Abstract
We investigated the mechanism of resistance to genital herpes simplex virus type 2 (HSV-2) infection in mice transfected with the murine alpha-1 interferon (IFN-α1) transgene. In situ transfection of mice with the IFN-α1 transgene resulted in an elevation in an IFN-responsive gene, RNA-dependent protein kinase (PKR), but not 2′,5′-oligoadenylate synthetases (OAS), in vaginal tissue. Coupled with the finding that mice lacking a functional PKR pathway were no longer resistant to genital HSV-2 infection following transfection with the IFN-α1 transgene in comparison to wild-type mice or mice lacking a functional OAS pathway, these results suggest that PKR is the dominant antiviral pathway activated by the IFN-α1 transgene.
A mouse model has been developed to study genital herpes simplex virus type 2 (HSV-2) infection and immune events associated with resistance to infection (5, 22). The basis of this model relies on progesterone-induced immunosuppression, which has an impact on Th1 development (19) and local HSV-2-specific immunoglobulin levels (14). Relative to the cellular immune response, neutrophils (16), NK cells (4), T lymphocytes (15, 23), and, to a lesser extent, B lymphocytes (7) contribute to controlling genital HSV-2 infection. Gamma interferon (IFN-γ), through the up-regulation of major histocompatibility complex class II antigen expression and recruitment of leukocytes, has also been found to be instrumental in the regulation of HSV-2 replication and spread in vaginal tissue (11, 18, 24, 25).
A tegument component of HSV-2, virion host shutoff (vhs) protein, is instrumental in the pathogenesis of HSV-2 (28) antagonizing the action of IFN in a double-stranded RNA-dependent protein kinase (PKR)-independent manner (20). However, IFN is effective in reducing HSV-2 replication in vitro (3), suggesting that IFN-inducible pathways can partially block HSV-2-mediated countermeasures. Previously, we reported that the administration of the murine IFN-α1 transgene into the vaginal lumen increased resistance to HSV-2 infection (12). The resistance was associated with a reduction in viral titer in the spinal cord and brain stem and enhanced survival.
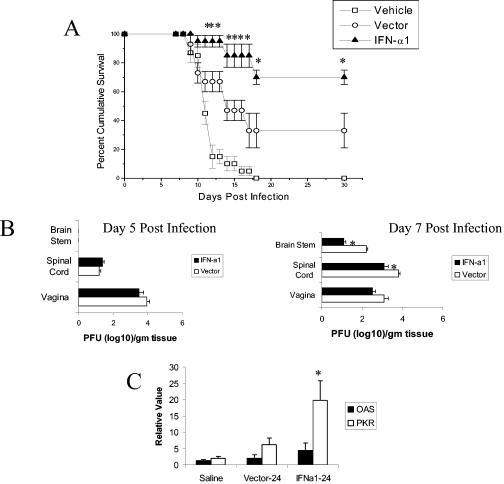
To gain further insight into the mechanism by which the IFN-α transgene operates, we conducted a study focusing on two prominent IFN-inducible, antiviral pathways, 2′,5′-oligoadenylate synthetases (OAS) and RNA-dependent protein kinase (PKR) (29). Initially, plasmid constructs with or without the murine IFN-α1 transgene (21) were applied to the vagina of Depo-Provera-treated ICR mice (22) 24 h prior to infection with HSV-2 (clinical isolate; New Orleans, LA), and the mice were monitored for (i) survival over 30 days, (ii) virus titer in infected tissue by plaque assay (12), and (iii) expression of OAS and PKR by real-time PCR (2). Consistent with previous results, mice administered the IFN-α1 transgene showed enhanced survival with genital HSV-2 infection in comparison to mice treated with the plasmid vector alone or vehicle (Fig. 1A). However, the plasmid alone afforded some degree of protection, since 33% of the plasmid-treated mice survived the infection compared to none of the vehicle control-treated mice (Fig. 1A). The enhanced survival associated with the IFN-α1 transgene-treated group corresponded with a significant reduction in the quantity of infectious virus recovered from the spinal cord and brain stem 7 days postinfection for the IFN-α1 transgene-treated group compared to the plasmid vector-treated mice (Fig. 1B). Although IFN-α is known to induce or up-regulate both OAS and PKR (29), only PKR mRNA was significantly elevated in the vaginal tissue of the IFN-α1 transgene-treated group compared to the plasmid vector-treated or vehicle-treated animals 24 h posttransfection (Fig. 1C). OAS mRNA levels in the vaginal tissue of all groups of treated animals were similar (Fig. 1C).
FIG. 1.
The murine IFN-α1 transgene enhances survival of HSV-2-infected mice. Depo-Provera-treated female ICR mice (n = 15/group) were intravaginally administered 100 μg of plasmid vector DNA (Vector) alone or plasmid containing the IFN-α1 transgene (IFN-α1). (A) Twenty-four hours posttreatment, the mice were infected intravaginally with 2,400 PFU of HSV-2/mouse, and they were monitored for survival over 30 days. Phosphate-buffered saline (Vehicle)-treated mice served as the control. This figure is a summary of three experiments; n = 5 mice/group/experiment. Bars represent standard errors of the means (SEM). *, P < 0.05, comparing the IFN-α1 transgene-treated to the vector-treated group, as determined by the nonparametric Mann-Whitney rank order test. (B) Depo-Provera-treated female ICR mice (n = 6/group) were intravaginally administered 100 μg of plasmid vector DNA (Vector) alone or plasmid containing the IFN-α1 transgene (IFN-a1). Twenty-four hours posttreatment, the mice were intravaginally infected with 2,400 PFU of HSV-2/mouse. On day 5 or 7 postinfection (p.i.), the mice were euthanized and the tissue was removed, processed, and assayed for viral titer by plaque assay. This is a summary of two experiments; n = 3 mice/group/experiment. Bars represent SEM. *, P < 0.05, comparing the IFN-α1 transgene-treated to vector-treated group as determined by analysis of variance and Tukey's post hoc t test. (C) Depo-Provera-treated mice (n = 5 to 7/group) were transfected with the IFN-α1 transgene (100 μg/vagina). Twenty-four hours posttransfection, the mice were euthanized and the vaginal tissue was removed. RNA extracted from the tissue was evaluated for OAS and PKR gene expression by real-time PCR. The results are a summary of two experiments. *, P < 0.05 comparing the IFN-α1-transfected tissue to vector-transfected or saline-treated vaginal tissue for PKR, as determined by analysis of variance and Tukey's post hoc t test.
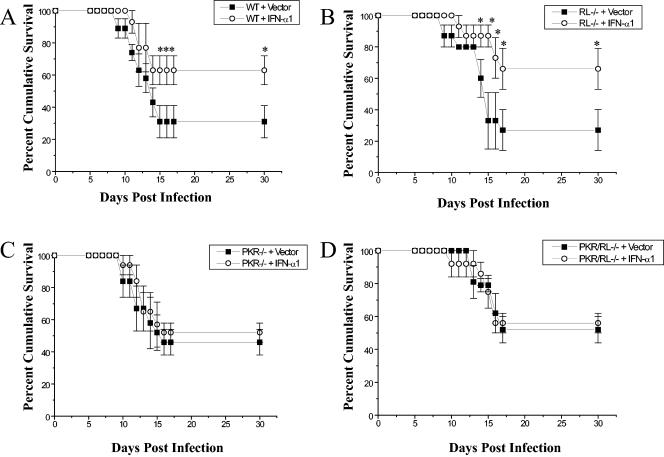
To further evaluate the contribution of OAS and PKR in genital HSV-2 infection, mice deficient in the downstream effector molecule of OAS, RNase L (RL−/−) (31), mice deficient in PKR (PKR−/−) (30), and mice devoid of both RNase L and PKR (PKR/RL−/−) (32) were infected with HSV-2 and monitored for the development of vaginal lesions and survival. Since the gene-deficient mice are on a C57BL/6 background, we also evaluated the antiviral efficacy of the IFN-α1 transgene with C57BL/6 wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME). The application of the IFN-α1 transgene into the lumen of mice subsequently infected with HSV-2 did not suppress the development of lesions in either C57BL/6 WT mice or mice deficient in IFN-responsive genes (data not shown). However, consistent with what we found using ICR outbred mice, C57BL/6 WT mice transfected with the IFN-α1 transgene showed enhanced survival compared to the plasmid vector-treated control WT animals infected with HSV-2 (Fig. 2A). Likewise, the absence of RNase L alone did not reduce the antiviral effect of the transgene (Fig. 2B). In contrast, for PKR−/− (Fig. 2C) or PKR/RL−/− (Fig. 2D) mice, the efficacy of the IFN-α transgene was lost in measuring cumulative survival. Along with our findings showing a selective increase in PKR mRNA expression in the vaginal tissue of IFN-α1-transfected recipient mice (Fig. 1C), the results point to a central role for the PKR pathway in controlling genital HSV-2 infection following transfection with the IFN-α transgene. A previous report using mice deficient in PKR found no significant increase in susceptibility to genital HSV-2 infection measuring viral titers in the central nervous system (20). However, these mice were on a 129 background and infected with a laboratory strain of HSV-2. Results from our lab find that mice deficient in PKR alone (on a C57BL/6 background) are also highly susceptible to the clinical isolate of HSV-2 as measured by cumulative survival, reinforcing the importance of PKR in genital HSV-2 infection (D. J. J. Carr, unpublished observation). CD4+ T lymphocytes from PKR-deficient mice have been reported to produce greater amounts of interleukin 4 (13), which suppresses IFN-α production by monocytes (9). Therefore, one interpretation of these findings is that PKR expression favors a Th1 profile that is conducive to the control of HSV-2 (17).
FIG. 2.
PKR is required for IFN-α1 transgene efficacy against genital HSV-2 infection. (A) Depo-Provera-treated female C57BL/6 mice (WT) (n = 15 to 20 mice/group) were intravaginally administered 100 μg of plasmid vector DNA alone (Vector) or plasmid containing the IFN-α1 transgene (IFN-α1). Twenty-four hours posttreatment, the mice were intravaginally infected with 2,400 PFU of HSV-2/mouse and monitored for survival. This figure is a summary of results of four experiments (n = 4 to 5 mice/group/experiment). (B) Conditions were the same as for panel A except that mice deficient in RNase L (RL−/−) (n = 15/group from three experiments) were employed. (C) Conditions were the same as for panel A except that mice deficient in PKR (PKR−/−) were evaluated (n = 13 from three experiments). (D) Conditions were the same as for panel A except that mice deficient in both RNase L and PKR (PKR/RL−/−) (n = 10 to 15/group from three experiments) were surveyed. *, P < 0.05 comparing the IFN-α1 transgene- to vector-transfected mice, as determined by the nonparametric Mann-Whitney rank order test.
The application of the IFN-α1 transgene into the vaginal lumen of C57BL/6 WT, RL−/−, PKR−/−, or PKR/RL−/− mice did not reduce the morbidity of infection as measured by the development of vaginal lesions. In our experiments, the development of these lesions begins to appear on day 5 postinfection. While we cannot rule out the contribution of the cytopathic effect elicited by the replicating virus in generating tissue pathology, it is equally likely that the host immune response plays a significant role in lesion development. The absence of a significant impact on vaginal lesion development by the IFN-α transgene but elevated survival rates and lower virus titer in the central nervous system following genital HSV-2 infection indicates that the efficacy is directed at sites distal from the original site of application. In fact, we have previously found that the plasmid traffics to the draining lymph node and spinal cord of mice within 48 h post-vaginal transfection and may, therefore, facilitate an effective host response to the infection at these sites (12).
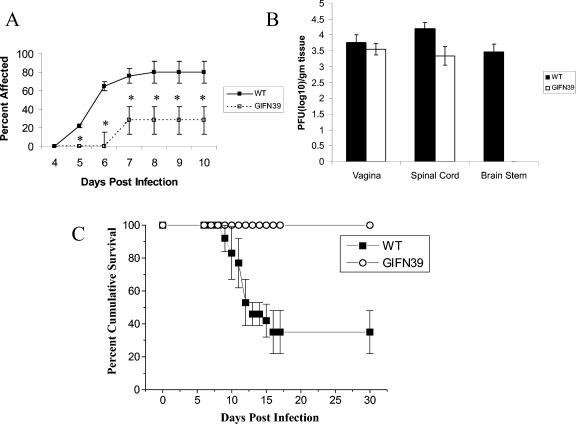
Since the in situ transfection of vaginal tissue with the IFN-α transgene would result in transient expression, we chose a more robust system of tissue-specific expression using a transgenic mouse in order to determine whether IFN-α could reduce lesion development. These transgenic mice (on a B6/129 background) express the IFN-α1 transgene under a glial fibrillary acidic protein (GFAP) promoter (GIFN39) and are highly resistant to virus infection (1). Following genital HSV-2 infection, B6/129 WT and GIFN39 transgenic mice were monitored for the appearance of vaginal lesions, virus titer in infected tissue, and cumulative survival. Unlike WT C57BL/6 mice transfected with the IFN-α1 transgene, GIFN39 transgenic mice showed a significant reduction in the appearance of genital lesions (Fig. 3A) and the absence of virus recovered in the brain stem (Fig. 3B) in comparison to the B6/129 WT counterparts. Moreover, more than 60% of the B6/129 WT mice succumbed to the infection, compared to 0% mortality for the GIFN39 transgenic animals (Fig. 3C). Collectively, the results point to the potent antiviral nature of the IFN-α1 transgene against genital HSV-2 infection.
FIG. 3.
Mice expressing the IFN-α1 transgene under the glial fibrillary acidic protein promoter are highly resistant to genital HSV-2 infection. Depo-Provera-treated female IFN-α1 transgenic mice (GIFN39) or nontransgenic B6/129 control mice (WT) (n = 13 to 18/group) were intravaginally infected with 2,400 PFU of HSV-2/mouse. (A) From day 4 to 10 p.i., the mice were monitored for perivaginal lesions and scored as positive or negative. *, P < 0.05 comparing the transfected to nontransfected mice or the GIFN39 to nontransgenic controls at the indicated time p.i. Results are expressed as means ± SEM. (B) Depo-Provera-treated female IFN-α1 transgenic mice (GIFN39) or nontransgenic B6/129 control mice (WT) (n = 3/group) were infected as described for panel A. At day 7 postinfection, the mice were euthanized and the vaginal tissue, spinal cord, and brain stem from the animals were isolated, homogenized, and assessed for infectious virus content by plaque assay using Vero cells. The results are representative of two experiments. (C) Depo-Provera-treated female IFN-α1 transgenic mice (GIFN39) or nontransgenic B6/129 control mice (WT) (n = 13 to 18/group) were infected as described for panel A and monitored for survival until day 30 p.i. Results are expressed as means ± SEM. The results are a summary of three experiments; n = 4 to 7 mice/group/experiment. *, P < 0.05, comparing the GIFN39 to nontransgenic controls.
In the ICR outbred mice, the application of the plasmid vector alone showed partial protection against HSV-2-mediated mortality. This protective effect may be linked to CpG motifs within the plasmid DNA that have previously been reported to enhance or protect against HSV-2 infection (8, 10, 26). The partial protection by the plasmid DNA may be the result of differences in the amounts of immunostimulatory DNA added. In the present study, 100 μg of DNA was administered in the vaginal lumen. By comparison, considerably more (60 to 100 μg) purified CpG-containing oligonucleotides were topically applied to mice in order to elicit a protective effect (10, 26). Since the antiviral efficacy of the IFN-α transgene was significantly enhanced compared to that found with the plasmid alone, we interpret these results to indicate that the resistance found in mice transfected with the transgene is independent of the immunostimulatory effects of CpG-containing motifs within the plasmid DNA backbone.
The present results reflect tissue tropism for some IFN-stimulatory genes in response to IFN-α1 transgene application. Although both OAS and PKR are detected in vaginal tissue, only PKR expression was enhanced following transfection with the IFN-α1 transgene. Collectively, the results illustrate the contribution of innate immunity in genital HSV-2 infection and the role of PKR in the antiviral efficacy associated with the IFN-α1 transgene. Since IFNs elicit a number of genes (6), determining which genes in addition to PKR regulate genital HSV-2 infection or other viral infections is critical to understanding the host response to infection and developing additional or alternative strategies to increase resistance of the host to the pathogen. As an example, IFN regulatory factor 1 (IRF-1), IRF-3, and IRF-7 employed as adjuvants have been found to bias T-cell responses and benefit the host in the production of antibody (27). Therefore, by identifying those unique genes that help establish an antiviral environment, it may be possible to directly introduce the candidate genes into tissue, reducing the unnecessary and sometimes unwarranted effects associated with exposure to IFN-α.
Acknowledgments
The work was supported by USPHS grants AI053108 (to D.J.J.C.), AI34039 (to B.R.G.W.), CA44059 (to R.H.S.), and NEI Core grant EY12190.
We thank Benitta John-Philip and Stephanie Wickham for their excellent technical support. We would also like to thank Peter Härle (University of Regensburg, Germany) for his technical assistance in the measurement of HSV-2 titers.
REFERENCES
- 1.Akwa, Y., D. E. Hassett, M.-L. Eloranta, K. Sandberg, E. Masliah, H. Powell, J. L. Whitton, F. E Bloom, and I. L. Campbell. 1998. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 161:5016-5026. [PubMed] [Google Scholar]
- 2.Al-khatib, K., B. R. G. Williams, R. H. Silverman, W. Halford, and D. J. J. Carr. 2003. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2′-5′-oligoadenylate synthetase-dependent RNase L are required for IFN-β-mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion culture. Virology 313:126-135. [DOI] [PubMed] [Google Scholar]
- 3.Arao, Y., Y. Ando, M. Narita, and T. Kurata. 1997. Unexpected correlation in the sensitivity of 19 herpes simplex virus strains to types I and II interferons. J. Interferon Cytokine Res. 17:537-541. [DOI] [PubMed] [Google Scholar]
- 4.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker, D. A., and S. A. Plotkin. 1978. Enhancement of vaginal infection in mice by herpes simplex virus type II with progesterone. Proc. Soc. Exp. Biol. Med. 158:131-134. [DOI] [PubMed] [Google Scholar]
- 6.De Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M Paranjape, R. H. Silverman, and B. R. G. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69:912-920. [PubMed] [Google Scholar]
- 7.Dudley, K. L., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 8.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 9.Gary-Guoy, H., P. Lebon, and A. H. Dalloul. 2002. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J. Interferon Cytokine Res. 22:653-659. [DOI] [PubMed] [Google Scholar]
- 10.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Erikkson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Härle, P., S. Noisakran, and D. J. J. Carr. 2001. The application of a plasmid DNA encoding IFN-α1 postinfection enhances cumulative survival of herpes simplex virus type 2 vaginally infected mice. J. Immunol. 166:1803-1812. [DOI] [PubMed] [Google Scholar]
- 13.Kadereit, S., H. Xu, T. M. Engeman, Y.-L. Yang, R. L. Fairchild, and B. R. G. Williams. 2000. Negative regulation of CD8+ T cell function by the IFN-induced and double-stranded RNA-activated kinase PKR. J. Immunol. 165:6896-6901. [DOI] [PubMed] [Google Scholar]
- 14.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159:460-466. [DOI] [PubMed] [Google Scholar]
- 16.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 18.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 19.Miyaura, H., and M. Iwata. 2002. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J. Immunol. 168:1087-1094. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noisakran, S., Campbell, I. L., and Carr, D. J. J. 1999. Ectopic expression of DNA encoding IFN-α1 in the cornea protects mice from herpes simplex virus type 1-induced encephalitis. J. Immunol. 162:4184-4190. [PubMed] [Google Scholar]
- 22.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. A. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 23.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parr, M. B., and E. L. Parr. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258:282-294. [DOI] [PubMed] [Google Scholar]
- 25.Parr, M. B., and E. L. Parr. 2000. Interferon-γ up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology 99:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki, S., R. R. Amara, W.-S. Yeow, P. M. Pitha, and H. L. Robinson. 2002. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 76:6652-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark, G. R., I. M. Kerr, B. R. G. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 30.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet and Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate synthetase-dependent RNase L. EMBO J. 14:6095-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, A., J. M. Paranjape, S. D. Der, B. R. Williams, and R. H. Silverman. 1999. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258:435-440. [DOI] [PubMed] [Google Scholar]