Abstract
Social prescribing is a model of care, usually in the community-setting, which aims to address people’s unmet social needs. Volunteers support primary health care and community-based care in non-medical roles. However, few studies focus on volunteers in social prescribing, therefore, aimed to synthesize the effect of health or peer volunteer-led interventions on psychosocial and behavioural outcomes for middle-aged and older adults with Type 2 Diabetes Mellitus (T2DM) to inform future work for volunteering in social prescribing. We followed Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and searched six databases and Google Scholar for peer-reviewed studies from 2013+ (last search May 16, 2024). We included randomized controlled trials (RCTs) from all languages, and synthesized data using the Cochrane’s Synthesis Without Meta-analysis (SWiM) guidelines; and assessed risk of bias using the "Risk of Bias 2 Tool". We identified nine RCTs (reported in 10 publications). Interventions aimed to promote self-management of T2DM, and study duration ranged from one to 46 months. Training for volunteers varied between one to 32 hours, and most volunteers were offered a stipend. For psychosocial outcomes, only one outcome on social support favoured the intervention group, with the remaining outcomes reporting no differences between study groups. For behaviour, six outcomes (from three studies) favoured the intervention group, and for three outcomes there were no differences between study groups. In conclusion, volunteers bring a unique perspective to health interventions, but volunteer training, matching and retention, as well as intervention mode and duration, and geographical context need to be thoughtfully considered as important implementation factors. This work generates ideas for future studies focused on volunteers and T2DM management and social prescribing.
Trial registration: PROSPERO registration: CRD42023453506.
Introduction
Volunteers, often due to altruistic motives, engage in unpaid non-profit activity that contributes significantly to society [1, 2]. In a health context, volunteers most often donate their time to structured organizations such as governments and public health agencies to deliver health messaging and behaviour change interventions in community-based settings [3, 4]. They provide unique and important, but limited roles within health interventions [3, 5]. Specifically, volunteers supplement the work of integrated care teams by providing social and emotional support [3, 5], bridging the gap between health providers and communities [3], leading physical activity or educational interventions [5], and acting as the “eyes and ears” [1] (p. 60S) of a care team [1, 5]. In such positions, volunteers can be effective at initiating behaviour change [6], improving psychosocial outcomes [5, 7, 8], and potentially reducing strain on the health care system [9, 10].
There are different groups that fall within the overarching category of volunteers, and these groups often play distinct roles, filling different gaps and tackling context-specific needs [1]. Two types of volunteers that are relevant to the community health context are community health volunteers and peer volunteers. Community health volunteers (who may also be referred to as community health workers, community health champions, lay health workers, promoters or promotoras) are a type of lay health worker who lack professional health training, but have been specifically instructed to promote health within their own communities [11, 12]. They are integral, particularly in low and middle-income countries, for achieving widespread and equitable healthcare coverage [11]. On the other hand, peer volunteers are an alternative to traditional health volunteers. They have “experiential knowledge of a specific behaviour or stressor and similar characteristics as the target population” [13] (p. 329), which is presumed to lead to more effective communication, providing behavioural and psychosocial benefits to both peers [14–16] and patients [15, 17–21]. Peer volunteers can be particularly beneficial for patients with noncommunicable diseases because they are thought to enhance social support, which is linked with a patient’s ability to self-manage their disease or condition [22]. The potential for peers to enhance social support is particularly relevant as public health agencies attempt to reduce social isolation in diverse and aging populations [20]. Henceforth, we will use the term “volunteers” as an inclusive term for all health volunteers; and the term “peers” to refer specifically to peer volunteers.
Social prescribing is a community-based model of health and social care that seeks to connect individuals with non-clinical resources aimed at addressing unmet social needs, sometimes connected to living with social risk factors related to the social determinants of health [23]. It is an area supported by volunteers [24]. It originated in the United Kingdom (UK), where it is a key part of the publicly-funded drive towards Universal Personalised Care [25], but has since migrated to other countries, including Canada [23]. However, social prescribing in Canada remains a developing field that receives funding on a case-by-case basis, and programs receive noteworthy contributions from volunteers [26].
In the UK, volunteers are often relied upon to develop one-to-one relationships with service users, while paid staff oversee the programs at a higher level [27]. In addition, there are studies which focus on the role of volunteering as a social prescription [16, 28, 29]; and emerging data on volunteers or the voluntary sector in social prescribing [30–32]. However, it remains difficult to recruit and train an adequate volunteer workforce, which can be challenging for paid staff and complicates the evaluation of the roles and effectiveness of the volunteers [24, 27]. As a result, although volunteers are used regularly by social prescribing programs globally, there is limited peer-reviewed evidence documenting their roles or effectiveness within the programs [33].
Social prescribing is an all-inclusive model of care, but can be particularly effective for people with long-term health conditions and people with complex social and mental health needs [25]. For example, people with Type 2 Diabetes Mellitus (T2DM) may benefit from social prescribing, since they commonly experience psychological and social barriers that make it more difficult to manage their condition [34]; and there are specific social prescribing programs focused on people living with diabetes [35–40]. People with T2DM also benefit from diabetes self-management education and support (DSMES), which aims to “give people with diabetes the knowledge, skills, and confidence to accept responsibility for their self-management” [41] (p. 2). The driving philosophy of DSMES is notable for its focus on allowing patients to set and regulate their own goals and behaviours, rather than the goals of a health care provider [42]. In philosophy and practice, DSMES closely reflects social prescribing (e.g., one-on-one personalized care, identifying barriers, referals to non-medical services, use of volunteers, can be community-based), and importantly, there is an abundance of peer-reviewed literature discussing the roles and effect of volunteers in DSMES [41]. Due to this similarity and the abundance of evidence about volunteers in diabetes self-management, this evidence could be used to inform the implementation of volunteers in social prescribing programs.
Accordingly, the primary objective of this review was to identify evidence from randomized controlled trials (RCTs) to synthesize the effect (on patients and volunteers) of one-on-one, community-based volunteer-led interventions for middle-aged and older adults living with T2DM to help inform social prescribing research and practice.
Methods
We conducted a rapid systematic review following standard methods, Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [43]. Our aim was to synthesize evidence for volunteers working in social prescribing. However, due to limited publications when we searched, we decided to search and summarize studies in an area which closely aligns with social prescribing: volunteers’ role in diabetes self-management education and support. We chose to conduct a rapid systematic review, which is a way to synthesize evidence using simplified or reduced methods for “the public, healthcare providers, researchers, policy makers, and funders in a systematic, resource efficient manner.” [44] (p. 3) For this rapid systematic review we followed most standard systematic review procedures, except we only included individual-RCTs, we did not conduct a forward and backward citation search of included studies, and we only included studies published within the last decade [44]. In addition, during the synthesis process, we aimed to identify information which may be relevant for end users to consider when developing volunteer training programs in social prescribing. We registered the protocol: PROSPERO registration: CRD42023453506.
Information sources and search strategy
We searched for individual-RCTs published between 1 January 2013 and 22 November 2023 in six sources [Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EBSCO Databases, Embase, Epistemonikos, and Web of Science], and we also conducted an advanced search in Google Scholar (keywords in title only). We updated the search on May 16, 2024. The full search strategy is outlined in S1 Table.
Selection process
Two reviewers (TI, MCA) independently screened the titles and abstracts (Level 1) to identify relevant citations in Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia (available at www.covidence.org.). Next, the same two reviewers independently screened the full texts (Level 2) to identify eligible studies. The two reviewers resolved differences through discussion.
Eligibility criteria
We used the following inclusion and exclusion criteria to define our search strategy.
Population
We included RCTs focused on middle-aged or older adults (mean age > 45) with T2DM. We excluded studies that included participants with pre-diabetes.
Intervention
We included studies providing one-on-one interventions delivered by volunteers; and included studies that did not provide compensation for volunteers, or gave volunteers a stipend or honorarium; but excluded studies where the intervention was delivered by paid health providers. We also excluded studies that described group-based interventions, or where interventions were delivered in hospitals.
Comparator
Any or none.
Outcomes
As we were interested in informing the health and social field of social prescribing (and not a specific health field, e.g., T2DM), we only included any studies that measured psychological (e.g., distress), social (e.g., social support), behavioural (e.g., physical activity), or quality of life outcomes. We also included studies that measured perceptions of the interventions, either from people receiving care or volunteers.
Time and type
We included individual-RCTs published in the last ten years (2013–2024).
Data collection process
Three reviewers (TI, JW, MCA) reviewed the final list of included studies and created the data extraction form in Covidence and Excel (Microsoft Corporation, Redmond, WA, USA). One author (TI) extracted the following data, and a second author checked it for accuracy (JW): general information (author last name, year of publication, location), description of participants receiving interventions (inclusion/exclusion criteria, demographics, sample size), volunteer description (demographics, role, training, content/setting of intervention, stipend), measured outcomes, findings, and adverse events. Prior to submitting the manuscript for peer-review, two reviewers rechecked tables for accuracy (HA, GSN).
Outcomes: Primary and secondary
Our primary outcomes included any psychological, social, or behaviour outcomes, for participants (e.g., patients) receiving interventions, or volunteers. Our secondary outcome was perceptions of the interventions, either from participants (receiving interventions) or volunteers.
Risk of bias assessment
Two reviewers (TI, MCA) independently assessed risk of bias using Version 2 of the Cochrane risk-of-bias tool for RCTs (RoB 2) [45] to determine if there were systematic errors due to the randomization process, deviations from the intervention, missing outcome data, measurement of the outcome, and selection of the reported findings. The two reviewers resolved any discrepancies through discussion.
Synthesis methods
Following Synthesis Without Meta-analysis (SWiM) guidelines [46], we reviewed extracted data and discussed similarities and differences across studies based on type of volunteer role, training, and outcomes. We calculated mean and standard deviation for relevant sociodemographic statistics, and then reviewed findings between intervention and control groups across studies, to summarize findings for the effect of a volunteer intervention on outcomes. Two reviewers (TI, MCA) independently reviewed and then met virtually several times to synthesize findings.
Changes to study protocol
We made a few changes to the protocol, for example, during the early phase of developing the search strategy we refined the research questions, and target databases we would search; and decided to limit the study design to only individual-RCTs.
Team composition and conflict of interest
Our team included university students, clinicians, people working in the non-profit sector, and researchers. None of the reviewers had authorship on any of the included studies.
Results
Study selection
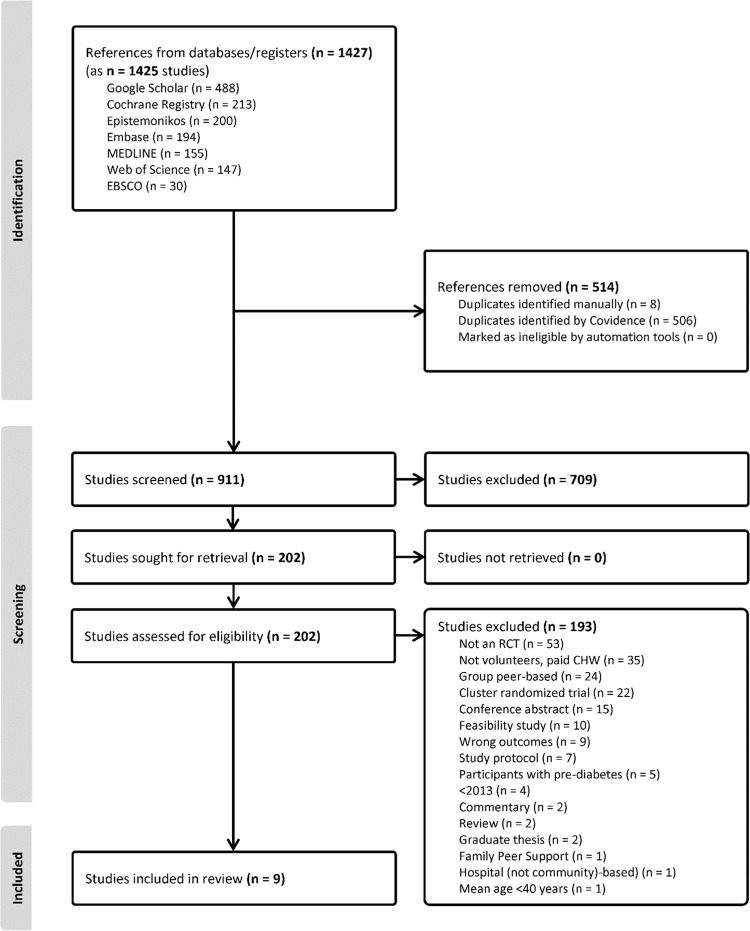
We identified 1427 citations (1425 studies) across sources. After removing duplicates, we screened 911 studies at Level 1 (title and abstract) and 202 studies at Level 2 (full text) review. We included nine studies (10 publications) [47–56]; and we provide a summary of the screening and selection process in Fig 1. The main reasons why studies were excluded were because of study design (not an individual RCT), people delivering the intervention were paid community workers, and the interventions were group-based. We excluded one study because the intervention included both group-based and one-on-one interventions [57]. We did not conduct a meta-analysis and we only reported data available within studies, or stated if data were not available. All studies were published in English.
Fig 1. PRISMA flow diagram [43] for the review.
Study characteristics
Studies were conducted in the following locations: USA (n = 3) [47, 50, 53] and one study each from Canada [55], Hong Kong [49, 56], India [54], Malaysia [52], Taiwan [48], and United Kingdom (UK) [51]. Patients’ mean (standard deviation; SD) age was 59.4 (9.3) years, on average 38% of participants were women, and the average number of years since T2DM diagnosis was 9.1 years (6.8) (Table 1).
Table 1. Summary of patients in the intervention and control groups, and relevant outcomes.
| Study Year Location | Sample, n = | N (%) Gender/Sex | Age, mean (SD) | Years with diabetes, mean (SD) | Intervention or Control Management Received | Study Outcomes |
|---|---|---|---|---|---|---|
|
Chan 2014 [49] Hong Kong Yeung 2018 [56] Hong Kong |
Control group (n = 316) | 177 (56.0) men 139 (44.0) women |
54.8 (8.6) | 9.6 (7.7) | The Joint Asia Diabetes Evaluation (JADE) program (patients received personalized reports and attended a 2-hour group empowerment class) | Depression (PHQ-9) [58] Diabetes-related distress (CDDS-15) [59] Diabetes self-care activities (SDSCA1-14) [60] Diabetes self-efficacy (DES-20) [61] Medication adherence (Morisky Medication Adherence Scale) [62] Psychological distress (DASS-21) [63] Quality of life (EQ-5D) [64] |
| Intervention group (n = 312) | 178 (57.1) men 134 (42.9) women |
54.5 (9.9) | 9.2 (7.8) | The JADE program + the Peer Support, Empowerment, and Remote Communication Linked by Information Technology (PEARL) program (2-hour group self-care class followed by one-on-one telephonic peer support) | ||
| Heisler 2019 [47] USA | Control group (n = 144) | 142 (98.6) male 2 (1.4) female |
62.1 (10.5) | 15.3 (9.9) | Face-to-face and telephonic peer support. Peers had access to consumer guides containing information about diabetes self-care | Perceived diabetes-specific social support (DSS) [65] |
| Intervention group (n = 146) | 141 (96.6) male 5 (3.4) female |
64.3 (9.7) | 15.0 (10.2) | Face-to-face and telephonic peer support. Peers had access to iDecide, a program that generates personalized feedback while sharing the same information as the consumer guides | ||
| Hsu 2021 [48] Taiwan | Control group (n = 33) | 22 (66.7) male 11 (33.3) female |
54.8 (6.9) | NA | 1–2 months of non-surgical periodontal treatment (full-mouth scaling, root planning, and oral hygiene instructions provided by a dental hygienist) | Attitudes towards periodontal health [48] Oral health quality of life (OHIP-14T) [66] Oral health-related knowledge [67] Oral self-care behaviours [48] |
| Intervention group (n = 35) | 18 (51.4) male 17 (48.6) female |
54.7 (6.1) | NA | Non-surgical periodontal treatment + a periodontal care curriculum taught by community health workers | ||
| Long 2020 [50] USA | Control group (n = 154) | 146 (94.8) male 8 (5.2) female |
60.6 (7.4) | 14.2 (8.0) | Usual care for T2DM | Depression (PHQ-2) [68] Diabetes distress (DDS2) [69] |
| Intervention group (n = 202) | 195 (96.5) male 7 (3.5) female |
59.6 (7.9) | 13.8 (9.1) | Usual care + telephonic peer support | ||
| Sampson 2021 [51] United Kingdom | Control group (n = 149) | 63 (42.3) male 86 (57.7) female |
63.5 (10.0) | *patients were screened and newly diagnosed with T2DM | 2-hour group education and behaviour change session | Diabetes quality of life (ADDQol) [70] Diabetes management self-efficacy (DMSES) [71] Diabetes treatment satisfaction (DTSQ) [72] Dietary behaviours (DBQ) [73] Physical activity (IPAQ) [74] Quality of life (EQ-5D) [75] Well-being (WBQ-12) [76] |
| Intervention group 1 (n = 142) | 61 (43.0) male 81 (57.0) female |
64.6 (10.1) | The Norfolk Diabetes Prevention Study (NDPS) intervention (six 2-hour group education and behaviour change sessions, followed by up to 15 2.5-hour group-based maintenance sessions) | |||
| Intervention group 2 (n = 141) | 57 (40.4) male 84 (59.6) female |
64.1 (9.9) | The NDPS intervention + telephonic peer support | |||
| Sazlina 2015 [52] Malaysia | Control group (n = 23) | 11 (47.8) men 12 (52.2) women |
63.0 (7.0) | 6.0 (9.0) | Usual care based on Malaysian guidelines for T2DM management (education about lifestyle modification, medication and self-care) | Exercise self-efficacy (SEES) [77] Perceived social support (MSPSS) [78] Physical activity (pedometer, weekly duration & frequency, PASE) [79] Psychological wellbeing (GHQ-12) [80] Quality of life (SF-12) [81] |
| Intervention group 1 (n = 23) | 14 (60.9) men 9 (39.1) women |
63.0 (8.0) | 10.0 (9.0) | Usual care + personalized feedback about physical activity patterns | ||
| Intervention group 2 (n = 23) | 12 (52.2) men 11 (47.8) women |
64.0 (7.0) | 9.0 (11.0) | Usual care + personalized feedback + face-to-face and telephonic peer support | ||
| Siminerio 2013 [53] USA | Control group (n = 32) | 15 (46.9) male 17 (53.1) female |
60.0 (12.0) | NA | Diabetes self-management education (DSME) provided by certified diabetes educators | Behavioural goal tracking [53] Emotional distress (PAID) [82] Empowerment (DES-SF) [83] Satisfaction [53] Self-care behaviours (SDSCA) [60] |
| Intervention group 1 (n = 38) | 16 (42.1) male 22 (57.9) female |
60.0 (10.0) | NA | DSME + telephonic diabetes self-management support (DSMS) provided by certified diabetes educators | ||
| Intervention group 2 (n = 36) | 17 (47.2) male 19 (52.8) female |
64.0 (10.0) | NA | DSME + telephonic DSMS provided by peers | ||
| Intervention group 3 (n = 35) | 14 (40.0) male 21 (60.0) female |
60.0 (13.4) | NA | DSME + telephonic DSMS provided by practice staff | ||
| Sreedevi 2017 [54] India | Control group (n = 26) | 26 (100.0) women | 51.92 (6.57) | 5.1 (3.04) | Usual care for T2DM | Quality of life (WHOQOL-BREF) [84] |
| Intervention group 1 (n = 31) | 31 (100.0) women | 51.97 (7.40) | 5.8 (2.78) | 24 60-minute yoga sessions led by an instructor | ||
| Intervention group 2 (n = 26) | 26 (100.0) women | 51.92 (8.32) | 5.34 (2.75) | Weekly face-to-face and telephonic peer support | ||
| Tang 2022 [55] Canada | Control group (n = 98) | 49 (50.0) male 49 (50.0) female |
58.5 (10.9) | 11.0 (11.5) | Usual care for T2DM | Depression (PHQ-9) [58] Diabetes distress (DDS17) [85] |
| Intervention group (n = 98) | 49 (50.0) male 49 (50.0) female |
60.5 (11.4) | 12.4 (11.1) | Face-to-face and telephonic peer support |
Abbreviations: ADDQol = Audit of Diabetes Dependent Quality of Life; CDDS-15 = Chinese Diabetes Distress Scale-15; DASS-21 = Depression Anxiety Stress Scale-21; DBQ = Dietary Behaviours Questionnaire; DDS = Diabetes Distress Scale; DES-20 = Diabetes Empowerment Scale-20; DES-SF = Diabetes Empowerment Scale-Short Form; DSS = Diabetes Support Scale; EQ5D = EuroQol-5D; IPAQ = International Physical Activity Questionnaire; MSPSS = Multidimensional Scale of Perceived Social Support; OHIP-14T = Oral Health Impact Profile-14; PAID = Problem Areas in Diabetes; PASE = Physical Activity Scale for the Elderly; PHQ-2 = Patient Health Questionnaire-2; PHQ-9 = Patient Health Questionnaire-9; SDSCA = Summary of Diabetes Self-Care Activities; WBQ-12 = Well-Being Questionnaire-12; WHOQOL-BREF = World Health Organization Quality of Life Scale
Volunteers were commonly referred to as peers (meaning they also had T2DM), with the exception of a single study that described volunteers as community health workers [48]; and they filled various roles such as educators, mentors, supporters, and facilitators (Table 2).
Table 2. Summary of the volunteers and the interventions.
| Study | Volunteer Description | Training | Intervention |
|---|---|---|---|
|
Chan 2014 [49]
Yeung 2018 [56] |
Peers: people with A1c < 8.0%. |
Time commitment: 32 hours Content: Tutorials, case sharing, reflections, role playing, games, and activities. Focused on mindset, empathic listening, questioning skills, and counselling skills. Addressed factors that could influence blood glucose level (e.g. diet, exercise), self-monitoring of blood glucose, sick day management, foot care, emotional support, resources for information, and clinical care. Materials: Tutorial notes, reference materials Ongoing supports: NA Assessment: Pre- and post-training evaluation of diabetes knowledge and psychological-behavioural measures |
Length of intervention: 12 months Time commitment: 2-hour face-to-face session, 15 minutes ≥ 12 phone calls Volunteer role: NA Patients per volunteer: 10 Volunteer-patient matching: NA Materials: Resource booklet, checklist for calls Mode: Telephone Stipend amount: US $500 |
| Heisler 2019 [47] | Peers: people with a history of poor glycemic control (A1c ≥ 8.0%), but whose most recent A1c was < 8.0% |
Time commitment: 2 hours (base training), 1 hour (iDecide training), 1.5 hour/month (follow-up meetings) Content: Motivational Interviewing-based communication skills (open-ended questions, rolling with resistance, eliciting ’change-talk’, and goal-setting and ’action planning’) Materials: iDecide (personally tailored diabetes medication decision aid) on iPads Ongoing supports: Monthly meetings with other peers to check-in and provide booster follow-up training Assessment: Self-assessment and random observation of phone calls by the study team |
Length of intervention: 6 months Time commitment: 2-hour face-to-face session, ≥ 1 phone call/week Volunteer role: During the initial face-to-face session, peers and patients identified a behavioural goal and an action plan and generated a list of questions and concerns for health care providers. During follow-up phone calls, peers and patients brainstormed solutions to barriers and set new goals and action steps Patients per volunteer: 1–5 Volunteer-patient matching: NA Materials: Diabetes medication guides, iDecide Mode: Face-to-face, telephone Stipend amount: NA |
| Hsu 2021 [48] | Community health workers: four people selected from the community. |
Time commitment: 4 hours Content: Periodontal disease and care, teaching and communication skills Materials: Training manual containing goals and contents of each lesson Ongoing supports: CHWs could contact research staff for support in the month prior to the intervention Assessment: Community health worker certification test |
Length of intervention: 1 month Time commitment: 30 minutes x 4 face-to-face lessons Volunteer role: Peers taught patients about effective toothbrushing methods and tools Patients per volunteer: NA Volunteer-patient matching: NA Materials: Slide presentations, toothbrushing tools Mode: Face-to-face Stipend amount: US $70 per patient |
| Long 2020 [50] | Peers: patients with a history of poor glycemic control, but whose most recent A1c was ≤ 7.5% OR previous study mentees (with no A1c restriction). |
Time commitment: 1 hour Content: Mentee’s story/motivations (to help set realistic goals and provide support), dealing with failure in an accepting manner, role-playing exercises, and sample questions Materials: NA Ongoing supports: Staff contacted peers 1 time/month to check in and provide training reinforcements Assessment: NA |
Length of intervention: 6 months Time commitment: ≥ 1 phone call/week Volunteer role: NA Patients per volunteer: 1 Volunteer-patient matching: Based on age, race/ethnicity, sex, and insulin use. Materials: NA Mode: Telephone Stipend amount: US $20 per month for each month peers attempted to contact their assigned mentee |
| Sampson 2021 [51] | Peers: people with T2DM. |
Time commitment: 14–17.5 hours Content: Impact of physical activity, diet, pre-diabetes, and lifestyle on T2DM. Also role-playing exercises Materials: NA Ongoing supports: NA Assessment: A mock call where the senior research associate assumed the role of trial participant to test peers in specific situation |
Length of intervention: 46 months Time commitment: ≥ 18 phone calls. Volunteer role: Peers and patients discussed progress, goal achievement, action planning, and barriers to coping Patients per volunteer: ≤ 7 Volunteer-patient matching: NA Materials: NA Mode: Telephone Stipend amount: £350 |
| Sazlina 2015 [52] | Peers: older adults (≥ 60 years) with successfully managed T2DM who lived in the same community as participants. |
Time commitment: 2 days Content: Interactive discussions, simulations, and role-plays to improve peers’ ability to provide support through telephone and face-to-face contacts Materials: NA Ongoing supports: Two fortnightly and two monthly debriefing meetings over the 12-week intervention Assessment: Assessments by the research team at monthly clinic visits with their peers |
Length of intervention: 3 months Time commitment: 3 face-to-face meetings, 3 phone calls Volunteer role: Peers and patients discussed barriers and motivations, and peers encouraged patients to become empowered to increase their physical activity to self-manage their diabetes Patients per volunteer: 3–5 Volunteer-patient matching: NA Materials: NA Mode: Face-to-face, telephone Stipend amount: NA |
| Siminerio 2013 [53] | Peers: patients who had previously attended diabetes self-management education selected based on their communication skills and willingness to participate (no A1c restriction). |
Time commitment: 2–3 hours Content: Information about active listening, empowerment, and behavioural approaches. Also role-playing exercises to practice skills Materials: NA Ongoing supports: Peers could contact a Certified Diabetes Educator or their Primary Care Provider Assessment: Human subject modules of the associated universities |
Length of intervention: 6 months Time commitment: ≥ 5 phone calls Volunteer role: Peers and patients engaged in a patient-centred discussion regarding the patient’s behavioural goal and barriers to achieving their goal Patients per volunteer: NA Volunteer-patient matching: NA Materials: Telephone scripts, behavioural goal forms Mode: Telephone Stipend amount: NA |
| Sreedevi 2017 [54] | Peers: three people with T2DM (RPG < 250 mg/dL) selected from the community based on their adherence to treatment, and capacity to be a successful mentor. |
Time commitment: 2 days Content: A physician explained diabetes, glycaemic control, and medications and their synergies with physical activity. A nutritionist explained the nutritional aspects of diabetes. A psychologist provided training in communication skills, empathy, and confidentiality Materials: Training manual (based on peers for progress handbook) Ongoing supports: NA Assessment: NA |
Length of intervention: 3 months Time commitment: 45–60 minutes x 1 face-to-face meeting/week, 1 phone call/week Volunteer role: During the initial session, peers collected treatment details and went over the functions of peer support. During follow-up sessions, peers and patients discussed diet, exercise, medication, emotional stress, diabetes symptoms, foot care, and more. During the final sessions, the peer conducted a final process assessment Patients per volunteer: 13–14 Volunteer-patient matching: NA Materials: Diary (for patients) Mode: Face-to-face, telephone Stipend amount: NA |
| Tang 2022 [55] | Peers: patients with A1c < 8.0%. |
Time commitment: 30 hours Content: Knowledge, skills, and strategies to address (1) assistance in daily self-management, (2) social and emotional support, and (3) linkage to clinical care. In particular, skills in motivating and empowering patients, active listening, goal-setting and action planning, and problem solving Materials: NA Ongoing supports: NA Assessments: Formative and summative assessments of five domains: diabetes-related knowledge, empowerment-based facilitation, active listening, goal-setting, and perceived self-efficacy. Also ’spot check’ phone calls made by the research team to peers |
Length of intervention: 12 months Time commitment: 1 face-to-face meeting, 29 phone calls Volunteer role: Peers and patients discussed challenges, feelings and questions about self-management, solved problems, and set goals and develop action plans Patients per volunteer: NA Volunteer-patient matching: Based on gender and geographical proximity. Materials: NA Mode: Face-to-face, telephone Stipend amount: CAD $400 following training, then $20 per participant per month [86] |
Abbreviations: CHW = Community Health Worker; NA = Not Available; T2DM = Type 2 Diabetes Mellitus
Study interventions were intended to promote patients’ ability for T2DM self-management in order to improve cardio-metabolic function and psychosocial well-being (Table 1). One study specifically aimed to improve oral health for patients with T2DM [48]. For our outcomes of interest, we extracted the following data: depression [48–50, 56], dietary behaviours [51], distress [49, 50, 53, 55, 56], empowerment [53], perceived social support [47, 52], physical activity [51, 52], quality of life [48, 49, 51, 52, 54, 56], self-care behaviours [48, 49, 53, 56], self-efficacy [49, 51, 52, 56], treatment satisfaction [51, 53], and well-being [51, 52] (Table 1). There were no studies which reported the effect or impact on the volunteers for the outcomes of interest for this review.
Three of the included studies discussed adverse events [50–52]; but there were no reported serious adverse events related to interventions.
Risk of bias studies
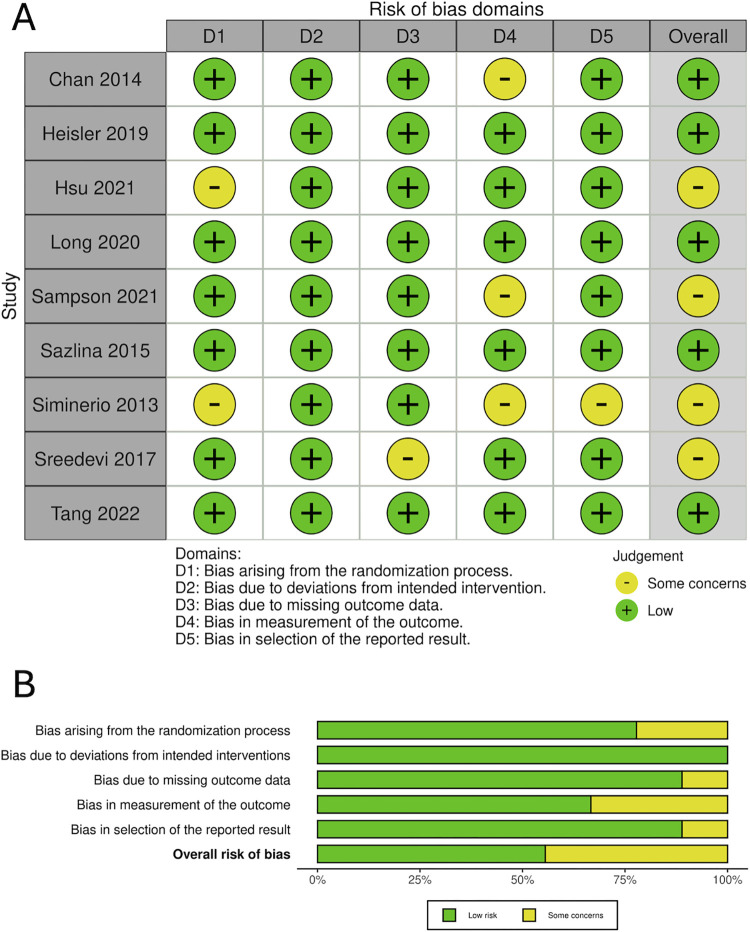
Overall, we rated five studies as low risk of bias [47, 49, 50, 52, 55], and some concerns for four studies [48, 51, 53, 54] (Fig 2A and 2B). Items where there were some concerns within studies included: measurement outcomes (n = 3), randomization (n = 2), missing data (n = 1), and selection of reported outcomes (n = 1).
Fig 2. Risk of bias.
(A) Summary of risk of bias for each study. (B) Overall risk of bias.
Results of syntheses
We provide details of the volunteer interventions in Table 2. The implementation of the programs varied in duration, contact time (dose), delivery mode, intervention content, volunteer training, and outcomes. The interventions ranged in duration between one and 46 months [mean (SD): 10.6 (13.8) months] and consisted of face-to-face meetings and/or telephone calls between volunteers and patients. Seven of the nine studies reported the content of their interventions, including: goal setting and action planning [47, 51, 53, 55], self-management education [48, 52, 54, 55], problem-solving [47, 51–53, 55], and discussions centred around empowerment and motivation [52]. In two of the nine studies [50, 55], researchers used pre-defined criteria to match volunteers with patients. In the remaining studies, the authors did not report how volunteers were matched with patients. Five of the nine studies [47, 49, 51, 52, 54] reported the number of patients matched with each volunteer: this number ranged from 1:1 to 1:14.
All included studies provided mandatory training for volunteers (Table 2.) The time commitment varied depending on the study, between one and 32 hours of training [mean (SD): 12.5 (11.9) hours], assuming that one full day of training is equal to eight hours. The training programs aimed to improve volunteers’ skills and knowledge in several areas: communication and listening [47–49, 53–55], disease self-management [48, 49, 51, 54, 55], behaviour change and goal-setting [47, 50, 51], and emotional and social support [50, 52, 53, 55]. Several studies employed role-playing in order to reinforce taught concepts [49–53].
Eight studies reported providing volunteers with a stipend to cover costs associated with participation in the study as well as to provide compensation for their substantial commitments. Of the eight studies, five specified the amount or rate of the stipend: US $500 [49]; US $70 per patient [48]; US $20 per month volunteers attempted to contact their mentee [50]; £350 [51]; and CAD $400 following training, then $20 per participant per month [55, 86]. Three studies reported providing a stipend, but not the amount [47, 52, 53]. Only one study did not report providing a stipend [54]. These results are summarized in Table 2.
Results from main analyses. Table 3provides a summary of findings for our outcomes of interest. Of 15 reported psychosocial outcomes, only one outcome (from one study) favoured the intervention group, and in 14 outcomes, there was no difference between the volunteer intervention and control groups. Of nine reported behavioural outcomes, six favoured the intervention group, and three studies reported no difference between groups.
Table 3. Overview of findings for outcomes of interest, comparing intervention groups who engaged with volunteers with a control condition.
| Outcome Type | Favors Volunteer Intervention Group | No Difference between Groups |
|---|---|---|
| Psychosocial | Perceived social support (MSPSS) [52] | Depression (PHQ-2) [50] Depression (PHQ-9) [49, 55] Diabetes distress (DDS) [50, 55] Diabetes quality of life (ADDQol) [51] Diabetes self-efficacy (DES-20) [49] Diabetes-related distress (CDDS-15) [49] Emotional distress (PAID) [53] Empowerment (DES-SF) [53] Oral-health quality of life (OHIP-14T) [48] Perceived diabetes-specific social support (DSS) [47] Psychological distress (DASS-21) [49] Psychological wellbeing (GHQ-12) [52] Quality of life (EQ-5D) [49, 51] Quality of life (WHOQOL-BREF) [54] Well-being (WBQ-12) [51] |
| Behavioural | Attitudes towards periodontal health [48] DBQ fat score scale at 24 months [51] Oral health-related knowledge [48] Oral self-care behaviours [48] Physical activity weekly duration & frequency (PASE) [52] Steps (pedometer) [52] |
Medication adherence [49] Physical activity (IPAQ, resistance questionnaire) [51] Self-care behaviours (SDSCA) [49, 53] |
Abbreviations: ADDQol = Audit of Diabetes Dependent Quality of Life; CDDS-15 = Chinese Diabetes Distress Scale-15; DASS-21 = Depression Anxiety Stress Scale-21; DBQ = Dietary Behaviours Questionnaire; DDS = Diabetes Distress Scale; DES-20 = Diabetes Empowerment Scale-20; DES-SF = Diabetes Empowerment Scale-Short Form; DSS = Diabetes Support Scale; EQ5D = EuroQol-5D; GHQ-12 = General Health Questionnaire-12; IPAQ = International Physical Activity Questionnaire; MSPSS = Multidimensional Scale of Perceived Social Support; OHIP-14T = Oral Health Impact Profile-14; PAID = Problem Areas in Diabetes; PASE = Physical Activity Scale for the Elderly; PHQ-2 = Patient Health Questionnaire-2; PHQ-9 = Patient Health Questionnaire-9; SDSCA = Summary of Diabetes Self-Care Activities; WBQ-12 = Well-Being Questionnaire-12; WHOQOL-BREF = World Health Organization Quality of Life Scale
Results from additional analyses
Chan and colleagues [49] reported peer support management (education and peer support) had no significant effect when compared with the control group management (education alone). However, in a sub-group analysis, which controlled for patients with elevated distress at baseline [Depression, Anxiety and Stress Scale—21 Items (DASS-21) ≥ 17], peer support improved distress and medication adherence, and reduced hospitalization when compared with the control group [49]. Second, Heisler and colleagues [47] compared two peer support groups. While there was no between-group difference, both groups had improvement in diabetes-specific social support [47]. Third, Siminerio and colleagues [53] reported a comparison between three groups who received diabetes self-management support from a person from one of the following groups: a certified diabetes educator; a peer; or a practice staff member. The authors reported the intervention delivered by certified diabetes educators resulted in significantly better empowerment scores compared with delivery by peers or practice staff [53]. Finally, Sampson and colleagues [51] measured diabetes management self-efficacy, but because their participants were newly diagnosed at the time of randomization, the authors did not include a baseline diabetes management self-efficacy measurement. In their exploratory analysis, the authors reported no significant unadjusted differences between groups at 12 and 24 months for this outcome.
Two of the nine studies measured satisfaction for patients alone [51] or volunteers and patients [53] (Table 2). Sampson and colleagues [51] used the Diabetes Treatment Satisfaction Questionnaire (DTSQ), but did not measure satisfaction at baseline, precluding them from adjusting their data for analysis. However, the authors reported no significant unadjusted differences between groups at 12 and 24 months. On the other hand, Siminerio and colleagues [53], who using a proprietary satisfaction survey, reported patient satisfaction of 100% in the educator group, 95% in the peer group, 75% in the practice staff group, and 74% in the usual (control) group. The authors also reported that peers felt satisfied with their training and experience, and would recommend being a peer to others.
Discussion
In this rapid systematic review, we identified nine RCTs from middle and high-income countries examining the effect of one-on-one, community-based volunteer-led interventions on the health and wellbeing of people living with T2DM, with the goal of informing practice and future research in social prescribing. Eight of the nine studies used peer volunteers [87, 88]–who were themselves living with T2DM–to provide self-management support that is often missing for people without well-developed personal support networks. Volunteers were trained in areas such as communication, behaviour change, and social support to deliver diabetes self-management interventions. However, the implementation of the programs varied in duration, contact time (dose), delivery mode, intervention content, volunteer training, and outcomes. Based on a qualitative summary of reported outcomes for participants, interventions did not have a substantial effect on psychosocial endpoints (e.g., depression, distress, quality of life). However, there were reported findings favouring volunteer interventions for social support and behaviour change, which generate hypotheses for future discovery. Overall, this rapid systematic review sheds light into how volunteers may be integrated into, and provide a meaningful contribution to, health and social models of care.
Social support and health
The results from this review highlight volunteers play an important role in health interventions, specifically via social support and/or other behaviour change strategies [89]. The included studies did not provide specific information on the behaviour change techniques (BCTs) used by volunteers, but there were instances of goal setting, action planning and social support. Researchers have defined several different types of social support, such as unspecified, practical, or emotional [89]. Other work has outlined five possible categories of social support in health interventions, specifically personal connection, support with activities of daily living, social and emotional support (including developing coping plans and strategies), navigation, and longitudinal support [90]. In this review, included studies did not provide detailed information on the type of social support, and only two of nine studies reported social support as an outcome. However, most likely, many volunteers provided support across most of the categories of social support, except for longitudinal support, as six of the nine studies were six months or less in duration.
Many of the studies were also not designed to compare the effect of volunteer vs. provider led programs, and therefore, we cannot comment on this directly. However, in a subgroup analysis, one study [53] compared volunteers and other study personnel (e.g., pooled data from several groups) with a health provider giving the same intervention: patient empowerment scores were significantly better from the group who received the intervention from the providers. This result may not be surprising, as volunteers may not have the same longitudinal care history with patients (as providers) so it may be challenging to establish the same rapport. In addition, there may be times when people prefer information from a provider compared with a volunteer [15]. That is, the timing and type of information delivery may be context and person dependent.
Implementation of volunteer programs
The lack of significant improvement in psychosocial outcomes due to volunteer support may be surprising. However, there are potential implementation factors to consider in these findings and in future interventions and research, such as: volunteer training, volunteer-patient matching, volunteer retention, intervention mode and duration, and geographical context.
Many volunteers do not have experience in the health and/or social care domain, and as a result they require adequate (consistent) training to equip them with the requisite skills and knowledge to be successful in their roles. What counts as adequate training is context and person dependent. In addition, training programs should be mindful that they do not require volunteers to commit too much time [91]. There was considerable variability in the length of training provided for volunteers in the included studies (1–32 hours). Despite this variability, positive behavioural results were observed for studies across the spectrum of training duration [48, 51, 52]; but not most psychosocial outcomes. However, without standardized training protocols and performance evaluations, these findings may not be generalizable or scalable. Future studies should consider implementing (and reporting) standardized training and evaluation to improve the consistency of outcomes.
Matching volunteers and patients can be completed randomly, or based on selective criteria (e.g., shared interests, geographic proximity, age, gender) which can improve rapport, and potentially lead to better outcomes for both volunteers (e.g., retention) and patients [20]. There may also be the need to reassign volunteers and patients to improve rapport [20]. One relevant matching criterion is disease or condition, which may be why eight of the nine studies in this review used peers (who, like patients, had T2DM) [47, 49–55]. In addition, only two of the included studies [50, 55] reported using additional volunteer-patient matching criteria (beyond disease or condition). However, neither study reported operational details for matching, nor reported social support outcomes. Future studies should consider defining (and reporting) criteria for volunteer-patient matching combined with ongoing evaluation of the volunteer-patient relationship (using social support or other measures) in one-to-one volunteer support programs.
Volunteers need to be recognized and valued; which may help with the sustainability of volunteer programs. Although a 2023 meta-analysis [92] identified values as the strongest predictor to volunteer; the authors also suggested understanding motivation to volunteering and regular communication [92] may support volunteer retention. Another factor which may influence the retention of volunteers in a health program is financial compensation [93]. Although they are not typically paid employees, volunteers might receive stipends to incentivize their commitment and cover basic costs incurred by their role, such as transportation [94]. Almost all of the included studies in this review provided a monetary reward to their volunteers, which can improve volunteer retention and perceived benefits [95], and may not ‘crowd-out’ intrinsic motivation [96]. Although improving volunteer retention may lead to more sustainable programs, there is a need for further research exploring whether monetary rewards have a measurable effect on patient outcomes, especially as they may modify the balance between intrinsic and extrinsic motivators. Given the current lack of evidence, our findings may not be generalizable to volunteer programs that do not provide a monetary reward.
Another important consideration when implementing a volunteer support program is the delivery mode and duration of the intervention. Previous work has shown that, while non-face-to-face contact can be beneficial in times when face-to-face contact is not possible (e.g., during COVID-19 lockdowns) [97], face-to-face contact is more effective at promoting wellbeing [98]. However, face-to-face contact can be time-consuming for volunteers and patients, depending on the meeting location, although this barrier could be overcome by matching volunteers with patients based on geographic proximity [20]. Perhaps, this is why only one of the included studies used exclusively face-to-face contact, while three studies were telephonic, and five studies used a combination of telephone and face-to-face contact. There was also considerable variability in the duration and frequency of the interventions, with studies ranging from one to 46 months in length, and meetings occurring weekly to once every two months. With positive behavioural results being observed at both ends of this spectrum, and in studies that were exclusively face-to-face and exclusively telephonic, it remains unclear what is the optimal format for intervention delivery. As a result, this rapid systematic review generates questions about the feasibility, acceptability and effects of delivery mode and duration of volunteer support interventions.
Volunteer interventions occur within local contexts, and so geographically variegated factors such as social and cultural norms, as well as healthcare systems must be considered during implementation. The studies included in this synthesis originated from a range of middle- and high-income countries, and across three continents (Asia, Europe, North America). Comparative research has attempted to classify healthcare systems by numerous variables, including expenditure, effectiveness, institutional boundaries, and disparities, but clear consensus has not been reached [99, 100]. Similarly, cultural variables are known to impact health in various ways. For example, work in Europe has shown that loneliness may have a greater negative impact on health in less individualistic, more collectivistic societies [101]. These (and other) variables may have influenced outcomes in the included studies, and we thus emphasize caution in generalizing findings across nations. Therefore, volunteer interventions should be adapted to local cultural contexts to enhance their effectiveness and produce valuable, context-specific evidence, although this warrants further investigation.
Volunteering and social prescribing
From the start of this review, our goal was to synthesize evidence on volunteers working in social prescribing. However, despite the important role of volunteers contributing to the success of social prescribing, few peer-reviewed studies are available. From this rapid systematic review, there are potential areas to explore related to implementation, for example, considering factors for volunteer recruitment, training, sustainability, and delivery and duration; as well as consistent evaluation of volunteer training, learning, effect on patients’ health and well-being, and impact of the program on volunteers. Importantly, “success” from the perspective of the volunteer (across outcomes) should be defined in collaboration with volunteers, and routinely evaluated and consistently reported.
Strengths and limitations
This work has many strengths. For example, we identified knowledge gaps for volunteers in health and lifestyle management; we conducted the study in accordance with PRISMA guidelines; and we built in redundancies by ensuring two reviewers completed key tasks independently, and that a second author reviewed all extracted data for accuracy. We also acknowledge several limitations with this study. For example, as a result of our narrow inclusion criteria, only nine studies (10 publications) were included in our review despite the large number of potential studies: A majority of potential studies were excluded due to study design, interventions delivered by professionals (not volunteers), or group-based interventions. However, we narrowed our inclusion criteria for several key reasons. Notably, we only included one-on-one interventions to replicate many social prescribing settings more closely, where care is typically delivered to individuals rather than groups [102, 103].
Another limitation of our review was the considerable variety of interventions and outcomes in the included studies. In particular, the control groups in the included studies received varied management strategies. Some groups received ‘usual care’, some received educational interventions that were also delivered to the intervention group, and others received alternate interventions. As a result, it was difficult to isolate the effect of volunteers from the effects of the alternate or supplemental interventions offered to patients. For instance, in a program where volunteer support is offered in addition to an educational program, it is possible that the educational program has already maximized the potential benefits, leaving little room for volunteers to provide any additional benefit. For this reason, it is important that future studies aim to standardize control group management, although we acknowledge that there will be inevitable variability owing to differences in local health context.
Finally, studies reported a wide variety of psychosocial and behavioural outcomes, and used a variety of scales and measures to record these outcomes. This heterogeneity limited our ability to combine data quantitatively and make general conclusions about the effect of volunteer interventions. We suggest future studies aim to standardize the outcomes used to assess volunteer interventions so that results can be directly compared and broader conclusions can be drawn. We also suggest that social support should be more regularly reported, as it may provide insights into the quality of the volunteer-patient relationship.
Conclusion
In this review, we addressed a knowledge gap and highlight the potential role of volunteers to support health behaviour change in diabetes self-management, and health and social models of care. We noted significant differences for some behavioral outcomes for participants, but only one study reported an outcome in favour of the volunteer-based intervention for social support, although this may be because this outcome was not routinely measured in all studies. We highlight the need to focus more on the implementation of volunteer training, matching and retention, as well as intervention mode and duration, and geographical context. Further, other areas for future consideration include evaluation (comprehensively defining and measuring success) of volunteer programs and testing the effect of the intervention on volunteers who deliver the program, while exploring potential mechanisms of action within the causal pathway of improving social support and health. This work can be applied to future studies to develop volunteer programs and evaluate the implementation, impact, and effect of volunteers for social prescribing.
Supporting information
(PDF)
The initial searches were completed in November 2023 and updated in May 2024.
(PDF)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
Dr. Esfandiari acknowledges the postdoctoral fellowship provided by the Edwin S.H. Leong Centre for Healthy Aging. Dr. Chudyk acknowledges the support of the Canadian Institutes of Health Research Patient-Oriented Research Awards—Transition to Leadership Stream—Phase 2 award (reference number 188352). Professor Ashe gratefully acknowledges the support of the Canada Research Chairs Program.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants received from the following sources: Social Sciences and Humanities Research Council (to MCA, GSN, RP, AC) and the University of British Columbia, and UBC Health Innovation Funding Investment (HIFI) Award (to MCA, GSN, RP, AC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Wit A, Mensink W, Einarsson T, Bekkers R. Beyond service production: volunteering for social innovation. Nonprofit and Voluntary Sector Quarterly. 2017;48(2_suppl):52S–71S. doi: 10.1177/0899764017734651 [DOI] [Google Scholar]
- 2.Güntert ST, Wehner T, Mieg HA. Definition of volunteer work and a model of volunteer activity. Organizational, Motivational, and Cultural Contexts of Volunteering: The European View. SpringerBriefs in Psychology: Springer, Cham; 2022. p. 1–10. [Google Scholar]
- 3.Firth A. Volunteering and managing change in the health sector. Perspectives in Public Health. 2013;133(5):240–1. doi: 10.1177/1757913913500413 . [DOI] [PubMed] [Google Scholar]
- 4.Vizeshfar F, Momennasab M, Yektatalab S, Iman MT. Empowering health volunteer’s through participatory action research in a comprehensive healthcare center. BMC Public Health. 2021;21(1):889. Epub 20210510. doi: 10.1186/s12889-021-10878-7 ; PubMed Central PMCID: PMC8112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaber J, Oliver D, Valaitis R, Cleghorn L, Lamarche L, Avilla E, et al. Experiences of integrating community volunteers as extensions of the primary care team to help support older adults at home: a qualitative study. BMC Family Practice. 2020;21(1):92. Epub 20200516. doi: 10.1186/s12875-020-01165-2 ; PubMed Central PMCID: PMC7231411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore A, Motagh S, Sadeghirad B, Begum H, Riva JJ, Gaber J, et al. Volunteer impact on health-related outcomes for seniors: a systematic review and meta-analysis. Canadian Geriatrics Journal. 2021;24(1):44–72. Epub 20210302. doi: 10.5770/cgj.24.434 ; PubMed Central PMCID: PMC7904324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handajani YS, Aryani A, Schroder-Butterfill E, Turana Y. Impact of trained volunteers’ services in caring for older persons with dementia: a systematic review. Psychogeriatrics. 2023;23(3):535–46. Epub 20230326. doi: 10.1111/psyg.12958 . [DOI] [PubMed] [Google Scholar]
- 8.Weber BA, Roberts BL, Yarandi H, Mills TL, Chumbler NR, Wajsman Z. The impact of dyadic social support on self-efficacy and depression after radical prostatectomy. Journal of Aging and Health. 2007;19(4):630–45. doi: 10.1177/0898264307300979 . [DOI] [PubMed] [Google Scholar]
- 9.Sales VL, Ashraf MS, Lella LK, Huang J, Bhumireddy G, Lefkowitz L, et al. Utilization of trained volunteers decreases 30-day readmissions for heart failure. Journal of Cardiac Failure. 2013;19(12):842–50. Epub 20131029. doi: 10.1016/j.cardfail.2013.10.008 . [DOI] [PubMed] [Google Scholar]
- 10.Scharlach AE. Estimating the value of volunteer-assisted community-based aging services: a case example. Home Health Care Services Quarterly. 2015;34(1):46–65. doi: 10.1080/01621424.2014.999902 . [DOI] [PubMed] [Google Scholar]
- 11.Ndu M, Andoniou E, McNally S, Olea Popelka F, Tippett M, Nouvet E. The experiences and challenges of community health volunteers as agents for behaviour change programming in Africa: a scoping review. Global Health Action. 2022;15(1):2138117. doi: 10.1080/16549716.2022.2138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Using lay health workers to improve access to key maternal and newborn health interventions2013 July 13, 2024:[6 p.]. Available from: https://iris.who.int/bitstream/handle/10665/85617/WHO_RHR_13.09_eng.pdf?sequence=1. [Google Scholar]
- 13.Dennis CL. Peer support within a health care context: a concept analysis. International Journal of Nursing Studies. 2003;40(3):321–32. doi: 10.1016/s0020-7489(02)00092-5 . [DOI] [PubMed] [Google Scholar]
- 14.McHugh JE, Lee O, Aspell N, Connolly L, Lawlor BA, Brennan S. Peer volunteer perspectives following a complex social cognitive intervention: a qualitative investigation. International Psychogeriatrics. 2016;28(9):1545–54. Epub 20160218. doi: 10.1017/S1041610216000144 . [DOI] [PubMed] [Google Scholar]
- 15.Peel NM, Warburton J. Using senior volunteers as peer educators: what is the evidence of effectiveness in falls prevention? Australasian Journal on Ageing. 2009;28(1):7–11. doi: 10.1111/j.1741-6612.2008.00320.x . [DOI] [PubMed] [Google Scholar]
- 16.Tierney S, Mahtani KR, Wong G, Todd J, Roberts N, Akinyemi O, et al. The role of volunteering in supporting well-being—what might this mean for social prescribing? A best-fit framework synthesis of qualitative research. Health & Social Care in the Community. 2022;30(2):e325–e46. Epub 20210802. doi: 10.1111/hsc.13516 . [DOI] [PubMed] [Google Scholar]
- 17.Buman MP, Giacobbi PR, Jr., Dzierzewski JM, Morgan AA, McCrae CS, Roberts BL, et al. Peer volunteers improve long-term maintenance of physical activity with older adults: a randomized controlled trial. Journal of Physical Activity and Health. 2011;8(s2):S257–S66. doi: 10.1123/jpah.8.s2.s257 . [DOI] [PubMed] [Google Scholar]
- 18.Ginis KA, Nigg CR, Smith AL. Peer-delivered physical activity interventions: an overlooked opportunity for physical activity promotion. Translational Behavioral Medicine. 2013;3(4):434–43. doi: 10.1007/s13142-013-0215-2 ; PubMed Central PMCID: PMC3830020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer PN, Heisler M, Piette JD, Rogers MA, Valenstein M. Efficacy of peer support interventions for depression: a meta-analysis. General Hospital Psychiatry. 2011;33(1):29–36. Epub 20101113. doi: 10.1016/j.genhosppsych.2010.10.002 ; PubMed Central PMCID: PMC3052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stathi A, Withall J, Agyapong-Badu S, Barrett E, Kritz M, Wills D, et al. Mobilising people as assets for active ageing promotion: a multi-stakeholder perspective on peer volunteering initiatives. BMC Public Health. 2021;21(1):150. Epub 20210118. doi: 10.1186/s12889-020-10136-2 ; PubMed Central PMCID: PMC7812118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webel AR, Okonsky J, Trompeta J, Holzemer WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. American Journal of Public Health. 2010;100(2):247–53. Epub 20091217. doi: 10.2105/AJPH.2008.149419 ; PubMed Central PMCID: PMC2804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Education & Behavior. 2003;30(2):170–95. doi: 10.1177/1090198102251030 . [DOI] [PubMed] [Google Scholar]
- 23.Canadian Institute for Social Prescribing. About social prescribing [Internet]. Canadian Institute for Social Prescribing; 2024. [August 14, 2024]. Available from: https://www.socialprescribing.ca/about-social-prescribing. [Google Scholar]
- 24.Foster A, Thompson J, Holding E, Ariss S, Mukuria C, Jacques R, et al. Impact of social prescribing to address loneliness: a mixed methods evaluation of a national social prescribing programme. Health & Social Care in the Community. 2021;29(5):1439–49. Epub 20201020. doi: 10.1111/hsc.13200 . [DOI] [PubMed] [Google Scholar]
- 25.NHS England. Social prescribing [Internet]. Leeds (UK): NHS England; [August 14, 2024]. Available from: https://www.england.nhs.uk/personalisedcare/social-prescribing/. [Google Scholar]
- 26.United Way BC. Rediscovering purpose, connection, and creativity in retirement years through social prescribing in British Columbia [Internet]. 2023. [cited August 14, 2024]. Available from: https://uwbc.ca/stories/2023/rediscovering-purpose-connection-and-creativity-in-retirement-years-through-social-prescribing-in-british-columbia/. [Google Scholar]
- 27.Holding E, Thompson J, Foster A, Haywood A. Connecting communities: A qualitative investigation of the challenges in delivering a national social prescribing service to reduce loneliness. Health & Social Care in the Community. 2020;28(5):1535–43. Epub 20200312. doi: 10.1111/hsc.12976 ; PubMed Central PMCID: PMC7496112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turk A, Tierney S, Wong G, Todd J, Chatterjee HJ, Mahtani KR. Self-growth, wellbeing and volunteering—implications for social prescribing: a qualitative study. SSM—Qualitative Research in Health. 2022;2:100061. doi: 10.1016/j.ssmqr.2022.100061 [DOI] [Google Scholar]
- 29.Nichol B, Wilson R, Rodrigues A, Haighton C. Exploring the effects of volunteering on the social, mental, and physical health and well-being of volunteers: an umbrella review. Voluntas: International Journal of Voluntary and Nonprofit Organizations. 2024;35(1):97–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zisman-Ilani Y, Hayes D, Fancourt D. Promoting social prescribing in psychiatry–using shared decision-making and peer support. JAMA Psychiatry. 2023;80(8):759–60. doi: 10.1001/jamapsychiatry.2023.0788 ; PubMed Central PMCID: PMC10529310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottershead R, Alonaizi N. Empowering social prescribing and peer support: a proposed therapeutic alliance against addiction and sustance misuse within the Middle East. Journal of Drug and Alcohol Research. 2022;11(11):236208. [Google Scholar]
- 32.Bertotti M, Frostick C, Hutt P, Sohanpal R, Carnes D. A realist evaluation of social prescribing: an exploration into the context and mechanisms underpinning a pathway linking primary care with the voluntary sector. Primary Health Care Research and Development. 2018;19(3):232–45. Epub 20171207. doi: 10.1017/S1463423617000706 ; PubMed Central PMCID: PMC5904290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tierney S, Wong G, Roberts N, Boylan AM, Park S, Abrams R, et al. Supporting social prescribing in primary care by linking people to local assets: a realist review. BMC Medicine. 2020;18(1):49. Epub 20200313. doi: 10.1186/s12916-020-1510-7 ; PubMed Central PMCID: PMC7068902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis J, Fischl AH, Beck J, Browning L, Carter A, Condon JE, et al. 2022 national standards for diabetes self-management education and support. Science of Diabetes Self-Management and Care. 2022;48(1):44–59. Epub 20220120. doi: 10.1177/26350106211072203 . [DOI] [PubMed] [Google Scholar]
- 35.Wildman J, Wildman JM. Evaluation of a community health worker social prescribing program among UK patients with type 2 diabetes. JAMA Network Open. 2021;4(9):e2126236. Epub 20210901. doi: 10.1001/jamanetworkopen.2021.26236 ; PubMed Central PMCID: PMC8411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner A, Hartfiel N, Lynch M, Jones AW, Edwards RT. Social return on investment of social prescribing via a diabetes technician for preventing type 2 diabetes progression. International Journal of Environmental Research and Public Health. 2023;20(12). Epub 20230607. doi: 10.3390/ijerph20126074 ; PubMed Central PMCID: PMC10298404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffatt S, Wildman J, Pollard TM, Gibson K, Wildman JM, O’Brien N, et al. Impact of a social prescribing intervention in North East England on adults with type 2 diabetes: the SPRING_NE multimethod study. Public Health Research (Southampton). 2023;11(2):1–185. doi: 10.3310/AQXC8219 . [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick SL. Social prescribing integrated in primary care enhances access to type 2 diabetes preventative approaches among high-risk patient population. Evidence Based Nursing. 2024;27(2):66. Epub 20240328. doi: 10.1136/ebnurs-2023-103765 . [DOI] [PubMed] [Google Scholar]
- 39.Calderon-Larranaga S, Greenhalgh T, Clinch M, Robson J, Dostal I, Eto F, et al. Unravelling the potential of social prescribing in individual-level type 2 diabetes prevention: a mixed-methods realist evaluation. BMC Medicine. 2023;21(1):91. Epub 20230313. doi: 10.1186/s12916-023-02796-9 ; PubMed Central PMCID: PMC10008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilkington K, Loef M, Polley M. Searching for real-world effectiveness of health care innovations: scoping study of social prescribing for diabetes. Journal of Medical Internet Research. 2017;19(2):e20. Epub 20170202. doi: 10.2196/jmir.6431 ; PubMed Central PMCID: PMC5314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Journal of the American Pharmacists Association. 2020;60(6):e1–e18. Epub 20200608. doi: 10.1016/j.japh.2020.04.018 . [DOI] [PubMed] [Google Scholar]
- 42.Skinner TC, Cradock S, Arundel F, Graham W. Four theories and a philosophy: self-management education for individuals newly diagnosed with type 2 diabetes. Diabetes Spectrum. 2003;16(2):75–80. doi: 10.2337/diaspect.16.2.75 [DOI] [Google Scholar]
- 43.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. International Journal of Surgery 2021;88:105906. Epub 20210329. doi: 10.1016/j.ijsu.2021.105906 . [DOI] [PubMed] [Google Scholar]
- 44.Garritty C, Hamel C, Trivella M, Gartlehner G, Nussbaumer-Streit B, Devane D, et al. Updated recommendations for the Cochrane rapid review methods guidance for rapid reviews of effectiveness. BMJ. 2024;384:e076335. doi: 10.1136/bmj-2023-076335 [DOI] [PubMed] [Google Scholar]
- 45.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. Epub 20190828. doi: 10.1136/bmj.l4898 . [DOI] [PubMed] [Google Scholar]
- 46.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. Epub 20200116. doi: 10.1136/bmj.l6890 ; PubMed Central PMCID: PMC7190266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heisler M, Choi H, Mase R, Long JA, Reeves PJ. Effectiveness of technologically enhanced peer support in improving glycemic management among predominantly African American, low-income adults with diabetes. Diabetes Educator. 2019;45(3):260–71. Epub 20190426. doi: 10.1177/0145721719844547 ; PubMed Central PMCID: PMC6750000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu YJ, Chen YH, Lin KD, Lee MY, Lee YL, Yu CK, et al. Clinical outcomes and oral health-related quality of life after periodontal treatment with community health worker strategy in patients with type 2 diabetes: a randomized controlled study. International Journal of Environmental Research and Public Health. 2021;18(16):8371. Epub 20210807. doi: 10.3390/ijerph18168371 ; PubMed Central PMCID: PMC8394731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JC, Sui Y, Oldenburg B, Zhang Y, Chung HH, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Internal Medicine. 2014;174(6):972–81. doi: 10.1001/jamainternmed.2014.655 . [DOI] [PubMed] [Google Scholar]
- 50.Long JA, Ganetsky VS, Canamucio A, Dicks TN, Heisler M, Marcus SC. Effect of peer mentors in diabetes self-management vs usual care on outcomes in US veterans with type 2 diabetes: a randomized clinical trial. JAMA Network Open. 2020;3(9):e2016369. Epub 20200901. doi: 10.1001/jamanetworkopen.2020.16369 ; PubMed Central PMCID: PMC7489832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampson M, Clark A, Bachmann M, Garner N, Irvine L, Howe A, et al. Effects of the Norfolk diabetes prevention lifestyle intervention (NDPS) on glycaemic control in screen-detected type 2 diabetes: a randomised controlled trial. BMC Medicine. 2021;19(1):183. Epub 20210819. doi: 10.1186/s12916-021-02053-x ; PubMed Central PMCID: PMC8375190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sazlina SG, Browning CJ, Yasin S. Effectiveness of personalized feedback alone or combined with peer support to improve physical activity in sedentary older Malays with type 2 diabetes: a randomized controlled trial. Frontiers in Public Health. 2015;3:178. Epub 20150713. doi: 10.3389/fpubh.2015.00178 ; PubMed Central PMCID: PMC4500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siminerio L, Ruppert KM, Gabbay RA. Who can provide diabetes self-management support in primary care? Findings from a randomized controlled trial. Diabetes Educator. 2013;39(5):705–13. Epub 20130619. doi: 10.1177/0145721713492570 . [DOI] [PubMed] [Google Scholar]
- 54.Sreedevi A, Unnikrishnan AG, Karimassery SR, Deepak KS. The effect of yoga and peer support interventions on the quality of life of women with diabetes: results of a randomized controlled trial. Indian Journal of Endocrinology and Metabolism. 2017;21(4):524–30. doi: 10.4103/ijem.IJEM_28_17 ; PubMed Central PMCID: PMC5477438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang TS, Afshar R, Elliott T, Kong J, Gill S. From clinic to community: a randomized controlled trial of a peer support model for adults with type 2 diabetes from specialty care settings in British Columbia. Diabetic Medicine. 2022;39(11):e14931. Epub 20220902. doi: 10.1111/dme.14931 . [DOI] [PubMed] [Google Scholar]
- 56.Yeung RO, Cai JH, Zhang Y, Luk AO, Pan JH, Yin J, et al. Determinants of hospitalization in Chinese patients with type 2 diabetes receiving a peer support intervention and JADE integrated care: the PEARL randomised controlled trial. Clinical Diabetes and Endocrinology. 2018;4(1):5. Epub 20180307. doi: 10.1186/s40842-018-0055-6 ; PubMed Central PMCID: PMC5842642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayala GX, Ibarra L, Cherrington AL, Parada H, Horton L, Ji M, et al. Puentes hacia una mejor vida (bridges to a better life): outcome of a diabetes control peer support intervention. The Annals of Family Medicine. 2015;13(Suppl 1):S9–S17. doi: 10.1370/afm.1807 ; PubMed Central PMCID: PMC4648133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x ; PubMed Central PMCID: PMC1495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ting RZ, Nan H, Yu MW, Kong AP, Ma RC, Wong RY, et al. Diabetes-related distress and physical and psychological health in Chinese type 2 diabetic patients. Diabetes Care. 2011;34(5):1094–6. Epub 20110311. doi: 10.2337/dc10-1612 ; PubMed Central PMCID: PMC3114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–50. doi: 10.2337/diacare.23.7.943 . [DOI] [PubMed] [Google Scholar]
- 61.Shiu AT, Martin CR, Thompson DR, Wong RY. Psychometric properties of the Chinese version of the diabetes empowerment scale. Psychology, Health & Medicine. 2006;11(2):198–208. doi: 10.1080/13548500500286845 . [DOI] [PubMed] [Google Scholar]
- 62.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007 . [DOI] [PubMed] [Google Scholar]
- 63.Taouk Moussa M, Lovibond P, Laube R. Psychometric properties of a Chinese version of the short Depression Anxiety Stress Scales (DASS21). Sydney (AU): New South Wales Transcultural Mental Health Centre: 2001. [Google Scholar]
- 64.Gao F, Ng GY, Cheung YB, Thumboo J, Pang G, Koo WH, et al. The Singaporean English and Chinese versions of the EQ-5D achieved measurement equivalence in cancer patients. Journal of Clinical Epidemiology. 2009;62(2):206–13. Epub 20080710. doi: 10.1016/j.jclinepi.2008.03.007 . [DOI] [PubMed] [Google Scholar]
- 65.Barrera M, Jr., Glasgow RE, McKay HG, Boles SM, Feil EG. Do Internet-based support interventions change perceptions of social support? An experimental trial of approaches for supporting diabetes self-management. American Journal of Community Psychology. 2002;30(5):637–54. doi: 10.1023/A:1016369114780 . [DOI] [PubMed] [Google Scholar]
- 66.Kuo H-C, Chen J-H, Wu J-H, Chou T-M, Yang Y-H. Application of the oral health impact profile (OHIP) among Taiwanese elderly. Quality of Life Research. 2011;20:1707–13. doi: 10.1007/s11136-011-9901-z [DOI] [PubMed] [Google Scholar]
- 67.Yuen HK, Wolf BJ, Bandyopadhyay D, Magruder KM, Salinas CF, London SD. Oral health knowledge and behavior among adults with diabetes. Diabetes Research and Clinical Practice. 2009;86(3):239–46. doi: 10.1016/j.diabres.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Medical Care. 2003;41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C . [DOI] [PubMed] [Google Scholar]
- 69.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Annals of Family Medicine. 2008;6(3):246–52. doi: 10.1370/afm.842 ; PubMed Central PMCID: PMC2384991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Quality of Life Research. 1999;8(1–2):79–91. doi: 10.1023/a:1026485130100 . [DOI] [PubMed] [Google Scholar]
- 71.Bijl JV, Poelgeest-Eeltink AV, Shortridge-Baggett L. The psychometric properties of the diabetes management self-efficacy scale for patients with type 2 diabetes mellitus. Journal of Advanced Nursing. 1999;30(2):352–9. doi: 10.1046/j.1365-2648.1999.01077.x . [DOI] [PubMed] [Google Scholar]
- 72.Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes/Metabolism Research and Reviews. 2002;18 Suppl 3(S3):S64-9. doi: 10.1002/dmrr.279 . [DOI] [PubMed] [Google Scholar]
- 73.Shannon J, Kristal AR, Curry SJ, Beresford SA. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiology, Biomarkers & Prevention. 1997;6(5):355–61. . [PubMed] [Google Scholar]
- 74.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB . [DOI] [PubMed] [Google Scholar]
- 75.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6 . [DOI] [PubMed] [Google Scholar]
- 76.Bradley C. The Well-being Questionnaire. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood Academic Publishers; 1994. [Google Scholar]
- 77.Resnick B, Luisi D, Vogel A, Junaleepa P. Reliability and validity of the self-efficacy for exercise and outcome expectations for exercise scales with minority older adults. Journal of Nursing Measurement. 2004;12(3):235–47. doi: 10.1891/jnum.12.3.235 . [DOI] [PubMed] [Google Scholar]
- 78.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52(1):30–41. [DOI] [PubMed] [Google Scholar]
- 79.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of Clinical Epidemiology. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4 . [DOI] [PubMed] [Google Scholar]
- 80.Goldberg DP, Williams P, University of London Institute of P. A user’s guide to the General Health Questionnaire: NFER-NELSON; 1988. [Google Scholar]
- 81.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003 . [DOI] [PubMed] [Google Scholar]
- 82.Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–60. doi: 10.2337/diacare.18.6.754 . [DOI] [PubMed] [Google Scholar]
- 83.Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self-efficacy. Diabetes Care. 2000;23(6):739–43. doi: 10.2337/diacare.23.6.739 . [DOI] [PubMed] [Google Scholar]
- 84.Sreedevi A, Cherkil S, Kuttikattu DS, Kamalamma L, Oldenburg B. Validation of WHOQOL-BREF in Malayalam and determinants of quality of life among people with type 2 diabetes in Kerala, India. Asia Pacific Journal of Public Health. 2016;28(1_suppl):62S–9S. doi: 10.1177/1010539515605888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31. doi: 10.2337/diacare.28.3.626 . [DOI] [PubMed] [Google Scholar]
- 86.Tang TS, Afshar R, Elliott T, Kong J, Gill S. Study protocol and baseline sample characteristics: from clinic to community: using peer support as a transition model for improving long-term diabetes-related health outcomes. Contemporary Clinical Trials. 2019;79:104–10. doi: 10.1016/j.cct.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 87.Sherifali D, da Silva LP, Dewan P, Cader FA, Dakhil Z, Gyawali B, et al. Peer support for type 2 diabetes management in low- and middle-income countries (LMICs): a scoping review. Global Heart. 2024;19(1):20. Epub 20240220. doi: 10.5334/gh.1299 ; PubMed Central PMCID: PMC10885823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu S, Leduc N, Moullec G. Type 2 diabetes peer support interventions as a complement to primary care settings in high-income nations: a scoping review. Patient Education and Counseling. 2022;105(11):3267–78. doi: 10.1016/j.pec.2022.08.010 [DOI] [PubMed] [Google Scholar]
- 89.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine. 2013;46(1):81–95. Epub 2013/03/21. doi: 10.1007/s12160-013-9486-6 . [DOI] [PubMed] [Google Scholar]
- 90.Evans M, Daaleman T, Fisher EB. Peer support for chronic medical conditions. In: Avery JD, editor. Peer Support in Medicine. Cham: Springer International Publishing; 2021. p. 49–69. [Google Scholar]
- 91.Schleiff MJ, Aitken I, Alam MA, Damtew ZA, Perry HB. Community health workers at the dawn of a new era: 6. recruitment, training, and continuing education. Health Research Policy and Systems. 2021;19(Suppl 3):113. Epub 20211012. doi: 10.1186/s12961-021-00757-3 ; PubMed Central PMCID: PMC8506097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou S, Kodama Muscente K. Meta-analysis of volunteer motives using the Volunteer Functions Inventory to predict volunteer satisfaction, commitment, and behavior. Nonprofit and Voluntary Sector Quarterly. 2022;52(5):1331–56. doi: 10.1177/08997640221129540 [DOI] [Google Scholar]
- 93.Sellon AM. Recruiting and retaining older adults in volunteer programs: best practices and next steps. Ageing International. 2014;39:421–37. [Google Scholar]
- 94.Moghaddam HR, Allahverdipour H, Matlabi H. Successful recruitment and retention strategies for women health volunteers: viewpoints of the volunteers’ supervisors and relevant researchers. Journal of Multidisciplinary Healthcare. 2018;11:621–34. Epub 20181026. doi: 10.2147/JMDH.S180544 ; PubMed Central PMCID: PMC6208547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McBride AM, Gonzales E, Morrow‐Howell N, McCrary S. Stipends in volunteer civic service: inclusion, retention, and volunteer benefits. Public Administration Review. 2011;71(6):850–8. doi: 10.1111/j.1540-6210.2011.02419.x [DOI] [Google Scholar]
- 96.Dallmeyer S, Breuer C, Feiler S. To pay or not to pay? The effects of monetary compensation on volunteers in sports clubs. Nonprofit and Voluntary Sector Quarterly. 2023;53(5):1181–204. doi: 10.1177/08997640231210456 [DOI] [Google Scholar]
- 97.Noguchi T, Hayashi T, Kubo Y, Tomiyama N, Ochi A, Hayashi H. Living alone and depressive symptoms among older adults in the COVID-19 pandemic: role of non-face-to-face social interactions. Journal of the American Medical Directors Association. 2023;24(1):17–21 e4. Epub 20221028. doi: 10.1016/j.jamda.2022.10.014 ; PubMed Central PMCID: PMC9613798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Newson M, Zhao Y, Zein ME, Sulik J, Dezecache G, Deroy O, et al. Digital contact does not promote wellbeing, but face-to-face contact does: a cross-national survey during the COVID-19 pandemic. New Media & Society. 2024;26(1):426–49. Epub 20211207. doi: 10.1177/14614448211062164 ; PubMed Central PMCID: PMC10758341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aspalter C. and Asian health care systems in comparative perspective. In: Aspalter C, Uchida Y, Gauld R, editors. Health Care Systems in Europe and Asia. 1 ed: Routledge; 2011. p. 167–90. [Google Scholar]
- 100.Beckfield J, Olafsdottir S, Sosnaud B. Healthcare systems in comparative perspective: classification, convergence, institutions, inequalities, and five missed turns. Annu Rev Sociol. 2013;39:127–46. Epub 20130517. doi: 10.1146/annurev-soc-071312-145609 ; PubMed Central PMCID: PMC5536857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beller J, Wagner A. Loneliness and health: the moderating effect of cross-cultural individualism/collectivism. Journal of Aging and Health. 2020;32(10):1516–27. doi: 10.1177/0898264320943336 [DOI] [PubMed] [Google Scholar]
- 102.Kilgarriff-Foster A O ’Cathain A. Exploring the components and impact of social prescribing. Journal of Public Mental Health. 2015;14(3):127–34. doi: 10.1108/jpmh-06-2014-0027 2177166732. [DOI] [Google Scholar]
- 103.Morse DF, Sandhu S, Mulligan K, Tierney S, Polley M, Giurca BC, et al. Global developments in social prescribing. BMJ Global Health. 2022;7(5):e008524. doi: 10.1136/bmjgh-2022-008524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The initial searches were completed in November 2023 and updated in May 2024.
(PDF)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.