Abstract
Egress of herpes capsids from the nucleus to the plasma membrane is a complex multistep transport event that is poorly understood. The current model proposes an initial envelopment at the inner nuclear membrane of capsids newly assembled in the nucleus. The capsids are then released in cytosol by fusion with the outer nuclear membrane. They are finally reenveloped at a downstream organelle before traveling to the plasma membrane for their extracellular release. Although the trans-Golgi network (TGN) is often cited as a potential site of reenvelopment, other organelles have also been proposed, including the Golgi, endoplasmic reticulum-Golgi intermediate compartment, aggresomes, tegusomes, and early or late endosomes. To clarify this important issue, we followed herpes simplex virus type 1 egress by immunofluorescence under conditions that slowed intracellular transport and promoted the accumulation of the otherwise transient reenvelopment intermediate. The data show that the capsids transit by the TGN and point to this compartment as the main reenvelopment site, although a contribution by endosomes cannot formally be excluded. Given that viral glycoproteins are expected to accumulate where capsids acquire their envelope, we examined this prediction and found that all tested could indeed be detected at the TGN. Moreover, this accumulation occurred independently of capsid egress. Surprisingly, capsids were often found immediately adjacent to the viral glycoproteins at the TGN.
The release of newly assembled herpesviruses requires passage through several host membranes by mechanisms that are poorly understood. Following their assembly and maturation in the nucleus, the capsids acquire a primary envelope by budding through the inner nuclear membrane (16, 58, 82) to end up in the perinuclear space, which is contiguous with the endoplasmic reticulum (ER) lumen. One model suggests these perinuclear virions escape the cell via the host biosynthetic pathway, which requires an obligatory transit through the Golgi (16, 44). However, the currently favored model proposes that the enveloped perinuclear capsids fuse with the outer nuclear membrane to produce naked cytosolic capsids (81, 82). These would in turn acquire a secondary envelope downstream from an intracellular compartment, before reaching the plasma membrane and being released extracellularly by a second fusion event. This reenvelopment model appears valid for several, if not all, members of the herpesvirus family and is supported by several approaches, including electron microscopy (EM), immunofluorescence, freeze fracture, lipid content, as well as analysis of the site of tegument addition and the use of various viral mutants (23, 53, 54).
Herpes simplex virus type 1 (HSV-1) is a member of the herpes family that has extensively been studied for egress. Unfortunately, its relatively short life cycle makes it difficult to analyze the vectorial movement of the virus during its rapid egress. Furthermore, EM analysis often gives a static snapshot without detailed information regarding the direction of transport or sequence of events. One way to circumvent these limitations is to synchronize the infection, for example, with the ts1201 (69), tsProt A (29), or V701 (71) strain. These mutants encode a thermosensitive UL26 protease, which is required for capsid maturation and DNA encapsidation (12, 29, 69, 73). Incubation at the nonpermissive temperature results in the accumulation of immature procapsids in the nucleus (12, 71). Upon incubation at the permissive temperature, mature capsids are formed and released in a tight synchronized wave (12, 37). Using this tool, Wilson and colleagues were able to identify an ATP requirement for capsid assembly and DNA packaging, a need for acidification of the endosomal/trans-Golgi network (TGN) compartments for viral egress and evidence supporting the secondary reenvelopment egress model (10, 11, 17, 37). An important feature of this approach is the expression and transport of the individual viral proteins to their normal intracellular locations at nonpermissive temperatures (72).
The reenvelopment model supposes the presence of an intermediate transient egress stage at an intracellular organelle where capsids acquire their secondary envelope. Several studies point to the TGN as the site of reenvelopment, including EM (30-32, 46) and immunofluorescence (92, 93) reports. This is also corroborated by the lipid composition of extracellular virions reportedly resembling that of the TGN/Golgi (89). In addition, Wilson and colleagues showed that HSV-1 biochemically copurifies with the TGN and/or endosomes during a synchronized infection (37). Finally, a number of viral proteins have been identified at the TGN (see below). However, the exact site of reenvelopment is unclear, since alternative sites have also been proposed, including the ER-Golgi intermediate compartment (76), post-Golgi vacuoles (39), tegusomes (74), aggresomes (59), and early (37) as well as late (8, 27) endosomes. Given the critical role of the reenvelopment compartment in the life cycle of the virus, it is essential to identify it to permit a detailed analysis of the mechanism of reenvelopment and consider potential alternative means to control herpes infections.
The cellular compartment used for reenvelopment contributes not only an envelope to herpesviruses but also several proteins. The egress model infers the presence at the site of reenvelopment of all the viral glycoproteins found on extracellular virions. Of the 11 glycoproteins present in the viral envelope (54), gB, gD, gE/gI, gK, gM, and perhaps gH have individually been reported at the TGN for HSV-1 and other members of the herpesvirus family (1-3, 6, 14, 25, 35, 42, 52, 56, 75, 83, 96, 97). However, not all studies used established cellular markers to confirm their precise locations. Furthermore, no study has collectively evaluated the presence of the different viral glycoproteins at the site of reenvelopment in the context of an infection.
HSV-1 glycoproteins, like host glycoproteins, are synthesized at the ER and travel through the Golgi and TGN before reaching their final destination. Various inhibitors of this pathway have been used in the past to perturb cellular and viral glycoproteins transport, including monensin (44, 88), tunicamycin (65, 88), and brefeldin A (18, 92). Furthermore, intracellular transport is a temperature-dependent process. For instance, all transport steps are normally arrested at 4°C (40, 47, 80). In the case of the biosynthetic pathway, incubation at 20°C reversibly and specifically stops newly made proteins in the TGN (34, 51, 77). Given the common route of transport between host and viral glycoproteins, viral egress should also be sensitive to a 20°C temperature shift. This was an interesting option, given the suggestion in the literature of the TGN as site of herpes reenvelopment. Although the biogenesis of the individual herpesvirus proteins does not necessarily define the site of reenvelopment, one predicts that the viral glycoproteins and capsids should meet there.
To better understand HSV-1 egress, we set out to examine the site of HSV-1 capsid reenvelopment and the concomitant presence of the viral glycoproteins. To this end, we first synchronized the infection with V701, encoding a thermosensitive protease in strain 17, and a 20°C chase to arrest the intracellular transport out of the TGN. The data show that the synchronization protocol worked as anticipated. Using established subcellular markers, we further found a prevalence of capsids at the TGN, while some colocalized with early endosomes. Surprisingly, we also found that a green fluorescent protein (GFP)-tagged wild-type (wt) virus (strain KOS) also accumulated at the TGN, pointing at the potency of the 20°C block to arrest capsid egress and the extension of the results to two different viral strains. In addition, all the viral glycoproteins tested were present at the TGN, confirming its likely importance as the main site of reenvelopment. However, in contrast to the other glycoproteins, gB and to some extent gL did not completely colocalize with the TGN46 marker. As expected, the accumulation of the viral glycoproteins at the TGN occurred independently of capsid egress. Surprisingly, while some capsids colocalized with both the TGN and viral glycoproteins, many seemed to be immediately adjacent. These results confirmed the above predictions and were consistent with the reenvelopment egress model.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney (BHK), Vero, and human 143B osteosarcoma tumor cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (Medicorp), 2 mM l-glutamine (Invitrogen), and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in 5% CO2. For 143B cells, 15 μg/ml 5-bromo-2′deoxyuridine (BUdR) (Sigma-Aldrich) was added to the medium, except upon infection. The HSV-1 V701 ts80-1C2 mutant (strain 17), supplied by Bruce Register and Jules A. Shafer (71), is identical to the well-characterized ts1201 mutant, which encodes a thermosensitive UL26 protease (69). The HSV-1 K26GFP mutant (strain KOS) carries a GFP-tagged capsid protein VP26 and was kindly provided by Phrasant Desai (21). Both viruses were propagated on BHK cells and titrated on Vero cells.
Antibodies and reagents.
Immunohistochemical staining was performed using antibodies against TGN46 (Serotec), EEA1 (given by Marino Zerial), LBPA (provided by Jean Gruenberg), SKL (obtained from Rick Rachubinski), and calnexin (Stressgen Biotechnologies). Anti-golgin-97, bovine serum albumin-complexed nitrobenzoxadiazole (NBD) labeled C6-ceramide, Lysotracker Red DND-99, and Alexa 350, 488, and 568 secondary antibodies were all from Molecular Probes. Viral glycoprotein antibodies were generously provided by various laboratories: monoclonal antibody (MAb) 1D3 (gD) was from Roselyn J. Eisenberg and Gary H. Cohen, while MAb 3104 (gI) was from Nigel Stow. David Johnson provided MAb 15βB2 (gB), 3114 (gE), and anti-syn1 (gK); LP11 (gH) was from Helena Browne; and MAb VIII 87-1 (gL) was from Patricia G. Spear. Finally, two distinct VP5 antibodies were used. MAb 8F5 (kindly provided by Jay Brown) is specific for VP5 present in mature capsids (86). Alternatively, the antibody ICP5 (Virusys Corporation) was used to detect total VP5 (i.e., both mature and immature capsids).
VSV G plasmid transfection.
143B cells were cultured on glass coverslips in DMEM without antibiotics or on BUdR until 60 to 70% confluence was reached. Cells in 24-well plates were then transfected with 0.8 μg pVSV G3 GFP, using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). This plasmid was obtained from Patrick Keller and encodes a GFP-tagged thermosensitive vesicular stomatitis virus G glycoprotein (VSV G tsO45). After an initial incubation of 4 h at the nonpermissive temperature (39°C) in transfection medium (i.e., without serum), the medium was replaced with medium containing 10% FBS, 2 mM l-glutamine, and 10 mM sodium butyrate (Research Chemicals, Ltd.). Cells were cultured for an additional 24 h at 39°C before they were switched to 20°C in CO2-independent medium supplemented with 4 mM l-glutamine for 2 h to allow the egress of VSV G tsO45 to the TGN. When indicated, VSV G tsO45 was chased out of the TGN by incubation at 31°C for various times. Cells were then fixed and permeabilized, and immunofluorescence staining was performed as below.
Infection.
Cells were plated in DMEM (without BUdR for 143B) on glass coverslips in 24-well tissue culture plates and grown overnight. Cells were mock treated or infected with HSV-1 K26GFP or HSV-1 V701 at a multiplicity of infection of 5 for 1 h at 37°C. Cells were then grown at 39°C (V701) or 37°C (K26GFP) for 7 h. Thirty minutes prior to downshift, 20 μg/ml cycloheximide (Sigma-Aldrich) was added to ensure a single pulse of viral assembly (12). Cells were then shifted to 20°C for the times indicated. To reverse the 20°C block, the samples were subsequently incubated at 31°C for various periods. Finally, 20 μM nocodazole (Sigma) was added at 11 hours postinfection (hpi) for 2 h when indicated. All samples were treated for immunofluorescence or analyzed by plaque assays as indicated below.
Plaque assays.
Infected 143B cells grown in 60-mm dishes were infected as above with V701 and incubated at 39°C for 7 h to accumulate the capsids in the nucleus and at 20°C for 6 h to chase them to the TGN. When indicated, the 20°C block was released at 31°C for 24 h. As a control, we incubated the cells at 39°C for 7 h, followed by a chase of 30 h at 31°C. In each case, the extracellular medium was collected and the virus was concentrated for 1 h at 39,000 × g. The viral pellet was resuspended in MNT (30 mM morpholineethanesulfonic acid [MES; Sigma], 100 mM NaCl [Fisher Scientific], and 20 mM Tris [Rose Scientific] at pH 7.4) and titrated on Vero cells.
Immunofluorescence staining.
Uninfected, infected, or transfected cells were washed in phosphate-buffered saline (PBS), fixed in 3% paraformaldehyde for 30 min on ice, washed with PBS, and neutralized with 50 mM NH4Cl in PBS for 30 min at room temperature (RT). Samples were permeabilized with 0.1% Triton X-100 for 4 min and blocked with 10% FBS in PBS for 20 min at RT before being washed and stained for 30 min at RT with primary antibodies diluted in 10% FBS-PBS. Cells were then washed with PBS, incubated for 20 min with secondary antibodies diluted in PBS, and washed again. Samples were mounted on glass slides in Mowiol containing 0.1 μg/ml Hoechst 33342 (Sigma-Aldrich) to stain the nuclei, except when triple colocalization experiments were performed, in which case the Hoechst was omitted.
Fluorescence microscopy and image processing.
Fluorescence microscopy was performed with an Axiophot wide-field fluorescence microscope (Zeiss) equipped with filters and a Retiga 1300 Camera (Q Imaging). The images were acquired and analyzed with Northern Eclipse imaging software (Empix Imaging). They were processed and assembled with Photoshop 6.0 (Adobe). To better reveal the colocalization between gB and the TGN, we performed a Boolean analysis of the images according to Douglas and colleagues (22). In brief, overlays were analyzed with the Boolean operator AND, whereby any green (i.e., gB+/TGN46−) or red-only (i.e., gB−/TGN46+) pixels were converted to black, while yellow pixels (i.e., colocalizing gB+/TGN46+) were left yellow. This was merely a means to visually enhance colocalization.
Confocal microscopy and Z-slices.
Confocal microscopy was performed with a Leica DM IRBE Inverted Microscope equipped with a Leica SP1 spectrometer and argon (488 nm), argon/krypton (568 nm), and helium/neon (647 nm) lasers. A set of 22 optical slices, collected at 126-nm steps for a combined thickness of 2.77 μm, was acquired with a 100× objective and a pinhole of 0.75. The images were processed as above.
RESULTS
Choice of cell line.
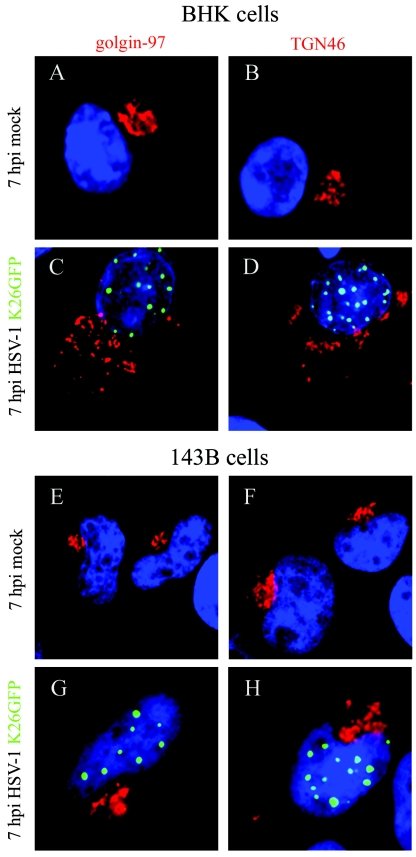
BHK cells are often used to propagate and study HSV-1, given their fast growth, susceptibility to HSV-1 infection, and good morphology by microscopy. However, a number of studies have indicated that infection by HSV-1 alters the integrity of the Golgi in some cell lines (4, 9), a phenomenon attributed to gK (26). It was not known, however, whether the infection also perturbs the TGN. Since it may be the site of capsid reenvelopment, we set out to evaluate if that compartment remains intact upon infection and hence whether BHK cells could be used for our studies. In parallel, we used 143B cells whose Golgi has been documented to resist disruption by HSV-1 (9). We therefore infected BHK and 143B cells with K26GFP virus, a GFP-labeled HSV-1 virus, for its easy monitoring by immunofluorescence microscopy. To minimize the impact of the infection on the morphology of the cells but to permit sufficient assembly of capsids, we chose to examine the cells at 7 hpi (12). As expected, the results indicated that the virus substantially perturbed the morphology of the Golgi in BHK, but not in 143B cells (Fig. 1). In BHK cells, the virus also disrupted the TGN, rendering the use of that cell line difficult for our assays. In contrast, the morphology of the TGN was relatively intact in infected 143B cells, at least at the times examined (Fig. 1). It should be noted that HSV-1 did partially disrupt the TGN in some 143B cells by 7 hpi and in most cells late in infection (data not shown). This nonetheless allowed us to pursue our study with the 143B cell line.
FIG.1.
Choice of cell line. To assess the disruption of the Golgi and TGN upon HSV-1 infection, BHK and 143B cells were mock treated or infected with HSV-1 K26GFP for 7 h at 37°C and labeled in parallel with golgin-97 (Golgi) or TGN46 (TGN) primary antibodies, followed with Alexa 568 secondary antibody. The samples were then examined by immunofluorescence (green, virus; red, Golgi or TGN). Comparison between BHK mock-infected cells (A and B) and BHK-infected cells (C and D) demonstrated a clear disruption of the Golgi and the TGN upon infection. In contrast, the morphology of the Golgi (E and G) and the TGN (F and H) were usually intact for 143B cells.
Block of intracellular transport at 20°C.
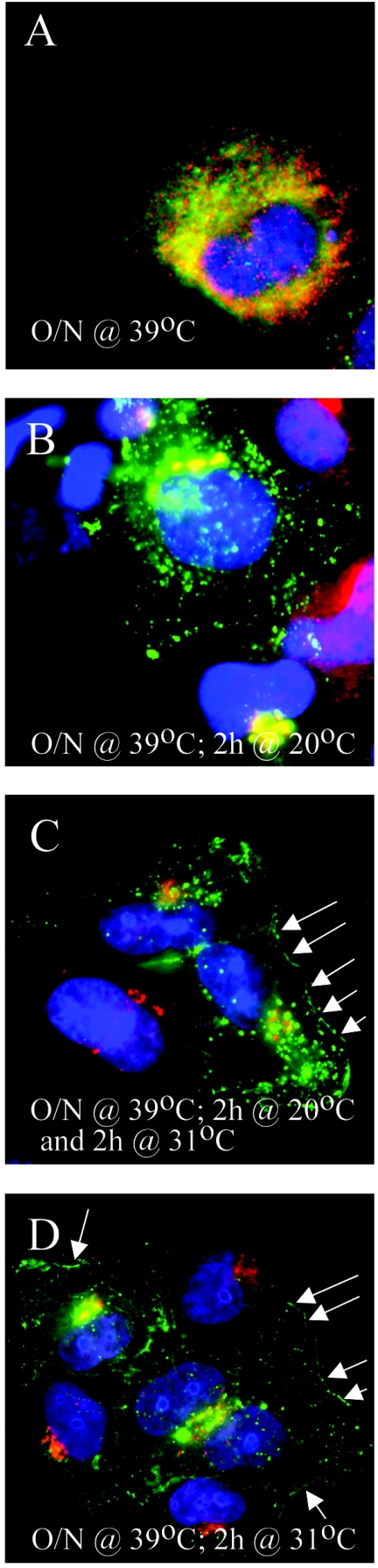
Given that newly synthesized HSV-1 glycoproteins travel though the biosynthetic pathway (see the introduction) and that HSV-1 capsids are often presumed to join this pathway at the TGN, this opens the possibility to trap the virus at that stage and facilitate their detection at this otherwise likely transient step. To first insure that it is possible to perform a block of intracellular transport in 143B cells, we examined a well-established marker for the biosynthetic pathway, namely, VSV G tsO45. This transmembrane mutant viral protein has been widely used to study membrane transport, because of its retention in the ER at 39°C, its accumulation in the TGN at 20°C, and its ability to move out of the TGN and reach the cell surface when the temperature is shifted to 31°C (13, 19, 34, 50, 68, 78, 85). We therefore transfected 143B cells with a plasmid-encoded GFP-tagged VSV G tsO45 and followed its transport at different temperatures by immunofluorescence. The data confirmed that VSV G accumulated at the ER at the nonpermissive temperature (Fig. 2A) and traveled to the TGN but failed to reach the plasma membrane at 20°C (Fig. 2B). In contrast, VSV G could reach the plasma membrane at 31°C (Fig. 2C and D), despite a significant remaining intracellular pool, consistent with the literature. The intracellular transport in 143B cells can therefore be modulated by a 20°C block.
FIG. 2.
Transport of the control VSV G tsO45 glycoprotein at different temperatures. 143B cells were transfected with GFP-tagged VSV G tsO45 and incubated at 39°C (nonpermissive temperature) for 24 h and examined immediately by immunofluorescence (A) or following a further 2 h at 20°C (B) or 31°C (D). (C) The cells were incubated at 39°C for 24 h and then at 20°C for 2 h and finally chasedat 31°C for another 2 h. The cells were fixed and stained for TGN46 (in red) (B to D) or with calnexin (an ER marker, also in red) (A). As expected, VSV G tsO45 was transported to the TGN at 20°C (B) and could reach the plasma membrane at 31°C (D, arrows). Upon release of the 20°C block, the block was reversed, and the protein reached the cell surface (C, arrows). Note that under these conditions, only a portion of VSV G tsO45 travels to the plasma membrane at the permissive temperature, as widely reported in the literature.
Kinetics of viral egress.
Considering that HSV-1 infections proceed very quickly, we wished to synchronize the viral egress to more readily identify the site of reenvelopment. To achieve this goal, we chose the mutant HSV-1 V701, which is identical to the ts1201 strain and encodes a thermosensitive protease. Building on our knowledge that egress of cellular and viral proteins out of the TGN is hampered in 143B cells by an incubation at 20°C (Fig. 2), we accumulated the capsids at 39°C and chased them at 20°C instead of the usual permissive temperature, in an attempt to slow down viral egress and accumulate the virus at the TGN. Our reasoning was that under these conditions, the virus might accumulate at the TGN if it was an important egress station, thus making them easier to detect. To monitor the TGN, we labeled it with TGN46, an established marker for this organelle (66, 67). To follow capsids exiting the nucleus, we used 8F5, an anti-VP5 monoclonal that only recognizes VP5 present on mature capsids (Fig. 3B and C) (86). However, to detect the immature capsids in the nucleus at 39°C, we used ICP5, a commercial antibody detecting all VP5 proteins (Fig. 3A). Figure 3 shows that the bulk of virus required a minimum of 4 h at 20°C to leave the nucleus (data not shown for shorter times). In some experiments, it was necessary to incubate the cells another hour or two to fully chase the virus, so we picked 6 h (Fig. 3C). Interestingly, the capsids seemed to accumulate at or near the TGN under these conditions.
FIG. 3.
Viral kinetics. 143B cells were infected with HSV-1 V701 for 7 h at the nonpermissive temperature (A to F). Following the accumulation of immature capsids in the nucleus (A), the capsids were chased out by incubation at 20°C for 4 and 6 h (B and C). To release the 20°C block, the cells were additionally incubated at 31°C for 2 to 4 h (D to F). The samples were then fixed and stained with antibodies specific for the TGN (red) and the viral capsid protein VP5 (green). The antibody ICP5 (detecting total VP5) was used in all panels, except in the experiments shown in panels B and C where the antibody 8F5 (detecting VP5 present in mature capsids only) was used. The arrows in panels E and F indicate the presence of virus at the periphery of the cells, as revealed by phase contrast (insets). For comparison, 143B cells were also infected with the wild-type GFP-labeled virus (K26GFP) and chased at 20°C for 4 and 6 h (G to I) (K26GFP is in green and TGN is in red). Note that both viruses showed a similar egress kinetic.
To ensure this colocalization was not an artifact, we chased the capsids out of the TGN with an additional incubation at 31°C and determined their intracellular location by immunofluorescence. Based on the results of Wilson and colleagues (12), we opted to examine the virus after a chase of 2 to 4 h at 31°C. The data show that the virus could indeed escape the TGN at 31°C and reach the periphery of the cells (Fig. 3D to F). Furthermore, to examine whether the 20°C block was specific to the V701 mutant, we infected the cells with the GFP-labeled wt virus (K26GFP) for 7 h at 37°C and chased it at 20°C for 4 or 6 h. To our surprise, the 20°C block also caused the accumulation of the wt virus at the TGN (Fig. 3G to I). Finally, we confirmed the reversibility of the 20°C block by plaque assay by measuring the virus released extracellularly. To insure that the bulk of the virus could escape the cells, we released the block with a longer incubation at 31°C. Figure 4 shows that hardly any virus escaped the cells after 6 h at 20°C. In contrast and consistent with our immunofluorescence data (Fig. 3), the virus escaped the TGN when further incubated at 31°C for 24 h (Fig. 4). The amount of virus in the medium nearly reached the level found in the control infection chased for the entire period at 31°C (i.e., 30 h). These results show that the 20°C block is reversible and that the capsids are not aberrantly accumulating at the TGN. This suggested that the TGN is a major egress station for HSV-1 and that the accumulation of the virus at the TGN was not due to the mutation in the protease or an artifact. Given our results, we used incubations of 7 h at 39°C (V701) or 37°C (K26GFP) followed by 6 h at 20°C as our standard protocol.
FIG. 4.
Titration of extracellular virus with Vero cells. 143B cells were incubated with HSV-1 V701 for 7 h at 39°C and then at 20°C for 6 h. The 20°C block was reversed by incubation at 31°C for 24 h. The control cells were incubated for 30 h at 31°C without the 20°C block. The extracellular medium was collected and titrated on Vero cells for 4 days at 31°C.
Site of reenvelopment.
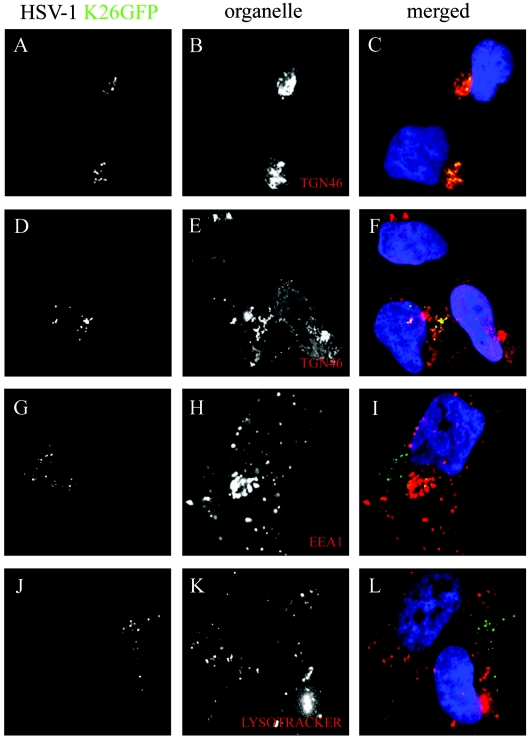
Our results already suggested that the virus transits by the TGN. To confirm this and identify the putative site(s) of reenvelopment, we examined the colocalization of HSV-1 capsids with various subcellular markers by immunofluorescence. Using our previously established kinetics and 20°C block, we confirmed our previous finding that capsids traveled to the TGN (Fig. 5A to C). As pointed out above, the TGN was not always tightly packed in 143B-infected cells. However, the capsids still colocalized with the TGN, even when dispersed (Fig. 5D to F). As an independent control, we used NBD-labeled ceramide, a fluorescent marker that stains lipids present in both the Golgi and TGN (48, 49, 61, 62) and found that the dye stained the region where the virus was found (data not shown). Thin-slice analysis (Z stacks) by confocal microscopy further confirmed the colocalization of V701 and K26GFP capsids with the TGN46 (data not shown). Despite these findings, some virus did not colocalize with either the nucleus or TGN. To address where they might be, we tested various other organelle markers. When we stained the early endosomes with an EEA1 antibody (Fig. 5G to I), we detected some minor colocalization of capsids with the EEA1 marker, suggesting a potential secondary reenvelopment site. Unfortunately, staining of peroxisomes or late endosomes was unsuccessful in these cells with the antibodies tested. However, staining of lysosomes with Lysotracker (Fig. 5J to L), the ER with calnexin, or the Golgi with golgin-97 failed to show any colocalization (data not shown), indicating that the capsids are not reaching intracellular compartments randomly. Clearly, while some virus perfectly colocalized with the TGN46 marker, some seem immediately adjacent to it, a phenomenon observed repeatedly (see, for example, the results shown in Fig. 3). To be sure that the capsids were at the TGN, we incubated the cells with nocodazole, a drug that disassembles microtubules and disperses the TGN (94). Figure 6 confirms the disruption of the TGN by the drug. More important, many capsids, although not all, were also redistributed and remained associated with the TGN46 marker. Taken together, these results implicate the TGN as an important compartment for HSV-1 reenvelopment, while early endosomes may represent a secondary site of reenvelopment.
FIG. 5.
Colocalization of capsids with the TGN. 143B cells were infected with wild-type K26GFP virus (in green), incubated for 7 h at 37°C, and chased at 20°C for 6 h. They were then fixed and stained for various subcellular markers (in red), including TGN46 (A to F), EEA1 (G to I) and Lysotracker (J to L).
FIG. 6.
Confirmation of the presence of capsids at the TGN by disruption of the TGN with nocodazole. To disrupt the TGN, mock-treated (A and B) or HSV-1 K26GFP-infected (C and D) 143B cells were treated with 20 μM nocodazole for the last 2 h of the 20°C chase and labeled with TGN46 primary antibodies (in red). Comparison between untreated (A and C) and treated (B and D) cells demonstrated a clear disruption of the TGN when nocodazole was added. K26GFP capsids (in green) largely remained associated with the TGN46 marker, even when the TGN was disrupted by the drug.
HSV-1 glycoproteins localize to the TGN at 20°C.
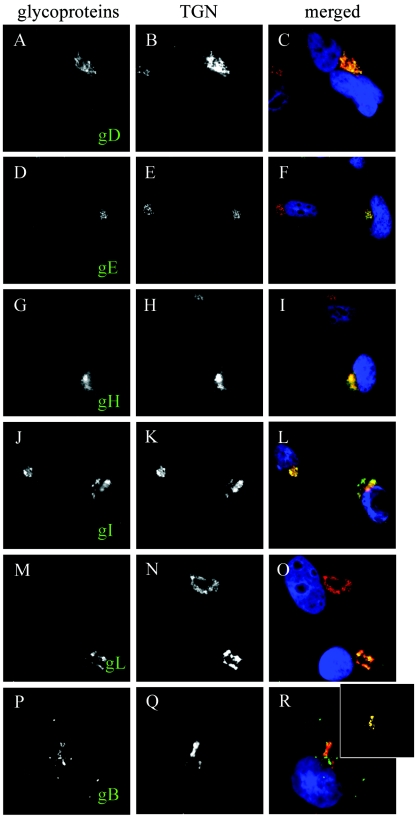
The current model of egress stipulates that the viral glycoproteins present in the final envelope should be present at the site of reenvelopment. To confirm this, we examined their localization by immunofluorescence. Antibodies reactive with various viral glycoproteins were used to stain infected 143B cells according to the viral kinetics established earlier (i.e., with the 20°C block). Immunofluorescence analysis revealed a clear colocalization of the HSV-1 glycoproteins gD, gE, gH, and gI with the TGN46 marker (Fig. 7). In addition, gB and to some extent gL also localized to the TGN, but not completely (see Fig. 7M to R). To confirm that the more weakly TGN-associated gB really colocalized with the TGN, we processed the overlays with a Boolean approach (22) to better reveal the colocalization signal. Hence, all overlapping green (capsids) and red (TGN) pixels were left untouched (i.e., yellow), while all other pixels were converted to black (see Materials and Methods for details). The data confirmed that while partial, a significant colocalization of gB with the TGN46 marker was nonetheless detectable (Fig. 7R, inset).
FIG. 7.
Presence of viral glycoproteins at the TGN. 143B cells were infected with V701 and stained with TGN46 (in red) and antibodies against various viral glycoproteins (in green), including gD (A to C), gE (D to F), gH (G to I), gI (J to L), gL (M to O), and gB (P to R). All glycoproteins showed a strong colocalization with TGN46, with the exception of gB and to some extent gL, which were also found elsewhere. For gB, which showed the weakest colocalization with the TGN, the overlay was treated by a Boolean analysis so that only the overlapping green and red pixels are shown, as described in Materials and Methods and Results (R, inset).
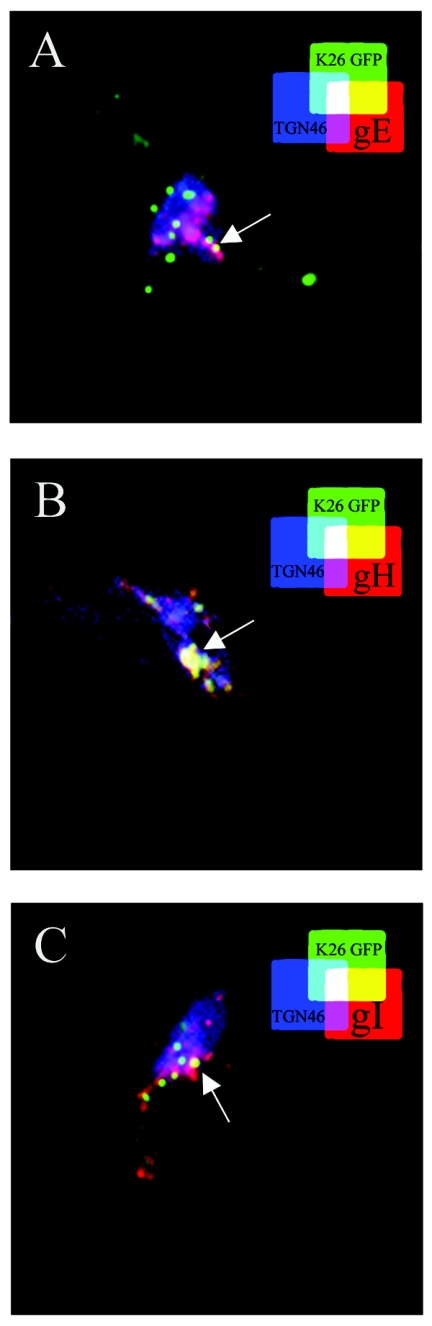
Given the presence of the viral capsids and glycoproteins at the TGN, we examined whether they colocalized with each other. Unfortunately, we could not perform triple-labeling experiments with V701, since two of the three antibodies were from the same species, and attempts to use the Zenon technology or direct antibody coupling (Molecular Probes) were not successful. Consequently, we used K26GFP and antibodies against TGN46 and the viral glycoproteins. Figure 8 confirms that the three viral glycoproteins tested localized extensively with TGN46. However, only a partial colocalization of the capsid, glycoprotein, and TGN was detected (Fig. 8). Thus, capsids and viral glycoproteins accumulated at the TGN, although not necessarily at the exact same site.
FIG. 8.
Colocalization of viral glycoproteins with capsids and TGN. 143B cells were infected with wild-type K26GFP virus (in green) to allow the three-color staining. Cells were incubated for 7 h at 37°C and chased for 6 h at 20°C. They were then fixed and stained with antibodies specific for the TGN (blue) and against viral glycoproteins gE (A), gH (B), or gI (C) in red. It should be noted that the three-color staining prohibited us from staining the nuclei and hence distinguish the capsids still trapped in the nucleus. The arrows show the site of partial colocalization of the capsids with both the TGN46 and glycoproteins (white labeling).
The viral glycoproteins travel to the TGN independently of capsid egress.
So far, the results were consistent with the reenvelopment of HSV-1 capsids by membranes of the TGN, where the viral glycoproteins accumulate. To examine whether the viral glycoproteins precede the capsids at the TGN, we examined their localization in the absence of capsid egress. To address this issue, we made use of the V701 mutant that expresses all viral proteins yet accumulates its newly formed capsids in the nucleus at the nonpermissive temperature. We examined the localization of the viral glycoproteins after an incubation of 7 h at 39°C but without the subsequent chase at 20°C. It should be noted that under these conditions, the capsids did not escape the nucleus (Fig. 3A). Our colocalization results clearly demonstrated that, once again, all the glycoproteins were present at the TGN (Fig. 9). Consistent with our previous results, gB was only partially present in the TGN (Fig. 9R, inset). Oddly, gL now repeatedly colocalized almost perfectly with the TGN (Fig. 9O).
FIG. 9.
Transport of the viral glycoproteins to the TGN independent of capsid egress. 143B cells were treated as described in the legend to Fig. 7, except that they were analyzed immediately after the incubation of 7 h at nonpermissive temperature (without any 20°C chase). They were stained with TGN46 (in red) and the viral markers (in green). All the glycoproteins localized at the TGN, although gB was also detected elsewhere as before. Unlike the results shown in Fig. 7, gL was only found at the TGN. The inset of panel R shows the Boolean analysis for gB, as performed in the results shown in Fig. 7.
DISCUSSION
The current model of herpesvirus egress stipulates that the viral capsids escape the nuclei by budding and fusion through the two nuclear membranes, thereby releasing naked capsids into the cytosol. The capsids then acquire a secondary and final envelope, which is found on the extracellular virions. Three simple predictions can be derived from this model, as follows. (i) The capsids acquire their envelope from a compartment other than the nucleus. (ii) The viral glycoproteins found in the extracellular virions should accumulate at the site of reenvelopment. (iii) The viral glycoproteins likely precede the capsid where they acquire their envelope.
This study examines the above predictions using a 20°C temperature chase to arrest the transport out of the TGN. We reasoned that if the virus uses the TGN for capsid reenvelopment, the capsids and glycoproteins should accumulate there, thereby promoting the likely transient accumulation of reenvelopment capsid intermediates. For these studies, we used the 143B cell line, since the morphology of both the Golgi and TGN proved relatively resistant to HSV-1 disruption under the conditions used (Fig. 1), a result which greatly facilitated the interpretation of the immunofluorescence images. We also confirmed that the 143B cell line behaved as expected when incubated at 20°C. To this end, we used the well-studied VSV G tsO45 protein (Fig. 2), known to be reversibly arrested at the TGN at 20°C when newly synthesized (see the introduction). Finally, we determined the kinetics of egress of V701 and found that capsids arrested in the nuclei at the nonpermissive temperature accumulated in the TGN at 20°C and could be chased to the cell surface at 31°C (Fig. 3A to F). Surprisingly, a wild-type virus encoding a normal UL26 protease behaved the same way as the V701 mutant (Fig. 3G to I), hinting at the potency of the 20°C block. This is important, since it shows that the results are not specific for the mutated V701 virus, applies to wt (albeit GFP-labeled) virus, two different viral strains (17 and KOS), and two different cell lines (see below). This accumulation was unlikely an artifact, as the block was reversible as determined by immunofluorescence (Fig. 3D to F) and plaque assays (Fig. 4). Furthermore, our conclusion is supported by other reports pointing at the TGN as site of reenvelopment for herpesviruses in the absence of a 20°C temperature block (30, 32, 33, 37, 45, 46, 79, 82, 89, 92, 96). In addition, this block has extensively been used since the mid-1980s with no report, to our knowledge, of artifactual localizations. It has, in fact, been instrumental in elucidating transport along the biosynthetic pathway.
With the above parameters and various subcellular markers, our data show a reproducible colocalization of the viral capsids with the TGN (Fig. 3 and 5). Two distinct markers, including TGN46 and NBD ceramide (a marker of lipids found in both the Golgi and the TGN) (48, 49, 61, 62), corroborated these findings. This was supported by confocal analyses (data not shown) and disruption of the TGN by nocodazole, where capsids remained associated with TGN46 (Fig. 6). Although the TGN was not always the tightly packed perinuclear structure one could hope for, the capsids were nonetheless found at or near the TGN46 marker whether the TGN was tightly packaged or not (Fig. 5). Finally, the capsids also colocalized with the much-dispersed TGN in BHK cells at 20°C (not shown). In contrast, capsids were not found in lysosomes, the ER, or the Golgi, indicating that the virus does not randomly acquire an envelope from other cellular compartments (Fig. 5 and data not shown). A few capsids seem to colabel with EEA1, a typical marker of early endosomes (57). We were intrigued by the possible presence of capsids at two different compartments. Given that the viral glycoproteins recycle to the TGN from the plasma membrane via endosomes (2, 5, 7, 15, 42, 60, 63, 70, 84, 87, 90, 91), it is possible that the capsids were recruited to early endosomes because of the presence of recycling viral glycoproteins. This would be consistent with the results of Wilson and colleagues, who found HSV-1 capsids in both the TGN and early endosome fractions (37). However, the partial colocalization of EEA1 and TGN46 in 143B cells complicates the interpretation of the results (data not shown). Taken together, these findings support the TGN as a major reenvelopment site, consistent with various studies implying the TGN as a source of the secondary envelope (30, 32, 33, 37, 45, 46, 79, 82, 89, 92, 96), while a secondary reenvelopment site at the early endosomes cannot formally be ruled out.
Given that the glycoproteins present on the extracellular virions need to be incorporated at the site of reenvelopment, we examined their cellular localization. Not surprisingly, the glycoproteins tested were all present at the TGN upon HSV-1 infection (Fig. 7). This confirms our results and extents previous publications that individually reported accumulation of HSV-1 glycoproteins at the TGN (3, 5, 14, 25, 52, 56). An interesting case was gB and to some extent gL, which were clearly found at the TGN but not as completely as for the other viral glycoproteins (Fig. 7). Presumably, their amount at the TGN is sufficient to support viral entry at the next round of infection into the neighboring cells. The significance of their accumulation in other compartments is unclear at present and may reflect additional functions. Moreover, we cannot readily explain the partial presence of gL at the TGN at 20°C (Fig. 7) but its near-complete colocalization at 39°C (Fig. 9). However, given that gL targeting to the TGN is dependent on its association with gH (41), it is possible that their association at 20°C may be incomplete.
We also examined the localization of the viral glycoproteins in the absence of capsid egress. We made use of the V701 mutant, whose capsid egress is severely impaired at the nonpermissive temperature, and examined whether the viral glycoproteins still migrated to the TGN without the 20°C chase. Importantly, all capsids are fully retained in the nucleus under these conditions (Fig. 3). The results confirmed that all viral glycoproteins examined were detectable at the TGN at 39°C in the context of an infection (Fig. 9). These results once again support the TGN as the site of reenvelopment. They are also consistent with the targeting of viral glycoproteins to the TGN in transfected cells (1, 14, 24, 96, 97) and the presence of TGN-targeting signals in some glycoproteins (2, 3, 24, 43, 52, 97). The possible transport of the glycoproteins independent of capsid egress in axons (38, 55, 56, 64) would also be consistent with this view but remains disputed (20). Taken together, the data strongly support the notion that the transport of the viral glycoproteins to the TGN is independent of capsid egress. Interestingly, Browne and colleagues (14) recently showed that gM targets several viral glycoproteins to the TGN. Similarly, some of the glycoproteins may contain information that promotes targeting of the capsids to the TGN. However, reports of the interaction between viral glycoproteins and teguments hint at an indirect targeting mechanism (28, 36, 53, 95).
Surprisingly, capsids colocalized with the TGN, but were often immediately adjacent to the TGN46 marker. This was evident by wide-field (Fig. 3 and 6) and confocal (data not shown) microscopy. Furthermore, in triple-labeling experiments, the capsids and glycoproteins did not always perfectly colocalize (Fig. 8). It may be that some capsids are in the lumen of the TGN, while others are in the process of reenvelopment. EM analyses are currently under way to examine these possibilities.
In conclusion, although we cannot rule out a contribution by endosomes, our results strongly hint at the TGN as the major site of reenvelopment, since both capsids and viral glycoproteins predominantly accumulate there. Moreover, the glycoproteins travel to the TGN independently of capsids egress, as expected from the current model of HSV-1 egress.
Acknowledgments
We thank Heidi McBride, Joël Lanoix, Sylvie LaBoissière, and Allégria Kessous for critical reading of the manuscript. We are indebted to Bruce Register, Jules A. Shafer, and Phrasant Desai for supplying viruses and to Patrick Keller for the VSV G ts045 construct. Special thanks to Michel Lauzon for his help with the confocal. Finally, we are grateful to Marino Zerial, Jean Gruenberg, Rick Rachubinski, Roselyn Eisenberg, Gary Cohen, Nigel Stow, David Johnson, Helena Browne, Patricia Spear, and Jay Brown for antibodies.
This work was supported by the Canadian Institutes of Health Research (CIHR grant MOP 12679) and establishment grants from the Canadian Foundation for Innovation and the Fonds de la recherche en santé du Québec. R.L. is the recipient of a CIHR scholarship.
REFERENCES
- 1.Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. [DOI] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avitabile, E., S. Di Gaeta, M. R. Torrisi, P. L. Ward, B. Roizman, and G. Campadelli-Fiume. 1995. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J. Virol. 69:7472-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beitia Ortiz de Zarate, I., K. Kaelin, and F. Rozenberg. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 72:4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 8.Brunetti, C. R., K. S. Dingwell, C. Wale, F. L. Graham, and D. C. Johnson. 1998. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J. Virol. 72:3330-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campadelli-Fiume, G., R. Brandimarti, C. Di Lazzaro, P. L. Ward, B. Roizman, and M. R. Torrisi. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 90:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi, J. H., and D. W. Wilson. 2000. ATP-dependent localization of the herpes simplex virus capsid protein VP26 to sites of procapsid maturation. J. Virol. 74:1468-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church, G. A., A. Dasgupta, and D. W. Wilson. 1998. Herpes simplex virus DNA packaging without measurable DNA synthesis. J. Virol. 72:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosson, P., I. de Curtis, J. Pouyssegur, G. Griffiths, and J. Davoust. 1989. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J. Cell Biol. 108:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crump, C. M., B. Bruun, S. Bell, L. E. Pomeranz, T. Minson, and H. M. Browne. 2004. Alphaherpesvirus glycoprotein M causes the relocalization of plasma membrane proteins. J. Gen. Virol. 85:3517-3527. [DOI] [PubMed] [Google Scholar]
- 15.Crump, C. M., C. H. Hung, L. Thomas, L. Wan, and G. Thomas. 2003. Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77:11105-11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darlington, R. W., and L. H. Moss III. 1968. Herpesvirus envelopment. J. Virol. 2:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta, A., and D. W. Wilson. 1999. ATP depletion blocks herpes simplex virus DNA packaging and capsid maturation. J. Virol. 73:2006-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta, A., and D. W. Wilson. 2001. Evaluation of the primary effect of brefeldin A treatment upon herpes simplex virus assembly. J. Gen. Virol. 82:1561-1567. [DOI] [PubMed] [Google Scholar]
- 19.de Curtis, I., and K. Simons. 1988. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc. Natl. Acad. Sci. USA 85:8052-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas, M. W., R. J. Diefenbach, F. L. Homa, M. Miranda-Saksena, F. J. Rixon, V. Vittone, K. Byth, and A. L. Cunningham. 2004. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 279:28522-28530. [DOI] [PubMed] [Google Scholar]
- 23.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 24.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster, T. P., J. M. Melancon, T. L. Olivier, and K. G. Kousoulas. 2004. Herpes simplex virus type 1 glycoprotein K and the UL20 protein are interdependent for intracellular trafficking and trans-Golgi network localization. J. Virol. 78:13262-13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster, T. P., G. V. Rybachuk, X. Alvarez, O. Borkhsenious, and K. G. Kousoulas. 2003. Overexpression of gK in gK-transformed cells collapses the Golgi apparatus into the endoplasmic reticulum inhibiting virion egress, glycoprotein transport, and virus-induced cell fusion. Virology 317:237-252. [DOI] [PubMed] [Google Scholar]
- 27.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218-232. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann III, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granzow, H., C. Birghan, T. C. Mettenleiter, J. Beyer, B. Kollner, and E. Mundt. 1997. A second form of infectious bursal disease virus-associated tubule contains VP4. J. Virol. 71:8879-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths, G., S. Pfeiffer, K. Simons, and K. Matlin. 1985. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J. Cell Biol. 101:949-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grose, C. 1990. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu. Rev. Microbiol. 44:59-80. [DOI] [PubMed] [Google Scholar]
- 36.Gross, S. T., C. A. Harley, and D. W. Wilson. 2003. The cytoplasmic tail of herpes simplex virus glycoprotein H binds to the tegument protein VP16 in vitro and in vivo. Virology 317:1-12. [DOI] [PubMed] [Google Scholar]
- 37.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horiuchi, H., R. Lippe, H. M. McBride, M. Rubino, P. Woodman, H. Stenmark, V. Rybin, M. Wilm, K. Ashman, M. Mann, and M. Zerial. 1997. A novel Rab5 GDP/GTP exchange factor complexed to rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90:1149-1159. [DOI] [PubMed] [Google Scholar]
- 41.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvis, M. A., T. R. Jones, D. D. Drummond, P. P. Smith, W. J. Britt, J. A. Nelson, and C. J. Baldick. 2004. Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication. J. Virol. 78:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones, F., and C. Grose. 1988. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J. Virol. 62:2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komuro, M., M. Tajima, and K. Kato. 1989. Transformation of Golgi membrane into the envelope of herpes simplex virus in rat anterior pituitary cells. Eur. J. Cell Biol. 50:398-406. [PubMed] [Google Scholar]
- 47.Lippe, R., M. Miaczynska, V. Rybin, A. Runge, and M. Zerial. 2001. Functional synergy between Rab5 effector rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol. Biol. Cell 12:2219-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipsky, N. G., and R. E. Pagano. 1983. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc. Natl. Acad. Sci. USA 80:2608-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipsky, N. G., and R. E. Pagano. 1985. A vital stain for the Golgi apparatus. Science 228:745-747. [DOI] [PubMed] [Google Scholar]
- 50.Lotti, L. V., M. R. Torrisi, M. C. Erra, and S. Bonatti. 1996. Morphological analysis of the transfer of VSV ts-045 G glycoprotein from the endoplasmic reticulum to the intermediate compartment in vero cells. Exp. Cell Res. 227:323-331. [DOI] [PubMed] [Google Scholar]
- 51.Matlin, K. S., and K. Simons. 1983. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34:233-243. [DOI] [PubMed] [Google Scholar]
- 52.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 54.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mu, F. T., J. M. Callaghan, O. Steele-Mortimer, H. Stenmark, R. G. Parton, P. L. Campbell, J. McCluskey, J. P. Yeo, E. P. Tock, and B. H. Toh. 1995. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270:13503-13511. [DOI] [PubMed] [Google Scholar]
- 58.Nii, S., C. Morgan, and H. M. Rose. 1968. Electron microscopy of herpes simplex virus. II. Sequence of development. J. Virol. 2:517-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nozawa, N., Y. Yamauchi, K. Ohtsuka, Y. Kawaguchi, and Y. Nishiyama. 2004. Formation of aggresome-like structures in herpes simplex virus type 2-infected cells and a potential role in virus assembly. Exp. Cell Res. 299:486-497. [DOI] [PubMed] [Google Scholar]
- 60.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagano, R. E. 1990. The Golgi apparatus: insights from lipid biochemistry. Biochem. Soc. Trans. 18:361-366. [DOI] [PubMed] [Google Scholar]
- 62.Pagano, R. E., M. A. Sepanski, and O. C. Martin. 1989. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J. Cell Biol. 109:2067-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pizer, L. I., G. H. Cohen, and R. J. Eisenberg. 1980. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J. Virol. 34:142-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ponnambalam, S., M. Girotti, M. L. Yaspo, C. E. Owen, A. C. Perry, T. Suganuma, T. Nilsson, M. Fried, G. Banting, and G. Warren. 1996. Primate homologues of rat TGN38: primary structure, expression and functional implications. J. Cell Sci. 109:675-685. [DOI] [PubMed] [Google Scholar]
- 67.Prescott, A. R., J. M. Lucocq, J. James, J. M. Lister, and S. Ponnambalam. 1997. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur. J. Cell Biol. 72:238-246. [PubMed] [Google Scholar]
- 68.Presley, J. F., N. B. Cole, T. A. Schroer, K. Hirschberg, K. J. Zaal, and J. Lippincott-Schwartz. 1997. ER-to-Golgi transport visualized in living cells. Nature 389:81-85. [DOI] [PubMed] [Google Scholar]
- 69.Preston, V. G., J. A. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 71.Register, R. B., and J. A. Shafer. 1996. A facile system for construction of HSV-1 variants: site directed mutation of the UL26 protease gene in HSV-1. J. Virol. Methods 57:181-193. [DOI] [PubMed] [Google Scholar]
- 72.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73:277-284. [DOI] [PubMed] [Google Scholar]
- 73.Rixon, F. J., A. M. Cross, C. Addison, and V. G. Preston. 1988. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J. Gen. Virol. 69:2879-2891. [DOI] [PubMed] [Google Scholar]
- 74.Roffman, E., J. P. Albert, J. P. Goff, and N. Frenkel. 1990. Putative site for the acquisition of human herpesvirus 6 virion tegument. J. Virol. 64:6308-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez, V., E. Sztul, and W. J. Britt. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-Golgi-intermediate compartment. J. Virol. 74:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saraste, J., and E. Kuismanen. 1984. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell 38:535-549. [DOI] [PubMed] [Google Scholar]
- 78.Scales, S. J., R. Pepperkok, and T. E. Kreis. 1997. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90:1137-1148. [DOI] [PubMed] [Google Scholar]
- 79.Siminoff, P., and M. G. Menefee. 1966. Normal and 5-bromodeoxyuridine-inhibited development of herpes simplex virus. An electron microscope study. Exp. Cell Res. 44:241-255. [DOI] [PubMed] [Google Scholar]
- 80.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 81.Smith, J. D. 1980. An additional role for the outer nuclear membrane in the morphogenesis of herpes simplex virus. Intervirology 13:312-316. [DOI] [PubMed] [Google Scholar]
- 82.Stackpole, C. W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J. Virol. 4:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toomre, D., P. Keller, J. White, J. C. Olivo, and K. Simons. 1999. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 112:21-33. [DOI] [PubMed] [Google Scholar]
- 86.Trus, B. L., W. W. Newcomb, F. P. Booy, J. C. Brown, and A. C. Steven. 1992. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc. Natl. Acad. Sci. USA 89:11508-11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Drunen Littel-van den Hurk, S., and L. A. Babiuk. 1985. Effect of tunicamycin and monensin on biosynthesis, transport, and maturation of bovine herpesvirus type-1 glycoproteins. Virology 143:104-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Genderen, I. L., R. Brandimarti, M. R. Torrisi, G. Campadelli, and G. van Meer. 1994. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology 200:831-836. [DOI] [PubMed] [Google Scholar]
- 90.Van Minnebruggen, G., H. W. Favoreel, and H. J. Nauwynck. 2004. Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex. J. Virol. 78:8852-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, and A. A. Gershon. 2000. Trafficking of varicella-zoster virus glycoprotein gI: T338-dependent retention in the trans-Golgi network, secretion, and mannose 6-phosphate-inhibitable uptake of the ectodomain. J. Virol. 74:6600-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang, W., and B. Storrie. 1998. Scattered Golgi elements during microtubule disruption are initially enriched in trans-Golgi proteins. Mol. Biol. Cell 9:191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu, Q., and R. J. Courtney. 1994. Chemical cross-linking of virion envelope and tegument proteins of herpes simplex virus type 1. Virology 204:590-599. [DOI] [PubMed] [Google Scholar]
- 96.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]