ABSTRACT
Objectives
Studies have shown that people living with multiple sclerosis (PwMS) were substantially impacted by the COVID‐19 pandemic. However, no study has compared the overall health‐related quality of life impact of the COVID‐19 pandemic on PwMS and the general population. Differences would have implications for crises/pandemic management policies. This study aimed to compare the prevalence and health‐related quality of life impact of COVID‐19‐related adversity (such as deteriorations in mental or physical health) in PwMS and the general population.
Methods
Cross‐sectional data were obtained from the How Is Your Life Australian general population study (comprising subsamples with and without chronic disease) and the Australian MS Longitudinal Study from August to October 2020. Health‐related quality of life was measured using health state utilities (HSUs; represented on a 0 [death] to 1 [full health] scale) generated by the EQ‐5D‐5L‐Psychosocial. COVID‐19‐related adversity was measured via specialized survey items. Descriptive and multivariable regression analyses were conducted.
Results
A total of 1020 general population individuals and 1635 MS participants entered the study (mean age 52.4 and 58.4; female 52.4% and 80.2%, respectively). COVID‐19‐related adversity prevalence was higher among PwMS compared to the general population with and without chronic diseases (PR: 1.430 [CI: 1.153, 1.774] and PR: 1.90 [CI: 1.56, 2.32], respectively). However, the HSU impact of COVID‐19‐related adversity was not dependent on disease status (p > 0.20, test for interaction).
Conclusion
This study found that PwMS were more likely to experience COVID‐19‐related adversity compared to the general population, though the health‐related quality‐of‐life impact was similar. This demonstrates that PwMS require additional support during national and global crises.
Keywords: COVID‐19, health state utility, multiple sclerosis, outcome measurement, quality of life
1. Introduction
1.1. Multiple Sclerosis in the Health Economics Context
Multiple sclerosis (MS) is a neurodegenerative disorder, currently without cure or certain etiology (McGinley, Goldschmidt, and Rae‐Grant 2021; Thompson, Baranzini, et al. 2018). Symptoms associated with MS are diverse and may include incoordination; motor, cognitive, sexual, bladder, and bowel dysfunction; sensory impairment; pain; and fatigue (Zhang et al. 2021). Consequently, people living with multiple sclerosis (PwMS) have varying experiences of the disease (Zhang et al. 2021). However, health‐related quality of life is often substantially impacted by the disease (J. A. Campbell, Ahmad, et al. 2023).
MS affected around 2.8 million people globally as of 2020 (Walton et al. 2020), and an estimated 33,335 Australians in 2021 (Ahmad et al. 2018; J. Campbell, van der Mei, Taylor, and Palmer 2023). Our comprehensive cost of illness study showed that the total, annual societal cost of MS in Australia was 2.45 billion Australian dollars in the 2021 calendar year (J. Campbell, van der Mei, Taylor, and Palmer 2023). The cost per person with MS per year was estimated at 73,457 Australian dollars (J. Campbell, van der Mei, Taylor, and Palmer 2023). MS‐related costs and global prevalence are predicted to increase at an accelerating rate (Walton et al. 2020; J. A. Campbell et al. 2020).
1.2. Health‐Related Quality of Life During the COVID‐19 Pandemic and Study Rationale
Several studies have contributed to our understanding of the effect of the COVID‐19 pandemic on health‐related quality of life. General population studies have frequently found significant impacts on psychological well‐being (Every‐Palmer et al. 2020; Grover et al. 2020; Huang and Zhao 2020; Karageorghis et al. 2021). Studies of chronic disease populations have identified limitations in access to health care as a principal vector through which the COVID‐19 pandemic has reduced quality of life. Regarding MS, most studies have focused on the mental health effects of the pandemic (Motolese et al. 2020; Costabile et al. 2021; Ramezani et al. 2021; Manacorda et al. 2021). While these studies have frequently concluded that PwMS were negatively impacted by the COVID‐19 pandemic, there is less conclusive evidence regarding whether PwMS were more affected than the general population. Upon examination of the literature, we found conflicting evidence presented across relevant studies, with some finding a difference (Yeni, Tulek, and Terzi. 2022) and others not (Motolese et al. 2020; Talaat et al. 2020; Altieri et al. 2022).
To our knowledge, only two studies have investigated how the COVID‐19 pandemic affected the broader health‐related quality of life of PwMS using a multiattribute utility instrument (namely the EQ‐5D‐5L [Ferreira et al. 2021] and the AQoL‐8D [Henson et al. 2024]). Using algorithms, these instruments convert patient‐reported responses into numerical scores, based on preestablished, country‐specific value sets (Drummond et al. 2015). These scores are known as health state utilities (HSUs) and are represented on a 0 (death) to 1 (full health) scale (Culyer 2014). HSU is an essential metric for clinical trials and cost‐utility studies, the latter of which informs resource allocation decisions (J. A. Campbell et al. 2016).
Importantly, no study has investigated whether the impact of the COVID‐19 pandemic differed between PwMS and the general population using HSUs. This is particularly significant as HSU is a uniquely holistic measure of health‐related quality of life (Drummond et al. 2015). A study using this measure may therefore contribute impactful evidence to the literature. Indeed, a recent systematic review, regarding differences in the psychological impact of the COVID‐19 pandemic on PwMS and healthy controls, called for further, related research (Altieri et al. 2022). This was due to the strong impact of the COVID‐19 pandemic on the general population's mental health and well‐being, which may have had an attenuating effect and led to the study's largely negative results. Moreover, we could identify no study that compared the impact of the COVID‐19 pandemic between PwMS and persons living with other chronic diseases generally.
1.3. Aims of the Study
This study aimed to determine if the impact of the COVID‐19 pandemic differed between PwMS and the general population (both with and without chronic diseases) through analysis of EQ‐5D‐5L‐Psychosocial HSUs. In doing so, it would contribute important information to a currently divided literature. If PwMS were more affected, this would indicate that they require additional resources to support their health‐related quality of life during pandemics and other isolating crises. Values determined by our study would be applicable in health economic simulation models that aim to evaluate the impact of and solutions to such crises.
2. Methods
2.1. Source of Study Participants
Participants living with MS (hereafter “PwMS sample”) were sourced from the Australian Multiple Sclerosis Longitudinal Study (AMSLS). The AMSLS is a representative, survey‐based cohort study that has been funded by MS Australia since 2002 (Taylor et al. 2013). Recruitment to the study is ongoing, and all study participants are required to provide informed consent before admittance. The AMSLS currently involves around 2600 active participants, with an estimated 96.0% of these participants diagnosed with MS per the McDonald criteria (Thompson, Banwell, et al. 2018). Ethics Approval for the AMSLS was received from the Tasmanian Health and Medical Human Research Ethics Committee (ethics approval number H0014183).
Participants from the Australian general population (hereafter “general population sample”) were drawn from the How is Your Life (HIYL) study. This study was conducted by Monash University's Centre for Health Economics. The HIYL study participants (aged 18 and over) were recruited through the global company Cint (www.cint.com) from its panel members. These participants were subsequently asked to complete an anonymous, online survey developed using Qualtrics (www.qualtrics.com). Recruitment for this study used a quota sampling method that was informed by the age and sex distributions in Australia. Ethics approval for the HIYL study was granted by the Monash University Human Research Ethics Committee (Project ID 8442).
2.2. Sources of Data
General population data were sourced from the HIYL online survey (conducted in September–October 2020). AMSLS data were sourced primarily from the 2020 Quality of Life survey (conducted August–October 2020), with additional data regarding education level and MS phenotype obtained from the 2018, 2019, and 2020 Disease Course surveys. Unique AMSLS research identifiers were used to link AMSLS data sources. All data used in this study were cross‐sectional, collected during a time of major lockdowns (isolation quarantines) in Australia (Henson et al. 2024) and in which moderate–high levels of distress were observed among the Australian population (Griffiths et al. 2022).
2.3. Measures
2.3.1. Health‐Related Quality of Life and the EQ‐5D‐5L‐Psychosocial Instrument
Health‐related quality of life was measured using the EQ‐5D‐5L‐Psychosocial multiattribute utility instrument (Chen and Olsen 2020). Table S1 displays the nine items of EQ‐5D‐5L‐Psychosocial and each of their five levels. The EQ‐5D‐5L‐Psychosocial comprises the five items of the EQ‐5D‐5L (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) and four bolted‐on, psychosocial items from the AQoL‐8D (vitality, sleep, personal relationships, and social isolation) (Chen and Olsen 2020). These psychosocial bolt‐ons were developed to improve the EQ‐5D‐5L's ability to capture mental and social aspects of health‐related quality of life (Chen and Olsen 2020); the EQ‐5D‐5L's relatively limited sensitivity to changes in psychosocial health has been discussed previously (Finch et al. 2017; Finch, Brazier, and Mukuria 2019; Yang et al. 2015). The added psychosocial questions were found to account for 45% of the explained variation in health‐related quality of life, as measured by the EuroQoL Visual Analogue Scale (measures health on a 0–100 numeric scale) (Chen and Olsen 2023).
As with other multiattribute utility instruments, the EQ‐5D‐5L‐Psychosocial represents health‐related quality of life on a 0 (death) to 1 (full health) scale. This instrument's Australian value set was developed by mapping AQoL‐8D utilities onto EQ‐5D‐5L‐Psychosocial health states (Chen and Olsen 2020). The new instrument has also been found to be interchangeable with the comprehensive, 35‐item AQoL‐8D while being substantially less burdensome (comprising nine items) (J. A. Campbell, Ahmad, et al. 2023). Additionally, the EQ‐5D‐5L‐Psychosocial instrument was recently validated, by our group, for use with MS and myalgic encephalomyelitis cohorts (J. A. Campbell, Ahmad, et al. 2023; Orji et al. 2023). Our study found that psychosocial domains of health are key contributors to the health‐related quality of life (and thus HSUs) of PwMS, especially the domains of sleep (Laslett et al. 2022; Braley and Boudreau 2016) and vitality (J. A. Campbell, Ahmad, et al. 2023).
Changes in HSUs can be evaluated using minimum important differences. A minimum important difference is the smallest change in HSU that is considered clinically meaningful and would support a change in disease management (Henson et al. 2022). In this study, we adopted a minimum important difference of 0.06 (Richardson et al. 2014). This minimal important difference has been used with the AQoL‐8D, which is interchangeable with EQ‐5D‐5L‐Psychosocial due to the instruments using the same value set. Similarly, EQ‐5D‐5L‐Psychosocial HSUs may also be evaluated against the AQoL‐8D population norm of 0.80 (Richardson et al. 2014).
2.3.2. Measures of COVID‐19‐Related Adversity
For the sample of PwMS, COVID‐19‐related adversity (composed of perceived diminutions in health) was determined using a specialized COVID‐19 questionnaire, embedded in the AMSLS 2020 Quality of Life survey (see Figure S1). This questionnaire (Cronbach's alpha of 0.864) specifically required participants to rank the influence of the COVID‐19 pandemic, in seven quality of life domains, on 0–5 Likert scales. The questionnaire's content was informed by the domains included in the AQoL‐8D multiattribute utility instrument. Following examination of responses, this scale was simplified to 0 for no adverse effect (0–1), 1 for a minor adverse effect (2), and 2 for a major adverse effect (3–5) on the basis of differences in mean HSUs associated with the response categories (Henson et al. 2024).
In the HIYL study, COVID‐19‐related adversity was measured using a modified version of the Personal Wellbeing Index instrument, with responses to the overall life satisfaction item being extracted. Using this item, an ordinal variable was constructed, which indicated if an HIYL participant had been (1) adversely affected, (2) not affected, or (3) beneficially affected by the COVID‐19 pandemic.
The measures of COVID‐19‐related adversity were reduced to a dichotomous indicator variable (to facilitate interstudy data compatibility) for which a value of 1 indicated the presence of COVID‐19‐related adversity. A conservative approach was adopted in constructing this indicator, whereby members of the PwMS sample were considered to have reported COVID‐19‐related adversity if they reported a major adverse effect in at least one domain of health. Further detail regarding this is presented in Appendix A of the Supporting Information.
2.3.3. Other Sociodemographic and Clinical Measures
Sociodemographic measures included age (stratified into the categories <35, 35–44, 45–54, 55–64, 65–74, >74), sex, education (secondary or less, occupational certificate or diploma, bachelor's degree, postgraduate degree), the Australian Bureau of Statistics’ Index of Relative Socioeconomic Advantage and Disadvantage (IRSAD; stratified by quartile [this variable also accounts for remoteness]), exposure to COVID‐19‐related lockdown (either metropolitan lockdown, rural lockdown, or neither), and Australian state of residence.
Clinical measures included MS‐related disability and MS phenotype (for the PwMS sample) and the presence of chronic diseases (for the general population sample). As in previous studies, MS‐related disability was mapped from the Patient Determined Disease Steps to the Expanded Disability Severity Scale (EDSS; 0–10) and categorized as no (0.0), mild (0.5–3.5), moderate (4.0–6.0), or severe disability (6.5–9.5) (J. A. Campbell, Ahmad, et al. 2023; Kobelt et al. 2006). Chronic diseases among the general population were grouped into the following classifications (which broadly reflect ICD‐10 Classifications/Codes) (World Health Organization 2015): psychological, musculoskeletal, respiratory, oncological, endocrinological, cardiovascular, gastrointestinal, neurological, sensory, and other. These data were used to classify two general population subgroups—those with and without chronic diseases. These subgroups were utilized in analyses.
Data were collected in functionally identical formats for both samples, except for COVID‐19‐related adversity (as discussed under Section 2.3.2). Differences in the characteristics of the samples’ participants are presented below. Importantly, adjustment for the above covariates, in regression, was utilized to facilitate intersample comparability.
2.4. Descriptive Analyses
Descriptive analyses involved the stratification of HSUs over key variables and sample/group membership. In all descriptive analyses, means and standard deviations were reported for continuous variables, while counts and proportions were adopted for categorical variables. Ordinal variables were summarized in both manners. All quantitative analyses were undertaken using Stata 17 (StataCorp, 2021).
2.5. Regression Analyses
Linear models, with HSU as the outcome variable, were used to identify differences in COVID‐19‐related adversity impacts and overall health‐related quality of life between the PwMS sample and the general population sample and subgroups. Despite the non‐normality of the HSU data (D'Agostino K 2 of 120.76, p < 0.01 [null hypothesis of normality]), model residuals were sufficiently gaussian (see Figure S2 for a related Q–Q plot and statistical test) that transformation of this dependent variable, prior to regressions, was not required to ensure valid statistical inferences, especially given our large sample size (n = 2656) (D'Agostino, Belanger, and D'Agostino 1990). In addition, log‐binomial regression models were estimated to investigate if COVID‐19‐related adversity was more prevalent in the PwMS sample compared to the general population sample and its subgroups. Log‐binomial models are analogous to logit models, except that they generate ratios of relative risk rather than odds.
Both univariable and multivariable regression models were estimated. Multivariable models were adjusted for all available covariates, including age, sex, employment status, education level, socioeconomic status by area, lockdown exposure (log‐binomial only), and reports of COVID‐19‐related adversity (linear only). Lockdown exposure and COVID‐19 adversity were not adjusted for simultaneously as they lie on the same causal pathway (as explained and evidenced elsewhere [Henson et al. 2024]), and COVID‐19 adversity was the subject of the log‐binomial regressions.
3. Results
3.1. Flow of Participants Into the Study
Figure 1 outlines the flow of participants into the study. Regarding the PwMS sample, 2513 active AMSLS participants were invited to participate in the AMSLS 2020 Quality of Life Survey. Of these, 1683 (67.0%) participants responded to the 2020 Quality of Life survey. EQ‐5D‐5L‐Psychosocial HSUs were generatable for 1635 of these participants (97.2%). Regarding the general population sample, 1172 people from the general population used the HIYL study survey link, with 1024 (87.4%) providing valid responses. Of these participants, three were excluded. This was as they reported having MS and their diagnoses could not be confirmed to be adherent to the McDonald criteria (Thompson, Banwell, et al. 2018).
FIGURE 1.
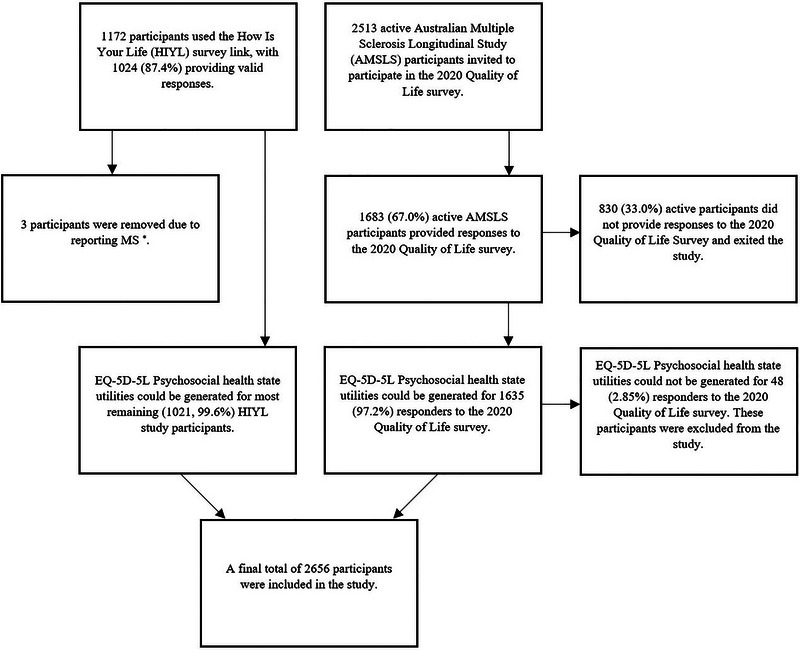
Flow of people living with multiple sclerosis and general population participants into the study. The asterisk indicates that HIYL participants self‐reporting MS were not transferred to the AMSLS dataset as their diagnoses could not be confirmed.
3.2. Comparison of the MS and General Population Samples
Table 1 displays the sociodemographic and clinical characteristics of the MS and general population study samples. PwMS were on average 6 years older (58.4 vs. 52.4); however, this difference did not exceed 1 SD from the mean age of either sample. More importantly, the PwMS sample had a higher proportion of females than the general population sample (80.2% vs. 52.4%). This result is expected as MS disproportionately affects females at a 3:1 ratio (Thompson, Baranzini, et al. 2018; Orton et al. 2006). Additionally, participants in the PwMS sample were more likely to possess postgraduate qualifications (17.4% vs. 10.5%) and live in higher socioeconomic status areas (34.2% vs. 25.1%).
TABLE 1.
Participant characteristics for the people living with multiple sclerosis (PwMS) and general population samples.
| Variables | PwMS sample (N = 1635) | General population sample (N = 1021) |
|---|---|---|
| Age: Mean (SD) | 58.4 (11.3) | 52.4 (17.0) |
| Sex: N (%) | ||
| Male | 320 (19.6) | 486 (47.6) |
| Female | 1315 (80.4) | 535 (52.4) |
| Education level: N (%) | ||
| Secondary school or less | 414 (25.3) | 290 (28.4) |
| Occupation certificate or diploma | 571 (34.9) | 362 (35.5) |
| Bachelor's degree | 354 (21.7) | 259 (25.4) |
| Postgraduate degree | 285 (17.4) | 108 (10.5) |
| Unknown | 11 (0.7) | 2 (0.2) |
| Socioeconomic status by area: N (%) | ||
| Well below average (first quantile) | 187 (11.4) | 182 (17.8) |
| Below average (second quantile) | 256 (15.7) | 179 (17.5) |
| Average (third quantile) | 297 (18.2) | 184 (18.0) |
| Above average (fourth quantile) | 334 (20.4) | 219 (21.5) |
| Well above average (fifth quantile) | 560 (34.2) | 256 (25.1) |
| Unknown | 1 (0.1) | 1 (0.1) |
| State: N (%) | ||
| Australian Capital Territory | 65 (4.0) | 13 (1.3) |
| New South Wales | 457 (28.0) | 317 (31.1) |
| Northern Territory | 2 (0.1) | 8 (0.8) |
| Queensland | 207 (12.7) | 206 (20.2) |
| South Australia | 165 (10.1) | 77 (7.5) |
| Tasmania | 94 (5.7) | 18 (1.8) |
| Victoria | 481 (29.4) | 273 (26.7) |
| Western Australia | 163 (10.0) | 108 (10.6) |
| Unknown | 1 (0.1) | 1 (0.1) |
| COVID‐19‐related adversity: N (%) | ||
| Reported adversity | 700 (42.8) | 300 (29.4) |
| Did not report adversity | 922 (56.4) | 721 (70.6) |
| Unknown | 13 (0.8) | 0 (0.0) |
| EDSS (PwMS sample): N (%) | ||
| No disability | 392 (24.0) | |
| Mild disability | 341 (20.8) | |
| Moderate disability | 588 (36.0) | |
| Major disability | 303 (18.5) | |
| Unknown | 11 (0.7) | |
| Phenotype (PwMS sample): N (%) | ||
| Relapse Onset | 1280 (78.3) | |
| Progressive Onset | 219 (13.4) | |
| Unknown | 136 (8.3) | |
| Chronic disease (general population sample): N (%) | ||
| Reported chronic disease | 602 (59.0) | |
| Did not report chronic disease | 364 (35.6) | |
| Unknown | 55 (5.4) | |
| Psychiatric | 250 (24.5) | |
| Musculoskeletal | 247 (24.2) | |
| Respiratory | 120 (11.8) | |
| Oncological | 28 (2.7) | |
| Endocrinological | 114 (11.2) | |
| Cardiovascular | 69 (6.8) | |
| Gastrological | 13 (1.2) | |
| Neurological | 40 (3.9) | |
| Sensory | 11 (1.1) | |
| Other | 14 (1.4) |
Note: Diseases classified as “other” were used for categories that would have applied to <10 participants including autoimmune, dermatological, and hematological, among others.
Abbreviation: EDSS, Expanded Disability Severity Scale.
A total of 59.0% of the general population sample reported chronic diseases, and 54.5% of the PwMS sample had moderate or severe MS‐related disability. Among participants reporting chronic diseases, 24.5% and 24.2% reported psychiatric and musculoskeletal conditions, respectively. Also represented in the general population sample were oncological (11.8%) and endocrinological (11.2%) disorders. Other types of conditions were disclosed by less than 10.0% of the general population sample.
3.3. Distributions of EQ‐5D‐5L‐Psychosocial HSUs by Sample and Subgroup Membership
Figure 2 contains a kernel density chart representing the distribution of each sample and subgroups’ HSUs, which are a measure of health‐related quality of life. The curve for the general population subgroup without chronic diseases shows that most members of this subgroup had HSUs above 0.700, with the highest density of HSUs around 0.850. The curves for the PwMS sample and the chronic disease subgroup illustrate that the distribution of HSUs for these groups is relatively similar, which implies comparability. Figure 2 is supported by Figures S3–S6, which provide individual histograms for each sample/subgroup's HSU distribution.
FIGURE 2.
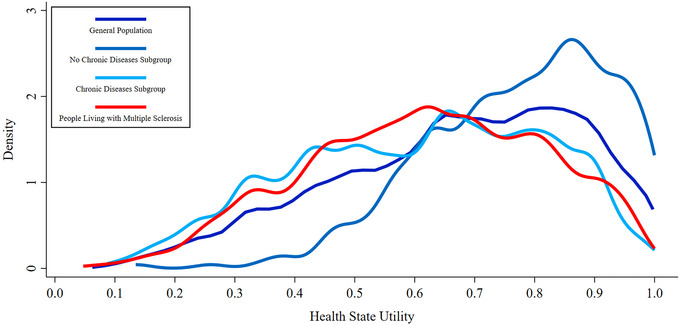
EQ‐5D‐5L‐Psychosocial health state utility kernel density estimates for all samples and subgroups.
3.4. Stratifications of EQ‐5D‐5L‐Psychosocial Item Scores by Sample and Subgroup Membership
Figure 3 (supported by Table S2) shows mean scores for the EQ‐5D‐5L‐Psychosocial items, stratified by sample and subgroup membership. Notably, PwMS scored worst, relative to the general population, in the domain of mobility, scoring 2.35 points on average (compared to 1.52 points, p < 0.01). Interestingly, all subgroups scored relatively poorly in the domains of vitality and sleep, compared to other domains.
FIGURE 3.
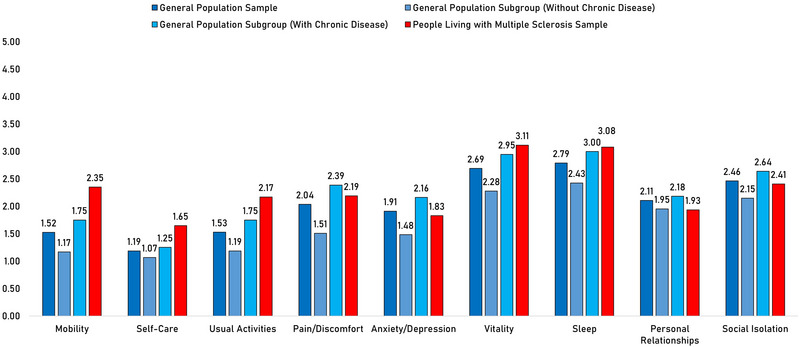
Column chart of mean EQ‐5D‐5L‐Psychosocial item scores for all samples and subgroups. Higher scores indicate greater disability.
3.5. Stratifications of Mean EQ‐5D‐5L‐Psychosocial HSUs by Key Measures
Table 2 demonstrates that mean HSUs for participants’ self‐reporting COVID‐19‐related adversity were lower in every sample and subgroup. Associated HSU decrements were consistently twice the 0.06 minimal important difference or greater. For example, the mean HSU of PwMS who reported COVID‐19‐related adversity was 0.512, compared to 0.671 for those who did not.
TABLE 2.
Mean health state utilities for the multiple sclerosis and general population samples with and without stratification by COVID‐19‐related adversity.
| Samples and subgroups | Health state utility: Mean (SD) | ||
|---|---|---|---|
| Overall | Not reporting COVID‐19‐related adversity | Reporting COVID‐19‐related adversity | |
| People living with multiple sclerosis sample | 0.616 (0.197) | 0.671 (0.185) | 0.512 (0.197) |
| General population sample | 0.669 (0.204) | 0.713 (0.191) | 0.565 (0.199) |
| General population without chronic disease | 0.776 (0.152) | 0.803 (0.141) | 0.684 (0.155) |
| General population with chronic disease | 0.606 (0.206) | 0.651 (0.197) | 0.512 (0.197) |
Table 3 displays mean HSUs stratified by disability severity, MS phenotypes, and chronic diseases (general population sample only). Across the categories of MS disability severity, mean HSU decreased from 0.791 to 0.501, a reduction almost five times the 0.06 minimum important difference. Table 3 also reveals that people living with progressive forms of MS had lower HSUs than people living with relapse onset MS (0.544 vs. 0.645), and among the chronic disease subgroup, persons with psychiatric conditions had the lowest HSUs (0.487, on average).
TABLE 3.
Health state utilities for the multiple sclerosis and general population samples stratified by clinical classifications.
| Sample and subgroup | Health state utility: Mean (SD) |
|---|---|
| People living with multiple sclerosis sample | |
| Disability severity (EDSS) | |
| No disability | 0.791 (0.139) |
| Mild disability | 0.661 (0.158) |
| Moderate disability | 0.534 (0.167) |
| Severe disability | 0.501 (0.501) |
| MS phenotype | |
| Relapse Onset | 0.645 (0.197) |
| Progressive Onset | 0.544 (0.205) |
| General population sample | |
| Chronic disease type | |
| Psychiatric | 0.487 (0.188) |
| Musculoskeletal | 0.593 (0.210) |
| Respiratory | 0.600 (0.221) |
| Oncological | 0.614 (0.185) |
| Endocrinology | 0.620 (0.211) |
| Cardiovascular | 0.590 (0.218) |
| Gastroenterology | 0.622 (0.203) |
| Neurology | 0.514 (0.234) |
| Sensory | 0.643 (0.218) |
| Other | 0.610 (0.203) |
Note: MS‐related disability severity was classified with the Expanded Status Disability Scale (EDSS) as no disability (EDSS = 0), mild disability (EDSS = 0.5–3.5), moderate disability (EDSS = 4.0–6.0), and severe disability (EDSS = 6.5–9.5). Chronic diseases classified as “other” were appropriate for categories that would have applied to less than 10 participants. These diseases included autoimmune, dermatological, and hematological diseases, among others.
3.6. Associations Between Sample and Subgroup Membership and COVID‐19‐Related Adversity Impacts
Table 4 provides regression results regarding the HSU impact of COVID‐19‐related adversity. These results demonstrate that the association between reported COVID‐19‐related adversity and HSU was not substantially modified by sample/subgroup membership (p > 0.10 for all tests for interaction). Specifically, COVID‐19‐related adversity was associated with a 0.129‐point reduction in HSU, on average, for all participants.
TABLE 4.
Univariable and multivariable linear regressions to determine the differences in the health state utility impact of COVID‐19‐related adversity.
| Linear regressions with health state utility as the outcome | ||||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| Coefficient [95% CI] | Coefficient [95% CI] | |||
| Two‐group comparison: | ||||
| General population | 0.000 | (Reference) | 0.000 | (Reference) |
| Multiple sclerosis | −0.041 | [−0.060, −0.022] | −0.050 | [−0.070, −0.030] |
| General population + COVID‐19‐related adversity | −0.148 | [−0.173, −0.122] | −0.150 | [−0.175, −0.124] |
| Multiple sclerosis + COVID‐19‐related adversity | −0.168 | [−0.187, −0.148] | −0.179 | [−0.200, −0.158] |
| Test for interaction between COVID‐19‐related adversity and multiple sclerosis | p = 0.200 for Wald test | |||
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Coefficient [95% CI] | Coefficient [95% CI] | |||
| Three‐group comparison: | ||||
| General population without chronic diseases | 0.000 | (Reference) | 0.000 | (Reference) |
| General population without chronic diseases + COVID‐19‐related adversity | −0.119 | [−0.164, −0.075] | −0.119 | [−0.163, −0.075] |
| General population with chronic diseases | −0.152 | [−0.180, −0.124] | −0.148 | [−0.176, −0.120] |
| General population with chronic diseases + COVID‐19‐related adversity | −0.291 | [−0.325, −0.257] | −0.287 | [−0.321, −0.254] |
| Multiple sclerosis | −0.131 | [−0.156, −0.107] | −0.143 | [−0.169, −0.117] |
| Multiple sclerosis + COVID‐19‐related adversity | −0.257 | [−0.284, −0.232] | −0.271 | [−0.298, −0.245] |
| Test for interaction between COVID‐19‐related adversity and general population with chronic disease | p = 0.463 for Wald test | |||
| Test for interaction between COVID‐19‐related adversity and multiple sclerosis | p = 0.710 for Wald test | |||
Note: Tests for interaction utilized results obtained through multivariable regression. All multivariable regressions were adjusted for age, sex, education, and socioeconomic index. All bolded results were significant at an α = 0.001 level.
As presented in Table 5, examination of self‐reported COVID‐19‐related adversity as an outcome revealed that PwMS were 1.496 (p < 0.001) times more likely to report COVID‐19‐related adversity compared to the total general population sample, and 1.901 (p < 0.001) times more likely than the general population subgroup without chronic diseases. Additionally, members of the general population with chronic diseases were 1.430 times (p < 0.001) more likely to report COVID‐19‐related adversity than those without chronic diseases.
TABLE 5.
Univariable and multivariable log‐binomial regressions to determine the associations between sample and subgroup membership and the prevalence of COVID‐19‐related adversity.
| Log‐binomial regressions with COVID‐19‐related adversity as the outcome | |||
|---|---|---|---|
| Univariable | Multivariable | ||
| Absolute proportion reporting COVID‐19‐related adversity | Prevalence ratio (95% CI) | Prevalence ratio (95% CI) | |
| Two‐group comparison | |||
| General population | 29.38% | 0.000 (Reference) | 0.000 (Reference) |
| Multiple sclerosis | 43.97% | 1.496 (1.341, 1.670) | 1.485 (1.315, 1.678) |
| Three‐group comparison | |||
| General population without chronic diseases | 23.90% | 0.000 (Reference) | 0.000 (Reference) |
| General population with chronic diseases | 32.06% | 1.342 (1.080, 1.667) | 1.430 (1.153, 1.774) |
| Multiple sclerosis | 43.97% | 1.839 (1.519, 2.227) | 1.901 (1.557, 2.321) |
Note: All multivariable regressions were adjusted for age, sex, education, socioeconomic index, and lockdown exposure. All bolded results were significant at an α = 0.001 level.
4. Discussion
We found that the prevalence of self‐reported COVID‐19‐related adversity, defined as a self‐perceived reduction in well‐being associated with the pandemic, was greater among people living with chronic diseases, especially MS. This adversity was associated with clinically meaningful reductions in health‐related quality of life. The magnitude of this effect was not modified by having a chronic disease, including MS.
4.1. Differences in the Health‐Related Quality‐of‐Life Impact of the COVID‐19 Pandemic Between PwMS and the General Population
We identified that a greater proportion of participants living with MS reported COVID‐19‐related adversity compared to the general population. As noted above, this adversity was associated with clinically meaningful reductions in health‐related quality of life. Our study also found that sample/subgroup membership did not modify the association between COVID‐19‐related adversity and HSU.
A review of the quality of life literature identified a study that used the MS‐specific, Multiple Sclerosis Quality of Life‐54 instrument to measure health‐related quality of life, and which supported our findings (Yeni, Tulek, and Terzi. 2022). It concluded that PwMS were more impacted by the COVID‐19 pandemic versus healthy controls. Interestingly, studies utilizing generic, symptom‐specific instruments yielded opposing results, finding no difference in the impact of the pandemic on PwMS. One such study used the Depression Anxiety Stress Score‐21 (Talaat et al. 2020), and another applied the Beck Depression Inventory‐II and the Generalised Anxiety Disease‐7, among other instruments (Motolese et al. 2020).
4.2. Potential Causes of Worse Pandemic Outcomes for PwMS and Suggested Interventions
Reviews of relevant literature indicated that emotional health was a key route through which the COVID‐19 pandemic may have impacted the health‐related quality of life of PwMS (Altieri et al. 2022; Zarghami et al. 2022). Specifically, PwMS may have felt particularly isolated during the pandemic (Henson et al. 2024; J. A. Campbell, van der Mei, Taylor, et al. 2023) and experienced greater anxiety regarding potential COVID‐19 infection (due to the use of immunosuppressive therapies) (Manacorda et al. 2021; Learmonth et al. 2022). The COVID‐19 pandemic's impact on self‐care routines may also have been a major contributor to reduced health‐related quality of life for PwMS. In particular, the pandemic led to worsened diet and exercise routines (Marck et al. 2021), as well as feelings of abandonment resulting from reduced access to self‐care and healthcare resources (Manacorda et al. 2021).
Interventions have been proposed in the literature that would counteract the negative impacts of the COVID‐19 pandemic—and other isolating crises—on PwMS. In particular, remote delivery of health services (i.e., telehealth, which may include meetings with healthcare professionals, counselling, or trainer‐led exercise routines) was recommended (Learmonth et al. 2022). Other studies have indicated that engendering effective coping strategies may represent a proactive method for improving crisis outcomes among PwMS (Morris‐Bankole and Ho 2021; Pakenham 2006). These studies also suggested that active coping strategies are likely to be the most efficacious.
4.3. Strengths and Limitations
The greatest strengths of this study were the large samples from which data were obtained. These samples were diverse in terms of sociodemographic and clinical factors. Therefore, study results may be generalisable to a variety of populations. Moreover, the general population subgroup with chronic diseases had an HSU distribution similar to the PwMS sample. This implies comparability between the two groups and thus internal validity in this respect.
Regarding limitations, the EQ‐5D‐5L‐Psychosocial does not yet have a tailored minimum important difference or population norm. However, this was ameliorated by the similarities between the output of the EQ‐5D‐5L‐Psychosocial and the AQoL‐8D, which are interchangeable and use the same value sets (J. A. Campbell, Ahmad, et al. 2023). Additionally, COVID‐19‐related adversity data were extracted for each sample using different items. Our conservative approach to determining the presence of COVID‐19‐related adversity in PwMS (supported by Appendix A), and our choice of a simple, dichotomous indicator to represent COVID‐19‐related adversity, acknowledged this limitation and were intended to limit its influence on study results.
5. Conclusions
The prevalence of COVID‐19‐related adversity was much greater among people living with chronic diseases, especially MS, than among the general population without chronic diseases. Reductions in health‐related quality of life, associated with COVID‐19‐related adversity, were consistently both statistically significant and clinically meaningful. Given our findings, it is apparent that during isolating crises, such as pandemics, the management of health‐related quality of life among PwMS may require greater per capita investment, compared to that which is necessary for either the general population or people living with other chronic diseases.
Author Contributions
Glen J. Henson: conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, validation. Ingrid van der Mei: data curation, methodology, resources, writing–review and editing, supervision. Bruce V. Taylor: methodology, writing–review and editing, supervision. Suzi B. Claflin: writing–review and editing, supervision. Andrew J. Palmer: writing–review and editing. Gang Chen: conceptualization, data curation, investigation, methodology, resources, writing–review and editing, validation. Julie A. Campbell: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, validation, supervision.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.70210
Supporting information
Supplementary Materials
Acknowledgments
We acknowledge the participants of the Australian Multiple Sclerosis Longitudinal Study and the participants of the How Is Your Life study. We also acknowledge all Australian Multiple Sclerosis Longitudinal Study staff and volunteers.
Open access publishing facilitated by University of Tasmania, as part of the Wiley ‐ University of Tasmania agreement via the Council of Australian University Librarians.
Gang Chen and Julie A. Campbell are joint senior authors.
Funding: This study was supported by Dr. Julie Campbell's MS Australia Research Fellowship (grant number 19–0702).
Data Availability Statement
Data is available, upon reasonable request, from the corresponding author.
References
- World Health Organization . 2015. International Statistical Classification of Diseases and Related Health Problems. 10th revision, 5th ed. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Ahmad, H. , Campbell J., van der Mei I., Taylor B., and Palmer A.. 2018. Health Economic Impact of Multiple Sclerosis in Australia in 2017: An Analysis of MS Research Australia's Platform—The Australian MS Longitudinal Study (AMSLS). North Sydney, Australia: Multiple Sclerosis Australia. https://www.msaustralia.org.au/wp‐content/uploads/2018/08/health‐economic‐impact‐of‐ms‐in‐australia‐in‐2017_ms‐research‐australia_web.pdf. [Google Scholar]
- Altieri, M. , Capuano R., Bisecco A., et al. 2022. “The Psychological Impact of Covid‐19 Pandemic on People With Multiple Sclerosis: A Meta‐Analysis.” Multiple Sclerosis and Related Disorders 61: 103774. 10.1016/j.msard.2022.103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley, T. J. , and Boudreau E. A.. 2016. “Sleep Disorders in Multiple Sclerosis.” Current Neurology and Neuroscience Reports 16, no. 5: 50. 10.1007/s11910-016-0649-2. [DOI] [PubMed] [Google Scholar]
- Campbell, J. , van der Mei I., Taylor B., and Palmer A.. 2023. Health Economic Impact of MS in 2021: An Interim Update of Prevalence, Costs and Costs of Illness From 2017 to 2021. North Sydney, Australia: Multiple Sclerosis Australia. https://www.msaustralia.org.au/wp‐content/uploads/2023/02/health‐economic‐impact‐of‐multiple‐sclerosis‐in‐australia‐in‐2021_final.pdf. [Google Scholar]
- Campbell, J. A. , Ahmad H., Chen G., et al. 2023. “Validation of the EQ‐5D‐5L and Psychosocial Bolt‐Ons in a Large Cohort of People Living with Multiple Sclerosis in Australia.” Quality of Life Research 32, no. 2: 553–568. 10.1007/s11136-022-03214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. A. , Palmer A. J., Venn A., Sharman M., Otahal P., and Neil A.. 2016. “A Head‐to‐Head Comparison of the EQ‐5D‐5L and AQoL‐8D Multi‐Attribute Utility Instruments in Patients Who Have Previously Undergone Bariatric Surgery.” Patient 9, no. 4: 311–322. 10.1007/s40271-015-0157-5. [DOI] [PubMed] [Google Scholar]
- Campbell, J. A. , Simpson S. Jr., Ahmad H., Taylor B. V., van der Mei I., and Palmer A. J.. 2020. “Change in Multiple Sclerosis Prevalence Over Time in Australia 2010–2017 Utilising Disease‐Modifying Therapy Prescription Data.” Multiple Sclerosis 26, no. 11: 1315–1328. 10.1177/1352458519861270. [DOI] [PubMed] [Google Scholar]
- Campbell, J. A. , van der Mei I., Taylor B. V., et al. 2023. “Using Qualitative Free‐Text Data to Investigate the Lived Experience of the COVID‐19 Pandemic for a Large Cohort of Australians With Different Multiple Sclerosis Related Disability Levels.” Journal of Neurology, Neurosurgery, and Psychiatry 94, no. 12: 975–983. 10.1136/jnnp-2022-330755. [DOI] [PubMed] [Google Scholar]
- Chen, G. , and Olsen J. A.. 2020. “Filling the Psycho‐Social Gap in the EQ‐5D: The Empirical Support for Four Bolt‐on Dimensions.” Quality of Life Research 29, no. 11: 3119–3129. 10.1007/s11136-020-02576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , and Olsen J. A.. 2023. “Extending the EQ‐5D: The Case for a Complementary Set of 4 Psycho‐Social Dimensions.” Quality of Life Research 32, no. 2: 495–505. 10.1007/s11136-022-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costabile, T. , Carotenuto A., Lavorgna L., et al. 2021. “COVID‐19 Pandemic and Mental Distress in Multiple Sclerosis: Implications for Clinical Management.” European Journal of Neurology 28, no. 10: 3375–3383. 10.1111/ene.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culyer, A. J. 2014. Encyclopedia of Health Economics. 1st ed. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- D'Agostino, R. B. , Belanger A., and D'Agostino R. B. Jr. 1990. “A Suggestion for Using Powerful and Informative Tests of Normality.” American Statistical 44, no. 4: 316–321. [Google Scholar]
- Drummond, M. F. , Sculpher M. J., Claxton K., Stoddart G. L., and Torrance G. W.. 2015. Methods for the Economic Evaluation of Health Care Programmes. 4th ed. Oxford, UK: Oxford University Press. [Google Scholar]
- Every‐Palmer, S. , Jenkins M., Gendall P., et al. 2020. “Psychological Distress, Anxiety, Family Violence, Suicidality, and Wellbeing in New Zealand During the COVID‐19 Lockdown: A Cross‐Sectional Study.” PLoS ONE 15, no. 11: e0241658. 10.1371/journal.pone.0241658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, L. N. , Pereira L. N., da Fé Brás M., and Ilchuk K.. 2021. “Quality of Life Under the COVID‐19 Quarantine.” Quality of Life Research 30, no. 5: 1389–1405. 10.1007/s11136-020-02724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch, A. P. , Brazier J. E., and Mukuria C.. 2019. “Selecting Bolt‐On Dimensions for the EQ‐5D: Examining Their Contribution to Health‐Related Quality of Life.” Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research 22, no. 1: 50–61. 10.1016/j.jval.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Finch, A. P. , Brazier J. E., Mukuria C., and Bjorner J. B.. 2017. “An Exploratory Study on Using Principal‐Component Analysis and Confirmatory Factor Analysis to Identify Bolt‐On Dimensions: The EQ‐5D Case Study.” Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research 20, no. 10: 1362–1375. 10.1016/j.jval.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Griffiths, D. , Sheehan L., Petrie D., van Vreden C., Whiteford P., and Collie A.. 2022. “The Health Impacts of a 4‐Month Long Community‐Wide COVID‐19 Lockdown: Findings From a Prospective Longitudinal Study in the State of Victoria, Australia.” PLoS ONE 17, no. 4: e0266650. 10.1371/journal.pone.0266650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover, S. , Sahoo S., Mehra A., et al. 2020. “Psychological Impact of COVID‐19 Lockdown: An Online Survey From India.” Indian Journal of Psychiatry 62, no. 4: 354–362. 10.4103/psychiatry.IndianJPsychiatry_427_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson, G. J. , Taylor B. V., van der Mei I., et al. 2022. “Protocol for a Systematic Review and Meta‐Analysis of Minimal Important Differences for Generic Multiattribute Utility Instruments.” BMJ Open 12, no. 10: e062703. 10.1136/bmjopen-2022-062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson, G. J. , van der Mei I., Taylor B. V., et al. 2024. “The Quality of Life Impact of the COVID‐19 Pandemic and Lockdowns for People Living with Multiple Sclerosis (MS): Evidence from the Australian MS Longitudinal Study.” Quality of Life Research 33, no. 6: 1675–1689. 10.1007/s11136-024-03620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , and Zhao N.. 2020. “Generalized Anxiety Disorder, Depressive Symptoms and Sleep Quality During COVID‐19 Outbreak in China: A Web‐Based Cross‐Sectional Survey.” Psychiatry Research 288: 112954. 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karageorghis, C. I. , Bird J. M., Hutchinson J. C., et al. 2021. “Physical Activity and Mental Well‐Being Under COVID‐19 Lockdown: A Cross‐Sectional Multination Study.” BMC Public Health 21, no. 1: 988. 10.1186/s12889-021-10931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobelt, G. , Berg J., Atherly D., and Hadjimichael O.. 2006. “Costs and Quality of Life in Multiple Sclerosis: A Cross‐Sectional Study in the United States.” Neurology 66, no. 11: 1696–1702. 10.1212/01.wnl.0000218309.01322.5c. [DOI] [PubMed] [Google Scholar]
- Laslett, L. L. , Honan C., Turner J. A., et al. 2022. “Poor Sleep and Multiple Sclerosis: Associations with Symptoms of Multiple Sclerosis and Quality of Life.” Journal of Neurology, Neurosurgery, and Psychiatry. Published ahead of print, July 27, 2022. 10.1136/jnnp-2022-329227. [DOI] [PubMed] [Google Scholar]
- Learmonth, Y. C. , Hunter A., Gibbs L., Walker D., Kermode A. G., and Marck C. H.. 2022. “The Impact of the Australian Black Summer Bushfires and the COVID‐19 Pandemic on Wellbeing in Persons With Multiple Sclerosis; Preparation for Future and Ongoing Crises.” Disability and Rehabilitation 45, no. 4: 630–643. 10.1080/09638288.2022.2037756. [DOI] [PubMed] [Google Scholar]
- Manacorda, T. , Bandiera P., Terzuoli F., et al. 2021. “Impact of the COVID‐19 Pandemic on Persons with Multiple Sclerosis: Early Findings From a Survey on Disruptions in Care and Self‐Reported Outcomes.” Journal of Health Services Research & Policy 26, no. 3: 189–197. 10.1177/1355819620975069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck, C. H. , Hunter A., Heritage B., et al. 2021. “The Effect of the Australian Bushfires and the COVID‐19 Pandemic on Health Behaviours in People With Multiple Sclerosis.” Multiple Sclerosis and Related Disorders 53: 103042. 10.1016/j.msard.2021.103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley, M. P. , Goldschmidt C. H., and Rae‐Grant A. D.. 2021. “Diagnosis and Treatment of Multiple Sclerosis: A Review.” Jama 325, no. 8: 765–779. 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- Morris‐Bankole, H. , and Ho A. K.. 2021. “The COVID‐19 Pandemic Experience in Multiple Sclerosis: The Good, the Bad and the Neutral.” Neurology and Therapy 10, no. 1: 279–291. 10.1007/s40120-021-00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motolese, F. , Rossi M., Albergo G., et al. 2020. “The Psychological Impact of COVID‐19 Pandemic on People with Multiple Sclerosis.” Frontiers in Neurology 11: 580507. 10.3389/fneur.2020.580507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orji, N. C. , Cox I. A., Jason L. A., et al. 2023. “Assessing Health State Utilities for People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Australia Using the EQ‐5D‐5L, AQoL‐8D and EQ‐5D‐5L‐Psychosocial Instruments.” Quality of Life Research 33, no. 1: 45–57. 10.1007/s11136-023-03498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton, S. M. , Herrera B. M., Yee I. M., et al. 2006. “Sex Ratio of Multiple Sclerosis in Canada: A Longitudinal Study.” Lancet Neurology 5, no. 11: 932–936. 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- Pakenham, K. I. 2006. “Investigation of the Coping Antecedents to Positive Outcomes and Distress in Multiple Sclerosis (MS).” Psychology & Health 21, no. 5: 633–649. 10.1080/14768320500422618. [DOI] [Google Scholar]
- Ramezani, N. , Ashtari F., Bastami E. A., et al. 2021. “Fear and Anxiety in Patients With Multiple Sclerosis During COVID‐19 Pandemic; Report of an Iranian Population.” Multiple Sclerosis and Related Disorders 50: 102798. 10.1016/j.msard.2021.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, J. , Iezzi A., Khan M. A., and Maxwell A.. 2014. “Validity and Reliability of the Assessment of Quality of Life (AQoL)‐8D Multi‐Attribute Utility Instrument.” Patient 7, no. 1: 85–96. 10.1007/s40271-013-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat, F. , Ramadan I., Aly S., and Hamdy E.. 2020. “Are Multiple Sclerosis Patients and Their Caregivers More Anxious and More Committed to Following the Basic Preventive Measures During the COVID‐19 Pandemic?” Multiple Sclerosis and Related Disorders 46: 102580. 10.1016/j.msard.2020.102580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, B. V. , Palmer A., Simpson S. Jr., et al. 2013. “Assessing Possible Selection Bias in a National Voluntary MS Longitudinal Study in Australia.” Multiple Sclerosis 19, no. 12: 1627–1631. 10.1177/1352458513481511. [DOI] [PubMed] [Google Scholar]
- Thompson, A. J. , Banwell B. L., Barkhof F., et al. 2018. “Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria.” Lancet Neurology 17, no. 2: 162–173. 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Thompson, A. J. , Baranzini S. E., Geurts J., Hemmer B., and Ciccarelli O.. 2018. “Multiple Sclerosis.” Lancet 391, no. 10130: 1622–1636. 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- Walton, C. , King R., Rechtman L., et al. 2020. “Rising Prevalence of Multiple Sclerosis Worldwide: Insights From the Atlas of MS, Third Edition.” Multiple Sclerosis 26, no. 14: 1816–1821. 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Rowen D., Brazier J., Tsuchiya A., Young T., and Longworth L.. 2015. “An Exploratory Study to Test the Impact on Three “Bolt‐On” Items to the EQ‐5D.” Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research 18, no. 1: 52–60. 10.1016/j.jval.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeni, K. , Tulek Z., and Terzi M.. 2022. “A Year With the Fear of COVID‐19 in Multiple Sclerosis Patients: Examination of Depression, Sleep Quality and Quality of Life Before and After the Pandemic.” Multiple Sclerosis and Related Disorders 57: 103370. 10.1016/j.msard.2021.103370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghami, A. , Hussain M. A., Campbell J. A., et al. 2022. “Psychological Impacts of COVID‐19 Pandemic on Individuals Living With Multiple Sclerosis: A Rapid Systematic Review.” Multiple Sclerosis and Related Disorders 59: 103562. 10.1016/j.msard.2022.103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Taylor B. V., Simpson S. Jr., et al. 2021. “Feelings of Depression, Pain and Walking Difficulties Have the Largest Impact on the Quality of Life of People With Multiple Sclerosis, Irrespective of Clinical Phenotype.” Multiple Sclerosis 27, no. 8: 1262–1275. 10.1177/1352458520958369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials
Data Availability Statement
Data is available, upon reasonable request, from the corresponding author.


