Abstract
In the acute stage of infection following sexual transmission of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV), virus-specific CD8+ T-lymphocyte responses partially control but do not eradicate infection from the lymphatic tissues (LTs) or prevent the particularly massive depletion of CD4+ T lymphocytes in gut-associated lymphatic tissue (GALT). We explored hypothetical explanations for this failure to clear infection and prevent CD4+ T-lymphocyte loss in the SIV/rhesus macaque model of intravaginal transmission. We examined the relationship between the timing and magnitude of the CD8+ T-lymphocyte response to immunodominant SIV epitopes and viral replication, and we show first that the failure to contain infection is not because the female reproductive tract is a poor inductive site. We documented robust responses in cervicovaginal tissues and uterus, but only several days after the peak of virus production. Second, while we also documented a modest response in the draining genital and peripheral lymph nodes, the response at these sites also lagged behind peak virus production in these LT compartments. Third, we found that the response in GALT was surprisingly low or undetectable, possibly contributing to the severe and sustained depletion of CD4+ T lymphocytes in the GALT. Thus, the virus-specific CD8+ T-lymphocyte response is “too late and too little” to clear infection and prevent CD4+ T-lymphocyte loss. However, the robust response in female reproductive tissues may be an encouraging sign that vaccines that rapidly induce high-frequency CD8+ T-lymphocyte responses might be able to prevent acquisition of HIV-1 infection by the most common route of transmission.
In the natural history of sexual transmission of human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV), systemic infection is established throughout the lymphatic tissues (LTs) within the first weeks after exposure. Viral production and viremia peak at this time and then decline, temporally coincident with the development of virus-specific CD8+ T-lymphocyte responses (5, 14, 16, 24). However, in contrast to many other viral infections, the immune system is unable to completely clear infection. Depletion of CD4+ T lymphocytes, which is particularly severe in the gut-associated lymphoid tissue (GALT) (6, 19, 29, 33), is demonstrable in this acute stage in both HIV-1 and SIV infections and will generally progress in all LT compartments to levels where AIDS-associated opportunistic infections and cancers are manifest unless infection is controlled with antiretroviral therapy.
Understanding why HIV and SIV win this struggle with host defenses might provide important insights into designing an effective HIV vaccine. To that end, we investigated three hypothetical explanations for the failure to prevent infection and CD4+ T-lymphocyte loss following vaginal exposure. (i) The female reproductive tract is a poor inductive site to mount a virus-specific immune response and cannot thus prevent dissemination and establishment of systemic infection. (ii) The host mounts a robust response but not in enough time to prevent widespread systemic dissemination and establishment of a persistent infection in LTs. (iii) The response is of insufficient magnitude relative to the number of infected cells to fully contain infection.
We investigated these hypotheses with the SIV rhesus macaque model of vaginal transmission of HIV-1 by determining the relationship between the immune response in tissue compartments and the kinetics and magnitude of infection in those compartments described in the accompanying report. We focused on the virus-specific CD8+ T-lymphocyte response because of several lines of evidence that suggest that these responses partially control viral replication during acute infection. (i) Antigen-specific T lymphocytes are temporally associated with a reduction in plasma viremia (5, 14, 16, 24). (ii) CD8 depletion results in high-level viral replication (18, 25). (iii) The emergence of escape variants indicates selective pressure exerted by the CD8+ T lymphocytes (3, 22). We specifically investigated the Mamu-A*01-restricted immunodominant epitopes, Gag181-189CM9 and Tat28-35SL8, because the response to these two epitopes accounts for the majority of detectable CD8+ T-lymphocyte responses in Mamu-A*01-positive macaques (21) and because Gag CM9-specific responses following intravenous transmission have been documented in the mucosal and lymphoid tissue compartments which are relevant to understanding the reasons why immune defenses fail to fully protect animals following intravaginal exposure. Veazey et al., for example, detected Gag CM9-specific CD8+ T lymphocytes in the blood and the intestinal mucosa at 2 and 3 weeks postintravenous infection at similar frequencies (30, 32). During the chronic phase of infection, virus-specific CD8+ T lymphocytes were detected at similar levels in peripheral blood mononuclear cells (PBMC) and secondary lymphoid tissues (16), and tetramer-staining lymphocytes were found at comparable, or higher, frequencies in the vagina and intestinal mucosa (26, 28, 32).
Here, we show first that CD8+ lymphocytes in female reproductive tissues do respond to infection at the portal of entry, but there is a surprisingly long lag between CD8+ T-lymphocyte responses to the immunodominant SIV epitopes and peak levels of infection at the portal of entry and all LT compartments. Second, the magnitude of the virus-specific CD8+ T-lymphocyte responses, while relatively robust at the portal of entry, was modest in LTs generally and low or undetectable in the GALT. The overall disparity in the timing and magnitude of immunodominant response with respect to propagation and dissemination of infection is thus a major factor in the failure to clear infection and prevent CD4+ T-cell loss, particularly preferential depletion of CD4+ T lymphocytes in the GALT.
MATERIALS AND METHODS
Monkeys, SIV inoculum, and infection status.
As described in the accompanying paper by Miller et al. (19a), eight Mamu-A*01 rhesus monkeys became systemically infected after intravaginal inoculation.
Isolation of PBMC.
PBMC were isolated from heparin- or EDTA-treated blood samples using Lymphocyte Separation medium (ICN Biomedicals, Aurora, OH). PBMC not used in fresh assays were frozen in 10% dimethyl sulfoxide (Sigma, St. Louis, MO)-90% fetal bovine serum (Gemini BioProducts, Calabasas, Calif.) and stored in liquid nitrogen for future analyses.
Lymphoid tissue collection and lymphocyte isolation.
At necropsy, blood, intestine, spleen, and peripheral and genital lymph node (LN) samples were collected. In addition, cell suspensions were prepared as previously described (17) from whole blood, spleen, and peripheral and genital lymph nodes for gamma interferon (IFN-γ) enzyme-linked immunospot assay and fluorescence-activated cell sorter (FACS) analysis by gentle dissection of lymph nodes with scalpels in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini BioProducts, Calabasas, CA) (complete RPMI) and passage of the cell homogenate through a cell strainer (Fisher, Pittsburgh, PA). The cells were washed twice by centrifugation for 10 min at 1,600 rpm. Spleen tissue samples were cut into small pieces and homogenized with a syringe plunger. The homogenate was passed through a cell strainer. Splenic lymphocytes were isolated by gradient centrifugation with Lymphocyte Separation medium from ICN Biomedicals (Aurora, OH), followed by two washes with complete RPMI. PBMC were isolated from whole blood with Lymphocyte Separation medium (ICN).
Isolation of lymphocytes from the cervix and vaginal mucosa.
At necropsy, fresh cervix and vagina were collected in complete RPMI medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin (Sigma, Inc., St Louis, MO,) and 1% amphotericin B (Sigma). Lymphocytes were isolated as previously described (17) from genital mucosal tissues by cutting the tissues into small pieces (maximum size, 10 by 10 by 5 mm) and placing them in 1.2 U/ml Dispase II (Boehringer Mannheim GmbH, Germany) in complete RPMI medium (3 ml per piece). The tissue-dispase II mixture was incubated in a water bath shaker (New Brunswick Scientific, Inc., Edison, NJ) for 2 h at 37°C and 200 rpm. After being rinsed, the epithelial layer was removed from the underlying tissue with forceps and scalpel. The subepithelial tissues were cut into 2- by 5-mm slices (each, approximately 2 mm thick) and incubated in cell release medium. Cell release medium is complete RPMI 1640 containing 25 mM HEPES (Sigma), 5 × 10−3 M β-mercaptoethanol (Sigma), 0.5 mg/ml collagenase (Sigma), 0.1 mg/ml DNase (Sigma), and 0.02 mg/ml ciprofloxacin-HCl. The tissue was incubated for 3 to 4 h in a shaking water bath at 37°C and 100 rpm. To obtain cell suspensions from the lamina propria, 40 ml of the submucosal tissue suspension was shaken vigorously in a tube for 90 s. The resulting cell suspension was then passed through a 100-mesh sterile stainless steel sieve to remove larger pieces of debris and centrifuged at 530 × g for 10 min. Mononuclear cells were subsequently isolated on a discontinuous Percoll (Pharmacia) gradient. Cells were resuspended in 40% Percoll in RPMI medium, layered onto 75% Percoll in phosphate-buffered saline (PBS), and centrifuged at 530 × g for 30 min using the no-brake setting. Cell viability, measured by trypan blue exclusion, was typically >95%.
Lymphocyte isolation from uterus.
After being extensively washed in PBS, the uterus was cut into 4- to 6-mm2 pieces. The tissues were digested in R10 (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 2 mM l-glutamine, 25 mM HEPES buffer, 50 μg/ml streptomycin, 50 U/ml penicillin) containing type IV collagenase (0.5 mg/ml) (Sigma) and DNase type IV (100 U/ml) (Sigma) for 2 h at 37°C. The supernatant was collected, and lymphocytes were enriched by density gradient centrifugation using a 90% to 40% isotonic Percoll gradient at 1,200 rpm for 30 min at room temperature. The lymphocytes were collected from the 90% to 40% Percoll interface. Cells were washed in R10.
Isolation of lymphocytes from GALT.
Sections of colon and jejunum, 6- to 8-in long, were cut into approximately 5-mm2 pieces and incubated in complete Hank's balanced salt solution (Sigma, St. Louis, MO) supplemented with 10% FCS, 25 mM HEPES buffer, and 1 mM EDTA at 37°C for 90 min. Supernatant, containing intraepithelial lymphocytes (IEL), was collected and run over glass wool loosely packed into a 30-ml syringe to remove large particulates. Flowthrough was washed twice with 30 ml of R10. To release the lamina propria lymphocytes (LPL), the remaining tissue was cut into small pieces of ∼1 mm2 each and incubated for 90 min at 37°C in R10 containing type IV collagenase (15 U/ml) (Sigma, St. Louis, MO) and DNase type IV (100 U/ml) (Sigma, St. Louis, MO). Supernatant was collected, run over loosely packed glass wool, and washed twice in 30 ml of R10. Both IEL and LPL were enriched from epithelial cells by centrifuging over a 90% to 40% isotonic Percoll gradient. Lymphocytes were collected from the 90% to 40% Percoll interface.
Intracellular cytokine staining of fresh lymphocytes.
Intracellular cytokine (ICS) assays were performed as previously described (12). Briefly, 5 × 105 to 1 × 106 freshly isolated lymphocytes were incubated with individual peptides at a concentration of 5 μg/ml along with 0.5 μg anti-CD28 (clone L293; BD Biosciences, San Diego, CA) and 0.5 μg anti-CD49d (clone 9F10; BD Pharmingen, San Diego, CA) for optimal costimulation in a total volume of 200 μl R-10. Staphylococcal enterotoxin B (10 μg/ml; Sigma, St. Louis, MO) was used as a positive control while R10, anti-CD28, and anti-CD49d alone were used as a negative control. After incubation for 1.5 h at 37°C 10 μg/ml of brefeldin A (Sigma, St. Louis, MO) was added to inhibit secretion of cytokines. The cells were further incubated for 5 h at 37°C. Cells were washed twice with FACS buffer (PBS plus 2% FCS) and stained with anti-CD8α-PerCP (clone SK1; Becton Dickinson) and anti-CD4-APC (clone SK3; Becton Dickinson) in 100 μl FACS buffer for 40 min at room temperature. After two washes, the cells were fixed with 2% paraformaldehyde (PFA)-PBS solution overnight at 4°C. After being washed twice with permeabilization buffer (0.1% saponin in FACS buffer) the cells were stained with anti-human IFN-γ-fluorescein isothiocyanate (FITC) monoclonal antibody (MAb) (clone 4S.B3; Pharmingen) and anti-human tumor necrosis factor alpha (TNF-α)-PE MAb (clone Mab11; Pharmingen) and incubated for 50 min at room temperature. Afterwards, the cells were washed twice with permeabilization buffer and fixed with 2% PFA-PBS. A total of 10,000 to 200,000 lymphocyte-gated events were acquired on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with FlowJo software (Treestar). All values are reported after subtraction of the background level staining.
Tetramer staining and FACS.
From 5 × 105 to 1 × 106 freshly isolated lymphocytes were suspended in a 100-μl volume of R10 and stained for 1 h at 37°C with Mamu-A*01/peptide tetramer labeled with phycoerythrin or antigen-presenting cell. After a 1-h incubation, the lymphocytes were stained for surface markers using anti-human CD3ɛ-FITC (SP34; Pharmingen) and anti-CD8α-PerCP (clone SK1; Becton Dickinson) and incubated for an additional 40 min at room temperature. The cells were washed twice with FACS buffer and fixed with 2% PFA-PBS. Sample data was acquired using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar). Background staining of CD3−/CD8+ lymphocytes in all tissues was typically ≤0.1%.
In situ tetramer (IST) staining.
Biotinylated Mamu-A*01/β2m/peptide molecules were produced with Gag (CTPYDINQM), Tat (TTPESANL), or irrelevant (FLPSDYFPSV) peptides at the National Institute of Allergy and Infectious Diseases tetramer facility. Tetramers were generated by adding six aliquots of FITC-labeled ExtraAvidin (Sigma) to biotinylated Mamu-A*01/β2m/peptide monomers over the course of 8 h to a final molar ratio of 4.5:1. IST staining was performed essentially as previously described (27). Fresh lymph nodes, spleen, uterus, and vaginal and cervical tissues were shipped on ice overnight in PBS or PBS with 100 μg/ml heparin (PBS-H). Tissues were cut into 0.5-cm pieces and embedded in 4% low-melt agarose. Tissue blocks were placed in a vibratome bath containing 0 to 4°C PBS-H, and 200-mm-thick sections were generated. Fresh sections were stained free floating in 1 ml of solution with four sections per well in 24-well tissue culture plates. Incubations were carried out at 4°C on a rocking platform. Tetramers were added at a concentration of 0.5 mg/ml with 2% normal goat serum (NGS) and 0.5 mg/ml mouse anti-human CD8 antibodies (Dako clone DK25) and incubated overnight. Sections were washed with chilled PBS-H and then fixed with 4% paraformaldehyde for 2 h at room temperature. Sections were again washed with PBS-H, incubated with rabbit anti-FITC antibodies (BioDesign) diluted 1:10,000 in PBS-H with 2% NGS, and incubated for 1 to 3 days. Sections were washed three times with PBS-H for at least 20 min and then incubated with Cy3-conjugated goat anti-rabbit antibodies and Alexa 488-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch), both diluted 1:1,000 in PBS-H with 2% NGS, for 1 to 3 days. Finally, sections were washed three times for at least 20 min in PBS-H, postfixed with 4% paraformaldehyde for 1 h, and then mounted on slides with warmed glycerol gelatin (Sigma) containing 4 mg/ml n-propyl galate. Stained sections were analyzed using a Bio-Rad 1000 confocal microscope.
RESULTS
CD8+ T-lymphocyte responses to immunodominant SIV epitopes lag behind peak levels of virus production at the portal of entry and LTs.
In the accompanying report, we show that infection is established beyond the portal of entry in the proximal and distal LTs by the end of the first week postintravaginal inoculation (PI). We found that viral replication peaked in all tissues and blood between days 10 and 14 PI and then declined by an order of magnitude or more by day 28 PI, the last time point examined.
Here, we investigated the relationship between propagation and spread of infection and the development of SIV-specific CD8+ T-lymphocyte responses to the Mamu-A*01-restricted immunodominant epitopes in Gag (Gag CM9) and Tat (Tat SL8) that comprise the majority of the detectable virus-specific CD8+ T-lymphocyte responses during acute infection of Mamu-A*01+ macaques (21). To make optimal use of Mamu-A*01+ macaques, we first bracketed the times virus-specific cellular immune responses were detectable in Mamu-A*01− macaques by an optimized enzyme-linked immunospot assay shown previously to be comparable in sensitivity to flow cytometric assays (23). We did not detect significant cellular immune responses until day 14 PI and then in only one of the two animals. By 21 days PI, virus-specific cellular immune responses were detectable in the reproductive and secondary lymphoid tissues (not shown). Therefore, we focused the detailed compartmental analysis in Mamu-A*01+ macaques at 13 to 28 days PI, spanning the peak through decay stages of declining viral loads and productively infected cells (19a).
We determined the location and magnitude of epitope-specific T lymphocytes by collecting tissue and isolating lymphocytes from the female reproductive tract (vagina, cervix, and uterus), the colon and jejunum, the proximal lymphoid tissues draining the site of inoculation (genital and inguinal lymph nodes), the distal lymphoid tissues (mesenteric lymph nodes, axillary lymph nodes, and spleen) and the blood. CD8+ T-lymphocyte responses against Gag CM9, Tat SL8, and two previously reported Mamu-A*01 restricted subdominant responses, Env233-241CL9 and Env620-628TL9 (10), were detected by in situ staining and FACS analysis using Mamu-A*01/peptide tetrameric complexes. Peptide-specific cytokine secretion was monitored by ICS using antibodies against IFN-γ and TNF-α. We present only data for responses against Gag CM9 and Tat SL8, because measurable responses to the Env epitopes were not detectable. Additionally, both IEL and LPL were isolated from the colon and jejunum, but little or no difference in the frequency of epitope-specific CD8+ T-lymphocyte responses was detected between these compartments. We therefore present only the responses from the LPL.
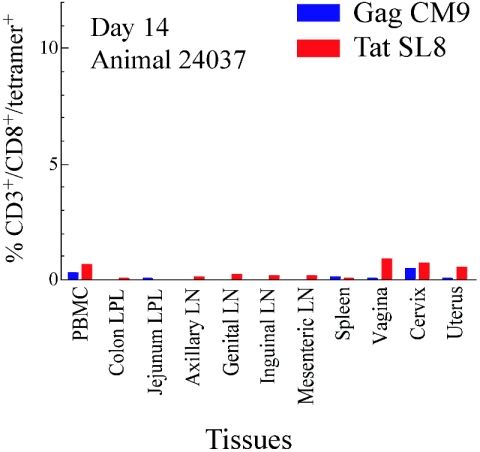
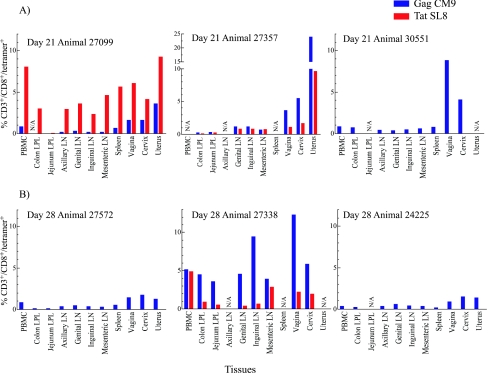
At 13 to 14 days PI, 4 days after the peak levels of virus in female reproductive tissues (19a), we detected low but significant responses against the Gag CM9 and Tat SL8 epitopes in the reproductive tract and in blood, but not elsewhere (Fig. 1) in one of two animals. At days 20 to 21 PI, we detected Gag CM9- and Tat SL8-specific responses at different levels in the female reproductive tract tissues and lymphatic tissue compartments in the three animals tested (Fig. 2 and Fig. 3A). The tissues of the female reproductive tract consistently had high frequencies of Gag CM9- and Tat SL8-specific CD8+ T lymphocytes (range, 1 to 5%, with a high of 24%). In contrast to this relatively robust response at the site of initial exposure, there were only modest responses in the draining and distal LNs and very low or undetectable responses in the GALT (Fig. 3A). Similarly, at days 27 to 28, the frequencies of tetramer-positive cells were highest in the reproductive tissues, followed by the draining and/or distal LNs, and relatively low in the colon and jejunum (Fig. 3B). IST staining of lymph nodes, spleen, vagina, and cervix from each animal similarly revealed Gag CM9- and Tat SL8-specific CD8+ lymphocytes in reproductive and lymphoid tissues at 20 to 21 and 27 to 28 but not 13 to 14 days PI (Fig. 4). Thus, particularly in GALT, the level of immunodominant response not only lagged well behind the peak of viral replication but was also the lowest of any LT compartment.
FIG. 1.
Low frequency of CD8+ T-lymphocyte responses at 14 days postintravaginal infection. The frequency of tetramer binding CD3+/CD8+ lymphocytes isolated from different lymphoid tissues is shown.
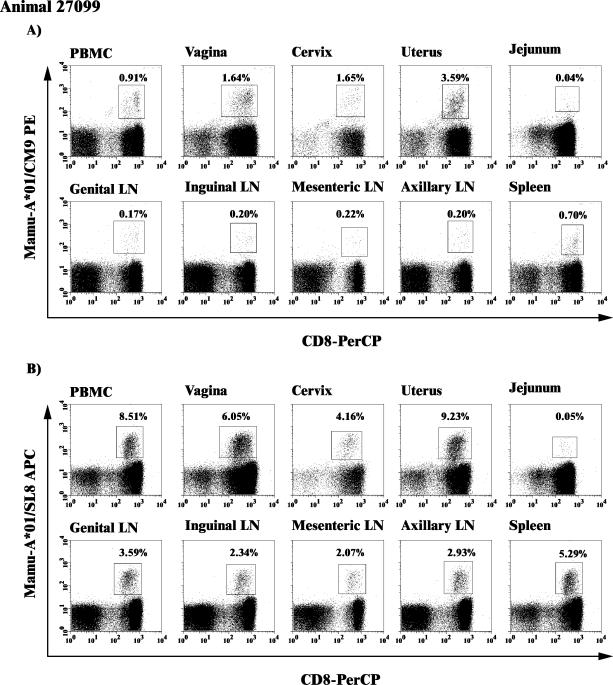
FIG. 2.
Mamu-A*01/Gag CM9 and/Tat SL8 tetramer binding CD3+/CD8+ lymphocytes in multiple lymphoid compartments. The frequency of Mamu-A*01/Gag CM9 (A) or Mamu-A*01/Tat SL8 (B) tetramer binding cells from multiple lymphoid tissues is shown. The dot plots are gated on CD3+ lymphocytes, but the frequencies are reported as the percentages of CD3+/CD8+ lymphocytes. Animal 27099 is shown as an example, as data from all animals were analyzed in this manner.
FIG. 3.
Frequency of CD3+/CD8+ lymphocyte responses against immunodominant SIV epitopes at 20 to 21 and 27 to 28 days postintravaginal infection. The frequency of tetramer binding CD3+/CD8+ lymphocytes isolated from different lymphoid tissues is shown at day 20 to 21 (A) and day 27 to 28 (B). Mamu-A*01/Tat SL8 tetramer staining is unavailable for samples from animals 30551, 27572, and 24225. From some tissues, tetramer staining was not available (N/A) because an insufficient number of lymphocytes were isolated or collected on the FACSCalibur for analysis.
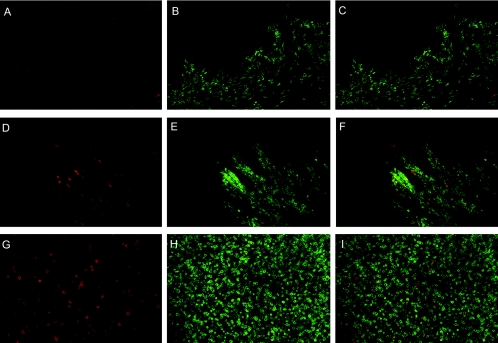
FIG. 4.
Comparison of IST staining in vagina at 13 days postinfection (dpi) (top panels) to 28 dpi (middle panels) and an example of tetramer-positive CD8+ T cells in lymph tissues (bottom panels). In each set of panels, the left image is Mamu-A*01 Gag181-189CM9 or Tat28-35SL8 tetramer stain (red), the middle image is CD8 antibody stain (green), and the right panel is a merged image of the red and green images. Panels A to C and D to F are representative images that show staining in vaginal epithelia at 13 dpi (animal 28035), and 28 dpi (27338), respectively. (G to I) Obturator lymph node from 20 dpi (27357). Images were collected using a 20× objective and are a z-series projection in which Z-scans were collected at 2-μm intervals into the tissue, with the vaginal epithelium images encompassing 10 μm of tissue and the lymph tissue image encompassing 20 μm of tissue.
We found no reproducible difference in the distribution pattern of the CD8+ T-lymphocyte responses against the two-immunodominant SIV epitopes in terms of their frequencies among the different tissue compartments. Furthermore, we found no evidence for higher epitope diversity with respect to the four SIV epitopes in the reproductive tissues and regional lymph nodes investigated compared to other mucosal sites or lymphoid tissues (data not shown).
Homing of IFN-γ-secreting SIV-specific CD8+ T lymphocytes to the cervicovaginal mucosa.
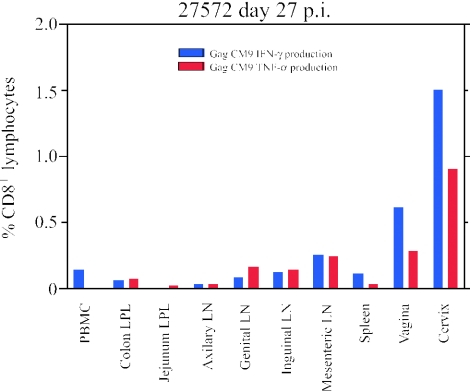
Because insufficient numbers of cells were recovered to directly measure cytotoxicity of freshly isolated CD8+ T lymphocytes from the female reproductive tissues and GALT, we evaluated the effector status and location of the SIV epitope-specific lymphocytes by ICS for production of IFN-γ and TNF-α. We detected cytokine-secreting cells in all but one of the animals, in larger numbers in the female reproductive tract than in the LNs and the GALT (Table 1), as illustrated for animal 27572 (Fig. 5). We speculate that the effects hormonal changes during the menstrual cycle have on effector function (8, 35) may be the reason that we detected tetramer-positive cells but not cytokine-secreting cells in one animal (24225).
TABLE 1.
Frequency of peptide-specific IFN-γ and TNF-α production by CD8+ lymphocytes among the different lymphoid and nonlymphoid tissues, as measured by intracellular staininga
| Animal | Necropsy (days P1) | PBMC
|
Colon LPL
|
Jejunum LPL
|
Axillary LN
|
Genital LN
|
Inguinal LN
|
Mesenteric LN
|
Spleen
|
Vagina
|
Cervix
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | (−) | CM9 | SL8 | ||
| 28035 | 13 | 0.02 | 0.10 | 0.01 | − | − | − | 0.05 | ≤0 | ≤0 | − | − | − | − | − | − | 0.09 | ≤0 | ≤0 | 0.06 | ≤0 | 0.04 | − | − | − | − | − | − | − | − | − |
| 24037 | 14 | 0.02 | 0.17 | 0.23 | 0.04 | 0.01 | 0.01 | 0.02 | ≤0 | ≤0 | 0.00 | 0.03 | 0.04 | 0.01 | ≤0 | 0.04 | 0.02 | 0.02 | 0.02 | 0.03 | 0.06 | 0.04 | 0.02 | 0.15 | 0.23 | 0.05 | ≤0 | 0.02 | 0.09 | ≤0 | 0.24 |
| 27357 | 20 | 0.14 | 1.94 | 0.71 | 0.30 | 0.42 | 0.17 | 0.06 | 0.08 | 0.23 | − | − | − | 0.08 | 0.58 | 0.42 | 0.14 | 0.50 | 0.61 | 0.02 | 0.15 | 0.16 | − | − | − | − | − | − | 0.94 | 1.52 | 1.27 |
| 27099 | 21 | 0.08 | 0.16 | 0.70 | − | − | − | 0.02 | ≤0 | 0.02 | 0.03 | 0.04 | 0.21 | 0.02 | 0.07 | 0.23 | 0.03 | 0.04 | 0.15 | 0.04 | 0.10 | 0.26 | 0.10 | 0.58 | 0.50 | 0.47 | 0.83 | 1.43 | − | − | − |
| 30551 | 21 | 1.04 | 1.05 | 0.99 | 2.24 | 2.29 | 2.49 | − | − | − | 0.18 | 2.54 | 2.55 | 0.09 | 1.72 | 1.97 | 0.34 | 1.40 | 2.41 | 0.21 | 2.12 | 3.38 | 0.27 | 1.05 | 1.60 | 0.18 | 5.80 | 10.24 | 0.26 | 7.58 | 10.38 |
| 24225 | 28 | 0.07 | 0.19 | 0.59 | 1.24 | 0.04 | 0.67 | − | − | − | 0.12 | ≤0 | 0.19 | 0.16 | 0.01 | 0.25 | 0.18 | ≤0 | ≤0 | 0.12 | 0.03 | 0.06 | 0.11 | 0.12 | 0.19 | 0.59 | ≤0 | 0.52 | 0.29 | 0.32 | 0.38 |
| 27572 | 27 | 2.22 | 0.14 | 0.53 | 0.02 | 0.06 | 0.14 | 0.02 | ≤0 | ≤0 | 0.01 | 0.03 | 0.03 | 0.02 | 0.08 | 0.06 | 0.03 | 0.12 | 0.01 | 0.34 | 0.25 | ≤0 | 0.04 | 0.11 | 0.21 | 0.20 | 0.62 | 0.34 | 0.13 | 1.50 | 0.92 |
| 27338 | 28 | 0.08 | 1.16 | 5.10 | 0.38 | 0.62 | 2.21 | 0.12 | 0.30 | − | − | − | − | 0.02 | 0.38 | 0.51 | 0.12 | 0.39 | 0.95 | 0.11 | 1.00 | 0.66 | − | − | − | 0.08 | 2.74 | 8.24 | 0.27 | 2.03 | 5.50 |
| 28035 | 13 | 0.06 | 0.11 | 0.03 | − | − | − | 0.11 | 0.03 | ≤0 | − | − | − | 0.10 | ≤0 | ≤0 | − | − | − | 0.08 | ≤0 | 0.07 | − | − | − | − | − | − | − | − | − |
| 24037 | 14 | 0.38 | ≤0 | ≤0 | 0.27 | ≤0 | ≤0 | 0.20 | ≤0 | 0.04 | 1.01 | ≤0 | ≤0 | 0.76 | ≤0 | 0.08 | 0.86 | ≤0 | ≤0 | 0.20 | 0.01 | 0.05 | 0.16 | 0.03 | 0.08 | 0.35 | ≤0 | ≤0 | 0.28 | ≤0 | 0.05 |
| 27357 | 20 | 0.58 | 1.64 | 0.37 | 0.34 | 0.22 | 0.17 | 0.40 | 0.11 | 0.18 | − | − | − | 0.20 | 0.22 | 0.18 | 0.31 | 0.18 | 0.33 | 0.20 | ≤0 | 0.02 | − | − | − | − | − | − | 0.59 | 1.42 | 1.28 |
| 27099 | 21 | 0.33 | 0.11 | 0.05 | − | − | − | 0.10 | ≤0 | 0.08 | 0.19 | ≤0 | 0.03 | 0.53 | ≤0 | ≤0 | 0.51 | ≤0 | ≤0 | 0.41 | ≤0 | ≤0 | 0.85 | ≤0 | 0.37 | 1.12 | 0.21 | 0.10 | − | − | − |
| 30551 | 21 | 1.46 | 0.49 | 0.17 | 2.25 | 1.16 | 1.00 | − | − | − | 0.58 | 1.85 | 1.36 | 0.55 | 0.82 | 0.90 | 0.81 | 0.76 | 1.35 | 0.77 | 1.13 | 1.60 | 0.77 | 0.45 | 0.73 | 0.67 | 2.76 | 4.53 | 0.81 | 6.89 | 8.57 |
| 24225 | 28 | 0.63 | 0.21 | 0.40 | 1.59 | ≤0 | 0.59 | − | − | − | 2.03 | ≤0 | 0.24 | 1.25 | ≤0 | 0.20 | 1.96 | ≤0 | ≤0 | 0.82 | 0.05 | ≤0 | 0.81 | 0.10 | 0.14 | 1.20 | ≤0 | 0.22 | 1.51 | ≤0 | ≤0 |
| 27572 | 27 | 2.41 | ≤0 | ≤0 | 0.38 | 0.07 | 0.14 | 0.09 | 0.02 | ≤0 | 0.12 | 0.03 | 0.03 | 0.12 | 0.16 | 0.03 | 0.09 | 0.14 | ≤0 | 0.80 | 0.24 | ≤0 | 0.27 | 0.03 | 0.11 | 0.51 | 0.28 | ≤0 | 0.27 | 0.90 | 0.41 |
| 27338 | 28 | 0.27 | 0.70 | 3.43 | 1.04 | ≤0 | 2.08 | 1.06 | 0.24 | − | − | − | − | 1.05 | ≤0 | 2.11 | 1.06 | 0.32 | 1.08 | 0.44 | 0.58 | 0.31 | − | − | − | 0.60 | 2.06 | 5.86 | 0.59 | 1.41 | 3.81 |
Peptide-specific responses are reported after background has been deducted. The column (−) is the frequency of nonspecific cytokine production detected in the medium-only negative control. The symbol − in the table represents samples not tested, and ≤0 represents samples that were at or below background. IFN-γ production was measured in the data shown in the first eight rows, and TNF-α production was measured in the data shown in the next eight rows.
FIG. 5.
Peptide-specific cytokine production. Cytokine-producing CD8+ lymphocytes are detected at higher frequencies in female reproductive tissues than in lymph nodes and GALT. Animal 27572 is used as an example.
DISCUSSION
In the accompanying report, we show that systemic infection is quickly established throughout the LTs and show in this report that this occurs before a significant virus-specific CD8+ T-lymphocyte response to the major immunodominant SIV epitopes has developed. Robust CD8+ T-lymphocyte responses against two immunodominant epitopes are detected only after the peak of viremia and then mainly in the female reproductive tract but not in the GALT. Moreover, while strong responses were detected in the three animals infected for 20 to 21 days, lower frequencies of tetramer-positive cells were observed in two of the three animals infected for 27 to 28 days, consistent with declining virus-specific CD8+ T-lymphocyte responses from a peak earlier in infection. The strikingly different virus-specific CD8+ T-lymphocyte response in the female reproductive tissues and the GALT is consistent with an increasing body of evidence that suggests that the mucosal immune system is a distinctly compartmentalized rather than a single system (15, 20, 34). We found relatively poor virus-specific responses in the GALT after intravaginal inoculation, in contrast to a previous study where relatively high frequencies of Gag CM9-specific CD8+ T lymphocytes were detected in the GALT during acute infection after intravenous inoculation (30). In our study, virus-specific CD8+ T lymphocytes were concentrated in the female reproductive tract. The relatively poor CD8+ T-lymphocyte response in GALT, compared to the response in the female reproductive tract, may be due, at least in part, to the preferential homing of virus-specific effector cells back to the initial site of infection (20). This would account for the higher frequency of epitope-specific responses in the female reproductive tissues and the decreased frequency of responses at more distal sites. For two reasons, we think that it is unlikely that the explanation for the low response is the loss of CD8+ T lymphocytes during isolation because of the susceptibility of GALT lymphocytes to undergo apoptosis. (i) The proportion of CD8+ T lymphocytes isolated from the intestinal tissues was high (65 to 90%). (ii) Using these same isolation procedures, we have detected high frequencies of tetramer-positive cells in GALT in chronically infected animals and in the acute stages of infection in animals infected intrarectally (unpublished data), similar to results previously described by other investigators (26).
The preferential depletion of CD4+ T lymphocytes in the GALT by SIV and HIV may reflect infection of larger numbers of susceptible CD4+ T-lymphocyte targets (2, 4, 31, 33). However, the poor to nonexistent virus-specific CD8+ T lymphocytes documented here may be an important contributing factor in the preferential CD4+ T-lymphocyte depletion in GALT.
The robust immune response following intravaginal inoculation was somewhat unexpected in light of a recent report that a recombinant poxvirus strain of modified vaccinia virus Ankara failed to elicit CD8+ T-lymphocyte responses in the female reproductive tract of mice when the vaccine was administered intravaginally but did elicit a response after intranasal inoculation (11). Here, we show that the lower female reproductive tract in primates can be an excellent antigen-specific CD8+ T-lymphocyte-priming site when exposed to replication-competent SIV.
This compartmental analysis reveals some of the potential reasons virus-specific CD8+ T-lymphocyte responses fail to fully contain infection in the LTs targeted by SIV and, by extension, HIV, because of the relatively low frequency of virus-specific CD8+ T lymphocytes in LTs after peak virus production. However, a vaccine-induced local recall response might control viral replication before the immune system must contend with large numbers of infected cells distributed throughout the LTs. Evidence that a local response might prevent transmission comes from studies in which antigen-specific IFN-γ-producing CD8+ T lymphocytes were detected in cervical tissue samples of women exposed to seropositive partners who remain seronegative with no detectable plasma viral loads (13).
In summary, we show here that SIV replicates to peak levels unchecked by a CD8+ T-lymphocyte response against immunodominant SIV epitopes for at least 1 week and, in the GALT for an additional 2 weeks following intravaginal transmission. CD4+ T lymphocytes in GALT thus lack protection from infection and the cytopathic effects of SIV replication for an extended period, which may be one reason that CD4+ T-lymphocyte depletion is greater in GALT than in other lymphoid organs. In this experimental model of the natural history of intravaginal transmission and acute infection, the immune response is too little, too late, just as it may be in preventing the establishment of a persistent HIV-1 infection (9). To be successful, a vaccine would clearly have to induce a much more rapid and robust recall response, not only in the reproductive tissues where infection begins but also throughout all LT compartments. Nonetheless, the robust response in female reproductive tissue is an encouraging sign that it may be possible to develop an effective vaccine that limits infection at the portal of entry.
Acknowledgments
We thank the Immunology Core Laboratory and Primate Services Unit of the California National Primate Research Center (CNPRC); the staff at the Wisconsin Primate Research Center (WPRC); Ding Lu, Tracy Rourke, Rino Dizon, and Blia Vang for technical assistance; and Colleen O'Neill and Tim Leonard for help in preparing the manuscript and figures.
This work was supported by NIH grants R01 AI48484 to A.H., U51 RR00169 to the CNPRC, R01 AI51239 and R01 AI51596 to C.J.M., and P51 RR00167 to the WPRC.
REFERENCES
- 1.Reference deleted.
- 2.Agace, W. W., A. I. Roberts, L. Wu, C. Greineder, E. C. Ebert, and C. M. Parker. 2000. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 30:819-826. [DOI] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Anton, P. A., J. Elliott, M. A. Poles, I. M. McGowan, J. Matud, L. E. Hultin, K. Grovit-Ferbas, C. R. Mackay, I. S. Y. Chen, and J. V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14:1761-1765. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Cohen, J. H., L. Danel, G. Cordier, S. Saez, and J. P. Revillard. 1983. Sex steroid receptors in peripheral T cells: absence of androgen receptors and restriction of estrogen receptors to OKT8-positive cells. J. Immunol. 131:2767-2771. [PubMed] [Google Scholar]
- 9.Davenport, M. P., R. M. Ribeiro, and A. P. Perelson. 2004. Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J. Virol. 78:10096-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furchner, M., A. L. Erickson, T. Allen, D. I. Watkins, A. Sette, P. R. Johnson, and C. M. Walker. 1999. The simian immunodeficiency virus envelope glycoprotein contains two epitopes presented by the Mamu-A*01 class I molecule. J. Virol. 73:8035-8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gherardi, M. M., E. Perez-Jimenez, J. L. Najera, and M. Esteban. 2004. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J. Immunol. 172:6209-6220. [DOI] [PubMed] [Google Scholar]
- 12.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 14.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkel, E. J., and E. C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16:1-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 17.Lu, F. X., K. Abel, Z. Ma, T. Rourke, D. Lu, J. Torten, M. McChesney, and C. J. Miller. 2002. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin. Exp. Immunol. 128:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Miller, C. J., Q. Li, K. Abel, E.-Y. Kim, Z.-M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79:9217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mora, J. R., M. R. Bono, N. Manjunath, W. Weninger, L. L. Cavanagh, M. Rosemblatt, and U. H. Von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424:88-93. [DOI] [PubMed] [Google Scholar]
- 21.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 23.Pahar, B., J. Li, T. Rourke, C. J. Miller, and M. B. McChesney. 2003. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J. Immunol. Methods 282:103-115. [DOI] [PubMed] [Google Scholar]
- 24.Reimann, K. A., K. Tenner-Racz, P. Racz, D. C. Montefiori, Y. Yasutomi, W. Lin, B. J. Ransil, and N. L. Letvin. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68:2362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz, J. E., R. S. Veazey, M. J. Kuroda, D. B. Levy, A. Seth, K. G. Mansfield, C. E. Nickerson, M. A. Lifton, X. Alvarez, A. A. Lackner, and N. L. Letvin. 2001. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood 98:3757-3761. [DOI] [PubMed] [Google Scholar]
- 27.Skinner, P. J., and A. T. Haase. 2002. In situ tetramer staining. J. Immunol. Methods 268:29-34. [DOI] [PubMed] [Google Scholar]
- 28.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 76:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 30.Veazey, R. S., M. C. Gauduin, K. G. Mansfield, I. C. Tham, J. D. Altman, J. D. Lifson, A. A. Lackner, and R. P. Johnson. 2001. Emergence and kinetics of simian immunodeficiency virus-specific CD8+ T cells in the intestines of macaques during primary infection. J. Virol. 75:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey, R. S., and A. A. Lackner. 1998. The gastrointestinal tract and the pathogenesis of AIDS. AIDS 12(Suppl. A):S35-S42. [PubMed] [Google Scholar]
- 32.Veazey, R. S., J. D. Lifson, J. E. Schmitz, M. J. Kuroda, M. Piatak, Jr., I. Pandrea, J. Purcell, R. Bohm, J. Blanchard, K. C. Williams, and A. A. Lackner. 2003. Dynamics of simian immunodeficiency virus-specific cytotoxic T-cell responses in tissues. J. Med. Primatol. 32:194-200. [DOI] [PubMed] [Google Scholar]
- 33.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weninger, W., N. Manjunath, and U. H. von Andrian. 2002. Migration and differentiation of CD8+ T cells. Immunol. Rev. 186:221-233. [DOI] [PubMed] [Google Scholar]
- 35.White, H. D., K. M. Crassi, A. L. Givan, J. E. Stern, J. L. Gonzalez, V. A. Memoli, W. R. Green, and C. R. Wira. 1997. CD3+ CD8+ CTL activity the within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J. Immunol. 158:3017-3027. [PubMed] [Google Scholar]