Abstract
Background
Since the mid-20th century, it has been argued by some that the transition from diploidy to polyploidy is an ‘evolutionary dead end’ in plants. Although this point has been debated ever since, multiple definitions of ‘dead end’ have been used in the polyploidy literature, without sufficient differentiation between alternative uses.
Scope
Here, we focus on the two most common conceptions of the dead-end hypothesis currently discussed: the ‘lowering diversification’ hypothesis and the ‘rarely successful’ hypothesis. We discuss the evidence for both hypotheses, and we use a recently developed method of inferring tip diversification rates to demonstrate tests for the effect of ploidy on diversification in Solanaceae.
Conclusions
We find that diversification rates in the family are not strongly correlated with ploidy or with the closely related trait of breeding system. We also outline recent work in the field that moves beyond the relatively simple question of whether polyploidy increases, decreases or does not significantly affect diversification rates in plants.
Keywords: Solanaceae, whole genome duplication, diversification, phylogenetic comparative methods, self-compatibility
INTRODUCTION
In a sense, we can assume that any species that originates, no matter how ‘successful’, will inevitably terminate in a dead end; >99% of all species that have ever lived have gone extinct (Jablonski, 2004), and all extant species have a non-zero probability of going extinct (Marshall, 2017). Even well-adapted species can fall victim to changes in their environment (Vrba, 1993) or being out-competed (Van Valen, 1973). Specific traits in such situations, which were once adaptive but became deleterious owing to environmental changes, are known as ‘maladaptations’ (Crespi, 2000). However, can the evolution of an adaptive trait itself lead to the demise of a population or species? Indeed, this strange phenomenon can occur in a variety of evolutionary scenarios. Examples include ‘evolutionary suicide’, in which traits that are adaptive at the level of the individual organism lead to the extinction of a population (Rankin and López-Sepulcre, 2005); ‘macroevolutionary self-destruction’, referring to traits that evolve frequently but are rapidly lost through extinction of their possessors (Bromham et al., 2016); and ‘evolutionary dead ends’, in which traits that are adaptive at the level of the population are deleterious at the level of species through the lowering of diversification rates. Support for the dead-end phenomenon has been found for a variety of traits, including narrow host range in tachinid flies (Day et al., 2016), fossoriality in snakes (Cyriac and Kodandaramaiah, 2018), shifts to self-compatibility in Solanaceae (Goldberg et al., 2010; but see Wright et al., 2013) and shifts to polyploidy in vascular plants (Mayrose et al., 2011).
The idea that polyploidy is a dead end has a long and contentious history in botany. The person most responsible for the idea is G. Ledyard Stebbins, who strongly shaped the history of polyploidy research with his extensive writings (see Soltis et al., 2014a). However, Stebbins’ conception of polyploidy as a dead end was very different from the definition given above. Instead of referring to evolutionary rates, he offered several different arguments, including that selection cannot work efficiently on polyploids owing to the masking of gene copies, that polyploidy is not causally related to the diversification of plants and that polyploidization produces no subsequent increases in morphological disparity or the evolution of any traits that might be called key innovations (Stebbins, 1950, 1971). In fact, Stebbins never even used the phrase ‘evolutionary dead end’ to refer to polyploidy (Soltis et al., 2014a); he did, however, apply the similar term ‘blind alley’ to self-fertilization, for which he offered another explanation; Stebbins (1957) defined a dead end as the result of an ‘unlucky accident’ in which a species acquired advantageous mutations but lost its ability to respond adequately to environmental pressures, in this case through outcrossing, and has thus increased its likelihood of extinction. The connection between Stebbins’ ideas about polyploidy and modern tests of the dead-end hypothesis that invoke evolutionary rates (e.g. Mayrose et al., 2011) thus appears to us as tenuous.
In contemporary polyploidy research, the power of the dead-end hypothesis to explain evolutionary rates in polyploid plants is controversial, owing to arguments about new methods of inferring diversification rates, in addition to the veracity of the dead-end hypothesis itself (Soltis et al., 2014b; Mayrose et al., 2015). Many botanists argue that polyploidy is not a dead-end trait at all and that, in fact, it has considerably shaped the macroevolutionary history of flowering plants (e.g. Soltis et al., 2009, 2014b; Heslop-Harrison et al., 2023). Additionally, there are now two dead-end hypotheses often lumped together into one: a ‘traditional’ dead-end hypothesis, in which polyploidy generally lowers diversification, and the ‘rarely successful’ hypothesis, in which most polyploids are dead ends, but some occasionally establish successfully and go on to diversify, sometimes greatly (Arrigo and Barker, 2012). The purpose of this review is to discuss these alternative formulations of dead-end hypotheses in polyploidy research and the evidence currently supporting each. To spur further research on polyploid diversification rates, we also perform the first study comparing tip diversification rates in polyploids and diploids, using Solanaceae as a case study. Finally, we outline emerging directions in the field that go beyond the dead-end hypothesis, emphasizing work that focuses on more complex questions about the relationship between ploidy and diversification.
THE ‘TRADITIONAL’ DEAD-END HYPOTHESIS
Since the advent of modern phylogenetic comparative methods, arguments for and against dead-end status almost invariably involve how polyploidy affects specific evolutionary rates, such as speciation, extinction, net diversification and transition rates among the ploidy states. Arguably, the most common definition of an evolutionary dead end is a trait that confers a short-term selective advantage to a species but in the long run leads to decreased net diversification (e.g. Mayrose et al., 2011, 2015). The first test of this hypothesis with phylogenetic comparative methods was conducted with sister-clade comparisons in Rosaceae (Vamosi and Dickinson, 2006), finding that polyploidy is associated with increased species richness in the clade. However, sister-clade comparisons do not use all the information available in a phylogeny, cannot distinguish increased speciation from decreased extinction, and thus are generally coarse-grained analyses of trait-dependent diversification. Hence, researchers in the field now tend to favour using state-dependent speciation and extinction (-SSE) models, which allow for estimates of diversification and transition rates, in addition to comparisons of rates between taxa possessing different characters. In examining the ‘traditional’ dead-end hypothesis, -SSE models can both infer how ploidy states affect diversification rates and infer rates of transition between ploidy states, because irreversibility of a state is often cited as a second criterion for a character state to be a dead end (Ng and Smith, 2014).
Mayrose et al. (2011) used binary state speciation and extinction (BiSSE; Maddison et al., 2007) models to show that polyploidy generally lowers diversification relative to diploidy. Although Soltis et al. (2014b) argued against their findings on the grounds that their results were confounded by clade age and size, Mayrose et al. (2015) responded by pointing out that BiSSE rates are scaled by time, thus avoiding these concerns. However, the authors did acknowledge that their analysis was limited to recently developed polyploids, or ‘neopolyploids’, in young clades, thus sidestepping the question of whether polyploidy contributed to diversification in deep time (e.g. Fawcett et al., 2009). Studies have since examined this question with newly developed -SSE models; for example, Landis et al. (2018) used Multi-State Speciation and Extinction (MuSSE) (FitzJohn, 2012), which extends BiSSE to allow for multiple characters with multiple states, to conclude that multiple rounds of polyploidization generally increases diversification rates across angiosperms.
With the development of Hidden State Speciation and Extinction (HiSSE) (Beaulieu and O’Meara, 2016), which allows for models in which diversification is controlled not only by observed traits but also by ‘hidden’ traits representing hypothetical, unobserved factors that also effect evolutionary rates, researchers were able to test whether ploidy controls diversification more than unconsidered influences. This was beneficial for hypothesis testing; if a model including a hidden trait is favoured and one finds that the hidden trait controls diversification rates more strongly than ploidy, one may then look to other traits often linked to ploidy, such as selfing (Barringer, 2007; but see Mable, 2004) or herbaceousness (Zenil-Ferguson et al., 2017). HiSSE was used by Zenil-Ferguson et al. (2019) to find that selfing does explain diversification better than ploidy, and by Han et al. (2020) to find that lineages with greater proportions of polyploids exhibit higher diversification rates.
LINKED TRAITS AND THE ‘RARELY SUCCESSFUL’ HYPOTHESIS
Although analyses of multiple traits using models such as MuSSE and HiSSE are certainly useful in that they have enabled joint analysis of ploidy and other traits, the effects of polyploidy qua polyploidy are often insufficiently distinguished from other evolutionary developments that frequently accompany polyploidization in formulations of the dead-end hypothesis. For example, given that polyploidy is believed to occur most frequently in perennial herbs (Stebbins, 1971; Zenil-Ferguson et al., 2017), and because perenniality might increase extinction rates (Soltis et al., 2013), polyploidy might thus lead indirectly to decreased diversification. It could be argued that unless ploidy shifts are causally linked to concomitant shifts in other traits or it can be shown that polyploidy causes decreased diversification in cases where such shifts do not occur, the label of ‘dead end’ seems inapplicable in this case.
Another example of such a trait is breeding system. Shifts to self-compatibility are frequently believed to accompany ploidy shifts, and they have been cited as a reason for polyploidy being an evolutionary dead end (Stebbins, 1950). What is interesting is that selfing has been used as evidence both for polyploids being dead ends and for polyploids exhibiting increased diversification in deep time. Although shifts to self-compatibility have been demonstrated to be linked to decreased diversification rates (Goldberg et al., 2010), selfing has also been cited as crucial to polyploids to overcome minority cytotype exclusion (Levin, 1975) and to survive mass extinctions and repopulate empty niches (Fawcett et al., 2009; Lohaus and Van de Peer, 2016; Freeling, 2017). The potential for such widely differing responses to environmental pressure at the species level is, in the case of polyploidy, the basis of the second dead-end hypothesis, the ‘rarely successful’ hypothesis (sensuArrigo and Barker, 2012).
In the ‘rarely successful’ hypothesis, most avenues terminate at dead ends, but some occasionally lead to diversification, sometimes at very high rates (see Fig. 1). Sessa (2019) uses the metaphor of the ‘Las Vegas strategy’, where plants that undergo polyploidization effectively ‘gamble’, with the possibility of gaining evolutionary advantages. Although this bet most often results in extinction, plants occasionally ‘win big’, and their gamble pays off with increased diversification. The aforementioned -SSE models can hint at whether this is the case; Román-Palacios et al. (2020) found that although polyploids exhibit similar net diversification (speciation–extinction) rates in Brassicaceae to diploids, they also exhibit higher turnover (speciation + extinction), which is a measure that suggests a higher frequency of both speciation and extinction events over evolutionary time (Vasconcelos et al., 2022a). Yet the ‘rarely successful’ hypothesis requires the deep time perspective noted to be missing in studies such as that of Mayrose et al. (2011); lowered diversification rates can be detected on relatively small and shallow trees, whereas finding ‘rare success’ in the descendants of certain polyploids requires the study of very large trees with deep roots. Candidates for ‘rarely successful’ polyploids include, possibly, the ancestors of all flowering plants and all seed plants (Jiao et al., 2011; but see Ruprecht et al., 2017). Additionally, the ‘traditional’ dead-end hypothesis requires only present-day ploidy data, whereas testing the ‘rarely successful’ hypothesis necessitates data about histories of ploidy hidden in the genomes of species that have since downsized and reorganized their genomes through the process of diploidization (see Dodsworth et al., 2016). Several rounds of so-called ‘palaeopolyploidy’ have been uncovered even in plants with very small genomes (Bowers et al., 2003). Interestingly, these are often clustered near times of major environmental stress (Vanneste et al., 2014; Novikova et al., 2018; Levin et al., 2020), and many bursts of diversification seem to occur after long ‘lags’, sometimes lasting millions of years (Tank et al., 2015; Landis et al., 2018).
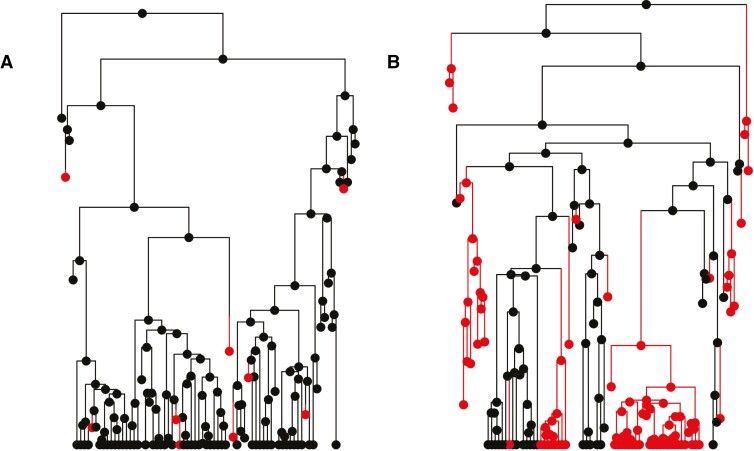
Fig. 1.
Simulated depictions of plant phylogenies expected under the ‘traditional’ dead-end hypothesis (A) and the ‘rarely successful’ hypothesis (B). Diploid taxa are represented in black and polyploids in red. (A) Under the ‘traditional’ dead-end hypothesis, polyploids appear frequently, but go extinct before they diversify. (B) Under the ‘rarely successful’ hypothesis, transitions to polyploidy followed by rapid extinction are frequent, but some polyploid clades can succeed and diversify.
TESTING POLYPLOID DIVERSIFICATION WITH TIP RATE CORRELATION
In looking at the progression of polyploid diversification research alongside the development of -SSE models, it appears that evidence for a significant association between ploidy and evolutionary rates wanes as models become more sophisticated. This is likely to be a result of both biology and modelling; the effects of ploidy are highly dependent on chance, evolutionary history and ecological context (Segraves, 2017; Meudt et al., 2021), and the addition of hidden states in -SSE models removes false-positive results that would otherwise be found in models such as BiSSE (Beaulieu and O’Meara, 2016; Caetano et al., 2018). In a recent systematic review of -SSE model studies performed on angiosperms, Helmstetter et al. (2023) showed that, in the three studies that have used HiSSE or its multi-state counterpart, MuHiSSE, to study ploidy, two analyses found that net diversification was higher for the polyploid state, whereas two other analyses found that it was lower. This is a very small number of studies, but depending on the clade being studied and whether ploidy state is classified based on neopolyploidy or palaeopolyploidy, finding support for the dead-end hypothesis might be analogous to the flipping of a coin.
Furthermore, it is currently difficult to conduct tests that explicitly compare support for the ‘traditional’ as opposed to the ‘rarely successful’ dead-end hypotheses within a single framework. One would need the ability to study flexibly the links between diversification rate shifts and both neopolyploidization and palaeopolyploidization events. Unfortunately, in standard -SSE analyses, information rapidly deteriorates as one moves from the tips to the root of a phylogeny (O’Meara and Beaulieu, 2021), hence ancient diversification estimates might be less reliable than ones close to the present. Additionally, dating ancient polyploidizations and placing them on phylogenies becomes more difficult with age (Vanneste et al., 2013), which means that correlating these with subsequent diversification shifts can be difficult. One method that could be useful for more accurately predicting which taxa are heading towards dead ends would be comparing the present-day ‘snapshots’ of evolutionary rates of diploids and polyploids. These methods quantify what are known as species-specific diversification rates or tip rates (Title and Rabosky, 2019), which have recently gained interest in studies of polyploidy (e.g. Testo and Sundue, 2018; Román-Palacios et al., 2020; Folk et al., 2024) but remain an under-used area with great potential (Soltis et al., 2019). Tip rates are calculated using the evolutionary history of each lineage, and it has been argued that, in the same way that a general diversification rate can be inverted for a waiting time, the reciprocal of a tip rate can be interpreted as a hypothesis for the time it will take for some speciation or extinction or other event to occur (Title and Rabosky, 2019; but see Vasconcelos et al., 2022a, b). Many simple model-free tip rate statistics are computationally inexpensive yet remarkably informative and reliable for determining traits that drive diversification through tip-rate correlation (Freckleton et al., 2008; Jetz et al., 2012; Harvey and Rabosky, 2018), including the phylometrics package (Bromham et al., 2016), which was designed explicitly to test dead-end hypotheses. In model-based comparative methods, increasing numbers of diversification models now include functions that allow the calculation of tip rates based on marginal reconstruction, including Bayesian Analysis of Macroevolutionary Mixtures (BAMM) (Rabosky, 2014) and Missing State Speciation and Extinction (MiSSE) (Vasconcelos et al., 2022a).
MiSSE, which is implemented in the hisse R package, is structurally similar to HiSSE except that no observed states are incorporated into models. Unlike other trait-free diversification models, such as Modeling Evolutionary Diversification Using Stepwise Akaike Information Criterion (MEDUSA) (Alfaro et al., 2009), MiSSE allows users to design custom models composed solely of hidden states that can vary the number of free parameters for turnover and extinction fraction. Tip rates calculated from model-averaged reconstructions can then be statistically analysed post hoc in a flexible manner, such as in regressions with various characters, after they have been corrected with phylogenetic independent contrasts (Felsenstein, 1985; see Vasconcelos et al., 2022a). Considering that the relative importance of ploidy as opposed to related factors, particularly self-compatibility, in shaping the evolutionary history of polyploid plants is contentious (see Zenil-Ferguson et al., 2019), MiSSE is a useful framework to study the dead-end hypothesis, because the flexibility of its post hoc analyses allows for rapid and extensive modifications of state assignments (e.g. polyploid vs. diploid, self-compatible vs. self-incompatible, or combinations of those states) and applications of phylogenetic comparative analyses. MiSSE is also a new tool and, to our knowledge, it has not yet been used to compare diversification between diploid and polyploid plants. Therefore, we analysed the Solanaceae phylogeny (Särkinen et al., 2013) with MiSSE to explore its potential and to compare its results with those obtained with HiSSE in a previous study on Solanaceae (Zenil-Ferguson et al., 2019), from which we also obtained ploidy data for extant tips.
For these analyses, models were determined using the model set-up function generateMiSSEGreedyCombinations, allowing for up to ten free turnover parameters and three free extinction fraction parameters within a single model. For all 30 models, we ran MiSSE using the MiSSEGreedy function, which uses a ‘greedy’ hill-climbing optimization routine, and afterwards pruned redundant models, which left us with 29 in total. We then performed model-averaged marginal reconstructions and calculated tip rates. To examine the relationships between tip rates and observed ploidy data, we performed phylogenetic ANOVA (Garland et al., 1993; Revell, 2012) and phylogenetic logistic regression (Ives and Garland, 2010), in addition to their non-phylogenetic statistical counterparts. We used these tests instead of the TipCorrelation function in MiSSE, which uses phylogenetic independent contrasts (Felsenstein, 1985), because our analysis required correlation of discrete characters with tip rates instead of continuous ones. Prior to all analyses, we removed ‘cherries’ from the phylogeny. ‘Cherries’ are sister tips that share the same branch length to their direct ancestor node, and MiSSE gives the option to remove them in its own TipCorrelation function because they inherit the same rate class probabilities and thus constitute pseudoreplicates (Vasconcelos et al., 2022a).
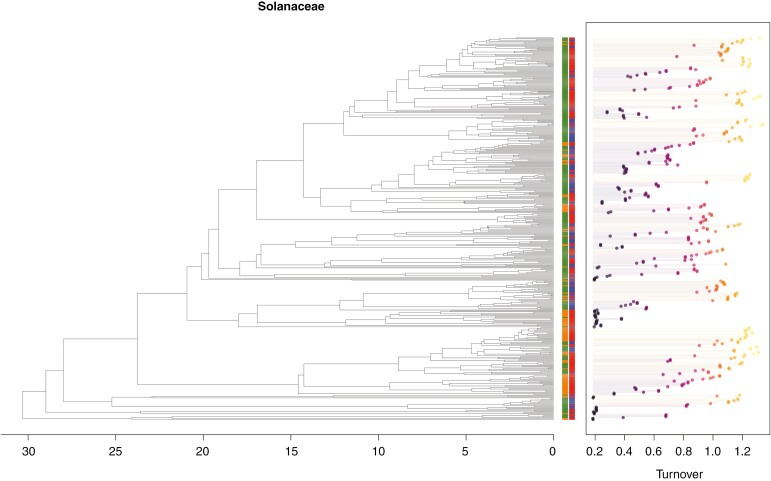
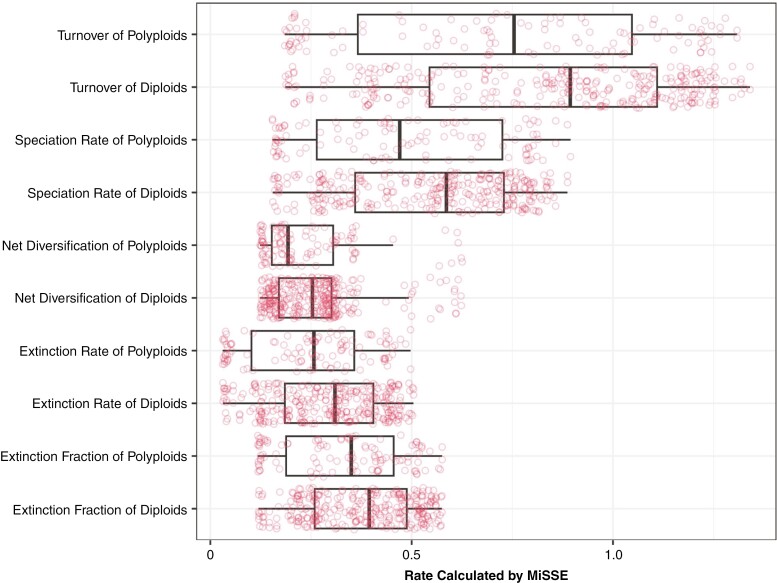
Ploidy states and tip turnover rates across the clade are depicted in Fig. 2. Note that we focus here on turnover because, again, it provides a measure of the frequency of events over evolutionary time. Visual inspection of rates calculated at diploid and polyploid tips indicates that diploids consistently exhibit higher average estimates not only for turnover, but also for all five different measures of tip-based diversification rates (Fig. 3). Although non-phylogenetic ANOVA and logistic regression indicated significant differences between the two groups (ANOVA: F = 5.822, P = 0.017; logit: P = 0.018), incorporating phylogenetic information resulted in no significant differences in turnover rates (phylogenetic ANOVA: F = 5.822, P = 0.481; phylogenetic logit: P = 0.957). In other words, ploidy states appeared to be clumped on the Solanaceae phylogeny, suggesting that it is less labile than observed tip turnover rates. We also detected no significant correlation between tip rates and either breeding system (phylogenetic ANOVA: F = 1.831, P = 0.549; phylogenetic logit: P = 0.291) or combinations of breeding system and ploidy states (phylogenetic ANOVA: F = 6.886, P = 0.458; Fig. 4).
Fig. 2.
Solanaceae phylogeny with tip states and turnover tip rates. Tip states for ploidy are shown in green (diploids) and orange (polyploids). Tip states for breeding system are shown in blue (self-incompatible) and red (self-compatible). Turnover rates for each species are shown on the right, with colour varying from black to light yellow as rates increase.
Fig. 3.
Boxplots comparing tip rates across ploidy states, including the underlying distributions depicted with red dots. Incorporating phylogenetic information in the summary statistics shows that differences between diploids and polyploids are not significant and might have arisen by chance.
Fig 4.
Boxplots comparing tip rates across breeding system states, including the underlying distributions depicted with red dots. As with differences in ploidy state (Fig. 3), incorporating phylogenetic information in the summary statistics shows that these differences are not significant and might have arisen by chance.
It is possible that, given that this study examines neopolyploids instead of the influence of ploidy in deeper evolutionary history, insufficient time has passed for polyploidy to affect diversification rates significantly. It is also possible that traits beyond ploidy and breeding system more strongly control tip diversification rates in Solanaceae. Although our findings regarding ploidy accord with those of Zenil-Ferguson et al. (2019), who conducted a HiSSE analysis with the same data, our analysis differs in that we found no significant correlation of either breeding system or combinations of ploidy and breeding system states with tip diversification rates in Solanaceae. These contrasting results might be attributable to our use of model averaging, unlike Zenil-Ferguson et al. (2019), who used model selection (for details, see Burnham and Anderson, 2002). Importantly, further studies on other clades are needed to determine whether our findings in Solanaceae are representative of diversification in other parts of the angiosperm tree of life.
EMERGING DIRECTIONS IN POLYPLOID DIVERSIFICATION RESEARCH
Phylogenomic approaches
The increasing availability of both species-level phylogenies and gene trees has spurred the development of phylogenomics as a discipline, one of the aims of which is to reconstruct the evolutionary history of genes in phylogenetic context (Eisen, 1998). One phenomenon heavily investigated in this field is gene tree discordance, which occurs when gene trees and species trees show different topologies and can be caused by genome multiplications (Linder and Rieseberg, 2004). Particularly interesting in this line of research is the possibility that whole genome multiplications are linked to bursts of evolutionary innovation, because discordance is also associated with morphological innovation in several clades (Stull et al., 2020; Parins-Fukuchi et al., 2021). This is especially interesting because polyploidization might cause innovation without also altering diversification rates (Rabosky, 2017; Larson et al., 2020). ‘Innovation without diversification’ might be a solution to the problem of lags, although it is also possible that dates estimated for ancient polyploidization events are inaccurate and that their theorized relationship is spurious (Smith et al., 2018; Larson et al., 2020). It might also explain how polyploidization frequently leads to no changes or even decreases in diversification rates, and why genome multiplications that have been linked to diversification rate increases are often accompanied by other innovations or transitions. How polyploidization might create innovation without necessarily altering diversification rates may be perplexing to many botanists, because diversification has been linked to innovations in so many cases (see Rabosky, 2017). Yet genome multiplication and subsequent reorganization can create novelty through gene duplication and gene loss (Clark, 2023).
Mixed-ploidy systems
What if the question is not as simple as comparing diversification rates in diploids and polyploids? It is possible that instead of comparing how polyploidy affects diversification across clades, we should also compare how its effects on diversification change over time. Huang et al. (2020) underscores the fact that diversification patterns are not cleanly related to individual polyploidization events but instead are remnants of polyploidization–diploidization cycles (Baduel et al., 2018). Owing to this, they argue that whole genome multiplications are not consistently related to diversification over time but instead should be viewed as a kind of ‘pump’ for species diversity. Another area of research that could lead to a decline in the invocation of the dead-end hypothesis is study of mixed-ploidy systems, which has gained interest recently (Kolář et al., 2017). Most research up to this point has compared diploid vs. polyploid diversification in species that are either one cytotype or another, and they can thus be categorized with binary traits. Mixed-ploidy species, in which taxa contain both diploid and polyploid individuals, have been shown to diversify at faster rates than single polyploids or diploids, probably because polymorphic species are able to speciate rapidly into both mixed and polyploid new species (Wei et al., 2018). In the genus Allium, the rate of speciation in mixed-ploidy systems is also related to the proportion of polyploids and diploids, being higher when the proportion of polyploids is higher (Han et al., 2020). Additionally, mixed-ploidy species occupy larger geographical/latitudinal ranges than single polyploids or diploids (Lobato-de Magalhães et al., 2021), and it is hypothesized that the recurrent production of triploid hybrids might produce beneficial novelty (Čertner et al., 2017). These findings suggest a role for conspecific diploids working together with polyploids in speciating and radiating geographically as opposed to merely competing, and they open the door to new work that moves beyond binary comparisons of diversification in polyploids and diploids.
CONCLUSIONS
With these directions in mind and with a greater focus on specific hypotheses in trait-dependent diversification modelling, it seems possible that polyploid research will soon leave discussions of dead ends behind. The effects of polyploidy are too intertwined with other traits, too ecologically dependent and too dependent on factors such as time and clade biology to be so easily categorized. Although the dead-end concept might have reached its own dead end, many roads extend ahead for future polyploid diversification research.
ACKNOWLEDGEMENTS
E.R.H. thanks Rosana Zenil-Ferguson, Thais Vasconcelos and James D. Boyko for helpful conversations and comments about this manuscript.
Contributor Information
Eric R Hagen, Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA; Department of Ecology & Evolutionary Biology, University of Toronto, Toronto, ON M5S 3B2, Canada.
Jeremy M Beaulieu, Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA.
FUNDING
This work was supported by the National Science Foundation [DEB-1916558 to J.M.B.].
LITERATURE CITED
- Alfaro ME, Santini F, Brock C, et al. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences of the United States of America 106: 13410–13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo N, Barker MS.. 2012. Rarely successful polyploids and their legacy in plant genomes. Current Opinion in Plant Biology 15: 140–146. [DOI] [PubMed] [Google Scholar]
- Baduel P, Bray S, Vallejo-Marin M, Kolář F, Yant L.. 2018. The ‘Polyploid Hop’: shifting challenges and opportunities over the evolutionary lifespan of genome duplications. Frontiers in Ecology and Evolution 6: 117. [Google Scholar]
- Barringer BC. 2007. Polyploidy and self‐fertilization in flowering plants. American Journal of Botany 94: 1527–1533. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, O’Meara BC.. 2016. Detecting hidden diversification shifts in models of trait-dependent speciation and extinction. Systematic Biology 65: 583–601. [DOI] [PubMed] [Google Scholar]
- Bowers JE, Chapman BA, Rong J, Paterson AH.. 2003. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422: 433–438. [DOI] [PubMed] [Google Scholar]
- Bromham L, Hua X, Cardillo M.. 2016. Detecting macroevolutionary self-destruction from phylogenies. Systematic Biology 65: 109–127. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR.. 2002. Model selection and multimodel inference. New York: Springer. [Google Scholar]
- Caetano DS, O’Meara BC, Beaulieu JM.. 2018. Hidden state models improve state-dependent diversification approaches, including biogeographical models. Evolution 72: 2308–2324. [DOI] [PubMed] [Google Scholar]
- Čertner M, Fenclová E, Kúr P, et al. 2017. Evolutionary dynamics of mixed-ploidy populations in an annual herb: dispersal, local persistence and recurrent origins of polyploids. Annals of Botany 120: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW. 2023. Genome evolution in plants and the origins of innovation. The New Phytologist 240: 2204–2209. [DOI] [PubMed] [Google Scholar]
- Crespi BJ. 2000. The evolution of maladaptation. Heredity 84: 623–629. [DOI] [PubMed] [Google Scholar]
- Cyriac VP, Kodandaramaiah U.. 2018. Digging their own macroevolutionary grave: fossoriality as an evolutionary dead end in snakes. Journal of Evolutionary Biology 31: 587–598. [DOI] [PubMed] [Google Scholar]
- Day EH, Hua X, Bromham L.. 2016. Is specialization an evolutionary dead end? Testing for differences in speciation, extinction and trait transition rates across diverse phylogenies of specialists and generalists. Journal of Evolutionary Biology 29: 1257–1267. [DOI] [PubMed] [Google Scholar]
- Dodsworth S, Chase MW, Leitch AR.. 2016. Is post-polyploidization diploidization the key to the evolutionary success of angiosperms? Botanical Journal of the Linnean Society 180: 1–5. [Google Scholar]
- Eisen JA. 1998. Phylogenomics: improving functional predictions for uncharacterized genes by evolutionary analysis. Genome Research 8: 163–167. [DOI] [PubMed] [Google Scholar]
- Fawcett JA, Maere S, Van De Peer Y.. 2009. Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proceedings of the National Academy of Sciences of the United States of America 106: 5737–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. The American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution 3: 1084–1092. [Google Scholar]
- Folk RA, Charboneau JLM, Belitz M, et al. 2024. Anatomy of a mega-radiation: biogeography and niche evolution in Astragalus. American Journal of Botany 111: e16299. [DOI] [PubMed] [Google Scholar]
- Freckleton RP, Phillimore AB, Pagel M.. 2008. Relating traits to diversification: a simple test. The American Naturalist 172: 102–115. [DOI] [PubMed] [Google Scholar]
- Freeling M. 2017. Picking up the ball at the K/Pg boundary: the distribution of ancient polyploidies in the plant phylogenetic tree as a spandrel of asexuality with occasional sex. The Plant Cell 29: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Dickerman AW, Janis CM, Jones JA.. 1993. Phylogenetic analysis of covariance by computer simulation. Systematic Biology 42: 265–292. [Google Scholar]
- Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igić B.. 2010. Species selection maintains self-incompatibility. Science 330: 493–495. [DOI] [PubMed] [Google Scholar]
- Han TS, Zheng QJ, Onstein RE, et al. 2020. Polyploidy promotes species diversification of Allium through ecological shifts. The New Phytologist 225: 571–583. [DOI] [PubMed] [Google Scholar]
- Harvey MG, Rabosky DL.. 2018. Continuous traits and speciation rates: alternatives to state-dependent diversification models. Methods in Ecology and Evolution 9: 984–993. [Google Scholar]
- Helmstetter AJ, Zenil-Ferguson R, Sauquet H, et al. 2023. Trait-dependent diversification in angiosperms: patterns, models and data. Ecology Letters 26: 640–657. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison JS, Schwarzacher T, Liu Q.. 2023. Polyploidy: its consequences and enabling role in plant diversification and evolution. Annals of Botany 131: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XC, German DA, Koch MA.. 2020. Temporal patterns of diversification in Brassicaceae demonstrate decoupling of rate shifts and mesopolyploidization events. Annals of Botany 125: 29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives AR, Garland T.. 2010. Phylogenetic logistic regression for binary dependent variables. Systematic Biology 59: 9–26. [DOI] [PubMed] [Google Scholar]
- Jablonski D. 2004. Extinction: past and present. Nature 427: 589. [DOI] [PubMed] [Google Scholar]
- Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO.. 2012. The global diversity of birds in space and time. Nature 491: 444–448. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, et al. 2011. Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97–100. [DOI] [PubMed] [Google Scholar]
- Kolář F, Čertner M, Suda J, Schönswetter P, Husband BC.. 2017. Mixed-ploidy species: progress and opportunities in polyploid research. Trends in Plant Science 22: 1041–1055. [DOI] [PubMed] [Google Scholar]
- Landis JB, Soltis DE, Li Z, et al. 2018. Impact of whole-genome duplication events on diversification rates in angiosperms. American Journal of Botany 105: 348–363. [DOI] [PubMed] [Google Scholar]
- Larson DA, Walker JF, Vargas OM, Smith SA.. 2020. A consensus phylogenomic approach highlights paleopolyploid and rapid radiation in the history of Ericales. American Journal of Botany 107: 773–789. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1975. Minority cytotype exclusion in local plant populations. Taxon 24: 35–43. [Google Scholar]
- Levin DA. 2020. Has the polyploid wave ebbed? Frontiers in Plant Science 11: 504318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder CR, Rieseberg LH.. 2004. Reconstructing patterns of reticulate evolution in plants. American Journal of Botany 91: 1700–1708. [PMC free article] [PubMed] [Google Scholar]
- Lobato-de Magalhães T, Murphy K, Efremov A, Chepinoga V, Davidson TA, Molina-Navarro E.. 2021. Ploidy state of aquatic macrophytes: global distribution and drivers. Aquatic Botany 173: 103417. [Google Scholar]
- Lohaus R, Van de Peer Y.. 2016. Of dups and dinos: evolution at the K/Pg boundary. Current Opinion in Plant Biology 30: 62–69. [DOI] [PubMed] [Google Scholar]
- Mable BK. 2004. Polyploidy and self‐compatibility: is there an association? New Phytologist 162: 803–811. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Midford PE, Otto SP.. 2007. Estimating a binary character’s effect on speciation and extinction. Systematic Biology 56: 701–710. [DOI] [PubMed] [Google Scholar]
- Marshall CR. 2017. Five palaeobiological laws needed to understand the evolution of the living biota. Nature Ecology & Evolution 1: 0165. [DOI] [PubMed] [Google Scholar]
- Mayrose I, Zhan SH, Rothfels CJ, et al. 2011. Recently formed polyploid plants diversify at lower rates. Science 333: 1257. [DOI] [PubMed] [Google Scholar]
- Mayrose I, Zhan SH, Rothfels CJ, et al. 2015. Methods for studying polyploid diversification and the dead end hypothesis: a reply to Soltis et al. (2014). New Phytologist 206: 27–35. [DOI] [PubMed] [Google Scholar]
- Meudt HM, Albach DC, Tanentzap AJ, et al. 2021. Polyploidy on islands: its emergence and importance for diversification. Frontiers in Plant Science 12: 637214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Smith SD.. 2014. How traits shape trees: new approaches for detecting character state-dependent lineage diversification. Journal of Evolutionary Biology 27: 2035–2045. [DOI] [PubMed] [Google Scholar]
- Novikova PY, Hohmann N, Van de Peer Y.. 2018. Polyploid Arabidopsis species originated around recent glaciation maxima. Current Opinion in Plant Biology 42: 8–15. [DOI] [PubMed] [Google Scholar]
- O’Meara BC, Beaulieu JM.. 2021. Potential survival of some, but not all, diversification methods. EcoEvoRxiv. doi: https://doi.org/ 10.32942/osf.io/w5nvd. [Preprint; not peer reviewed.] [DOI] [Google Scholar]
- Parins-Fukuchi CT, Stull GW, Smith SA.. 2021. Phylogenomic conflict coincides with rapid morphological innovation. Proceedings of the National Academy of Sciences of the United States of America 118: e2023058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9: e89543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL. 2017. Phylogenetic tests for evolutionary innovation: the problematic link between key innovations and exceptional diversification. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 372: 20160417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin DJ, López‐Sepulcre A.. 2005. Can adaptation lead to extinction? Oikos 111: 616–619. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Román-Palacios C, Molina-Henao YF, Barker MS.. 2020. Polyploids increase overall diversity despite higher turnover than diploids in the Brassicaceae. Proceedings Biological Sciences 287: 20200962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht C, Lohaus R, Vanneste K, et al. 2017. Revisiting ancestral polyploidy in plants. Science Advances 3: e1603195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Särkinen T, Bohs L, Olmstead RG, Knapp S.. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves KA. 2017. The effects of genome duplications in a community context. The New Phytologist 215: 57–69. [DOI] [PubMed] [Google Scholar]
- Sessa EB. 2019. Polyploidy as a mechanism for surviving global change. The New Phytologist 221: 5–6. [DOI] [PubMed] [Google Scholar]
- Smith SA, Brown JW, Yang Y, et al. 2018. Disparity, diversity, and duplications in the Caryophyllales. The New Phytologist 217: 836–854. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, et al. 2009. Polyploidy and angiosperm diversification. American Journal of Botany 96: 336–348. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Mort ME, Latvis M, et al. 2013. Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. American Journal of Botany 100: 916–929. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Visger CJ, Soltis PS.. 2014a. The polyploidy revolution then…and now: Stebbins revisited. American Journal of Botany 101: 1057–1078. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Segovia-Salcedo MC, Jordon-Thaden I, et al. 2014b. Are polyploids really evolutionary dead-ends (again)? A critical reappraisal of Mayrose et al. (2011). New Phytologist 202: 1105–1117. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Folk RA, Soltis DE.. 2019. Darwin review: angiosperm phylogeny and evolutionary radiations. Proceedings of the Royal Society B: Biological Sciences 286: 20190099. [Google Scholar]
- Stebbins GL. 1950. Variation and evolution in plants. New York: Columbia University Press. [Google Scholar]
- Stebbins GL. 1957. Self fertilization and population variability in the higher plants. The American Naturalist 91: 337–354. [Google Scholar]
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Edward Arnold. [Google Scholar]
- Stull GW, Soltis PS, Soltis DE, Gitzendanner MA, Smith SA.. 2020. Nuclear phylogenomic analyses of asterids conflict with plastome trees and support novel relationships among major lineages. American Journal of Botany 107: 790–805. [DOI] [PubMed] [Google Scholar]
- Tank DC, Eastman JM, Pennell MW, et al. 2015. Nested radiations and the pulse of angiosperm diversification: increased diversification rates often follow whole genome duplications. The New Phytologist 207: 454–467. [DOI] [PubMed] [Google Scholar]
- Testo WL, Sundue MA.. 2018. Are rates of species diversification and body size evolution coupled in the ferns? American Journal of Botany 105: 525–535. [DOI] [PubMed] [Google Scholar]
- Title PO, Rabosky DL.. 2019. Tip rates, phylogenies and diversification: what are we estimating, and how good are the estimates? Methods in Ecology and Evolution 10: 821–834. [Google Scholar]
- Vamosi JC, Dickinson TA.. 2006. Polyploidy and diversification: a phylogenetic investigation in Rosaceae. International Journal of Plant Sciences 167: 349–358. [Google Scholar]
- Vanneste K, Van de Peer Y, Maere S.. 2013. Inference of genome duplications from age distributions revisited. Molecular Biology and Evolution 30: 177–190. [DOI] [PubMed] [Google Scholar]
- Vanneste K, Baele G, Maere S, Van de Peer Y.. 2014. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous–Paleogene boundary. Genome Research 24: 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen L. 1973. A new evolutionary law. Evolutionary Theory 1: 1–30. [Google Scholar]
- Vasconcelos T, O’Meara BC, Beaulieu JM.. 2022a. A flexible method for estimating tip diversification rates across a range of speciation and extinction scenarios. Evolution 76: 1420–1433. [DOI] [PubMed] [Google Scholar]
- Vasconcelos T, O’Meara BC, Beaulieu JM.. 2022b. Retiring ‘cradles’ and ‘museums’ of biodiversity. The American Naturalist 199: 194–205. [DOI] [PubMed] [Google Scholar]
- Vrba ES. 1993. Turnover-pulses, the Red Queen, and related topics. American Journal of Science 293: 418–452. [Google Scholar]
- Wei R, Ree RH, Sundue MA, Zhang XC.. 2018. Polyploidy and elevation contribute to opposing latitudinal gradients in diversification and species richness in lady ferns (Athyriaceae). bioRxiv. doi: https://doi.org/ 10.1101/351080. [Preprint; not peer reviewed.] [DOI] [Google Scholar]
- Wright SI, Kalisz S, Slotte T.. 2013. Evolutionary consequences of self-fertilization in plants. Proceedings Biological Sciences 280: 20130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenil-Ferguson R, Ponciano JM, Burleigh JG.. 2017. Testing the association of phenotypes with polyploidy: an example using herbaceous and woody eudicots. Evolution 71: 1138–1148. [DOI] [PubMed] [Google Scholar]
- Zenil‐Ferguson R, Burleigh JG, Freyman WA, Igić B, Mayrose I, Goldberg EE.. 2019. Interaction among ploidy, breeding system and lineage diversification. New Phytologist 224: 1252–1265. [DOI] [PubMed] [Google Scholar]