Abstract
The tumor suppressor gene PTEN is a phosphoinositide phosphatase that is inactivated by deletion and/or mutation in diverse human tumors. Wild-type PTEN is expressed both in the cytoplasm and nucleus in normal cells, with a preferential nuclear localization in differentiated or resting cells. To elucidate the relationship between PTEN′s subcellular localization and its biologic activities, we constructed different PTEN mutants that targeted PTEN protein into different subcellular compartments. Our data show that the subcellular localization patterns of a PTEN (ΔPDZB) mutant versus a G129R phosphatase mutant were indistinguishable from those of wild-type PTEN. In contrast, the Myr-PTEN mutant demonstrated an enhanced association with the cell membrane. We found that nuclear PTEN alone is capable of suppressing anchorage-independent growth and facilitating G1 arrest in U251MG cells without inhibiting Akt activity. Nuclear compartment-specific PTEN-induced growth suppression is dependent on possessing a functional lipid phosphatase domain. In addition, the down-regulation of p70S6K could be mediated, at least in part, through activation of AMP-activated protein kinase in an Akt-independent fashion. Introduction of a constitutively active mutant of Akt, Akt-DD, only partially rescues nuclear PTEN-mediated growth suppression. Our collective results provide the first direct evidence that PTEN can contribute to G1 growth arrest through an Akt-independent signaling pathway.
The tumor suppressor gene MMAC1/PTEN (henceforth referred to as PTEN) was identified independently by three groups (39, 41, 65). Deletions in or mutations of PTEN are frequent occurrences in high-grade glial, advanced prostate, breast, endometrial, and kidney tumors, as well as in small-cell lung carcinoma and melanoma (6, 63, 74). Germ line mutations of the PTEN gene have also been linked to several hamartomatous syndromes, including Cowden disease, Bannayan-Zonana syndrome (also known as Bannayan-Riley-Ruvalcaba syndrome), Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndromes. PTEN encodes a 403-amino-acid protein that is a member of the protein tyrosine phosphatase family. In addition to its N-terminal catalytic tyrosine phosphatase domain (IHCXXGXXRS/T), other domains/motifs have been discovered, including a tensin/auxillin homology domain overlapping the phosphatase domain (41, 65), a calcium-independent C2 domain (37), two PEST motifs, and a PDZ (PSD95, Dlg, and ZO1) binding domain (PDZB) at the C terminus (2). Although it shares extensive homology with members of the protein tyrosine phosphatase family, PTEN′s primary physiologic substrates are phosphatidylinositols (PtdIns) phosphorylated at the D3 position (PtdIns-3,4-P2 and PtdIns-3,4,5-P3) (47), which are products of phosphoinositide 3-kinases (PI3K). PTEN can thus antagonize PI3K-dependent signaling pathways that are specifically involved in cell growth, apoptosis, transcription/translation, glucose metabolism, and cell migration. Ectopic expression of wild-type PTEN in PTEN-null tumor cell lines results either in G1 growth arrest, anoikis, or apoptosis, depending on the cell type (13, 46, 51, 81), and inhibits important biologic properties such as development of metastases (12). In contrast, PTEN mutants with abrogated phosphatase activity, such as C124S, G129R, and R130G, lose their tumor suppressing ability. Interestingly, one mutant, G129E, which is frequently observed in Cowden disease and in occasional sporadic cancers, lacks lipid phosphatase activity but retains PTPase activity against the polypeptide substrate poly (Glu-Tyr) (18, 51). However, Tamura et al. (69) showed that the G129E mutant retained the ability to inhibit integrin-mediated cell migration, spreading, and tumor cell invasion, and provided evidence that these properties were due to dephosphorylation of FAK. Moreover, PTEN was also recently shown to inhibit cell migration through its C2 domain, independent of its lipid phosphatase activity (57). These data suggest that although lipid phosphatase is critical to many PTEN functions, PTEN regulates biologic functions that are independent of this activity.
PTEN is expressed primarily in the cytoplasm of many tumor cells, including thyroid, endocrine, pancreas, and primary cutaneous melanomas (21, 53, 78). In contrast, PTEN is expressed both in the cytoplasm and in the nucleus of normal cells, with a preferential nuclear localization in differentiated or resting cells (15, 21, 36, 53, 78). Nuclear localization of PTEN is increased during neuronal differentiation and is required for the survival of differentiating neuronal cells (35). Similarly, activated PI3K has been shown to translocate to the nucleus (49), and functional PIP3 has also been detected inside the nucleus (70). An increased level of nuclear PTEN is associated with G0-G1 in MCF-7 cells (22), and these authors postulated that nuclear PTEN could be directly involved in regulating cell cycle progression. Thus, nuclear PTEN may have growth-regulatory roles that are distinctive from those of cytoplasmic PTEN. This study examines the relationship between the biologic function and subcellular localization of PTEN, with an emphasis on characterizing nuclear PTEN. Our data show that nuclear PTEN suppresses tumorigenicity in and facilitates the G1 arrest of U251MG glioma cells without down-regulating Akt phosphorylation/activation or cell invasiveness. Growth suppression induced by nuclear PTEN is mediated, at least in part, through down-regulation of p70S6K phosphorylation/activation via activation of AMP-activated protein kinase (AMPK) in an Akt-independent fashion. In addition, the intact lipid phosphatase domain is indispensable for nuclear PTEN to fully exert its growth-suppressing activities. Collectively, these results suggest that PTEN is biologically functional in the nucleus and mediates growth suppression via signaling mechanisms than differ from those in cytoplasmic PTEN.
MATERIALS AND METHODS
Cell culture and transfection.
Mouse astrocytes, NHA (E6/E7/hTERT) and the glioblastoma cell lines U251MG, LN18, and LN229 were maintained in Dulbecco's modified Eagle's medium-F-12 (high glucose) medium supplemented with 10% fetal bovine serum. Plasmids were transfected into U251MG cells with FuGENE6 (Roche) according to the manufacturer's protocol.
Plasmid construction.
The pLNCX-PTEN wild type (WT) and mutants were constructed by initially using a PCR strategy for cloning into the BamHI/EcoRI sites of the pBS (SK+) vector and were subsequently subcloned into the NotI/SalI sites of the pLNCX retroviral vector (Clontech). Briefly, PTEN mutants were generated by PCR with DeepVent thermal-stable polymerase (NEB) using 5′ primers containing NLS (nuclear localization signals) from the MDV oncogene MEQ (RRKKRK) (43) or a myristoylation signal from the Rasheed sarcoma virus Gag protein (MKGSLTTH) (30). These were used to construct mutants with enhanced nuclear localization or association with the plasma membrane. A ΔPDZB mutant was created with 3′ primers bearing TKV/TVD mutations to disrupt PTEN′s binding to PDZ domain-containing proteins. Meanwhile, the pCMV-PTEN (ER) was generated by digesting pBS (KS+)-PTEN (ΔPDZB) with NcoI (blunt end)/SalI and subcloning it into a pCMV/myc/ER vector (Invitrogen) at SalI (blunt end)/XhoI sites to direct expression to the endoplasmic reticulum.
Indirect immunofluorescence and confocal laser scanning microscopy.
Immunofluorescence staining was performed as described previously (45). Briefly, cells were seeded at a concentration of 2 × 105 cells per well in six-well plates with coverslips inside and left overnight. The following day, media were aspirated and the cells were washed with phosphate-buffered saline (PBS) once before being fixed with 3.7% formaldehyde in PBS for 20 min. After another PBS wash, the cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min followed by blocking with 3% bovine serum albumin-0.1% Tween 20-PBS for 1 h. Cells were then incubated with mouse primary antibodies against PTEN (immunoglobulin G [IgG]; BD Bioscience) or PIP2 (IgM; Echelon) for 1 h. After 2 washes with PBS (0.1% Tween 20), the cells were incubated with the secondary antibodies conjugated with fluorescein isothiocyanate (FITC) or Texas Red (Molecular Probes) for 1 h, and the cells were examined and analyzed with an Olympus FluoView (60× objective) confocal laser scanning microscope.
Cell invasion assay.
The invasion of U251MG cells in vitro was measured by passage of the cells through Matrigel-coated transwell inserts (50) in Costar transwells. Briefly, transwell inserts with 8-μm pores were coated with 200 μl of 0.78 mg/ml Matrigel in cold serum-free medium. The cells were trypsinized, and 2 × 105 cells in 700 μl of cell suspension were added in duplicate wells. After 24 h of incubation, the cells that passed through the filter into the lower wells were stained using a hema-3 stain kit (Fisher Scientific) and the cells in 5 fields were counted under a microscope and expressed as a 100% set for vector-transfected U251MG cells that migrated to the lower part of the chamber.
Colony-forming assay.
Anchorage-independent growth was measured by soft agar colony assay to evaluate transforming potential (44). Briefly, this assay was performed in six-well plates with a base of 2 ml of medium containing 1% fetal bovine serum with 0.5% Bacto agar (Difco). Cells were seeded in 2 ml of medium containing 1% fetal bovine serum with 0.35% agar at 1 × 104 or 5 × 104 cells/ml and layered onto the base. The number of colonies was scored under a microscope after 2 weeks.
Flow cytometry cell cycle analysis.
U251MG cells were maintained in Dulbecco's modified Eagle's medium-F-12 media containing 1% fetal bovine serum for 3 days, trypsinized, and washed with cold PBS twice, followed by fixation with cold 70% ethanol. Flow cytometry cell cycle analysis was performed by the Cancer Cell Biology Core at The University of Texas M. D. Anderson Cancer Center.
Subcellular fractionations.
Nuclear and cytoplasmic fractions of U251MG-PTEN clones were separated using an NE-PER nuclear and extraction reagent kit according the manufacturer's specifications (Pierce).
Western blotting.
Cells were washed with ice-cold phosphate-buffered saline and lysed in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM MgCl2, 150 mM NaCl, 1 mM EGTA, 20 mM NaF, 10 mM Na4P2O7 (sodium pyrophosphate), 10% glycerol, 1% Triton X-100, 3 mM benzamidine, 10 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 10 μg/ml aprotinin, 5 mM iodoacetic acid, and 2 μg/ml leupeptin to prepare whole-cell lysates. Lysates were clarified by centrifugation at 14,000 × g for 5 min. Proteins equivalent to 5 × 105 cells per lane were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted to polyvinylidene difluoride (PVDF) membranes (Millipore). The PVDF membranes were then probed with rabbit polyclonal antibodies against Akt, phospho-Akt (S473), phospho-Erk1/2, p70S6K, phospho-p70S6K (T389), mTOR, phospho-mTOR (S2448), phospho-TSC2 (T1462), AMPK, phospho-AMPK (T172), S6, phospho-S6 (S235/236), 4E-BP1, phospho-4E-BP1 (T36/46), GSK3β, phospho-GSK3β (S9) (Cell Signaling Technology), TSC1, and TSC2 and with monoclonal antibodies (MAbs) against PTEN (Santa Cruz), poly(ADP-ribose) polymerase 1 (Oncogene Research Products), hemagglutinin (HA) tag (Cell Signaling Technology), and IgM anti-PIP2 (Echelon). Specific proteins were detected by enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech) following incubation with horseradish peroxidase-conjugated secondary antibodies.
Statistics.
Statistical analysis was performed using an unpaired (equal variance) t test. Data are presented as means ± standard deviations (SD). Each group was compared with a vector control; a P value of <0.05 is considered significant.
RESULTS
Expression of PTEN mutants targeted to subcellular compartments.
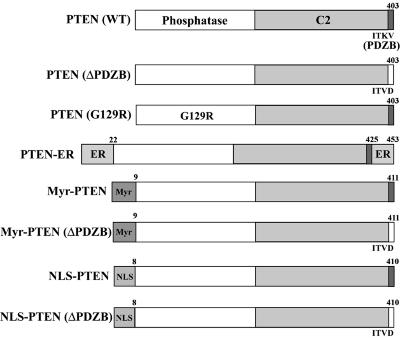
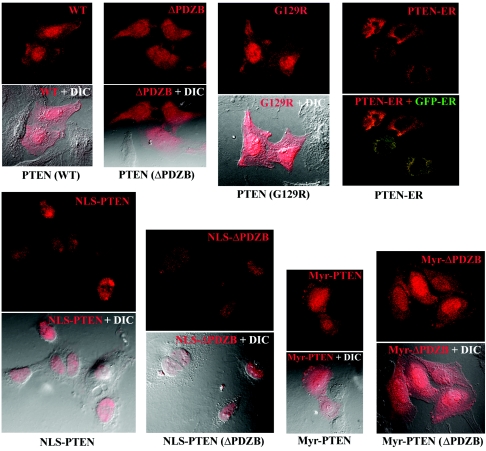
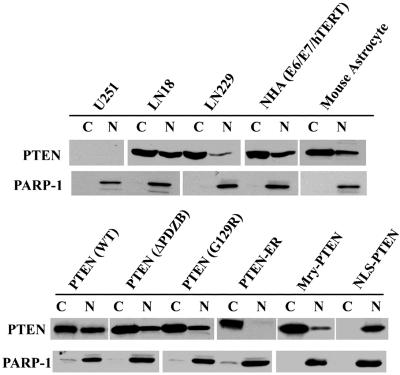
PTEN has been shown to localize to the plasma membrane, cytoplasm, and nucleus of the cell. To understand the biologic functions of PTEN in various subcellular compartments, specific mutants were constructed via PCR in which localization motifs were added to target PTEN to different subcellular compartments, including the plasma membrane (targeted by addition of an N-terminal Myr myristoylation signal), nucleus (through an N-terminal NLS), and endoplasmic reticulum (ER) (by addition of N- and C-terminal ER retention motifs). Additionally, a TKV/TVD mutation was introduced to disrupt PTEN′s PDZ binding activity (ΔPDZB). These constructs (illustrated in Fig. 1), termed pLNCX (for WT), ΔPDZB, Myr, Myr (ΔPDZB), NLS, NLS (ΔPDZB), and G129R or pCMV-ER retroviral vectors, were transfected into PTEN-null U251MG cells via lipofection (FuGENE6). Localization of the wild-type and mutant gene products was determined by immunostaining followed by analysis with a FluoView confocal microscope. Representative staining patterns are shown in Fig. 2. Differential interference contrast was used to enhance cellular structure (Fig. 2, bottom pictures of each construct). Wild-type PTEN was expressed in the plasma membrane, cytoplasm, and nucleus, consistent with the reported distribution of PTEN in log-phase cells. In contrast, mutants with nuclear and endoplasmic reticular targeting domains were found primarily in the targeted cellular compartment. Interestingly, the ΔPDZB and G129R phosphatase mutants had patterns of localization in the cytoplasm and nucleus that were similar to those observed for wild-type PTEN. This observation suggests that the PDZ binding domain and functional phosphatase domain are not required for nuclear localization of PTEN. As expected, Myr-PTEN demonstrated an increased association with the plasma membrane. However, a significant amount of the Myr-PTEN mutant was also found in the nucleus. Interestingly, the addition of an exogenous NLS completely abolished PTEN′s cytoplasmic translocation, as observed with the NLS and NLS (ΔPDZB) mutants. To confirm the effects of mutant PTEN on nuclear localization, immunoblotting of PTEN was performed following cell fractionation. As shown in Fig. 3, PTEN is detected both in the nucleus and cytoplasm of different cell lines expressing wild-type PTEN at various degrees, verifying that the subcellular distribution of PTEN in the nucleus and cytoplasm is a general physiological phenomenon rather than an artificial effect as a result of exogenous expression. In addition, the cell fractionation on PTEN mutants also corroborates the immunostaining data. As PTEN lacks a canonical NLS domain, these findings suggest that its nuclear localization is mediated by other domains in the molecule.
FIG. 1.
Schematic diagram of PTEN localization mutants. To target PTEN into different subcellular compartments, specific localization signals were inserted into PTEN′s sequence via PCR or molecular cloning as described in Materials and Methods. These were: N-terminal Myr (8-amino-acid [aa] myristoylation motif, MKGSLTTH) targeting the plasma membrane, N-terminal NLS (7 aa, M+RRKKRK) targeting the nucleus, and ER retention motifs (21 N-terminal aa containing an ER signal peptide and 28 C-terminal aa containing an ER retention signal) targeting the ER. In addition, TKV/TVD mutations were introduced to abolish PTEN′s PDZ binding ability.
FIG. 2.
Subcellular localization of PTEN mutants. To examine subcellular localization of PTEN mutants, U251MG cells were transfected with expression vectors for the mutants shown in Fig. 1 and clonal populations were obtained as described in Materials and Methods. Immunostaining was performed with a MAb to PTEN, followed by confocal microscopy, as described. Differential interference contrast was used to depict cellular contrast; in the case of PTEN-ER, localization in the ER was verified by successful transfection of the pCMV-GFP-ER construct.
FIG. 3.
Substantiation of PTEN′s localization with subcellular fractionation. Immunoblotting with a MAb to PTEN was performed on cellular fractions extracted from cell lines expressing WT PTEN and U251MG PTEN mutant clones, as indicated (C, cytoplasm; N, nucleus), to corroborate the immunostaining results. Immunoblotting with MAb against poly(ADP-ribose) polymerase 1 (PARP-1), a nucleus-specific protein, showed minimum cross-contamination between the cytoplasmic and nuclear fractions.
Nuclear PTEN is capable of suppressing anchorage-independent growth.
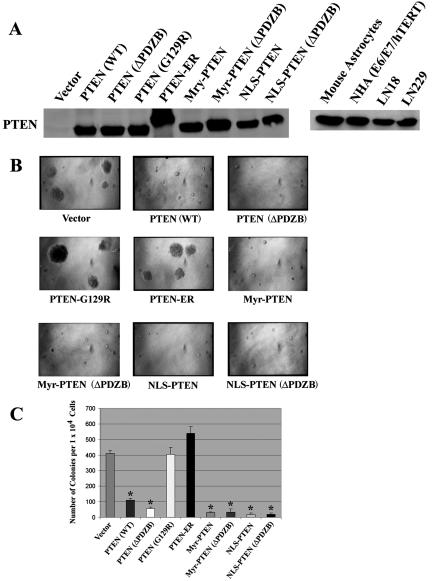
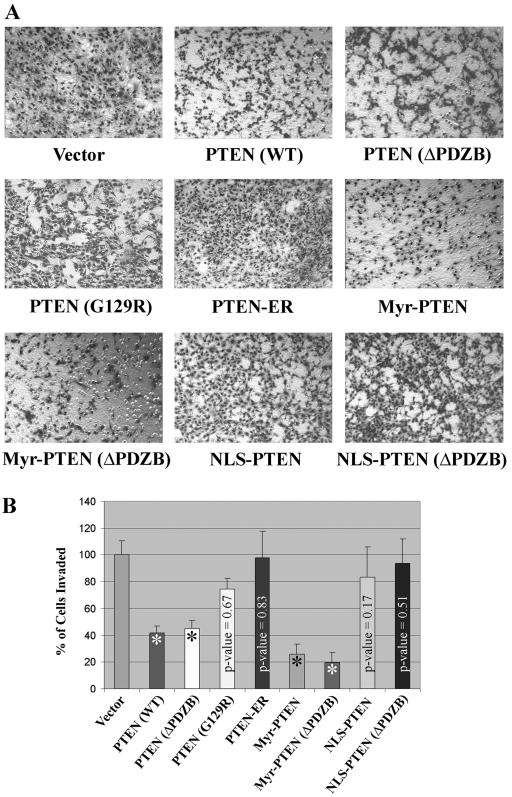
Several stable transfectants from each PTEN mutant expressing comparable protein levels to those of human cell lines harboring wild-type PTEN (39, 64, 79) were selected for further characterization. Functional assays were conducted on 3 to 5 clones from each construct and repeated a minimum of three times. The expression of PTEN was equivalent in all clones (Fig. 4A). First, a soft agar colony-forming assay was performed in the presence of 1% fetal bovine serum to measure anchorage-independent growth as a marker for the transforming potential of each clone. As shown in Fig. 4B, PTEN (WT) significantly reduced the soft agar colony-forming ability relative to vector-transfected controls. Vector-transfected controls produced numbers of soft agar colonies equivalent to those produced by nontransfected parental cells (not shown). PTEN (G129R)-, and PTEN-ER-transfected U251MGcells exhibited a capacity for anchorage-independent growth similar to that pLNCX vector-transfected U251MG cells. In contrast, ΔPDZB-, Myr-, Myr (ΔPDZB)-, NLS-, and NLS (ΔPDZB)-transfected U251MG clones had a significant (P < 10−5) reduction (∼75% to 95%) in the number of soft agar colonies. These experiments corroborate previously published observations showing that a functional phosphatase domain is required, but a PDZB domain is dispensable, for PTEN′s anchorage-independent growth-suppressing activity (19, 34, 72). Interestingly, when PTEN is misdirected into the ER, its growth-suppressing activity is lessened considerably, despite the fact that its phosphatase domain remains intact. However, when PTEN is directed into the nucleus, its growth-suppressing activity is restored.
FIG. 4.
Nuclear PTEN is capable of suppressing anchorage-independent growth. (A) PTEN localization mutants expressing comparable protein levels as determined by immunoblotting were selected for functional analysis, as described in Materials and Methods. The migration of the PTEN-ER protein was slower than the other PTEN mutants due to the larger size of ER retention motifs, as illustrated in Fig. 1. (B) The effect of PTEN and its mutants on anchorage-independent growth was assessed using a soft agar colony assay. Selected stable U251MG transfectants were seeded into six-well plates in duplicate at a concentration of 5 × 104/well in the presence of 1% serum. (C) The number of colonies was scored under a microscope after 2 weeks. The results shown are the averages of the results from three experiments (means ± SD). An unpaired (equal variance) t test was performed on all PTEN and PTEN mutant clones compared to the vector control. The P values of PTEN (WT), PTEN (ΔPDZB), Myr, Myr (ΔPDZB), NLS, and NLS (ΔPDZB) clones are statistically significant (*, P < 10−5). In addition, the size of their soft agar colonies appeared significantly smaller than those of vector colonies.
Growth suppression induced by nuclear PTEN is mediated by facilitating G1 accumulation.
Wild-type PTEN has been shown to elicit G1 growth arrest (18, 46, 58, 85), anoikis (13, 46), and apoptosis (46, 76, 77) depending upon cell type. PTEN-mediated G1 arrest in glioblastoma cells has been well documented (1, 10, 18, 23, 40). We therefore compared the effects of PTEN mutants on the proliferation of U251MG cells. The results are displayed in Fig. 5A. NLS-PTEN and NLS-PTEN (ΔPDZB) inhibited cell growth in transfected U251MG cells in the presence of 1% serum as did the PTEN (WT), ΔPDZB, Myr, and Myr (ΔPDZB) clones (Fig. 5A). To further investigate whether the disruption in cell cycle progression is responsible for growth suppression, the cell cycle profiles of various PTEN clones were analyzed using flow cytometry. In the presence of 5% serum, PTEN clones failed to induce significant G1 arrest in U251MG cells, an observation that corroborates previous reports (18). Conversely, in the presence of 1% serum, G1 accumulation was statistically significantly increased (to nearly 20%; P values of <0.05) in U251MG cells in all PTEN clones except for G129R and ER mutants (Fig. 5B). These findings were similar to findings from the soft agar colony-forming assays described above. Taken together, the results demonstrate that nuclear PTEN-induced growth suppression is mediated, at least in part, through enhanced G1 accumulation.
FIG. 5.
Nuclear PTEN-induced growth suppression is mediated through facilitating G1 accumulation. (A) Proliferation rates of cells expressing PTEN mutants. Equal numbers of cells (1 × 105) were seeded in 60-mm dishes in the presence of 1% serum, and cells were subsequently counted at days 2 through 7 after seeding. The results shown are the averages of the results from four separate experiments (means ± SD). (B) G1 arrest induced by PTEN mutants. Stable U251MG transfectants were maintained in medium containing 1% serum for 3 days and collected for analysis of the cell cycle profile using flow cytometry. The results shown are the averages of the results from five separate experiments (means ± SD).
Nuclear PTEN does not inhibit cell invasion.
Cellular invasiveness is one of the hallmarks of brain tumor cells. Benign astrocytoma and malignant glioblastoma cells are notorious for their ability to extensively invade the normal brain parenchyma surrounding a tumor. To determine the effects of PTEN localization on invasion, a Matrigel cell invasion assay was used. Both PTEN (WT) and ΔPDZB reduced the invasiveness of U251MG cells by ∼55% to 60% compared with vector-transfected U251MG cells (Fig. 6). The expression of the Myr and Myr-ΔPDZB forms of PTEN inhibited cell invasion to an even greater degree (by ∼75% to 80%), suggesting that the association between PTEN and the cell membrane enhances its antagonizing effect on PI3K/Akt-mediated cellular invasiveness. The ER clone had virtually no effect on cell invasion. Likewise, NLS, NLS (ΔPDZB), and G129R did not affect invasion significantly (P values = 0.172, 0.511, and 0.675, respectively).
FIG. 6.
Nuclear PTEN does not inhibit cell invasion. (A) A Matrigel invasion assay was performed using a transwell membrane coated with 0.7 mg/ml of Matrigel matrix. Cells were seeded at a concentration of 2 × 105 cells/well overnight. Cells that invaded the bottom side of the membrane were fixed and stained using a hema-3 kit. (B) The number of invaded cells in vector clones was set at 100%. The results shown are the averages of the results from five individual experiments (means ± SD). Only PTEN (WT), ΔPDZB, Myr, and Myr (ΔPDZB) clones showed statistically significant inhibition of cell invasion compared to the vector control (*, P < 10−6).
Nuclear PTEN leads to p70S6K inactivation without down-regulating Akt.
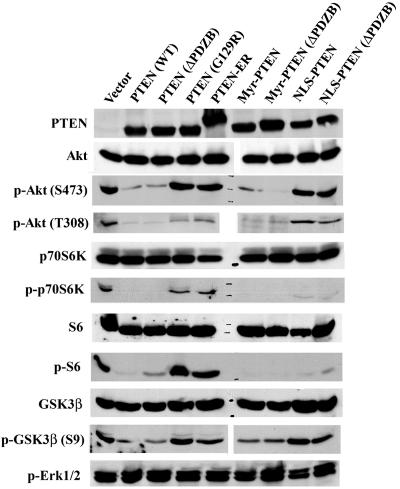
To determine the mechanism by which nuclear PTEN suppressed cell growth, Western blotting was used to detect some of the signaling molecules involved in cell survival and apoptosis phosphorylation and/or expression, activities that are potentially regulated by the interplay between PI3K and PTEN. Specifically, the phosphorylation statuses of Akt, p70S6K, GSK3β, S6, and Erk1/2 were determined. The expression of PTEN protein was also analyzed independently to ensure that its level of expression remained similar to that of the original clones. As shown in Fig. 7, Erk1/2 was not down-regulated in any of the PTEN clones, a finding that is in accord with results from previous studies (13, 14, 72, 73). In contrast, as expected, Akt phosphorylation was markedly reduced in PTEN (WT)-, ΔPDZB-, Myr-, and Myr (ΔPDZB)-transfected U251MG clones. There was, however, no significant difference in Akt phosphorylation levels among vector, G129R, ER, NLS, and NLS (ΔPDZB)-expressing clones. The activity of Akt was further evaluated and validated by the analysis of its substrate, GSK3β. This observation demonstrates that when PTEN is unable to reach the plasma membrane, its ability to inhibit Akt phosphorylation is considerably diminished. In contrast to Akt phosphorylation, the phosphorylation of p70S6K and its substrate, S6, was significantly down-regulated in U251MG cells expressing only a nuclear form of PTEN, implying that nuclear PTEN-mediated p70S6K down-regulation is Akt independent. These studies further suggest that nuclear PTEN-mediated inactivation of p70S6K could be at least partly responsible for the increased G1 growth arrest seen in cells that express the nuclear form of PTEN.
FIG. 7.
Nuclear PTEN inhibits p70S6K activation without down-regulating Akt. Cell lysates equivalent to 5 × 105 cells from stable clones grown in the presence of 1% serum for 3 days were loaded into each well on an sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto PVDF membranes. The filters were blotted with antibodies against PTEN, Akt, phospho-Akt (S473), phospho-Akt (T308), p70S6K, phospho-p70S6K (T389), S6, phospho-S6 (S235/236), GSK3β, phospho-GSK3β (S9), and phospho-Erk1/2, respectively, followed by horseradish peroxidase-conjugated secondary antibodies and visualized by the ECL reaction.
An intact lipid phosphatase domain is required for nuclear PTEN-mediated growth suppression.
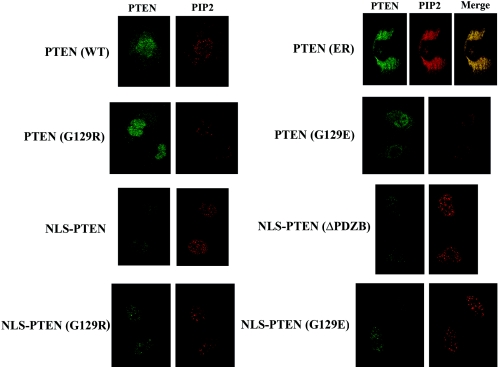
Since nuclear PTEN suppresses cell growth without down-regulating Akt, we wanted to determine whether lipid phosphatase activity is required for PTEN to exert its biologic functions in the nucleus. To this end, we constructed NLS-PTEN (G129R) and NLS-PTEN (G129E) mutants and transfected them into U251MG cells by using the same procedures used for the previously used constructs. Several stable clones were selected for their exclusive PTEN expression in the nucleus. To indirectly assess lipid phosphatase activity, we immunostained cells with PIP2-specific antibodies and measured the PIP2 signal in the nucleoplasm, excluding nuclear speckles. This method was chosen because most of the PIP2 in the nucleus is localized inside the nuclear speckles (spliceosomes) and is generated by phosphatidylinositol phosphate kinase (7). As shown in Fig. 8, PIP2 levels correlate with PTEN′s lipid phosphatase activity using these criteria. We feel that this is a fairly accurate measure of the effect of PTEN lipid phosphatase on PIP2 generation. However, using this measure as a quantitative assay for determining PTEN′s lipid phosphatase activity would be made difficult by virtue of free PIP2 (not protected by proteins) being washed away during fixation/permeabilization. In the case of the PTEN-ER clone, the majority of PIP2 is detected in the ER, since PTEN-ER is restricted in this cellular fraction. As summarized in Table 1, similar to NLS-PTEN, both the NLS-G129R and NLS-G129E mutants did not inhibit the same degree of cell invasiveness shown by transfected U251MG cells. Further, anchorage-independent growth was not suppressed and G1 accumulation was not enhanced in NLS-G129R- and NLS-G129E-transfected U251MG cells, in contrast to NLS-PTEN cells. These data suggest that even though nuclear PTEN cannot down-regulate Akt, it still requires a functional lipid phosphatase domain to exert growth-suppressing activity.
FIG. 8.
Assessment of in vivo lipid phosphatase activity of PTEN mutants. Stable transfectants were first immunostained with MAb against PTEN and then with FITC-conjugated goat anti-mouse IgG, followed by mouse IgM anti-PIP2 and then Texas red-conjugated goat anti-mouse IgM (μ chain) to avoid cross-reactivities of secondary antibodies. The fluorescence signals were analyzed by an Olympus FluoView LSM confocal microscope.
TABLE 1.
Lipid phosphatase domain is required for nuclear PTEN-mediated growth suppression of U251MG cells
| U251MG cell type | % Soft agar colony formationa | % G1 arrestb | % Cell invasionc |
|---|---|---|---|
| Vector | 100.0 ± 6.5 | 62.5 ± 2.5 | 100.0 ± 10.2 |
| NLS-PTEN | 5.4 ± 2.3 | 82.5 ± 2.5 | 84.6 ± 11.9 |
| NLS-PTEN (G129R) | 94.8 ± 9.7 | 68.0 ± 1.0 | 87.3 ± 15.4 |
| NLS-PTEN (G129E) | 93.3 ± 6.2 | 68.5 ± 0.5 | 88.7 ± 16.1 |
Cells (5 × 104/well) were seeded and colonies were measured as described in the legend to Fig. 4A. The percentage of soft agar colonies in vector-transfected U251MG clones was set at 100%.
G1 arrest was measured by flow cytometry as described in the legend to Fig. 5.
Matrigel invasion assays were performed by a modified Boyden chamber assay as described in the legend to Fig. 6.
Nuclear PTEN up-regulates AMPK.
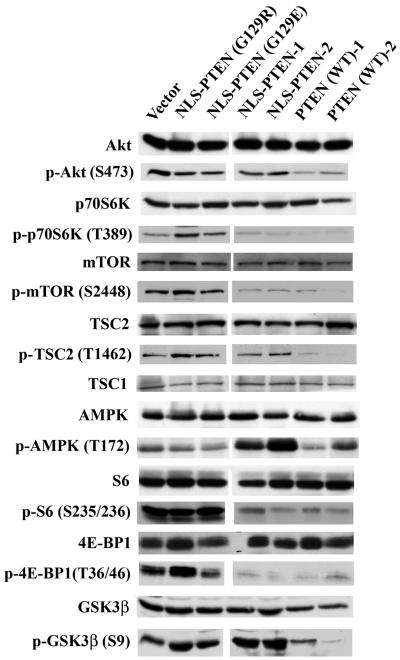
The canonical pathway of activation of p70S6K involves a PI3K/Akt/mTOR signaling cascade. However, p70S6K can also be regulated via Akt-independent signaling pathways. Our preliminary results suggested that nuclear PTEN is capable of down-regulating p70S6K without interfering with Akt activity (Fig. 7). Consequently, we analyzed the expression and phosphorylation patterns of a panel of signaling molecules, including mTOR, TSC2 (tuberin), TSC1 (hamartin), S6, 4E-BP1, GSK3β, and AMPK to dissect the mechanisms involved in Akt-independent nuclear PTEN-mediated p70S6K down-regulation. As shown in Fig. 9, the decreased phosphorylation of Akt substrates GSK3β and TSC2 could only be observed in PTEN (WT) clones; these results corroborate our findings that Akt is inactivated only by PTEN (WT) and not by nuclear PTEN. Down-regulation of mTOR and its other substrate, 4E-BP1, is found in both PTEN (WT) and nuclear PTEN clones, suggesting that nuclear PTEN-induced down-regulation of p70S6K is most likely also mediated through inactivation of mTOR. The inactivation of p70S6K is also consistent with the phosphorylation level of its substrate, S6. However, TSC2 phosphorylation is not affected by nuclear PTEN, and the expression level of TSC1 remains constant by any of the PTEN clones. We therefore examined other signaling molecules capable of down-regulating mTOR/p70S6K without the release of Akt-mediated TSC2 inactivation. Interestingly, we found that AMPK is persistently up-regulated in nuclear PTEN clones but not in PTEN (WT) clones. AMPK has been shown to activate TSC2 to bypass Akt-dependent inactivation via phosphorylation (11, 27). Thus, the activation of AMPK may, at least in part, account for nuclear PTEN-induced down-regulation of p70S6K. It should be noted that there is partial activation of AMPK in PTEN (WT) clones, since wild-type PTEN is expressed in both the cytoplasm and nucleus. Although LKB1 has been shown to be the main activator of AMPK (25, 62, 80), AMPK can also be phosphorylated by ATM through Akt-dependent activation of ARK5, an ATM upstream regulator (68). Consequently, the phosphorylation of AMPK would be abridged by PTEN in the cytoplasm through inactivation of Akt. This likely would contribute to our observation that AMPK activation is more prominent in nuclear PTEN than in PTEN (WT) clones. To validate the specificity of nuclear PTEN-mediated AMPK phosphorylation, we performed the following experiment using U251MG cells. As shown in Fig. 10, there is very little phosphorylation of AMPK in U251MG cells that have undergone serum deprivation (0.1% serum for 3 days), the phosphorylation is significantly elevated after serum release, which is at least in part due to Akt-mediated activation of AMPK, as discussed above. The phosphorylation of AMPK is conspicuously down-regulated in U251MG cells treated with the PI3K inhibitor LY294002 but not with the MEK1 inhibitor PD98059 in the presence of 10% serum. Conversely, phosphorylation of AMPK is further enhanced in U251MG cells treated with two known AMPK activators, 5-aminoimidazole-4-carbozamide-1-β-4 ribofuranoside (67) and carbonyl cyanide m-chlorophenylhydrazone (71), in the presence of 10% serum.
FIG. 9.
AMPK is activated by nuclear PTEN. Western blotting was performed on lysates of cells expressing the indicated mutants as described in Materials and Methods using antibodies against Akt, phospho-Akt (S473), p70S6K, phospho-p70S6K (T389), mTOR, phospho-mTOR (S2448), TSC2, phospho-TSC2 (T1462), TSC1, AMPK, phospho-AMPK (T172), S6, phospho-S6 (S235/236), 4E-BP1, phospho-4E-BP1 (T36/46), GSK3β, and phospho-GSK3β (S9), followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
FIG. 10.
Regulation of AMPK phosphorylation in U251MG cells. U251MG cells were either serum starved for 3 days and released in 10% serum or treated with 10 μM PD98059, 20 μM LY294002, 10 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP), and 2 mM 5-aminoimidazole-4-carbozamide-1-β-4 ribofuranoside (AICAR) in the presence of 10% serum overnight. Cell lysates were subsequently collected to perform immunoblotting using antibodies against AMPK and phospho-AMPK (T172), followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
The Akt constitutively active mutant, Akt-DD, only partially rescues nuclear PTEN-mediated growth suppression.
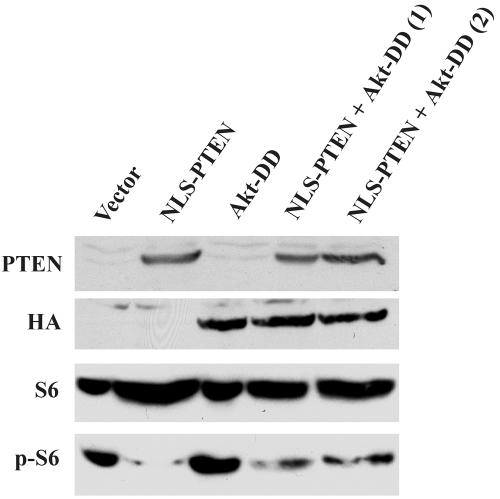
We postulated that nuclear PTEN is capable of mediating cell growth suppression without down-regulating Akt, and our observations have supported our notion. However, to definitely prove our hypothesis, we cotransfected U251MG cells with pLNCX-NLS-PTEN and HA-tagged pcDNA3-Akt-DD constructs to see whether the constitutively active mutant of Akt, Akt-DD, could override nuclear PTEN-mediated growth suppression. Several stable clones were selected for further soft agar colony assay and cell cycle analysis in the presence of 1% serum under the same conditions as the preceding experiments were performed. As summarized in Table 2, NLS-PTEN suppresses anchorage-independent growth and enhances G1 accumulation. On the other hand, Akt-DD alone significantly increases the soft agar colony formation and reduces G1 accumulation. However, in two clones in which nuclear PTEN and Akt-DD are coexpressed, nuclear PTEN-mediated growth suppression is only partially rescued by Akt-DD. Figure 11 shows that nuclear PTEN is still able to suppress the phosphorylation of S6 to a large extent despite the presence of Akt-DD. Taken together, in congruence with our hypothesis, the constitutively active mutant of Akt, Akt-DD, does not override nuclear PTEN-mediated growth suppression.
TABLE 2.
A constitutively active Akt mutant, Akt-DD, only partially rescues nuclear PTEN-mediated growth suppression
FIG. 11.
Phosphorylation of S6 remains suppressed by nuclear PTEN in the presence of Akt-DD. Cell lysates were collected from U251MG clones expressing the pLNCX vector, NLS-PTEN, Akt-DD, or NLS-PTEN plus Akt-DD grown in the presence of 1% serum. Western blotting was performed using antibodies against PTEN, HA tag, S6, and phospho-S6, followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
DISCUSSION
Recent studies have demonstrated that wild-type PTEN is expressed both in the cytoplasm and nucleus. PTEN is preferentially expressed in the nuclei of differentiated or resting cells. In addition, enhanced nuclear PTEN expression is associated with G0-G1 in MCF-7 cells (22). We also observed a similar cell cycle-dependent localization of PTEN in NIH 3T3 cells and mouse astrocytes (data not shown). Current evidence suggests that sequestration of PTEN in the nucleus may play an important role in regulating cell cycle progression. However, potential function(s) of nuclear PTEN signaling in regulating physiological activities have remained largely undefined. To study such putative biologic functions of nuclear PTEN, we used the U251MG PTEN-null cell line and constructed mutants that would be localized to specific cellular compartments, thus allowing us to compare the signaling pathways regulated with the biologic effects of PTEN directed to these compartments. We found that nuclear PTEN alone is capable of suppressing anchorage-independent growth and facilitating G1 arrest in U251MG cells in the absence of Akt down-regulation. In addition, the intact lipid phosphatase domain is necessary for PTEN to fully exert its growth-suppressing effects in the nucleus. Our collective results provide the first direct evidence that nuclear PTEN can contribute to G1 growth arrest through an Akt-independent signaling pathway.
The ability of PTEN to regulate the Akt pathway depends both on its phosphatase activity and access to phospholipid substrates. PTEN has been shown to bind to cell membrane phospholipids through a Ca2+-independent C2 domain (37). Because the C2 domain is closely associated with the PTEN phosphatase domain, it may serve to position the catalytic site correctly with respect to its substrates, conferring substrate specificity. Mutagenesis of basic residues within the C2 domain reduced the tumor suppressor activity of PTEN without interfering with its enzymatic activity in vitro (37). Analysis of PTEN-related proteins has led to similar conclusions. PTEN2 (TPTE), a testis-specific PTEN homolog that is localized to the Golgi apparatus, has been recently identified (9, 82). Enzymatic analysis of PTEN2 revealed substrate specificity similar to that of PTEN, with a preference for the dephosphorylation of the phosphatidylinositol 3,5-phosphate phospholipid, a known mediator of vesicular trafficking. However, PTEN2 is not involved in the down-regulation of PI3K/Akt pathways. TPIP α, another PTEN homologue, is restricted in subcellular localization to the ER (75) and also does not inhibit Akt phosphorylation/activation. Analogous to these findings, our results show that the PTEN-ER mutant is defective in suppressing anchorage-independent growth (Fig. 4), cell proliferation (Fig. 5), cell invasion (Fig. 6), and Akt activation (Fig. 7), since its lipid phosphatase activity is confined to the ER (Fig. 8). Together, these findings suggest that differential and/or modified subcellular localization of PTEN family members does not affect their phosphatase activity per se but rather alters their accessibility to substrates.
In this study, we demonstrate that the ability of PTEN to regulate cell invasion can be dissociated from PTEN′s disparate functions in the nucleus. Invasiveness is mainly regulated by PI3K/Akt signaling cascades by affecting a variety of signaling pathways, including activating Rac1 and cdc42 GTPases (42), down-regulating the expression of E-cadherin (24) and RhoB (29), increasing matrix metalloproteinase 2 (MMP-2) (52) and MMP-9 (31) production, or up-regulating the synthesis of MT1-MMP (membrane type 1 MMP), a major activator of MMP-2 (83). Indeed, abrogation of PI3K/Akt signaling pathways by reintroduction of wild-type PTEN into PTEN-null glioma cells invariably reduced their invasiveness (32, 33, 35). While several reports have suggested that in some cell types PTEN may inhibit cell invasion by a mechanism that is independent of its lipid phosphatase activity (20, 69) or its phosphatase domain altogether (48), in U251MG cells, PTEN appears to regulate invasiveness through its lipid phosphatase activity (33). In addition, although p70S6K has been implicated as playing auxiliary roles in cell migration (reviewed in reference 5), it is not directly involved in regulating cell invasion. In the conditions under which invasion was assayed in this study, Akt remained active due to growth factor autocrine loops in U251MG cells. Thus, expression of NLS-PTEN did not reduce cell invasiveness, since Akt was not down-regulated even though p70S6K was inactivated. Taken together, our data suggest that in the cytoplasm PTEN exerts its effects (including anti-invasion) through a PI3K/Akt pathway, but in the nucleus, PTEN exerts additional Akt-independent tumor suppressive effects.
As shown in Fig. 12, Akt is known to directly phosphorylate TSC2 (26, 55), abrogating the suppression of a TSC1/TSC2 complex on an mTOR kinase activator, Rheb (Ras homologue enriched in brain) (60, 66, 84). Activated mTOR restores phosphorylation of the T389 residue in p70S6K by inhibiting PP2A's phosphatase activity (54). Our results demonstrate that nuclear PTEN down-regulates mTOR and p70S6K in an Akt-independent manner. Recent studies have shown that the activation of AMPK by LKB1 or ATP depletion leads to phosphorylation and activation of TSC2, which overrides Akt inhibition (11, 27). Alternatively, the kinase activity of mTOR can also be triggered by PI3K/Akt-independent stimuli, such as nutrients/amino acids, or phosphatidic acid (16). Our observation of activated AMPK in NLS-PTEN clones suggests that this enzyme may play a key role in suppressing mTOR activity but does not exclude the involvement of other Akt-independent signaling pathways. Additionally, p70S6K is a mitogen-activated serine/threonine (S/T) kinase that is required for cell growth and cell cycle progression (reviewed in reference 56). Although mTOR-mediated phosphorylation of the T389 residue has been shown to be essential for p70S6K's kinase activity, the precise mechanisms that result in T389 phosphorylation are not understood. It has been hypothesized that TSC complex-mediated p70S6K inactivation is independent of mTOR (28), and as illustrated in Fig. 12, cdk1/cdc2 down-regulation of T389 phosphorylation has also been shown (61). Although the kinase(s) responsible for T389 phosphorylation have yet to be identified in vivo, mTOR (8), mTOR-related kinase (38), NEK6/7 (4), and even p70S6K itself have been suggested as potential candidates (59). Thus, nuclear PTEN could also inhibit p70S6K phosphorylation through the pathways listed above in addition to the inhibition of mTOR activity that we observed. Finally, whether nuclear PTEN activates AMPK via LKB1, either by depleting the ATP pool or by other pathways, requires further investigation.
FIG. 12.
Nuclear PTEN down-regulates mTOR/p70S6K, bypassing PI3K/Akt signaling pathways. Despite the fact that mTOR-mediated p70S6K phosphorylation at the T389 residue is regulated primarily by Akt through TSC2 phosphorylation, other pathways have also been shown to regulate mTOR and/or p70S6K activities. Accordingly, nuclear PTEN can down-regulate mTOR/p70S6K through alternative mechanisms without interfering with Akt activation.
The present study and many others show that PTEN is a versatile tumor suppressor that exhibits biologic properties in addition to its signature function of down-regulating the PI3K/Akt signaling cascade through lipid phosphatase activity. Although we have discovered a novel biologic property of PTEN in the nucleus, the mechanisms involved in nuclear PTEN-mediated growth suppression remain to be fully elucidated. PTEN could mediate G1 arrest through its interaction with p53, as postulated by Freeman et al. (17). However, U251MG harbors mutant p53, making this mechanism an unlikely scenario in these cells. Another possibility is that the PTEN may be engaged in growth suppression through inhibition of MSP58 in the nucleus (F. Furnari, personal communication). On the other hand, activated PI3K has been shown to translocate to the nucleus, and functional PIP3 has also been detected inside the nucleus. Most recently, Ahn et al. (3) showed that nuclear PI3K mediates the antiapoptotic activity of nerve growth factor in the isolated nuclei of PC12 cells. Thus, nuclear PTEN could potentially regulate novel nuclear PI3K-dependent signaling pathways that are independent from activation of Akt. Future studies on nuclear PTEN are likely to reveal new mechanisms for PI3K-mediated growth regulation.
Acknowledgments
We thank Kenne Turner for technical assistance and Joann Aaron for critical reading and editing of the manuscript. We are also indebted to T.-J. Liu, Candelaria Gomez-Manzano, Juan Fueyo, Dimpy Koul, and Maria-Magdalena Georgescu for invaluable input; to Ken Hess for statistical analysis; to Ken Aldape for the NHA (E6/E7/hTERT) cell line; to James R. Woodgett for the pcDNA3-Akt-DD construct; and to Karen Ramirez and Tiffany Lafortune for assistance in flow cytometry cell cycle analysis.
This study was supported by grants from NCI/NIH (RO1 CA56041 to W.K.A.Y.), Gilliland Foundation (to W.K.A.Y.), University Cancer Foundation/UTMDACC (to J.-L.L.), and Cancer Center Core (CA16672).
REFERENCES
- 1.Adachi, J., K. Ohbayashi, T. Suzuki, and T. Sasaki. 1999. Cell cycle arrest and astrocytic differentiation resulting from PTEN expression in glioma cells. J. Neurosurg. 9:822-830. [DOI] [PubMed] [Google Scholar]
- 2.Adey, N. B., L. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. [PubMed] [Google Scholar]
- 3.Ahn, J. Y., R. Rong, X. Liu, and K. Ye. 2004. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J. 23:3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belham, C., M. J. Comb, and J. Avruch. 2001. Identification of the NIMA family kinases NEK6/7 as regulators of the p70 ribosomal S6 kinase. Curr. Biol. 11:1155-1167. [DOI] [PubMed] [Google Scholar]
- 5.Berven, L. A., and M. F. Crouch. 2000. Cellular function of p70S6K: a role in regulating cell motility. Immunol. Cell Biol. 78:447-451. [DOI] [PubMed] [Google Scholar]
- 6.Bonneau, D., and M. Longy. 2000. Mutations of the human PTEN gene. Hum. Mutat. 16:109-122. [DOI] [PubMed] [Google Scholar]
- 7.Boronenkov, I. V., J. C. Loijens, M. Umeda, and R. A. Anderson. 1998. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell 9:3547-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett, P. E., R. K. Barrow, N. A. Cohen, S. H. Snyder, and D. M. Sabatini. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 95:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, H., C. Rossier, M. A. Morris, H. S. Scott, A. Gos, A. Bairoch, and S. Antonarakis. 1999. A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y. Hum. Genet. 105:399-409. [DOI] [PubMed] [Google Scholar]
- 10.Cheney, I. W., S. T. Neuteboom, M. T. Vaillancourt, M. Ramachandra, and R. Bookstein. 1999. Adenovirus-mediated gene transfer of MMAC1/PTEN to glioblastoma cells inhibits S phase entry by the recruitment of p27Kip1 into cyclin E/CDK2 complexes. Cancer Res. 59:2318-2323. [PubMed] [Google Scholar]
- 11.Corradetti, M. N., K. Inoki, N. Bardeesy, R. A. DePinho, and K. L. Guan. 2004. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 18:1533-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies, M. A., S. J. Kim, N. U. Parikh, Z. Dong, C. D. Bucana, and G. E. Gallick. 2002. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin. Cancer Res. 8:1904-1914. [PubMed] [Google Scholar]
- 13.Davies, M. A., D. Koul, H. Dhesi, R. Berman, T. J. McDonnell, D. McConkey, W. K. Yung, and P. A. Steck. 1999. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 59:2551-2556. [PubMed] [Google Scholar]
- 14.Davies, M. A., Y. Lu, T. Sano, X. Fang, P. Tang, R. LaPushin, D. Koul, R. Bookstein, D. Stokoe, W. K. Yung, G. B. Mills, and P. A. Steck. 1998. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res. 58:5285-5290. [PubMed] [Google Scholar]
- 15.Deleris, P., D. Bacqueville, S. Gayral, L. Carrez, J. P. Salles, B. Perret, and M. Breton-Douillon. 2003. SHIP-2 and PTEN are expressed and active in vascular smooth muscle cell nuclei, but only SHIP-2 is associated with nuclear speckles. J. Biol. Chem. 278:38884-38891. [DOI] [PubMed] [Google Scholar]
- 16.Fang, Y., M. Vilella-Bach, R. Bachmann, A. Flanigan, and J. Chen. 2001. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294:1942-1945. [DOI] [PubMed] [Google Scholar]
- 17.Freeman, D. J., A. G. Li, G. Wei, H. H. Li, N. Kertesz, R. Lesche, A. D. Whale, H. Martinez-Diaz, N. Rozengurt, R. D. Cardiff, X. Liu, and H. Wu. 2003. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3:117-130. [DOI] [PubMed] [Google Scholar]
- 18.Furnari, F. B., H. J. Huang, and W. K. Cavenee. 1998. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 58:5002-5008. [PubMed] [Google Scholar]
- 19.Georgescu, M. M., K. H. Kirsch, T. Akagi, T. Shishido, and H. Hanafusa. 1999. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl. Acad. Sci. USA 96:10182-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gildea, J. J., M. Herlevsen, M. A. Harding, K. M. Gulding, C. A. Moskaluk, H. F. Frierson, and D. Theodorescu. 2004. PTEN can inhibit in vitro organotypic and in vivo orthotopic invasion of human bladder cancer cells even in the absence of its lipid phosphatase activity. Oncogene 23:6788-6797. [DOI] [PubMed] [Google Scholar]
- 21.Gimm, O., A. Perren, L. P. Weng, D. J. Marsh, J. J. Yeh, U. Ziebold, E. Gil, R. Hinze, L. Delbridge, J. A. Lees, G. L. Mutter, B. G. Robinson, P. Komminoth, H. Dralle, and C. Eng. 2000. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 156:1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginn-Pease, M. E., and C. Eng. 2003. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 63:282-286. [PubMed] [Google Scholar]
- 23.Gottschalk, A. R., D. Basila, M. Wong, N. M. Dean, C. H. Brandts, D. Stokoe, and D. A. Haas-Kogan. 2001. p27Kip1 is required for PTEN-induced G1 growth arrest. Cancer Res. 61:2105-2111. [PubMed] [Google Scholar]
- 24.Grille, S. J., A. Bellacosa, J. Upson, A. J. Klein-Szanto, F. van Roy, W. Lee-Kwon, M. Donowitz, P. N. Tsichlis, and L. Larue. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172-2178. [PubMed] [Google Scholar]
- 25.Hawley, S. A., J. Boudeau, J. L. Reid, K. L. Mustard, L. Udd, T. P. Makela, D. R. Alessi, and D. G. Hardie. 2003. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648-657. [DOI] [PubMed] [Google Scholar]
- 27.Inoki, K., T. Zhu, and K. L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577-590. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke, A., J. Hartkamp, M. Saitoh, W. Roworth, T. Nobukuni, A. Hodges, J. Sampson, G. Thomas, and R. Lamb. 2002. Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J. Cell Biol. 159:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, K., J. Sun, J. Cheng, J. Y. Djeu, S. Wei, and S. Sebti. 2004. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 24:5565-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. R., A. D. Cox, P. A. Solski, B. Devadas, S. P. Adams, R. M. Leimgruber, R. O. Heuckeroth, J. E. Buss, and J. I. Gordon. 1990. Functional analysis of protein N-myristoylation: metabolic labeling studies using three oxygen-substituted analogs of myristic acid and cultured mammalian cells provide evidence for protein-sequence-specific incorporation and analog-specific redistribution. Proc. Natl. Acad. Sci. USA 87:8511-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, D., S. Kim, H. Koh, S. O. Yoon, A. S. Chung, K. S. Cho, and J. Chung. 2001. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15:1953-1962. [DOI] [PubMed] [Google Scholar]
- 32.Kotelevets, L., J. van Hengel, E. Bruyneel, M. Mareel, F. van Roy, and E. Chastre. 2001. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J. Cell Biol. 155:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koul, D., R. Parthasarathy, R. Shen, M. A. Davies, S. A. Jasser, S. K. Chintala, J. S. Rao, Y. Sun, E. N. Benvenisite, T. J. Liu, and W. K. Yung. 2001. Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene 20:6669-6678. [DOI] [PubMed] [Google Scholar]
- 34.Koul, D., S. A. Jasser, Y. Lu, M. A. Davies, R. Shen, Y. Shi, G. B. Mills, and W. K. Yung. 2002. Motif analysis of the tumor suppressor gene MMAC/PTEN identifies tyrosines critical for tumor suppression and lipid phosphatase activity. Oncogene 21:2357-2364. [DOI] [PubMed] [Google Scholar]
- 35.Kubiatowski, T., T. Jang, M. B. Lachyankar, R. Salmonsen, R. R. Nabi, P. J. Quesenberry, N. S. Litofsky, A. H. Ross, and L. D. Recht. 2001. Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J. Neurosurg. 95:480-488. [DOI] [PubMed] [Google Scholar]
- 36.Lachyankar, M. B., N. Sultana, C. M. Schonhoff, P. Mitra, W. Poluha, S. Lambert, P. J. Quesenberry, N. S. Litofsky, L. D. Recht, R. Nabi, S. J. Miller, S. Ohta, B. G. Neel, and A. H. Ross. 2000. A role for nuclear PTEN in neuronal differentiation. J. Neurosci. 20:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J. O., H. Yang, M. M. Georgescu, A. Di Cristofano, T. Maehama, Y. Shi, J. E. Dixon, P. Pandolfi, N. P. Pavletich. 1999. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323-334. [DOI] [PubMed] [Google Scholar]
- 38.Lehman, J. A., V. Calvo, and J. Gomez-Cambronero. 2003. Mechanism of ribosomal p70S6 kinase activation by GM-CSF in neutrophils: cooperation of a MEK-related, T421/S424-kinase and a rapamycin-sensitive, mTOR-related, T389-kinase. J. Biol. Chem. 278:28130-28138. [DOI] [PubMed] [Google Scholar]
- 39.Li, D. M., and H. Sun. 1997. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 57:2124-2129. [PubMed] [Google Scholar]
- 40.Li, D. M., and H. Sun. 1998. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc. Natl. Acad. Sci. USA 95:15406-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, J., C. Yen, D. Liaw, K. Podsypanina, S. Bose, S. I. Wang, J. Puc, C. Miliaresis, L. Rodgers, R. McCombie, S. H. Bigner, B. C. Giovanella, M. Ittamann, B. Tycko, H. Hibshoosh, M. H. Wigler, and R. Parsons. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943-1947. [DOI] [PubMed] [Google Scholar]
- 42.Liliental, J., S. Y. Moon, R. Lesche, R. Mamillapalli, D. Li, Y. Zheng, H. Sun, and H. Wu. 2000. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10:401-404. [DOI] [PubMed] [Google Scholar]
- 43.Liu, J.-L., L. F. Lee, Y. Ye, Z. Qian, and H.-J. Kung. 1997. Nucleolar and nuclear localization properties of a herpesvirus bZIP oncoprotein, MEQ. J. Virol. 71:3188-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, J.-L., Y. Ye, L. F. Lee, H.-J. Kung. 1998. Transforming potential of the herpesvirus oncoprotein MEQ: morphological transformation, serum-independent growth, and inhibition of apoptosis. J. Virol. 72:388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, J.-L., M. D. Hebert, Y. Ye, D. J. Templeton, H.-J. Kung, and A. G. Matera. 2000. Cell cycle-dependent localization of the CDK2-cyclin E complex in Cajal (coiled) bodies. J. Cell Sci. 113:1543-1552. [DOI] [PubMed] [Google Scholar]
- 46.Lu, Y., Y. Z. Lin, R. LaPushin, B. Cuevas, X. Fang, S. X. Yu, M. A. Davies, H. Khan, T. Furui, M. Mao, R. Zinner, M. C. Hung, P. Steck, K. Siminovitch, and G. B. Mills. 1999. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 18:7034-7045. [DOI] [PubMed] [Google Scholar]
- 47.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375-13378. [DOI] [PubMed] [Google Scholar]
- 48.Maier, D., G. Jones, X. Li, A. H. Schonthal, O. Gratzl, E. G. van Meir, and A. Merlo. 1999. The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 59:5479-5482. [PubMed] [Google Scholar]
- 49.Metjian, A., R. L. Roll, A. D. Ma, and C. S. Abrams. 1999. Agonists cause nuclear translocation of phosphatidylinositol 3-kinase gamma. A Gbetagamma-dependent pathway that requires the p110gamma amino terminus. J. Biol. Chem. 274:27943-27947. [DOI] [PubMed] [Google Scholar]
- 50.Mohanam, S., R. Sawaya, I. McCutcheon, F. Ali-Osman, D. Boyd, and J. S. Rao. 1993. Modulation of in vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res. 53:4143-4147. [PubMed] [Google Scholar]
- 51.Myers, M. P., I. Pass, I. H. Batty, J. Van der Kaay, J. P. Stolarov, B. A. Hemmings, M. H. Wigler, C. P. Downes, and N. K. Tonks. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95:13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park, B. K., X. Zeng, and R. I. Glazer. 2001. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 61:7647-7653. [PubMed] [Google Scholar]
- 53.Perren, A., P. Komminoth, P. Saremaslani, C. Matter, S. Feurer, J. A. Lees, P. U. Heitz, and C. Eng. 2000. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am. J. Pathol. 157:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson, R. T., B. N. Desai, J. S. Hardwick, and S. L. Schreiber. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin-associated protein. Proc. Natl. Acad. Sci. USA 96:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potter, C. J., L. G. Pedraza, and T. Xu. 2002. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4:658-665. [DOI] [PubMed] [Google Scholar]
- 56.Pullen, N., and G. Thomas. 1997. The modular phosphorylation and activation of p70s6k. FEBS Lett. 410:78-82. [DOI] [PubMed] [Google Scholar]
- 57.Raftopoulou, M., S. Etienne-Manneville, A. Self, S. Nicholls, and A. Hall. 2004. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303:1179-1181. [DOI] [PubMed] [Google Scholar]
- 58.Ramaswamy, S., N. Nakamura, F. Vazquez, D. B. Batt, S. Perera, T. M. Roberts, and W. R. Sellers. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romanelli, A., V. C. Dreisbach, and J. Blenis. 2002. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J. Biol. Chem. 277:40281-40289. [DOI] [PubMed] [Google Scholar]
- 60.Saucedo, L. J., X. Gao, D. A. Chiarelli, L. Li, D. Pan, and B. A. Edgar. 2003. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 5:566-571. [DOI] [PubMed] [Google Scholar]
- 61.Shah, O. J., S. Ghosh, and T. Hunter. 2003. Mitotic regulation of ribosomal S6 kinase 1 involves Ser/Thr, Pro phosphorylation of consensus and non-consensus sites by Cdc2. J. Biol. Chem. 278:16433-16442. [DOI] [PubMed] [Google Scholar]
- 62.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 101:3329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simpson, L., and R. Parsons. 2001. PTEN: life as a tumor suppressor. Exp. Cell Res. 264:29-41. [DOI] [PubMed] [Google Scholar]
- 64.Sonoda, Y., T. Ozawa, Y. Hirose, K. D. Aldape, M. McMahon, M. S. Berger, and R. O. Pieper. 2001. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 61:4956-4960. [PubMed] [Google Scholar]
- 65.Steck, P. A., M. A. Pershouse, S. A. Jasser, W. K. A. Yung, H. Lin, A. Ligon, L. A. Langford, M. L. Baumgard, T. Hattier, T. Davis, C. Frye, R. Hu, B. Swedlund, D. H. F. Teng, and S. V. Tavtigian. 1997. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15:356-362. [DOI] [PubMed] [Google Scholar]
- 66.Stocker, H., T. Radimerski, B. Schindelholz, F. Wittwer, P. Belawat, P. Daram, S. Breuer, G. Thomas, and E. Hafen. 2003. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 5:559-566. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan, J. E., K. J. Brocklehurst, A. E. Marley, F. Carey, D. Carling, and R. K. Beri. 1994. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353:33-36. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki, A., G. Kusakai, A. Kishimoto, J. Lu, T. Ogura, M. F. Lavin, and H. Esumi. 2003. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 278:48-53. [DOI] [PubMed] [Google Scholar]
- 69.Tamura, M., J. Gu, T. Takino, and K. M. Yamada. 1999. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 59:442-449. [PubMed] [Google Scholar]
- 70.Tanaka, K., K. Horiguchi, T. Yoshida, M. Takeda, H. Fujisawa, K. Takeuchi, M. Umeda, S. Kato, S. Ihara, S. Nagata, and Y. Fukui. 1999. Evidence that a phosphatidylinositol 3,4,5-trisphosphate-binding protein can function in nucleus. J. Biol. Chem. 274:3919-3922. [DOI] [PubMed] [Google Scholar]
- 71.Thors, B., H. Halldorsson, and G. Thorgeirsson. 2004. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 573:175-180. [DOI] [PubMed] [Google Scholar]
- 72.Tolkacheva, T., and A. M. Chan. 2000. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene 19:680-689. [DOI] [PubMed] [Google Scholar]
- 73.van Golen, C. M., T. S. Schwab, K. M. Ignatoski, S. P. Ethier, and E. L. Feldman. 2001. PTEN/MMAC1 overexpression decreases insulin-like growth factor-I-mediated protection from apoptosis in neuroblastoma cells. Cell Growth Differ. 12:371-378. [PubMed] [Google Scholar]
- 74.Waite, K. A., and C. Eng. 2002. Protean PTEN: form and function. Am. J. Hum. Genet. 70:829-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker, S. M., C. P. Downes, and N. R. Leslie. 2001. TPIP: a novel phosphoinositide 3-phosphatase. Biochem. J. 360:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weng, L. P., W. M. Smith, P. L. Dahia, U. Ziebold, E. Gil, J. A. Lees, and C. Eng. 1999. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 59:5808-5814. [PubMed] [Google Scholar]
- 77.Weng, L. P., O. Gimm, J. B. Kum, W. M. Smith, X. P. Zhou, D. Wynford-Thomas, G. Leone, and C. Eng. 2001. Transient ectopic expression of PTEN in thyroid cancer cell lines induces cell cycle arrest and cell type-dependent cell death. Hum. Mol. Genet. 10:251-258. [DOI] [PubMed] [Google Scholar]
- 78.Whiteman, D. C., X. P. Zhou, M. C. Cummings, S. Pavey, N. K. Hayward, and C. Eng. 2002. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int. J. Cancer 99:63-67. [DOI] [PubMed] [Google Scholar]
- 79.Wick, W., F. B. Furnari, U. Naumann, W. K. Cavenee, and M. Weller. 1999. PTEN gene transfer in human malignant glioma: sensitization to irradiation and CD95L-induced apoptosis. Oncogene 18:3936-3943. [DOI] [PubMed] [Google Scholar]
- 80.Woods, A., S. R. Johnstone, K. Dickerson, F. C. Leiper, L. G. Fryer, D. Neumann, U. Schlattner, T. Wallimann, M. Carlson, and D. Carling. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13:2004-2008. [DOI] [PubMed] [Google Scholar]
- 81.Wu, X., K. Senechal, M. S. Neshat, Y. E. Whang, and C. L. Sawyers. 1998. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 95:15587-15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu, Y., D. Dowbenko, M. T. Pisabarro, L. Dillard-Telm, H. Koeppen, and L. A. Lasky. 2001. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J. Biol. Chem. 276:21745-21753. [DOI] [PubMed] [Google Scholar]
- 83.Zhang, D., and P. Brodt. 2003. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 22:974-982. [DOI] [PubMed] [Google Scholar]
- 84.Zhang, Y., X. Gao, L. J. Saucedo, B. Ru, B. A. Edgar, and D. Pan. 2003. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 5:578-581. [DOI] [PubMed] [Google Scholar]
- 85.Zhu, X., C. H. Kwon, P. W. Schlosshauer, L. H. Ellenson, and S. J. Baker. 2001. PTEN induces G(1) cell cycle arrest and decreases cyclin D3 levels in endometrial carcinoma cells. Cancer Res. 61:4569-4575. [PubMed] [Google Scholar]