Abstract
Pseudorabies virus (PRV) glycoprotein E (gE) is a type I viral membrane protein that facilitates the anterograde spread of viral infection from the peripheral nervous system to the brain. In animal models, a gE-null mutant infection spreads inefficiently from presynaptic neurons to postsynaptic neurons (anterograde spread of infection). However, the retrograde spread of infection from post- to presynaptic neurons remains unaffected. Here we show that gE is required for wild-type localization of viral structural proteins in axons of infected neurons. During a gE-null PRV infection, a subset of viral glycoproteins, capsids, and tegument proteins enter and localize to the axon inefficiently. This defect is most obvious in the distal axon and growth cones. However, axonal entry and localization of other viral membrane proteins and endogenous cellular proteins remains unaffected. Neurons infected with gE-null mutants produce wild-type levels of viral structural proteins and infectious virions in the cell body. Our results indicate that reduced axonal targeting of viral structural proteins is a compelling explanation for the lack of anterograde spread in neural circuits following infection by a gE-null mutant.
Neuroinvasion, by definition, describes the spread of an infection from sites in the periphery to the central nervous system (CNS) (spinal cord or brain). Viral infections of the CNS often lead to fatal encephalitis and are difficult to treat even with efficacious antiviral drugs (45). Members of the Alphaherpesvirinae subfamily such as herpes simplex virus (HSV) type 1 (HSV-1) and HSV-2 and varicella-zoster virus are neurotropic parasites of the peripheral nervous system (PNS) in their natural hosts. Unlike most other neurotropic viruses, alphaherpesviruses are rarely neuroinvasive in their natural hosts (e.g., humans for HSV and swine for pseudorabies virus [PRV]) despite an invariable infection of the PNS. After initial infection of the peripheral tissue, alphaherpesvirus virions will invade and establish a latent but reactivateable infection at the PNS neurons where they reside for life (34). This characteristic infection program occurs in the natural host without significant pathogenesis and without spread to the CNS. For healthy individuals, reactivation of latent infection usually results in the reinfection of peripheral tissue at the initial site of entry. Occasionally, for unknown reasons, productive replication continues unabated in the PNS and infection spreads to the CNS, causing lethal infection of the brain (13, 47). The molecular mechanisms that regulate this potentially devastating infection of the nervous system are poorly understood.
To study the mechanisms of directional spread of alphaherpesviruses in neurons, we focused our attention on an attenuated PRV strain called Bartha (24). PRV Bartha is selectively neuroinvasive. It is unable to invade the CNS by sensory (efferent) routes, and once in the CNS, it spreads only from postsynaptic to presynaptic neurons in a circuit-specific manner (4). This discriminatory neuroinvasiveness is due largely to a deletion in the Bartha genome, which removes the coding sequences of three PRV membrane proteins: glycoprotein E (gE), gI, and Us9. Deletion of any one of these three genes results in the selective neuroinvasive phenotype as well as a lower virulence (2, 6, 40). Since PRV Bartha replicates like wild-type virus in cell lines and primary cultured neurons, gE, gI, and Us9 most likely function specifically in regulating the directional spread of PRV infection from presynaptic to postsynaptic neurons (anterograde spread of infection). In animal models tested, all three viral mutants are also attenuated compared to a wild-type infection (2, 22, 38, 40).
The PRV gE and gI membrane proteins have distinct topologies. PRV gE is a type I transmembrane glycoprotein and is known to interact with gI, also a type I transmembrane protein, to form a heterodimer via ectodomain interactions (44). Current thinking is that the gE/gI proteins are multifunctional and appear to play a role in efficient cell-to-cell spread in nonneuronal cells, anterograde spread in synaptically connected neurons, species-specific binding of immunoglobulin G as Fc receptors, and mediators of full virulence in animal infections (17).
PRV gE is required for efficient cell-cell spread of infection but does not affect production of infectious extracellular virions, since replication and single-step growth of gE null mutants is normal (29). Indeed, gE and gI proteins are considered nonessential for replication in cell culture. Work from several laboratories indicates that the gE/gI complex plays distinct roles in secondary envelopment (31) and targeting and/or release of virions at cell-cell junctions (20). In HSV-1, the cytoplasmic domain of gE targets mature virions to basolateral junctions where spread to a neighboring cell occurs. In addition, expression of a truncated gE and gI at cell junctions decrease the spread of HSV between keratinocytes (9). Taken together, all these results indicate that gE protein is essential in promoting efficient targeting of virions and subsequent spread of infection at the basolateral cell-cell junctions.
A prevailing hypothesis states that gE is required at cell-cell junctions, e.g., synaptic junctions, to promote trans-neuronal spread of infection from presynaptic to postsynaptic neurons (13, 28). In this hypothesis, the absence of gE would result in accumulation of virions at the axon terminals on the presynaptic side of the terminal. An alternative hypothesis is that the defect in gE-null infections occurs in the neuron cell body rather than at axon terminals. Since gE has been shown to target mature virions to the cell-cell junction in epithelial cells, it is conceivable that a sorting or axonal entry defect would prevent the virion from being transported to the axon terminal and subsequently infect the postsynaptic neuron. In either model, the gE-null defect affects only the anterograde spread of virions from pre- to postsynaptic neurons and not the production of virions in the neuron soma or the retrograde spread of infection from post- to presynaptic neurons. To test the hypotheses, we cultured sympathetic neurons and infected them with PRV. In the absence of gE, we show a dramatic reduction of viral glycoproteins (gB, gD, and gC) in axons. Furthermore, viral capsid (VP5, VP26) and tegument (VP22) proteins also fail to enter axons efficiently. This surprising result suggests that gE plays a central role in entry of and/or targeting a subset of viral proteins to axons and explains why anterograde spread of infection via axons is compromised in gE-null mutant infections of animal models.
MATERIALS AND METHODS
Virus strains and cells.
All PRV strains used in the study were grown and passaged in a porcine kidney cell line (PK15). The replication defective adenovirus that expresses chick NgCAM (AdNgCAM) was kindly provided by the Mellman laboratory (46) upon receiving permission from the Sonderegger laboratory (43). AdNgCAM was passaged on a Swiss 3T3 cell line (American Type Culture Collection). The wild-type strain of PRV used in this study is called PRV Becker (33), while the gE-null strain (PRV 91) is isogenic with PRV Becker, as previously described. Another strain of gE-null PRV (PRV 758) was produced from a PRV infectious clone (37) carrying a deletion of the gE open reading frame. Both PRV 91 and PRV 758 were constructed using the parental plasmid pALM91 (6). Both mutants share identical phenotypes and do not express gE, as confirmed by Western blot analyses. All experiments involving gE-null PRV refer to the PRV 758 strain unless otherwise stated. PRV 758R (gE-null revertant) was constructed from PRV 758. PRV 107 was previously made and characterized (38). PRV 756 (gE-ectodomain null) was constructed from a plasmid pALM93, which was previously published (44). Briefly, pALM93 was digested with StuI and MluI to liberate a fragment of the PRV genome that contains gE and its flanking region. This fragment was cloned into pGEM5zf(+) and designated pTH27. To remove the gE ectodomain, pTH27 was digested with XcmI and SrfI and replaced with a PCR-amplified fragment that contains an 8-amino-acid FLAG epitope tag (amino acids DYKDDDK) with a GSG linker (pTH31; forward oligonucleotide, 5′-GGAGCCCAAGATGACGTTGGCCGAGC-3′; reverse oligonucleotide, 5′-TCCACTTCCTTTGTCATCGTCGTCCTTGTAGTCGGTCGTCTCGGCGGAGAG-3′). pTH31 was subsequently digested with FspI and NsiI, subcloned into the bacterial artificial chromosome transfer vector (pTH36), and sequenced, and allelic exchange was carried out to transfer the gE deletion into the bacterial artificial chromosome infectious clone (36) (pTH55). Infectious virions were harvested via electroporation of pTH55 in PK15 cells. PRV 181 expresses dual fluorescent fusion proteins monomeric red fluorescent protein (mRFP)-VP26 and green fluorescent protein (GFP)-VP22 (11). All three dual fluorescent fusion mutants bearing gE mutations were constructed by recombining the viral genomes of PRV 181 with either PRV 758 (gE null), PRV 107 (gE cytoplasmic tail null), or PRV 756 (gE ectodomain null) via coinfection and then subsequently screening for viral progeny that express both fluorescent fusion proteins in the absence of gE.
Neuronal cultures.
Sympathetic neurons from rat superior cervical ganglia were harvested from embryonic day 15.5 to 16.5 rat embryos (Hilltop Labs, Inc.). Culture conditions and dissection methods were previously described by Ch'ng et al. 2005 (7), modified from Tomishima and Enquist (42). Delta TPG glass-bottom dishes (Bioptechs, Inc.) were coated with 100 μg/ml poly-dl-ornithine (Sigma-Aldrich) and 10 μg/ml of natural mouse laminin (Invitrogen). The neuron cultures were maintained in Dulbecco's modified Eagle (Gibco) and Ham's F-12 (Gibco) in a 1:1 ratio and supplemented with 10 mg/ml of bovine serum albumin (BSA; Sigma-Aldrich), 4.6 mg/ml glucose (J. T. Baker), 100 μg/ml of holo-transferrin (Sigma-Aldrich), 16 μg/ml of putrescine (Sigma-Aldrich), 10 μg/ml of insulin (Sigma-Aldrich), 2 mM l-glutamine (Gibco), 50 μg/ml or units of penicillin and streptomycin (Gibco), 30 nM selenium (Sigma-Aldrich), 20 nM progesterone (Sigma-Aldrich), and 100 ng/μm of nerve growth factor 2.5S (Invitrogen). Nonneuronal cells were eliminated with 1 μM/ml of cytosine-β-d-arabinofuranoside. All experimental protocols related to animal use have been approved by The Institutional Animal Care and Use Committee (IACUC) of the Princeton University Research Board under IACUC protocol number 1453-AR2.
Antibodies.
Mouse monoclonal antibody (MAb) specific for SV2 and cNgCAM (8D9) were purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. The mouse MAb against actin (AC-40) was purchased from Sigma (A-4990), and the antibodies that recognize mannosidase II were purchased from Covance (53FC3). Mouse MAbs that recognize PRV-specific proteins, gB (M2) and gC (M1), were described in a previous study (14). The rabbit polyclonal antiserum specific for gE was also described in a previous study (40). IN-13 mouse MAb that recognizes VP5 was a gift from H. Rhiza (Federal Research Center for Viruses Diseases for Animals, Tubingen, Germany). The pooled gD mouse MAb was a kind gift from G. Cohen (Department of Microbiology, University of Pennsylvania). The Us9-specific rabbit polyclonal antiserum was generated by A. Brideau and was previously described (3). The goat polyclonal antiserum raised against Us2 was a gift from B. Banfield (Department of Microbiology, University of Colorado Health Sciences Center). The rabbit polyclonal antiserum against VP22 and the mouse MAb (3C10) that recognizes the major capsid protein VP5 were gifts from T. del Rio and A. Flood from the Enquist lab. The horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary immunoglobulin G antibodies used in the Western blots were purchased from Kierkegaard and Perry Labs, Inc. All secondary fluorophores used in immunofluorescence experiments were Alexa Fluor dyes (Molecular Probes).
Viral infections.
Protocols for viral infection of neurons have been described by Ch'ng et al. (7). All PRV infections of neuron cultures were carried out under high multiplicities of infection (MOI) unless otherwise stated. Briefly, neurons were cultured on Delta TPG dishes for approximately 2 to 3 weeks prior to any experiment. The viral inoculum was diluted in 2% fetal bovine serum in Dulbecco's modified Eagle (Gibco) and overlaid on the neuronal culture for 1 h in a humidified 37°C incubator. After 1 h, the viral inoculum was removed and replaced with neuron medium. Infections usually lasted for 14 to 16 h before the samples were fixed and processed for immunofluorescence. For experiments using AdNgCAM, neuron cultures were first infected at a high MOI for 24 h with AdNgCAM using the standard infection protocols described above. At 24 h postinfection (hpi), a superinfection was carried out with either a mock sample or samples infected with PRV Becker or gE-null PRV for another 15 h. Cultures were fixed and processed for immunofluorescence.
Fluorescence and immunofluorescence microscopy.
All fluorescence experiments were carried out based on previously described protocols (7). Briefly, neuron cultures were infected for 14 to 16 hpi, washed once with phosphate-buffered saline (PBS), fixed for 10 min with 3.2% paraformaldehyde (PFA), and rinsed three times with PBS containing 3% BSA (PBS-BSA). For direct fluorescence experiments, the process was completed, a drop of Aqua Poly/Mount (Polysciences, Inc.) mounting medium was added to the floor of the dish, and a coverslip was placed on top of the drop. The samples were left overnight for the Aqua Poly/Mount to harden. For indirect immunofluorescence studies, neurons were permeabilized with 3% BSA and 0.5% saponin after 3.2% PFA fixation. The permeabilized cells were then incubated with primary antibodies for 1 h followed by the secondary antibodies for another 1 h. After each incubation, the neurons were rinsed three times with PBS-3% BSA and 0.5% saponin. After the final rinse, a drop of Aqua Poly/Mount was added to the floor of the dish and a coverslip was placed on top of the drop. Samples were allowed to dry prior to any microscopy work. For nonpermeabilized surface staining of infected cultures, detergent was omitted from each step of the protocol described above. All confocal micrographs were collected with a Zeiss LSM510 laser scanning microscope with either a 40× or 63× oil objective. The confocal sections obtained were either through the focal plane of the cell body or the focal plane of the axon, depending on experimental parameters. All images were processed with Adobe Photoshop (version 6.0) and assembled in Adobe Illustrator (version 9.0).
Kinetics of PRV infection in neuronal cultures.
Neuron cultures were allowed to mature for 3 weeks before being infected at a high MOI with wild-type PRV or PRV 91 as described in the previous section with one additional step: after the neuron cultures were incubated for 1 h in viral inoculum, the inoculum was removed and incubated with a citrate wash (40 mM citrate, pH 3, 10 mM KCl, 0.135 M NaCl) for 1 min. The citrate wash inactivated the input virus. After the incubation, the citrate wash was removed and the infected cultures were rinsed twice with PBS and replaced with the original culture medium. For each virus, samples were collected at four time points, t = 0, 10, 16, and 24 hpi, with the sample collected at t = 0 being the first sample collected immediately after the citrate wash. At each time point, the medium was removed, the infected cell bodies were harvested, and titers were determined on PK15 cells. Note that while infectious particles can be detected in the medium, we did not present the extracellular titer data because the accurate PFUs cannot be determined due to the affinity of extracellular particles to the surface of the dish.
Western blot analysis of infected neuronal cultures.
Neuron cultures were plated at high density (approximately one ganglion/dish) on 35-mm tissue culture dishes (Falcon). The cultures were maintained for 1 month to allow growth and expansion of the neuritic network. After 1 month, the cultures were infected with wild-type PRV, PRV gE null, and PRV gE null revertant according to the protocols described in the section above. At 16 hpi, the infected neurons were washed once with PBS and harvested. The harvested neurons were pelleted, lysed with loading buffer, boiled for 10 min, and then sheared with a 26-gauge needle before being loaded in equal volumes and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein gel was transferred onto a nylon membrane and Western blotted for various viral structural proteins. Cellular actin served as a loading control.
Quantitation of viral glycoproteins.
Neuron cultures were grown, infected, and processed for indirect immunofluorescence as described above. For quantitation purposes, all images were collected with a Nikon Eclipse TE-300 inverted epifluorescence microscope with a 20× objective lens. Ten images were collected for each viral infection, and five images were collected for each mock infection. To ensure that the axonal densities were comparable between images, each image collected contained approximately 10 neuron cell bodies. All images were converted to grayscale and inverted using Adobe Photoshop. To quantify the immunofluorescence intensity of the axonal network, software called Scion Image for Windows (version 4.0.2; Scion Corp.) was used. A density threshold was uniformly applied to all the images, and a filter was applied to block any signals coming from the neuron cell body. The average pixel density was calculated and plotted on a bar graph. Error bars reflect ±2 standard errors of the means.
RESULTS
Subcellular localization of viral gE in dissociated sympathetic neurons during wild-type PRV infection.
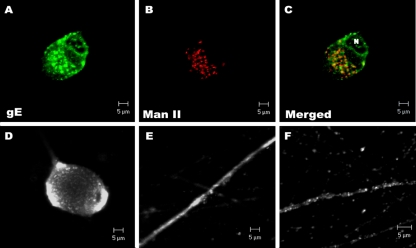
Dissociated superior cervical ganglia neuron cultures were infected with PRV Becker, fixed with PFA, and labeled with gE-specific antibodies. At 4 hpi, gE can easily be detected in the cell bodies but is absent in axons (unpublished data). At 14 hpi, gE is abundant in both cell bodies and axons. Confocal micrographs collected at the plane of the neuron cell body show that gE is predominantly localized to the Golgi (Fig. 1A). Antibodies specific for mannosidase II, a cis-medial Golgi marker, colocalized with gE in the cell bodies (Fig. 1B and C). In nonpermeabilized samples, gE is readily detected on the surfaces of cell bodies (Fig. 1D) and the surfaces of axons (Fig. 1E). Unlike the permeabilized immunofluorescence staining, surface expression of gE is uniform and nonpunctate. In axons, gE appears as individual, punctate, immunoreactive vesicles, the distribution of gE immunoreactivity within the length of the axon varies with certain regions more heavily labeled with gE than others (Fig. 1F).
FIG. 1.
Steady-state localization of gE in infected neuron cultures. Cultured neurons were infected with wild-type PRV, and at 14 hpi, neurons were labeled with various antibodies in permeabilized (A, B, C, F) or nonpermeabilized (D, E) neurons. Antibodies specific for gE were used to determine steady-state localization of gE (A, D to F), while antibodies raised against mannosidase II (Man II) were used as cis and medial Golgi markers (B). (C) Colocalization of gE (green) and mannosidase II (red) is shown in the merged image. N, nucleus.
gE is required for wild-type axonal localization of viral structural glycoproteins.
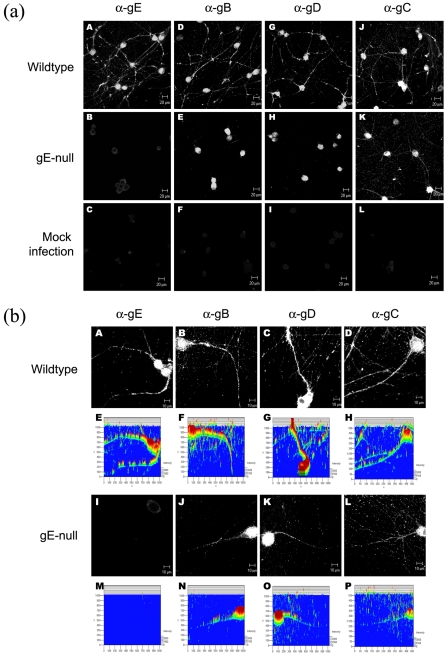
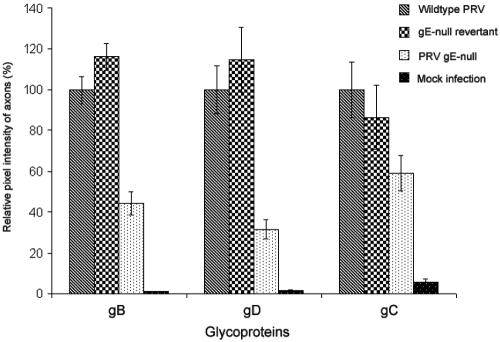
Recently, Tomishima and Enquist reported that the type II, tail-anchored viral membrane protein Us9 was essential for axonal localization of viral glycoproteins (42). They also stated that gE mutants had no such effect. However, when we developed quantitative techniques to measure glycoprotein content in axons, we found that neurons infected with gE-null PRV expressed significantly lower amounts of viral glycoproteins in axons compared to a wild-type infection (Fig. 2a). The apparent disparity in these results and those of Tomishima and Enquist is resolved upon closer examination of the proximal and distal regions of the axon. In wild-type PRV-infected neurons, glycoproteins are found in both the proximal and distal regions of the axon (Fig. 2b, A to D). However, in gE-null mutant-infected neurons, glycoproteins localize primarily to the proximal segment of the axon (Fig. 2b, I to L), while the intensity of the immunofluorescence signal rapidly fades away in the distal segment of the axon (Fig. 2b, E to H and M to P). Our findings are consistent with the published observations of Tomishima and Enquist that glycoproteins in gE-null mutant-infected neurons are localized mainly to the proximal segment of the axon. To further confirm the gE-null phenotype, we quantitated the immunofluorescence intensity of glycoproteins in axons (as described in Materials and Methods). Neurons infected with wild-type and gE-null revertant viruses express similar amounts of axonal glycoproteins. In contrast, neurons infected with gE-null mutant virus have reduced amounts of gB, gD, and to a lesser extent, gC, in their axons (Fig. 3).
FIG.2.
gE is required for wild-type axonal localization of viral glycoproteins. (a) Cultured neurons were infected with either wild-type PRV (A, D, G, J) or gE-null PRV (B, E, H, K) or mock infected (C, F, I, L). At 14 hpi, cells were fixed, permeabilized, and labeled with antibodies specific for gE (A, B, C), gB (D, E, F), gD (G, H, I), and gC (J, K, L). α-, anti-. (b) The same experiment as for panel a, but confocal images were obtained at a higher magnification. 3-D representations of the immunofluorescence signals were obtained using Zeiss LSM 510 software (E to H and M to P) to compare the intensity of axonal staining in a wild-type and gE-null infection.
FIG. 3.
Quantitation of viral glycoproteins in infected axons. Quantitation of viral glycoproteins in axons was carried out for wild-type, gE-null, gE-null revertant, and mock-infected neuron cultures. At 14 hpi, samples were fixed and processed for immunofluorescence and images were collected via epifluorescence microscopy. Using protocols described in Methods and Materials, we calculated the relative immunofluorescence intensity of the glycoproteins in axons.
gE is not required for wild-type axonal localization of two membrane-associated, nonglycosylated viral proteins.
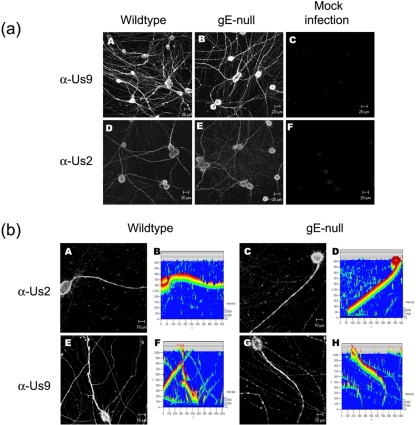
As Us9 is required for axonal localization of viral glycoproteins, it is important to demonstrate that the expression or subcellular localization of Us9 protein is not affected by PRV gE-null mutant infections. Figure 4a (A to C) indicates that Us9 localization in wild type- and gE-null mutant-infected neurons is similar, if not identical. Indeed, in either infection, Us9 is easily detected throughout the neuronal network. A closer examination of individual neurons confirms that subcellular distribution of Us9 in cell bodies and axons is unchanged during a gE-null mutant infection (Fig. 4b, E to H). Viral glycoproteins like gB, gC, gD, and gE are posttranslationally modified in the Golgi apparatus, but nonglycosylated viral membrane proteins are also well known. Us9 is a type II tail-anchored integral membrane protein (1), while Us2 associates with the plasma membrane through prenylation of its CAAX motif (8). Interestingly, both Us9 and Us2 localize to the axonal compartment independent of gE expression (Fig. 4a). A magnified image of individual neurons reveals that subcellular distribution of Us9 and Us2 appears identical in either wild type- or gE-null mutant-infected neurons (Fig. 4b).
FIG. 4.
gE is not required for wild-type axonal localization of viral membrane proteins. (a) Cultured neurons were infected with either wild-type PRV (A, D) or gE-null PRV (B, E) or mock infected (C, F). At 14 hpi, cells were fixed, permeabilized, and labeled with antibodies that are specific for viral membrane proteins Us9 (A, B, C) or Us2 (D, E, F). α-, anti-. (b) This experiment is similar that described for panel a, except the confocal images were magnified. Both wild-type PRV (A, E) and gE-null PRV (C, G) infections were labeled with antibodies directed against Us9 (E, G) and Us2 (A, C). 3-D reconstructions of the confocal images are also shown (B, D, F, H).
gE expression does not disrupt axonal localization of cellular proteins.
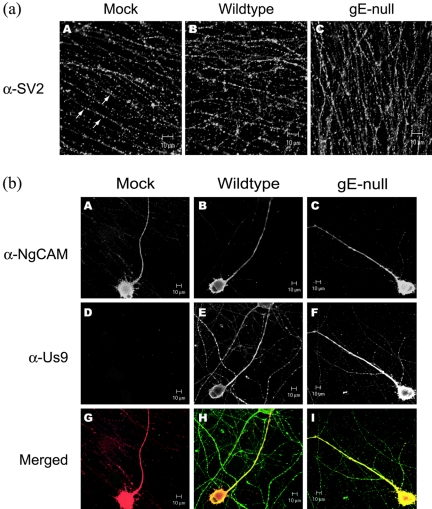
While a PRV infection affects various cellular processes, the neuronal secretory pathway is unaffected during infection and host proteins are capable of entering axons independent of gE expression. For example, the synaptic vesicle protein (SV2) is detected as punctate, vesicular structures in the axonal network of all three infected neuron cultures (Fig. 5a). Similarly, the localization of an ectopically expressed axonal protein, NgCAM, a well-characterized cell adhesion molecule, is unaffected by PRV infection. Neurons were initially infected with a defective adenovirus vector that expresses the chicken isoform of NgCAM for 24 h. About 20% of the neurons in the culture infected by the adenovirus expressed NgCAM. After 24 h, the neurons were superinfected with wild-type or gE-null PRV or mock infected. Fourteen hours after superinfection, neurons were indirectly labeled with antibodies specific for NgCAM and Us9. Figure 5b shows that both Us9 and NgCAM are found in abundance in both cell bodies and axons independent of gE expression. Taken together, these two experiments indicate that the neuronal secretory pathway is not disrupted during infection and gE does not affect axonal localization of endogenous and exogenously expressed neuronal proteins.
FIG. 5.
gE does not affect localization of cellular proteins. (a) Cultured neurons were mock infected (A) or infected with either wild-type PRV (B) or gE-null PRV (C). At 14 hpi, cells were fixed, permeabilized, and labeled with antibodies that recognize SV2 (α-SV2). The arrows indicate the presence of individual synaptic vesicles within the neuritic network. (b) Cultured neurons were initially infected with a replication-defective adenovirus expressing a chick isoform of the neural-glia cell adhesion molecule (cNgCAM). At 24 h after the adenoviral infection, the neuron cultures were then mock infected (A, D, G) or superinfected with either wild-type PRV (B, E, H) or gE-null PRV (C, F, I). The cultures were further incubated for another 14 h before being fixed, permeabilized, and labeled with antibodies specific for cNgCAM (A, B, C) and Us9 (D, E, F) as controls for infection. Merged images (G, H, I) show localization of Us9 (green) and cNgCAM (red).
gE is required for wild-type axonal localization of viral tegument protein VP22 and capsid proteins VP5 and VP26.
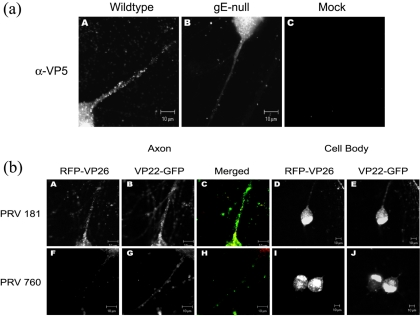
PRV DNA is packaged in an icosahedral capsid structure within the nucleus prior to localization to axons for long-distance transport. However, it is unclear how tegument proteins enter and are transported in axons. Tegument proteins could enter axons and be transported within vesicles, in association with capsids, with glycoproteins, or a combination of all three modes of targeting and transport. Previously, Tomishima and Enquist reported that only viral glycoproteins require Us9 to enter axons, while capsids and tegument proteins entered axons independent of Us9 expression. In contrast, we discovered that not only glycoproteins but also capsids and some tegument proteins require gE to enter axons efficiently. At 4 hpi with a wild-type virus, capsid protein VP5 was detected primarily in the nuclei of cell bodies but not in axons (unpublished data). At 14 hpi with a wild-type virus, VP5 is found localized to the cell body and the entire length of the axon. Capsid proteins were usually visible as uniform punctate structures in axons (Fig. 6a). In gE-null PRV mutant-infected neurons, capsid proteins filled the nuclei and cell body but were markedly reduced in axons (Fig. 6a, A to C) Like glycoproteins, capsid proteins can still be detected in axons during gE-null mutant infections, but the amount is markedly reduced compared to wild-type infection and visible primarily in the proximal segment of the axon. To confirm these findings, we constructed recombinant viruses that express fluorescent fusion proteins. First, we built PRV 181 by fusing red fluorescence protein (RFP) and enhanced GFP to the amino termini of capsid protein VP26 and tegument protein VP22, respectively. Both RFP-VP26 and GFP-VP22 are packaged into virions and behave like wild-type virus in cell cultures (11). To produce a gE-null mutant bearing the fluorescent fusion proteins, we crossed PRV 181 with a gE-null virus, creating PRV 760. Next, we infected cultured neurons with PRV 181 and PRV 760 and imaged live neurons using confocal microscopy (see Materials and Methods). As expected, we observed that during the early stages of infection, RFP-VP26 and GFP-VP22 localize primarily to the nuclei and cannot be detected in axons (unpublished data). By 14 hpi for PRV 181, both VP26 and VP5 fusion proteins were visible throughout the cell body and the axon compartment (Fig. 6b, A to E). In contrast, by 14 hpi with PRV 760, the cell body was filled with both fusion proteins (Fig. 6b, I and J), but neither RFP-VP26 nor GFP-VP22 was visible in the distal axons (Fig. 6b, F to H). A small number of capsids can be observed in the proximal axon, but most remained in the cell body, leading to a subtle increase in the mRFP signal in the cytoplasm of PRV 760-infected neurons. Next, we constructed PRV 759, a gE-null mutant virus that expresses only RFP-VP26. As with PRV 760, we observed a significant reduction of RFP-VP26 in axons (unpublished data). Based on the experiments above, we conclude that gE is required for efficient wild-type localization of viral capsids and at least one tegument protein to axons.
FIG. 6.
gE is required for wild-type axonal localization of viral capsid proteins and viral tegument protein VP22. (a) gE is required for wild-type axonal localization of viral capsid VP5. Cultured neurons were infected with either wild-type PRV (A) or gE-null PRV (B) or mock infected (C). At 14 hpi, cells were fixed, permeabilized, and labeled with antibodies specific for the viral capsid protein VP5 (α-VP5). (b) Cultured neurons were infected with either PRV 181 (A to E) or PRV 760 (F to J). At 14 hpi, cells were fixed and confocal images were collected for RFP-VP26 (A, F, D, I) or GFP-VP22 (B, G, E, J) either through the plane of the axon (A, C, F, H) or through the plane of the neuron cell body (D, E, I, J).
Neurons infected with a gE-null mutant produce wild-type levels of viral structural proteins.
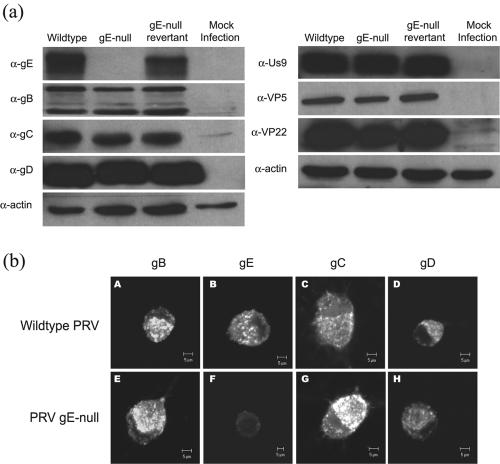
Cultured neurons were infected with wild-type PRV or gE-null mutant or gE-null revertant virus or were mock infected. After 18 hpi, whole-cell lysates were loaded in equal volumes, analyzed via SDS-PAGE, and subsequently Western blotted for selected viral structural proteins (Fig. 7a). The steady-state expression of viral glycoproteins (gB, gC, and gD), viral capsid (VP5), viral tegument (VP22), and viral membrane protein (Us9) are identical in all infections. In addition, the relative ratio of mature versus immature glycoproteins produced during a gE-null mutant infection is comparable to that of a wild-type infection. In data not shown, we found that the amount of gI protein in infected cells was unaffected. We conclude that production and processing of viral structural proteins are not affected during a gE-null mutant infection and that posttranslational processing of viral glycoproteins in the Golgi compartment is independent of gE expression.
FIG. 7.
Viral structural proteins are expressed at wild-type levels in infected neurons and have wild-type localization in the neuron cell bodies. (a) Steady-state expression of viral structural proteins during infection of cultured neurons was determined via Western blots. Cultured neurons were infected at a high MOI with wild-type PRV, gE-null PRV, gE-null revertant PRV or mock infected. At 16 hpi, the infected neuron lysates were harvested, analyzed via SDS-PAGE, and Western blotted for viral glycoproteins (gE, gB, gD, and gC), membrane protein (Us9), and tegument (VP22) and capsid (VP5) proteins. The total amount of actin served as a loading control. (b) Steady-state expression of viral glycoproteins in cell bodies was determined for wild-type PRV- and gE-null PRV-infected neurons. At 14 hpi, cultures were labeled with antibodies specific for gB (A, E), gE (B, F), gC (C, G), and gD (D, G). α-, anti-.
Localization of viral glycoproteins in neuron cell bodies is indistinguishable in wild type- and gE-null mutant-infected neurons.
To ensure that the absence of gE does not alter the localization of other viral proteins, neurons were infected with the wild type or gE-null mutants and stained for viral gB, gC, gD, and gE. Confocal images were taken at the plane of the cell bodies. Figure 7b (A to D) depicts a typical wild-type subcellular distribution of glycoproteins in the Golgi compartment. These images are similar, if not identical, to those observed during a gE-null mutant infection (Fig. 7b, E to H). While the subcellular distributions of glycoproteins in cell bodies appear normal, the overall intensity of glycoproteins within the cell bodies is slightly elevated during a gE-null mutant infection. This observation may reflect the bottleneck created by the inability of glycoproteins to enter axons.
Absence of gE has no effect on attachment, entry, or replication of PRV in neurons.
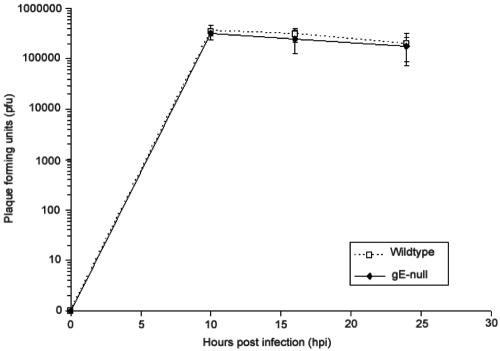
gE has not been reported to function during attachment, entry, or replication of PRV in epithelial cells. To confirm these results in neurons, we infected neurons at a high multiplicity of infection with the wild type or gE-null mutants and measured the amount of PFU recovered. Cultures were briefly treated with a citric acid buffer after the 1-h incubation to eliminate the infectivity of any extracellular virions. At various time points after infection, the amount of newly produced intracellular infectious particles was determined on a monolayer of PK15 cells as described in Materials and Methods. As shown in Fig. 8, both wild-type and gE-null mutant infection produce identical amounts of infectious particles.
FIG. 8.
The kinetics of infectious virus production after a gE-null infection are identical to those for a wild-type infection in cultured neurons. Cultured neurons were infected with either wild-type PRV or gE-null PRV. At 0, 10, 16, and 24 hpi, the amount of infectious intracellular virus produced by the neurons was harvested and titers were determined on PK15 cells. This experiment was done in triplicate, and the standard deviations for each time point were calculated and shown as error bars on the graph.
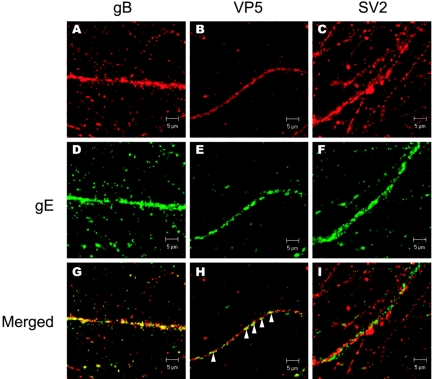
gE colocalizes with virion proteins but not with SV2.
To examine whether viral capsids and glycoproteins are being packaged in synaptic vesicles, neurons were infected with wild-type PRV, fixed, and stained with antibodies specific for gE along with antibodies specific for either gB, VP5, or SV2. Distinct puncta of immunofluorescence was visible along the extent of each infected axon. While colocalization of gE with any one protein is evident to a certain extent, the degree of colocalization varies with each individual protein. For example, gE and gB have similar axonal localization profiles and their immunofluorescence signals often overlap in the entire axonal compartment (Fig. 9A, D, and G). gE and VP5 immunofluorescence signals also partially colocalized at distinct regions in axons (Fig. 9B, E, and H) but to a lesser extent than gE and gB colocalization. In contrast, gE and SV2 rarely colocalized in axons (Fig. 9C, F, and I).
FIG. 9.
gE colocalizes with viral glycoproteins and capsids but not with synaptic vesicle protein. Cultured neurons were infected with wild-type PRV for 14 h, fixed, permeabilized, and labeled with antibodies that recognize gE (D to F) and either gB (A), VP5 (B), or SV2 (C). Merged images are shown in panels G to I. Arrowheads indicate colocalization of gE and VP5 in axons.
Both gE protein domains function in targeting viral proteins to axons.
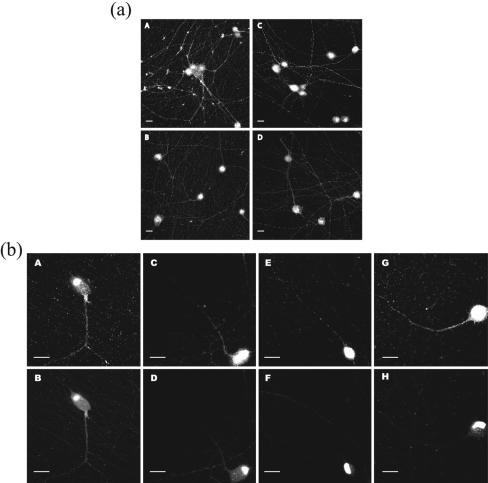
The gE cytoplasmic tail domain is essential for endocytic retrieval of the protein from the plasma membrane and contributes to gE-promoted virulence (38, 39). This domain is also required for efficient cell-cell spread in plaque formation and reduces the kinetics of anterograde trans-neuronal spread (39). The contribution of the gE ectodomain to any of these phenotypes has not been studied. Accordingly, we infected neurons with mutant viruses that express a truncated gE protein lacking the cytoplasmic tail (PRV 107) or the gE ectodomain (PRV 756). When compared to a wild-type infection, neurons infected with both PRV 107 and PRV 756 had comparable amounts of gB in the cell body but reduced amounts of gB in axons (Fig. 10a). Nevertheless, we consistently observed a slight increase in gB antigen in axons after infection with PRV107 mutant than with the gE null mutant. The data implicate both gE protein domains in the efficient localization of glycoproteins to axons.
FIG. 10.
The gE ectodomain and cytoplasmic tail are required for efficient targeting of viral proteins to axons. (a) Cultured neurons were infected with wild-type PRV (A), PRV 758 (B), PRV 107 (C), and PRV 756 (D) for 14 h, fixed, permeabilized, and labeled with antibodies that recognize gB. (b) Cultured neurons were infected with PRV 181 (A, B), PRV 760 (C, D), PRV 763 (E, F), and PRV 762 (G, H) for 14 h, fixed, and mounted on glass slides. Fluorescence images for both RFP-VP26 (top row; A, C, E, and G) and GFP-VP22 (bottom row; B, D, F, and H) were captured via confocal microscopy. Bars, 20 μm.
To visualize capsid and tegument proteins in axons without resorting to antibody detection methods, we recombined the various gE mutations into the dual-fluorescent fusion virus PRV 181 (RFP-VP26/GFP-VP22). To accomplish this task, we coinfected PRV 181 with PRV 107, PRV 758, and PRV 756 individually and isolated recombinant dual-fluorescent fusion virions with their corresponding deletions in gE. Cultured neurons were then infected with the dual-fluorescent gE mutants PRV 760 (gE null), PRV 762 (gE cytoplasmic tail null), and PRV 763 (gE ectodomain null), fixed, and compared with PRV 181-infected neurons (Fig. 10b). In neurons infected with PRV 181, capsids are localized in axons and in growth cones in abundance. However, in PRV 760-infected neurons, the capsids in axons are dramatically reduced and cannot be detected in growth cones. With neurons infected with PRV 762 and PRV 763, capsids were also dramatically reduced in both axons and growth cones, although the amount in PRV 762-infected neurons appears to be slightly higher. As expected, neurons infected with each of the three gE mutants also have little to no tegument protein GFP-VP22 in the axons. The data confirm our previous findings that both the gE cytoplasmic tail and the gE ectodomain play a role in axonal targeting of viral proteins. While the severity of the axonal targeting defect in the gE mutant infection has not yet been rigorously characterized in animal models, it is likely that both domains contribute to wild-type function of gE in sorting or targeting of viral cargo into axons.
DISCUSSION
In nonneuronal cells, it is thought that final (secondary) envelopment of PRV virions occurs when cytoplasmic capsids bud into a specialized vesicular compartment to acquire a mature envelope. This compartment has been postulated to be part of the trans-Golgi network, a late endosome, or a specialized viral compartment (27). In this model, the mature virion leaves the compartment in a transport vesicle and exits the cell via exocytosis. In PNS neurons, secondary envelopment presumably occurs in the cell body with release of infectious virions. However, in addition to local release of virions, the infection must spread to the CNS or back to the surface via long-distance movement in axons. The nature of this long-distance movement of infection via axons remains contentious. Electron microscopy studies suggest that mature virions produced from cell bodies move through the axons in transport vesicles (5, 10, 11, 15, 16, 21, 23, 26, 48). However, several studies indicate that virions are not transported fully assembled, but rather, separate structural components, or subassemblies, are moved in axons (30, 32, 42). From these studies, the authors concluded that unenveloped capsids and viral glycoproteins are transported independently in axons and secondary envelopment of these subassemblies subsequently occurs in distal sites of the axon and axon terminals.
In this study, we show that gE is required for efficient entry of viral glycoproteins, capsids, and at least one tegument protein into axons. This defect becomes more pronounced in distal axons and growth cones. The neuronal secretory pathway remains functional in wild-type and gE-null mutant infections. Our findings for gE-null and Us9-null mutant viruses are consistent with the hypothesis that spread of infection from neurons occurs in two distinct and independent pathways in cultured neurons (12, 13, 28, 32). One pathway involves a gE-, gI-, and Us9-independent assembly and release of virions from the neuronal cell body. The other pathway involves axonal entry of viral proteins required for long-distance spread of infection via axons. The efficiency of axonal entry of these viral proteins depends on gE, gI, and Us9.
Most studies on compartmentalization of axonal proteins focus on the sorting of individual proteins. What makes viral proteins such as gE and Us9 remarkable and noteworthy is not only must they enter axons themselves but they also must direct and regulate entry of virus structural proteins into axons. So, how does gE function in localizing viral cargo to axons? Based on our current understanding of polarized trafficking of axonal proteins, we speculate that gE functions in a cell body sorting compartment that is part of the trans-Golgi network and associated with sites of viral assembly in the neuron cell body. In this specialized compartment, gE may assume several roles such as recruiting and localizing viral glycoproteins and assembled capsids to budding sites where they will exit the compartment in transport vesicles destined for the axon. Alternatively, gE might bind the cellular machinery normally required for axonal targeting, such as the fast axonal transport complex. Whether gE actively recruits viral proteins to the compartment or interacts directly with proteins involved in axonal transport, the loss of gE ultimately will block a pre- to postsynaptic transmission of infection while having no affect on the production of extracellular virions from the cell body.
Our data support the proposal that viral capsids are transported in membrane vesicles rather than as unenveloped capsids. The gE protein is inserted in lipid membranes yet regulates axonal entry of viral capsids. It is hard to envision how gE could regulate capsid entry into axons if the capsids were not associated with membrane vesicles. A long-standing idea was that herpes virions might be transported in synaptic vesicles carrying neurotransmitters. This idea seems unlikely to be valid, as we have been unable to prove colocalization of synaptic vesicle proteins such as SV2 and VAMP2 with viral structural proteins. The nature of the membranes surrounding PRV virion components remains to be discovered. All of our findings are consistent with the idea that not all viral proteins or protein complexes moved into axons need be localized to the same compartment. Viral membrane proteins are often inserted into axonal membranes independently and found on the axolemma, and some tegument protein and capsid structures are often found in separate complexes in axons (11, 25).
Our initial experiments to localize the domains of the gE protein required for the axonal sorting function indicated that both the cytoplasmic tail and ectodomain are required for efficient targeting of viral cargo to axons. A loss of either domain resulted in reduced axonal localization of viral proteins, particularly to the distal regions of the axon. These data are consistent with published work using gE cytoplasmic tail mutants and our own unpublished data from rat eye infection studies indicating that both gE domains affect rate, or efficiency of spread, of infection to second-order retinorecipient neurons (38; unpublished data). The gE cytoplasmic domain encodes several sorting signals including the canonical tyrosine-based “YXXΦ” endocytosis motif at amino acids 478 and 517, an aspartate-rich acidic cluster domain, and dileucine motifs (41). It might be expected that loss of the cytoplasmic tail may prevent gE-mediated targeting of viral cargo to axons. Similarly, the gE ectodomain may also harbor motifs responsible for axonal targeting, as has been described for the type 1 membrane axonal protein called NgCAM. Indeed, like gE, NgCAM has axonal targeting motifs in both the cytoplasmic and ectodomains (35). Alternatively, the gE ectodomain might function indirectly by aiding the gE cytoplasmic tail in directing viral proteins into axons by forming a complex with gI (19, 40, 44). Since the cytoplasmic domains of either gE or gI are required for efficient pre- to postsynaptic spread of infection, formation of a stable complex through ectodomain interactions may facilitate wild-type spread of the virus by either cytoplasmic domain (38).
It is important to stress that absence of gE does not completely abolish localization of viral structural components in axons. The gE, gI, and Us9 proteins appear to have an additive effect in axonal targeting (unpublished data). This finding was first observed in vivo in PRV Bartha-infected neurons where the defect in axonal localization of viral proteins was markedly severe, with essentially no viral structural proteins detected in the axons (Fig. 3 and data not shown) (6). The gE-null defect in entry and transport of viral proteins is most prominent in the distal regions of the axon at varicosities and at growth cones because the phenotype is amplified by requiring that viral proteins travel the entire length of the axon. While our results indicate that gE is required for axonal targeting of viral proteins, we have not yet identified the viral proteins needed at the axon terminals to promote transmission of infection from a pre- to postsynaptic neuron. In fact, it may be that gE/gI proteins could have a role at the presynaptic terminal to promote spread to the connected neuron, but the effect in the cell body is epistatic on this putative downstream function.
The relevance of our findings must be understood in the light of neuronal cell biology. Nearly all features of neuronal specialization depend on the proper localization of membrane proteins, whether they be neurotransmitter receptors, ion channels, transporters, or adhesion molecules. Proteins must be accurately sorted to the somatodendritic or axonal compartments. One serious impediment to understanding this fundamental sorting process is that no one predominant mechanism functions across all cell types. What is true for an epithelial cell is not always true for a neuron (18). The initial idea that axonal sorting signals were identical to apical membrane sorting signals is true for some proteins but not others. Important questions remain: do herpesvirus virion proteins override intrinsic neuronal sorting machinery or do these viral proteins carry their own neuronal sorting signals? The answers will be most useful for virologists and neurobiologists alike.
Acknowledgments
We thank M. Tomishima for expertise in culturing rat sympathetic neurons, A. Flood, L. Pomerantz, and C. Hengartner for valuable manuscript preparation advice, and J. Goodhouse for technical help with the confocal microscopy. We thank Roger Tsien for the mRFP clone used to construct PRV 181 and its derivatives.
This work was supported by the National Institute of Neurological Disorders and Stroke (NIH-NINDS; grant no. 1RO1 33506).
REFERENCES
- 1.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 72:4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brideau, A. D., J. P. Card, and L. W. Enquist. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 74:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 74:4549-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brittle, E. E., A. E. Reynolds, and L. W. Enquist. 2004. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 78:12951-12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 8.Clase, A. C., M. G. Lyman, T. del Rio, J. A. Randall, C. M. Calton, L. W. Enquist, and B. W. Banfield. 2003. The pseudorabies virus Us2 protein, a virion tegument component, is prenylated in infected cells. J. Virol. 77:12285-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, W. J., and D. C. Johnson. 2003. Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread. J. Virol. 77:2686-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook, M. L., and J. G. Stevens. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7:272-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enquist, L. W. 1994. Infection of the mammalian nervous system by pseudorabies virus (PRV). Semin. Virol. 5:221-231. [Google Scholar]
- 13.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 14.Hampl, H., T. Ben-Porat, L. Ehrlicher, K. O. Habermehl, and A. S. Kaplan. 1984. Characterization of the envelope proteins of pseudorabies virus. J. Virol. 52:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill, T. J., and H. J. Field. 1973. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. J. Gen. Virol. 21:123-133. [DOI] [PubMed] [Google Scholar]
- 16.Hill, T. J., H. J. Field, and A. P. Roome. 1972. Intra-axonal location of herpes simplex virus particles. J. Gen. Virol. 15:233-235. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, L. 1994. Glycoprotein E of pseudorabies virus and homologous proteins in other alphaherpesvirinae. Arch. Virol. 137:209-228. [DOI] [PubMed] [Google Scholar]
- 18.Jareb, M., and G. Banker. 1998. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron 20:855-867. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensson, K., B. Ghetti, and H. M. Wisniewski. 1974. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 69:189-201. [DOI] [PubMed] [Google Scholar]
- 22.Kritas, S. K., H. J. Nauwynck, and M. B. Pensaert. 1995. Dissemination of wild-type and gC-, gE-, and gI-deleted mutants of Aujeszky's disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J. Gen. Virol. 76:2063-2066. [DOI] [PubMed] [Google Scholar]
- 23.LaVail, J. H., K. S. Topp, P. A. Giblin, and J. A. Garner. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 24.Lomniczi, B., M. L. Blankenship, and T. Ben-Porat. 1984. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J. Virol. 49:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luxton, G., W. Haverlock, S. Coller, K., S. E. Antinone, A. Pincetic, and G. S. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettenleiter, T. C. 2003. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 92:197-206. [DOI] [PubMed] [Google Scholar]
- 29.Mettenleiter, T. C., C. Schreurs, F. Zuckermann, and T. Ben-Porat. 1987. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J. Virol. 61:2764-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixdorf, R., B. G. Klupp, and T. C. Mettenleiter. 2001. Role of the cytoplasmic tails of pseudorabies virus glycoproteins B, E and M in intracellular localization and virion incorporation. J. Gen. Virol. 82:215-226. [DOI] [PubMed] [Google Scholar]
- 32.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platt, K. B., C. J. Mare, and P. N. Hinz. 1979. Differentiation of vaccine strains and field isolates of pseudorabies (Aujeszky's disease) virus: thermal sensitivity and rabbit virulence markers. Arch. Virol. 60:13-23. [DOI] [PubMed] [Google Scholar]
- 34.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 35.Sampo, B., S. Kaech, S. Kunz, and G. Banker. 2003. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 37:611-624. [DOI] [PubMed] [Google Scholar]
- 36.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. USA 97:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirabassi, R. S., and L. W. Enquist. 2000. Role of the pseudorabies virus gI cytoplasmic domain in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J. Virol. 74:3505-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tirabassi, R. S., R. A. Townley, M. G. Eldridge, and L. W. Enquist. 1997. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J. Virol. 71:6455-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt, L., R. J. Giger, U. Ziegler, B. Kunz, A. Buchstaller, W. Hermens, M. G. Kaplitt, M. R. Rosenfeld, D. W. Pfaff, J. Verhaagen, and P. Sonderegger. 1996. Continuous renewal of the axonal pathway sensor apparatus by insertion of new sensor molecules into the growth cone membrane. Curr. Biol. 6:1153-1158. [DOI] [PubMed] [Google Scholar]
- 44.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitley, R. J., and J. W. Gnann. 2002. Viral encephalitis: familiar infections and emerging pathogens. Lancet 359:507-513. [DOI] [PubMed] [Google Scholar]
- 46.Winckler, B., P. Forscher, and I. Mellman. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397:698-701. [DOI] [PubMed] [Google Scholar]
- 47.Wozniak, M. A., S. J. Shipley, M. Combrinck, G. K. Wilcock, and R. F. Itzhaki. 2005. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients. J. Med. Virol. 75:300-306. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, T., S. Otani, and H. Shiraki. 1973. Ultrastructure of herpes simplex virus infection of the nervous system of mice. Acta Neuropathol. (Berlin) 26:285-299. [DOI] [PubMed] [Google Scholar]