnenburg JL. 2011. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe 10:336–347 10.1016/j.chom.2011.10.002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol 66:1654–1661 10.1128/AEM.66.4.1654-1661.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chassard C, Bernalier-Donadille A. 2006. H2 and acetate transfers during xylan fermentation between a butyrate-producing xylanolytic species and hydrogenotrophic microorganisms from the human gut. FEMS Microbiol Lett 254:116–122 10.1111/j.1574-6968.2005.00016.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Duncan SH, Louis P, Flint HJ. 2004. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol 70:5810–5817 10.1128/AEM.70.10.5810-5817.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lozupone CA, Hamady M, Cantarel BL, Coutinho PM, Henrissat B, Gordon JI, Knight R. 2008. The convergence of carbohydrate active gene repertoires in human gut microbes. Proc Natl Acad Sci USA 105:15076–15081 10.1073/pnas.0807339105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, Verbeke K, Raes J. 2017. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. [Epub ahead of print.] 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tap J, Furet J-P, Bensaada M, Philippe C, Roth H, Rabot S, Lakhdari O, Lombard V, Henrissat B, Corthier G, Fontaine E, Doré J, Leclerc M. 2015. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol 17:4954–4964 10.1111/1462-2920.13006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Segata N. 2015. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr Biol 25:R611–R613 10.1016/j.cub.2015.05.040. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Heiman ML, Greenway FL. 2016. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab 5:317–320 10.1016/j.molmet.2016.02.005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clayton JB, Vangay P, Huang H, Ward T, Hillmann BM, Al-Ghalith GA, Travis DA, Long HT, Tuan BV, Minh VV, Cabana F, Nadler T, Toddes B, Murphy T, Glander KE, Johnson TJ, Knights D. 2016. Captivity humanizes the primate microbiome. Proc Natl Acad Sci USA 113:10376–10381 10.1073/pnas.1521835113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong LC, Holmes BA, Cotillard A, Habi-Rachedi F, Brazeilles R, Gougis S, Gausserès N, Cani PD, Fellahi S, Bastard J-P, Kennedy SP, Doré J, Ehrlich SD, Zucker J-D, Rizkalla SW, Clément K. 2014. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLoS One 9:e109434 10.1371/journal.pone.0109434. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Upadhyaya B, McCormack L, Fardin-Kia AR, Juenemann R, Nichenametla S, Clapper J, Specker B, Dey M. 2016. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci Rep 6:28797 10.1038/srep28797. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.West NP, Christophersen CT, Pyne DB, Cripps AW, Conlon MA, Topping DL, Kang S, McSweeney CS, Fricker PA, Aguirre D, Clarke JM. 2013. Butyrylated starch increases colonic butyrate concentration but has limited effects on immunity in healthy physically active individuals. Exerc Immunol Rev 19:102–119. [PubMed] [PubMed] [Google Scholar]
- 100.Hooda S, Boler BMV, Serao MCR, Brulc JM, Staeger MA, Boileau TW, Dowd SE, Fahey GCJ Jr, Swanson KS. 2012. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr 142:1259–1265 10.3945/jn.112.158766. [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. 2006. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243 10.1097/00004836-200603000-00015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 102.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227 10.1136/gut.28.10.1221. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA. 2017. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol 595:541–555 10.1113/JP272613. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J, Martínez I, Walter J, Keshavarzian A, Rose DJ. 2013. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23:74–81 10.1016/j.anaerobe.2013.06.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 105.Yang J, Rose DJ. 2014. Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation. Nutr Res 34:749–759 10.1016/j.nutres.2014.08.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 106.Roediger WE. 1980. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21:793–798 10.1136/gut.21.9.793. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dahl WJ, Stewart ML. 2015. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J Acad Nutr Diet 115:1861–1870 10.1016/j.jand.2015.09.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 108.Cummings JH, Macfarlane GT. 1991. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 70:443–459 10.1111/j.1365-2672.1991.tb02739.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 109.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. 2007. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73:1073–1078 10.1128/AEM.02340-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ, Duthie GG, Flint HJ. 2011. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 93:1062–1072 10.3945/ajcn.110.002188. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Windey K, De Preter V, Verbeke K. 2012. Relevance of protein fermentation to gut health. Mol Nutr Food Res 56:184–196 10.1002/mnfr.201100542. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.Sonnenburg JL, Xu J, Leip DD, Chen C-H, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955–1959 10.1126/science.1109051. [PubMed] [DOI] [PubMed] [Google Scholar]
- 113.Earle KA, Billings G, Sigal M, Lichtman JS, Hansson GC, Elias JE, Amieva MR, Huang KC, Sonnenburg JL. 2015. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18:478–488 10.1016/j.chom.2015.09.002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339–1353.e21 10.1016/j.cell.2016.10.043. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambeau KV, McRorie JWJ Jr. 2017. Fiber supplements and clinically proven health benefits: how to recognize and recommend an effective fiber therapy. J Am Assoc Nurse Pract 29:216–223. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18:190–195 10.1038/oby.2009.167. [PubMed] [DOI] [PubMed] [Google Scholar]
- 117.Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, Moriyama EH, Copeland JK, Kumar S, Green B, Geddes K, Pezo RC, Navarre WW, Milosevic M, Wilson BC, Girardin SE, Wolever TMS, Edelmann W, Guttman DS, Philpott DJ, Martin A. 2014. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158:288–299 10.1016/j.cell.2014.04.051. [PubMed] [DOI] [PubMed] [Google Scholar]
- 118.Lupton JR. 2004. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr 134:479–482. [PubMed] [DOI] [PubMed] [Google Scholar]
- 119.Du H, van der A DL, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, Tjønneland A, Overvad K, Jakobsen MU, Boeing H, Buijsse B, Masala G, Palli D, Sørensen TI, Saris WH, Feskens EJ. 2010. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr 91:329–336 10.3945/ajcn.2009.28191. [PubMed] [DOI] [PubMed] [Google Scholar]
- 120.Ben Q, Sun Y, Chai R, Qian A, Xu B, Yuan Y. 2014. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology 146:689–699.e6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Kunzmann AT, Coleman HG, Huang W-Y, Kitahara CM, Cantwell MM, Berndt SI. 2015. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr 102:881–890 10.3945/ajcn.115.113282. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. 2014. The role of short-chain fatty acids in health and disease. Adv Immunol 121:91–119 10.1016/B978-0-12-800100-4.00003-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 123.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. 2009. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69:2826–2832 10.1158/0008-5472.CAN-08-4466. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wanders D, Graff EC, Judd RL. 2012. Effects of high fat diet on GPR109A and GPR81 gene expression. Biochem Biophys Res Commun 425:278–283 10.1016/j.bbrc.2012.07.082. [PubMed] [DOI] [PubMed] [Google Scholar]
- 125.Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. 2010. Colonic gene expression in conventional and germfree mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg 14:449–461 10.1007/s11605-009-1045-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 126.Le Poul E, Loison C, Struyf S, Springael J-Y, Lannoy V, Decobecq M-E, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. 2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278:25481–25489 10.1074/jbc.M301403200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 127.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. 2003. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–11319 10.1074/jbc.M211609200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 128.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H, Westerterp M. 2010. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 11:251–270 10.1111/j.1467-789X.2010.00714.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samuel BS, Shaito A, Motoike T, Rey FE, Bäckhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. 2008. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105:16767–16772 10.1073/pnas.0808567105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61:364–371 10.2337/db11-1019. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. 1987. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 28:166–170 10.1136/gut.28.2.166. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. 2002. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650–654 10.1038/nature00887. [PubMed] [DOI] [PubMed] [Google Scholar]
- 133.Wei Y, Mojsov S. 1995. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358:219–224 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 134.Merchenthaler I, Lane M, Shughrue P. 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 . [PubMed] [DOI] [PubMed] [Google Scholar]
- 135.Schjoldager BT, Mortensen PE, Christiansen J, Ørskov C, Holst JJ. 1989. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci 34:703–708 10.1007/BF01540341. [PubMed] [DOI] [PubMed] [Google Scholar]
- 136.Näslund E, Bogefors J, Skogar S, Grybäck P, Jacobsson H, Holst JJ, Hellström PM. 1999. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol 277:R910–R916. [PubMed] [DOI] [PubMed] [Google Scholar]
- 137.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. 2004. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA 101:1045–1050 10.1073/pnas.2637002100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJH, Smith DM, Arch JRS. 2010. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584:2381–2386 10.1016/j.febslet.2010.04.027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 139.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD. 2014. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611 10.1038/ncomms4611. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. 2014. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96 10.1016/j.cell.2013.12.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 141.Delaere F, Duchampt A, Mounien L, Seyer P, Duraffourd C, Zitoun C, Thorens B, Mithieux G. 2013. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab 2:47–53 10.1016/j.molmet.2012.11.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. 2011. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035 10.1073/pnas.1016088108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517 10.2337/db08-1637. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Freitag J, Berod L, Kamradt T, Sparwasser T. 2016. Immunometabolism and autoimmunity. Immunol Cell Biol 94:925–934 10.1038/icb.2016.77. [PubMed] [DOI] [PubMed] [Google Scholar]
- 145.Hajer GR, van Haeften TW, Visseren FLJ. 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971 10.1093/eurheartj/ehn387. [PubMed] [DOI] [PubMed] [Google Scholar]
- 146.Kellow NJ, Coughlan MT, Reid CM. 2014. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr 111:1147–1161 10.1017/S0007114513003607. [PubMed] [DOI] [PubMed] [Google Scholar]
- 147.Whitehead A, Beck EJ, Tosh S, Wolever TMS. 2014. Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutr 100:1413–1421 10.3945/ajcn.114.086108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beserra BTS, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MGF, Trindade EBSM. 2014. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr 34:845–858. [PubMed] [DOI] [PubMed] [Google Scholar]
- 149.Ning H, Van Horn L, Shay CM, Lloyd-Jones DM. 2014. Associations of dietary fiber intake with long-term predicted cardiovascular disease risk and C-reactive protein levels (from the National Health and Nutrition Examination Survey Data [2005-2010]). Am J Cardiol 113:287–291 10.1016/j.amjcard.2013.09.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 150.Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, Mo M, Zhang H, Zhao Y. 2014. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol 29:79–88 10.1007/s10654-013-9876-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 151.Kreymann B, Williams G, Ghatei MA, Bloom SR. 1987. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 2:1300–1304 10.1016/S0140-6736(87)91194-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 152.Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S. 1989. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes 38:902–905 10.2337/diab.38.7.902. [PubMed] [DOI] [PubMed] [Google Scholar]
- 153.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. 2003. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144:5149–5158 10.1210/en.2003-0323. [PubMed] [DOI] [PubMed] [Google Scholar]
- 154.Holz GGI IV, Kühtreiber WM, Habener JF. 1993. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 361:362–365 10.1038/361362a0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, Morrison DJ, Preston T, Wallis GA, Tedford C, Castañera González R, Huang GC, Choudhary P, Frost G, Persaud SJ. 2017. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab 19:257–265 10.1111/dom.12811. [PubMed] [DOI] [PubMed] [Google Scholar]
- 156.Röder PV, Wu B, Liu Y, Han W. 2016. Pancreatic regulation of glucose homeostasis. Exp Mol Med 48:e219 10.1038/emm.2016.6. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clore JN, Stillman J, Sugerman H. 2000. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes 49:969–974 10.2337/diabetes.49.6.969. [PubMed] [DOI] [PubMed] [Google Scholar]
- 158.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. 1992. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90:1323–1327 10.1172/JCI115997. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud D-J, Bakker BM. 2015. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 64:2398–2408 10.2337/db14-1213. [PubMed] [DOI] [PubMed] [Google Scholar]
- 160.Wolever TM, Spadafora P, Eshuis H. 1991. Interaction between colonic acetate and propionate in humans. Am J Clin Nutr 53:681–687. [PubMed] [DOI] [PubMed] [Google Scholar]
- 161.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen J-L, Tian H, Li Y. 2008. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149:4519–4526 10.1210/en.2008-0059. [PubMed] [DOI] [PubMed] [Google Scholar]
- 162.Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, Venema K. 2012. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest 42:357–364 10.1111/j.1365-2362.2011.02590.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 163.Gregor MF, Hotamisligil GS. 2011. Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445 10.1146/annurev-immunol-031210-101322. [PubMed] [DOI] [PubMed] [Google Scholar]
- 164.Jiao J, Xu J-Y, Zhang W, Han S, Qin L-Q. 2015. Effect of dietary fiber on circulating C-reactive protein in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Food Sci Nutr 66:114–119 10.3109/09637486.2014.959898. [PubMed] [DOI] [PubMed] [Google Scholar]
- 165.North CJ, Venter CS, Jerling JC. 2009. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur J Clin Nutr 63:921–933 10.1038/ejcn.2009.8. [PubMed] [DOI] [PubMed] [Google Scholar]
- 166.Piya MK, Harte AL, McTernan PG. 2013. Metabolic endotoxaemia: is it more than just a gut feeling? Curr Opin Lipidol 24:78–85 10.1097/MOL.0b013e32835b4431. [PubMed] [DOI] [PubMed] [Google Scholar]
- 167.Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Groop P-H, Lehto M, FinnDiane Study Group. 2011. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34:1809–1815 10.2337/dc10-2197. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hawkesworth S, Moore SE, Fulford AJ, Barclay GR, Darboe AA, Mark H, Nyan OA, Prentice AM. 2013. Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr Diabetes 3:e83 10.1038/nutd.2013.24. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. 2008. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87:1219–1223. [PubMed] [DOI] [PubMed] [Google Scholar]
- 170.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772 10.2337/db06-1491. [PubMed] [DOI] [PubMed] [Google Scholar]
- 171.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. 2011. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60:2775–2786 10.2337/db11-0227. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. 2011. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6:e20944 10.1371/journal.pone.0020944. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103 10.1136/gut.2008.165886. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. 2015. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–671 10.1016/j.chom.2015.03.005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, Campbell EL, Kuhn KA, Furuta GT, Colgan SP, Glover LE. 2015. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 26:2252–2262 10.1091/mbc.E14-07-1194. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peng L, He Z, Chen W, Holzman IR, Lin J. 2007. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res 61:37–41 10.1203/01.pdr.0000250014.92242.f3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 177.Chen H, Wang W, Degroote J, Possemiers S, Chen D, De Smet S, Michiels J. 2015. Arabinoxylan in wheat is more responsible than cellulose for promoting intestinal barrier function in weaned male piglets. J Nutr 145:51–58 10.3945/jn.114.201772. [PubMed] [DOI] [PubMed] [Google Scholar]
- 178.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine M-P, Aubert J-P, Laboisse C, Cherbut C, Hoebler C. 2004. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol 287:G1168–G1174 10.1152/ajpgi.00219.2004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 179.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. 2009. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 420:211–219 10.1042/BJ20082222. [PubMed] [DOI] [PubMed] [Google Scholar]
- 180.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. 2014. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139 10.1016/j.immuni.2013.12.007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CCD, Graham M, Elinav E, Flavell RA. 2015. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163:1444–1456 10.1016/j.cell.2015.10.072. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zeng H, Chi H. 2015. Metabolic control of regulatory T cell development and function. Trends Immunol 36:3–12 10.1016/j.it.2014.08.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cipolletta D. 2014. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 142:517–525 10.1111/imm.12262. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573 10.1126/science.1241165. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455 10.1038/nature12726. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450 10.1038/nature12721. [PubMed] [DOI] [PubMed] [Google Scholar]
- 187.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. 2015. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8:80–93 10.1038/mi.2014.44. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166 10.1038/nm.3444. [PubMed] [DOI] [PubMed] [Google Scholar]
- 189.Schwarz A, Bruhs A, Schwarz T. 2017. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol 137:855–864 10.1016/j.jid.2016.11.014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 190.Ji J, Shu D, Zheng M, Wang J, Luo C, Wang Y, Guo F, Zou X, Lv X, Li Y, Liu T, Qu H. 2016. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep 6:24838 10.1038/srep24838. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chang PV, Hao L, Offermanns S, Medzhitov R. 2014. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111:2247–2252 10.1073/pnas.1322269111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kamp ME, Shim R, Nicholls AJ, Oliveira AC, Mason LJ, Binge L, Mackay CR, Wong CHY. 2016. G protein-coupled receptor 43 modulates neutrophil recruitment during acute inflammation. PLoS One 11:e0163750 10.1371/journal.pone.0163750. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vinolo MAR, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. 2011. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22:849–855 10.1016/j.jnutbio.2010.07.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 194.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286 10.1038/nature08530. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, Cong Y. 2017. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10:946–956 10.1038/mi.2016.114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim M, Qie Y, Park J, Kim CH. 2016. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20:202–214 10.1016/j.chom.2016.07.001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. 2015. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43:304–317 10.1016/j.immuni.2015.07.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 198.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. 2007. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50:2374–2383 10.1007/s00125-007-0791-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 199.He B, Nohara K, Ajami NJ, Michalek RD, Tian X, Wong M, Losee-Olson SH, Petrosino JF, Yoo S-H, Shimomura K, Chen Z. 2015. Transmissible microbial and metabolomic remodeling by soluble dietary fiber improves metabolic homeostasis. Sci Rep 5:10604 10.1038/srep10604. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, Layden BT. 2015. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol 29:1055–1066 10.1210/me.2015-1007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. 2015. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes 39:424–429 10.1038/ijo.2014.153. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj J, Bell JD, Bell-Anderson K, Mackay CR, Ghatei MA, Bloom SR, Frost G, Bewick GA. 2017. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab 6:48–60 10.1016/j.molmet.2016.10.011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. 2016. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G-protein coupled receptor 41. Physiol Genomics 48:826–834 10.1152/physiolgenomics.00089.2016. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. 2015. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734 10.1038/ncomms7734. [PubMed] [DOI] [PubMed] [Google Scholar]
- 205.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141 10.1016/j.cell.2014.03.011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Coates ME. 1975. Gnotobiotic animals in research: their uses and limitations. Lab Anim 9:275–282 10.1258/002367775780957296. [PubMed] [DOI] [PubMed] [Google Scholar]
- 207.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germfree mice. Proc Natl Acad Sci USA 104:979–984 10.1073/pnas.0605374104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schwabe RF, Jobin C. 2013. The microbiome and cancer. Nat Rev Cancer 13:800–812 10.1038/nrc3610. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sonnenburg JL, Bäckhed F. 2016. Diet-microbiota interactions as moderators of human metabolism. Nature 535:56–64 10.1038/nature18846. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, Brand-Miller JC. 2014. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr 99:813–833 10.3945/ajcn.113.074252. [PubMed] [DOI] [PubMed] [Google Scholar]
- 211.Food and Drug Administration. 2016. Code of Federal Regulations Title 21. Subpart E: Specific Requirements for Health Claims. Report no. 21CFR101. FDA, College Park, MD. [Google Scholar]
- 212.EFSA Panel on Dietetic Products Nutrition and Allergies. 2015. Scientific opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA J 13:3951 10.2903/j.efsa.2015.3951. [DOI] [Google Scholar]
- 213.Clarke ST, Green-Johnson JM, Brooks SPJ, Ramdath DD, Bercik P, Avila C, Inglis GD, Green J, Yanke LJ, Selinger LB, Kalmokoff M. 2016. β2-1 fructan supplementation alters host immune responses in a manner consistent with increased exposure to microbial components: results from a double-blinded, randomised, cross-over study in healthy adults. Br J Nutr 115:1748–1759 10.1017/S0007114516000908. [PubMed] [DOI] [PubMed] [Google Scholar]
- 214.Lambert JE, Parnell JA, Tunnicliffe JM, Han J, Sturzenegger T, Reimer RA. 2017. Consuming yellow pea fiber reduces voluntary energy intake and body fat in overweight/obese adults in a 12-week randomized controlled trial. Clin Nutr 36:126–133 10.1016/j.clnu.2015.12.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 215.Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonnalagadda S, Koecher K, Karl JP, Thomas M, Dolnikowski G, Li L, Saltzman E, Wu D, Meydani SN. 2017. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr 105:635–650 10.3945/ajcn.116.146928. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. 2017. Prebiotic reduces body fat and alters intestinal microbiota in children with overweight or obesity. Gastroenterology. [Epub ahead of print.] 10.1053/j.gastro.2017.05.055. [PubMed] [DOI] [PubMed] [Google Scholar]
- 217.Collado Yurrita L, San Mauro Martín I, Ciudad-Cabañas MJ, Calle-Purón ME, Hernández Cabria M. 2014. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr Hosp 30:244–252. [PubMed] [DOI] [PubMed] [Google Scholar]
- 218.Brighenti F. 2007. Dietary fructans and serum triacylglycerols: a meta-analysis of randomized controlled trials. J Nutr 137(Suppl):2552S–2556S. [PubMed] [DOI] [PubMed] [Google Scholar]
- 219.Liu F, Prabhakar M, Ju J, Long H, Zhou HW. 2017. Effect of inulin-type fructans on blood lipid profile and glucose level: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 71:9–20 10.1038/ejcn.2016.156. [PubMed] [DOI] [PubMed] [Google Scholar]
- 220.Fechner A, Kiehntopf M, Jahreis G. 2014. The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J Nutr 144:599–607 10.3945/jn.113.186858. [PubMed] [DOI] [PubMed] [Google Scholar]
- 221.Buttó LF, Haller D. 2017. Functional relevance of microbiome signatures: the correlation era requires tools for consolidation. J Allergy Clin Immunol 139:1092–1098 10.1016/j.jaci.2017.02.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 222.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982 10.1016/j.cmet.2015.10.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 223.Freeland KR, Wolever TMS. 2010. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr 103:460–466 10.1017/S0007114509991863. [PubMed] [DOI] [PubMed] [Google Scholar]
- 224.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Olde Damink SWM, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE. 2016. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 130:2073–2082 10.1042/CS20160263. [PubMed] [DOI] [PubMed] [Google Scholar]
- 225.Livingston KA, Chung M, Sawicki CM, Lyle BJ, Wang DD, Roberts SB, McKeown NM. 2016. Development of a publicly available, comprehensive database of fiber and health outcomes: rationale and methods. PLoS One 11:e0156961 10.1371/journal.pone.0156961. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Saura-Calixto F. 2011. Dietary fiber as a carrier of dietary antioxidants: an essential physiological function. J Agric Food Chem 59:43–49 10.1021/jf1036596. [PubMed] [DOI] [PubMed] [Google Scholar]
- 227.Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S, Thielecke F, Gallo MA, Scalfi L, Fogliano V. 2015. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr 101:251–261 10.3945/ajcn.114.088120. [PubMed] [DOI] [PubMed] [Google Scholar]
- 228.Quirós-Sauceda AE, Palafox-Carlos H, Sáyago-Ayerdi SG, Ayala-Zavala JF, Bello-Perez LA, Alvarez-Parrilla E, de la Rosa LA, González-Córdova AF, González-Aguilar GA. 2014. Dietary fiber and phenolic compounds as functional ingredients: interaction and possible effect after ingestion. Food Funct 5:1063–1072 10.1039/C4FO00073K. [PubMed] [DOI] [PubMed] [Google Scholar]
- 229.Jacobs DR Jr, Tapsell LC. 2007. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev 65:439–450. [DOI] [PubMed] [Google Scholar]
- 230.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. 2013. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24:1415–1422 10.1016/j.jnutbio.2013.05.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 231.O’Keefe SJD, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PGB, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. 2015. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 6:6342 10.1038/ncomms7342. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jenkins DJ, Kendall CW, Popovich DG, Vidgen E, Mehling CC, Vuksan V, Ransom TP, Rao AV, Rosenberg-Zand R, Tariq N, Corey P, Jones PJ, Raeini M, Story JA, Furumoto EJ, Illingworth DR, Pappu AS, Connelly PW. 2001. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 50:494–503 10.1053/meta.2001.21037. [PubMed] [DOI] [PubMed] [Google Scholar]
- 233.Kendall CW, Esfahani A, Jenkins DJ. 2010. The link between dietary fibre and human health. Food Hydrocoll 24:42–48 10.1016/j.foodhyd.2009.08.002. [DOI] [Google Scholar]
- 234.Delcour JA, Aman P, Courtin CM, Hamaker BR, Verbeke K. 2016. Prebiotics, fermentable dietary fiber, and health claims. Adv Nutr 7:1–4 10.3945/an.115.010546. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Martínez I, Perdicaro DJ, Brown AW, Hammons S, Carden TJ, Carr TP, Eskridge KM, Walter J. 2013. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl Environ Microbiol 79:516–524 10.1128/AEM.03046-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. 2015. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:26191. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bindels LB, Segura Munoz RR, Gomes-Neto JC, Mutemberezi V, Martínez I, Salazar N, Cody EA, Quintero-Villegas MI, Kittana H, de Los Reyes-Gavilán CG, Schmaltz RJ, Muccioli GG, Walter J, Ramer-Tait AE. 2017. Resistant starch can improve insulin sensitivity independently of the gut microbiota. Microbiome 5:12 10.1186/s40168-017-0230-5. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Burkitt DP, Walker ARP, Painter NS. 1972. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 2:1408–1411 10.1016/S0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 239.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. 2016. Population-level analysis of gut microbiome variation. Science 352:560–564 10.1126/science.aad3503. [PubMed] [DOI] [PubMed] [Google Scholar]
- 240.Cameron-Smith D, Collier GR, O’Dea K. 1994. Effect of soluble dietary fibre on the viscosity of gastrointestinal contents and the acute glycaemic response in the rat. Br J Nutr 71:563–571 10.1079/BJN19940163. [PubMed] [DOI] [PubMed] [Google Scholar]
- 241.Dikeman CL, Murphy MR, Fahey GCJ Jr. 2006. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr 136:913–919. [PubMed] [DOI] [PubMed] [Google Scholar]
- 242.Ferguson LR, Harris PJ. 2001. Adsorption of carcinogens by dietary fiber, p 207–213. In Cho SS, Dreher ML (ed), Hand-book of Dietary Fiber. Marcel Dekker, New York, NY. 10.1201/9780203904220.ch14 [PubMed] [DOI] [Google Scholar]
- 243.Gunness P, Gidley MJ. 2010. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct 1:149–155 10.1039/c0fo00080a. [PubMed] [DOI] [PubMed] [Google Scholar]
- 244.Anderson JW, Baird P, Davis RHJ Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. 2009. Health benefits of dietary fiber. Nutr Rev 67:188–205 10.1111/j.1753-4887.2009.00189.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 245.Li T, Chiang JYL. 2014. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983 10.1124/pr.113.008201. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shepherd R, Shepherd R. 2002. Resistance to changes in diet. Proc Nutr Soc 61:267–272 10.1079/PNS2002147. [PubMed] [DOI] [PubMed] [Google Scholar]
- 247.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. 2009. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137:588–597 10.1053/j.gastro.2009.04.046. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, Shi P. 2013. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics 14:669 10.1186/1471-2164-14-669. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


