Abstract
TRIP6 (thyroid receptor-interacting protein 6), also known as ZRP-1 (zyxin-related protein 1), is a member of the zyxin family that has been implicated in cell motility. Previously we have shown that TRIP6 binds to the LPA2 receptor and associates with several components of focal complexes in an agonist-dependent manner and, thus, enhances lysophosphatidic acid (LPA)-induced cell migration. Here we further report that the function of TRIP6 in LPA signaling is regulated by c-Src-mediated phosphorylation of TRIP6 at the Tyr-55 residue. LPA stimulation induces tyrosine phosphorylation of endogenous TRIP6 in NIH 3T3 cells and c-Src-expressing fibroblasts, which is virtually eliminated in Src-null fibroblasts. Strikingly, both phosphotyrosine-55 and proline-58 residues of TRIP6 are required for Crk binding in vitro and in cells. Mutation of Tyr-55 to Phe does not alter the ability of TRIP6 to localize at focal adhesions or associate with actin. However, it abolishes the association of TRIP6 with Crk and p130cas in cells and significantly reduces the function of TRIP6 to promote LPA-induced ERK activation. Ultimately, these signaling events control TRIP6 function in promoting LPA-induced morphological changes and cell migration.
Lysophosphatidic acid (LPA) associates with the G protein-coupled LPA receptors and induces actin rearrangement, focal adhesion assembly, and cell migration through a Rho-dependent, integrin-mediated signaling pathway (24). Similar to several other ligands for G protein-coupled receptors, LPA induces a transient increase of Src kinase activity (21) and rapid tyrosine phosphorylation of a number of proteins involved in cell adhesion and migration, such as FAK, p130cas, and paxillin (31). More and more evidence has shown that tyrosine phosphorylation of these signaling molecules plays a critical role in recruiting active signaling molecules into multiprotein complexes (23). Subsequently, these activated proteins coordinately regulate cell adhesion, migration, and downstream signaling events involved in cell proliferation, survival, and apoptosis (5). Using cells from knockout mice and advanced imaging technology, several tyrosine kinases and phosphatases have been demonstrated to modulate the dynamic processes of cell adhesion and migration. For example, FAK-null cells and SYF cells lacking c-Src, Fyn, and Yes of the Src family kinases show reduced cell migration (13, 17). Similarly, overexpression of the protein tyrosine phosphatase PTP-PEST, a phosphatase for tyrosine-phosphorylated p130cas, leads to defective cell migration (1). Moreover, tyrosine phosphorylation of paxillin and p130cas and the activation of extracellular signal-regulated kinases (ERKs) and MLCK (myosin light-chain kinase) have been implicated in FAK/Src-mediated adhesion turnover and disassembly (37). Thus, these signaling molecules utilize tyrosine phosphorylation as a mechanism to coordinately regulate cell adhesion and migration.
TRIP6 (thyroid receptor-interacting protein 6), also known as ZRP-1 (zyxin-related protein 1), is a member of the zyxin family that has been implicated in the regulation of actin dynamics and signal transduction involved in cell adhesion and migration (22, 40, 42). Originally discovered as an interacting protein of the nuclear thyroid hormone receptor in a yeast two-hybrid system (18), TRIP6 was later identified as a focal adhesion molecule with the capability to shuttle between cell surface and nucleus (36). The zyxin family members, including zyxin, TRIP6, LPP (lipoma preferred partner), and Ajuba, possess a proline-rich region and nuclear export signals at their N terminus and three LIM domains at their carboxyl terminus (3, 14, 22, 26). The LIM domains (named by the initials of three homeodomain proteins, Lin-11, Isl-1, and Mec-3) contain two cysteine-rich zinc finger motifs, which are critical for protein-protein interaction (2). Several LIM domain-containing proteins, such as the zyxin family members, paxillin, and other related proteins, have been shown to localize at focal adhesion plaques and associate with actin cytoskeleton (9, 19, 28, 40). They serve as scaffold or adaptor proteins for the assembly of multiple protein complexes involved in actin rearrangement, cell adhesion, and motility.
Previously it has been shown that zyxin and TRIP6 associate with the Cas family members, including p130cas and CasL/HEF1, and may cooperate in cell motility (42). Very recently, we have demonstrated that LPA stimulation promotes the recruitment of TRIP6 to the activated LPA2 receptor and induces the association of TRIP6 with the components of focal complexes, including paxillin, p130cas, FAK, and c-Src (40). Overexpression of TRIP6 enhances LPA-induced cell migration; in contrast, suppression of the endogenous TRIP6 expression by its specific small interfering RNA (siRNA) inhibits it in SKOV-3 ovarian cancer cells (40). Similarly, another zyxin family member, LPP, has been reported to promote epidermal growth factor-induced cell migration in vascular smooth muscle cells (11). In contrast to zyxin knockout mice, which do not exhibit significant phenotypes (12), the Ajuba-null cells show a reduction of tyrosine phosphorylation of p130cas, Crk, and Dock180 at nascent focal complexes, which leads to the inhibition of Rac activation and impaired cell migration (27). All of these results suggest that these zyxin family members are involved in cell motility. However, the detailed mechanism(s) by which these zyxin family members regulate cell migration is not yet clear.
Despite all of the data suggesting a role for TRIP6 in LPA-induced cell migration, very little is known about how TRIP6 function is regulated in this dynamic process. In the present study, we demonstrate that c-Src mediates LPA-induced TRIP6 phosphorylation at the Tyr-55 residue. This phosphorylation does not regulate its focal adhesion targeting or association with actin cytoskeleton. However, it is critical for the recruitment of Crk and p130cas and for c-Src-mediated ERK activation. Ultimately, these signaling events regulate the process of LPA-induced morphological changes and cell migration.
MATERIALS AND METHODS
Plasmid construction and site-directed mutagenesis.
To express recombinant glutathione S-transferase (GST) fusion proteins of TRIP6-Y55F and TRIP6-P58A mutants in Escherichia coli, site-directed mutagenesis (Promega) was performed using pGEX-6P-3-TRIP6 (40) as the template. The nucleotides TAC encoding Tyr-55 and nucleotides CCA encoding Pro-58 were replaced with TTC and GCG, respectively. The cDNA sequences were verified by automatic DNA sequencing. These mutant cDNA sequences were then inserted into pCMV-Tag2A, pCMV-Tag3A (Stratagene), and pEGFP-C1 (Clontech), respectively, such that they were tagged in frame with a FLAG epitope, a MYC epitope, or a green fluorescent protein (GFP) at its N terminus. The pSUPER vector (6) was used to direct the expression of an siRNA of mouse TRIP6 (designated pSUPER-siTRIP6), which specifically targets the 19-nucleotide sequences of mouse TRIP6, 5′-GAAACTGGTGCATGACATG-3′. The clone containing full-length cDNA sequences of Crk I was purchased from the IMAGE Consortium, amplified by PCR, and inserted into pEGFP-C1, pCMV-Tag2B, and pGEX-6P-3, respectively. The entire sequences were verified by automatic DNA sequencing.
Phosphorylation of TRIP6 by c-Src in vitro and in cells.
pGEX-6P-3-TRIP6 was transformed into E. coli BL21(DE3)(LysS). GST-TRIP6 was purified and further digested with PreScission protease (Amersham Biosciences) to cleave GST. Purified TRIP6 was phosphorylated by recombinant p60c-Src (Upstate Biotechnology Inc.) at 30°C for 30 min and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel slices containing phosphorylated TRIP6 were cut out and digested in situ with trypsin. After purification, the phosphorylation sites of TRIP6 were identified by mass spectrometry analysis (Protein Chemistry Core Facility, Baylor College of Medicine). Similarly, 1 μg of TRIP6 or TRIP6-Y55F was purified and incubated with recombinant p60c-Src. The phosphoproteins were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and detected with a horseradish peroxidase (HRP)-conjugated antiphosphotyrosine antibody (PY20H; Santa Cruz Biotechnology).
To detect tyrosine phosphorylation of TRIP6 in cells, NIH 3T3 cells, Src-null SYF cells, or c-Src-reconstituted SYF+c-Src cells (American Type Culture Collection) were transiently transfected with an empty vector or the expression vector of TRIP6 or TRIP6-Y55F. Cells were starved in 0.1% fatty acid-free bovine serum albumin (BSA)-containing Dulbecco's modified Eagle's medium (DMEM) for 8 h or overnight. Cells were incubated without or with 1 or 2 μM LPA for various times and harvested in lysis buffer (1% Triton X, 10% glycerol, 150 mM NaCl, 10 mM HEPES, 1 mM EDTA, 1 mM EGTA) supplemented with a mixture of protease inhibitors and phosphatase inhibitors. The endogenous or epitope-tagged TRIP6 was immunoprecipitated with a TRIP6-specific monoclonal antibody (Transduction Laboratories) or epitope-specific antibodies and resolved by SDS-PAGE. The tyrosine-phosphorylated TRIP6 was detected by immunoblotting using an HRP-conjugated antiphosphotyrosine antibody (RC20H [Transduction Laboratories] or PY20H [Santa Cruz Biotechnology]).
In vitro binding of Crk I with c-Src-phosphorylated TRIP6.
To examine the effect of c-Src-mediated phosphorylation on the binding of TRIP6 with Crk I in vitro, GST, GST-Crk I, GST-TRIP6, GST-TRIP6-Y55F, and GST-TRIP6-P58A were expressed in E. coli and purified by immobilizing the proteins on glutathione-Sepharose 4B beads (Amersham Biosciences). Crk I was further purified by cleaving GST with PreScission protease (Amersham Biosciences). GST and GST fusion proteins of TRIP6, TRIP6-Y55F, and TRIP6-P58A were phosphorylated by c-Src in vitro. Following reaction, GST fusion protein-immobilized glutathione beads were washed four times to remove c-Src. These beads were then incubated with purified Crk I for 3 h at 4°C. Crk I pulled down by GST fusion proteins was resolved by SDS-PAGE, and the immunoblot was probed with a Crk-specific antibody (Transduction Laboratories).
Cellular coimmunoprecipitation and LPA-induced ERK activation.
Cellular coimmunoprecipitation was performed as described previously (40). To determine LPA-induced ERK activation, transfected SYF+c-Src cells were starved overnight and then stimulated with 2 μM LPA for 5 or 10 min. The whole-cell lysates were subjected to SDS-PAGE, and the immunoblot was probed with an anti-phospho-ERK polyclonal antibody (Promega). The blot was then stripped and reprobed with an anti-ERK2 monoclonal antibody (Transduction Laboratories) to ensure equal expression of total ERKs. The MEK1-K97A expression vector (30) was cotransfected or not with pEGFP or pEGFP-TRIP6 into SKOV-3 cells. The expression of MEK1 and MEK1-K97A was detected by probing the immunoblot with a MEK1-specific antibody (Transduction Laboratories). The activation of ERKs was quantitated using NIH Image J software.
Immunocytochemistry and time-lapse imaging of live cells.
LPA-promoted colocalization of TRIP6 or TRIP6-Y55F with MYC-FAK, vinculin, paxillin, or actin was performed in SYF+c-Src cells or NIH 3T3 cells as described before (40). For live cell imaging, SYF+c-Src cells or SYF cells expressing different GFP constructs as indicated were plated on glass-bottomed 35-mm tissue culture dishes (MatTek Corp.). After starvation for 2 h in DMEM containing 0.1% fatty acid-free BSA and 10 mM HEPES, pH 7.4, GFP-positive cells were visualized with an Olympus IX70 inverted fluorescence microscope using a 100× objective. Ten micromolar LPA was then added at the center of the plates, and the images of GFP-positive cells were acquired every 20 or 30 s for 30 to 40 min with a Photometrics 1400 charge-coupled device camera under the control of IPLab software (Scanalytics, Inc.).
Transwell cell migration assays.
SKOV-3 cells expressing GFP, GFP-TRIP6, or GFP-TRIP6-Y55F in the absence or presence of MEK1-K97A were subjected to a transwell cell migration assay as described previously (40). The bottom of the transwell membrane was coated with 10 μg/ml fibronectin overnight before the experiment. Cells at a density of 150,000/well were placed in the upper chamber, 2 μM LPA was added or not to the lower chamber, and the cells were allowed to migrate for 6 h. After removing the nonmigrated cells from the top surface, the migrated GFP-positive cells on the whole filter were counted by fluorescence microscopy. Meanwhile, an aliquot of cells was placed on the coverslips, and GFP-positive cells in each field were counted to estimate transfection efficiency. The relative migration rate was defined as the increase of migrated cells compared to migrated GFP control cells in the absence of LPA treatment and has been adjusted by transfection efficiency.
RESULTS
c-Src mediates LPA-induced phosphorylation of TRIP6 at the Tyr-55 residue.
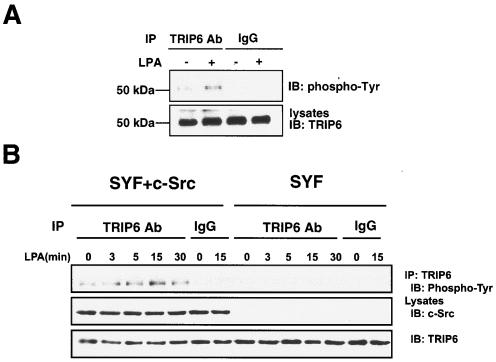
To explore whether the function of TRIP6 is regulated by LPA-dependent tyrosine phosphorylation, we first investigated LPA-dependent tyrosine phosphorylation of TRIP6 in NIH 3T3 fibroblasts, which express high levels of TRIP6. Indeed, we found that LPA stimulation for 15 min promoted tyrosine phosphorylation of endogenous TRIP6 in NIH 3T3 cells (Fig. 1A).
FIG. 1.
LPA induces tyrosine phosphorylation of endogenous TRIP6 in NIH 3T3 and SYF+c-Src cells, but not SYF cells. A. NIH 3T3 cells were starved overnight in DMEM containing 0.1% fatty acid-free BSA. After stimulation with 1 μM LPA or not for 15 min, endogenous TRIP6 in the whole-cell lysates as shown in the bottom panel was immunoprecipitated (IP) with an anti-TRIP6 monoclonal antibody or a mouse immunoglobulin G (IgG). The immunoblot (IB) was probed with an HRP-conjugated antiphosphotyrosine antibody (RC20H). B. Mouse embryonic fibroblasts lacking Src, Fyn, and Yes (SYF cells) or reconstituted with c-Src (SYF+c-Src cells) were starved for 8 h and then treated with LPA for the indicated times. The endogenous TRIP6 was immunoprecipitated with a TRIP6 antibody or a mouse IgG, and the immunoblot was probed with an HRP-conjugated antiphosphotyrosine antibody (PY20H). The bottom two panels show the expression of c-Src and TRIP6 in the whole-cell lysates, respectively.
Previously we have demonstrated that LPA promotes the interaction of TRIP6 with two tyrosine kinases involved in cell adhesion and migration, including c-Src and FAK (40). Because TRIP6 contains proline-rich sequences in the N-terminal region that allow it to bind to SH3 domain-containing proteins, such as Src, we speculated that this kinase might be a potential candidate for mediating TRIP6 phosphorylation in cells. Therefore, we compared tyrosine phosphorylation of endogenous TRIP6 in SYF mouse embryonic fibroblasts lacking Src, Fyn, and Yes and SYF+c-Src fibroblasts that have been reconstituted with c-Src. Compared to NIH 3T3 cells, these SYF+c-Src cells express a slightly higher level of c-Src (data not shown). As shown in Fig. 1B, tyrosine phosphorylation of TRIP6 was steadily detected in SYF+c-Src cells but not in Src-deficient SYF cells. It reached the highest level after LPA stimulation for 15 min. This result indicates that Src family kinases are responsible for TRIP6 phosphorylation in vivo.
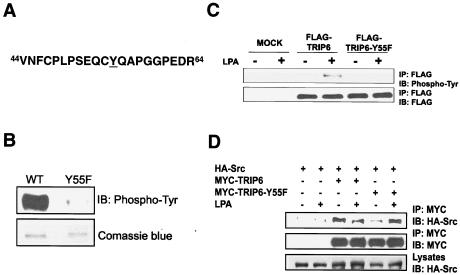
We then set out to map the relevant sites of TRIP6 phosphorylation. The recombinant TRIP6 purified from E. coli was subjected to phosphorylation by the active c-Src kinase in vitro, digested with trypsin, and sequenced. The result revealed that TRIP6 is predominantly phosphorylated by c-Src at Tyr-55 (Fig. 2A). Another minor phosphorylation site of TRIP6 is Tyr-123. However, mutation of Tyr-123 to Phe did not alter c-Src-mediated TRIP6 phosphorylation in vitro or in cells (data not shown).
FIG. 2.
TRIP6 is phosphorylated at the Tyr-55 residue by c-Src. A. Tryptic phosphopeptide of TRIP6. Purified TRIP6 was phosphorylated by c-Src in vitro, resolved by SDS-PAGE, and digested with trypsin in situ. After purification, the phosphorylation sites of TRIP6 were identified by mass spectrometry analysis. The phosphorylated Tyr-55 residue is underlined. B. Mutation of Tyr-55 to Phe eliminates phosphorylation of TRIP6 in vitro. Purified wild-type (WT) or Y55F mutant of TRIP6 was phosphorylated by c-Src in vitro. The immunoblot was probed with an HRP-conjugated antiphosphotyrosine antibody (PY20H). Equal loading of purified proteins is shown by Coomassie blue staining. C. LPA-induced TRIP6 phosphorylation is eliminated by mutation of Tyr-55 to Phe. SYF+c-Src cells alone or those expressing FLAG-tagged TRIP6 or TRIP6-Y55F were starved for 8 h and then treated without or with LPA for 15 min. TRIP6 in the whole-cell lysates was immunoprecipitated with the anti-FLAG M2 monoclonal antibody-conjugated agarose beads, and the immunoblot was probed with an HRP-conjugated antiphosphotyrosine antibody (top panel). The blot was then stripped and reprobed with an anti-FLAG polyclonal antibody to detect immunoprecipitated TRIP6 or TRIP6-Y55F (bottom panel). D. Tyr-55 mutation does not affect the association of TRIP6 with c-Src. HEK 293T cells expressing HA-c-Src alone or with either MYC-TRIP6 or MYC-TRIP6-Y55F were starved overnight and then stimulated with LPA for 15 min. TRIP6 in the whole-cell lysates was immunoprecipitated with the anti-MYC monoclonal antibody-conjugated agarose beads. The immunoblot was probed with an anti-HA polyclonal antibody to detect coimmunoprecipitated c-Src (top panel). The blot was stripped and reprobed with an anti-MYC polyclonal antibody to detect immunoprecipitated TRIP6 or TRIP6-Y55F (middle panel). The bottom panel shows the expression of HA-c-Src in the whole-cell lysates.
Next, we generated a TRIP6 mutant, in which Tyr-55 was substituted with Phe, and compared the ability of c-Src to phosphorylate the wild type and a Y55F mutant of TRIP6 in vitro. As shown in Fig. 2B, this single mutation greatly eliminated tyrosine phosphorylation of TRIP6. To confirm the assignment of Tyr-55 as the physiologically relevant site of TRIP6 phosphorylation, FLAG-tagged wild-type or Y55F mutant TRIP6 was expressed in SYF+c-Src cells, and LPA-dependent tyrosine phosphorylation of these proteins was examined. The result showed that LPA-induced tyrosine phosphorylation of TRIP6 was virtually eliminated by mutation of Tyr-55 to Phe (Fig. 2C), although this mutation did not alter the ability of TRIP6 to associate with c-Src (Fig. 2D). Taken together from these results, we conclude that c-Src mediates LPA-induced TRIP6 phosphorylation at the Tyr-55 residue.
The capability of TRIP6 to target to focal adhesions and colocalize with actin is not affected by Tyr-55 mutation.
To investigate how Tyr-55 phosphorylation regulates the function of TRIP6 in LPA signaling, we first examined LPA-dependent recruitment of TRIP6 and the TRIP6-Y55F mutant to focal adhesions and colocalization with actin cytoskeleton. Previously we have shown that TRIP6 is predominantly cytosolic or forms small clusters at sites of adhesions in SKOV-3 cells after serum starvation overnight (40). Similarly, we have found that by serum starvation overnight, both TRIP6 and TRIP6-Y55F were diffusely distributed in the cytoplasm in SYF+c-Src cells or NIH 3T3 cells. Only a small amount of TRIP6 or TRIP6-Y55F colocalized with vinculin, paxillin, and transfected MYC-FAK in SYF+c-Src cells (see Fig. S1A, C, and E in the supplemental material), or with actin in NIH 3T3 cells (see Fig. S1G in the supplemented material). With LPA stimulation for 15 min, substantial amounts of TRIP6 and TRIP6-Y55F have been found to associate with endogenous vinculin, paxillin, and transfected MYC-FAK in SYF+c-Src cells (see Fig. S1B, D, and F in the supplemental material), and colocalize with actin in NIH 3T3 cells (see Fig. S1H in the supplemental material). Thus, the function of TRIP6 in LPA-induced focal adhesion targeting and actin colocalization is not changed by this point mutation. The localization of vinculin, paxillin, transfected MYC-FAK, and actin in GFP control cells treated with LPA or not is shown in Fig. S2 in the supplemental material.
Tyr-55 phosphorylation of TRIP6 regulates its binding to Crk and p130cas.
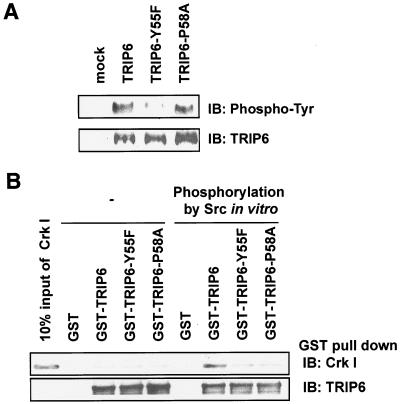
The majority of Src substrates identified so far contain SH2 or SH3 binding domains, or both, which are important for protein-protein interaction during the organization of signaling pathways. When phosphorylated, the phosphotyrosine motifs bind with a high degree of specificity to SH2 domains or phosphotyrosine binding domains contained in different proteins (25). Several such molecules, including p130cas, paxillin, c-Cbl, and Gab1, utilize pYXXP motifs to bind the Crk SH2 domain (10). Here, phosphotyrosine and proline residues are critical for this interaction. Analogously, the Y55-Q-A-P motif of human TRIP6 (Fig. 2A) or Y55-Q-P-P motif of mouse TRIP6 may conform to a binding site for the Crk SH2 domain once Tyr-55 is phosphorylated by c-Src. To test this hypothesis, we first examined the importance of the pY55-Q-A-P58 motif of TRIP6 in Crk I binding in vitro. Thus, GST fusion proteins of wild-type TRIP6, TRIP6-Y55F mutant, and TRIP6-P58A mutant were subjected to in vitro phosphorylation by a recombinant c-Src kinase. Following the reaction, c-Src was removed by extensive washing, and the GST fusion proteins, phosphorylated or not, were used to pull down recombinant Crk I, which contains one SH2 domain and one SH3 domain. As shown in Fig. 3A, c-Src phosphorylated TRIP6 and the TRIP6-P58A mutant equally well, which was greatly eliminated by Y55F mutation. Strikingly, only the c-Src-phosphorylated TRIP6 was able to pull down Crk I, but not nonphosphorylated TRIP6 or the TRIP6-Y55F mutant (Fig. 3B). Although the TRIP6-P58A mutant was phosphorylated by c-Src, it was unable to bind Crk I (Fig. 3B). We further examined the association of TRIP6 with Crk I in HEK 293T cells. In all of the following experiments using GFP constructs to perform coimmunoprecipitation, GFP did not nonspecifically bind to the antibody-conjugated agarose beads (data not shown). As shown in Fig. 4A, LPA promoted the interaction of TRIP6 with Crk I in HEK 293T cells only when c-Src was coexpressed. In contrast to wild-type TRIP6, the TRIP6-Y55F mutant was unable to associate with Crk I in HEK 293T cells even if c-Src was overexpressed (Fig. 4B). Similarly, mutation of Pro-58 to Ala disrupted the interaction of TRIP6 with Crk I in c-Src-expressing HEK 293T cells (see Fig. S3 in the supplemental material). These results indicate that c-Src-mediated phosphorylation of TRIP6 at Tyr-55 renders TRIP6 capable of binding to Crk in vitro and in cells.
FIG. 3.
Mutation of Tyr-55 or Pro-58 abolishes c-Src-dependent TRIP6 binding to purified Crk I in vitro. A. c-Src phosphorylates wild-type TRIP6 and TRIP6-P58A, but not TRIP6-Y55F, in vitro. Purified TRIP6, TRIP6-Y55F, or TRIP6-P58A was phosphorylated by recombinant c-Src kinase in vitro. The immunoblot was probed with an HRP-conjugated antiphosphotyrosine antibody (top panel). The blot was stripped and reprobed with an anti-TRIP6 antibody (bottom panel). B. GST or GST fusion protein of TRIP6, TRIP6-Y55F, or TRIP6-P58A was phosphorylated by c-Src or not in vitro. Following the reaction, c-Src was removed from GST fusion proteins by washing the beads four times, and these GST fusion proteins were incubated with purified recombinant Crk I. Crk I pulled down by GST fusion proteins was detected with a Crk-specific antibody. The first lane is 10% input of Crk I used in this experiment.
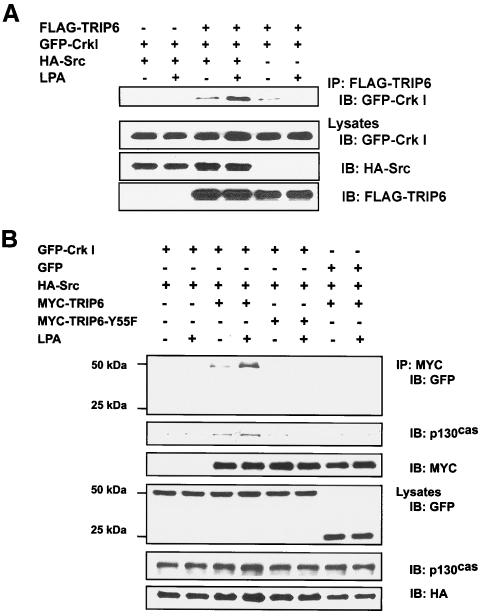
FIG. 4.
LPA promotes the association of Crk I and p130cas with TRIP6, but not TRIP6-Y55F, in HEK 293T cells overexpressing c-Src. A. LPA-dependent interaction of Crk I with TRIP6 requires c-Src. GFP-Crk I was expressed without or with FLAG-TRIP6 and hemagglutinin (HA)-c-Src in HEK 293T cells as indicated. Cells were starved overnight and then treated with 2 μM LPA or not for 15 min. TRIP6 in the whole-cell lysates was immunoprecipitated with the anti-FLAG M2 monoclonal antibody-conjugated agarose beads, and the immunoblot was probed with an anti-GFP polyclonal antibody to detect coimmunoprecipitated Crk I (top panel). The bottom three panels show the expression of GFP-Crk I, HA-c-Src, and FLAG-TRIP6 in the whole-cell lysates, respectively. B. Crk I and p130cas are present in the immunoprecipitates of TRIP6, but not that of TRIP6-Y55F, in HEK 293T cells overexpressing HA-c-Src. HA-c-Src was coexpressed with GFP-Crk I or GFP and either MYC-TRIP6 or MYC-TRIP6-Y55F in HEK 293T cells as indicated. Cells were treated with LPA or not as described above. MYC-TRIP6 or MYC-TRIP6-Y55F in the whole-cell lysates was immunoprecipitated with the anti-MYC (9E10) monoclonal antibody-conjugated agarose beads. The immunoblot was detected with an anti-GFP polyclonal antibody followed by a p130cas-specific monoclonal antibody. The blot was then stripped and reprobed with an anti-MYC polyclonal antibody to detect immunoprecipitated TRIP6 or TRIP6-Y55F. The bottom three panels show the expression of GFP-Crk I, GFP, endogenous p130cas, and HA-c-Src in the whole-cell lysates.
Previously it had been shown that LIM domains 1 and 2 of TRIP6 associate with the CAS (Crk-associated substrate) family members, including p130cas and CasL/HEF, in a yeast two-hybrid screen (42). We then demonstrated that LPA stimulation promotes the interaction of TRIP6 with p130cas (40). Because p130cas also binds Crk through Src-dependent phosphorylation at multiple YXXP motifs of substrate domains (5), it is important to examine if TRIP6, Crk, and p130cas are present in a macromolecular complex by LPA stimulation. Indeed, both GFP-Crk I and endogenous p130cas were coimmunoprecipitated by wild-type TRIP6, but not TRIP6-Y55F, in an LPA-dependent manner (Fig. 4B). These results suggest that LPA stimulation promotes the c-Src-dependent complex formation among TRIP6, Crk, and p130cas. The endogenous p130cas could not be detected in TRIP6-Y55F immunoprecipitates, suggesting that although LIM domains 1 and 2 are responsible for a direct binding of TRIP6 to p130cas, phosphorylation at Tyr-55 by c-Src is also important for TRIP6 to form a stable complex with p130cas. It should be noted that in contrast to our previous finding that LPA induces the association of TRIP6 with p130cas (40), here we were unable to detect endogenous p130cas in the immunoprecipitates of TRIP6 if Crk I was not coexpressed (Fig. 4B, lanes 7 and 8). This result may suggest that the binding affinity between TRIP6 and p130cas is very weak in HEK 293T cells, but the complex formation can be promoted through c-Src-dependent Crk binding. We further examined the effect of TRIP6 on the association of Crk and p130cas in SYF cells and SYF+c-Src cells by overexpressing a TRIP6-specific siRNA. As shown in Fig. S4 in the supplemental material, the endogenous p130cas was present in FLAG-Crk I immunoprecipitates only in SYF+c-Src cells, but not in SYF cells, indicating that c-Src is required for this binding. However, this coimmunoprecipitation was not significantly altered by suppression of TRIP6 expression with a TRIP6-specific siRNA (see Fig. S4, lanes 9 and 10, in the supplemental material), suggesting that the association of Crk and p130cas is not affected by TRIP6.
LPA-stimulated ERK activation is regulated by tyrosine phosphorylation of TRIP6.
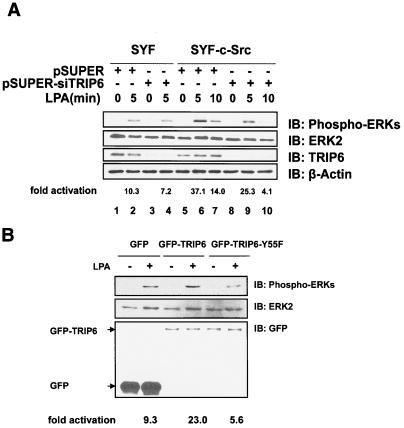
Many G protein-coupled receptors, including the LPA receptors, mediate Ras-dependent activation of ERK cascades. Substantial evidence has shown that recruitment and activation of the Src family kinases regulate these signaling pathways (20). In addition to mediating mitogenic signaling, activated ERKs have been shown to regulate adhesion turnover and cell migration (34, 37). Phosphorylation of MLCK by the activated ERK can modulate actomyosin contractility (15) and promote adhesion disassembly (37). Moreover, the activated Gi/o-ERK pathway has been shown to contribute to LPA-induced pancreatic tumor cell migration (33). To examine if TRIP6 is involved in these signaling pathways, we knocked down the expression of endogenous TRIP6 in SYF and SYF+c-Src fibroblasts by a mouse TRIP6-specific siRNA and assessed its effect on LPA-induced ERK activation. As shown in Fig. 5A, the Src-deficient SYF cells exhibited a lower level of LPA-activated ERKs compared to SYF+c-Src cells (top panel, compare lanes 2 and 6). The TRIP6-specific siRNA significantly knocked down the expression of endogenous TRIP6 (third panel) and reduced LPA-induced ERK activation more dramatically in SYF+c-Src cells (top panel, compare lanes 6 and 7 with lanes 9 and 10) than in SYF cells (top panel, compare lanes 2 and 4), suggesting that TRIP6 is involved in c-Src-mediated ERK activation. This effect was apparently regulated by Tyr-55 phosphorylation, since LPA-induced ERK activation was enhanced in SYF+c-Src cells overexpressing TRIP6 but was reduced in that overexpressing TRIP6-Y55F mutant (Fig. 5B).
FIG. 5.
Phosphorylation of TRIP6 at Tyr-55 regulates LPA-induced ERK activation in SYF+c-Src cells. A. Suppression of endogenous TRIP6 expression reduces LPA-induced ERK activation in SYF+c-Src cells. SYF or SYF+c-Src cells were transfected with either pSUPER or pSUPER-siTRIP6. Cells were starved for 8 h and then treated with LPA for 5 or 10 min. Equal amounts of whole-cell lysates were subjected to SDS-PAGE. The immunoblot was probed with an anti-phospho-ERK polyclonal antibody to detect activated ERKs (top panel). The immunoblot was then stripped and reprobed with an anti-ERK2 monoclonal antibody to detect total ERKs (second panel). The third panel shows the expression of TRIP6 in the whole-cell lysates, which was dramatically inhibited by a TRIP6-specific siRNA. The immunoblot shown in the third panel was then stripped and reprobed with an anti-β-actin antibody to ensure equal loading of the proteins (bottom panel). The LPA-stimulated increase of ERK phosphorylation is quantitated and has been adjusted by total ERK expression using NIH Image J software. B. LPA-induced ERK activation is reduced by overexpression of TRIP6-Y55F in SYF+c-Src cells. LPA-induced ERK activation was examined in SYF+c-Src cells expressing GFP, GFP-TRIP6, or GFP-TRIP6-Y55F as described above (top two panels). The immunoblot was probed with an anti-GFP polyclonal antibody to detect the expression of GFP, GFP-TRIP6, and GFP-TRIP6-Y55F in the whole-cell lysates (bottom panel). The activation of ERKs by LPA stimulation is quantitated and has been adjusted by total ERK expression in the whole-cell lysates.
The function of TRIP6 to promote LPA-induced morphological changes and cell migration is regulated by c-Src-mediated Tyr-55 phosphorylation.
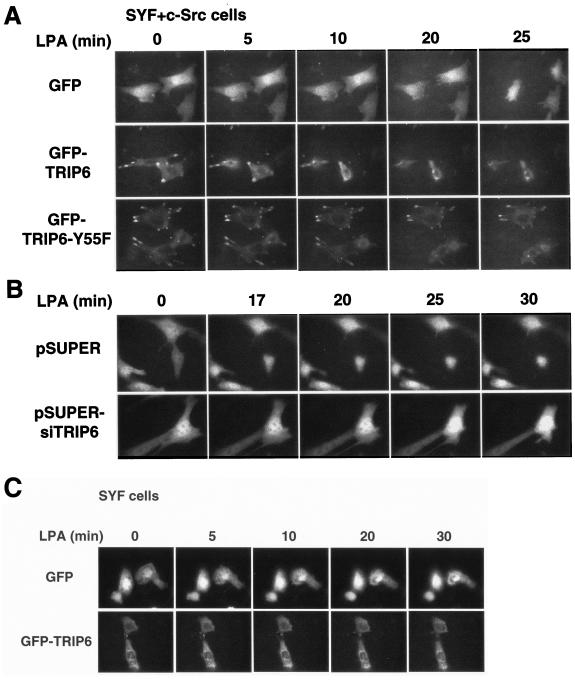
LPA stimulation results in cytoskeletal rearrangement with a concomitant change of cell morphology through the activation of Rho GTPases (24). To explore the role of TRIP6 in LPA-stimulated morphological changes, SYF+c-Src fibroblasts expressing GFP, GFP-TRIP6, or GFP-TRIP6-Y55F were plated on glass-bottomed dishes, starved for 2 h, and then stimulated with LPA. The images of live cells were acquired by time-lapse fluorescence microscopy. As shown in Fig. 6A, soon after LPA stimulation for ∼5 to 10 min, GFP-expressing cells started to retract their cell bodies and completely rounded up after LPA treatment for 22.8 ± 1.0 min (n = 6). Subsequently, they displayed cell surface blebbing and pseudopodium formation. Strikingly, cells expressing GFP-TRIP6 exhibited rapid morphological changes and became rounded soon after LPA stimulation for 8.6 ± 1.1 min (n = 9 in three independent experiments; P < 0.01 versus GFP control; Student's t test). In contrast, cells expressing GFP-TRIP6-Y55F required a much longer time to round up, which could be from 16 to 35 min (29.8 ± 2.8 min; n = 5 in three independent experiments; P < 0.01 versus GFP-TRIP6-expressing cells). Moreover, with the concomitant changes of cell morphology, wild-type TRIP6 at sites of adhesions disappeared much faster than TRIP6-Y55F, as shown in videos S1 and S2 in the supplemental material.
FIG. 6.
Phosphorylation of TRIP6 at Tyr-55 regulates LPA-induced morphological changes. A. LPA-stimulated morphological changes are promoted by TRIP6, but not TRIP6-Y55F, in SYF+c-Src cells. SYF+c-Src cells expressing GFP, GFP-TRIP6, or GFP-TRIP6-Y55F were seeded on glass-bottomed 35-mm dishes. After starvation for 2 h, cells were stimulated with 10 μM LPA and the images of GFP-positive live cells were acquired every 30 s for 30 to 40 min using an inverted fluorescence microscope under the control of IPLab software. Data shown are a comparison of the images captured at various times. B. Suppression of endogenous TRIP6 expression slows the process of LPA-induced morphological changes. A similar experiment as that described for panel A was performed in SYF+c-Src cells harboring pEGFP with either pSUPER or pSUPER-siTRIP6. C. Time-lapse imaging of SYF cells expressing GFP or GFP-TRIP6 was performed as described above. Results shown in panels A to C are representative of three to five independent experiments.
To verify the physiological function of TRIP6 in LPA-induced morphological changes, we further knocked down the expression of endogenous TRIP6 by its specific siRNA. In this experiment, SYF+c-Src cells harboring pEGFP with either pSUPER or pSUPER-siTRIP6 were stimulated with LPA, and time-lapse fluorescence microscopy of the GFP-positive cells was performed. The result showed that cells harboring pEGFP and the pSUPER empty vector completely rounded up after LPA stimulation for 18.7 ± 1.3 min (n = 5); however, suppression of endogenous TRIP6 expression by its specific siRNA slowed the process to 26.3 ± 2.5 min (n = 6 in three independent experiments; P < 0.05 versus pSUPER-expressing cells) (Fig. 6B). All of these findings suggest that TRIP6 is involved in the dynamic process of LPA-induced morphological changes. Moreover, phosphorylation of TRIP6 at Tyr-55 regulates this activity in SYF+c-Src cells.
If c-Src-mediated phosphorylation of TRIP6 is important for its function in LPA-induced morphological changes, expression of wild-type TRIP6 in Src-null SYF cells would not promote this process. To test this hypothesis, SYF cells expressing GFP or GFP-TRIP6 were stimulated with LPA, and time-lapse imaging was performed. As shown in Fig. 6C, the GFP control SYF cells rounded up soon after LPA stimulation. Compared to the elongated shapes of SYF+c-Src fibroblasts, the morphology of SYF cells was more square-like. Thus, we did not observe a dramatic retraction of cell bodies in these cells. Interestingly, overexpression of TRIP6 in SYF cells did not promote LPA-induced morphological changes as it did in SYF+c-Src cells, and it even inhibited LPA-induced cell rounding. The turnover of TRIP6 at the sites of adhesions seemed much slower in SYF cells than in SYF+c-Src cells. All of these results suggest that c-Src-mediated phosphorylation of TRIP6 regulates LPA-induced morphological changes and possibly affects the turnover of TRIP6 at sites of adhesions as well.
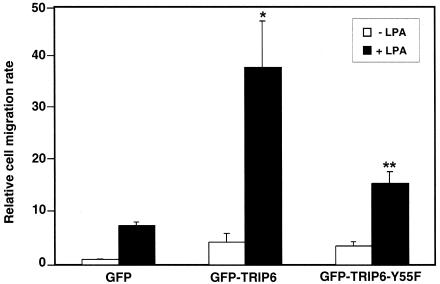
Previously it was shown that coupling of tyrosine-phosphorylated p130cas and the Crk SH2 domain is critical for cell motility (7, 16). Since Tyr-55 mutation disrupts the coupling of TRIP6 to Crk and p130cas and reduces LPA-induced ERK activation, it might affect LPA-induced cell migration. To understand how Tyr-55 mutation affects TRIP6 function in LPA-induced cell migration, GFP, GFP-TRIP6, or the GFP-TRIP6-Y55F mutant was transiently expressed in SKOV-3 cells, and LPA-induced cell migration was assessed. As shown in Fig. 7, in the absence of ligand stimulation, the relative cell migration rate was slightly higher in cells overexpressing GFP-TRIP6 and the GFP-TRIP6-Y55F mutant than the GFP control cells. After LPA stimulation, cell migration was highly increased by overexpression of TRIP6. However, the ability of TRIP6 to promote LPA-induced cell migration was significantly reduced by Tyr-55 mutation. Similar results were observed in NIH 3T3 cells and SYF+c-Src cells (data not shown). This finding suggests that phosphorylation of TRIP6 at Tyr-55 promotes LPA-induced cell migration. However, the function of TRIP6 in this event is also regulated by another mechanism(s), since TRIP6-Y55F does not act as a dominant-negative mutant.
FIG. 7.
Tyr-55 phosphorylation of TRIP6 regulates its function in LPA-induced cell migration. SKOV-3 cells were transiently transfected with pEGFP, pEGFP-TRIP6, or pEGFP-TRIP6-Y55F. After washing twice with 1% fatty acid-free BSA-containing DMEM, cells were subjected to transwell cell migration assays. Two micromolar LPA was added or not in the lower chamber of the transwells, and cells were allowed to migrate for 6 h. Nonmigrated cells from the top surface were removed. The filter was fixed, and GFP-positive cells that migrated to the bottom surface were counted by fluorescence microscopy. The relative cell migration rate in each sample is defined as the fold increase of migrated cells compared to the migrated GFP-expressing cells in the absence of LPA stimulation and has been adjusted by transfection efficiency of GFP expression. Data shown are the means ± standard errors of three independent experiments. *, P < 0.05 versus LPA-stimulated GFP control cells; **, P < 0.05 versus LPA-stimulated GFP-TRIP6-expressing cells (Student's t test).
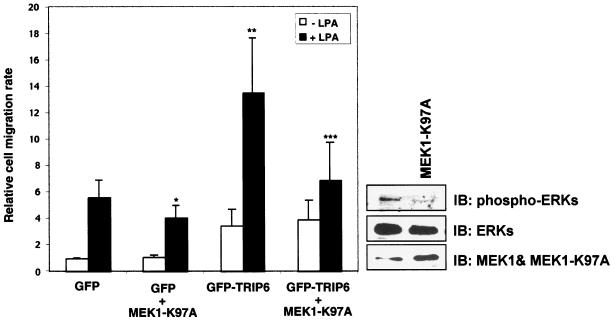
The role of activated ERK has been shown to play a critical role in LPA-induced migration of human pancreatic cancer cells (33). To investigate if the effect of TRIP6 in promoting LPA-induced cell migration is due to the activation of ERKs or its coupling to Crk and p130cas, or both, we employed a dominant-negative MEK1-K97A mutant to inhibit phosphorylation of ERKs and then examined its effect on TRIP6 function in LPA-stimulated migration of SKOV-3 cells. As shown in Fig. 8, overexpression of the MEK1-K97A mutant was able to inhibit ERK phosphorylation and reduce LPA-induced cell migration in the GFP control SKOV-3 cells. In the absence of LPA, the basal levels of migrated cells were slightly higher in TRIP6-expressing cells, which were not altered by MEK1-K97A. This is not surprising, since the levels of activated ERKs are already very low under serum-free conditions. Interestingly, the effect of TRIP6 in promoting LPA-induced chemotaxis was partially reduced by overexpression of MEK1-K97A. However, MEK1-K97A did not act as a dominant-negative mutant in blocking LPA-induced cell migration. These results suggest that the effect of TRIP6 in promoting LPA-induced cell migration is partly due to the activated ERKs; however, other TRIP6-mediated signaling pathways, such as the recruitment of Crk and p130cas, also contribute to its function in cell migration.
FIG. 8.
The dominant-negative MEK1-K97A mutant reduces LPA-induced cell migration in SKOV-3 cells expressing GFP or GFP-TRIP6. SKOV-3 cells were transfected with pEGFP or pEGFP-TRIP6 without or with pcDNA3-MEK1-K97A. LPA-stimulated transwell cell migration was performed as described in the legend for Fig. 7. The results shown are the means ± standard errors of four independent experiments. *, P < 0.05 versus LPA-stimulated GFP control cells; **, P = 0.05 versus LPA-stimulated GFP control cells; ***, P = 0.05 versus LPA-stimulated GFP-TRIP6-expressing cells (Student's t test). At the right lower corner is a representative immunoblot showing the expressional levels of phospho-ERKs, total ERKs, and MEK1 with MEK1-K97A in the whole-cell lysates.
DISCUSSION
Cell migration is a dynamic process that requires a tight coordination of various signaling molecules involved in cell adhesion assembly and disassembly. The fundamental role of FAK-Src signaling pathways in adhesion turnover and cell migration has been demonstrated previously (37, 38). Several substrates for the FAK-Src complex, including paxillin and p130cas, have been shown to be involved in this dynamic process. Analogously, the function of TRIP6 is regulated by c-Src-mediated phosphorylation. It is likely that a coordinated cycling of tyrosine-phosphorylated and -dephosphorylated TRIP6 mediates its association and dissociation with Crk, p130cas, and other adhesion molecules, which in turn regulates the recruitment and turnover of TRIP6 at sites of adhesions. In conjunction with other signaling events, they orchestrate the dynamic process of adhesion formation and turnover and thus control LPA-induced cell rounding and migration.
The zyxin family members share a high degree of sequence homology in the carboxyl-terminal LIM domains; however, the N-terminal proline-rich sequences are much less homologous (22). The Tyr-55 residue is only present in TRIP6 and not other zyxin family members. Thus, they must be regulated either by phosphorylation at other sites or by totally different mechanisms. Whether c-Src-mediated phosphorylation is only specific to TRIP6 but not other zyxin family members remains to be determined. However, our results do reveal some analogies between TRIP6 and another LIM domain-containing focal adhesion molecule, paxillin. First, tyrosine phosphorylation of paxillin is also induced by LPA stimulation (31). Second, this phosphorylation is not required for their localization to focal adhesions; instead, it mediates the recruitment of Crk and promotes their disassembly at the sites of adhesions (35, 37). Consequently, it regulates their functions in cell motility. However, there are also some distinctions between these two molecules. It has been reported that FAK, in association with c-Src, phosphorylates paxillin at Tyr-31 and Tyr-118 (4, 29). Although LPA induces the association of TRIP6 with FAK (40), overexpression of FAK barely increases tyrosine phosphorylation of TRIP6 (data not shown). Instead, TRIP6 is predominantly phosphorylated at Tyr-55 by c-Src (Fig. 2). Previously it was shown that paxillin associates with the protein tyrosine phosphatase PTP-PEST (32). This binding may be necessary for the targeting of PTP-PEST to focal adhesions to facilitate dephosphorylation of the main PTP-PEST substrate, p130cas (1, 8, 32). However, paxillin does not seem to be a direct substrate for PTP-PEST (1). Although it has been shown that the third LIM domain and carboxyl-terminal region of TRIP6 bind to the second PDZ domain of human PTP-1E and its mouse homologue, PTP-BL, the functional significance for this interaction is not known (22). It is also not clear whether TRIP6 is a substrate for PTP-1E or PTP-BL in cells.
In this report, we demonstrate that overexpression of TRIP6, but not the Y55F mutant of TRIP6, promotes LPA-induced morphological changes in SYF+c-Src cells (Fig. 6A). In contrast, overexpression of TRIP6 in SYF cells markedly retards the whole process (Fig. 6C). These results suggest that the effect of TRIP6 in LPA-induced morphological changes is dependent on c-Src-mediated phosphorylation. Since the non-tyrosine-phosphorylated TRIP6 is able to target to focal adhesions and colocalize with actin in SYF cells, it is possible that it forms stable complexes with other signaling molecules, which results in more mature adhesions, thereby perturbing adhesion disassembly. Thus, the balance between the expression of phosphorylated and dephosphorylated TRIP6 would be important for normal control of its function in adhesion turnover and cell motility.
The aberrant regulation of Src family kinases has been shown to associate with the initiation and progression of various cancers. For example, in late-stage ovarian cancers in which the levels of LPA are elevated (41), the Src family kinases are also overexpressed and activated (39). Whether LPA-induced tyrosine phosphorylation of TRIP6 is aberrantly regulated by the activated Src family kinases during ovarian cancer progression and whether it contributes to ovarian tumor invasion and metastasis remain to be investigated in the future.
Supplementary Material
Acknowledgments
We thank Albert Tousson at UAB for the technical assistance in time-lapse fluorescence microscopy and Richard G. Cook at Baylor College of Medicine for tryptic phosphopeptide sequencing.
This work was supported by National Institutes of Health grant CA100848 (to F.-T.L).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Angers-Loustau, A., J. F. Cote, A. Charest, D. Dowbenko, S. Spencer, L. A. Lasky, and M. L. Tremblay. 1999. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144:1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach, I. 2000. The LIM domain: regulation by association. Mech. Dev. 91:5-17. [DOI] [PubMed] [Google Scholar]
- 3.Beckerle, M. C. 1997. Zyxin: zinc fingers at sites of cell adhesion. Bioessays 19:949-957. [DOI] [PubMed] [Google Scholar]
- 4.Bellis, S. L., J. T. Miller, and C. E. Turner. 1995. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J. Biol. Chem. 270:17437-17441. [DOI] [PubMed] [Google Scholar]
- 5.Bouton, A. H., R. B. Riggins, and P. J. Bruce-Staskal. 2001. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene 20:6448-6458. [DOI] [PubMed] [Google Scholar]
- 6.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 7.Cho, S. Y., and R. L. Klemke. 2000. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 149:223-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote, J. F., C. E. Turner, and M. L. Tremblay. 1999. Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J. Biol. Chem. 274:20550-20560. [DOI] [PubMed] [Google Scholar]
- 9.Drees, B. E., K. M. Andrews, and M. C. Beckerle. 1999. Molecular dissection of zyxin function reveals its involvement in cell motility. J. Cell Biol. 147:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feller, S. M. 2001. Crk family adaptors-signalling complex formation and biological roles. Oncogene 20:6348-6371. [DOI] [PubMed] [Google Scholar]
- 11.Gorenne, I., R. K. Nakamoto, C. P. Phelps, M. C. Beckerle, A. V. Somlyo, and A. P. Somlyo. 2003. LPP, a LIM protein highly expressed in smooth muscle. Am. J. Physiol. Cell Physiol. 285:C674-C685. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman, L. M., D. A. Nix, B. Benson, R. Boot-Hanford, E. Gustafsson, C. Jamora, A. S. Menzies, K. L. Goh, C. C. Jensen, F. B. Gertler, E. Fuchs, R. Fassler, and M. C. Beckerle. 2003. Targeted disruption of the murine zyxin gene. Mol. Cell. Biol. 23:70-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 14.Kanungo, J., S. J. Pratt, H. Marie, and G. D. Longmore. 2000. Ajuba, a cytosolic LIM protein, shuttles into the nucleus and affects embryonal cell proliferation and fate decisions. Mol. Biol. Cell 11:3299-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. de Lanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemke, R. L., J. Leng, R. Molander, P. C. Brooks, K. Vuori, and D. A. Cheresh. 1998. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140:961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, J. W., H. S. Choi, J. Gyuris, R. Brent, and D. D. Moore. 1995. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol. Endocrinol. 9:243-254. [DOI] [PubMed] [Google Scholar]
- 19.Li, B., L. Zhuang, M. Reinhard, and B. Trueb. 2003. The lipoma preferred partner LPP interacts with alpha-actinin. J. Cell Sci. 116:1359-1366. [DOI] [PubMed] [Google Scholar]
- 20.Luttrell, L. M., S. S. Ferguson, Y. Daaka, W. E. Miller, S. Maudsley, G. J. Della Rocca, F. Lin, H. Kawakatsu, K. Owada, D. K. Luttrell, M. G. Caron, and R. J. Lefkowitz. 1999. Beta-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283:655-661. [DOI] [PubMed] [Google Scholar]
- 21.Luttrell, L. M., B. E. Hawes, T. van Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 22.Murthy, K. K., K. Clark, Y. Fortin, S. H. Shen, and D. Banville. 1999. ZRP-1, a zyxin-related protein, interacts with the second PDZ domain of the cytosolic protein tyrosine phosphatase hPTP1E. J. Biol. Chem. 274:20679-20687. [DOI] [PubMed] [Google Scholar]
- 23.Panetti, T. S. 2002. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 7:d143-d150. [DOI] [PubMed] [Google Scholar]
- 24.Panetti, T. S., M. K. Magnusson, O. Peyruchaud, Q. Zhang, M. E. Cooke, T. Sakai, and D. F. Mosher. 2001. Modulation of cell interactions with extracellular matrix by lysophosphatidic acid and sphingosine 1-phosphate. Prostaglandins 64:93-106. [DOI] [PubMed] [Google Scholar]
- 25.Pawson, T. 2004. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116:191-203. [DOI] [PubMed] [Google Scholar]
- 26.Petit, M. M., R. Mols, E. F. Schoenmakers, N. Mandahl, and W. J. Van de Ven. 1996. LPP, the preferred fusion partner gene of HMGIC in lipomas, is a novel member of the LIM protein gene family. Genomics 36:118-129. [DOI] [PubMed] [Google Scholar]
- 27.Pratt, S. J., H. Epple, M. Ward, Y. Feng, V. M. Braga, and G. D. Longmore. 2005. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J. Cell Biol. 168:813-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaller, M. D. 2001. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459-6472. [DOI] [PubMed] [Google Scholar]
- 29.Schaller, M. D., and J. T. Parsons. 1995. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol. Cell. Biol. 15:2635-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seger, R., D. Seger, A. A. Reszka, E. S. Munar, H. Eldar-Finkelman, G. Dobrowolska, A. M. Jensen, J. S. Campbell, E. H. Fischer, and E. G. Krebs. 1994. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J. Biol. Chem. 269:25699-25709. [PubMed] [Google Scholar]
- 31.Seufferlein, T., and E. Rozengurt. 1994. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. Signaling pathways and cross-talk with platelet-derived growth factor. J. Biol. Chem. 269:9345-9351. [PubMed] [Google Scholar]
- 32.Shen, Y., G. Schneider, J. F. Cloutier, A. Veillette, and M. D. Schaller. 1998. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 273:6474-6481. [DOI] [PubMed] [Google Scholar]
- 33.Stahle, M., C. Veit, U. Bachfischer, K. Schierling, B. Skripczynski, A. Hall, P. Gierschik, and K. Giehl. 2003. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J. Cell Sci. 116:3835-3846. [DOI] [PubMed] [Google Scholar]
- 34.Stupack, D. G., S. Y. Cho, and R. L. Klemke. 2000. Molecular signaling mechanisms of cell migration and invasion. Immunol. Res. 21:83-88. [DOI] [PubMed] [Google Scholar]
- 35.Turner, C. E. 2000. Paxillin and focal adhesion signalling. Nat. Cell. Biol. 2:E231-E236. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Y., and T. D. Gilmore. 2001. LIM domain protein Trip6 has a conserved nuclear export signal, nuclear targeting sequences, and multiple transactivation domains. Biochim. Biophys. Acta 1538:260-272. [DOI] [PubMed] [Google Scholar]
- 37.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154-161. [DOI] [PubMed] [Google Scholar]
- 38.Webb, D. J., J. T. Parsons, and A. F. Horwitz. 2002. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat. Cell Biol. 4:E97-E100. [DOI] [PubMed] [Google Scholar]
- 39.Wiener, J. R., T. C. Windham, V. C. Estrella, N. U. Parikh, P. F. Thall, M. T. Deavers, R. C. Bast, G. B. Mills, and G. E. Gallick. 2003. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol. Oncol. 88:73-79. [DOI] [PubMed] [Google Scholar]
- 40.Xu, J., Y. J. Lai, W. C. Lin, and F. T. Lin. 2004. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J. Biol. Chem. 279:10459-10468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, Y., Z. Shen, D. W. Wiper, M. Wu, R. E. Morton, P. Elson, A. W. Kennedy, J. Belinson, M. Markman, and G. Casey. 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 280:719-723. [DOI] [PubMed] [Google Scholar]
- 42.Yi, J., S. Kloeker, C. C. Jensen, S. Bockholt, H. Honda, H. Hirai, and M. C. Beckerle. 2002. Members of the zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J. Biol. Chem. 277:9580-9589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.