Abstract
Background
Postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) can affect many organ systems. However, temporal changes during the coronavirus disease 2019 (Covid-19) pandemic, including the evolution of SARS-CoV-2, may have affected the risk and burden of PASC. Whether the risk and burden of PASC have changed over the course of the pandemic is unclear.
Methods
We used health records of the Department of Veterans Affairs to build a study population of 441,583 veterans with SARS-CoV-2 infection between March 1, 2020, and January 31, 2022, and 4,748,504 noninfected contemporaneous controls. We estimated the cumulative incidence of PASC at 1 year after SARS-CoV-2 infection during the pre-delta, delta, and omicron eras of the Covid-19 pandemic.
Results
Among unvaccinated persons infected with SARS-CoV-2, the cumulative incidence of PASC during the first year after infection was 10.42 events per 100 persons (95% confidence interval [CI], 10.22 to 10.64) in the pre-delta era, 9.51 events per 100 persons (95% CI, 9.26 to 9.75) in the delta era, and 7.76 events per 100 persons (95% CI, 7.57 to 7.98) in the omicron era (difference between the omicron and pre-delta eras, −2.66 events per 100 persons [95% CI, −2.93 to −2.36]; difference between the omicron and delta eras, −1.75 events per 100 persons [95% CI, −2.08 to −1.42]). Among vaccinated persons, the cumulative incidence of PASC at 1 year was 5.34 events per 100 persons (95% CI, 5.10 to 5.58) during the delta era and 3.50 events per 100 persons (95% CI, 3.31 to 3.71) during the omicron era (difference between the omicron and delta eras, −1.83 events per 100 persons; 95% CI, −2.14 to −1.52). Vaccinated persons had a lower cumulative incidence of PASC at 1 year than unvaccinated persons (difference during the delta era, −4.18 events per 100 persons [95% CI, −4.47 to −3.88]; difference during the omicron era, −4.26 events per 100 persons [95% CI, −4.49 to −4.05]). Decomposition analyses showed 5.23 (95% CI, 4.97 to 5.47) fewer PASC events per 100 persons at 1 year during the omicron era than during the pre-delta and delta eras combined; 28.11% of the decrease (95% CI, 25.57 to 30.50) was attributable to era-related effects (changes in the virus and other temporal effects), and 71.89% (95% CI, 69.50 to 74.43) was attributable to vaccines.
Conclusions
The cumulative incidence of PASC during the first year after SARS-CoV-2 infection decreased over the course of the pandemic, but the risk of PASC remained substantial even among vaccinated persons who had SARS-CoV-2 infection in the omicron era. (Supported by the Department of Veterans Affairs.)
Postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC), also called “long Covid,” can affect many organ systems.1,2 The risk of PASC appears to increase with greater severity of infection and with the presence of preexisting medical conditions, and the risk appears to decrease after coronavirus disease 2019 (Covid-19) vaccination.3,4 However, SARS-CoV-2 has changed during the Covid-19 pandemic. Although PASC has been documented with different SARS-CoV-2 variants, it has been postulated that variation in viral characteristics and other factors, including the introduction of Covid-19 vaccines, may have contributed to a reduction in the risk of PASC during the course of the pandemic. However, whether and to what extent the risk of PASC has changed during the Covid-19 pandemic is unclear. Thus, a comparison of the risk and burden of PASC during the main eras of the pandemic, including the era before the delta variant became the dominant lineage (pre-delta era), the era when the delta variant was dominant (delta era), and the era when the omicron variant was dominant (omicron era), is needed.5 Such a comparison may deepen our understanding of the epidemiology of PASC, inform the public health response, guide the prioritization of research, and improve clinical care.5
In this study, we used the Department of Veterans Affairs Health Care System databases to build a study population of 441,583 veterans with SARS-CoV-2 infection between March 1, 2020, and January 31, 2022, and 4,748,504 noninfected contemporaneous controls. Persons in the study were followed for 1 year to estimate the risk and burden of PASC during the pre-delta, delta, and omicron eras.
Methods
Cohort
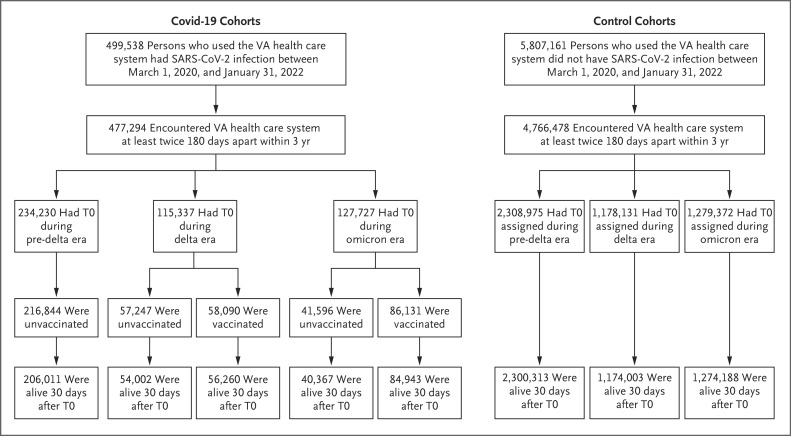
We used databases of the Veterans Affairs Health Care System to select 441,583 veterans with SARS-CoV-2 infection between March 1, 2020, and January 31, 2022, who were alive 30 days after T0 (defined below) for inclusion into one of five cohorts: no vaccination and SARS-CoV-2 infection during the pre-delta era (206,011 persons), no vaccination and SARS-CoV-2 infection during the delta era (54,002 persons), vaccination and SARS-CoV-2 infection during the delta era (56,260 persons), no vaccination and SARS-CoV-2 infection during the omicron era (40,367 persons), and vaccination and SARS-CoV-2 infection during the omicron era (84,943 persons). Three era-specific control cohorts of persons without SARS-CoV-2 infection between March 1, 2020, and January 31, 2022, who were alive 30 days after T0 were also selected: a pre-delta era control cohort (2,300,313 persons), a delta era control cohort (1,174,003 persons), and an omicron era control cohort (1,274,188 persons) (Figure 1). T0 for persons in the Covid-19 cohorts was defined as the date of SARS-CoV-2 infection. T0 for persons in the control cohorts was assigned according to the distribution of T0 in the Covid-19 cohorts. Additional details about the selection of the study participants are provided in the Methods section of the Supplementary Appendix, available with the full text of this article at NEJM.org. The representativeness of the study participants is shown in Table S1 in the Supplementary Appendix.
Figure 1. Study Cohorts.
T0 for persons in the coronavirus disease 2019 (Covid-19) cohorts was defined as the date of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. T0 for persons in the control cohorts was assigned according to the distribution of T0 in the Covid-19 cohorts. VA denotes Veterans Affairs.
The Covid-19 cohorts were followed until death from any cause, 1 year after T0, or SARS-CoV-2 reinfection, whichever occurred first. The noninfected era-specific control cohorts were followed until death from any cause, 1 year after T0, or the first SARS-CoV-2 infection, whichever occurred first.
This study used data from the VA Covid-19 Shared Data Resource. The institutional review board of the Department of Veteran Affairs of St. Louis Health Care System approved the study and granted a waiver of informed consent. The contributions of the authors are described in the Supplementary Appendix.
Outcomes
We prespecified a list of health outcomes that have been previously described as postacute sequelae of SARS-CoV-2 infection (Table S2).1,6-12 Outcomes were defined on the basis of multiple data domains, including International Classification of Diseases, 10th Revision, diagnosis codes, laboratory values, and prescription medications. An incident health outcome was defined as an outcome that first occurred between 30 days and 1 year after T0 and had not been present before T0. The health outcomes were classified according to 10 disease categories — cardiovascular, coagulation and hematologic, fatigue, gastrointestinal, kidney, mental health, metabolic, musculoskeletal, neurologic, and pulmonary. The risk of PASC is presented for each disease category individually and overall. An event within an outcome category was defined as the occurrence of any incident individual health outcome under that category.
We also used the Global Burden of Disease study methods to estimate the number of disability-adjusted life-years due to PASC.6,13-15 Disability-adjusted life-years account for the number of outcome events and their influence on overall health.6,13-15
Covariates
Covariates were selected with the use of a directed acyclic graph that was built on the basis of previously published data; covariate data were collected during the 3-year period before baseline (Fig. S1).3,7-11,16,17 Sociodemographic variables included age, race (White, Black, or other), sex as reported by the participant, area deprivation index (a measure of socioeconomic disadvantage), smoking status (current, former, or never), and use of long-term care services. Laboratory measurements and vital signs included the estimated glomerular filtration rate, systolic and diastolic blood pressures, and body-mass index. Coexisting conditions included cancer, cardiovascular disease, chronic lung disease, coronary artery disease, dementia, diabetes, hyperlipidemia, human immunodeficiency virus infection, immune dysfunction, liver diseases, and peripheral artery diseases. To account for potential differences in health behavior and health resource use, we also adjusted for the number of outpatient visits and hospital admissions, blood panel tests, medications received, Medicare outpatient visits and hospital admissions, and immunizations and for the receipt of seasonal influenza vaccine. Covariates related to health behavior and health resource use were measured during the third year before T0, which was before the beginning of the pandemic, and separately during the year before T0. We also estimated and adjusted for the predicted probability of undergoing testing for Covid-19 during each era conditional on covariate data before the beginning of the pandemic. Missing data, including missing estimated glomerular filtration rates (for 6.82% of participants), missing body-mass indexes (for 6.62% of participants), and missing blood pressures (for 1.56% of participants), were imputed with the use of a predictive mean matching method based on multivariate imputation by chained equations.18 Continuous variables were transformed into restricted cubic spline functions with knots at the 5th, 35th, 65th, and 95th percentiles to account for potential nonlinear relationships between covariates and cohorts.19
Statistical Analysis
The baseline characteristics of the five Covid-19 cohorts and the three control cohorts are reported as means and standard deviations for continuous variables and as numbers and percentages for categorical variables. Pairwise absolute standardized differences across the cohorts were used to assess between-group differences in baseline characteristics, with a value of less than 0.1 considered to be evidence of good covariate balance.
To achieve balance on baseline characteristics across the eight cohorts, overlap weighting for multiple groups was used.20 For each participant, probabilities of being assigned to each of the eight cohorts were estimated with the use of logistic-regression analysis conditional on baseline characteristics. The overlap weight was then constructed as the inverse probability of being assigned to the cohort in which the participant was enrolled divided by the summation of the inverse probabilities of being assigned to each of the eight cohorts.
Weighted generalized estimating equations with Poisson regression were used to estimate the cumulative incidence of PASC in each of the eight cohorts. The risk and burden of PASC in each Covid-19 cohort were then estimated on the basis of the difference in these measures between the Covid-19 cohorts and their corresponding era-specific control cohorts. Comparisons between the Covid-19 cohorts with respect to the risk and burden of PASC during the first year after infection were then conducted, and findings are reported as the incidence rate ratio, cumulative incidence, and disability-adjusted life-years per 100 persons. We also estimated the risk and burden of PASC in combinations of cohorts, including the pre-delta era cohort and the unvaccinated delta era cohort, both delta era cohorts, both omicron era cohorts, and the pre-delta era cohort and both delta era cohorts, on the basis of the proportion composition of the five independent Covid-19 cohorts in these combined cohorts.
We then conducted decomposition analyses to understand the distributional contribution of era and vaccines to changes in the cumulative incidence of PASC. Details on decomposition analyses are provided in the Methods section of the Supplementary Appendix.
We conducted multiple sensitivity analyses to test the robustness of the results. These analyses included alternative approaches to the exposure definition, follow-up definition, modeling assumptions, covariate adjustment, and outcome definition. We also conducted analyses of the sensitivity of the results to the influence of unmeasured confounding and misclassification of exposure. In addition, we assessed incident neoplasm as a negative outcome control.21 Details about the sensitivity analyses and the negative outcome control analysis are provided in the Methods section of the Supplementary Appendix.
We obtained 95% confidence intervals of the estimates from the 2.5th and 97.5th percentiles of parametric bootstrapping with 1000 simulations generated with the use of a generalized estimating equations–based covariance matrix. SAS Enterprise Guide, version 8.3 (SAS Institute), was used for data management and analyses; R, version 4.3.0 (R Foundation for Statistical Computing), was used for data visualization.
Results
Cohorts
The demographic and clinical characteristics of the eight cohorts before and after weighting are presented in Tables S3 and S4, respectively. Assessment of the standardized mean differences for all baseline characteristics in the weighted cohorts showed that all differences were less than 0.1, which suggests that good balance was achieved.
Cumulative Incidence of PASC
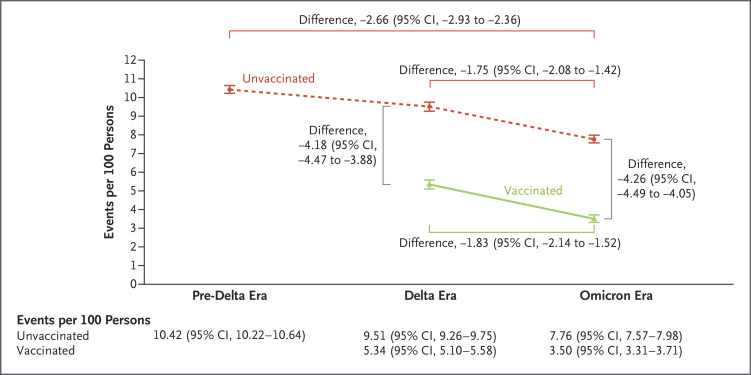
Among unvaccinated persons, the cumulative incidence of PASC at 1 year after SARS-CoV-2 infection was 10.42 events per 100 persons (95% confidence interval [CI], 10.22 to 10.64) during the pre-delta era, 9.51 events per 100 persons (95% CI, 9.26 to 9.75) during the delta era, and 7.76 events per 100 persons (95% CI, 7.57 to 7.98) during the omicron era. The cumulative incidence of PASC at 1 year was lower during the omicron era than during the pre-delta era (difference, −2.66 events per 100 persons; 95% CI, −2.93 to −2.36) and the delta era (difference, −1.75 events per 100 persons; 95% CI, −2.08 to −1.42), corresponding to an incidence rate ratio of 0.74 (95% CI, 0.72 to 0.77) and 0.82 (95% CI, 0.79 to 0.85), respectively (Figure 2 and Tables S5 and S6).
Figure 2. Cumulative Incidence of Postacute Sequelae of SARS-CoV-2 Infection (PASC) in the Pre-Delta, Delta, and Omicron Eras According to Vaccination Status.
Shown is the cumulative incidence of PASC at 1 year after SARS-CoV-2 infection among unvaccinated persons during the pre-delta, delta, and omicron eras and among vaccinated persons during the delta and omicron eras. Also shown are selected comparisons (presented as differences on the absolute scale) within and between the cohorts. 𝙸 bars indicate 95% confidence intervals.
Among vaccinated persons, the cumulative incidence of PASC at 1 year after infection was 5.34 events per 100 persons (95% CI, 5.10 to 5.58) during the delta era and 3.50 events per 100 persons (95% CI, 3.31 to 3.71) during the omicron era (difference, −1.83 events per 100 persons [95% CI, −2.14 to −1.52]; incidence rate ratio, 0.66 [95% CI, 0.61 to 0.71]) (Figure 2). The cumulative incidence of PASC at 1 year was lower among vaccinated persons than among unvaccinated persons during the delta era (difference, −4.18 events per 100 persons [95% CI, −4.47 to −3.88]); incidence rate ratio, 0.56 [95% CI, 0.54 to 0.59]) and the omicron era (difference, −4.26 events per 100 persons [95% CI, −4.49 to −4.05]; incidence rate ratio, 0.45 [95% CI, 0.43 to 0.47]).
Analyses of PASC according to disease category showed a lower risk of sequelae in most disease categories but a higher risk of gastrointestinal, metabolic, and musculoskeletal disorders during the omicron era than during the pre-delta and delta eras combined. Among vaccinated persons, nearly all disease categories showed a lower cumulative incidence of PASC and none showed a higher cumulative incidence of PASC during the omicron era than during the delta era (Table 1 and Table S7). Pairwise comparisons according to era and vaccination status are presented in the Supplementary Appendix.
Table 1. Incidence of Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection (PASC) According to Disease Category, Vaccination Status, and Pandemic Era.*.
| Disease Category | Unvaccinated: Omicron Era vs. Pre-Delta and Delta Eras Combined | Vaccinated: Omicron Era vs. Delta Era | ||
|---|---|---|---|---|
| Difference in Cumulative Incidence (95% CI) | Incidence Rate Ratio (95% CI) | Difference in Cumulative Incidence (95% CI) | Incidence Rate Ratio (95% CI) |
|
| events per 100 persons at 1 yr | events per 100 persons at 1 yr | |||
| Cardiovascular | −0.46 (−0.61 to −0.30) | 0.80 (0.75 to 0.87) | −0.48 (−0.65 to −0.30) | 0.67 (0.58 to 0.78) |
| Coagulation and hematologic | −0.01 (−0.13 to 0.12) | 1.00 (0.94 to 1.06) | −0.57 (−0.72 to −0.42) | 0.63 (0.56 to 0.71) |
| Fatigue | −0.29 (−0.37 to −0.19) | 0.85 (0.81 to 0.90) | 0.02 (−0.08 to 0.12) | 1.03 (0.90 to 1.17) |
| Gastrointestinal | 0.39 (0.23 to 0.56) | 1.14 (1.08 to 1.21) | 0.08 (−0.11 to 0.27) | 1.04 (0.94 to 1.16) |
| Kidney | −0.17 (−0.31 to −0.03) | 0.78 (0.63 to 0.96) | −0.17 (−0.33 to 0.00) | 0.67 (0.44 to 1.00) |
| Mental health | −0.75 (−0.91 to −0.58) | 0.77 (0.73 to 0.82) | −0.50 (−0.69 to −0.30) | 0.81 (0.75 to 0.88) |
| Metabolic | 0.19 (0.06 to 0.32) | 1.14 (1.05 to 1.25) | −0.30 (−0.45 to −0.15) | 0.66 (0.53 to 0.81) |
| Musculoskeletal | 0.13 (0.01 to 0.26) | 1.08 (1.00 to 1.17) | −0.04 (−0.19 to 0.11) | 0.97 (0.88 to 1.08) |
| Neurologic | −0.37 (−0.53 to −0.20) | 0.88 (0.83 to 0.93) | −0.31 (−0.49 to −0.12) | 0.79 (0.68 to 0.91) |
| Pulmonary | −1.15 (−1.27 to −1.03) | 0.74 (0.72 to 0.77) | −0.88 (−1.01 to −0.74) | 0.67 (0.62 to 0.71) |
| Any PASC | −2.47 (−2.73 to −2.20) | 0.76 (0.74 to 0.78) | −1.83 (−2.14 to −1.52) | 0.66 (0.61 to 0.71) |
Models were adjusted for age; race (White, Black, or other); sex as reported by the participant; area deprivation index; smoking status (current, former, or never); use of long-term care services; estimated glomerular filtration rate; systolic and diastolic blood pressures; body-mass index; the presence of cancer, cardiovascular disease, chronic lung disease, coronary artery disease, dementia, diabetes, hyperlipidemia, human immunodeficiency virus infection, immune dysfunction, liver diseases, and peripheral artery disease; the number of outpatient visits, hospital admissions, blood panel tests, medications received, Medicare outpatient visits and hospital admissions, and immunizations; the receipt of seasonal influenza vaccine; and the predicted probability of undergoing testing for Covid-19 during each era.
Decomposition Analyses of the Influence of Era and Vaccines
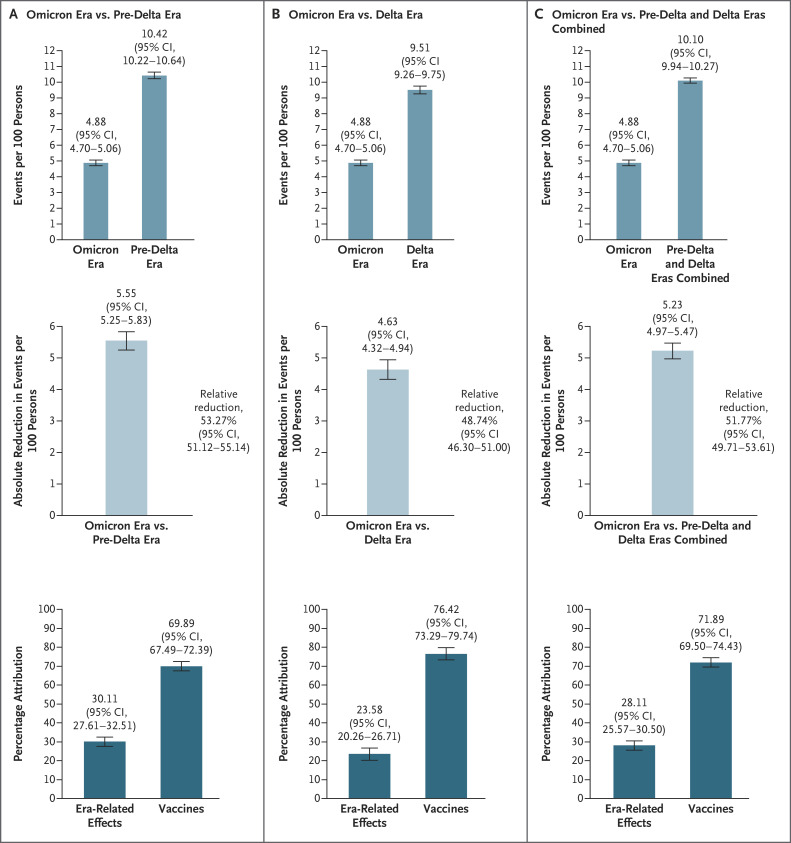
We conducted decomposition analyses (see the Methods section in the Supplementary Appendix) to understand the distributional contribution of era (i.e., temporal changes, including changes in SARS-CoV-2) and vaccines to changes in the cumulative incidence of PASC in the study population over the course of the pandemic (Figure 3 and Table S8). Our analysis showed 5.23 (95% CI, 4.97 to 5.47) fewer PASC events per 100 persons at 1 year after infection during the omicron era than during the pre-delta and delta eras combined. This represented a decrease of 51.77% (95% CI, 49.71 to 53.61), of which 28.11% (95% CI, 25.57 to 30.50) was attributable to changes related to era and 71.89% (95% CI, 69.50 to 74.43) was attributable to vaccines. During the omicron era, 30.11% (95% CI, 27.61 to 32.51) of the decrease in the cumulative incidence from the pre-delta era and 23.58% (95% CI, 20.26 to 26.71) of the decrease from the delta era was attributable to era-related changes, and 69.89% (95% CI, 67.49 to 72.39) of the decrease from the pre-delta era and 76.42% (95% CI, 73.29 to 79.74) of the decrease from the delta era was attributable to vaccines. Additional decomposition analyses were based on counterfactual scenarios in which the vaccination rate during the omicron era varied from 10% to 90% (Table S8).
Figure 3. Decomposition Analyses of the Influence of Pandemic Era and Vaccines on PASC.
Shown in Panel A is the cumulative incidence of PASC at 1 year after SARS-CoV-2 infection in the omicron and pre-delta cohorts (top), the absolute reduction in the cumulative incidence in the omicron cohort as compared with the pre-delta cohort (middle), and the percentage of the reduction in the cumulative incidence that was attributable to era-related effects (e.g., changes in the characteristics of SARS-CoV-2) and vaccines (bottom). Shown in Panel B is the cumulative incidence of PASC at 1 year in the omicron and delta cohorts (top), the absolute reduction in the cumulative incidence in the omicron cohort as compared with the delta cohort (middle), and the percentage of the reduction that was attributable to era-related effects and vaccines (bottom). Shown in Panel C is the cumulative incidence of PASC at 1 year in the omicron cohort and the pre-delta and delta cohorts combined (top), the absolute reduction in the cumulative incidence in the omicron cohort as compared with the pre-delta and delta cohorts combined (middle), and the percentage of the reduction that was attributable to era-related effects and vaccines (bottom). Data include all the vaccinated and unvaccinated persons in the study. 𝙸 bars indicate 95% confidence intervals.
Disability-Adjusted Life-Year Burden Due to PASC
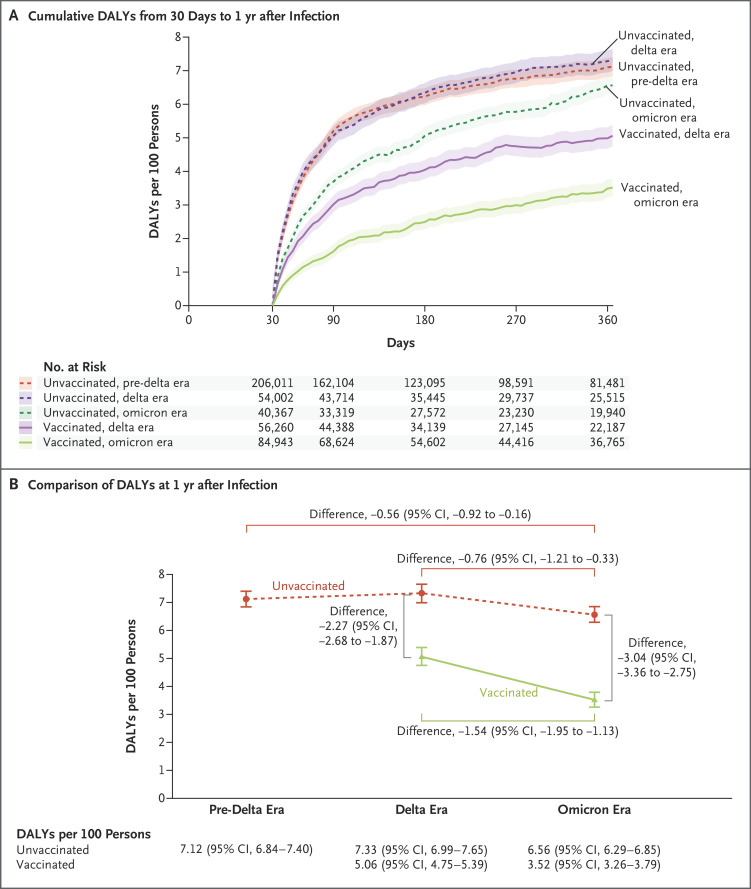
Estimates of the disability-adjusted life-year burden due to PASC during the first year after infection are provided in Figure 4, Figure S2, and Tables S9 and S10.
Figure 4. Disability-Adjusted Life-Years Due to PASC in the Pre-Delta, Delta, and Omicron Eras According to Vaccination Status.
Shown in Panel A are cumulative disability-adjusted life-years (DALYs) due to PASC per 100 persons from 30 days to 1 year after infection among unvaccinated persons during the pre-delta, delta, and omicron eras and among vaccinated persons during the delta and omicron eras. Shaded areas indicate 95% confidence intervals. Shown in Panel B are DALYs due to PASC per 100 persons at 1 year among unvaccinated persons during the pre-delta, delta, and omicron eras and among vaccinated persons during the delta and omicron eras and selected comparisons (presented as differences on the absolute scale) within and between the cohorts. 𝙸 bars indicate 95% confidence intervals.
Sensitivity Analyses and Negative Outcome Control
We conducted several sensitivity analyses (see the Supplementary Appendix) to challenge the robustness of our findings. The results of all sensitivity analyses were consistent with those of the main analyses (Table S11). E values, which are used to assess the potential effects of unmeasured confounding, were estimated to provided measures of the strength of association (independent of the covariates already in the models) needed between a putative confounder and both exposure and outcome to fully explain the results. An analysis of the influence of possible exposure misclassification (i.e., misclassification of persons with SARS-CoV-2 infection as controls) showed results that appeared to be consistent with those of the primary analyses. The analysis of a negative outcome control led to results consistent with pretest expectations (Table S12).
Discussion
In this study involving 441,583 veterans with SARS-CoV-2 infection and 4,748,504 noninfected contemporaneous controls, the cumulative incidence of PASC at 1 year after infection decreased over the course of the pandemic, from a high of 10.42 cases per 100 persons (95% CI, 10.22 to 10.64) among unvaccinated persons during the pre-delta era to a low of 3.50 cases per 100 persons (95% CI, 3.31 to 3.71) among vaccinated persons during the omicron era. However, even after this substantial decrease, the cumulative incidence of PASC at 1 year among vaccinated persons during the omicron era was not negligible. The large number of infected persons during the omicron era, the large numbers of ongoing new infections and reinfections, and the poor uptake of vaccination may translate into a high number of persons with PASC.15,16
Decomposition analyses of the change in the cumulative incidence of PASC showed that 28.11% (95% CI, 25.57 to 30.50) of the decrease was attributable to era-related effects (i.e., changes in the pathogenicity of the virus and other temporal effects) and that 71.89% (95% CI, 69.50 to 74.43) was attributable to Covid-19 vaccines. These findings suggest that vaccine uptake will be key to maintaining the lower cumulative incidence of PASC relative to earlier phases of the pandemic.
The reduced risk of sequelae in most disease categories but increased risk of gastrointestinal and metabolic disorders among unvaccinated people with SARS-CoV-2 infection during the omicron era suggest a differential nonmonolithic shift in the phenotypic features of PASC as a consequence of changes in the characteristics of SARS-CoV-2, the introduction of Covid-19 vaccines, and other temporal effects.5 This observation aligns with the hypothesis that although postacute infectious illnesses may share a common etiologic driver — an infectious agent as the initial trigger — their phenotypic features may differ owing to the influence of multiple factors, including not only the characteristics of the virus but also epidemiologic and contextual factors that influence the development of postacute sequelae.5,22-25
This study has several limitations. The study population consisted predominantly of older White men. The eligibility criteria, including demographic characteristics, coexisting conditions, and vaccination rates, may not be representative of the general population. Owing to its observational nature, the study is subject to biases, including residual confounding and misclassification bias. The cumulative incidence in each era of the pandemic was estimated among persons who were alive (i.e., at risk for infection) during that era. Although we adjusted for a large number of covariates from multiple data domains to balance the characteristics across cohorts from different eras, quantified the threat of confounding with the use of E values, conducted a large array of sensitivity analyses to assess the robustness of our findings, and successfully assessed a negative outcome control, residual biases may still exist and bias the estimation of the era-related effects.
Although the Veterans Affairs data resources comprehensively capture Covid-19 test results from a broad array of data sources (from within and outside the Veterans Affairs Health Care System), we cannot rule out the possibility that undiagnosed Covid-19 cases or SARS-CoV-2–positive test results were not captured by the databases; persons with these characteristics would have been misclassified as noninfected controls. However, analyses of various misclassification scenarios in the control population showed results that were similar to those in the primary analyses. The results reflect the risk of PASC among persons who tested positive for SARS-CoV-2 as compared with the control cohort, which included persons with no known infection (no known positive test for SARS-CoV-2). Persons with undiagnosed SARS-CoV-2 infection may have milder or asymptomatic disease and different risks of PASC than reported here. We did not balance the cohorts at the time of vaccination, and we cannot exclude the possibility that vaccinated persons were misclassified as unvaccinated persons, which may have underestimated the benefit of vaccines.
The era-related effect reflects the net effect of multiple potential drivers of PASC, including changes in the pathogenicity of SARS-CoV-2 and other temporal changes that may affect the rate of PASC, including improved medical care and the use of antivirals. We decomposed the effects of era and vaccination on PASC, an assessment that reflects the net contribution of sequelae in 10 disease categories; however, each disease category or individual sequela may be affected differently by era and vaccination. We assessed the contribution of vaccines but did not assess the effects of the type and number of doses of vaccine. We examined the influence of the initial infection and did not examine the burden of PASC among persons with repeat infection. Although the results from the primary analyses were consistent with those derived from sensitivity analyses, a holistic interpretation of the findings would necessarily incorporate all the results from all the models along with their uncertainty — specifically, the assumptions that underlie each model.
Our study has several strengths. We leveraged the breadth and depth of the vast health care databases of the Department of Veterans Affairs to include persons who had SARS-CoV-2 infection during the pre-delta, delta, and omicron eras. To estimate the cumulative incidence of PASC and assess the contextual factors associated with the development of PASC in each era, we constructed three era-specific noninfected contemporaneous control cohorts. We then evaluated differences in PASC estimates between eras and according to vaccination status within eras. We considered a broad array of potential confounders, used advanced statistical methods to estimate the burden of PASC in each era, and performed decomposition analyses to disentangle effects related to era and vaccines. We provided estimates of the cumulative incidence of PASC and the number of disability-adjusted life-years due to PASC in each Covid-19 cohort. We also provided measures of the difference between cohorts on both the relative scale (incidence rate ratio) and the absolute scale (differences in cumulative incidence and disability-adjusted life-years).
Our study showed that temporal effects and vaccines contributed to a substantial decrease in the burden of PASC over the course of the pandemic. However, a substantial residual risk of PASC remains among vaccinated persons who had SARS-CoV-2 infection during the omicron era.
Supplementary Appendix
Disclosure Forms
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. government.
This article was published on July 17, 2024, at NEJM.org.
Footnotes
Supported by a grant from the Department of Veterans Affairs (to Dr. Al-Aly).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259-264. [DOI] [PubMed] [Google Scholar]
- 2.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022;28:1461-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun 2021;12:6571-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Topol E. Solving the puzzle of long Covid. Science 2024;383:830-832. [DOI] [PubMed] [Google Scholar]
- 6.Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med 2023;29:2347-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 2022;28:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol 2022;10:311-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022;376:e068993-e068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 2020;371:m4677-m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, Choi T, Al-Aly Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect Dis 2024;24:239-255. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post–COVID-19 condition. JAMA Intern Med 2023;183:554-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y, Choi T, Al-Aly Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 2023;381:e074572-e074572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wulf Hanson S, Abbafati C, Aerts JG, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 2022;28:2398-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol 2021;32:2851-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219-242. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer, 2001. [Google Scholar]
- 20.Li F, Li F. Propensity score weighting for causal inference with multiple treatments. Ann Appl Stat 2019;13:2389-2415 (https://projecteuclid.org/journals/annals-of-applied-statistics/volume-13/issue-4/Propensity-score-weighting-for-causal-inference-with-multiple-treatments/10.1214/19-AOAS1282.full?tab=ArticleLink). [Google Scholar]
- 21.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med 2022;28:911-923. [DOI] [PubMed] [Google Scholar]
- 23.Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol 2023;23:618-634. [DOI] [PubMed] [Google Scholar]
- 24.Ford ND, Slaughter D, Edwards D, et al. Long COVID and significant activity limitation among adults, by age — United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb Mortal Wkly Rep 2023;72:866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022;399:2263-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.