Abstract
In the current global AIDS pandemic, more than half of new human immunodeficiency virus type 1 (HIV-1) infections are acquired by women through intravaginal HIV exposure. For this study, we explored pathogenesis issues relevant to the development of effective vaccines to prevent infection by this route, using an animal model in which female rhesus macaques were exposed intravaginally to a high dose of simian immunodeficiency virus (SIV). We examined in detail the events that transpire from hours to a few days after intravaginal SIV exposure through week 4 to provide a framework for understanding the propagation, dissemination, and establishment of infection in lymphatic tissues (LTs) during the acute stage of infection. We show that the mucosal barrier greatly limits the infection of cervicovaginal tissues, and thus the initial founder populations of infected cells are small. While there was evidence of rapid dissemination to distal sites, we also show that continuous seeding from an expanding source of production at the portal of entry is likely critical for the later establishment of a productive infection throughout the systemic LTs. The initially small founder populations and dependence on continuous seeding to establish a productive infection in systemic LTs define a small window of maximum vulnerability for the virus in which there is an opportunity for the host, vaccines, or other interventions to prevent or control infection.
The AIDS pandemic, already the most widespread pandemic in recorded human history, has claimed the lives of millions and continues relatively unabated for want of an effective vaccine or other means of prevention. Especially urgent is the need for effective vaccines and microbicides to prevent the vaginal transmission of human immunodeficiency virus type 1 (HIV-1), as women now account for close to 60 percent of newly acquired infections in Africa (27).
The simian immunodeficiency virus (SIV)/rhesus monkey model of vaginal HIV transmission is clearly relevant to this objective. It has been used extensively to test vaccines (1, 7, 12, 18, 22) and microbicides (16, 17, 19, 25, 28) designed to prevent vaginal transmission. Moreover, pathogenesis studies relevant to the design and testing of vaccine and microbicide candidates that would be impossible in humans can be undertaken by use of this animal model. This model uses SIV, a primate lentivirus that is closely related to HIV (5) and that can be efficiently transmitted to macaques by vaginal inoculation of cell-free inocula (11, 20, 21). Further, the rhesus monkey is similar to humans with regard to the populations of target cells (10) and the physiology (4) and immunology (8, 9) of the female genital tract.
Here we describe the use of this animal model to address the following two critical issues for the development of vaccines to prevent systemic infection following intravaginal transmission: the role of local propagation in establishing systemic infection and the dynamics of spread to the lymphatic tissues (LTs). The intravaginal inoculation model is not only obviously essential to addressing the first issue but also likely essential for the other as well because of differences after intravenous and intravaginal SIV inoculation in the timing of the establishment and peak of productive systemic infections. The detection of LT infection and peak p27 antigenemia occur later after intravaginal inoculation (11, 29) than after intravenous inoculation, consistent with a brief period when replication is largely confined to the portal of entry and when host defenses are in the most favorable position to prevent the establishment of a persistent systemic infection.
In this report, we focus on infection at the portal of entry and the spread and establishment of productive infection in the LTs. In an accompanying report, we focus on the immune response in the cervicovaginal and LT compartments. Together, our studies reveal the formidable challenges the host faces in containing infection after it is established throughout the LTs and describe the brief period at the portal of entry when a robust vaccine-induced mucosal immune response might be able to eliminate the relatively smaller numbers of productively infected cells.
MATERIALS AND METHODS
Animals.
All animals used for this study were adult female rhesus macaques (Macaca mulatta) that were housed at the California National Primate Research Center in accordance with the regulations of the American Association of Accreditation of Laboratory Animal Care standards. All animals were negative for antibodies to HIV type 2, SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 at the time the study was initiated. When necessary, animals were anesthetized with 10 mg/kg ketamine hydrochloride (Parke-Davis, Morris Plains, N.J.) or 0.7 mg/kg tiletamine HCl and zolazepan (Telazol; Fort Dodge Animal Health, Fort Dodge, Iowa) injected intramuscularly.
Intravaginal SIV inoculation.
All monkeys used for this study were inoculated intravaginally with SIV twice in a single day, with a 4-hour interval between inoculations. Twenty-one animals were necropsied between days 0 and 10 after intravaginal SIVmac251 inoculation (Table 1). Eight Mamu-A01*-positive monkeys were necropsied 13 to 28 days after intravaginal inoculation with SIVmac239 (Table 2). The stocks had titers of 105 50% tissue culture infective doses (TCID50)/ml, as determined by end-point dilution culture on CEMX174 cells. One milliliter of virus stock was used for each intravaginal inoculation. We have previously shown that two intravaginal inoculations with SIVmac251 resulted in systemic infection in 15 of 16 rhesus monkeys (11), while two intravaginal inoculations with UCD-SIVmac239 resulted in systemic infection in 12 of 16 rhesus monkeys (1). Although some investigators have reported differences in the long-term clinical outcomes of SIVmac239 and SIVmac251 infections of rhesus monkeys, these two viruses are closely related and we have not seen any difference in the patterns of plasma viremia in the first several months after intravaginal inoculation (1, 11). Thus, we think that using these viruses sequentially in order to explore the virology and immune response during the acute phase of SIV infection is appropriate.
TABLE 1.
SIV RNA and DNA in tissues of rhesus monkeys inoculated intravaginally with SIVmac251 or AT-inactivated SIVmac239 and in uninfected animals
| Animal no. and inoculum | Time of necropsy (days p.i.) | Plasma vRNAe | Genital tracta
|
Genital LNsb
|
Systemic lymphoid tissuesc
|
Intestinal tractd
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VRNAf | VDNAg | MS RNAh | vRNA | vDNA | MS RNA | vRNA | vDNA | MS RNA | vRNA | VDNA | |||
| SIVmac 251i | |||||||||||||
| 29699 | 2 h | <125 | 2.0 × 103 | + | − | ND | ND | ND | ND | ND | ND | ND | ND |
| 23053 | 2 h | <125 | 2.0 × 103 | − | − | ND | ND | ND | ND | ND | ND | ND | ND |
| 25103 | 1 | <125 | 5.0 × 103 | + | − | ND | ND | ND | ND | ND | ND | ND | ND |
| 26937 | 1 | <125 | + | − | − | − | − | − | − | − | − | ND | |
| 31426 | 1 | <125 | 4.0 × 103 | − | − | − | − | − | − | − | − | − | ND |
| 31937 | 1 | <125 | 1.0 × 104 | 8.0 × 104 | − | − | − | − | − | − | − | − | ND |
| 30127 | 1 | <125 | 4.1 × 102 | − | − | − | − | − | 3 × 102 | − | − | − | ND |
| 31529 | 1 | <125 | − | − | − | − | − | − | − | + | 3 × 102 | ND | |
| 30201 | 2 | <125 | + | − | − | + | − | − | − | − | 7 × 102 | − | |
| 28018 | 3 | <125 | − | − | − | − | − | 1.0 × 103 | − | + | − | ND | |
| 28420 | 3 | <125 | 9.0 × 103 | + | − | − | − | − | − | − | − | − | ND |
| 31373 | 4 | <125 | 3.4 × 102 | 3.5 × 105 | − | − | + | − | − | 3.6 × 102 | − | − | 7.2 × 102 |
| 31433 | 4 | <125 | − | − | − | − | − | − | − | + | − | ND | |
| 31385 | 4 | 1.5 × 103 | − | − | − | − | − | − | − | − | − | − | |
| 34443 | 5 | <125 | − | − | − | − | − | − | 8.5 × 103 | − | − | 2.1 × 103 | |
| 30991 | 6 | 2.3 × 104 | 9.0 × 103 | 1.9 × 103 | − | 1.0 × 105 | 5.0 × 103 | 2.0 × 104 | 5.0 × 103 | ND | 2.0 × 102 | + | |
| 31523 | 6 | 7.3 × 104 | 4.0 × 104 | 2.9 × 104 | − | 8.0 × 104 | + | 4.8 × 104 | + | ND | 1.0 × 103 | 5.0 × 103 | |
| 26222 | 7 | 2.6 × 106 | 9.0 × 105 | + | + | 3.0 × 105 | + | + | 3.4 × 106 | + | + | 1.0 × 105 | ND |
| 34498 | 8 | 2.5 × 106 | 3.2 × 105 | + | + | 1.0 × 105 | + | + | 2.0 × 105 | + | + | 1.0 × 103 | + |
| 28013 | 9 | 2.5 × 106 | 6.0 × 105 | + | + | 2.0 × 106 | + | + | 7.0 × 105 | + | + | 7.0 × 105 | ND |
| 24818 | 10 | 9.9 × 106 | 5.0 × 105 | + | ND | 7.0 × 105 | + | − | 2.0 × 107 | + | − | 1.0 × 106 | + |
| SIVmac AT-2j | |||||||||||||
| 27438 | 1 | <125 | 1.0 × 104 | − | ND | − | − | ND | 2.5 × 102 | − | ND | − | − |
| 27776 | 2 | <125 | − | − | ND | − | − | ND | − | − | ND | − | − |
| 32109 | 3 | <125 | − | − | ND | − | − | ND | − | − | ND | − | − |
| 31098 | 4 | <125 | − | − | ND | − | − | ND | − | − | ND | − | − |
| Uninfectedk | |||||||||||||
| 34572 | 1 | ND | − | ND | ND | − | ND | ND | − | ND | − | ND | |
| 34550 | 1 | ND | − | ND | ND | − | ND | ND | 170 | ND | 800 | ND | |
| 31931 | 1 | ND | − | ND | ND | − | ND | ND | 130 | ND | 180 | ND | |
The genital tract includes three sites in the vagina and one site in the cervix. Results from the tissue samples with the highest vRNA levels are shown.
Genital lymph nodes are the iliac and obturator LNs. Results from the tissue samples with the highest vRNA levels are shown.
Systemic lymphoid tissues are the axillary, inguinal, and mesenteric LNs and the spleen. Results from the tissue samples with the highest vRNA levels are shown.
The intestinal tract is the jejunum, ileum, and colon. Results from the tissue samples with the highest vRNA levels are shown.
SIV RNA copies/μl plasma (125 copies is the lower limit of detection) (see Materials and Methods).
SIV RNA copies/μg of tissue RNA (see Materials and Methods).
Positive (+) or negative (−) detection of SIV DNA by nested PCR or number of SIV DNA copies/μg of tissue RNA by real-time PCR (see Materials and Methods).
Multiply spliced SIV RNA copies/μg of tissue RNA (see Materials and Methods). ND, not done.
Age-matched female rhesus monkeys inoculated intravaginally with SIVmac239 (105 TCID50/ml and 109 vRNA copies/ml).
Age-matched female rhesus monkeys inoculated intravaginally with AT-2-inactivated SIVmac239 (0 TCID50/ml and 1010 vRNA copies/ml).
Age-matched female rhesus monkeys that were never exposed to SIV.
TABLE 2.
SIV RNA and DNA in tissues of Mamu A01*-positive rhesus monkeys inoculated intravaginally with SIVmac239
| Animal no.i | Time of necropsy (days p.i.) | Plasma vRNAe | Genital tracta
|
Genital LNsb
|
Systemic lymphoid tissues (includes mesenteric LNs)c
|
Intestinal tractd
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vRNAf | vDNAg | MS RNAh | vRNA | vDNA | MS RNA | vRNA | vDNA | MS RNA | vRNA | VDNA | |||
| 28035 | 13 | 6.8 × 106 | 8.0 × 105 | + | − | 1.4 × 106 | + | + | 1.4 × 107 | + | + | 1.2 × 106 | ND |
| 24037 | 14 | 2.3 × 108 | 5.6 × 105 | + | − | 9.0 × 104 | + | − | 2.7 × 106 | + | + | 2.0 × 105 | ND |
| 27327 | 20 | 8.1 × 106 | 1.5 × 105 | + | + | 3.0 × 105 | + | + | 1.1 × 106 | + | + | 1.8 × 105 | ND |
| 27099 | 21 | 6.8 × 106 | 4.2 × 104 | + | − | 4.0 × 105 | + | + | 1.7 × 106 | + | + | 2.8 × 105 | + |
| 30551 | 21 | 3.6 × 107 | 1.9 × 105 | + | − | 3.0 × 106 | + | − | 1.4 × 106 | + | + | 7.7 × 105 | ND |
| 24225 | 28 | 1.5 × 106 | 2.1 × 105 | + | + | 7.0 × 105 | + | + | 2.2 × 106 | + | + | 7.6 × 104 | ND |
| 27572 | 28 | 7.6 × 105 | 5.3 × 104 | + | + | 4.4 × 105 | + | + | 2.0 × 105 | + | + | 2.3 × 105 | ND |
| 27338 | 28 | 1.8 × 106 | 1.5 × 104 | + | + | 2.0 × 105 | + | + | 1.0 × 106 | + | + | 1.1 × 105 | ND |
The genital tract equals three sites in the vagina and one site in the cervix. Results from the tissue samples with the highest vRNA levels are shown.
Genital lymph nodes are the iliac and obturator LNs. Results from the tissue samples with the highest vRNA levels are shown.
Systemic lymphoid tissues are the axillary, inguinal, and mesenteric LNs and the spleen. Results from the tissue samples with the highest vRNA levels are shown.
The intestinal tract is the jejunum, ileum, and colon. Results from the tissue samples with the highest vRNA levels are shown.
SIV RNA copies/μl plasma (125 copies is the lower limit of detection) (see Materials and Methods).
SIV RNA copies/μg of tissue RNA (see Materials and Methods).
Positive (+) or negative (−) detection of SIV DNA by nested PCR (see Materials and Methods).
Multiply spliced SIV RNA copies/μg of tissue RNA (see Materials and Methods).
Age-matched female rhesus monkeys were inoculated intravaginally with SIVmac239 (105 TCID50/ml and 109 vRNA copies/ml).
To evaluate the potential contribution of the viral RNA (vRNA) in the inoculum to the vRNA detected in tissues of animals inoculated with live virus, we inoculated four additional animals intravaginally with aldithriol-2 (AT-2)-inactivated SIVmac239, using the same inoculation schedule as that for the animals inoculated with live SIVmac. Whole AT-2-inactivated SIVmac239 was provided by the AIDS Vaccine Program, SAIC Frederick, Inc., National Cancer Institute-Frederick, Frederick, Md.
Tissue collection and RNA isolation.
At the time of euthanasia, blood, vaginal, cervical, spleen, jejunum, colon, and axillary mesenteric and genital (obturator/iliac) lymph node (LN) samples were collected. Tissue samples were stored in RNAlater (Ambion, Austin, Tex.) at −20°C until RNA preparation. This process removed any adherent mucus from the tissue samples. Prior to RNA isolation, the tissue samples were homogenized by use of a Power homogenizer (PowerGen, 7 mm × 195 mm; Fisher Scientific). The total RNA was isolated by the use of Trizol (Invitrogen, Grand Island, N.Y.) according to the manufacturer's protocol. The organic phase was saved and stored at −80°C for subsequent DNA isolation. The purified RNA was used to determine tissue viral RNA levels by a branched DNA (bDNA) assay.
In addition, cell suspensions were prepared from vaginal mucosae, spleens, and lymph nodes. Tissue cell suspensions from lymph nodes were prepared by gently dissecting the lymph nodes with scalpels in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini BioProducts, Calabasas, Calif.) (complete RPMI) and then passing the cell homogenate through a cell strainer (Fisher, Pittsburgh, Pa.). The cells were washed twice by centrifugation for 10 min at 1600 rpm. Spleen tissue samples were cut into small pieces and homogenized by use of a syringe plunger. The homogenate was passed through a cell strainer. Splenic lymphocytes were isolated by gradient centrifugation with lymphocyte separation medium from ICN Biomedicals (Aurora, Ohio), followed by two washes with complete RPMI. Peripheral blood mononuclear cells were isolated from whole blood by the use of lymphocyte separation medium (ICN Biomedicals). Cell suspensions were stored in liquid nitrogen in 10% dimethyl sulfoxide-90% fetal bovine serum.
DNA isolation.
DNAs were isolated from the organic phase collected during the Trizol RNA isolation procedure. A polyacryl carrier (Molecular Research Center, Cincinnati, Ohio) was added to all samples to ensure the maximum recovery of DNA. DNAs were isolated by an alternative DNA isolation procedure (back extraction buffer method) described by Molecular Research Center, Inc., according to the manufacturer's protocol.
Virus load measurement.
Tissue RNA samples were analyzed for viral RNA (vRNA) by a quantitative branched DNA (bDNA) assay (2), and RNA levels are reported as viral RNA copy numbers per μg of total tissue RNA. The detection limit of this assay is 125 copies of vRNA/ml of plasma. Although some reverse transcription-PCR (RT-PCR) assays are more sensitive, they are also more limited in the quantity of tissue RNA that can be sampled. We chose to use the bDNA assay because we could easily analyze at least 2 to 10 μg of total tissue RNA and could thus sample a larger and more representative fraction of the collected tissues. To evaluate the specificity of the bDNA assay, we collected tissue samples from three animals that had not been exposed to SIV. Excluding one spurious result that could not be repeated upon reanalysis, the average value produced by the bDNA assay for the tissues of uninfected animals was 113 vRNA copies/μg tissue RNA. Based on these results, we set a cutoff of 200 copies/μg tissue RNA to consider a sample vRNA positive based on the bDNA assay, which is the mean vRNA copy number (113 vRNA copies/μg tissue RNA) in uninfected tissues + 2 standard deviations of the mean. Furthermore, all positive samples with vRNA levels of <104 copies/μg tissue RNA were tested at least three separate times in the bDNA assay. If a sample was consistently positive for vRNA based on the bDNA assay results, then the highest level found was reported. If a sample was inconsistently positive for vRNA based on the above criteria, it was considered negative (Tables 1 and 2).
Viral DNA-specific PCR.
The presence of proviral DNA was determined by the use of a nested PCR method as described previously (15, 22) or by real-time PCR-based amplification of the SIV gag gene. Real-time PCR assays were carried out in 96-well Optical plates (Applied Biosystems, Foster City, Calif.) in 25-μl reaction volumes containing 5 μl of DNA (100 ng) and 20 μl of master mix (Applied Biosystems). All sequences were amplified by using the 7900 default amplification program, which was 2 min at 50°C and 10 min at 95°C followed by 50 cycles of 15 s at 95°C and 1 min at 60°C. The presence of viral DNA was determined based on the amplification of SIV gag from the input sample. Since low frequencies of SIV-infected cells were expected at the early time points, a total of 106 cell equivalents were analyzed for the presence of viral DNA. The genomic DNA from a single cell contains two copies of the CCR5 gene, and thus, by coamplifying the CCR5 gene from the same sample, we could determine the number of cell equivalents. CCR5 copy numbers were calculated based on a standard curve that was generated by using 10-fold dilutions (range, 101 to 106 copies) of a plasmid carrying the CCR5 gene (data not shown). The CCR5-expressing plasmid pc.Rh-CCR5 was obtained through the NIH AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, from Preston Marx. The following primer pair and probe were used to amplify the CCR5 sequence (5′-3′): forward primer, CCA TGC AGG TGA CAG AGA CTC T; reverse primer, TCT CCC CGA CAA AGG CAT AG; and probe, TAMRA-TGA CAC ACT GCT GCA TGA ACC CCA-FAM.
The copy numbers of SIV gag and SIV env were calculated based on standard curves spanning a dynamic range of 101 to 106 copies. The lower detection limit of the assay was 10 copies. The SIVgag plasmid contained the SIV gag gene, which was amplified by PCR (primer sequences, 5′-GAG ATG GGC GTG AGA AAC TCC-3′ and 5′-ATA TGA GCA GTG ACT ACT GGT CTC CTC-3′) from the p239SPSP5′ plasmid obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, from R. Desrosiers. The PCR product was cloned into the pCR2.1 vector by use of a pCR2.1 Topo cloning kit (Invitrogen) according to the manufacturer's protocol, and the gag gene was then subcloned into the pBAC-1 plasmid via EcoRI restriction sites. The following sequences were used to amplify the SIV gag gene (5′-3′): forward primer, GGG AGA TGG GCG TGA GAA A; reverse primer, CGT TGG GTC GTA GCC TAA TTT T; probe, TAMRA-TCA TCT GCT TTC TTC CCT GAC AAG ACG GA-FAM. The SIVenv plasmid pVP2 nef+ was a gift from P. Luciw (UC Davis) and contains the SIVmac239 env, tat, rev, and nef (without a premature stop codon) genes and 3′ long terminal repeat. The methods were optimized, and the ongoing accuracy of the assay was tested for every run by the use of negative (uninfected CEMx174 cells) and positive (SIVmac251-infected CEMx174 cells) control samples (data not shown). The results were analyzed with SDS 7900 system software, version 2.1 (Applied Biosystems). Note that in samples that tested positive for vDNA not all wells necessarily tested positive for the presence of viral DNA. Thus, the average vDNA copy number in the vDNA-positive wells was calculated for each sample, and the results for each sample are reported as log10 vDNA copies per 1 × 106 cells.
Viral RNA-specific PCR.
The presence of vRNA was determined by real-time RT-PCR-based amplification of the SIV gag and multiply spliced SIV (MS SIV) sequences. The RNA copy numbers of the SIV gag and MS SIV templates were calculated based on standard curves spanning a dynamic range of 101 to 106 copies. For the MS SIV standard, the purified RNAs from cultured peripheral blood mononuclear cells infected with SIVmac239 for 48 h were reverse transcribed to cDNAs by use of the EK7r primer (5′-CGA CCG GCT CCT CCC CAG TA-3′) and Thermoscript (Invitrogen), and these cDNAs were then used in a first PCR with EK1 (5′-GCT CTG CGG AGA GGC TGG C-3′) and EK7r and in a second PCR with EK2 (5′-GAG GTT CTC TCC AGC ACT AGC-3′) and EK4 (5′-TGA ATA CAG AGC GAA ATG CAG-3′). For the first round of amplification, we used 1 unit of Platinum High Fidelity Taq DNA polymerase (Invitrogen, Calif.) in 2 mM MgSO4, an 800 mM concentration of each deoxynucleoside triphosphate, a 200 μM concentration of each primer, and either 5 μl of cDNA or 1 μg of genomic DNA template in a total volume of 50 μl. After an initial hot activation at 94°C for 1 min, the cycling profile was 94°C for 30 s, 55°C for 30 s, and 68°C for 5 min for 35 cycles, with a final extension at 68°C for 10 min. Second-round amplification was performed by using 5 μl of the first-round PCR product, with a cycling profile of 94°C for 20 s, 55°C for 30 s, and 68°C for 4 min 30 s for 35 cycles, with a final extension at 68°C for 10 min. The PCR products were cloned into the pCR-TOPO2.1 vector (Invitrogen) and screened for the MS SIV sequence. For standard templates, SIV gag and the MS SIV plasmid were transcribed to RNAs by use of the T7 promoter and a MEGAscript T7 kit (Ambion), and the RNAs were then purified and quantified. The primers and probe used for SIV gag-specific real-time RT-PCR were the same as those used for SIV gag DNA-specific real-time PCR. MS SIV real-time RT-PCR was performed with the primers SP-F (5′-CCG AAA AAG GCT AAG GCT AAT ACA-3′) and SP-R (5′-CAG TTG GCG GAT CAG GAA ATG-3′) and the probe SP-Probe F (5′-FAM-AGA AGG CGG TGG CAA CAG CTC CTG-TAMRA-3′), with TaqMan One-Step RT-PCR master mix (Applied Biosystems, Calif.) and a heat profile of 50°C for 30 min, 95°C for 10 min, and 50 cycles of 95°C for 15 s and 60°C for 1 min.
ISH.
For in situ hybridization (ISH), SIV RNAs were detected in cells of formalin-fixed and paraffin-embedded tissues as previously described (29, 30). In brief, 6- to 8-μm sections were cut and adhered to silanized slides. After deparaffinization in xylene, rehydration in phosphate-buffered saline, and permeabilization by treatment of the sections with HCl, digitonin, and proteinase K, the sections were acetylated and hybridized to 35S-labeled SIV-specific riboprobes. After washing and digestion with ribonucleases, the sections were coated with nuclear track emulsion, exposed, developed, and counterstained with Giemsa stain.
ISH/TSA/ELF for detection of virions.
Virions were detected in tissue sections by ISH/tyramide signal amplification/enzyme-linked fluorescence (ISH/TSA/ELF) as recently described (30). After deparaffinization in xylene, slides were placed in ethanol with 0.5% H2O2, rehydrated through a graded series of ethanol, incubated in 0.2 M HCl for 10 min, and digested at room temperature for 15 min with pepsin (150 μg/ml) in 20 mM HCl. After washing in phosphate-buffered saline and fixation in 2% paraformaldehyde for 15 min, the slides were acetylated and dehydrated through a graded series of ethanol. Digoxigenin-labeled SIV-specific RNA probes were hybridized overnight at 45°C in 50% formamide, 5% dextran sulfate, 1 mM EDTA, 20 mM HEPES (7.5), 0.1% sodium dodecyl sulfate, and 50 μg/ml yeast RNA. After being washed in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 45°C, in 2× SSC-50% formamide at 50°C, and in 0.1 M Tris (7.5), 0.15 M NaCl at 37°C, the slides were digested for 60 min at 37°C with 30 μg/ml RNase A in Tris-NaCl buffer and washed in 2× SSC at 37°C for 15 min. Bound digoxigenin-labeled RNA probes were detected by tyramide signal amplification. Slides were placed in a blocking solution for 1 h (TNB solution, 2% sheep serum, and 25 mM NH4SCN) and then incubated overnight at 4°C with a 1:1,500 dilution of anti-digoxigenin-peroxidase conjugate (Roche) in TNB, sheep serum, and 25 mM NH4SCN. After being washed four times in TNT (Tris, NaCl, Tween 20), the slides were incubated for 7 min at 26°C with a solution of biotinyl tyramine in amplification diluent (Perkin-Elmer) containing 25% dextran 500 (Pharmacia) to reduce the diffusion of reacted tyramine. After three washes with TNT, avidin biotinyl-peroxidase (ABC Elite; Vector Labs) diluted in TNB was added to the slides for 30 min. Three washes in TNT completed one round of amplification. The amplification step was repeated with biotinyl tyramine, the ABC reagent, and biotinyl tyramine. The slides were then incubated for 30 min with streptavidin-alkaline phosphatase diluted 1:60 in blocking buffer (Molecular Probes) and were washed three times for 15 min in wash buffer. The slides were incubated with ELF 97 substrate (Molecular Probes), stained with Hoechst 33342, washed with TNT for 24 h to 3 days to reduce or eliminate nonspecific precipitates of the ELF substrate, and mounted in Aquapolymount (Polysciences).
RESULTS
Small residue of tissue-associated SIVmac251 despite exposure to a large dose of SIV.
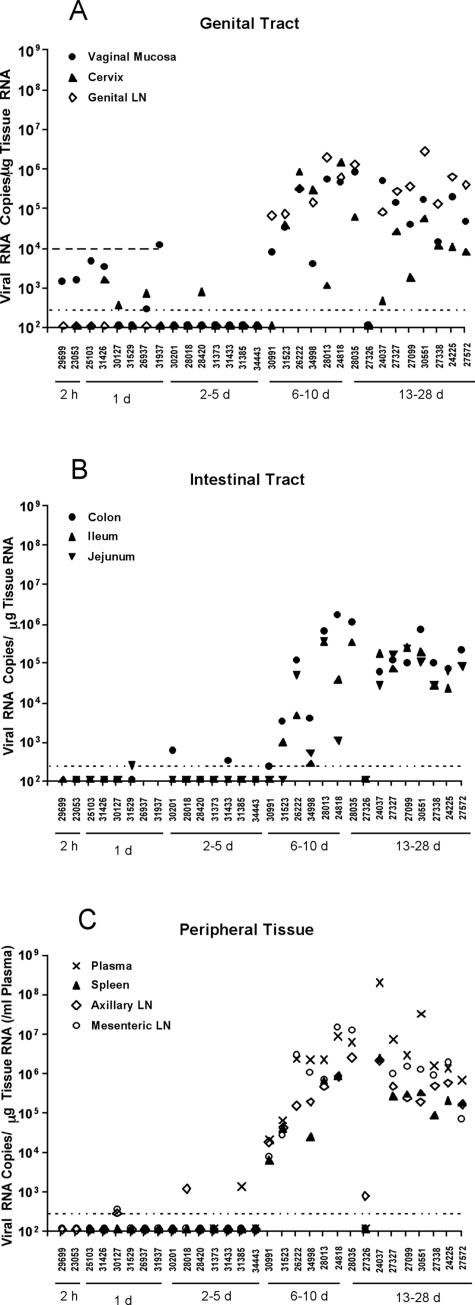
We evaluated the clearance of the SIV inoculum, its tissue penetration, and the initiation of replication and dissemination postinoculation (p.i.) by the following three measures: (i) the amount of vRNA in the inoculum and the amount of vRNA detectable in cervicovaginal tissues, (ii) the detection of vDNA in tissues, and (iii) the detection of vRNA and cells in tissues. We found that the inoculum contained 109 (bDNA) copies of SIV RNA per ml. Animals were thus exposed to ∼109 virions that were mainly defective, since the titer of replication-competent virus was only 105 TCID50/ml. This high dose was required to produce systemic infection in a large proportion (>80%) of animals by this route because of the large numbers of defective virions and the effectiveness of the barrier mechanisms described below at reducing the exposure of target cells to infectious virus.
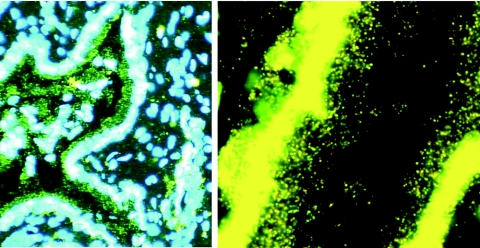
We detected from a few hundred to at most 104 copies of SIV RNA per μg of cervical and vaginal tissue from two animals at 2 h and five of six animals 1 day after intravaginal exposure to 2 × 109 copies of viral RNA in the live SIVmac251 inoculum (Table 1; Fig. 1). Thus, most of the inoculum was diluted in and cleared from cervicovaginal fluids, and only a small residue remained, e.g., entrapped in cervical mucus (Fig. 2). The exposure of CD4+ T cells, dendritic cells (DCs), and macrophages to relatively small numbers of infectious virions accounts, we think, for the high dose of virus needed to infect animals by this route. It also follows that initially there will be few productively infected cells at the portal of entry. The low frequency of SIV RNA-positive cells and the low levels of vRNA detectable in the genital tissues in the first week of infection, as described below, are in accord with this interpretation.
FIG. 1.
vRNA levels in genital (A) and intestinal tract (B) tissues, peripheral lymphoid tissues (C), and plasma samples (C) of rhesus monkeys after intravaginal SIV inoculation. The bDNA assay (seeMaterials and Methods) results for the indicated tissues are displayed vertically above the animal numbers along the x axis. The animals are grouped by the timing of necropsy, as indicated by the solid horizontal lines under the animal numbers. The horizontal dotted line indicates the mean vRNA level + 2 standard deviations in the tissues of SIV-naïve monkeys and represents the cutoff value for designating positive samples (see Materials and Methods). The upper horizontal dashed line in panel A indicates the vRNA level found in the genital tract tissue of a monkey (27438) that was necropsied 24 h after intravaginal AT-2-inactivated SIVmac239 inoculation. Thus, for monkeys necropsied <48 h after intravaginal SIV inoculation, vRNA levels below this dashed line may represent virion RNA in the inoculum (see Results).
FIG. 2.
Residual virions from the inoculum trapped in cervical mucus. Sections of a cervix obtained 24 h after intravaginal exposure to SIV were cut and hybridized to SIV-specific riboprobes, and the SIV RNA signal from virions or infected cells was amplified by TSA/ELF as described in Materials and Methods. Viral RNA was not detected in cells but was detected in virions, as shown in the mucus adhering to the epithelium of the endocervical gland shown. (Left) Original magnification, ×100. The image brightness and contrast were adjusted to reveal Hoechst-counterstained nuclei and histology. (Right) Magnification, ×1,000. The image was adjusted to reveal virions at the mucosal surface.
Days 1 to 3: limited penetration of the mucosal barrier, small founder populations of infected cells, and rapid dissemination from the portal of entry.
To understand the relationship between the vRNA representing the residual inoculum and the vRNA produced by infected cells, we measured the vRNA in animals inoculated intravaginally with 1 ml of AT-inactivated SIVmac239 (1010 copies of SIV RNA per ml, as measured by a bDNA assay). The dose of AT-inactivated SIVmac239 used for these inoculations was approximately 10-fold larger than the dose of SIV used for live virus inoculations to ensure that we could conservatively estimate the contribution of residual inoculum virions to the vRNA levels detected in the tissues of animals inoculated with live SIV. For one monkey that was necropsied 24 h after AT-inactivated SIV inoculation, 104 copies of vRNA/μg tissue RNA were detected in the vaginal mucosa. However, vRNA was never detected in any tissues of animals necropsied >24 h after AT-inactivated SIVmac239 inoculation (Table 1). Thus, we concluded that the vRNA detected in the cervicovaginal tissues of animals necropsied ≤24 h after the intravaginal inoculation of live SIVmac251 represented virion RNA from the residual inoculum. vDNA, MS vRNA, or SIV RNA-positive cells were never detected in animals exposed to AT-2-inactivated SIVmac239, and therefore we took their detection as evidence of infected cells in the tissues.
Based on the above results, we interpreted the detection of vDNA in the vagina of one of two monkeys necropsied at 2 h p.i. (Table 1) as evidence that SIV had already infected small numbers of cells. We similarly interpreted the detection of vDNA in cervicovaginal tissues of five of nine animals from days 1 to 3 and of vRNA at >102.2 in cervicovaginal tissues of one of three animals from days 2 to 3 p.i. as evidence of infection of small numbers of cells at the portal of entry.
We detected vRNA in axillary LNs at 24 h p.i. for one animal inoculated with AT-2-inactivated SIV. On days 1 to 3 after intravaginal SIVmac251 inoculation, vRNA, MS vRNA, or vDNA- but not vRNA-positive cells were detected in the systemic LTs of four of eight animals (Table 1). We interpreted these results as evidence of pathways for the rapid dissemination of both the virus in the inoculum and infected cells from the portal of entry.
Collectively, these findings suggest that productively infected cells during the first 3 days of SIV infection are mainly found in cervicovaginal tissues, but even there they are present only in very small numbers. These small founder populations are most consistently detectable by PCR for SIV DNA and the bDNA assay for vRNA and less consistently detectable by assays of MS vRNA, likely reflecting the relative sensitivities of the assays for tissue samples and the difference in the amount of sample that can be analyzed by each assay. The negative results of the extensive search for SIV vRNA-positive cells by ISH likely reflects the highly focal nature of the infection and the small founder populations of infected cells (see below). This examination of the early hours after intravaginal exposure to virus also provides evidence of the rapid dissemination from the portal of entry of extremely small quantities of virus and/or infected cells.
Days 4 and 5: focal productive infection in cervicovaginal tissues and detection of systemic dissemination.
By day 4, there was evidence of a productive infection in cervicovaginal tissues, with vRNA-positive cells (Fig. 3), and of hematogenous dissemination-viremia (1,500 copies of vRNA/ml of plasma) in one animal (31385). However, the infection in the genital tract was highly focal, as illustrated in Fig. 3, with just a single focus of SIV RNA-positive cells in 1 of 20 sections examined. Intraepithelial SIV RNA-positive cells were detectable in this focus of infection, as previously described (6, 29). vRNA and vDNA were not detected in separate samples of the cervix from animal 31385. In addition, regardless of the bDNA or PCR results, SIV RNA-positive cells were not detected in any tissue from the other 14 animals that were necropsied prior to 6 days p.i. The discordance between the results of the ISH, bDNA and PCR assays for tissue samples from animals necropsied in the first 5 days p.i. is consistent with a highly focal infection that is difficult to adequately sample. Furthermore, the detection of vDNA and MS vRNA in LTs from two other animals on days 4 and 5 p.i. (Table 1) is also consistent with a disseminated systemic infection in which the number of productively infected cells and the level of viral RNA in each infected cell are below the threshold of detection by ISH.
FIG. 3.
Highly focal productive infection in an endocervix 4 days after intravaginal SIV inoculation. (A) Montage of images (magnification, ×100) of a single section of endocervix, among 20 sections examined from monkey 31385, in which one focus (encircled) of SIV RNA-positive cells was detectable. (B) SIV RNA-positive cells at a higher magnification. The double-headed arrow points to two SIV RNA-positive cells that appear to be intraepithelial lymphocytes. The single-headed arrow points to a focal collection of SIV RNA-positive cells in the endocervical mucosa. SIV RNA was detected by ISH with radiolabeled riboprobes. In the developed radioautographs viewed with transmitted light, the SIV RNA-positive cells appear black because of the large numbers of silver grains overlying the cell. Original magnification, ×100.
Days 6 to 10: establishment of productive systemic infection and peak virus production in tissues.
By day 6, the evidence of an established productive infection in systemic LTs was unequivocal, with readily detectable vDNA, vRNA, and vRNA-positive cells in the cervicovaginal mucosa and draining and systemic LTs and with detectable vRNA in the plasma (Table 1). We detected SIV vDNA and vRNA (103 to 106 copies/μg of tissue RNA) in all of the genital and distal tissues and in the plasma of four of four animals and MS vRNA in three of four animals examined between days 7 and 10 p.i., with the highest vRNA levels measured in the plasma and tissues of the animal necropsied on day 10 p.i. (Fig. 1; Table 1).
Productive infection spreads from the portal of entry to distal lymphatic tissue compartments.
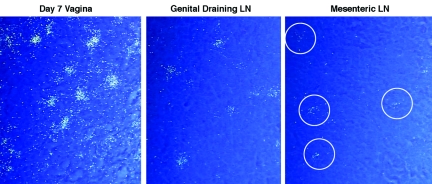
While there may be rapid routes of dissemination to distal sites (see Discussion), we expected a productive infection in cervicovaginal tissues to first cause the spread of virus and infected cells to the genital draining LNs. Subsequently, the virus and infected cells would reach the bloodstream via the lymphatics and thoracic duct and then be hematogenously disseminated to distal LNs, the spleen, the gut-associated lymphoid tissue (GALT), and the mesenteric LNs that drain the GALT. Consequently, the relative amount of time that cells would have been infected would decrease from the cervicovaginal tissue to the mesenteric LNs, and the numbers of copies of vRNA per cell would be in descending order as well. We provide evidence that this is the case in Fig. 4, as the vRNA copy number per cell can be estimated for the same radioautographic exposure from the proportional number of silver grains overlying vRNA-positive cells after ISH with radiolabeled riboprobes. We found that at 7 days p.i., the numbers of silver grains per cell in the tissues of monkey 26222 decreased in the predicted order of vagina > genital LNs > mesenteric LNs (Fig. 4). We observed similar gradients in grain counts for the two animals that were necropsied on day 6 p.i. Furthermore, while the tissue viral load bears a complex relationship with the number of infected cells (reflecting the density of target cell populations) and the duration of infection, we noted that for two of three animals necropsied on day 6 or 7 p.i., the vRNA levels were higher in the genital LNs than in distal LTs.
FIG. 4.
Relationship between duration of infection and SIV RNA levels in cells at 7 days p.i. ISH was performed with radiolabeled SIV-specific riboprobes. In developed radioautographs viewed in epipolarized light, silver grains appear white. The number of grains is proportional to the number of copies of SIV RNA per cell, which in turn is related to the length of time the cell has been infected. By this criterion, the encircled cells in the mesenteric LNs were the most recently infected. All tissues were from monkey 26222.
Days 13 to 28: decreasing viral load in late-stage infection.
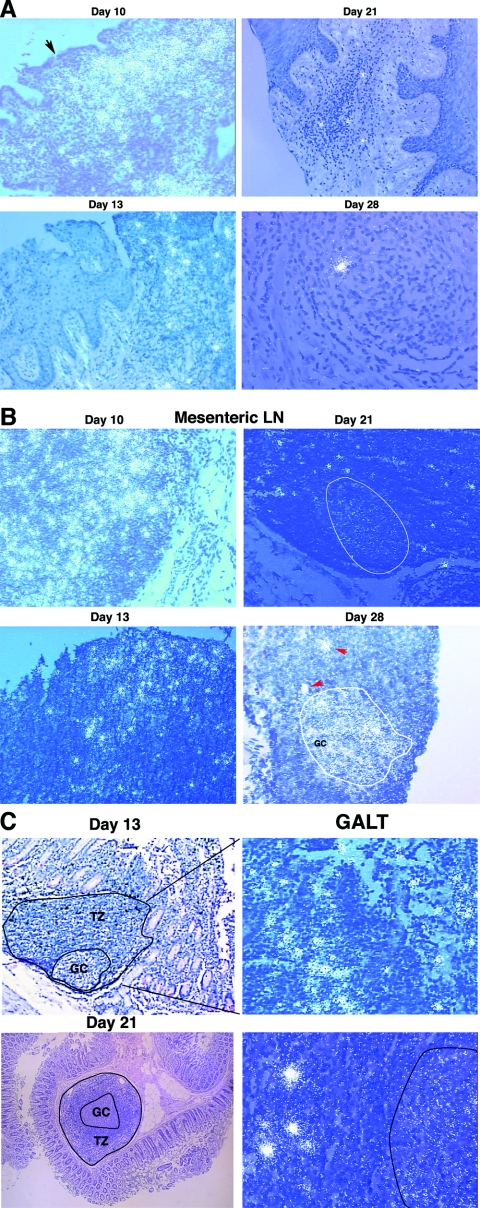
We investigated the subsequent course of SIV infection in Mamu-A01*-positive rhesus macaques in order to determine the relationship between viral replication and the SIV-specific immune response to immunodominant epitopes, using tetramer reagents specific for this prevalent allele, as described in the accompanying paper. We detected SIV vRNA (103 to 107 copies/μg of tissue RNA) and vDNA in all of the genital and distal tissues, including the plasma, of the eight animals (Table 2). For one animal that was necropsied on day 13, the vRNA levels in the plasma and tissues were close to the levels found in the tissues of the animal necropsied on day 10. For animals necropsied at 28 days, the vRNA levels in the plasma and tissues were lower, by about an order of magnitude, than those in the animals necropsied on day 14 p.i. The number of infected cells/mm2 in the cervicovaginal tissues and the LT compartment increased to a peak on day 10, as described above, and then declined 50-fold in parallel with the vRNA levels (Fig. 1, 5, and 6A to C). The abrupt decline in infected cells between days 10 and 14 p.i. was not likely due to the CD8+ T-cell response to immunodominant SIV epitopes because, as shown in the accompanying paper, there was no detectable immune response to these epitopes on day 13 and only a small response on day 14.
FIG. 5.
Frequency of SIV RNA-positive cells in mesenteric LNs. SIV RNA-positive cells detected by ISH were enumerated in sections of defined areas.
FIG. 6.
Examples of SIV RNA-positive cells in cervicovaginal and lymphatic tissues and of virions associated with follicular dendritic cells (FDCs) in LTs. SIV RNA in cells and virions was detected by ISH with 35S-labeled riboprobes. In the developed radioautographs illuminated with epipolarized light, SIV RNA-positive cells appear as bright ob-jects and FDC-associated virions appear as bright silver grains overlying follicles. (A) Cervical and vaginal tissues. On day 10, there were dense collections of SIV RNA-positive cells in inflammatory foci in the submucosa of the endocervix. The arrow points to a break in the overlying epithelium. SIV RNA-positive cells progressively decreased in frequency from days 13 to 28 and were usually associated with small foci of inflammation. (B) LNs. In mesenteric and other LNs, there were numerous SIV RNA-positive cells on day 10. Their numbers then declined. A faint signal from FDC-associated virion RNA in follicles (encircled) was detectable by day 21 and increased by day 28 in the encircled germinal center of a follicle. The red arrows each point to SIV RNA-positive cells in the adjacent T-cell zone. (C) GALT. On day 13, dense collections of SIV RNA-positive cells in the follicular aggregate of this section from the jejunum were evident in the T-cell zone (TZ; enlarged view, right panel). On day 21, FDC-associated virion RNA was detectable in the outlined germinal center (GC), in addition to SIV RNA-positive cells (right panel).
DISCUSSION
In this and the accompanying study, we evaluated key factors likely to affect the ability of vaccine-induced immunity to prevent clinical HIV disease by determining the speed of viral dissemination from the vaginal portal of entry, the time required to establish a productive infection in the primary target lymphoid organs, and the magnitude of productive infection vis-à-vis the magnitude of the immune response in the SIV/rhesus monkey model of vaginal HIV transmission.
Implications of small founder populations of infected cells at transmission.
By using three criteria for productive infection (the detection of vDNA, multiply spliced vRNA, and SIV RNA-positive cells), we found that there was only a low level of productive SIV infection, limited mainly to the genital tract, 1 to 3 days after intravaginal inoculation of a high dose of SIV. Because we detected relatively little SIV RNA in cervical and vaginal tissues after intravaginal exposure to 2 × 109 copies of vRNA, we concluded that most of the inoculum was diluted and cleared in cervicovaginal fluids or retained in small amounts entrapped in cervical mucus. Thus, despite the large dose of virus used, the mucosal barrier, the mucus-trapping mechanisms, and the high ratio of defective to replication-competent virions limited the exposure of susceptible CD4+ T cells, DCs, and macrophages in the lamina propria to relatively small numbers of infectious virions. This accounts for the high dose of SIV needed to consistently infect animals by intravaginal inoculation.
The several-orders-of-magnitude reduction in the number of virions that penetrated the mucosal barrier should have resulted in very small founder populations of productively infected cells at the portal of entry. This prediction was supported by the findings that SIV RNA-positive cells were rare and the levels of viral RNA were very low in the genital tissues of animals necropsied from 24 to 96 h p.i.
We see four implications of the initially small size of infected cell populations at the portal of entry. First, modest improvements in barrier integrity might result in further reductions that are sufficient to prevent infection altogether. This explanation is consistent with the observations that increased vaginal SIV transmission due to exogenous progesterone and decreased vaginal SIV transmission due to exogenous estrogen are associated with thinning and thickening of the vaginal epithelium, respectively (13, 23). Second, this initial period of infection is one of maximum vulnerability when the outcome of infection for the virus hangs in the balance. It must sufficiently expand the founder population to sustain the infection when target cells may be limiting in the dispersed lymphocyte populations in the genital submucosa. Otherwise, the infection will burn out, because the basic reproductive rate cannot be maintained at or >1 (R0 ≥ 1). Third, the early stage of infection with a small founder population of infected cells is the time when the host defenses will have the fewest infected cells to eliminate. Although we show in the accompanying report that the small numbers of infected cells apparently do not send a sufficiently strong danger signal to elicit a robust mucosal SIV-specific CD8+ T-cell response at these early stages of infection, a more rapid and robust memory T-cell response induced in vaccinated animals might enable host defenses to clear the infection. Fourth, while cell tropisms, coreceptor usage, and host factors likely play roles in the reduction of virus genetic diversity at transmission, that reduction may also reflect the small fraction of the viral quasispecies in the inoculum that gains access to and infects cells in the submucosa.
Rapid dissemination from the portal of entry.
We detected vRNA at distal sites at 24 h p.i. in monkeys inoculated with AT-2-inactivated SIV, and there was evidence of both virus and infected cells, albeit at very low levels, at sites distal to the genital tract in animals exposed to live virus. We see two potential mechanisms for such rapid dissemination. First, inactivated viral particles might rapidly gain access to systemic lymphoid tissues after intravaginal SIV inoculation through lymphovascular anastamoses (3). Second, there is also good evidence that DCs can rapidly convey virions from the portal of entry. HIV-1 is transported by vaginal Langerhans cells to the draining lymph nodes of mice within 24 h p.i. (14), and SIV infection moves from the site of intradermal injection to the draining LNs within 16 h (26). Furthermore, SIV-infected cells (presumably Langerhans cells) are present in the subcapsular sinuses of the genital tract lymph nodes of rhesus monkeys 18 and 24 h after intravaginal inoculation (6).
If these mechanisms for rapid dissemination consistently and immediately established productive infection at distal sites, there would be little hope, in our view, that a vaccine could prevent infection. However, as we discuss below, the continued expansion of infection at the portal of entry is the engine that drives the process of continuous seeding of viruses and infected cells distally, thereby enhancing the prospects for the successful establishment of a productive infection in the systemic LTs. It follows that limiting the productive infection locally would still have the best chance of preventing or limiting a productive systemic infection.
Delayed systemic SIV replication despite rapid dissemination as model of infection.
Despite the fact that vDNA, vRNA, and vRNA-positive cells were detected in the distal systemic lymphoid tissues of monkeys necropsied from 1 to 4 days p.i., there was little evidence of active viral replication in these tissues until 6 days p.i. The delay in systemic replication may be related to a failure to reach the threshold of virus required to establish a productive infection in these LTs until 5 to 6 days p.i. We think the data on the dynamics and magnitude of productive infection following intravaginal inoculation fit a model (Fig. 7) in which two interrelated processes operate simultaneously. The infection grows in strength at the portal of entry, releasing limited amounts of virus and infected cells via lymphatic drainage and then the bloodstream until a threshold of sustainable infection is achieved at the distal sites. However, during this early seeding of distal sites, the low level of infection, while detectable, is insufficient to establish a productive infection for several reasons. First, many infections will be abortive because of the infection of cells by defective viruses or the infection of cells that are unable to sustain infection. For example, resting CD4+ T cells may be in a state in which they cannot be infected or can be infected but either become latently infected or produce too little virus to sustain infection. Second, the infected cells at distal sites may be too spatially dispersed to achieve the critical mass necessary to sustain the infection. Third, innate immune mechanisms in distal LTs may be able to contain the infection at this stage when there are so few infected cells.
FIG. 7.
Model of delayed systemic SIV replication despite rapid dissemination. In the first 3 days p.i., the relatively few infected cells produce enough virus and infected cells to sustain the infection locally. The virus, infected cells, and DCs carrying the virus also disseminate the infection to distal sites, but the level of virus replication is insufficient to sustain a productive infection (R0 ≥ 1). On days 4 to 6 p.i., increased virus production continues at the portal of entry and now is of sufficient strength in all systemic LTs to exceed the threshold required to maintain a productive infection. Arrows indicate the direction and degree (thickness) of virus dissemination.
In this model of delayed systemic SIV replication despite rapid dissemination, the expansion of infection at the portal of entry and seeding of the draining lymph nodes increase the number of cells releasing virus. The increased cumulative virus production (virus and infected cells) then achieves a sufficient magnitude at distal sites to establish the critical mass of virus and infected cells required to maintain the basic reproductive rate at or above 1, the sustainable infection threshold. At this point, the increased number of infected cells and virus in the tissues has also exceeded a second, higher threshold of detection set by the limits of the experimental methods in assay sensitivity and tissue sampling power.
This model can account for the apparent conflict between the detection of infection at distal sites soon after intravaginal SIV exposure and the later times at which infection can be documented at distal sites and is consistent with and supported by ISH results. Local expansion will lead to the oldest infections at the portal of entry, the detection of vRNA-positive cells there and not elsewhere at day 4, and an age of infection of cells in the same order as the routes of spread to genital draining LNs and peripheral blood, with subsequent seeding of the spleen, GALT, and peripheral LNs. The model can accommodate the detection of infection at low levels at distal sites at much earlier times, because even the few virions and infected cells that disseminate at early times can initiate infections in distal LTs.
Peak and decline of virus production and target cell exhaustion.
SIV RNA levels peaked in all tissues between days 10 and 21 p.i., and by day 28, the vRNA levels had declined at least 10-fold, with the decreases in numbers of vRNA-positive cells being even more dramatic (50-fold decrease in mesenteric LNs). We discuss in the accompanying paper the contributions of the SIV-specific CD8+ T-cell response to this decline and have discussed elsewhere the importance of the exhaustion of an initially relatively large pool of ostensibly resting CD4+ T-cell targets to this decline (30).
Vaccine implications.
Although the establishment of a productive systemic infection 6 days after intravaginal SIV exposure, as documented in these studies, is faster than has been reported in other studies (29), there are precedents for vaccines preventing clinically manifested disease from viral infections with similar dynamics. After the experimental inoculation of rhesus monkeys, measles virus disseminates from the conjunctiva and oropharynx to systemic lymphoid tissues in 7 days (15). While the highly effective live-attenuated measles vaccine does not prevent infection in this animal model, the vaccine dramatically reduces measles virus replication and dissemination and completely prevents the clinical signs of disease (15). Thus, the rapid dissemination of SIV, and by extension HIV, from mucosal portals of entry to target lymphoid tissues is not necessarily an insurmountable obstacle for HIV vaccine development. In fact, several groups have shown that attenuated lentiviral vaccines can protect against an intravaginal challenge with pathogenic SIV (1, 7, 22), with protection defined as either immune control of challenge virus replication or the complete inability to detect the challenge virus in the peripheral blood. After vaccination with SIV Δnef delivered to the tonsils of rhesus macaques, the vaccine virus was detectable in the tonsils of all vaccinees, but 7 to 10 days after a tonsillar SIVmac239 challenge, the challenge virus was only detected at this portal of entry in 4 of 10 monkeys (24). Thus, SIV infection was blocked at an early stage within the tonsillar mucosa that was exposed to the challenge virus, and this was associated with a significant increase in γδ T cells and mature dendritic cells in the tonsils relative to those of unvaccinated controls (24).
It remains to be shown that a similar early block of virus replication and dissemination occurs in attenuated vaccine models of vaginal HIV-1 transmission, but vaccine-mediated protection from systemic viral dissemination after vaginal HIV-1 exposure should be possible with a vaccine that induces robust and rapid recall immune responses, particularly at the portal of entry, when the ratio of effector cells to target cells will be most favorable.
Acknowledgments
We thank Jake Estes, Tim Schacker, and Cavan Reilly for helpful discussions; Ding Lu, Blia Vang, Kristen Bost, David Bennet, Linda Hirst, and Rino Dizon of the Immunology Core Laboratory and Primate Services Units at the CNPRC for excellent technical assistance; and Tim Leonard and Colleen O'Neill for help with the figures and the manuscript.
This work was supported by Public Health Service grants U51 RR00169, from the National Center for Research Resources, and R01 AI51239 and R01 AI51596 and by a consortial R01 grant (AI 48484) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dailey, P. J., M. Zamround, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. Presented at the 13th Annual Symposium on Nonhuman Primate Models of AIDS, Monterey, Calif.
- 3.Fokin, A. A., F. Robicsek, and T. N. Masters. 2000. Transport of viral-size particulate matter after intravenous versus intralymphatic entry. Microcirculation 7:357-365. [PubMed] [Google Scholar]
- 4.Hendrickx, A. G., and M. A. Cukierski. 1987. Reproductive and developmental toxicology in nonhuman primates, p. 73-88. In C. R. Graham (ed.), Preclinical safety of biotechnology products intended for human use. Alan R. Liss, Inc., New York, N.Y. [PubMed]
- 5.Hirsch, V., N. Riedel, and J. I. Mullins. 1987. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell 49:307-319. [DOI] [PubMed] [Google Scholar]
- 6.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu, F. X., Z. Ma, S. Moser, T. G. Evans, and C. J. Miller. 2003. Effects of ovarian steroids on immunoglobulin-secreting cell function in healthy women. Clin. Diagn. Lab. Immunol. 10:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, F. X., K. Abel, Z. Ma, T. Rourke, D. Lu, J. Torten, M. McChesney, and C. J. Miller. 2002. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin. Exp. Immunol. 128:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, Z., F. X. Lu, M. Torten, and C. J. Miller. 2001. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin. Immunol. 100:240-249. [DOI] [PubMed] [Google Scholar]
- 11.Marthas, M. L., D. Lu, M. C. Penedo, A. G. Hendrickx, and C. J. Miller. 2001. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retrovir. 17:1455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx, P. A., A. Gettie, J. H. Eldridge, J. K. Staas, R. M. Gilley, J. M. Mulligan, J. Mestecky, and R. W. Compans. 1992. Presented at the Tenth Annual Symposium on Nonhuman Primate Models of AIDS, San Juan, Puerto Rico.
- 13.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 14.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McChesney, M. B., C. J. Miller, P. A. Rota, Y. D. Zhu, L. Antipa, N. W. Lerche, R. Ahmed, and W. J. Bellini. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 233:74-84. [DOI] [PubMed] [Google Scholar]
- 16.Miller, C. J. 1994. Use of the SIV/rhesus macaque system to test virucides designed to prevent the sexual transmission of HIV, p. 213-223. In C. K. Mauck, M. Cordero, H. Gablenick, J. Speiler, and R. Rivera (ed.), Barrier contraceptives. Wiley-Liss, New York, N.Y.
- 17.Miller, C. J., N. J. Alexander, A. Gettie, A. G. Hendrickx, and P. A. Marx. 1992. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil. Steril. 57:1126-1128. [PubMed] [Google Scholar]
- 18.Miller, C. J., N. J. Alexander, and M. B. McChesney. 1994. Vaccines to prevent sexually transmitted diseases: the challenge of generating protective immunity at genital mucosal surfaces, p. 193-212. In G. L. Ada (ed.), Strategies in vaccine design. R.G. Landes Co., Austin, Tex.
- 19.Miller, C. J., N. J. Alexander, S. Sutjipto, A. G. Hendrickx, M. Jennings, and P. A. Marx. 1990. Effect of virus dose and nonoxynol-9 on the genital transmission of SIV in rhesus macaques. J. Med. Primatol. 19:401-409. [PubMed] [Google Scholar]
- 20.Miller, C. J., N. J. Alexander, S. Sutjipto, A. A. Lackner, A. G. Hendrickx, A. Gettie, L. J. Lowenstine, M. Jennings, and P. A. Marx. 1989. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, C. J., M. Marthas, J. Torten, N. J. Alexander, J. P. Moore, G. F. Doncel, and A. G. Hendrickx. 1994. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, C. J., M. B. McChesney, X. S. Lü, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with SHIV are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, S. M., G. B. Baskin, and P. A. Marx. 2000. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J. Infect. Dis. 182:708-715. [DOI] [PubMed] [Google Scholar]
- 24.Tenner-Racz, K., C. S. Hennig, K. Uberla, H. Stoiber, R. Ignatius, J. Heeney, R. M. Steinman, and P. Racz. 2004. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proc. Natl. Acad. Sci. USA 101:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 26.Wang, Y. C., S. S. Kim, D. Lu, X. J. You, S. Joye, H. Fan, and C. J. Miller. 2004. Use of a replication-defective vector to track cells initially infected by SIV in vivo: infected mononuclear cells rapidly appear in the draining lymph node after intradermal inoculation of rhesus monkeys. AIDS Res. Hum. Retrovir. 20:1300-1307. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2004. HIV AIDS epidemic update. World Health Organization, Geneva, Switzerland.
- 28.Wyand, M. S., K. H. Manson, C. J. Miller, and A. R. Neurath. 1999. Effect of 3-hydroxyphthaloyl-beta-lactoglobulin on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 43:978-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Z.-Q., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of simian and human immunodeficiency viruses in two distinguishable populations of CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Z.-Q., S. Wietgrefe, Q. Li, M. D. Shore, L. Duan, C. Reilly, J. D. Lifson, and A. T. Haase. 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA 101:5640-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]