Abstract
Background and Aims
Leaf variegation is common in plants and confers diverse adaptive functions. However, its genetic underpinnings remain largely unresolved; this is particularly true for variegation that arises through modified leaf tissue structure that affects light reflection. White clover is naturally polymorphic for structure-based white leaf mark variegation. It therefore provides a useful system in which to examine the genetic basis of this phenotype and to assess potential costs to photosynthetic efficiency resulting from modified leaf structures. In this study, we sought to map the loci controlling the white leaf mark in white clover and to evaluate the relationship between white leaf mark, leaf thickness and photosynthetic efficiency.
Methods
We generated a high-density genetic linkage map from an F3 mapping population, using reference genome-based single nucleotide polymorphism markers. White leaf mark was quantified through detailed phenotypic evaluations alongside leaf thickness to test how tissue thickness might affect the variegation phenotype. Mapping of quantitative trait loci was performed to characterize their genetic basis. Photosynthetic efficiency measurements were used to test for physiological trade-offs between variegation and photosynthetic output.
Key Results
The V locus, a major gene responsible for the white leaf mark polymorphism, was mapped to the distal end of chromosome 5, and several modifier loci were also mapped that contribute additively to the intensity of variegation. The presence and intensity of white leaf mark were associated with greater leaf thickness; however, increased variegation did not affect photosynthetic efficiency detectably.
Conclusions
We have successfully mapped the major locus governing the white leaf mark in white clover, along with several modifier loci, revealing a complex basis for this structure-based variegation. The apparent absence of compromised photosynthesis in variegated leaves challenges the notion that variegation creates fitness trade-offs between photosynthetic efficiency and other adaptive functions. This finding suggests that other factors might maintain the white leaf mark polymorphism in white clover.
Keywords: Leaf variegation, white clover (Trifolium repens L.), leaf mark polymorphism, leaf thickness, plasticity, V locus, photosynthetic efficiency, quantitative trait locus mapping
INTRODUCTION
Polymorphism in leaf variegation, characterized by heterogeneous colour patterns on leaves, is observed across diverse natural and cultivated settings. Leaf variegation can arise from pigment-related factors, such as anthocyanin accumulation or chlorophyll deficit, or from structural attributes of tissue organization, such as the expansion of intercellular air spaces that can alter light reflection (Zhang et al., 2020). Beyond its horticultural appeal, various adaptive functions and mechanisms underlying leaf variegation polymorphism have been suggested. The presence of leaf variegation could facilitate herbivore deterrence (Charles, 1968; Cahn and Harper, 1976b; Campitelli et al., 2008; Soltau et al., 2008; Lev-Yadun, 2014), aid in thermoregulation (Ganders et al., 1980; Shelef et al., 2019), provide ultraviolet protection (Koski and Ashman, 2015) and/or enhance pollinator attraction (Song et al., 2018). Conversely, the presence of leaf variegation could compromise photosynthetic efficiency owing to a reduction in light absorption or reduced chloroplast content within tissue (reviewed by Menzies et al., 2015). Despite its potential roles in adaptation, the genetic mechanisms underlying naturally occurring leaf variegation, especially structure-based coloration, are largely unknown (Zhang et al., 2020).
White clover (Trifolium repens L.) serves as an ideal system in which to investigate the genetic basis of leaf variegation and its potential adaptive value. This species is naturally polymorphic for several leaf variegation traits, including white leaf mark, red fleck and red midrib (Brewbaker, 1955; Carnahan et al., 1955; Brewbaker and Carnahan, 1956; Corkill, 1971). In addition, these polymorphisms are found not only in white clover but also in several other Trifolium species (Attila, 1987; Tan and Collins, 1987; Abdi et al., 2020) and in the closely related genus Medicago (McComb, 1974). The study of leaf variegation in white clover could therefore have generalizability beyond this single species.
Classical genetics studies in white clover over the last 70 years have revealed that the white leaf mark polymorphism, a structure-based variegation pattern (Carnahan et al., 1955), is governed primarily by a single locus (the V locus); the pigment-based colour polymorphisms, including red fleck and red midrib, are governed by an unlinked locus (R) (Brewbaker, 1955; Carnahan et al., 1955; Corkill, 1971). The presence of either variegation pattern is dominant to its absence, with multiple dominant alleles at both loci conferring slightly different variegation patterns. Although the R locus has been mapped successfully (Barrett et al., 2004; Tashiro et al., 2010), the genomic location of the white mark V locus has remained unknown despite recent mapping attempts (Tashiro et al., 2010). The lack of success in mapping the V locus thus far could be attributable to the limited resolution of the microsatellite-based linkage map markers used until recently in white clover (Barrett et al., 2004; Tashiro et al., 2010; Isobe et al., 2012; Griffiths et al., 2013), in addition to the plasticity of expression of the leaf mark phenotype (reflecting developmental stage, lighting conditions or other environmental factors) (Smith, 1986; Kim et al., 2012), which can challenge accurate trait scoring. Based on the previous documentation that white leaf mark is a structure-based variegation caused by enlarged intercellular or sub-epidermal spaces (Carnahan et al., 1955), we hypothesize that the presence and the intensity of white leaf mark trait could be associated with greater leaf thickness.
Leveraging recent advances in genomic resource availability for white clover (Santangelo et al., 2023; Kuo et al., 2024), we revisited the white leaf mark in this species using a combination of genome-wide high-density single nucleotide polymorphism (SNP) markers and comprehensive phenotyping data across developmental stages in an F3 mapping population. Our study had three objectives. Firstly, we aimed to document the white leaf mark polymorphism using a quantitative measure of the phenotype, accounting for the plasticity associated with leaf developmental stages and intra-individual variation and assessing its potential relationship to leaf thickness. Secondly, we aimed to map the quantitative trait loci (QTLs) for white leaf mark and leaf thickness. If the presence of white leaf mark is associated with greater leaf thickness, we would expect QTLs for the two phenotypes to show some degree of overlap. Thirdly, we aimed to test for any association of the white leaf mark with decreases in photosynthetic efficiency, because, in principle, the white leaf mark could compromise photosynthetic efficiency owing to a reduction of mesophyll cell density or chlorophyll content within the variegated tissues [e.g. as documented in Arabidopsis mutant lines (Rodermel, 2002)].
MATERIALS AND METHODS
Mapping population
An F3 mapping population (‘DG’, n = 500) was constructed from a cross of two wild North American white clover samples, DMN_010 (BioSample: SAMN34157026) and GFL_007 (BioSample: SAMN37329216, SAMN34116011). DMN_010 has no white leaf marks on any leaves at any developmental stages. In contrast, GFL_007 has white leaf marks on all leaves at all developmental stages. The two parents were cross-pollinated bidirectionally by hand. An F2 population was constructed by intercrossing the F1 generation (n = 50–100 plants) with solitary bees (Crown Bees, Woodinville, WA, USA) in enclosed cages in the greenhouse (Olsen et al., 2021; Wright et al., 2022). The F3 population (n = 500) was constructed by randomly intercrossing the F2 population by hand. The gametophytic self-incompatibility feature of white clover prevented plants from self-pollination while allowing some sib-crossing to occur. All plants were cultivated in the Washington University in St. Louis greenhouse facilities in 3.5 inch (88.9 mm) square plastic pots (KORD products, Twinsburg, OH, USA), containing Berger BM6 all-purpose growing medium (Hummert™ International, Earth City, MO, USA), in standard lighting and temperature conditions (16 h–8 h, light–dark photoperiod, with 140 μmol supplemental lighting as needed; seasonal temperature range of approximately 18–28 °C) and a daily watering schedule.
Genotyping
Library preparation and sequencing
Genomic DNA was extracted from 100 mg young leaf tissue samples from F3 plants. Liquid nitrogen-frozen leaf samples were homogenized with steel beads using a homogenizer. Then, the DNA was extracted using a protocol modified from Whitlock et al. (2008). Genotyping-by-sequencing (GBS) library preparation followed a modification of the protocol of Elshire et al. (2011). Specifically, the DNA (100 ng) was digested with ApeKI and ligated to our customized barcoding adaptors (Olsen et al., 2021). The ligation product was cleaned and size selected by AMPure XP beads (1.2 × volume beads added to the ligation product) (Beckman Coulter, Brea, CA, USA). Then, each sample was used individually in PCR amplification. Each amplified product was quantified by Qubit dsDNA HS Assay Kits (Thermo Fisher Scientific, Waltham, MA, USA), and samples were adjusted to the same concentration before pooling. A final clean-up and size selection were conducted by 0.8 × volume AMPure XP beads. Paired-end sequencing (150-bp reads) was performed with each library in a unique lane using the Illumina Hi-Seq 2500 platform (Novogene Corp., Chula Vista, CA, USA). The detailed step-by-step GBS protocol is provided in Supplementary Data File S1.
SNP calling and filtering
The GBS SNP dataset was generated by the fast-GBS v.2.0 pipeline (Torkamaneh et al., 2020). A minimum of six reads was set as the required minimum for an SNP call. The raw output was prefiltered to include only SNPs with <0.5 missing rate. Remaining missing data were imputed by Beagle v.5.4 with default settings (Browning et al., 2021). The imputed output was filtered for homozygosity in the parental accessions, minor allele frequency > 0.2, and P-value > 0.01 in a Hardy–Weinberg genotype frequency test.
Phenotyping
Leaf mark phenotyping
The white leaf mark trait was phenotyped independently three times for each accession (summer 2020, spring 2021 and summer 2022) by two different people in order to eliminate potential biases caused by different developmental stages, intra-individual variation and human error. The leaf mark trait was categorized into five ranks: ‘0’, no observable leaf mark on any leaves at any time; ‘1’, weakly visible leaf mark on some or all leaves in at least one phenotyping trial; ‘2’, weak and medium-intensity leaf marks on some or all leaves in all trials; ‘3’, medium and strongly visible leaf marks on some or all leaves in all trials; and ‘4’, strongly visible leaf mark on all leaves in all trials. See Fig. 1A for representative photographs of leaf mark variation among individual leaves; see Supplementary Data Fig. S1 for examples of leaf mark ranks for different F3 plants. Any data incongruencies between the measurement takers were rechecked to minimize phenotyping error.
Fig. 1.
Phenotypic variation in white leaf mark intensity and leaf thickness. (A) DG mapping population design and examples of different intensities of the white leaf mark. (B) Numbers of plants of the different leaf mark ranks. (C) Observed and expected numbers of plants of different leaf mark ranks based on the genotype of the V locus (chr_05.48106478), where the A allele is from GFL_007 and the B allele is from DMN_010. Horizontal black bars indicate observed counts for each V locus genotype. (D) Saturated leaf thickness. Each point is the mean value of four leaves from a single plant. The mean leaf thickness of the parents is shown by grey lines.
Leaf thickness phenotyping
To assess the correlation between leaf thickness and white leaf mark intensity, leaf thickness was measured after leaf saturation in distilled water overnight, a point at which cells contained their maximum water content. The protocol was modified from Afzal et al. (2017). Four healthy and mature leaves were cut from each plant, and the petioles with the cut surfaces were immediately placed in distilled water. Leaflets were kept above the water surface to avoid water infiltration into intercellular spaces. The leaves and distilled water were contained in a 50 mL centrifuge tube with lid, and the sealed tubes were kept in the dark at 4 °C overnight for complete saturation. The next day, leaves were blotted dry with tissue paper. The lamina thickness of the top leaflet of a trifoliate leaf was then measured using a thickness gauge (code no. 547-526S; Mitutoyo, Japan). The midrib was avoided for leaf thickness measurements.
Leaf physiology
To investigate the physiological effects of different levels of white leaf mark, we sampled 33 individuals randomly from the DG F3 mapping population (the same population used in QTL mapping) and 34 individuals from a second F3 mapping population, ‘GS’. The GS F3 mapping population was created using the same crossing protocol as DG but with the parental genotypes GFL_007 and STL_0701 (BioSample: SAMN34157027). Like DMN_010, the parent STL_0701 has no leaf marks on any leaves at any developmental stages. At least three leaves per individual were measured for all phenotyped accessions. Measurements, including net photosynthesis rate, stomatal conductance, intercellular CO2 concentration and water-use efficiency, were taken with top leaflets of the trifoliate leaves using a 1-cm-diameter chamber in a LICOR-6400XT system (LI-COR Biosciences, Lincoln, NE, USA), in summer 2021, at 10:00–15:00 h, with an LED light source (1100 µmol m−2 s−1) and controlled CO2 flow (400 µmol s−1). The leaflet was placed at the centre of the chamber. If the leaflet size was smaller than the chamber, the measurements were calibrated based on the leaflet area inside the chamber. The block temperature (23–30 °C) and reference relative humidity (40–60 %) were set to the ambient environment. After the LICOR-6400XT measurement, the leaf was cut from the plant, and the leaf thickness was measured immediately. Then, the leaf water potential was measured by a PMS pressure chamber (model 1000; Albany, OR, USA). The leaf area was measured by scanned images. All raw data for photosynthesis-related measurements are available in Supplementary Data File S2.
QTL mapping
Prior to QTL mapping, the genetic distance between all SNP markers was estimated by ASMap (Taylor and Butler, 2017), and any markers showing abnormal recombination rates (>150 cM) in juxtaposition were removed manually. The chromosomal locations and physical order of the markers were based on our recently generated high-quality reference genome (Kuo et al., 2024). QTL mapping was conducted in the R/qtl package using the normal model and the EM method with 1000 permutations (Broman et al., 2003). The genetic map with phenotypes in ‘cross’ object (.csv format) is available in Supplementary Data File S3.
RESULTS
Leaf phenotypes
Based on the phenotyping protocol, the DG parental accession GFL_007 was classified for white leaf mark as rank 3 (medium and strong leaf marks on some or all leaves at all developmental stages), whereas the other parental accession, DMN_010, was classified as rank 0 (no observable leaf mark on any leaves at any time) (Fig. 1A). In the F3 population, 197 plants were scored as rank 0, with the remaining 303 plants showing different degrees of white leaf mark intensity ranging from rank 1 to 4 (Fig. 1B). We found that individuals with the leaf mark present (rank > 0) varied in their plasticity of the phenotype (i.e. the extent to which the white leaf mark was expressed consistently across all leaves at all developmental stages in a plant). In general, plants at early developmental stages, when the young leaves were rarely covered by other leaves, had the most clearly observable white leaf mark patterns. In the later developmental stages, white leaf mark usually became less observable, but it could be restored by vigorous pruning, which exposed the regrowing young leaves to the light again (Supplementary Data Fig. S2). This observation suggests that plasticity of white leaf mark is dependent on light exposure during leaf development.
We examined the leaf thickness of the parental accessions and F3 progeny of the DG mapping population (396 plants measured in total) to test the hypothesis that the intensity of leaf mark is associated with greater leaf thickness. For the parental accessions, the leaf thickness of the rank 3 parent, GFL_007 (179.33 ± 9.45 μm; mean ± s.d.; n = 15), was significantly greater than that of the rank 0 parent, DMN_010 (133.93 ± 9.67 μm; n = 15) (t = −13.01, d.f. = 28, P = 2.19 × 10−13). In their F3 progeny, leaf thickness showed a high broad-sense heritability (H2 = 0.61) and differed significantly between plants with different white leaf mark ranks (F2,393 = 6.17, P = 2.31 × 10−3). Specifically, plants with a more intense white leaf mark showed greater leaf thickness (rank 4, 175.52 ± 17.31 μm) than the plants with medium-intensity (ranks 1 + 2 + 3, 164.47 ± 17.25 μm) or no leaf mark (rank 0, 165.05 ± 15.91 μm).
QTL mapping
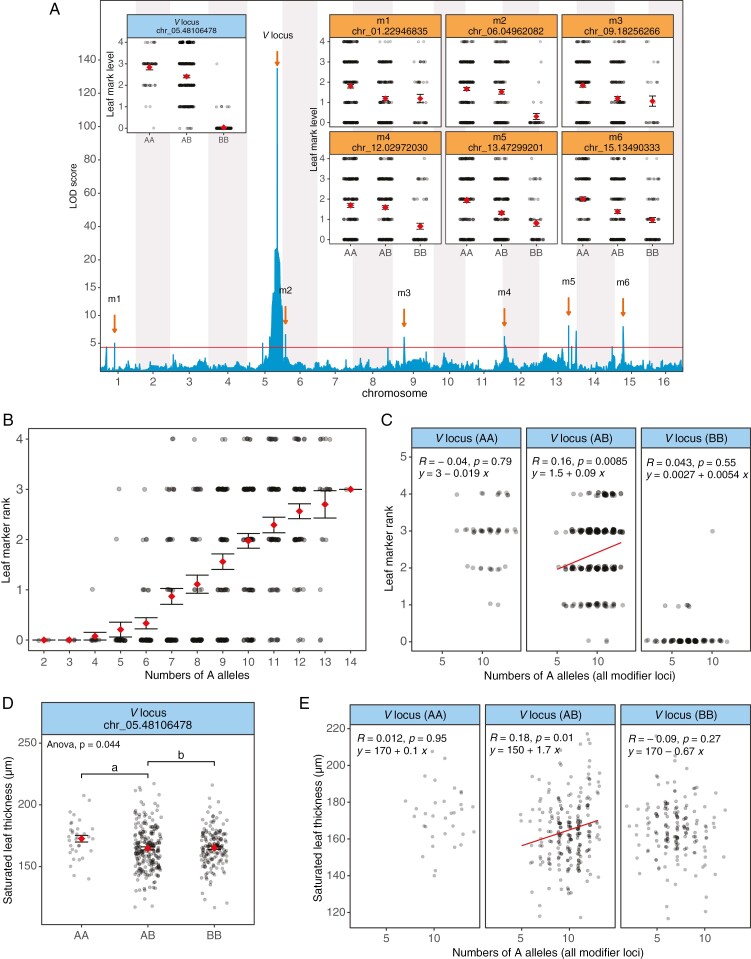
Using the F3 progeny of the DG cross, we generated a high-density linkage map with 6343 segregating SNP markers (28 893.88 cM, 4.55 cM per marker). For the white leaf mark polymorphism, QTL mapping revealed a major QTL on the distal end of chromosome 5 [chr_05:48106478, logarithm of the odds (LOD) = 134.87, percentage of variance explained (PVE) = 70.94 %]. We infer that this major QTL corresponds to the previously described but unmapped V leaf mark locus (Brewbaker, 1955; Carnahan et al., 1955; Brewbaker and Carnahan, 1956; Corkill, 1971). In addition, we detected six minor-effect modifier QTLs on different chromosomes (LOD = 5.00–8.12, PVE = 4.50–7.21 %). The seven QTLs jointly can explain 72.04 % of the total variance. For all seven QTLs, the GFL_007 parental allele (designated the ‘A’ allele) increased the intensity of leaf mark phenotype (Fig. 2A).
Fig. 2.
Genetics of white leaf mark and leaf thickness polymorphisms. (A) Logarithm of the odds (LOD) scan for the white leaf mark polymorphism. In addition to the major peak on chromosome 5 (chr_05.48106478, LOD = 134.87), which is inferred to correspond to the V locus, there are six minor peaks on different chromosomes with LOD scores greater than the significance threshold (LOD > 4.48, P < 0.05). Only one peak of the highest LOD score per chromosome is labelled. Inset panels show the phenotypic effects of the V locus genotypes (blue label, upper left) and the modifier loci (orange label, right). (B) Additive effect of ‘A’ alleles (pooled for the V locus and all six modifier loci) on leaf mark rank. (C) Additive effect of the six modifier loci on white leaf mark in the context of the different genotypes of the V locus. (D) Saturated leaf thickness by V locus genotype. Tukey multiple comparison was conducted at α = 0.05. (E) Saturated leaf thickness, additive effect of the six modifier loci in the context of the different genotypes of the V locus.
We found a high consistency between the leaf mark phenotype and the genotype of the major QTL on chromosome 5 (the inferred V locus). Notably, when merging the leaf mark ranks into three categories of intensity [no mark (rank 0); medium (rank 1, 2, 3); and strong (rank 4)], the number of plants in each category is highly consistent with counts of ‘AA’, ‘AB’ and ‘BB’ genotypes at this locus (χ2 = 6, d.f. = 4, P = 0.20) (Fig. 1C). Specifically, the number of plants classified as having no leaf mark (rank 0, n = 197) is exactly the same as the number with the ‘BB’ genotype at the V locus; the number classified as medium intensity (rank 1, 2, 3; n = 263) is slightly higher than the number with the ‘AB’ genotype (n = 254); and the number classified as strongly marked (rank 4, n = 40) is slightly lower than the number with the ‘AA’ genotype (n = 49). Although there are a few incongruencies between the leaf mark phenotype and the corresponding genotype at the V locus (Supplementary Data File S3), this overall pattern demonstrates a clear pattern of incomplete dominance at the V locus, and it confirms the QTL mapping results that the V locus alone can account for much of the observed leaf mark variation.
Taking into consideration the phenotypic contributions of the V locus plus the six modifier loci, we found that the leaf mark intensity is directly correlated with the number of the ‘A’ alleles across all seven loci; this indicates that the loci contribute additively to the leaf mark trait (Fig. 2B). This pattern of additivity is especially clear when the V locus is heterozygous (Fig. 2C), suggesting a pattern of incomplete dominance at the major-effect V locus, and with the modifier loci collectively contributing to the intensity of the leaf mark trait to a lesser degree.
Based on our finding that the intensity of the leaf mark phenotype is associated with greater leaf thickness (Fig. 1D), we tested for overlap between the leaf mark QTLs and the leaf thickness QTLs. However, despite a clear difference in the leaf thickness of the parents (Fig. 1D) and the high broad-sense heritability of this trait in the mapping population (H2 = 0.61), we failed to detect any significant QTLs related to the leaf thickness variation. It is possible that leaf thickness is a polygenic trait, with multiple QTLs contributing a small effect that falls below the significance threshold for detection.
Given that we could not directly compare leaf thickness QTLs with the leaf mark QTLs, we instead tested for differences in leaf thickness among the different genotypes of the seven leaf mark QTLs. For the V locus, ‘AA’ plants had significantly greater leaf thickness (172.61 ± 15.45 μm) than ‘BB’ plants (165.21 ± 15.55 μm) (Fig. 2D) (F2,393 = 3.1434, P = 0.0442). Likewise, the different genotypes of the modifier loci showed a significant association with leaf thickness, but only when the V locus was heterozygous (Fig. 2E). These findings indicate that there is an association between leaf mark intensity and leaf thickness and that the effect of the modifier loci on the leaf mark intensity and leaf thickness variation is V locus dependent.
Photosynthetic efficiency
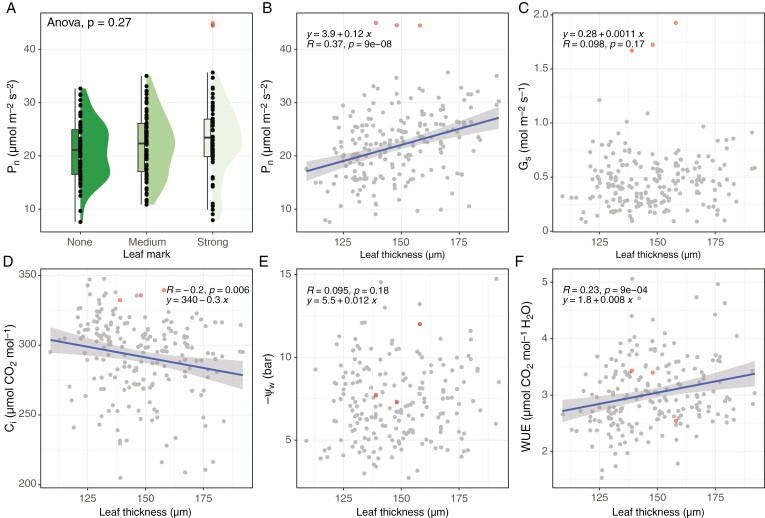
In addition to the association with greater leaf thickness, we hypothesized that the presence of white leaf mark could compromise the photosynthetic efficiency owing to an increase in intercellular spaces or reduced chloroplast content. However, we observed no decline of net photosynthesis rate (Pn) with increasing leaf mark intensity. Instead, we found a non-significant trend in the opposite direction, with the Pn of the strong leaf mark leaves (22.72 ± 6.74 μmol m−2 s−2) being greater than that of the medium-intensity (21.88 ± 5.83 μmol m−2 s−2) and no-leaf-mark (20.99 ± 5.39 μmol m−2 s−2) leaves (ANOVA, F2,194 = 1.32, P = 0.27; Fig. 3A). Similar to Pn, we did not detect any significant differences of stomatal conductance (F2,194 = 0.79, P = 0.46), intercellular CO2 concentration (F2,197 = 0.87, P = 0.42), water-use efficiency (F2,197 = 0.29, P = 0.75) or water potential (F2,195 = 2.03, P = 0.13) between different levels of leaf mark intensity (Supplementary Fig. S3).
Fig. 3.
Physiological parameters of the leaves with different levels of white leaf mark intensity and leaf thickness. (A) Net photosynthesis rate of leaves with different white leaf mark levels. (B–F) Associations with leaf thickness: (B) net photosynthesis rate (Pn); (C) stomatal conductance (Gs); (D) intercellular CO2 concentration (Ci); (E) water potential (−ΨW); and (F) water-use efficiency (WUE). The three red data points showing abnormally high net photosynthesis rate were excluded from all but water potential statistical analyses.
Because the intensity of the white leaf mark was found to be positively associated with leaf thickness (Fig. 2D, E), we also tested for associations between leaf thickness and net photosynthesis rate. We found a significantly positive association for these two traits (Pn, F1,195 = 30.87, P = 8.97 × 10−8; Fig. 3B). We investigated this relationship further by examining leaf stomatal conductance; this revealed that leaf thickness has no effect on stomatal conductance (F1,195 = 1.90, P = 0.17; Fig. 3C) but has a significantly negative effect on intercellular CO2 concentration (F1,195 = 7.73, P = 5.97 × 10−3; Fig. 3D). Given that the leaf thickness has no detectable effect on the water status of the tested leaves (F1,193 = 1.60, P = 0.21; Fig. 3E), these findings together suggest that the leaf thickness is positively associated with water-use efficiency (F1,195 = 11.37, P = 8.98 × 10−4; Fig. 3F). They also suggest that at a comparable cellular water level, the leaves with greater thickness, possibly associated with a loose cellular arrangement or additional layers of cells in a leaf, can fix more CO2 per unit of water use.
DISCUSSION
The inheritance and genetics of leaf mark variation in white clover have been studied for many decades (Brown, 1947), but the characteristic white leaf mark polymorphism (also referred to as the V mark or crescent mark) has remained largely uncharacterized with respect to its genetic basis and potential effects on other aspects of the leaf phenotype. Here we have successfully mapped the loci that control the white leaf mark polymorphism, and we have investigated its relationship to other aspects of the plant phenotype, including leaf thickness and physiology (photosynthetic efficiency).
Consistent with classical genetics studies (Brewbaker, 1955; Carnahan et al., 1955; Corkill, 1971), we found that the white leaf mark polymorphism is controlled primarily by a single major-effect locus (the V locus), which we map to the distal end of chromosome 5, with the leaf mark-conferring allele (‘A’) showing incomplete dominance over the no-leaf-mark allele (‘B’) in our mapping population (Fig. 2A). Additionally, we discovered that the modifier loci contribute additively to the intensity of the leaf mark (Fig. 2B), particularly when the V locus is heterozygous (Fig. 2C). Moreover, the presence and greatest intensity of the leaf mark are associated with greater leaf thickness (Fig. 1D), with ‘A’ alleles at the V locus and modifier loci also being positively associated with this phenotype (Fig. 2D, E). With respect to physiology, we found no evidence that the white leaf mark compromises photosynthesis. Instead, leaves with greater thickness were correlated with a higher Pn and enhanced water-use efficiency, irrespective of the presence of the leaf mark trait (Fig. 2B, F).
Unlike many model species, where white-coloured leaf variation has been studied using chimeric albino mutants or other genotypes with unstable chloroplast development (Sakamoto, 2003), the white leaf mark in white clover has long been known to be a nuclear-encoded stable and non-chimeric polymorphism (Brewbaker, 1955; Carnahan et al., 1955; Corkill, 1971). However, despite >60 years of research into the genetics of the white leaf mark polymorphism (Brewbaker, 1955; Carnahan et al., 1955; Corkill, 1971; Tashiro et al., 2010), the corresponding V locus has remained unmapped until this study. The success of the present study can be attributed to the use of sequencing-based genetic markers (Olsen et al., 2021; Kuo et al., 2024), which offer greater density and precision than previously used microsatellite markers (Tashiro et al., 2010), and a repeated phenotyping process at multiple stages of leaf development to account for the phenotypic plasticity, which has not been addressed in previous studies of the white leaf mark polymorphism.
The V locus, mapped to chromosome 5, explains 70.94 % of the white leaf mark variation in DG F3 progeny of our mapping population. Our finding that this locus shows incomplete dominance, with at least one copy of the functional (‘A’) allele at the V locus required for the leaf mark to develop, is consistent with observations of older classical genetics studies (Brewbaker, 1955; Carnahan et al., 1955; Corkill, 1971). The present study also suggests that the intensity of white leaf mark is likely to be dependent on intricate interactions involving other genetic loci and that it is likely to be associated with leaf structural development.
In addition to the heritable component, we also observed extensive plasticity in the white leaf mark phenotype. Our observations of leaf phenotypes at different developmental stages indicate that the white leaf mark is most evident at the early developmental stages. As plants matured, the white leaf mark became less observable in some genotypes (Supplementary Fig. S2). However, the evident leaf mark could reappear during periods of leaf regrowth, suggesting that this plasticity is dependent on light exposure. A similar phenomenon appears to have been documented indirectly in field observations of white clover by Cahn and Harper (1976a), who reported that the frequency of leaf mark-bearing genotypes decreased with increasing grass length in a mixed planting (although the authors interpreted this phenotypic shift as evidence of differential herbivore pressure on variegated leaves rather than developmental plasticity).
Our finding that the intensity of leaf mark is associated with leaf thickness suggests that leaf thickness can modify the intensity of the white leaf mark, especially when the V locus is heterozygous. Although no QTLs were detected for leaf thickness, we found that the genotypes of the V locus and leaf mark modifier loci were associated with leaf thickness variation. The effect direction was consistent with that of the leaf mark, with the ‘A’ allele contributing to the increase of leaf mark and leaf thickness (Fig. 2D, E). This correlation illustrates the phenotypic interactions between leaf mark and leaf thickness development and suggests potential pleiotropic effects of the V locus and modifier loci in controlling multiple aspects of leaf development.
Various balancing selection hypotheses have been proposed for the maintenance of the white leaf mark polymorphism in white clover and other species that are polymorphic for this trait, most of them related to potential fitness trade-offs between the effectiveness of the leaf mark in herbivore avoidance and the costs of compromised photosynthetic efficiency (Esteban et al., 2008; Menzies et al., 2015). Herbivore avoidance mechanisms could include the leaf mark serving as aposematic coloration to warn herbivores of cyanogenic toxicity (Lev-Yadun, 2014, 2021) or as a dazzling effect that could hinder the ability of herbivores to locate the leaves (Lev-Yadun, 2014) or as a disruptive coloration to make the leaves appear small or damaged to lower the foraging preferences (Cahn and Harper, 1976b). Conversely, the presence of the leaf mark could be associated with compromised photosynthetic efficiency owing to the presence of atypical palisade cells and increased intercellular and subepidermal spaces (Carnahan et al., 1955; Esteban et al., 2008).
In our analyses of the white leaf mark polymorphism in white clover, we observed no decrease in photosynthetic efficiency between leaves with and without the white leaf mark, suggesting a minimal fitness cost for a white leaf mark-bearing genotype. Similar results of uncompromised photosynthetic efficiency have been reported in naturally variegated Begonia species (Sheue et al., 2012) and in multiple plants of horticultural origin (Konoplyova et al., 2008; Zhang et al., 2019). These findings together suggest that structure-based colour patterning is a highly efficient way for plants to develop variegation patterns, with negligible costs for photosynthetic output. They also suggest that the presence of the white leaf mark polymorphism in white clover involves mechanisms more complex than a simple fitness trade-off between herbivore avoidance and photosynthetic efficiency.
CONCLUSION
Important advances of this study include mapping the white clover V locus and detailed documentation of associations between white leaf mark, leaf thickness and photosynthetic efficiency. In addition, we not only documented the plasticity of the leaf mark intensity in white leaf mark-bearing genotypes, but we also documented the additive effects of the V locus and the six modifier loci, which might also pleiotropically control leaf thickness development. Physiologically, we found no evidence that the white leaf mark compromised photosynthetic efficiency. Instead, the significant positive correlations we detected between the white leaf mark intensity, leaf thickness and photosynthetic efficiency highlight that selective explanations based on simple fitness trade-offs cannot fully account for the observed trait variation.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
File S1: step-by-step protocol of GBS library preparation. File S2: raw data of photosynthetic efficiency measurement. File S3: linkage map and all phenotypic data (a .csv table in ‘cross’ format for R/qtl package). Figure S1: examples of white leaf mark rank classification. Figure S2: variable intensity of the white leaf mark in a single plant. Figure S3: physiological parameters of leaves with different white leaf mark intensity.
ACKNOWLEDGEMENTS
We thank the Washington University in St. Louis greenhouse staff, especially Michael Dyer, Michael Stephan and Hammy Sorkin, for care of plants used in the study. We thank Linda Small for help with the phenotyping process in the greenhouse. We thank David Goad for providing suggestions in the QTL mapping and interpretation of results.
Contributor Information
Wen-Hsi Kuo, Department of Biology, Washington University in St. Louis, St. Louis, MO, USA.
Eimear Cunningham, Department of Biology, Washington University in St. Louis, St. Louis, MO, USA.
Emily Guo, Department of Biology, Washington University in St. Louis, St. Louis, MO, USA.
Kenneth M Olsen, Department of Biology, Washington University in St. Louis, St. Louis, MO, USA.
FUNDING
W.-H.K. was supported by a William H. Danforth Plant Science Graduate Research Fellowship and a Division of Biology and Biomedical Sciences (DBBS) fellowship at Washington University in St. Louis; additional funding was provided through a Taiwan Ministry of Education Scholarship. K.M.O. received funding through a grant from the U.S. National Science Foundation (IOS-1557770).
AUTHOR CONTRIBUTIONS
W.-H.K. designed and conducted the experiment, analysed the data, interpreted the results and wrote the manuscript. E.C. grew and maintained the plants and recorded the white leaf mark and photosynthesis data. E.G. grew and maintained the plants and recorded the leaf thickness data. E.C. and E.G. were undergraduate students on Washington University’s Bio 500 course (Undergraduate Independent Research). K.M.O. conceived the project, interpreted the results and edited the manuscript. All authors read and approved the final manuscript.
DATA AVAILABILITY
Raw GBS reads for DG F3 linkage map construction may be found under NCBI SRA BioProject: PRJNA1027808.
LITERATURE CITED
- Abdi AI, Nichols PGH, Kaur P, Wintle BJ, Erskine W.. 2020. Morphological diversity within a core collection of subterranean clover (Trifolium subterraneum L.): lessons in pasture adaptation from the wild. PLoS One 15: e0223699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal A, Duiker SW, Watson JE.. 2017. Leaf thickness to predict plant water status. Biosystems Engineering 156: 148–156. [Google Scholar]
- Attila TS. 1987. V-leaf marking in a Trifolium ambiguum Bieb. germplasm collection. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 17: 77–84. [Google Scholar]
- Barrett B, Griffiths A, Schreiber M, et al. 2004. A microsatellite map of white clover. Theoretical and Applied Genetics 109: 596–608. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL. 1955. V-leaf markings of white clover. Journal of Heredity 46: 115–123. [Google Scholar]
- Brewbaker JL, Carnahan HL.. 1956. Leaf marking alleles in white clover: uniform nomenclature. Journal of Heredity 47: 103–104. [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA.. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- Brown K. 1947. Inheritance of patterned leaf marking in white clover. Meeting of American Society of Agronomy, Cincinnati, OH, Nov.17–20. [Google Scholar]
- Browning BL, Tian X, Zhou Y, Browning SR.. 2021. Fast two-stage phasing of large-scale sequence data. American Journal of Human Genetics 108: 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn MG, Harper JL.. 1976a. The biology of the leaf mark polymorphism in Trifolium repens L. 1. Distribution of phenotypes at a local scale. Heredity 37: 309–325. [Google Scholar]
- Cahn MG, Harper JL.. 1976b. The biology of the leaf mark polymorphism in Trifolium repens L. 2. Evidence for the selection of leaf marks by rumen fistulated sheep. Heredity 37: 327–333. [Google Scholar]
- Campitelli BE, Stehlik I, Stinchcombe JR.. 2008. Leaf variegation is associated with reduced herbivore damage in Hydrophyllum virginianum. Botany 86: 306–313. [Google Scholar]
- Carnahan HL, Hill HD, Hanson AA, Brown KG.. 1955. Inheritance and frequencies of leaf markings in white clover. Journal of Heredity 46: 109–114. [Google Scholar]
- Charles AH. 1968. Some selective effects operating on white- and red-clover in swards. Grass and Forage Science 23: 20–25. [Google Scholar]
- Corkill L. 1971. Leaf markings in white clover. Journal of Heredity 62: 307–310. [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, et al. 2011. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6: e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R, Fernandez-Marin B, Becerril JM, Garcia-Plazaola JI.. 2008. Photoprotective implications of leaf variegation in E. dens-canis L. and P. officinalis L. Journal of Plant Physiology 165: 1255–1263. [DOI] [PubMed] [Google Scholar]
- Ganders FR, Griffiths AJF, Carey K.. 1980. Natural selection for spotted leaves: parallel morph ratio variation in three species of annual plants. Canadian Journal of Botany 58: 689–693. [Google Scholar]
- Griffiths AG, Barrett BA, Simon D, et al. 2013. An integrated genetic linkage map for white clover (Trifolium repens L.) with alignment to Medicago. BMC Genomics 14: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe SN, Hisano H, Sato S, et al. 2012. Comparative genetic mapping and discovery of linkage disequilibrium across linkage groups in white clover (Trifolium repens L.). G3 2: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kang SW, Pak CH, Kim MS.. 2012. Changes in leaf variegation and coloration of English ivy and polka dot plant under various indoor light intensities. HortTechnology 22: 49–55. [Google Scholar]
- Konoplyova A, Petropoulou Y, Yiotis C, Psaras GK, Manetas Y.. 2008. The fine structure and photosynthetic cost of structural leaf variegation. Flora 203: 653–662. [Google Scholar]
- Koski MH, Ashman TL.. 2015. Floral pigmentation patterns provide an example of Gloger’s rule in plants. Nature Plants 1: 14007. [DOI] [PubMed] [Google Scholar]
- Kuo W-H, Wright SJ, Small LL, Olsen KM.. 2024. De novo genome assembly of white clover (Trifolium repens L.) reveals the role of copy number variation in rapid environmental adaptation. BMC Biology 22: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Yadun S. 2014. Potential defence from herbivory by ‘dazzle effects’ and ‘trickery coloration’ of leaf variegation. Biological Journal of the Linnean Society 111: 692–697. [Google Scholar]
- Lev-Yadun S. 2021. Avoiding rather than resisting herbivore attacks is often the first line of plant defence. Biological Journal of the Linnean Society 134: 775–802. [Google Scholar]
- McComb J. 1974. Leaf marks in Medicago, with special reference to their inheritance in Medicago truncatula. Australian Journal of Botany 22: 67–80. [Google Scholar]
- Menzies IJ, Youard LW, Lord JM, et al. 2015. Leaf colour polymorphisms: a balance between plant defence and photosynthesis. Journal of Ecology 104: 104–113. [Google Scholar]
- Olsen KM, Goad DM, Wright SJ, et al. 2021. Dual‐species origin of an adaptive chemical defense polymorphism. New Phytologist 232: 1477–1487. [DOI] [PubMed] [Google Scholar]
- Rodermel S. 2002. Arabidopsis variegation mutants. Arabidopsis Book 1: e0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W. 2003. Leaf-variegated mutations and their responsible genes in Arabidopsis thaliana. Genes & Genetic Systems 78: 1–9. [DOI] [PubMed] [Google Scholar]
- Santangelo JS, Battlay P, Hendrickson BT, et al. 2023. Haplotype-resolved, chromosome-level assembly of white clover (Trifolium repens L., Fabaceae). Genome Biology and Evolution 15: evad146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelef O, Summerfield L, Lev-Yadun S, et al. 2019. Thermal benefits from white variegation of Silybum marianum leaves. Frontiers in Plant Science 10: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheue CR, Pao SH, Chien LF, Chesson P, Peng CI.. 2012. Natural foliar variegation without costs? The case of Begonia. Annals of Botany 109: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP. 1986. Ecology of a leaf color polymorphism in a tropical forest species: habitat segregation and herbivory. Oecologia 69: 283–287. [DOI] [PubMed] [Google Scholar]
- Soltau U, Dötterl S, Liede-Schumann S.. 2008. Leaf variegation in Caladium steudneriifolium (Araceae): a case of mimicry? Evolutionary Ecology 23: 503–512. [Google Scholar]
- Song B, Stocklin J, Armbruster WS, Gao Y, Peng D, Sun H.. 2018. Reversible colour change in leaves enhances pollinator attraction and reproductive success in Saururus chinensis (Saururaceae). Annals of Botany 121: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BH, Collins WJ.. 1987. Multi-allelic nature of the locus controlling leaf marking in subterranean clover. Australian Journal of Agricultural Research 38: 547–558. [Google Scholar]
- Tashiro RM, Han Y, Monteros MJ, Bouton JH, Parrott WA.. 2010. Leaf trait coloration in white clover and molecular mapping of the red midrib and leaflet number traits. Crop Science 50: 1260–1268. [Google Scholar]
- Taylor J, Butler D.. 2017. R Package ASMap: efficient genetic linkage map construction and diagnosis. Journal of Statistical Software 79: 1–29.30220889 [Google Scholar]
- Torkamaneh D, Laroche J, Belzile F.. 2020. Fast-GBS v2.0: an analysis toolkit for genotyping-by-sequencing data. Genome 63: 577–581. [DOI] [PubMed] [Google Scholar]
- Whitlock R, Hipperson H, Mannarelli M, Burke T.. 2008. A high-throughput protocol for extracting high-purity genomic DNA from plants and animals. Molecular Ecology Resources 8: 736–741. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Goad DM, Gross BL, Muñoz PR, Olsen KM.. 2022. Genetic trade‐offs underlie divergent life history strategies for local adaptation in white clover. Molecular Ecology 31: 3742–3760. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu Z, Song H, Chen M, Cheng S.. 2019. Protective role of leaf variegation in Pittosporum tobira under low temperature: insights into the physio-biochemical and molecular mechanisms. International Journal of Molecular Sciences 20: 4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-H, Zeng J-C, Wang X-M, Chen S-F, Albach DC, Li H-Q.. 2020. A revised classification of leaf variegation types. Flora 272: 151703. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw GBS reads for DG F3 linkage map construction may be found under NCBI SRA BioProject: PRJNA1027808.