Abstract
Objective
Predicting neonatal survival is essential for targeting interventions to reduce neonatal mortality. Pacific Islanders have been underrepresented in existing prediction tools and have unique, maternal obesity-related risk factors for both preterm birth and neonatal mortality. Using neonatal sex, birth weight, and gestational age, we developed a graphical tool for neonatal survival among Pacific Islander singletons in the United States.
Methods
Birth-infant death data files from the United States National Center for Health Statistics were used (2014–2018). Pacific Islander mothers and singletons without congenital anomalies born between 22–36 gestational weeks were included. Poisson regression models were used to predict neonatal mortality (<28 days of life) rate including neonatal sex, birth weight, and gestational age in weeks as predictors. Predicted survival rates in the graphical tool were calculated as "1 minus mortality rate”.
Results
Of the 5192 included neonates, the neonatal mortality rate was 2.0%; 43.5% of mothers had pre-pregnancy obesity, and 16.5% of neonates were born large-for-gestational age. Birth weight and gestational age had a non-linear association with neonatal death, and their interaction was included in the model. Retaining neonatal sex, models with gestational age at birth or both birth weight and gestational age at birth performed better than the model with birth weight only.
Conclusion
This is the first graphical tool for neonatal survival prediction among preterm-born Pacific Islander singletons in the United States. Using only neonatal sex, birth weight, and gestational age, this graphical tool is a straightforward reference for survival among groups of neonates with similar characteristics.
Introduction
Neonatal mortality (death within the first 28 days of life [1]) in the US declined from 4.1 per 1 000 live births in 2011 to 3.7 in 2019 (most recently available data from March of Dimes) [2]. However, during the same period, the prevalence of preterm birth (PTB, live birth at <37 gestational weeks [3]) increased from 9.8% to 10.2% [4], and the prevalence of low birth weight (LBW) increased from 8.1% to 8.3% [5]. Gestational age (GA) and birth weight (BW) are two important indicators for neonatal mortality and have been used in the development of tools to predict neonatal survival [6–8]. Although not used as a basis for individual care decisions, prediction of neonatal survival rate for groups of infants with certain characteristics may be informative for clinicians treating populations at high risk. These prediction modelling outcomes can provide population-based estimates of neonatal survival rate to further support clinicians’ assessments [9]. Graphical prognosis tools, including the original ‘Draper Grid’, are most commonly used for clinical reference, given their simple visualization of expected sex-specific survival based on gestational week and birth weight [6, 10, 11].
Racial disparities in infant mortality [2] (2017–2019: Black, 10.8 per 1 000 live births; White, 4.6 per 1 000 live births), preterm birth [4] (2018–2020: Black, 14.2%; White, 9.2%), and LBW [5] (2018–2020: Black, 13.8%; White, 7.0%) are evident in the US. One group currently underrepresented in perinatal research are Pacific Islanders. Data on the health of Pacific Islanders in the US is sparse, and frequent aggregation with Asian Americans exacerbates their underrepresentation. To our knowledge, no prognosis tool has either included a significant proportion of Pacific Islanders in their reference population, nor has a tool been specifically developed for this group. Current neonatal survival rate prediction tools have generally been designed for specific populations, like for Canadians, Australians, or for residents in the United Kingdom, for example [6, 7, 11–13]. Those tools do not transport well across populations due to different population characteristics and clinical practice variation [7, 14]. Based on trends observed in other populations, the unique health profile of Pacific Islanders—a high prevalence of overweight/obesity and obesity-related non-communicable diseases [15, 16] before and during pregnancy—is expected to place them at high risk of both preterm birth and neonatal death [17, 18]. Given their underrepresentation and frequent aggregation with other groups, understanding of the BW by GA distribution among Pacific Islanders is also limited, with studies reporting both higher prevalence of macrosomia among term births compared to other ethnic groups, and also greater incidence of low birth weight [19]. Here we aimed to develop a graphical tool for the prediction of survival rate among Pacific Islanders born between 22–36 weeks gestation in the US using neonatal sex, BW, and GA.
Materials and methods
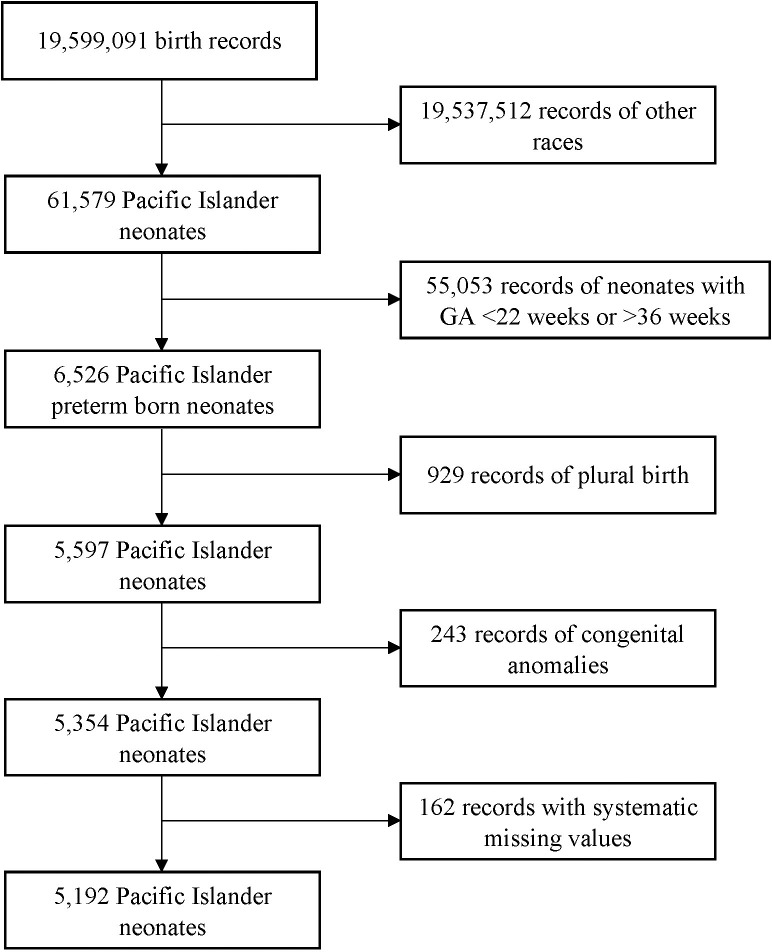
We used 2014–2018 cohort linked birth-infant death data files (corresponding to deaths that occurred between 2014–2019) from the US National Center for Health Statistics (NCHS) [20]. Our study population included preterm-born neonates (22–36 gestational weeks) whose mothers identified as Native Hawaiian or Other Pacific Islander. Plural births, neonates with congenital anomalies, and blank records with maternal race only were excluded. In total, data from n = 5192 neonates were included for the prediction of survival (Fig 1).
Fig 1. Flowchart of the sample selection from the 2014–2018 birth cohort birth-infant death data files.
GA, gestational age.
Exposure
Demographic characteristics of included mother-neonate dyads were obtained from birth and death certificates. Individual characteristics included GA (measured by obstetric estimate [the best estimate based on ultrasound-confirmed LMP, LMP (in the absence of early ultrasound] or early ultrasound [in the absence of LMP)]) at birth, degree of prematurity (extreme PTB 22–27 weeks, very PTB 28–31 weeks, moderate to late PTB 32–36 weeks) [3], BW and BW classification (I: LBW <2500 g, normal BW 2500–4000 g, macrosomia >4000 g [21]; II: small-for-GA [SGA], appropriate-for-GA [AGA], and large-for-GA [LGA], based on the 2017 US BW percentiles for singletons [22]), and neonatal sex at birth. Maternal characteristics included ethnicity (Hawaiian, Guamanian, Samoan, Other Pacific Islander), age (<20 years, 20–34 years, > = 35 years), pre-pregnancy body mass index (BMI, underweight <18.5, normal 18.5–24.9, overweight 25.0–29.9, obesity I 30.0–34.9, obesity II 35.0–39.9, extreme obesity III > = 40.0). In order to ensure the largest available sample size for analyses, missing values for pre-pregnancy BMI (5.3%) were imputed with maximum likelihood via expectation maximization (EM; one imputed dataset) [23].
Outcomes
Our primary outcome is neonatal mortality which is defined as death within the first 28 days of life [1]. Predicted survival rates in the graphical tool were based on "1 minus mortality rate”. Other birth outcomes including five-minute Appearance, Pulse, Grimace, Activity and Respiration (APGAR) score [24] and final route and method of birth were also reported to characterize the study population.
Statistical analysis
We first described the general preterm birth prevalence among all Pacific Islander neonates and neonatal mortality rate among Pacific Islander preterm singletons without congenital anomalies in the US 2014–2018 birth cohort. To characterize the study population, we then examined neonatal sex-distributed basic demographic characteristics, five-minute APGAR score and final route and method of birth. Data were presented as numbers and percentages for categorical variables, and as medians with lower (Q1) and upper (Q3) quartiles for non-normally distributed continuous variables. Differences by neonatal sex were examined using x2 tests or Fisher’s exact tests for categorical variables and with exact Wilcoxon two-sample tests for continuous variables. Monte Carlo estimations of exact P-values were presented when continuous variables were not normally distributed [25]. We also described the frequency distribution of included neonates by both BW (in increments of 250 g) and GA (in increments of 1 week) stratified by sex (S1 Fig).
Poisson regression models (link = log) were used to predict the neonatal mortality rate using neonatal sex at birth, BW, and GA in weeks as predictors. We tested their independent effects, as well as their statistical interactions (retained in the model at a two-sided α of 0.10). The final three models, adjusted by neonatal sex, were BW-based only, GA-based only, and BW with GA and their interaction. In the context of risk prediction, any multicollinearity between BW and GA would not influence the mortality rate predictions and their precision or the goodness-of-fit statistics [26]. The model fit of competing models was assessed by Akaike Information Criterion (AIC) [27]. Due to the curve-linear association between log-scaled mortality rate and predictors BW and GA, the latter two were transformed to the square root form prior to being used in the regressions in order to preserve the linear combination of regression parameters in the models. Overdispersion was assessed by Pearson Chi-square / degree of freedom (>2 indicating overdispersion [28]); the scaling parameter was set to 1 when overdispersion occurred. The plot of the observed moving sum of residuals against the predicted values assessed the functional form of our regression models [29], with a large probability indicating a correctly specified model for the mean response. Predictive accuracy was evaluated using Receiver Operating Characteristic (ROC) curves [30] which measure how well the model discriminates between neonates with or without mortality (>0.80 indicating a good discrimination), as well as with decile calibration plots [31] which measure how well the predicted probabilities of mortality agree with the observed probabilities of mortality. The internal validity of the models was assessed using bootstrapping with 500-samples with replacement [32]. Predicted neonatal survival rates were calculated from “1 minus neonatal mortality rate” and were presented as Draper Grids [10]. The presented predicted survival rate in each cell is at the midpoint of the cell (week of GA plus 0.5 week and BW midpoint). For ease of interpretation, the US 3rd, 10th, 25th, 50th, 75th, 90th, 97th BW percentiles for GA [22] were added to the plot. Analyses were performed using R software (version 3.6.2) [33], RStudio [34] and SAS software version 9.4 (SAS Institute Inc.). Per Yale University institutional review board policy, no research ethics board approval was required for the use of this deidentified, public data.
Results
A total of 5192 preterm-born (22–36 gestational weeks) Pacific Islander neonates without congenital anomalies were included in survival rate prediction models, including 2428 females (46.8%) and 2764 males (53.2%). The averaged preterm birth prevalence among the study population was 9.3%. Within the study population, the neonatal mortality rate among preterm-born singletons without congenital anomalies was 20.8 per 1 000 preterm births.
Length of gestation and BW were not observed to be different between female and male Pacific Islander neonates (Table 1). Among female neonates, 7.5% were SGA and 17.8% were LGA, and among male neonates, 8.5% were SGA and 15.3% were LGA, using the 2017 US reference data for singletons [22], which indicated that Pacific Islander neonates had a relatively larger BW compared to US neonates of other racial/ethnic groups. A more detailed distribution of the study population by BW and GA stratified by sex is presented in S1 Fig. Most Pacific Islander mothers identified as ‘Other’ Pacific Islanders (>60.0%). Adolescent mothers were 6.9% of all included mothers, and the percentage of mothers with advanced age (> = 35 years) was 20.0%. Of the included mothers, 29.6% had a pre-pregnancy BMI indicative of overweight, and 43.5% had obesity. Most preterm-born neonates included in the study population had a five-minute APGAR score of 9–10. The majority were born via spontaneous vaginal birth (55.8%), although Caesarean births accounted for 41.8% of all births.
Table 1. Characteristics of Included Pacific Islander mother-neonate dyads in the US, 2014–2018 birth cohort.
| Characteristics | Female (n = 2 428) | Male (n = 2 764) | P-value | |||
|---|---|---|---|---|---|---|
| N or median | % or Q1-Q3 | N or median | % or Q1-Q3 | |||
| Neonatal death | 41 | 1.7 | 65 | 2.4 | 0.09 | |
| Gestational age (median, Q1-Q3, week) | 34.1 | 32.2–36.0 | 34 | 32.1–35.9 | 0.21 | |
| Preterm birth categories | ||||||
| Extreme PTB (22–27 weeks) | 134 | 5.5 | 133 | 4.8 | ||
| Very PTB (28–31 weeks) | 184 | 7.6 | 240 | 8.7 | ||
| Moderate to Late PTB (32–36 weeks) | 2110 | 86.9 | 2391 | 86.5 | 0.2 | |
| Birth weight categories (I, median, Q1-Q3, g) | 2456.9 | 1951.5–2962.4 | 2482.6 | 1983.8–2981.4 | 0.1 | |
| Low birth weight (<2500 g) | 1146 | 47.2 | 1276 | 46.2 | ||
| Normal weight (2500–4000 g) | 1241 | 51.1 | 1446 | 52.3 | ||
| Macrosomia (>4000 g) | 41 | 1.7 | 42 | 1.5 | 0.64 | |
| Birth weight categories (II) | ||||||
| Small for gestational age | 181 | 7.5 | 235 | 8.5 | ||
| Appropriate for gestational age | 1814 | 74.7 | 2105 | 76.2 | ||
| Large for gestational age | 433 | 17.8 | 424 | 15.3 | 0.03 | |
| Maternal ethnicity | ||||||
| Hawaiian | 241 | 9.9 | 254 | 9.2 | ||
| Guamanian | 283 | 11.7 | 307 | 11.1 | ||
| Samoan | 403 | 16.6 | 498 | 18 | ||
| Other Pacific Islanders | 1501 | 61.8 | 1705 | 61.7 | 0.46 | |
| Maternal age (median, Q1-Q3, year) | 28.6 | 24.3–32.8 | 28.6 | 24.2–32.9 | 0.95 | |
| <20 years | 165 | 6.8 | 194 | 7 | ||
| 20–34 years | 1785 | 73.5 | 2011 | 72.8 | ||
| > = 35 years | 478 | 19.7 | 559 | 20.2 | 0.83 | |
| Maternal pre-pregnancy BMI (median, Q1-Q3, km/m2) | 29.7 | 24.9–34.5 | 29.8 | 24.9–34.7 | 0.81 | |
| Underweight (<18.5) | 64 | 2.6 | 66 | 2.4 | ||
| Normal (18.5–24.9) | 586 | 24.1 | 680 | 24.6 | ||
| Overweight (25.0–29.9) | 730 | 30.1 | 806 | 29.2 | ||
| Obesity I (30.0–34.9) | 557 | 22.9 | 627 | 22.7 | ||
| Obesity II (35.0–39.9) | 282 | 11.6 | 333 | 12.1 | ||
| Extreme Obesity III (> = 40.0) | 209 | 8.6 | 252 | 9.1 | 0.92 | |
| Five-minute APGAR scorea | ||||||
| A score of 0–3 | 55 | 2.3 | 69 | 2.5 | ||
| A score of 4–6 | 131 | 5.5 | 185 | 6.7 | ||
| A score of 7–8 | 689 | 28.7 | 736 | 26.8 | ||
| A score of 9–10 | 1529 | 63.6 | 1758 | 64 | 0.15 | |
| Final route and method of birtha | ||||||
| Spontaneous | 1369 | 56.4 | 1530 | 55.4 | ||
| Forceps | 16 | 0.7 | 24 | 0.9 | ||
| Vacuum | 27 | 1.1 | 53 | 1.9 | ||
| Caesarean | 1015 | 41.8 | 1157 | 41.9 | 0.09 | |
Q1, lower quartile; Q3, upper quartile; BMI, body mass index; GWG, gestational weight gain.
a Missing value for labelled variables: five-minute APGAR score (n = 40), final route and method of birth (n = 1).
Parameter estimates, model fit statistics, and model prediction statistics are presented for three neonate sex-adjusted competing models: GA-based, BW-based, and BW-GA-based models (Table 2). For descriptions of models where additional interactions were tested, see S1 File. The value of Pearson Chi-square/DF indicates that overdispersion was evident in the BW-based model, but not in the GA-based and BW-GA models. Comparing the AIC values, the GA-based and BW-GA-based models had the smallest values, indicating their better fit to the data than the BW-based model (Table 3).
Table 2. Poisson regression models for neonatal mortality rate among selected Pacific Islander neonates in the 2014–2018 US birth cohort.
| Model | Formula | Pearson Chi-square/DF |
|---|---|---|
| GA-based model | 1.007 | |
| BW-based modela | 2.799 | |
| BW-GA-based model | 0.920 |
GA, gestational age. BW, birth weight. DF, degree of freedom.
a Scaling parameter for labelled models was set to 1 due to overdispersion (Pearson Chi-square/DF>2).
Table 3. Parameter estimates, model fit, and predicted accuracy of Poisson regression models for neonatal mortality rate among selected Pacific Islander neonates in the 2014–2018 US birth cohort.
| Model | Coef | Est | SE | 95% CI | P-value | AIC | Non-bootstrapped | Bootstrapped | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ROC-AUC | ROC-AUC 95% CI | ROC-AUC | ROC-AUC 95% CI | ||||||||
| GA-based model | β 0 | 19.827 | 1.175 | 17.525 | 22.129 | <0.001 | 676.524 | 0.897 | 0.859–0.935 | 0.897 | 0.895–0.899 |
| β 1 | -0.274 | 0.200 | -0.665 | 0.117 | 0.170 | ||||||
| β 2 | -4.258 | 0.228 | -4.704 | -3.811 | <0.001 | ||||||
| BW-based modela | β 0 | 3.168 | 0.581 | 2.029 | 4.307 | <0.001 | 715.439 | 0.879 | 0.834–0.923 | 0.879 | 0.877–0.882 |
| β 1 | -0.413 | 0.334 | -1.067 | 0.241 | 0.216 | ||||||
| β 2 | -0.168 | 0.017 | -0.201 | -0.135 | <0.001 | ||||||
| BW-GA- based model | β 0 | 26.125 | 5.083 | 16.164 | 36.087 | <0.001 | 676.927 | 0.895 | 0.856–0.935 | 0.896 | 0.893–0.898 |
| β 1 | -0.295 | 0.201 | -0.689 | 0.098 | 0.141 | ||||||
| β 2 | -5.238 | 1.011 | -7.219 | -3.256 | <0.001 | ||||||
| β 3 | -0.307 | 0.167 | -0.634 | 0.020 | 0.066 | ||||||
| β 4 | 0.051 | 0.029 | -0.006 | 0.107 | 0.078 | ||||||
Coef, coefficient. Est, estimate. SE, standard error. 95% CI, 95% confidence interval. AIC, Akaike information criterion. ROC-AUC, area under the Receiver Operating Characteristic curve. GA, gestational age. BW, birth weight. DF, degree of freedom.
a Scaling parameter for labelled models was set to 1 due to overdispersion (Pearson Chi-square/DF>2).
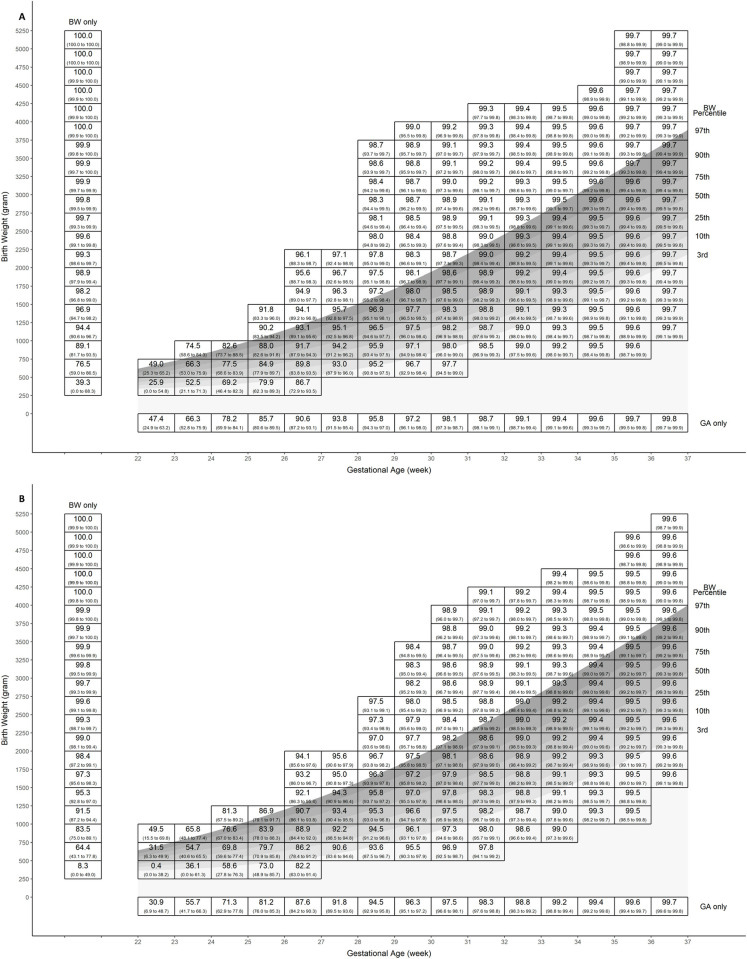
The observed moving sum of residuals plot for BW-GA-based model (p = 0.82) depicted a random pattern indicating that the log-link for the mean response as well as the selected covariates adequately captured neonatal mortality, but not so for the BW-based (p = 0.001) and GA-based (p = 0.09) models (S2 Fig). For the ROC value, all three models had a value >0.80 indicating a good discrimination, which was also confirmed in the bootstrapped sample (Table 3), with the highest performance by GA-based and BW-GA-based models. For the decile calibration plot (S3 and S4 Figs), using the non-bootstrapped sample, all three models had a wider 95% confidence interval (CI) of the agreement between the predicted and observed probabilities at higher probabilities of mortality, but in both the non-bootstrapped and the bootstrapped samples, the BW-GA-based model had the best calibration. Predicted survival rates from the BW-GA-based model are presented in Fig 2 stratified by neonatal sex. Female neonates had a relatively higher survival rate compared to male neonates, although the 95% confidence intervals overlapped given the same BW and GA.
Fig 2. Grid of predicted neonatal survival rate according to sex, birth weight, and gestational age (BW-GA-based model).
The point estimate in each cell is the predicted survival rate at the midpoint of the cell. The numbers in parentheses are lower and upper limit of predicted survival rate within each cell. A, female plot. B, male plot. BW, birth weight; GA, gestational age.
Discussion
To our knowledge, this is the first study to predict neonatal survival rate among Pacific Islanders in the US. Using obstetric estimate as the GA measurement method, the averaged preterm birth prevalence for singletons without congenital anomalies was 9.3% among Pacific Islander neonates from the 2014–2018 birth cohort in the US, which is higher than the prevalence among the general US population (7.6% to 8.1% using the same dataset, 2014–2018 with the same GA measurement method) [35]. Within our Pacific Islander population, the neonatal mortality rate among preterm-born singletons without congenital anomalies was 20.8 per 1 000 preterm births, which is similar to the rate among comparable White preterm-born singletons (19.2 per 1 000 preterm births) in the US [35]. As we hypothesized, the prevalence of pre-pregnancy obesity (BMI > = 30) among Pacific Islander women (43.5%) was higher than among non-Hispanic White (39.8%) women in the US [36]. Likely as an associated complication, Pacific Islander neonates also had a relatively larger BW compared to neonates of other races based on a large proportion of the study population being born LGA (16.5%).
Among Pacific Islander singletons without congenital anomalies, GA was the most important predictor of neonatal death. When using BW without GA, the increased value of AIC, the decreased value of ROC-AUC (for non-bootstrapped and bootstrapped samples), as well as a systematic trend on the observed moving sum of residuals, indicated the poorer performance of BW alone in predicting neonatal survival among infants born to Pacific Islander women. This is consistent with another study, conducted in Australia and New Zealand, which also reported a decreased discriminatory power when BW was included in the model for neonatal mortality [7]. This is to be expected given the heterogeneity in weight present at any given week of gestation. While the GA model has the best fit (smallest AIC), the model that also incorporates BW and their interaction shows better calibration while maintaining the same discriminatory power, indicating the value of birthweight when used in combination with GA. Even though neonatal sex did not reach the statistical threshold for the alpha-level, it was still retained in our model due to different birth weight for gestational age at birth [22] and neonatal mortality rate [37] by sex. Furthermore, it is plausible that with additional years of data from NCHS, a larger sample size will confirm the clinical importance of neonatal sex on neonatal survival and clear the statistical threshold in this population of infants.
We did not attempt to compare the performance of the model developed here to previously published tools that describe mortality or survival among other populations [6, 7, 10–13]. Several previous studies [7, 14] suggest that such tools do not transport well across populations due to differences in population characteristics (such as the level of obesity among Pacific Islanders), clinical practice variation, diagnosis and case mix differences, and chance variation. This further underlines the importance of population-based specific tools for neonatal survival prediction.
Several limitations should be noted. Like any other model, our predictions of neonatal survival should not be used for diagnosis in any individual neonate. Meanwhile, the small sample size of extreme preterm births (n = 267) included in our study population may also influence our accuracy at the earliest gestational ages. Plural births and neonates with congenital anomalies were not included and are at a much higher risk of neonatal death compared to singletons or neonates without congenital anomalies [38]. Given our sample size, we did not have sufficient power to develop survival predictions for these groups and efforts should be made to repeat this analyses with additional years of data, building sufficient sample size to inform estimates for this group. Fetal growth among plural births is much more complicated and discordant growth may occur and influence perinatal outcomes [39]. Death due to congenital anomalies usually occurs very early in gestation [40], and may be recorded as a stillbirth or termination of pregnancy, both of which were not included in the linked infant datafiles.
The major strength of our study is that we used only three simple predictors for developing this prediction plot. Similar graphical neonatal death prognosis tools have been used widely in other populations [6, 10, 11] indicating the accuracy of using these three predictors only. Presenting our estimates in the style of Draper Grid is straight-forward and serves as a reference for healthcare providers conducting parent consultations [10, 11] or for health policy makers [41, 42]. Moreover, we used the most recent national-level data for Pacific Islanders in the US with GA measured by obstetric estimate, which increased the reliability of our results [43].
Conclusions
Pacific Islander mothers have a relatively higher prevalence of preterm birth, neonatal mortality rate, pre-pregnancy obesity, and LGA compared to White mothers in the US. To address underrepresentation of this group, and considering their unique risks for preterm birth, we developed a race-specific graphical tool to predict neonatal survival for Pacific Islander singletons without congenital anomalies born preterm in the US. With additional validation, this graphical tool may serve as a straight-forward reference to support discussions and decisions by clinicians or health policy makers.
Supporting information
(DOCX)
A, female plot. B, male plot. BW, birth weight; GA, gestational age.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
Acknowledgments
The authors would like to thank Lesley McCowan, Tormod Rogne, and James Dziura, who gave feedback on an earlier version of this manuscript.
Abbreviations
- APGAR
Appearance, Pulse, Grimace, Activity and Respiration
- AGA
Appropriate for gestational age
- AIC
Akaike Information Criterion
- BMI
Body mass index
- BW
Birth weight
- DF
Degree of freedom
- GA
Gestational age
- LGA
Large for gestational age
- LBW
Low birth weight
- Q1
Lower quartile
- ROC
Receiver Operating Characteristic
- SGA
Small for gestational age
- US
United States
- Q3
Upper quartile
- WHO
World Health Organization
Data Availability
This is a public dataset provided by the US National Center for Health Statistics. Data access: https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm#Births.
Funding Statement
This work was supported by US National Institutes of Health [(PI: Hawley, NLH, grant number R03HD093993)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Newborn Mortality. Available from: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-mortality-report-2021. Published 2022. Accessed July 2nd, 2022.
- 2.March of Dimes Perinatal Data Center. Mortality and morbidity. Available from: https://www.marchofdimes.org/peristats/data?reg=99&top=6&stop=107&lev=&slev=1&obj=1. Published 2020. Accessed July 13, 2022.
- 3.World Health Organization. Preterm birth. Available from: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth. Published 2018. Accessed Sept, 2020.
- 4.March of Dimes Perinatal Data Center. A profile of prematurity in United States. Available from: https://www.marchofdimes.org/peristats/tools/prematurityprofile.aspx?reg=99. Accessed July 13, 2022.
- 5.March of Dimes Perinatal Data Center. Birthweight. Available from: https://www.marchofdimes.org/peristats/data?top=4&lev=1&stop=43®=99&obj=1&slev=1. Accessed December 25, 2022.
- 6.Cole TJ, Hey E, Richmond S. The PREM score: a graphical tool for predicting survival in very preterm births. Arch Dis Child Fetal Neonatal Ed. 2010;95(1):F14–F19. doi: 10.1136/adc.2009.164533 [DOI] [PubMed] [Google Scholar]
- 7.Evans N, Hutchinson J, Simpson J, et al. Prenatal predictors of mortality in very preterm infants cared for in the Australian and New Zealand Neonatal Network. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F34–F40. doi: 10.1136/adc.2006.094169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel JP, Chawanpaiboon S, Moller A-B, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Gooi A, Oei J, Lui K. Attitudes of Level II obstetricians towards the care of the extremely premature infant: a national survey. J Paediatr Child Health. 2003;39(6):451–455. doi: 10.1046/j.1440-1754.2003.00187.x [DOI] [PubMed] [Google Scholar]
- 10.Draper ES, Manktelow B, Field DJ, et al. Prediction of survival for preterm births by weight and gestational age: retrospective population based study. Br Med J. 1999;319(7217):1093–1097. doi: 10.1136/bmj.319.7217.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manktelow BN, Seaton SE, Field DJ, et al. Population-based estimates of in-unit survival for very preterm infants. Pediatrics. 2013;131(2):e425–e432. doi: 10.1542/peds.2012-2189 [DOI] [PubMed] [Google Scholar]
- 12.Shah PS, Xiang YY, Synnes A, et al. Prediction of survival without morbidity for infants born at under 33 weeks gestational age: a user-friendly graphical tool. Arch Dis Child Fetal Neonatal Ed. 2012;97(2):F110–F115. doi: 10.1136/archdischild-2011-300143 [DOI] [PubMed] [Google Scholar]
- 13.Mubiri P, Nambuya H, Kajjo D, et al. Birthweight and gestational age‐specific neonatal mortality rate in tertiary care facilities in Eastern Central Uganda. Health Sci Rep. 2020;3(4):e196. doi: 10.1002/hsr2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson J, Evans N, Gibberd R, et al. Analysing differences in clinical outcomes between hospitals. BMJ Qual Saf. 2003;12(4):257–262. doi: 10.1136/qhc.12.4.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services Office of Minority Health. Obesity and Native Hawaiians/Pacific Islanders. Available from: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=85. Accessed July 13, 2022.
- 16.Aitaoto N, Ichiho HM. Assessing the health care system of services for non-communicable diseases in the US-affiliated Pacific Islands: a Pacific regional perspective. Hawaii J Med Public Health. 2013;72(5 Suppl 1):106. [PMC free article] [PubMed] [Google Scholar]
- 17.Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. Jama. 2014;311(15):1536–1546. doi: 10.1001/jama.2014.2269 [DOI] [PubMed] [Google Scholar]
- 18.McDonald SD, Han Z, Mulla S, et al. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Br Med J. 2010;341. doi: 10.1136/bmj.c3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suss R, Mahoney M, Arslanian KJ, et al. Pregnancy health and perinatal outcomes among Pacific Islander women in the United States and US Affiliated Pacific Islands: Protocol for a scoping review. PLoS One. 2022;17(1):e0262010. doi: 10.1371/journal.pone.0262010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US National Center for Health Statistics. Vital Statistics Online Data Portal. Available from: https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm#Births. Accessed March 15, 2022.
- 21.Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 22.Aris IM, Kleinman KP, Belfort MB, et al. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics. 2019;144(1). doi: 10.1542/peds.2019-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim C. Imputing Missing Data using SAS®. Available from: https://support.sas.com/resources/papers/proceedings15/3295-2015.pdf. Accessed July 14, 2022.
- 24.AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON FETUS NEWBORN, AMERICAN COLLEGE OF OBSTETRICIANS, GYNECOLOGISTS COMMITTEE ON OBSTETRIC PRACTICE, et al. The Apgar Score. Pediatrics. 2015;136(4):819–822.
- 25.Hope AC. A simplified Monte Carlo significance test procedure. J R Stat Soc Series B Stat Methodol. 1968;30(3):582–598. [Google Scholar]
- 26.Lieberman MG, Morris JD. The precise effect of multicollinearity on classification prediction. Multiple Linear Regression Viewpoints. 2014;40(1):5–10. [Google Scholar]
- 27.Akaike H. Information Theory and an Extension of the Maximum Likelihood Principle. 1973. [Google Scholar]
- 28.Cameron AC, Trivedi PK. Regression analysis of count data: Cambridge university press; 2013. [Google Scholar]
- 29.Gordon Johnston YS. Let the data speak: new regression diagnostics based on cumulative residuals. Available from: https://support.sas.com/resources/papers/proceedings/proceedings/sugi28/255-28.pdf. Accessed February 8, 2023. [Google Scholar]
- 30.Wicklin R. Create and compare ROC curves for any predictive model. Available from: https://blogs.sas.com/content/iml/2018/11/14/compare-roc-curves-sas.html. Published 2018. Accessed February 8, 2023. [Google Scholar]
- 31.Wicklin R. Decile calibration plots in SAS. Available from: https://blogs.sas.com/content/iml/2018/05/16/decile-calibration-plots-sas.html. Published 2018. Accessed February 8, 2023.
- 32.Efron B, Tibshirani R. Improvements on cross-validation: the 632+ bootstrap method. J Am Stat Assoc. 1997;92(438):548–560. [Google Scholar]
- 33.The R Project for Statistical Computing. Available from: https://www.r-project.org/. Accessed February 17, 2023.
- 34.RStudio. Available from: https://posit.co/. Accessed February 17, 2023.
- 35.Wu B, Taylor S, Shabanova V, et al. Birth-Based vs Fetuses-at-Risk Approaches for Assessing Neonatal Mortality Rate by Race. JAMA Pediatr. 2023;177(6):633–635. doi: 10.1001/jamapediatrics.2023.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig M. Hales MDC, Cheryl D. Fryar, Cynthia L. Ogden. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Available from: https://www.cdc.gov/nchs/products/databriefs/db360.htm#:~:text=(Figure%202).-,The%20prevalence%20of%20obesity%20was%20lowest%20among%20non%2DHispanic%20Asian,higher%20than%20all%20other%20groups. Published 2020. Accessed July 29, 2022. [PubMed]
- 37.Boghossian NS, Geraci M, Edwards EM, et al. Sex differences in mortality and morbidity of infants born at less than 30 weeks’ gestation. Pediatrics. 2018;142(6). doi: 10.1542/peds.2018-2352 [DOI] [PubMed] [Google Scholar]
- 38.Bachilova S, Czuzoj-Shulman N, Abenhaim HA. Effect of maternal and pregnancy risk factors on early neonatal death in planned home births delivering at home. J Obstet Gynaecol Can. 2018;40(5):540–546. doi: 10.1016/j.jogc.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 39.Breathnach FM, Malone FD. Fetal growth disorders in twin gestations. Semin Perinatol; 2012: Elsevier. p. 175–181. doi: 10.1053/j.semperi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 40.Boyle B, Addor M-C, Arriola L, et al. Estimating global burden of disease due to congenital anomaly: an analysis of European data. Arch Dis Child Fetal Neonatal Ed. 2018;103(1):F22–F28. doi: 10.1136/archdischild-2016-311845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holloway RG, Benesch CG, Burgin WS, et al. Prognosis and decision making in severe stroke. Jama. 2005;294(6):725–733. doi: 10.1001/jama.294.6.725 [DOI] [PubMed] [Google Scholar]
- 42.Hemingway H, Croft P, Perel P, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. Br Med J. 2013;346. doi: 10.1136/bmj.e5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin JA, Osterman M, Kirmeyer SE, et al. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep. 2015;64(5):1–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
A, female plot. B, male plot. BW, birth weight; GA, gestational age.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, GA-based model. B, BW-based model. C, BW-GA-based model. BW, birth weight; GA, gestational age. All models are adjusted by neonatal sex.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
A, Model 1. B, Model 2. C, Model 3. D, Model 4. E, Model 5.
(TIF)
Data Availability Statement
This is a public dataset provided by the US National Center for Health Statistics. Data access: https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm#Births.