Abstract
PURPOSE
Revumenib, an oral, small molecule inhibitor of the menin-lysine methyltransferase 2A (KMT2A) interaction, showed promising efficacy and safety in a phase I study of heavily pretreated patients with KMT2A-rearranged (KMT2Ar) acute leukemia. Here, we evaluated the activity of revumenib in individuals with relapsed/refractory (R/R) KMT2Ar acute leukemia.
METHODS
AUGMENT-101 is a phase I/II, open-label, dose-escalation and expansion study of revumenib conducted across 22 clinical sites in five countries (ClinicalTrials.gov identifier: NCT04065399). We report results from the phase II, registration-enabling portion. Individuals age ≥30 days with R/R KMT2Ar acute leukemia or with AML and nucleophosmin 1 (NPM1) mutation were enrolled. Revumenib was administered once every 12 hours, at 163 mg (95 mg/m2 if weight <40 kg) with a strong cytochrome P450 inhibitor, in 28-day cycles. The primary end points were the rate of complete remission (CR) or CR with partial hematologic recovery (CR + CRh) and safety. At a prespecified interim analysis, safety was assessed in all KMT2Ar treated patients; efficacy was assessed in those with centrally confirmed KMT2Ar. The separate NPM1 cohort of the trial is ongoing.
RESULTS
From October 1, 2021, to July 24, 2023, N = 94 patients (median [range] age, 37 [1.3-75] years) were treated. Grade ≥3 adverse events included febrile neutropenia (37.2%), differentiation syndrome (16.0%), and QTc prolongation (13.8%). In the efficacy-evaluable patients (n = 57), the CR + CRh rate was 22.8% (95% CI, 12.7 to 35.8), exceeding the null hypothesis of 10% (P = .0036). Overall response rate was 63.2% (95% CI, 49.3 to 75.6), with 15 of 22 patients (68.2%) having no detectable residual disease.
CONCLUSION
Revumenib led to high remission rates with a predictable safety profile in R/R KMT2Ar acute leukemia. To our knowledge, this trial represents the largest evaluation of a targeted therapy for these patients.
INTRODUCTION
Acute leukemia arises from genetic alterations in hematopoietic cells that lead to a block in differentiation during hematopoiesis and unbridled cellular proliferation.1 Rearrangements of the lysine methyltransferase 2A (KMT2A) gene, located at chromosome locus 11q23, occur in up to 10% of acute leukemias in children and adults, with higher incidence in certain types of infant and childhood acute leukemias.2,3 AML, ALL, or mixed phenotype acute leukemia (MPAL) with KMT2A rearrangements (KMT2Ar) are associated with drug resistance and poor prognosis.4,5 The proportion of patients who achieve remission after three or more lines of therapy is <10% in adults.5
CONTEXT
Key Objective
To evaluate the efficacy and safety of revumenib, an oral, small molecule inhibitor of the menin-lysine methyltransferase 2A (KMT2A) interaction, in individuals with relapsed/refractory (R/R) KMT2A-rearranged acute leukemia in the phase II, registration-enabling portion of the AUGMENT-101 trial.
Knowledge Generated
Treatment with revumenib resulted in rates of complete remission (CR) or CR with partial hematologic recovery (CR +CRh) that exceeded the null hypothesis of expected remission rates for individuals with R/R KMT2A-rearranged acute leukemia. Along with a predictable safety profile, these results suggest that revumenib monotherapy provides clinical benefit beyond that of current therapies.
Relevance (C.F. Craddock)
The menin inhibitor revumenib is an active and tolerable agent in KMT2A-rearranged AML confirming the importance of this new class of drug in molecularly defined sub-populations of patients with R/R acute leukemias. Future trials examining the activity of revumenib in newly diagnosed AML are now indicated.
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
KMT2Ar lead to oncogenic fusion proteins that upregulate leukemogenic homeobox (HOX) genes and their DNA-binding cofactor Meis homeobox 1 (MEIS1).6,7 The scaffolding protein menin is a crucial mediator of the pathogenesis of KMT2Ar leukemia. Menin is essential for binding of the KMT2A protein complex to the HOX gene promoter.8 In preclinical models, disruption of the menin-KMT2A interaction reverses this aberrant gene expression, leading to hematopoietic differentiation and an antileukemic effect.9,10 This dependency on the menin-KMT2A interaction is shared by acute leukemia with a nucleophosmin 1 (NPM1) mutation, the most common genetic alteration in adults with AML, in addition to other less common leukemia subtypes.11-15 Despite an increased understanding of the mechanisms governing development of KMT2Ar acute leukemia, no targeted therapies are approved for it to date; most patients receive conventional chemotherapy or venetoclax combinations, with an allogenic hematopoietic cell transplantation in consolidation, and experience suboptimal outcomes.5
Revumenib is a potent, oral, small molecule inhibitor of the menin-KMT2A interaction. In a phase I clinical study, treatment with revumenib had an acceptable safety profile and preliminary evidence of efficacy in patients with relapsed or refractory (R/R) KMT2Ar or NPM1-mutated acute leukemias and showed hallmarks of hematopoietic differentiation.4 Here, we report the results of the pivotal phase II, registration-enabling clinical study of revumenib in a unique cohort of patients with R/R KMT2Ar acute leukemia, which represents, to our knowledge, the largest trial to date of a targeted treatment in this subset of patients.
METHODS
Study Design
AUGMENT-101 is a phase I/II, open-label, dose-escalation and expansion study of revumenib for patients with R/R leukemias with KMT2Ar or NPM1 mutation. The phase II began on October 1, 2021, and is ongoing across 22 clinical sites in five countries (ClinicalTrials.gov identifier: NCT04065399). Here, we report results from a prespecified interim analysis of patients with KMT2Ar enrolled in the phase II portion of the trial.
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization. The protocol and amendments were approved by the competent authorities and institutional review board or ethics committee at participating centers, and all patients or their legal guardians provided written informed consent. Important changes to the methods to expand eligibility were to adjust the design to include children and to allow patients to resume revumenib treatment post-transplant. Throughout the study, an independent data monitoring committee monitored safety and efficacy according to predefined parameters detailed in the protocol and provided recommendations for continuation or termination of the study (see Data Supplement for details). The details of the study design are provided in the study protocol.
Patients
Eligible patients included those age 30 days and older with an Eastern Cooperative Oncology Group performance-status score of 0-2 for adults (on a scale from 0 to 5, with higher scores indicating greater disability) or a Karnofsky/Lansky score of more than 50 for children (on a scale from 0 to 100, with lower scores indicating greater disability) and bone marrow blasts ≥5% or reappearance of blasts in peripheral blood. Patients with primary refractory (persistent leukemia following intensive induction chemotherapy) or relapsed refractory (unresponsive to most recent salvage treatment) KMT2Ar acute leukemia of any lineage were allowed on study, including those with AML, ALL, or MPAL. KMT2Ar status was based on local laboratory testing with central confirmation by fluorescence in situ hybridization (split signal).16 There was no restriction on the number or types of prior therapies, and patients with post-transplant relapse could be enrolled as early as 60 days from their prior hematopoietic stem cell transplant (HSCT). Patients with CNS disease at the most recent relapse were eligible for enrollment if no active CNS disease remained present at study entry.
Procedures
Treatment with revumenib was continued until lack of response after up to four cycles, disease progression, unacceptable adverse events (AEs), or withdrawal of consent. Maintenance therapy with revumenib after allogeneic HSCT was allowed until disease progression or unacceptable toxicity. Revumenib was administered orally in either capsule or liquid formulation every 12 hours in 28-day continuous cycles with dose adjustment by body surface area for patients weighing less than 40 kg. Because revumenib is a substrate of cytochrome P450 3A4 (CYP3A4), the dose used with a strong CYP3A4 inhibitor (CYP3A4i) was 163 mg (95 mg/m2 if <40 kg) once every 12 hours, which included all patients in this analysis, or 276 mg (160 mg/m2 if <40 kg) once every 12 hours without a strong CYP3A4i. These were the recommended phase II doses identified in the phase I of the study on the basis of prespecified protocol criteria on safety, tolerability, and pharmacokinetic data.4 Hydroxyurea for cytoreduction, intrathecal chemotherapy for CNS prophylaxis, and steroids for differentiation syndrome were allowed concomitantly with revumenib. Guidelines for managing AEs of special interest are described in the study protocol.
Study End Points and Assessments
The primary end points were the rate of complete remission (CR) or CR with partial hematologic recovery (CR + CRh) and the evaluation of safety and tolerability of revumenib. Secondary end points included overall response rate, duration of remission, and overall survival. Responses were assessed by the investigators according to the European LeukemiaNet response criteria.17 Details on secondary end points and response definitions are included in the Data Supplement.
AEs were collected from the time of the first revumenib dose until 30 days after the last dose, including maintenance therapy, and were graded using the Common Terminology Criteria for Adverse Events version 5.0. Differentiation syndrome, an expected on-target effect of inducing differentiation of leukemia cells into normal hematopoietic cells, was considered an AE of special interest. Other AEs of special interest included prolongation of the QT interval and peripheral neuropathy. Guidelines for managing AEs of special interest were provided to the investigators and included adjustment of the revumenib dose, administration of corticosteroids after suspicion of differentiation syndrome, and electrolyte repletion for QTc prolongation. Additional details are provided in the study protocol.
Baseline alterations were evaluated to determine if a particular KMT2A translocation or comutation was associated with response. Bone marrow–derived DNA from 40 patients from the efficacy population, collected before start of revumenib treatment, was genomically profiled on the Hematologic Malignancies panel from Genomic Testing Cooperative (Irvine, CA). Somatic short variants were called and analyzed according to manufacturer's instructions.
Transcriptional changes following one cycle of revumenib were evaluated for target genes of interest. Samples from bone marrow aspirates (RNA) were isolated using the Promega simplyRNA kit and quantified using the NanoDrop 2000. Total RNA quality and molecular weight distribution were evaluated using the Agilent 2100 Bioanalyzer. Multiplex gene analysis was performed using a custom-designed QuantiGene assay manufactured by Thermo Fisher Scientific. The custom assay included seven genes of interest (HOXA9; MEIS1; PBX homeobox 3 [PBX3]; fms related receptor tyrosine kinase 3 [FLT3]; integrin subunit alpha M; CD14; alanyl aminopeptidase, membrane) and five housekeeping genes (phosphoglycerate kinase 1, beta-2-microglobulin, ribosomal protein L13a, RNA polymerase II subunit A, hypoxanthine phosphoribosyltransferase 1). For analysis, phosphoglycerate kinase 1 and hypoxanthine phosphoribosyltransferase 1 were selected for use as the genes because of variability seen in the other housekeepers. The raw data were analyzed using Thermo Fisher Scientific QuantiGene Plex Data Analysis software to generalize normalized expression data. All steps were performed by Flagship Biosciences.
Statistical Analysis
The sample size was estimated on the basis of the number of adults in the efficacy-evaluable population, defined as patients who received at least one dose of study drug, were centrally confirmed for KMT2Ar status, and had at least 5% blasts in bone marrow at baseline. The number of efficacy-evaluable adults was determined on the basis of the minimax version of Simon's two-stage design, in which 64 adult efficacy-evaluable patients would provide 90% power for the target CR + CRh rate of 25% at a one-sided significance level of 2.5% with null hypothesis of CR + CRh rate of 10% or less. A futility interim analysis was planned to occur when the first 38 efficacy-evaluable adults with AML had 6 months of follow-up or discontinued therapy, a time point prospectively selected to allow sufficient time for response and durability of response to the drug to be evaluated. On the basis of Simon's two-stage design, if <5 responders were observed in these initial 38 adult evaluable patients, enrollment would be discontinued; otherwise, enrollment would continue until 64 adult evaluable patients were enrolled. Additionally, an interim analysis for superiority/efficacy for the primary end point (CR + CRh rate) in adults and children with KMT2Ar acute leukemia was conducted at the planned futility interim analysis. When the first 38 efficacy-evaluable patients with AML were enrolled, there were 57 adult and pediatric efficacy-evaluable patients with KMT2Ar acute leukemia, which formed the efficacy-evaluable population for the interim analysis (Data Supplement, Fig S1). The superiority/efficacy boundary at the interim analysis was set at 12 or more responders with CR + CRh.
Safety was summarized for all patients with KMT2Ar acute leukemia who received at least one dose of revumenib (safety population). No formal statistical hypothesis testing for safety analyses was conducted. Time-to-event end points were estimated using the Kaplan-Meier method, and descriptive statistics were used for other clinical or laboratory variables, with subgroup analyses performed for efficacy.
Role of the Funding Source
The sponsor, Syndax Pharmaceuticals, Inc, provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of the data in collaboration with the investigators. The first draft of the manuscript was written by the first and last authors with assistance by medical writers funded by the sponsor and input from all authors. Subsequent drafts were revised in collaboration with all the authors and the sponsor, all of whom vouch for the accuracy of the data.
RESULTS
From October 1, 2021, to July 24, 2023, 94 patients with KMT2Ar acute leukemia received at least one dose of revumenib and were included in the safety population (Fig 1). Patient demographics and baseline characteristics are provided in Table 1. These included 78 patients (83.0%) with AML, 14 (14.9%) with ALL, and two (2.1%) with acute leukemia of ambiguous lineage. The median age was 37 years (range, 1.3-75.0), with 71 adults and 23 children (<18 years). The median age of adults was 44 years (range, 18.0-75.0), and the median age of pediatric patients was 4.0 years (range, 1.3-17.0). Patients were heavily pretreated, with 43.6% of patients having received three or more prior lines of therapy (median, 2; range, 1-11). Many patients (57.4%) had no response to their most recent salvage treatment, and 19.1% had primary refractory leukemia. Half of the total population had a prior HSCT, with 7% having had more than one prior transplant. About two thirds of patients were previously treated with the B-cell lymphoma (BCL2) inhibitor venetoclax. Among the safety population, 57 patients had met the interim analysis efficacy-evaluable population criteria (Fig 1); the median duration of treatment was 10.0 weeks (range, 1-36; Fig 2).
FIG 1.

Flow diagram for participants with relapsed or refractory KMT2Ar acute leukemia who received revumenib. At the time of the interim analysis for the phase II KMT2Ar population, 95 patients were enrolled in the KMT2Ar cohorts. An additional 92 patients had been screened for inclusion in the study but failed the screening. The reasons for screening failure are listed in the Data Supplement (Table S4). aUpon review following data cutoff, these patients were confirmed to have elevated bone marrow blasts and were therefore included in the safety population. KMT2Ar, lysine methyltransferase 2A rearranged; PR, partial remission.
TABLE 1.
Demographic and Clinical Characteristics of the Patients at Baseline
| Parameter | Efficacy Population (n = 57)a | Safety Population (N = 94)b |
|---|---|---|
| Age, years, median (range) | 34.0 (1.3-75.0) | 37.0 (1.3-75.0) |
| <18, No. (%) | 13 (22.8) | 23 (24.5) |
| ≥18 to <65, No. (%) | 37 (64.9) | 58 (61.7) |
| ≥65, No. (%) | 7 (12.3) | 13 (13.8) |
| Sex, No. (%) | ||
| Female | 33 (57.9) | 56 (59.6) |
| Male | 24 (42.1) | 38 (40.4) |
| Race, No. (%) | ||
| Black or African American | 4 (7.0) | 4 (4.3) |
| Asian | 6 (10.5) | 8 (8.5) |
| White | 43 (75.4) | 68 (72.3) |
| Multiple | 0 | 2 (2.1) |
| Unknown | 4 (7.0) | 12 (12.8) |
| Primary refractory, No. (%) | 14 (24.6) | 18 (19.1) |
| Relapse refractory, No. (%) | 32 (56.1) | 54 (57.4) |
| Acute leukemia type, No. (%) | ||
| AML | 49 (86.0) | 78 (83.0) |
| ALL | 7 (12.3) | 14 (14.9) |
| Acute leukemia of ambiguous lineage | 1 (1.8) | 2 (2.1) |
| KMT2A translocation, No. (%) | ||
| t(9;11) (p22;q23) | 11 (19.3) | 21 (22.3) |
| t(11;19) (q23;p13) | 13 (22.8) | 17 (18.1) |
| t(10;11) (p12;q23) | 7 (12.3) | 12 (12.8) |
| t(6;11) (q27;q23) | 7 (12.3) | 8 (8.5) |
| t(4;11) (q21;q23) | 2 (3.5) | 7 (7.4) |
| t(1;11)c | 2 (3.5) | 2 (2.1) |
| Fusion partner unknown | 13 (22.8) | 24 (25.5) |
| Other translocations | 2 (3.5) | 3 (3.2) |
| Co-occurring mutations, No. (%) | ||
| RAS | 9 (15.8) | 12 (12.8) |
| FLT3 | 5 (8.8) | 7 (7.4) |
| TP53 | 4 (7.0) | 5 (5.3) |
| Prior lines of therapy, No., median (range) |
2 (1-11) | 2 (1-11) |
| 1, No. (%) | 17 (29.8) | 25 (26.6) |
| 2, No. (%) | 14 (24.6) | 28 (29.8) |
| ≥3, No. (%) | 26 (45.6) | 41 (43.6) |
| Prior venetoclax, No. (%) | 41 (71.9) | 61 (64.9) |
| Prior HSCT, No. (%) | 26 (45.6) | 47 (50.0) |
NOTE. Data cutoff: July 24, 2023.
Abbreviations: FLT3, fms-related tyrosine kinase 3; HSCT, hematopoietic stem cell transplant; KMT2A, lysine methyltransferase 2A; RAS, rat sarcoma virus; TP53, tumor protein p53.
The efficacy population includes patients with centrally confirmed KMT2A-rearranged status and ≥5% blasts in bone marrow at baseline.
Defined as patients with KMT2A-rearranged acute leukemia having received at least one dose of revumenib.
Additional information not reported.
FIG 2.
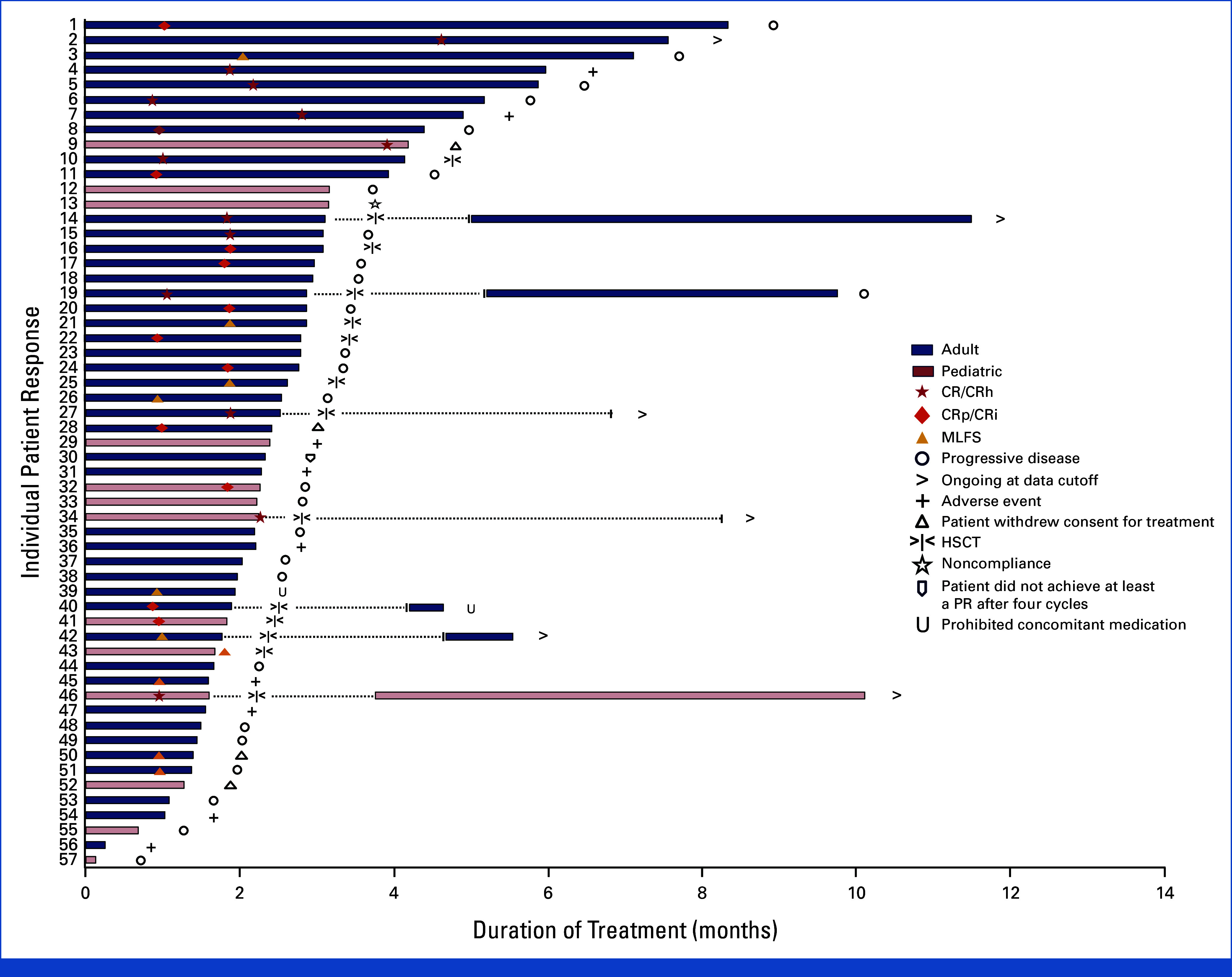
Duration of treatment. Treatment course of revumenib in patients with KMT2Ar relapsed or refractory acute leukemia. Time to response, duration of treatment, and patient status by the data cutoff date for patients in the efficacy population (n = 57). Dotted lines indicate patients who resumed post-transplant maintenance at the time of data cutoff. CR, complete remission; CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; HSCT, hematopoietic stem cell transplant; KMT2Ar, lysine methyltransferase 2A-rearranged; MLFS, morphological leukemia-free state; PR, partial remission.
A total of 93 patients (98.9%) had treatment-emergent AEs with revumenib. The most common AEs are listed in Table 2. Treatment-emergent AEs leading to dose reduction or discontinuation occurred in 9.6% and 12.8% of patients, respectively. Treatment-emergent AEs that led to treatment discontinuation included septic shock (2%), respiratory failure (2%), intracranial hemorrhage (1%), sudden death (1%), AML (1%), cardiac failure (1%), febrile neutropenia (1%), myocardial ischemia (1%), pneumonia (1%), nausea (1%), and vomiting (1%). With the exception of nausea and vomiting, these AEs would generally not respond to dose modification. No patients discontinued revumenib because of prolongation of the QTc interval or differentiation syndrome. AEs leading to death (grade 5) were reported in 14 of 94 patients (14.9%) while receiving revumenib or within 30 days of the last dose (Table 2; Data Supplement, Table S1).
TABLE 2.
Summary of AEs
| AE | Safety Populationa (N = 94) |
|---|---|
| Any AE, No. of patients (%) | 93 (98.9) |
| AEs that occurred in ≥20% of patients (grouped term), No. of patients (%) | |
| Nausea | 42 (44.7) |
| Febrile neutropenia | 36 (38.3) |
| Diarrhea | 33 (35.1) |
| Edema | 30 (31.9) |
| Vomiting | 29 (30.9) |
| Neutropenia | 28 (29.8) |
| Transaminitis | 27 (28.7) |
| Differentiation syndrome | 26 (27.7) |
| Hypokalemia | 26 (27.7) |
| Epistaxis | 25 (26.6) |
| QTc prolongation | 24 (25.5) |
| Thrombocytopenia | 22 (23.4) |
| Abdominal pain | 22 (23.4) |
| Rash | 22 (23.4) |
| Anemia | 21 (22.3) |
| Constipation | 21 (22.3) |
| Decreased appetite | 21 (22.3) |
| Fatigue | 20 (21.3) |
| Any grade ≥3 AE, No. of patients (%) | 86 (91.5) |
| AEs grade ≥3 (grouped term) that occurred in ≥10% of patients, No. of patients (%) | |
| Febrile neutropenia | 35 (37.2) |
| Neutropenia | 27 (28.7) |
| Thrombocytopenia | 20 (21.3) |
| Anemia | 17 (18.1) |
| Differentiation syndrome | 15 (16.0) |
| QTc prolongation | 13 (13.8) |
| Sepsis | 11 (11.7) |
| Hypokalemia | 10 (10.6) |
| AEs leading to dose modification, No. (%) | |
| Dose reduction | 9 (9.6) |
| Dose interruption | 41 (43.6) |
| Drug discontinuation | 12 (12.8) |
| Death | 14 (14.9) |
NOTE. Data cutoff: July 24, 2023.
Abbreviations: AE, adverse event; KMT2A, lysine methyltransferase 2A.
Defined as patients with KMT2A-rearranged acute leukemia having received at least one dose of revumenib.
Differentiation syndrome (any grade) occurred in 26 patients (27.7%); 14 (14.9%) had grade 3 events and one (1.1%) had a grade 4 event. The median time to initial onset and median duration of the initial event of differentiation syndrome were 10 days (range, 3-41) and 12 days (range, 3-31). Differentiation syndrome led to interruption of revumenib in seven patients. All cases were treated with corticosteroids, with the addition of hydroxyurea for associated leukocytosis in six of the 26 patients. QTc prolongation (any grade) occurred in 24 patients (25.5%), with 13.8% patients experiencing grade 3 and no grade 4 or fatal events. The median time to initial onset was 8.0 days (range, 1-72), with a median duration of initial event of 1.0 day (range, 1-8). QTc prolongation resulted in dose interruption and reduction in 11 and four patients, respectively. Events of peripheral neuropathy were infrequent (3.2%), and all were less than grade 2.
In the efficacy population, the rate of CR + CRh was 22.8% (n = 13/57; 95% CI, 12.7 to 35.8), exceeding the null hypothesis defined per protocol of 10% (one-sided P = .0036) and surpassing the predefined interim analysis efficacy boundary. At the recommendation of the independent data monitoring committee that reviewed efficacy and safety, enrollment of patients with KMT2Ar disease was stopped early for efficacy. Measurable residual disease (MRD) was done locally by flow cytometry. MRD status was available for only 22 patients (Data Supplement, Table S2); an MRD-negative response by local assessment was observed in 7 of 10 evaluable patients with CR + CRh.
The overall response rate was 63.2% (95% CI, 49.3 to 75.6; Table 3). Responses were rapid, with a median time to first overall response of 0.95 months (range, 0.9-2.0) and a median time to CR + CRh of 1.9 months (range, 0.9-4.6). The median duration of CR + CRh was 6.4 months (95% CI, 3.4 to not reached; Fig 3A). With a median follow-up of 6.1 months (range, 0.3-18.6), the median overall survival was 8.0 months (95% CI, 4.1 to 10.9; Fig 3B).
TABLE 3.
Response
| Parameter | Efficacy Population (n = 57) |
|---|---|
| Overall response rate, No. (%)a | 36 (63.2) |
| 95% CI | 49.3 to 75.6 |
| Time to first response, months, median (range) | 0.95 (0.9-2.0) |
| Duration of response, months, median (range) | 4.3 (1.9-NR) |
| CR + CRh rate, No. (%) | 13 (22.8) |
| 95% CI | 12.7 to 35.8 |
| P value, one-sided | 0.0036 |
| Time to first CR + CRh, months, median (range) | 1.87 (0.9-4.6) |
| Duration of CR + CRh, months, median (95% CI) | 6.4 (3.4 to NR) |
| CRc, No. (%)b | 25 (43.9) |
| 95% CI | 30.7 to 57.6 |
| Best response, No. (%) | |
| CR | 10 (17.5) |
| CRh | 3 (5.3) |
| CRi | 1 (1.8) |
| CRp | 11 (19.3) |
| Morphological leukemia-free state | 10 (17.5) |
| Partial remission | 1 (1.8) |
| Progressive disease | 4 (7.0) |
| No response | 14 (24.6) |
| Otherc | 3 (5.3) |
| MRD negative rate within evaluable patientsd | |
| Within CR + CRh, No. (%) | 7/10 (70.0) |
| Within CRc, No. (%) | 15/22 (68.2) |
| Time to negative MRD status for patients with CR + CRh, months, median (range) | 1.08 (1.0-3.9) |
NOTE. Data cutoff: July 24, 2023.
Abbreviations: CR, complete remission; CRc, composite complete remission; CRh, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery; CRp, complete remission with incomplete platelet recovery; MRD, measurable residual disease.
Overall response rate was defined as CRc + morphological leukemia-free state + partial remission.
Composite CR defined as CR + CRh + CRi + CRp.
Includes patients without postbaseline disease assessment.
MRD done locally by flow cytometry; not all patients had MRD status reported. Available flow cytometry details for reported patients can be found in the Data Supplement (Table S2).
FIG 3.

Duration of response and OS. The data cutoff date for this analysis was July 24, 2023. (A) The Kaplan-Meier estimate of duration of CR + CRh in 13 patients with CR + CRh. Duration of CR + CRh was defined as the date of first documented CR + CRh to the first documented confirmed relapse or death. The at-risk population at any specific time point was defined as patients with CR + CRh, up to that time point, who had not experienced relapse, death, or a censoring event. (B) The Kaplan-Meier estimate of overall survival in patients with KMT2Ar acute leukemia in the efficacy population. CR, complete remission; CRh, CR with partial hematologic recovery; KMT2Ar, lysine methyltransferase 2A-rearranged; NR, not reached: OS, overall survival.
Among patients who achieved a response, 14 (38.9%) received an allogeneic HSCT, with eight of these transplants occurring in patients attaining a reduction in the bone marrow blast percentage to <5% without complete or partial hematologic recovery. Of the 14 transplant recipients, seven resumed revumenib after transplant. The duration of maintenance therapy with post-transplant revumenib ranged from 1 to 199 days at the time of the data cutoff, with five of the seven patients remaining on revumenib at the time of the data cutoff date (Fig 2).
Responses were observed across all major subgroups, including patients with or without prior transplant, R/R disease, and varying numbers of prior lines of treatment (Data Supplement, Fig S2); however, this study was not powered to evaluate differences among subgroups. Of the five patients in the efficacy population with CNS disease at the most recent relapse, one responded and had a best response of morphological leukemia-free state. Responses were observed across a broad age range with the youngest responder age 1 year and oldest age 75 years (both CRs). Exploratory analysis with limited sample sizes demonstrated efficacy for KMT2Ar across various translocation partners (Data Supplement, Table S3). There was no clear response pattern by comutation in other genes (Data Supplement, Fig S3). Transfusion independence, defined as any transfusion-free period lasting for at least 56 consecutive days, was demonstrated in treated patients (Data Supplement, Fig S4). Of note, 11 of the 14 patients who proceeded to transplant did so within 56 days after achieving their best response, limiting their ability to demonstrate transfusion independence during the available follow-up.
When transcriptional changes were evaluated in patients following one cycle of revumenib, downregulation of the critical leukemogenic target genes MEIS1, HOXA9, PBX3, and FLT3 (a putative transcriptional target of MEIS1) was observed while expression of genes associated with differentiation (CD11b, CD14) was markedly increased (Data Supplement, Fig S5).
DISCUSSION
R/R KMT2Ar acute leukemia has a poor prognosis, with <10% of patients achieving a remission with currently available therapy. In this phase II study, the oral menin inhibitor revumenib led to substantially greater rates of remission compared with historical controls in patients with high-risk, R/R KMT2Ar acute leukemia. The prespecified primary study end point was met, with a rate of CR + CRh of 22.8% (95% CI, 12.7 to 35.8). Although sample sizes were small, efficacy was observed across subgroups, including age, lineage, prior venetoclax, and KMT2A translocation partner. The high overall response rate of 63.2% reflects the clearance of blasts in most patients on treatment and allowed a subset of patients with morphological responses without a full or partial hematologic recovery to proceed to an allogeneic HSCT. These results represent a robust, unique data set supporting revumenib monotherapy for R/R KMT2Ar leukemia and are further supported by consistency with the phase I data.
Revumenib was well tolerated. All episodes of grade 3 QTc prolongation resolved with dose adjustment and without treatment discontinuation. Although the rate of investigator-reported differentiation syndrome was higher than previously reported,4 especially the occurrence of grade 3 events, this may indicate an improved recognition of this syndrome. Notably, differentiation syndrome was successfully managed with corticosteroids and hydroxyurea for leukocytosis, and there were no treatment discontinuations because of this AE.
The response rates, durability of response, overall survival, and high fraction of patients proceeding to potentially curative therapy observed in this cohort of patients with KMT2Ar acute leukemia, along with the observed safety profile, demonstrate that revumenib monotherapy provides clinical benefit beyond that of current therapies. Preclinical data and the safety profile of revumenib suggest its potential to form rational combinations with partner agents, such as BCL2 or FLT3 inhibitors, or other epigenetic therapies.18-20 Preliminary data suggest tolerability and high rates of remission in patients who receive the combination of a hypomethylating agent, venetoclax, and revumenib (SAVE trial).21 There are currently multiple phase I and II studies investigating revumenib in combination with FLT3 inhibitors (gilteritinib, midostaurin) and in leukemias with genetic alterations associated with upregulation of HOX.22-24 Additional studies combining revumenib with standard-of-care induction and consolidation chemotherapy are ongoing or planned.
The study design and small number of patients limit any comparisons between subgroups. Given that KMT2Ar leukemia subtype is rare, inclusion of a comparator arm was not feasible, allowing only for comparison with historical controls.5,25 Mutational analyses at progression were not done in this study so the role of resistance mutations in any outcome is unknown. Of note, the trial design exemplifies the successful inclusion of children very early in drug development and is in line with fostering age inclusive research.26
In conclusion, treatment with the menin inhibitor revumenib provided clinical benefit and a low rate of discontinuation for AEs indicating a predictable safety profile. This benefit is demonstrated in refractory patients where currently available therapies are used with a palliative intent, whereas nearly a quarter of patients with resistant leukemia receiving revumenib on this study proceeded to a potentially curative HSCT. This study is ongoing in its evaluation of NPM1-mutated AML.
ACKNOWLEDGMENT
The authors would like to thank the study participants, their families, all investigational site members involved in this study, and Alex Bataller, MD, PhD for assistance with the gene expression figure. Writing and editorial support were provided under the direction of the authors by Ella A. Kasanga, PhD, PMP, and Stephanie Roulias, ELS, of MedThink SciCom, and funded by Syndax Pharmaceuticals, Inc.
Investigators are listed in the Appendix (online only).
APPENDIX. LIST OF INVESTIGATORS
Listed in alphabetical order by institution
Alfred Hospital (Andrew Wei, Shaun Fleming); Children's Healthcare of Atlanta at Egleston (Himalee Sabins); City of Hope National Medical Center (Ibrahim Aldoss); City of Hope Phoenix (Tibor Kovacsovics); Dana-Farber Cancer Institute (Richard Stone); Emory University School of Medicine (Martha Arellano); Florida Cancer Specialists (Manish Patel); H. Lee Moffitt Cancer Center & Research Institute (Chetasi Talati); Hackensack University Medical Center (Jing Chen); Hadassah Medical Center (Boaz Nachmias); Hôpital Lyon Sud (Mael Heiblig); Hospital Universitario y Politécnico La Fe (Pau Montesios Fernandez); Instituto Catalán de Oncología-Hospital Duran i Reynals (Montserrat Arnan-Sangerman); Memorial Sloan Kettering Cancer Center (Eytan M. Stein); Montefiore Einstein Comprehensive Cancer Center (Ioannis Mantzaris); Ohio State University (James S. Blachly); Oregon Health & Science University Knight Cancer Institute (Elie Traer); Princess Máxima Center for Pediatric Oncology (C. Michael Zwaan); Rabin Medical Center (Ofir Wolach); Regional Cancer Center (Istituto Romagnolo per lo Studio dei Tumori “Dino Amadori”-IRST IRCCS) (Giovanni Marconi); Royal Melbourne Hospital (Ashish Bajel); Sir Charles Gairdner Hospital (Carolyn S. Grove); Stanford University School of Medicine (Gabriel N. Mannis); University Health Network-Princess Margaret Cancer Center, Myeloma Clinic (Andre Schuh); University of Chicago Medicine (Michael Thirman); University of Cincinnati College of Medicine (John Byrd); University of Colorado School of Medicine (Christine M. McMahon); University of Iowa Stead Family Children's Hospital (David S. Dickens); University of Pennsylvania (Alexander Perl); University of Sydney (Matthew Greenwood); University of Texas MD Anderson Cancer Center (Ghayas C. Issa); University of Toronto-The Hospital for Sick Children (James Whitlock); University of Utah Huntsman Cancer Institute (Paul J. Shami); Washington University School of Medicine (John DiPersio).
Ghayas C. Issa
Consulting or Advisory Role: Novartis, Kura Oncology, Syndax, NuProbe, AbbVie, Sanofi
Research Funding: Novartis (Inst), Syndax (Inst), Kura Oncology (Inst), Merck, Cullinan Oncology, Astex Pharmaceuticals (Inst), NuProbe (Inst)
Ibrahim Aldoss
Honoraria: Amgen, AbbVie, Kite, a Gilead company, Agios, Jazz Pharmaceuticals, Syndax, Takeda
Consulting or Advisory Role: Amgen
Speakers' Bureau: Pfizer
Research Funding: AbbVie, Macrogenics
Michael J. Thirman
Consulting or Advisory Role: AstraZeneca, Genentech, AbbVie, Adaptive Biotechnologies, Celgene, Pharmacyclics, CVS
Research Funding: AbbVie (Inst), Syndax (Inst), Merck (Inst), TG Therapeutics (Inst), Nurix (Inst)
Expert Testimony: Apotex
John DiPersio
Stock and Other Ownership Interests: Magenta Therapeutics, WUGEN, Inc
Consulting or Advisory Role: Rivervest, Magenta Therapeutics, SPARC, hC Bioscience
Research Funding: Macrogenics (Inst), WUGEN, Inc (Inst), BiolineRx (Inst), NeoImmuneTech (Inst), Incyte (Inst)
Patents, Royalties, Other Intellectual Property: CD7 and CD2 Knockout for CART to CD7 and CD2, Duvelisib for treatment of cytokine release syndrome (CRS), NT-17 to enhance CART Survival, Novel WU mobilizing compounds (Inst), Selection of IMPDH Mutant Stem Cells, IFNg, upregulate MHCII for relapsed AML, Dextran based molecules to detect CAR-T cells, Combining integrin inhibitor with chemokine binders, 016131, JAK and calcineurin inhibition, solid organ transplant, VLA4, gro-b, Triple Combination—CXCR2, VLA-4, gro-b, Targeting IFNR/CSCR3 in GvHD, WU/SLU compounds VLA4 and CXCR2 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/213768
James S. Blachly
Consulting or Advisory Role: AstraZeneca, AbbVie, Kite, a Gilead company, AstraZeneca, Astellas Pharma, Innate Pharma, MingSight, Syndax
Patents, Royalties, Other Intellectual Property: Sequencing Technology patent pending (Inst)
Travel, Accommodations, Expenses: Oxford Nanopore Technologies
Gabriel N. Mannis
Consulting or Advisory Role: AbbVie/Genentech, Astellas Pharma, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Macrogenics, SERVIER, Rigel, WUGEN, Inc, Immunogen, Syndax, Orbital Therapeutics
Research Funding: Astex Pharmaceuticals (Inst), Glycomimetics (Inst), Jazz Pharmaceuticals (Inst), Forty Seven (Inst), Gilead Sciences (Inst), Syndax (Inst), Immune-Onc Therapeutics (Inst), Immunogen (Inst), Bristol Myers Squibb/Celgene (Inst)
Alexander Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Daiichi Sankyo, AbbVie, Celgene/Bristol Myers Squibb, Syndax, Genentech, Foghorn Therapeutics, Rigel, Curis, Schrodinger
Research Funding: Astellas Pharma (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Syndax (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Astellas Pharma
David S. Dickens
Consulting or Advisory Role: tempus, inc, Amgen
Research Funding: syndax (Inst)
Christine M. McMahon
Consulting or Advisory Role: TG Therapeutics, AbbVie, Kura Oncology
Research Funding: Syros Pharmaceuticals (Inst), Syndax (Inst)
Elie Traer
Honoraria: Binay Foundation
Consulting or Advisory Role: Astellas Pharma, AbbVie, Daiichi Sankyo/UCB Japan, Rigel, SERVIER
Research Funding: AstraZeneca, Prelude Therapeutics, Schrodinger, Incyte
Patents, Royalties, Other Intellectual Property: Patent for human marrow organoid model held by myself OHSU. Also on patient for ex vivo drug prediction assay with OHSU
C. Michel Zwaan
Consulting or Advisory Role: Takeda (Inst), Pfizer (Inst), AbbVie (Inst), Jazz Pharmaceuticals (Inst), Incyte (Inst), Novartis (Inst), Kura Oncology (Inst), Gilead Sciences (Inst)
Research Funding: Takeda (Inst), AbbVie/Genentech (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst), Kura Oncology (Inst), Daiichi Sankyo (Inst)
Carolyn S. Grove
Consulting or Advisory Role: Otsuka (Inst)
Speakers' Bureau: Astellas Pharma (Inst)
Richard Stone
Honoraria: Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Novartis, Takeda, Syntrix Biosystems, Syndax, BerGenBio, Janssen, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Jazz Pharmaceuticals, Epizyme, Aptevo Therapeutics, AvenCell, Kura Oncology, Rigel, Celularity, Ligand, Lava Therapeutics, Hermavant, Redona Therapeutics, Curis Oncology, Daiichi Sankyo Europe GmbH
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Paul J. Shami
Stock and Other Ownership Interests: JSK Therapeutics
Consulting or Advisory Role: RJH Biosciences, Daiichi Sankyo Inc
Research Funding: Chimerix (Inst), Amgen (Inst), Aptevo Therapeutics (Inst), Ono Pharmaceutical (Inst), Chimerix (Inst), Syndax
Uncompensated Relationships: JSK Therapeutics
Matthew Greenwood
Honoraria: Amgen, SERVIER
Research Funding: Amgen (Inst), SERVIER (Inst)
Neerav Shukla
Consulting or Advisory Role: Illumina
Tibor Kovacsovics
Honoraria: Rigel, Servier
Consulting or Advisory Role: Takeda, Kite, a Gilead company, Astellas Pharma, Zentalis, Kedrion Biopharma
Research Funding: Novartis (Inst), Glycomimetics (Inst), Gilead Sciences (Inst), Amgen (Inst), AbbVie (Inst), Janssen Oncology (Inst), Syndax (Inst)
Yu Gu
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Rebecca G. Bagley
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Kate Madigan
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax
Patents, Royalties, Other Intellectual Property: Patent pending US 0091183A1 The present invention features, inter alia, methods of treating (a) a patient who has been diagnosed with a hematopoietic cancer or (b) a population of patients who have been diagnosed with a hematopoietic cancer with a fixed dose of tamibarotene or a pharmaceutically acceptable salt thereof. The tamibarotene is administered daily, for a prescribed number of days, at 8-14 (eg, 12 mg/day) regardless of the patient's weight or body surface area, and may be administered as the sole therapeutic agent or in combination with one or more of the additional therapeutic agents described in the patent. This patent is pending and has not yet been granted. The drug remains experimental in the US at this time
Travel, Accommodations, Expenses: Syndax
Yakov Chudnovsky
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Consulting or Advisory Role: Eutropics
Huy Van Nguyen
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Research Funding: Syndax
Travel, Accommodations, Expenses: Syndax
Nicole McNeer
Employment: Syndax, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Syndax
Patents, Royalties, Other Intellectual Property: Compositions for enhancing targeted gene editing and methods of use thereof. Patent number: 11136597. Date: October 2021. Applicants: Yale University, Carnegie Mellon University. Inventors: W. Mark Saltzman, Peter Glazer, Raman Bahal, Nicole Ali McNeer, Danith H. Ly, Elias Quijano. Compositions and methods for treatment of cystic fibrosis. United States Patent Application No. 15/998,613, filed August 16, 2018. Applicants: Yale University. Inventors: Peter Glazer, W. Mark Saltzman, Marie Egan, Nicole Ali McNeer
Travel, Accommodations, Expenses: Syndax, AstraZeneca
Eytan M. Stein
Stock and Other Ownership Interests: Auron Therapeutics
Consulting or Advisory Role: Novartis, Janssen, Bristol Myers Squibb/Celgene, Agios, Menarini, Genentech, Genesis Pharma, AbbVie, Neoleukin Therapeutics, Gilead Sciences, Syndax, OnCusp Therapeutics, Immunogen, CTI BioPharma Corp, Foghorn Therapeutics, SERVIER, Calithera Biosciences, Daiichi Sankyo, Aptose Biosciences, Ono Pharmaceutical, Blueprint Medicines, GEMoaB, Jnana Therapeutics, Debiopharm Group
Research Funding: Eisai (Inst), Bristol Myers Squibb/Celgene (Inst), Bayer (Inst), agios (Inst), BioTheryX (Inst), Syros Pharmaceuticals (Inst), SERVIER (Inst), Foghorn Therapeutics (Inst), Syndax (Inst), Gilead Sciences (Inst), Cleave Biosciences (Inst), Prelude Therapeutics (Inst), Loxo/Lilly (Inst)
No other potential conflicts of interest were reported.
See accompanying Understanding the Pathway, p. 85
PRIOR PRESENTATION
Presented in part at the American Society of Hematology 2023, San Diego, CA, December 9-12, 2023; American Society of Pediatric Hematology/Oncology 2024, Seattle WA, April 3-6, 2024; Academy of Managed Care Pharmacy 2024, New Orleans, LA, April 15-18, 2024; and European Hematology Association 2024, Madrid, Spain, June 13-16, 2024.
SUPPORT
Supported by Syndax Pharmaceuticals, Inc.
CLINICAL TRIAL INFORMATION
NCT04065399 (AUGMENT-101)
G.C.I. and I.A. contributed equally to this work.
Contributor Information
Collaborators: Andrew Wei, Shaun Fleming, Himalee Sabins, Ibrahim Aldoss, Tibor Kovacsovics, Richard Stone, Martha Arellano, Manish Patel, Chetasi Talati, Jing Chen, Boaz Nachmias, Mael Heiblig, Pau Montesios Fernandez, Montserrat Arnan-Sangerman, Eytan M. Stein, Ioannis Mantzaris, James S. Blachly, Elie Traer, C. Michael Zwaan, Ofir Wolach, Giovanni Marconi, Ashish Bajel, Carolyn S. Grove, Gabriel N. Mannis, Andre Schuh, Michael Thirman, John Byrd, Christine M. McMahon, David S. Dickens, Alexander Perl, Matthew Greenwood, Ghayas C. Issa, James Whitlock, Paul J. Shami, and John DiPersio
DATA SHARING STATEMENT
Syndax Pharmaceuticals, Inc, (Waltham, MA) is committed to data sharing that advances science and medicine while protecting patient privacy. The data supporting the findings of this study, including the study protocol and statistical analysis plan, are available within the article and its Data Supplement. Any additional data requests from qualified scientific researchers are available upon reasonable request. Deidentified participant data are available to request after all trial prespecified analyses have been completed and after the indication studied has been approved in the United States and European Union, whichever is later. Access is provided after a research proposal has been approved by an appropriate review committee and after receipt of a signed data sharing agreement. Additional details may be requested at: https://syndax.com/contact-us/.
AUTHOR CONTRIBUTIONS
Conception and design: Ghayas C. Issa, Michael J. Thirman, John DiPersio, Richard Stone, Branko Cuglievan, Yu Gu, Kate Madigan, Nicole McNeer, Eytan M. Stein
Financial support: Nicole McNeer
Administrative support: John DiPersio, Nicole McNeer
Provision of study materials or patients: Ibrahim Aldoss, James S. Blachly, Alexander Perl, Christine M. McMahon, C. Michel Zwaan, Carolyn S. Grove, Richard Stone, Paul J. Shami, Matthew Greenwood, Nicole McNeer
Collection and assembly of data: Ghayas C. Issa, Ibrahim Aldoss, Michael J. Thirman, John DiPersio, Martha Arellano, James S. Blachly, Gabriel N. Mannis, Alexander Perl, David S. Dickens, Christine M. McMahon, Elie Traer, C. Michel Zwaan, Carolyn S. Grove, Paul J. Shami, Ioannis Mantzaris, Matthew Greenwood, Neerav Shukla, Branko Cuglievan, Tibor Kovacsovics, Yu Gu, Rebecca G. Bagley, Kate Madigan, Yakov Chudnovsky, Huy Van Nguyen, Nicole McNeer, Eytan M. Stein
Data analysis and interpretation: Ghayas C. Issa, Ibrahim Aldoss, Michael J. Thirman, John DiPersio, Martha Arellano, James S. Blachly, Gabriel N. Mannis, David S. Dickens, Christine M. McMahon, C. Michel Zwaan, Richard Stone, Neerav Shukla, Branko Cuglievan, Tibor Kovacsovics, Yu Gu, Rebecca G. Bagley, Kate Madigan, Yakov Chudnovsky, Huy Van Nguyen, Nicole McNeer, Eytan M. Stein
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Menin Inhibition With Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ghayas C. Issa
Consulting or Advisory Role: Novartis, Kura Oncology, Syndax, NuProbe, AbbVie, Sanofi
Research Funding: Novartis (Inst), Syndax (Inst), Kura Oncology (Inst), Merck, Cullinan Oncology, Astex Pharmaceuticals (Inst), NuProbe (Inst)
Ibrahim Aldoss
Honoraria: Amgen, AbbVie, Kite, a Gilead company, Agios, Jazz Pharmaceuticals, Syndax, Takeda
Consulting or Advisory Role: Amgen
Speakers' Bureau: Pfizer
Research Funding: AbbVie, Macrogenics
Michael J. Thirman
Consulting or Advisory Role: AstraZeneca, Genentech, AbbVie, Adaptive Biotechnologies, Celgene, Pharmacyclics, CVS
Research Funding: AbbVie (Inst), Syndax (Inst), Merck (Inst), TG Therapeutics (Inst), Nurix (Inst)
Expert Testimony: Apotex
John DiPersio
Stock and Other Ownership Interests: Magenta Therapeutics, WUGEN, Inc
Consulting or Advisory Role: Rivervest, Magenta Therapeutics, SPARC, hC Bioscience
Research Funding: Macrogenics (Inst), WUGEN, Inc (Inst), BiolineRx (Inst), NeoImmuneTech (Inst), Incyte (Inst)
Patents, Royalties, Other Intellectual Property: CD7 and CD2 Knockout for CART to CD7 and CD2, Duvelisib for treatment of cytokine release syndrome (CRS), NT-17 to enhance CART Survival, Novel WU mobilizing compounds (Inst), Selection of IMPDH Mutant Stem Cells, IFNg, upregulate MHCII for relapsed AML, Dextran based molecules to detect CAR-T cells, Combining integrin inhibitor with chemokine binders, 016131, JAK and calcineurin inhibition, solid organ transplant, VLA4, gro-b, Triple Combination—CXCR2, VLA-4, gro-b, Targeting IFNR/CSCR3 in GvHD, WU/SLU compounds VLA4 and CXCR2 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/213768
James S. Blachly
Consulting or Advisory Role: AstraZeneca, AbbVie, Kite, a Gilead company, AstraZeneca, Astellas Pharma, Innate Pharma, MingSight, Syndax
Patents, Royalties, Other Intellectual Property: Sequencing Technology patent pending (Inst)
Travel, Accommodations, Expenses: Oxford Nanopore Technologies
Gabriel N. Mannis
Consulting or Advisory Role: AbbVie/Genentech, Astellas Pharma, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Macrogenics, SERVIER, Rigel, WUGEN, Inc, Immunogen, Syndax, Orbital Therapeutics
Research Funding: Astex Pharmaceuticals (Inst), Glycomimetics (Inst), Jazz Pharmaceuticals (Inst), Forty Seven (Inst), Gilead Sciences (Inst), Syndax (Inst), Immune-Onc Therapeutics (Inst), Immunogen (Inst), Bristol Myers Squibb/Celgene (Inst)
Alexander Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Daiichi Sankyo, AbbVie, Celgene/Bristol Myers Squibb, Syndax, Genentech, Foghorn Therapeutics, Rigel, Curis, Schrodinger
Research Funding: Astellas Pharma (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Syndax (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Astellas Pharma
David S. Dickens
Consulting or Advisory Role: tempus, inc, Amgen
Research Funding: syndax (Inst)
Christine M. McMahon
Consulting or Advisory Role: TG Therapeutics, AbbVie, Kura Oncology
Research Funding: Syros Pharmaceuticals (Inst), Syndax (Inst)
Elie Traer
Honoraria: Binay Foundation
Consulting or Advisory Role: Astellas Pharma, AbbVie, Daiichi Sankyo/UCB Japan, Rigel, SERVIER
Research Funding: AstraZeneca, Prelude Therapeutics, Schrodinger, Incyte
Patents, Royalties, Other Intellectual Property: Patent for human marrow organoid model held by myself OHSU. Also on patient for ex vivo drug prediction assay with OHSU
C. Michel Zwaan
Consulting or Advisory Role: Takeda (Inst), Pfizer (Inst), AbbVie (Inst), Jazz Pharmaceuticals (Inst), Incyte (Inst), Novartis (Inst), Kura Oncology (Inst), Gilead Sciences (Inst)
Research Funding: Takeda (Inst), AbbVie/Genentech (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst), Kura Oncology (Inst), Daiichi Sankyo (Inst)
Carolyn S. Grove
Consulting or Advisory Role: Otsuka (Inst)
Speakers' Bureau: Astellas Pharma (Inst)
Richard Stone
Honoraria: Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Novartis, Takeda, Syntrix Biosystems, Syndax, BerGenBio, Janssen, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Jazz Pharmaceuticals, Epizyme, Aptevo Therapeutics, AvenCell, Kura Oncology, Rigel, Celularity, Ligand, Lava Therapeutics, Hermavant, Redona Therapeutics, Curis Oncology, Daiichi Sankyo Europe GmbH
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Paul J. Shami
Stock and Other Ownership Interests: JSK Therapeutics
Consulting or Advisory Role: RJH Biosciences, Daiichi Sankyo Inc
Research Funding: Chimerix (Inst), Amgen (Inst), Aptevo Therapeutics (Inst), Ono Pharmaceutical (Inst), Chimerix (Inst), Syndax
Uncompensated Relationships: JSK Therapeutics
Matthew Greenwood
Honoraria: Amgen, SERVIER
Research Funding: Amgen (Inst), SERVIER (Inst)
Neerav Shukla
Consulting or Advisory Role: Illumina
Tibor Kovacsovics
Honoraria: Rigel, Servier
Consulting or Advisory Role: Takeda, Kite, a Gilead company, Astellas Pharma, Zentalis, Kedrion Biopharma
Research Funding: Novartis (Inst), Glycomimetics (Inst), Gilead Sciences (Inst), Amgen (Inst), AbbVie (Inst), Janssen Oncology (Inst), Syndax (Inst)
Yu Gu
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Rebecca G. Bagley
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Kate Madigan
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax
Patents, Royalties, Other Intellectual Property: Patent pending US 0091183A1 The present invention features, inter alia, methods of treating (a) a patient who has been diagnosed with a hematopoietic cancer or (b) a population of patients who have been diagnosed with a hematopoietic cancer with a fixed dose of tamibarotene or a pharmaceutically acceptable salt thereof. The tamibarotene is administered daily, for a prescribed number of days, at 8-14 (eg, 12 mg/day) regardless of the patient's weight or body surface area, and may be administered as the sole therapeutic agent or in combination with one or more of the additional therapeutic agents described in the patent. This patent is pending and has not yet been granted. The drug remains experimental in the US at this time
Travel, Accommodations, Expenses: Syndax
Yakov Chudnovsky
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Consulting or Advisory Role: Eutropics
Huy Van Nguyen
Employment: Syndax
Stock and Other Ownership Interests: Syndax
Research Funding: Syndax
Travel, Accommodations, Expenses: Syndax
Nicole McNeer
Employment: Syndax, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Syndax
Patents, Royalties, Other Intellectual Property: Compositions for enhancing targeted gene editing and methods of use thereof. Patent number: 11136597. Date: October 2021. Applicants: Yale University, Carnegie Mellon University. Inventors: W. Mark Saltzman, Peter Glazer, Raman Bahal, Nicole Ali McNeer, Danith H. Ly, Elias Quijano. Compositions and methods for treatment of cystic fibrosis. United States Patent Application No. 15/998,613, filed August 16, 2018. Applicants: Yale University. Inventors: Peter Glazer, W. Mark Saltzman, Marie Egan, Nicole Ali McNeer
Travel, Accommodations, Expenses: Syndax, AstraZeneca
Eytan M. Stein
Stock and Other Ownership Interests: Auron Therapeutics
Consulting or Advisory Role: Novartis, Janssen, Bristol Myers Squibb/Celgene, Agios, Menarini, Genentech, Genesis Pharma, AbbVie, Neoleukin Therapeutics, Gilead Sciences, Syndax, OnCusp Therapeutics, Immunogen, CTI BioPharma Corp, Foghorn Therapeutics, SERVIER, Calithera Biosciences, Daiichi Sankyo, Aptose Biosciences, Ono Pharmaceutical, Blueprint Medicines, GEMoaB, Jnana Therapeutics, Debiopharm Group
Research Funding: Eisai (Inst), Bristol Myers Squibb/Celgene (Inst), Bayer (Inst), agios (Inst), BioTheryX (Inst), Syros Pharmaceuticals (Inst), SERVIER (Inst), Foghorn Therapeutics (Inst), Syndax (Inst), Gilead Sciences (Inst), Cleave Biosciences (Inst), Prelude Therapeutics (Inst), Loxo/Lilly (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pieters R, De Lorenzo P, Ancliffe P, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international phase III randomized study. J Clin Oncol. 2019;37:2246–2256. doi: 10.1200/JCO.19.00261. [DOI] [PubMed] [Google Scholar]
- 3. Driessen EM, de Lorenzo P, Campbell M, et al. Outcome of relapsed infant acute lymphoblastic leukemia treated on the interfant-99 protocol. Leukemia. 2016;30:1184–1187. doi: 10.1038/leu.2015.246. [DOI] [PubMed] [Google Scholar]
- 4. Issa GC, Aldoss I, DiPersio J, et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature. 2023;615:920–924. doi: 10.1038/s41586-023-05812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Issa GC, Zarka J, Sasaki K, et al. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J. 2021;11:162. doi: 10.1038/s41408-021-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorsteinsdottir U, Kroon E, Jerome L, et al. Defining roles for HOX and MEIS1 genes in induction of acute myeloid leukemia. Mol Cell Biol. 2001;21:224–234. doi: 10.1128/MCB.21.1.224-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu BD, Hess JL, Horning SE, et al. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama A, Somervaille TC, Smith KS, et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 9. Grembecka J, He S, Shi A, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012;8:277–284. doi: 10.1038/nchembio.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krivtsov AV, Evans K, Gadrey JY, et al. A Menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019;36:660–673.e11. doi: 10.1016/j.ccell.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uckelmann HJ, Kim SM, Wong EM, et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. 2020;367:586–590. doi: 10.1126/science.aax5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heikamp EB, Henrich JA, Perner F, et al. The menin-MLL1 interaction is a molecular dependency in NUP98-rearranged AML. Blood. 2022;139:894–906. doi: 10.1182/blood.2021012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Libbrecht C, Xie HM, Kingsley MC, et al. Menin is necessary for long term maintenance of meningioma-1 driven leukemia. Leukemia. 2021;35:1405–1417. doi: 10.1038/s41375-021-01146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barajas JM, Rasouli M, Umeda M, et al. Acute myeloid leukemias with UBTF tandem duplications are sensitive to Menin inhibitors Blood 143:619-630, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rasouli M, Blair H, Troester S, et al. The MLL–Menin interaction is a therapeutic vulnerability in NUP98-rearranged AML. HemaSphere. 2023;7:e935. doi: 10.1097/HS9.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Burg M, Beverloo HB, Langerak AW, et al. Rapid and sensitive detection of all types of MLL gene translocations with a single FISH probe set. Leukemia. 1999;13:2107–2113. doi: 10.1038/sj.leu.2401595. [DOI] [PubMed] [Google Scholar]
- 17. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter BZ, Mak PY, Mu H, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8:355ra117. doi: 10.1126/scitranslmed.aag1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dzama MM, Steiner M, Rausch J, et al. Synergistic targeting of FLT3 mutations in AML via combined menin-MLL and FLT3 inhibition. Blood. 2020;136:2442–2456. doi: 10.1182/blood.2020005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soto-Feliciano YM, Sánchez-Rivera FJ, Perner F, et al. A molecular switch between mammalian MLL complexes dictates response to Menin–MLL inhibition. Cancer Discov. 2023;13:146–169. doi: 10.1158/2159-8290.CD-22-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa GC, Cuglievan B, DiNardo CD, et al. Early results of the phase I/II study investigating the all-oral combination of the Menin inhibitor revumenib (SNDX-5613) with decitabine/cedazuridine (ASTX727) and venetoclax in acute myeloid leukemia (SAVE) Blood 142:58-602023 [Google Scholar]
- 22.SNDX-5613 and Gilteritinib for the Treatment of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia and Concurrent MLL-Rearrangement or NPM1 Mutation. https://classic.clinicaltrials.gov/ct2/show/NCT06222580 [Google Scholar]
- 23.Revumenib in Combination With 7+3 + Midostaurin in AML. https://classic.clinicaltrials.gov/ct2/show/NCT06313437 [Google Scholar]
- 24.A Phase II Study of the Menin Inhibitor Revumenib in Leukemia Associated With Upregulation of HOX Genes. https://classic.clinicaltrials.gov/ct2/show/NCT06229912 [Google Scholar]
- 25. Richard-Carpentier G, Kantarjian HM, Tang G, et al. Outcomes of acute lymphoblastic leukemia with KMT2A (MLL) rearrangement: The MD Anderson experience. Blood Adv. 2021;5:5415–5419. doi: 10.1182/bloodadvances.2021004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaspar N, Marshall LV, Binner D, et al. Joint adolescent-adult early phase clinical trials to improve access to new drugs for adolescents with cancer: Proposals from the multi-stakeholder platform-ACCELERATE. Ann Oncol. 2018;29:766–771. doi: 10.1093/annonc/mdy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Syndax Pharmaceuticals, Inc, (Waltham, MA) is committed to data sharing that advances science and medicine while protecting patient privacy. The data supporting the findings of this study, including the study protocol and statistical analysis plan, are available within the article and its Data Supplement. Any additional data requests from qualified scientific researchers are available upon reasonable request. Deidentified participant data are available to request after all trial prespecified analyses have been completed and after the indication studied has been approved in the United States and European Union, whichever is later. Access is provided after a research proposal has been approved by an appropriate review committee and after receipt of a signed data sharing agreement. Additional details may be requested at: https://syndax.com/contact-us/.


