Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax oncoprotein can repress the transcriptional activity of the tumor suppressor protein p53. However, it remains controversial whether Tax requires NF-κB factors/activity and/or p300/CBP in order to inactivate p53 function. To address this issue, we have investigated Tax's effect on p53's transcriptional activation in IκB-kinase-deficient mouse embryonic fibroblasts (MEFs); some of which are entirely silent for Tax-induced NF-κB activity. We found that, in IKKα−/−, IKKβ−/−, and IKKγ−/− MEFs, p53 activation of a prototypic responsive plasmid (pG13-luciferase) was repressed by wild-type Tax. Curiously, p53's activity in MEFs was also repressed by a p300/CBP-binding deficient Tax protein. Our results highlight the complex nature of Tax-mediated repression of p53- activity, which requires further investigation.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent for adult T-cell leukemia (ATL) (22, 33). While the mechanism of tumorigenesis by HTLV-1 is not fully understood, the viral Tax protein is strongly implicated in the transformation of cells (7, 8, 16, 23, 28). Tax has pleiotropic effects on host cell gene expression and activates both CREB/ATF (reviewed in reference 3) and NF-κB (26) pathways.
Tumor suppressor p53 is a DNA-binding transcription factor which plays a key role in the response of cells to DNA damage and transformation (31). Approximately 50% of all cancers have mutations in the TP53 gene (which encodes p53). Relevant to HTLV-1, it was first observed that p53 mutation is present in only a small fraction of patients with ATL and that within ATL cells the p53 protein appears to be stabilized (27). Subsequently, many investigators have independently demonstrated that p53's transcriptional activity is suppressed by the HTLV-1 Tax oncoprotein (1, 2, 15, 18, 24, 30).
Relevant to the mechanism of p53 inactivation by Tax, there are two schools of thought. One subscribes to the concept that Tax competes with p53 for the binding of the p300/CBP transcriptional coactivator (2, 29). The second suggests that Tax acts through an NF-κB/RelA(p65) activation pathway to perturb p53 function (19, 20). In this study, we have interrogated mouse embryonic fibroblasts (MEFs) genotypically deficient in IKKα (IKKα−/−), IKKβ (IKKβ−/−), and IKKγ (IKKγ−/−) for Tax-p53 functional interplay. We also asked whether in the same three MEFs, a Tax mutant (K88A), previously shown to be incapable of binding CBP, remains proficient for suppressing p53's transcriptional activity.
Tax repressed p53's transcriptional activity in IKKα−/−, IKKβ−/−, and IKKγ−/− MEFs.
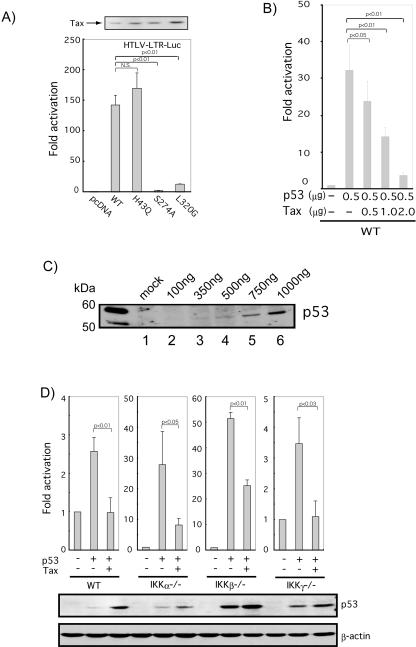
Previous findings showed that Tax can repress the transcriptional activity of p53 in various transformed cell lines. We began our present work by reconfirming Tax's repressive activity in HeLa cells. Accordingly, we transfected HeLa cells with a luciferase reporter plasmid containing 13 copies of a p53 consensus binding site (pG13-Luc) with a p53 expression plasmid, alone or with p53 plus a second expression plasmid for Tax (WT; Fig. 1A, left). As expected, p53 robustly activated the expression of pG13-Luc, while the further addition of Tax repressed activation by approximately 70% (Fig. 1A). Indeed, p53's activity was repressed by Tax in a dose-dependent manner (Fig. 1B). As controls, we verified the expression and activity of three Tax mutants (to be used in later transfections) by Western blotting and by assays which employed HTLV-long terminal repeat (LTR)-Luc (Fig. 1A). A dose-dependent expression of transfected p53 plasmid in HeLa cells was also verified (Fig. 1C).
FIG. 1.
Repression of p53's transcriptional activity in IKK-deficient MEFs by Tax. (A) Cells were transfected with 1 μg of Tax or indicated Tax mutants, and cell lysates were subjected to immunoblotting using polyclonal anti-Tax antibody (upper panel) or luciferase assay (bottom panel). For the luciferase assay, HeLa cells were cotransfected with 0.5 μg of HTLV-LTR-Luc and 0.5 μg of either Tax (WT) or a Tax mutant (H43Q, S274A, or L320G), respectively. (B) HeLa cells were cotransfected with 0.1 μg of reporter plasmid pG13-Luc, 0.5 μg of p53, and progressively increased amounts of wild-type Tax plasmid as indicated. (C) Expression of p53 in HeLa cells from transfected plasmids. p53 expression vectors, at the indicated amounts, were transfected into HeLa cells, and expression of protein was monitored by Western blotting. (D) Wild-type MEFs (WT) and IKKα−/−, IKKβ−/−, and IKKγ−/− MEFs were cotransfected in parallel with combinations of 0.1 μg of pG13-Luc, 0.5 μg of p53 expression plasmid, and 0.5 μg of Tax. Total transfected DNA was adjusted to the same amount with empty carrier plasmid. Luciferase assays were performed 24 h after transfection (upper panel). Cell lysates from a representative experiment were subjected to immunoblotting using anti-p53 (DO-1) and anti-β-actin (lower panel). All luciferase activities were normalized to β-galactosidase (β-Gal) readouts from a cotransfected cytomegalovirus-β-Gal plasmid. Levels of activation were based on the division of normalized luciferase values after activation by the normalized basal luciferase value. Results represent averages ± standard deviations from at least three independent experiments. P values are indicated.
We next proceeded to investigate Tax-p53 interaction in MEFs which are individually knocked out for IKKα, IKKβ, or IKKγ. We chose this approach because the IKKα/β/γ complex is critically important for downstream nuclear NF-κB activation (34) and because others have suggested a role for Tax-induced NF-κB activation in repressing p53 function (19, 20). Currently, there is wide agreement that Tax directly binds IKKγ (4, 9, 13) and that in IKKγ−/− settings NF-κB cannot be activated by tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), or Tax (11, 32).
We cotransfected pG13-Luc, p53, and Tax in the indicated combinations into IKKα−/−, IKKβ−/−, and IKKγ−/− MEFs (Fig. 1D). Since all of our assays in the MEFs were done side by side under identical conditions, we could compare and observe that the basal activity of MEF-endogenous p53 varied, in fact, quite a bit between WT, IKKα−/−, IKKβ−/−, and IKKγ−/− cells (Fig. 1D, first bar of each graph). Basal expression of pG13-Luc was lower (approximately 5- to 10-fold; data not shown) in IKKα−/− and IKKβ−/− cells than in either WT or IKKγ−/− cells, and correspondingly activation by p53 was commensurately much higher (Fig. 1D). Nonetheless, expression of exogenous p53 consistently elevated pG13-Luc (Fig. 1C, second bar of each graph) expression to decent levels in all cells. In all cases, we further observed that this activation was repressed by Tax (Fig. 1D, third bar of each graph). Consistent with previous reports which correlated stabilization of p53 by Tax with inactivation of p53 activity (24, 27), immunoblotting analyses of the transfected cells showed slightly higher steady-state amounts of p53 in the presence of Tax (Fig. 1D; middle panel, p53). These analyses indicate that the decreases seen in p53's transcriptional activity cannot be trivially explained by reduced p53 protein expression.
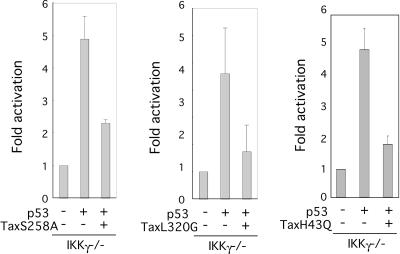
Elsewhere, we noted that Tax has residual NF-κB-activating activities in both IKKα−/− and IKKβ−/− MEFs; by comparison, its activation of NF-κB is completely silent in IKKγ−/− cells (unpublished observations). We, thus, focused our analyses of NF-κB requirement for p53 repression using IKKγ−/− cells. To provide an additional degree of stringency, we tested the TaxS258A mutant, a form of Tax previously characterized to be CREB active but NF-κB defective (25). We found that in IKKγ−/− MEFs, TaxS258A did, in fact, repress p53-activation of pG13-Luc (Fig. 2, left). Hence, although NF-κB activation was thwarted through two independent constraints (i.e., a cellular defect enforced through IKKγ−/− and a viral defect stipulated by using an NF-κB-incompetent Tax mutant), p53 activation of pG13-Luc was still suppressed by Tax. This result (Fig. 2, left) posits that NF-κB activation is dispensable for Tax's repression of p53-activity.
FIG. 2.
Repression of p53's transcriptional activity in IKKγ−/− MEFs by Tax mutants. IKKγ−/− cells were cotransfected with the indicated combinations of 0.1 μg of pG13-Luc, 0.5 μg of p53, 0.5 μg of Tax S258A (left), L320G (middle), and H43Q (right). Luciferase assays were performed 24 h later. Results are average values ± standard deviations from at least three independent experiments.
In parallel, we used another mutant, TaxL320G (25), as a control for TaxS258A. Whereas TaxS258A is NF-κB− CREB+, TaxL320G is NF-κB+ CREB−. One possible interpretation of the above TaxS258A result is that suppression of p53 activity requires CREB activation by Tax. However, when we analyzed the TaxL320G results in IKKγ−/− MEFs, we were surprised to see comparably strong suppression of p53 activation (Fig. 2, middle). We also analyzed a third mutant, TaxH43Q. Previously, Fu and colleagues have delineated that TaxH43Q cannot bind protein phosphatase 2A (PP2A) and failed to activate NF-κB (5). Here, we found that TaxH43Q, nevertheless, remained competent for suppressing p53's transcriptional activity (Fig. 2, right). Taken together, our results argue that in IKKγ−/− cells neither NF-κB (Fig. 2, left and right panels) nor CREB (Fig. 2, middle panel) activity appears to be needed for Tax's repression of p53-pG13-Luc activation.
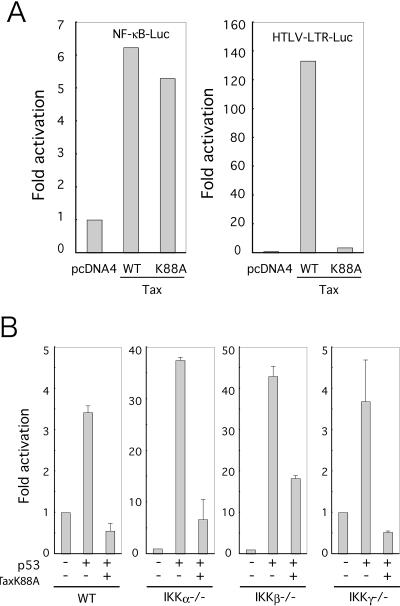
TaxK88A, a p300/CBP-binding defective mutant, repressed p53 function.
Although TaxL320G cannot functionally activate CREB, it remains competent for physical association with CREB and p300/CBP (6). To test directly the hypothesis that p300/CBP sequestration by Tax is a mechanism for suppressing p53's transcriptional activity, we next checked, in MEFs, the TaxK88A mutant previously shown by Harrod and colleagues to be defective in p300/CBP-binding (10). We confirmed that TaxK88A is NF-κB active (Fig. 3A, left); and, consistent with its p300/CBP binding defect, TaxK88A could not activate the HTLV-1 LTR (Fig. 3A, right). Provocatively, in WT, IKKα−/−, IKKβ−/−, and IKKγ−/− MEFs, we found that TaxK88A repressed p53's activation of pG13-Luc (Fig. 3B).
FIG. 3.
A TaxK88A mutant deficient for CBP binding still repressed p53's transcriptional activity. (A) Cells were cotransfected with 0.5 μg of NF-κB-Luc or HTLV-LTR-Luc and either 0.5 μg of Tax or Tax K88A, as indicated. (B) Wild-type MEF (WT), IKKα−/−, IKKβ−/−, and IKKγ−/− cells were cotransfected with 0.1 μg of pG13-Luc, 0.5 μg of p53, and 0.5 μg of TaxK88A. Luciferase assays were performed 24 h later. Results represent averages +/− standard deviations from at least three independent experiments.
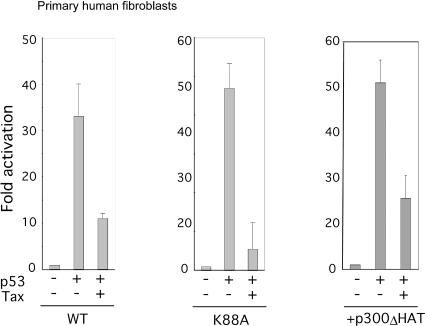
It is possible that the TaxK88A results are idiosyncratic to mouse embryo fibroblasts and harbor no general implication for the behavior of Tax in primary nontransformed human cells. To address this question, we obtained primary human fibroblasts from the American Type Culture Collection and tested for functional interplay between TaxK88A and p53. In human fibroblasts, WT Tax suppressed p53 activation of pG13-Luc (Fig. 4, left), and TaxK88A also did so comparably (Fig. 4, middle). Thus, the ability of TaxK88A to influence p53 activity is conserved from MEFs to human primary cells. To further establish that a functional interplay between Tax and p300/CBP may not be required for the former to suppress p53's transcriptional activity, we employed the p300ΔHAT protein. Previously, we found that p300ΔHAT is dominant negative for Tax's interaction with p300/CBP (17). At concentration of p300ΔHAT which suppressed the ability of Tax to activate HTLV-1 LTR (data not shown), we found that Tax nevertheless repressed p53 activity in primary human fibroblasts (Fig. 4, right). This result further supports the notion that the p300/CBP-interaction may be dispensable for Tax's action on p53 in human cells.
FIG. 4.
Tax-p300/CBP interaction appears not to be needed to repress p53 activity in human primary fibroblasts. Human primary fibroblast cells were cotransfected with indicated combinations of 0.25 μg of pG13-Luc, 0.25 μg of p53, and 0.5 μg of either Tax (WT) or the TaxK88A mutant. In the right panel 3 μg of p300ΔHAT shown previously to be dominant negative for Tax-p300/CBP interaction (17) was employed. Luciferase assays were performed 24 h later. Results represent averages ± standard deviations from at least three independent transfections.
Here, using IKK-deficient MEFs plus two NF-κB-deficient Tax mutants we accrued evidence that repression of p53 by Tax is independent of NF-κB signaling (Fig. 1 and 2). Astonishingly, we also found that, in IKK-deficient MEFs and in human primary fibroblasts, CREB- and p300/CBP interaction by Tax also seems to be unnecessary for repressing p53 activity (Fig. 3 and 4). Our results here using primary mouse and human fibroblasts add further complexity to the Tax-p53 interaction which cannot be easily explained by currently proposed mechanistic models.
After this work was completed and during the writing of the manuscript, we came across a paper from Jeong and colleagues suggesting that Tax repression of p53 is operative in IKKα−/− MEFs but not in IKKβ−/− MEFs (12). Perplexingly, these investigators did not test IKKγ−/− MEFs, which in our experience is the most stringent cell that restricts Tax's ability to activate NF-κB. The results of Jeong and coworkers in IKKα−/− agree with our findings, but their results in IKKβ−/− do not (Fig. 1 and 3). There are several possible reasons for the difference. First, Jeong et al. used an MDM2-Luc reporter as a p53-responsive surrogate, while we used the arguably more sensitive Vogelstein reporter, pG13-Luc, which contains 13 consecutive p53-binding sites (14). Second, we assayed for suppression by Tax of transfected p53; by contrast, Jeong et al. relied on Tax suppression of presumptively active MEF-endogenous p53. This difference in approach is significant because, as we have noted above, the basal activity of p53 is extremely low in IKKα−/− and IKKβ−/− MEFs. In fact, p53 protein is undetectable by immunoblotting in MEF cells (Fig. 1D, bottom), and we have found that interpretations of perturbations based on low basal luciferase readouts are frequently unreliable. Moreover, in the absence of data, it was unclear whether the ambient expression of MDM2-Luc in IKKβ−/− cells reported by Jeong et al. was in fact p53 dependent and not simply driven by TATAA-specific basal transcription factors. Nevertheless, despite these differences in detail, both we and Jeong et al. (12) arrived at the same major conclusion that Tax activation of NF-κB is dispensable for suppression of p53 function. We want to provide a word of caution that our findings stem from overexpression of Tax. Whether the results accurately reflect Tax effects from integrated provirus requires further verification.
If not NF-κB activation or CBP sequestration, how then does Tax suppress p53 function? We profess that we do not know the answer; however, we can offer a preliminary speculation. Previously, Pise-Masison et al. have reported that p53 is phosphorylated at Ser15 and Ser 392 in HTLV-1-transformed cells (21), while Ariumi et al. (2) demonstrated instead Tax-dependent phosphorylation of p53 at Ser 6, 9, 20, 33, and 37 and at Ser 15 only after DNA damage. In agreement with these independent findings, we have found a Tax-associated kinase which specifically phosphorylates p53; and on our two-dimensional gel analyses Tax-dependent phosphorylation of p53 correlates with repression of function (data not shown). As yet, we have not identified this Tax-associated kinase, and we do not yet know whether it has a role(s) in either NF-κB or CREB/CBP signaling.
Acknowledgments
We thank C.-Z. Giam and B. Vogelstein for plasmids and M. Schmidt-Supprian and I. M. Verma for sharing IKK-deficient MEFs. We also thank Lan Lin and Anthony Elmo for help with manuscript preparation.
REFERENCES
- 1.Akagi, T., H. Ono, N. Tsuchida, and K. Shimotohno. 1997. Aberrant expression and function of p53 in T-cells immortalized by HTLV-I Tax1. FEBS Lett. 406:263-266. [DOI] [PubMed] [Google Scholar]
- 2.Ariumi, Y., A. Kaida, J. Y. Lin, M. Hirota, O. Masui, S. Yamaoka, Y. Taya, and K. Shimotohno. 2000. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 19:1491-1499. [DOI] [PubMed] [Google Scholar]
- 3.Azran, I., Y. Schavinsky-Khrapunsky, and M. Aboud. 2004. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu, Z. L., Y. A. Shin, J. M. Yang, J. A. DiDonato, and D. W. Ballard. 1999. IKKγ mediates the interaction of cellular IκB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 274:15297-15300. [DOI] [PubMed] [Google Scholar]
- 5.Fu, D., Y. Kuo, B. Liu, K.-T. Jeang, and C.-Z. Giam. 2003. Human T-lymphotropic virus type I Tax activates I-kB kinase by inhibiting I-kB-associated serine/threonine protein phosphatase 2A. J. Biol. Chem. 278:1487-1493. [DOI] [PubMed] [Google Scholar]
- 6.Goren, I., O. J. Semmes, K. T. Jeang, and K. Moelling. 1995. The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J. Virol. 69:5806-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassmann, R., C. Dengler, I. Muller-Fleckenstein, B. Fleckenstein, K. McGuire, M. C. Dokhelar, J. G. Sodroski, and W. A. Haseltine. 1989. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 86:3351-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman, W. J., J. T. Kimata, F. H. Wong, M. Zutter, T. J. Ley, and L. Ratner. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 92:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhaj, E. W., and S. C. Sun. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911-22914. [DOI] [PubMed] [Google Scholar]
- 10.Harrod, R., Y. L. Kuo, Y. Tang, Y. Yao, A. Vassilev, Y. Nakatani, and C. Z. Giam. 2000. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 275:11852-11857. [DOI] [PubMed] [Google Scholar]
- 11.Iha, H., K. V. Kibler, V. R. Yedavalli, J. M. Peloponese, K. Haller, A. Miyazato, T. Kasai, and K. T. Jeang. 2003. Segregation of NF-κB activation through NEMO/IKKγ by Tax and TNFα: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 22:8912-8923. [DOI] [PubMed] [Google Scholar]
- 12.Jeong, S. J., C. A. Pise-Masison, M. F. Radonovich, H. U. Park, and J. N. Brady. 2004. A novel NF-B pathway involving IKKβ and p65/RelA Ser-536 phosphorylation results in p53 inhibition in the absence of NF-B transcriptional activity. J. Biol. Chem. 280:10326-10332. [DOI] [PubMed] [Google Scholar]
- 13.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IκB kinase gamma. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 14.Kern, S. E., J. A. Pietenpol, S. Thiagalingam, A. Seymour, K. W. Kinzler, and B. Vogelstein. 1992. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256:827-830. [DOI] [PubMed] [Google Scholar]
- 15.Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J. Fullen, M. V. Lorenzi, A. Cara, C. Nicot, C. Giam, and G. Franchini. 1998. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 72:8852-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 17.Okada, M., and K.-T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 76:12564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pise-Masison, C. A., K. S. Choi, M. Radonovich, J. Dittmer, S. J. Kim, and J. N. Brady. 1998. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 72:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pise-Masison, C. A., R. Mahieux, H. Jiang, M. Ashcroft, M. Radonovich, J. Duvall, C. Guillerm, and J. N. Brady. 2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol. Cell. Biol. 20:3377-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pise-Masison, C. A., R. Mahieux, M. Radonovich, H. Jiang, J. Duvall, C. Guillerm, and J. N. Brady. 2000. Insights into the molecular mechanism of p53 inhibition by HTLV type 1 Tax. AIDS Res. Hum. Retroviruses 16:1669-1675. [DOI] [PubMed] [Google Scholar]
- 21.Pise-Masison, C. A., M. Radonovich, K. Sakaguchi, E. Appella, and J. N. Brady. 1998. Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J. Virol. 72:6348-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzatti, R., J. Vogel, and G. Jay. 1990. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol. Cell. Biol. 10:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid, R. L., P. F. Lindholm, A. Mireskandari, J. Dittmer, and J. N. Brady. 1993. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene 8:3029-3036. [PubMed] [Google Scholar]
- 25.Semmes, O. J., and K.-T. Jeang. 1992. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J. Virol. 66:7183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, S. C., and D. W. Ballard. 1999. Persistent activation of NF-κB by the tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene 18:6948-6958. [DOI] [PubMed] [Google Scholar]
- 27.Takemoto, S., R. Trovato, A. Cereseto, C. Nicot, T. Kislyakova, L. Casareto, T. Waldmann, G. Torelli, and G. Franchini. 2000. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14(ARF)-MDM2 loop in ex vivo and cultured adult T-cell leukemia/lymphoma cells. Blood 95:3939-3944. [PubMed] [Google Scholar]
- 28.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van, P. L., K. W. Yim, D. Y. Jin, G. Dapolito, A. Kurimasa, and K.-T. Jeang. 2001. Genetic evidence of a role for ATM in functional interaction between human T-cell leukemia virus type 1 Tax and p53. J. Virol. 75:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Orden, K., J. P. Yan, A. Ulloa, and J. K. Nyborg. 1999. Binding of the human T-cell leukemia virus Tax protein to the coactivator CBP interferes with CBP-mediated transcriptional control. Oncogene 18:3766-3772. [DOI] [PubMed] [Google Scholar]
- 31.Vousden, K. H., and X. Lu. 2002. Live or let die: the cell's response to p53. Nat. Rev. Cancer 2:594-604. [DOI] [PubMed] [Google Scholar]
- 32.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandi, E., and M. Karin. 1999. Bridging the gap: composition, regulation, and physiological function of the IκB kinase complex. Mol. Cell. Biol. 19:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]