Abstract
Background:
Childhood obesity is associated with faster linear growth; nonetheless, its benefit to the mature height of Indonesian children is questionable. This study aimed to evaluate the relationship between adiposity and height growth of Indonesian children, adolescents, and young adults aged 7 to 23 years.
Methods:
Height and skinfolds at triceps, subscapular, suprailiac, and calf were measured in 2,520 children, adolescents, and young adults aged 7 to 23 years (boys = 1,116, girls = 1,404). Central adiposity (subscapular and suprailiac skinfolds) and peripheral adiposity (triceps and calf skinfolds) were projected against heights in each age group. The ANCOVA test and partial correlation were used for statistical analysis.
Results:
With the exception of ages 8 to 12 years, boys were always taller than girls after controlling for age and central or peripheral adiposity. Boys with higher central and peripheral adiposity were taller than their peers up to the age of 17 (r = 0.30–0.72, P < 0.05, P < 0.01). Girls with central adiposity grew taller than their thinner peers until the age of 14 (r = 0.17–0.50, P < 0.05, P < 0.01), whereas girls with peripheral adiposity benefit from this advantage over a more extended period of time. Afterward, adiposity did not offer any benefit on heights.
Conclusions:
Children with high adiposity who were taller at an earlier age have no significant advantage over their thinner peers in terms of adult height.
Keywords: Central adiposity, children, height, peripheral adiposity, young adults
Introduction
The prevalence of obesity in childhood has increased dramatically in recent decades. The increase has also been related to the rise in the risk of developing cardiometabolic and other comorbidities earlier in life.[1,2,3] However, those were not the only consequences of childhood obesity. The risk of childhood and adolescent obesity consistent with the World Health Organization (WHO) definition[4]—that is, excessiveness of adiposity—are almost similar. Excess adiposity in youth is a marker of increased cardiometabolic risk in adolescents and adults,[5,6] adult obesity persistence, and increased risk of death.[5] Meanwhile, adiposity in children and adolescents was strongly associated with pubertal development stages. Early pubertal development stages in females and males indicated a higher prevalence of central adiposity. The unfavorable effects of early maturation are, therefore, related to weight gain and obesity.[7]
Children with obesity are frequently taller for their age. They had accelerated linear growth and advanced skeletal maturation and tended to mature earlier than lean children.[8] Pubertal growth patterns, including earlier pubertal onset timing, smaller pubertal intensity, and shorter pubertal spurt duration, had a negative association with mature height, while, at the same time, resulting in a higher risk for overweight and obesity in late adolescence.[5] A review study has reported that childhood obesity is associated with accelerated linear growth with effects on early puberty and impaired adult height.[9] Nonetheless, research relating adiposity, either central or peripheral, to height growth is scarce. This study aimed to examine the relationship between adiposity and height of Indonesian children, adolescents, and young adults aged 7 to 23 years.
Materials and Methods
Subjects
As many as 2,520 children and young adults (1,116 boys and 1,404 girls) aged 7 to 23 years were recruited in cross-sectional studies in Yogyakarta Province, Indonesia, between 2015 and 2018. The inclusion criteria were boys and girls aged 7 to 23 years, Javanese, good health, and living in Yogyakarta Province, Indonesia. Those with mental and physical disabilities were barred from participating in the study. The sample size was defined using the cluster sampling method with schools as the clusters. This study used a secondary analysis of those several studies. Therefore, the sample size was not determined.
Measurements
Height and skinfold thickness of the triceps, subscapular, suprailiac, and calf were measured using the International Society for the Advancement of Kinanthropometry protocol.[10] Anthropometric tools were used to measure height to the nearest 0.1 cm (GPM, Switzerland, Ltd). Skinfold thickness was measured on the triceps, subscapular, suprailiac, and calf using a Harpenden skinfold caliper to the nearest 0.2 mm. Central adiposity was defined as the sum of the subscapular and suprailiac skinfold (SS), whereas peripheral adiposity was defined as the sum of the triceps and calf skinfold (TC).
Statistical analyses
An ANCOVA analysis with age, central, and peripheral adiposity as covariates was used to assess mean differences between sexes stratified by age groups. The correlation between adiposity and heights was evaluated using partial correlation analysis while controlling for age and the statistical power (1-β) was defined. SS and TC were plotted against height. SPSS (SPSS Version 25, SPSS Inc, Chicago, IL) was used for all statistical analyses, and P < 0.05 was considered statistically significant.
Results
Height between genders is compared with age and either total SS or TC as covariates in Table 1. They cannot be combined into a single model because the correlation between the sum of SS and TC is greater than 0.80. Except for children aged 8 to 12, boys were always taller than girls after controlling for age and central or peripheral skinfolds (P < 0.01).
Table 1.
Height differences between genders were compared using an ANCOVA with age (years) and either the sum of subscapular and suprailiac (SS) or the sum of triceps and calf (TC) skinfold as covariates
| Age (years) | N | Boys | Girls | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Boys (1,116) | Girls (1,404) | Means | 95% CI | Means | 95% CI | |
| 7 | 45 | 43 | 118.7** | 117.4–120.1 | 114.6 | 113.2–116.0 |
| 8 | 42 | 36 | 121.2 | 119.7–122.7 | 120.0 | 118.4–121.6 |
| 9 | 46 | 43 | 126.6 | 124.9–128.2 | 124.9 | 123.2–126.6 |
| 10 | 35 | 39 | 129.3 | 127.5–131.1 | 131.1 | 129.4–132.8 |
| 11 | 47 | 57 | 135.7 | 133.8–137.6 | 137.8 | 136.0–139.5 |
| 12 | 48 | 56 | 145.0 | 142.7–147.4 | 146.3 | 144.1–148.4 |
| 13 | 181 | 214 | 152.5** | 151.5–153.5 | 150.1 | 149.2–151.0 |
| 14 | 235 | 331 | 158.8** | 157.9–159.6 | 151.9 | 151.2–152.6 |
| 15 | 154 | 184 | 161.9** | 160.9–162.9 | 152.5 | 151.6–153.4 |
| 16 | 40 | 66 | 166.4** | 164.7–168.2 | 154.3 | 153.0–155.6 |
| 17 | 28 | 53 | 168.9** | 166.6–171.3 | 153.6 | 151.9–155.2 |
| 18 | 15 | 36 | 166.2** | 163.2–169.2 | 154.0 | 152.2–155.7 |
| 19 | 23 | 31 | 167.0** | 165.1–168.9 | 156.1 | 154.5–157.7 |
| 20 | 61 | 77 | 169.3** | 167.9–170.8 | 154.7 | 153.4–156.0 |
| 21 | 68 | 72 | 166.4** | 165.0–167.8 | 155.2 | 153.8–156.5 |
| 22 | 48 | 66 | 167.2** | 165.4–169.1 | 156.1 | 154.5–157.6 |
**P < 0.01; CI: confidence interval; because the sum of subscapular and suprailiac (SS) and the sum of triceps and calf (TC) skinfold had correlations >0.80, they could not be combined in a single model
Table 2 depicts the partial correlation between skinfold thickness and age-adjusted height. SS and TC were significantly and positively correlated with height in boys aged 7 to 17 years (r = 0.30–0.72, P < 0.05, P < 0.01), except for age 12 years. On the other hand, girls showed a mostly significant positive correlation with central adiposity only until age 14 and peripheral adiposity until the age of 16 years, with r ranging from 0.17 to 0.50 (P < 0.05, P < 0.01). The statistical power was good overall from 7 to 15 years, except for 9 and 12 years, particularly in boys. These findings suggest that adiposity remains positively correlated with height until a later age in boys than in girls, as also shown in the statistical power (1-β), which was greater in boys than in girls.
Table 2.
Analysis of partial correlations between skinfold thicknesses and height, adjusted for age (years)
| Age (years) | n | SS (r Pearson) | TC (r Pearson) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Boys | Girls | Boys | 1-β (%) | Girls | 1-β (%) | Boys | 1-β (%) | Girls | 1-β (%) | |
| 7 | 45 | 43 | 0.51** | 95.74 | 0.42** | 81.59 | 0.51** | 95.74 | 0.50** | 93.93 |
| 8 | 42 | 36 | 0.47** | 89.58 | 0.46** | 82.40 | 0.40** | 76.21 | 0.39* | 66.78 |
| 9 | 46 | 43 | 0.32* | 59.30 | 0.24 | 34.59 | 0.37* | 72.94 | 0.28 | 45.13 |
| 10 | 35 | 39 | 0.72** | 99.94 | 0.38* | 67.97 | 0.66** | 99.50 | 0.41* | 75.26 |
| 11 | 47 | 57 | 0.64** | 99.91 | 0.36** | 79.70 | 0.63** | 99.87 | 0.31* | 66.04 |
| 12 | 48 | 56 | 0.21 | 30.26 | 0.22 | 37.48 | 0.08 | 08.40 | 0.27* | 52.84 |
| 13 | 181 | 214 | 0.41** | 99.99 | 0.35** | 99.96 | 0.34** | 99.72 | 0.31** | 99.66 |
| 14 | 235 | 331 | 0.30** | 99.71 | 0.17** | 87.56 | 0.19** | 83.53 | 0.19** | 93.68 |
| 15 | 154 | 184 | 0.36** | 99.64 | 0.13 | 42.20 | 0.32** | 98.33 | 0.17* | 63.87 |
| 16 | 40 | 66 | 0.33* | 55.88 | 0.18 | 30.65 | 0.27 | 39.83 | 0.26* | 56.60 |
| 17 | 28 | 53 | 0.48* | 75.64 | −0.03 | 05.50 | 0.45* | 69.16 | 0.10 | 11.00 |
| 18 | 15 | 36 | 0.37 | 28.25 | −0.04 | 05.58 | 0.39 | 31.12 | −0.10 | 08.93 |
| 19 | 23 | 31 | 0.34 | 36.43 | −0.18 | 16.38 | 0.25 | 21.33 | −0.29 | 36.01 |
| 20 | 61 | 77 | 0.12 | 15.20 | 0.17 | 31.75 | 0.13 | 17.05 | 0.28 | 70.16 |
| 21 | 68 | 72 | 0.18 | 31.45 | −0.04 | 06.27 | 0.28 | 64.59 | −0.12 | 17.17 |
| 22 | 48 | 66 | 0.21 | 30.26 | 0.05 | 06.83 | 0.10 | 10.39 | −0.10 | 12.60 |
*P < 0.05; **P < 0.01; SS = sum of subscapular and suprailiac skinfold; TC = sum of triceps and calf skinfold
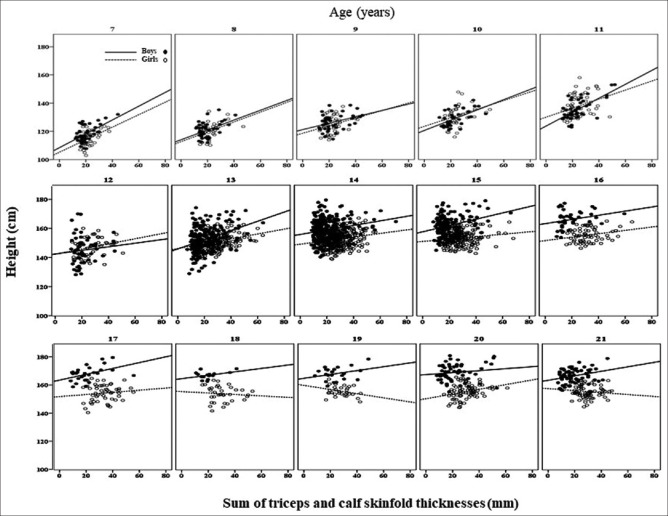
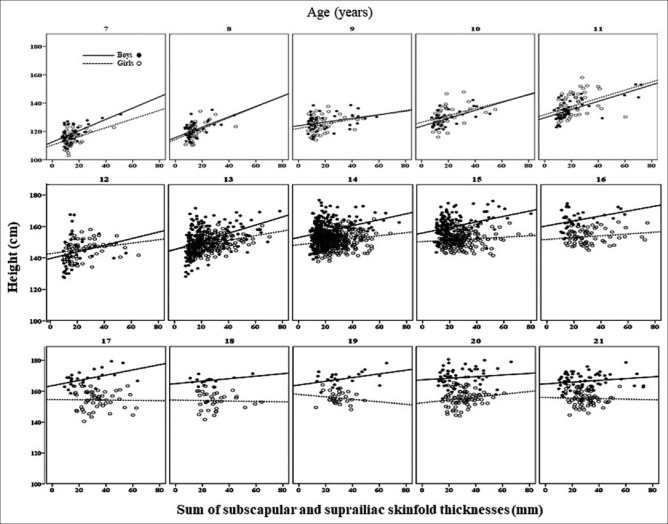
The scatter plots of SS and TC versus height are shown in Figures 1 and 2. These graphs do not include young people aged 22–23 but exhibit a similar trend to those aged 21. At younger ages (7–11 years), boys and girls showed nearly identical positive correlations between central adiposity and height [Figure 1]. Beginning at age 12, the association between central adiposity and height differed between boys and girls. Boys still showed positive correlations, while the correlations in girls gradually declined. At older age, the association in both sexes gradually declined, reaching a negative association at adult height for girls, implying shorter stature in the centrally adipose women. The height pattern in relation to peripheral adiposity [Figure 2] resembles that of central adiposity.
Figure 1.
Scatter plots of the sum of subscapular and suprailiac skinfold (SS) against height in children and young adults. The gaps of regression lines between boys (compact line) and girls (dash line) show greater starting at the age of 13 years and above. The R2 values of the regression lines from 7 to 21 years in boys vs. in girls are 0.33 vs. 0.15 (7 y), 0.28 vs. 0.21 (8 y), 0.10 vs. 0.07 (9 y), 0.38 vs. 0.13 (10 y), 0.42 vs. 0.14 (12 y), 0.05 vs. 0.06 (12 y), 0.18 vs. 0.13 (13 y), 0.09 vs. 0.03 (14 y), 0.11 vs. 0.01 (15 y), 0.12 vs. 0.01 (16 y), 0.22 vs. - (17 y), 0.14 vs. – (18 y), 0.16 vs. 0.03 (19 y), 0.01 vs. 0.03 (20 y), 0.03 vs. 0.001 (21 y), 0.02 vs. 0.004 (22 y)
Figure 2.
Scatter plots of the sum of triceps and calf skinfold (TC) against height in children and young adults. The gaps of regression lines between boys (compact line) and girls (dash line) show greater starting at the age of 13 years and above. The R2 values of the regression lines from 7 to 21 years in boys vs. in girls are 0.29 vs. 0.22 (7 y), 0.19 vs. 0.16 (8 y), 0.14 vs. 0.08 (9 y), 0.32 vs. 0.12 (10 y), 0.40 vs. 0.10 (12 y), 0.01 vs. 0.07 (12 y), 0.13 vs. 0.10 (13 y), 0.04 vs. 0.04 (14 y), 0.09 vs. 0.03 (15 y), 0.07 vs. 0.07 (16 y), 0.20 vs. 0.01 (17 y), 0.15 vs. 0.01 (18 y), 0.09 vs. 0.08 (19 y), 0.02 vs. 0.08 (20 y), 0.08 vs. 0.01 (21 y), - vs. 0.01 (22 y)
Discussion
Our study indicates that, in our population, boys and girls achieve the same height at around 8 to 12 years. Boys had greater height than girls after the age of 13. Furthermore, boys with central and peripheral adiposity were taller than their peers up to the age of 17 years (r = 0.30–0.72, P < 0.05, P < 0.01). Afterward, adiposity did not give any advantage in height. Girls with central adiposity were taller than their slimmer peers only until the age of 14 (r = 0.17–0.50, P < 0.05, P < 0.01). In contrast, girls with peripheral adiposity have this advantage for a longer period. Afterward, adiposity did not give any benefit for height. It even seemed to be a liability because adipose women tended to be shorter than their slimmer peers.
Our findings suggest that children with high central or peripheral adiposity, despite their seemingly taller stature at a younger age, would probably not have an advantage over their slimmer peers in terms of adult height. The finding is consistent with previous studies that childhood and adolescent obesity posed accelerated linear growth that would impair adult height.[9]
The pattern of height increment values versus peripheral adiposity is similar to central adiposity. The height gap, however, widens with increase in adiposity and age up to 21 years of age. We propose that children with greater adiposity may attain greater height until age 13 but then attain the same height as those with less adiposity. Almost similar findings were reported in obese Indian boys.[11] The rapid growth in adolescents is thought to be caused by the onset of puberty.[9,12,13] Elongation of the long bones in the growth plates drives height growth,[14] which is held in place by a complex network of nutritional, cellular, paracrine, and endocrine factors. Growth velocity increases during the pubertal growth spurt then decreases and may even be zero after epiphyseal fusion. During puberty, estrogen stimulates the GH–IGF-I axis, accelerating pubertal growth.[9,15] However, when a critical estrogen level is reached, epiphyseal closure and growth arrest occur. As puberty approaches its end, senescence occurs on the growth plates. The height of the growth plate gradually decreases, beginning with the proliferative and hypertrophic zones.[16]
Childhood obesity, in particular, can accelerate puberty in girls while delaying puberty in boys.[13] It may explain why, in our study, children with more adiposity reached greater heights only up to 13 years, but at a later age, they reached the same heights as those with less adiposity. Accelerated weight gain is frequently associated with a similar acceleration in height velocity, and overweight children have slightly advanced bone ages during early puberty.[13] These findings could be attributed to growth plate maturation due to increased insulin-like growth factor (IGF)-1 bioavailability and early estrogenization. As a result, despite accelerated bone age and linear growth during puberty, children with obesity generally attain adult-expected heights, possibly due to early estrogen-associated epiphyseal closure.[9,13] Nonetheless, the onset time of puberty is not the most vital factor influencing adult height. Earlier puberty onset, smaller puberty intensity, and shorter puberty spurt duration are associated with the risks for lower mature height and higher obesity incidence in late adolescence.[15] Relative shortness during childhood and less pubertal height gain may also result in shorter adulthood, as reported in Saudi boys.[9]
Our findings support the disadvantages of pediatric obesity by demonstrating that high adiposity children have a rapid increase in height during childhood rather than an advantage in mature height. In addition, studies show that excessive adiposity and weight gain during childhood are associated with CVD risks, cardiometabolic morbidity, and mortality.[1,2,6,17,18,19] Further study may need to include an assessment of pubertal development stages since early pubertal in children indicated a higher prevalence of central adiposity[7,20] and to look at skinfold thickness and height growth at early infancy to determine adiposity rebound (AR), as children may have higher adiposity later in life and cardiometabolic risk.
The strengths of our study include a diverse age range from 7 to 23 years and a large sample size. However, the cross-sectional design limited our ability to describe the growth process across all variables. Several studies have also indicated that parents’ height may influence children’s height.[21,22,23] Unfortunately, the data were not evaluated in our research. Furthermore, we cannot recommend that our findings be generalized to national representatives because participants were only Yogyakarta residents; however, the sample was a Javanese population, Indonesia’s largest ethnicity.
In summary, our study discovered that at the ages between 8 and 12 years, boys and girls had almost similar heights, but at the ages of 13 and up, boys were taller than girls. Boys with central and peripheral adiposity were taller from a young age than their peers up to 17 years and up to 14 years in girls. Finally, children with high central and peripheral adiposity from an early age do not exceed their slimmer peers regarding adult height. It is essential to educate people who have high adiposity during the growth period, which is not advantageous in terms of their mature height despite putting them at risk of obesity-related health problems.
Ethical approval
This study was approved by the Medical and Health Research Ethics Committee of Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia. The parents of the children provided written informed consent, while adolescents to young adults 12 years and over gave also their self-informed consent.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to the principals and staff of the primary, secondary, and high schools that participated in this study. We appreciate the participation of all students in this study. We would also like to thank the undergraduate students and medical doctors who assisted us in gathering data.
Funding Statement
This research was supported by a Research Grant from the Faculty of Medicine, Public Health, and Nursing at Universitas Gadjah Mada for the academic year 2015–2018.
References
- 1.Kassem E, Na’amnih W, Shapira M, Ornoy A, Muhsen K. Comparison between school-age children with and without obesity in nutritional and inflammation biomarkers. J Clin Med. 2022;11:6973. doi: 10.3390/jcm11236973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horesh A, Tsur AM, Bardugo A, Twig G. Adolescent and childhood obesity and excess morbidity and mortality in young adulthood-A systematic review. Curr Obes Rep. 2021;10:301–10. doi: 10.1007/s13679-021-00439-9. [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396:1511–24. doi: 10.1016/S0140-6736(20)31859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Obesity and Overweight. Available from: https://www.who.int/news-room/fact- sheets/detail/obesity-and-overweight .
- 5.Chen L, Su B, Zhang Y, Ma T, Liu J, Yang Z, et al. Association between height growth patterns in puberty and stature in late adolescence: A longitudinal analysis in Chinese children and adolescents from 2006 to 2016. Front Endocrinol (Lausanne) 2022;13:882840. doi: 10.3389/fendo.2022.882840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung ST, Onuzuruike AU, Magge SN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411:166–83. doi: 10.1111/nyas.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adami F, Benedet J, Takahashi LAR, da Silva Lopes A, da Silva Paiva L, de Vasconcelos FAG. Association between pubertal development stages and body adiposity in children and adolescents. Health Qual Life Outcomes. 2020;18:93. doi: 10.1186/s12955-020-01342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: A systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14:1266. doi: 10.3390/ijerph14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalitin S, Gat-Yablonski G. Associations of obesity with linear growth and puberty. Horm Res Paediatr. 2022;95:120–36. doi: 10.1159/000516171. [DOI] [PubMed] [Google Scholar]
- 10.Marfell-Jones M, Olds T, Stewart A, Carter L. Canbera: International Society for the Advancement of Kinanthropometry: Canberra; 2006. International Standards for Anthropometric Assessment. [Google Scholar]
- 11.Chatterjee S, Chatterjee P, Bandyopadhyay A. Skinfold thickness, body fat percentage and body mass index in obese and non-obese Indian boys. Asia Pac J Clin Nutr. 2006;15:231–5. [PubMed] [Google Scholar]
- 12.Javed A, Jumean M, Murad MH, Okorodudu D, Kumar S, Somers VK, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatric Obes. 2015;10:234–44. doi: 10.1111/ijpo.242. [DOI] [PubMed] [Google Scholar]
- 13.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr. 2011;75:383–91. doi: 10.1159/000327788. [DOI] [PubMed] [Google Scholar]
- 15.Shim K. Pubertal growth and epiphyseal fusion. Ann Pediatr Endocrinol Metab. 2015;20:8–12. doi: 10.6065/apem.2015.20.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ağırdil Y. The growth plate: A physiologic overview. EFORT Open Rev. 2020;5:498–507. doi: 10.1302/2058-5241.5.190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araújo J, Ramos E. Paediatric obesity and cardiovascular risk factors: A life course approach. Porto Biomed J. 2017;2:102–10. doi: 10.1016/j.pbj.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19:79. doi: 10.1186/s12933-020-01052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajamoorthi A, LeDuc CA, Thaker VV. The metabolic conditioning of obesity: A review of the pathogenesis of obesity and the epigenetic pathways that “program” obesity from conception. Front Endocrinol (Lausanne) 2022;13:1032491. doi: 10.3389/fendo.2022.1032491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Hu Y, Yang Z, Gong Z, Zhang S, Liu X, et al. Overweight/obesity in childhood and the risk of early puberty: A systematic review and meta-analysis. Front Pediatr. 2022;10:795596. doi: 10.3389/fped.2022.795596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatun W, Rasheed S, Alam A, Huda TM, Dibley MJ. Assessing the intergenerational linkage between short maternal stature and under-five stunting and wasting in Bangladesh. Nutrients. 2019;11:1818. doi: 10.3390/nu11081818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Kim R, Vollmer S, Subramanian SV. Factors associated with child stunting, wasting, and underweight in 35 low- and middle-income countries. JAMA Netw Open. 2020;3:e203386. doi: 10.1001/jamanetworkopen.2020.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Ma C, Yang L, Xi B. Association of parental height with offspring stunting in 14 low- and middle-income countries. Front Nutr. 2021;8:650976. doi: 10.3389/fnut.2021.650976. [DOI] [PMC free article] [PubMed] [Google Scholar]