Abstract
p63 is a member of the p53 tumor suppressor gene family, which regulates downstream target gene expression by binding to sequence-specific response elements similar to those of p53. By using oligonucleotide expression microarray analysis and analyzing the promoters of p63-induced genes, we have identified novel p63-specific response elements (p63-REs) in the promoter regions of EVPL and SMARCD3. These p63-REs exhibit characteristic differences from the canonical p53-RE (RRRCWWGYYY) in both the core-binding element (CWWG) as well as the RRR and/or YYY stretches. Luciferase assays on mutagenized promoter constructs followed by electromobility shift analysis showed that p53 preferentially activates and binds to the RRRCATGYYY sequence, whereas p63 preferentially activates RRRCGTGYYY. Whereas EVPL protein is highly expressed in epithelial cells of the skin and pharynx in the p63+/+ mouse, it is undetectable in these tissues in the p63−/− mouse. Our results indicate that p63 can regulate expression of specific target genes such as those involved in skin, limb, and craniofacial development by preferentially activating distinct p63-specific response elements.
p63 is a member of the p53 tumor suppressor gene family. Similar to p53, p63 is a transcription factor that activates target genes through sequence-specific DNA binding (35, 41, 43, 52, 56). It has been shown that expression of p21waf-1, MDM2, and BAX are induced by TAp63s through binding to p53 response elements (p53-REs) (45). In spite of their structural similarities, p63 functions differ greatly from those of p53. The most striking difference is the apparent involvement of p63 in skin and limb development. The p63 knockout mouse exhibits skin and limb defects as well as craniofacial abnormalities (29, 57). On the other hand, the p53 knockout mouse develops normally but is prone to suffering from various cancers from an early age (7). Heterozygous p63 germ line mutations cause several skin and other developmental disorders (1, 3, 17, 28, 53). On the other hand, germ line mutations of p53 cause Li-Fraumeni syndrome, in which affected individuals are exceptionally prone to developing cancer (26). p63 complements p53-dependent apoptosis induced by DNA damage. However, p63 itself induces apoptosis to a lesser extent than p53 (12, 42).
These differences may be due to the differential regulation of target genes by p53 and p63. The p53 and p63 proteins can bind to two or more tandem repeats of RRRCWWGYYY (p53-RE) or some other motifs and subsequently activate target gene expression (5, 9, 54, 56). In the case of the 14-3-3σ promoter, p53 and p63 differentially bind to two distinct response elements (55). Until now, a number of genes have been reported to be targets of p63 and its close relative, p73, such as JAG1, JAG2, IL4R, ΔNp73, AQP3, and REDD1 (11, 30, 39, 40, 59). However, p63-specific response elements (p63-REs) have not yet been defined. Thus, the specific mechanism of gene activation exhibited by p63 and its distinction from that exhibited by p53 remain unclear.
In order to clarify the regulatory mechanism of p63-specific target gene activation, we first performed oligonucleotide microarray analysis on a 293 human embryonic kidney cell line which inducibly expresses TAp63γ, the most potent transactivating p63 isoform. From the microarray data, we identified more than 100 highly induced genes and searched for p53-type response elements in their 5′-flanking promoter regions. Among 25 promoters cloned and examined, 5 were activated more than fivefold by p53 and/or TAp63γ, and two of these, the EVPL (Envoplakin) and SMARCD3 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily D, member 3) promoters, were specifically activated by TAp63γ but not by p53. Through promoter analysis, we observed that p63-REs remarkably differ from canonical p53-REs. Furthermore, luciferase assays, in vivo DNA-protein binding analysis, and electromobility shift analysis (EMSA) demonstrated differential binding and activation of specific response elements for p53 and TAp63γ. Our data indicate a mechanism for the distinction of specific target gene activation by p53 and p63.
MATERIALS AND METHODS
Inducible cell lines.
To generate stable clones, 293 cells were transfected with Flp-in target site vector pFRT/LacZeo (Invitrogen, Carlsbad, CA). Resulting clones were transfected with pcDNA6/TR, and clones were selected by Blasticidine S resistance and confirmed by immunoblotting with a monoclonal antibody to TetR (MoBiTec, Goettingen, Germany). Finally, the pcDNA5/FRT/TO-TAp63γ expression construct was introduced along with pOG44 plasmid into previously identified clones, and resulting cells were selected by hygromycine.
Cells, transient transfection, and luciferase assay.
293, DLD1, and Saos2 cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Transient transfection and luciferase assays were previously described (34). Briefly, DLD1 cells were transfected using an MBS Mammalian Transfection kit (Stratagene, La Jolla, CA) and pH was carefully adjusted to obtain more than 50% transfection efficiency. For luciferase analysis, 1 μg of expression vector and 200 ng of pGL3-Basic reporter plasmid (Promega, Madison, WI) containing target gene promoters, response elements, or their mutated derivatives were cotransfected by calcium phosphate into Saos2 cells in 24-well plates. Data reflect the fold change in luciferase activity in experimental cells over cells cotransfected with empty pGL3-Basic and pcDNA3.1-Hygro vectors unless otherwise stated in the figure legend. Means and standard deviations were calculated after three independent transfections.
Plasmids.
PCR amplified ABCB6 (−1539 to −126), ADRB (−289 to +384), AXL (−459 to +34), BAL (−1226 to −18), BIK (−998 to −137), BPAG2 (−553 to +38), CSPG4 (−217 to +76), DEPP (−339 to +21), DFFB (−348 to +34), ENIGMA (−197 to +20), EVPL (−274 to +101), FXYD2 (−155 to +135), GAS6 (−1364 to −176), GGT2 (−823 to +229), GRN (−260 to +18), HSD17B1 (−272 to +316), IMP13 (−439 to +31), ITGA2B (−256 to +44), KIAA0954 (−186 to +138), MRF1 (−515 to +47), P8 (−421 to +60), PTPN3 (−257 to +36), PROCR (−1122 to +10), RALGPS1A (−95 to +7), and SMARCD3 (−282 to −49) promoter regions were cloned into the MluI and XhoI or HindIII site of pGL3-Basic. KOD (Toyobo, Osaka, Japan), Herculase (Stratagene), or PCR SuperMix High Fidelity (Invitrogen) was used for PCR amplification. 5′ Deletion mutants of EVPL and SMARCD3 promoters and other p63-REs were also cloned into the MluI and XhoI sites of pGL3-Basic. The PCR primers used for promoter cloning are unpublished; all sequences are available on request. The plasmids containing response elements used in Fig. 2, 4, 5, 7, and unpublished data were constructed by annealing oligonucleotide pairs containing response elements as shown in the figures and cloning into the MluI and XhoI sites of pGL3-Basic. The complete open reading frames of p53 and each isoform of p63 were cloned into the BamHI and XhoI sites of pcDNA3.1-Hygro (Invitrogen).
FIG. 2.
Detailed reporter analysis for EVPL and SMARCD3 promoters. (A) Each deletion construct of the EVPL promoter plasmid was cotransfected with p53, TAp63γ, or pcDNA3.1 into Saos2 cells. “ratio” indicates relative fold activation between −274 and +101, −182 and +101, −182 and +101, and −123 and +101. The response element for p53 is located between −274 and −182. The EVPL promoter has two p63-REs located between −274 to −182 and −182 to −123. (B) Each response element was cloned into the pGL3-Basic plasmid and cotransfected with p53 or TAp63γ. The response elements located between −274 and −182 and −181 to −122 were designated RE1 and RE2, respectively. RE1 is activated by both p53 and TAp63γ, and RE2 is specifically activated by TAp63γ. (C) The deletion constructs of the SMARCD3 reporter were cotransfected with p53 or TAp63γ. The TAp63γ-RE is located between −282 and −179. Underlined bases in panels B and C are mismatches from the p53 consensus sequence. (D) The EVPL and SMARCD3 promoter plasmids were cotransfected with p53, TAp63α, TAp63β, TAp63γ, ΔNp63α, ΔNp63β, or ΔNp63γ expression vector, and luciferase activity was measured. The color scale is the same as in Fig. 1. (E) Chromatin immunoprecipitation analysis using ectopically expressed FLAG-tagged p53 and TAp63γ. After transfection of p53 or TAp63γ into DLD1 cells, cells were fixed with formaldehyde and immunoprecipitated with FLAG antibody. p63, but not p53, precipitated the EVPL and SMARCD3 promoter fragment. p21waf-1 was used as a positive control for both p53 and TAp63γ. (F) EVPL and SMARCD3 were induced by TAp63γ but not p53 expression in DLD1 cells. Ab, antibody.
FIG. 4.
Reporter analysis for various response element plasmids. Each oligonucleotide was cloned into the pGL3-Basic plasmid and cotransfected with p53 or TAp63γ into Saos2 cells. Boxes indicate the core sequences (one in each half site), and the same two bases indicated on the left were introduced into both core sequences. For example, AA in panel A represents GGGCAAGCTCGGGCAAGCCC. The introduced mismatches and nucleotide gaps (B and C) were based on the p63-RE in the WNT4 promoter (unpublished data). (A) RRR and YYY stretches are in perfect match (PM) with those in the p53-RE. (B) Three mismatches (MM3, underlined) were introduced into RRR and YYY stretches. (C) Three mismatches (underlined) were introduced into RRR and YYY stretches, and a 1-nucleotide gap was introduced between the half sites (MM3G1).
FIG. 5.
CGTG-containing response elements are preferably activated by TAp63γ. (A) Seventeen different CGTG-containing response elements were cloned into pGL3-Basic, and p53 and TAp63γ transactivation was examined. The mismatched bases (underlined) are based on response elements from FXYD2 and WNT4. (B) Additional response element sequences which were found within ∼1.5 kb of exon 1 in TAp63γ candidate target gene promoters by computational search as described in Materials and Methods. The candidate response elements lacking a gap between the two half sites were cloned into pGL3-Basic and luciferase activity was measured in response to p53 and TAp63γ. MM, numbers of mismatches in RRR and YYY stretches. *, EVPL-RE2 has seven mismatches in RRR and YYY stretches among three half sites.
FIG. 7.
p53 activation is recovered by mutating the core DNA binding element to CATG. (A) Mutated variants of EVPL's RE2 were cloned into the pGL3-Basic plasmid, and luciferase activity was measured after cotransfection with p53 or TAp63γ. RE2Mut7 and RE2Mut8 were mutated in two bases in the YYY stretch in the middle half site. RE2Mut9 was mutated in the core element of the middle half site (CAGG→CATG), and RE2Mut10 was mutated in the 5′ half site (CTGG→CATG). RE2Mut11 had the core element of both 5′ and middle half sites mutated to CATG. RE2Mut12 had the same mutations as RE2Mut11, with additional mismatches in the YYY stretch of the middle half site (AGG→CCC). (B) The core domain of the 5′ half site of SMARCD3-RE was mutated (CGTG→CATG), generating SMARCD3RE-Mut1. The luciferase activity in response to p53 recovered to 80% of the activity in response to TAp63γ. (C) EMSA for p53 and TAp63γ with EVPL-RE2Mut7 and EVPL-RE2Mut11 probes. Consistent with the luciferase assay results, EVPL-RE2Mut7 bound to TAp63γ but not to p53, whereas EVPL-RE2Mut11 bound to both TAp63γ and p53. Boldface letters indicate mutated bases, and underlined letters indicate mismatched bases. Ab, antibody.
Microarray analysis.
Hu95A arrays (Affymetrix, Santa Clara, CA) were used for expression analysis. Experimental procedures were performed according to the manufacturer's protocol. Briefly, total RNA was extracted from 293 cells before and after 12 h of p63 induction using the RNeasy Midi kit (QIAGEN, Hilden, Germany). Double-strand cDNA was synthesized and labeled using Superscript Choice System (Invitrogen). cRNA was synthesized using the BioArray RNA Transcript Labeling kit (ENZO, Farmingdale, NY). Hybridization was performed overnight at 55°C. Washing and antibody reactions were performed on the GeneChip Fluidics Station 400. Microarray Suite software was used for data analysis.
Reverse transcription-PCR (RT-PCR).
The First Strand cDNA Synthesis kit (Invitrogen) was used for reverse transcription. Semiquantitative PCR was performed with recombinant Taq polymerase (Invitrogen) as follows: 24 to 30 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 30 s. Primer sequences are not published.
Western blotting.
Total protein was extracted from cells using sample lysis buffer (50 M Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 6% [vol/vol] β-mercaptoethanol, and 10% glycerol) and run on a 9% SDS-polyacrylamide gel. Proteins were transferred onto a nitrocellulose membrane (Schleicher & Schuell, Limerick, Ireland) and blocked with 3% skim milk-phosphate-buffered saline (PBS)-Tween 20. Monoclonal 4A4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect p63. Anti-mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase was used as secondary antibody (Amersham-Pharmacia, Buckinghamshire, England), and chemiluminescent signals were detected by Supersignal WestPico Chemiluminescent Substrate kit (Pierce, Rockford, IL).
Immunohistochemistry.
p63+/− mice (Jackson Laboratory, Bar Harbor, ME) were mated, and homozygous mutants were produced. Embryos (18.5 days postcoitum) were formalin fixed and embedded in paraffin. Immunohistochemical detection was performed by the avidin-biotin-peroxidase complex method using Vectastain ABC kits (Vector Laboratories, Burlingame, CA). Antigen retrieval was achieved by 5 cycles of boiling and cooling in 0.1 M citric acid and 0.1 M trisodium citrate. Slides were incubated with monoclonal anti-p63 4A4 antibody (Santa Cruz Biotechnology) (1:100) or polyclonal anti-EVPL M-20 (Santa Cruz Biotechnology) (1:100) antibody at 4°C overnight. The sections were developed with diaminobenzidine and counterstained with hematoxylin.
Computational search for p53-type response elements.
Putative p53-type response elements were identified using the Possum (http://zlab.bu.edu/∼mfrith/possum/) and GenomeNet motif search (http://motif.genome.jp/) strategies. Putative response elements were selected by the following criteria: (i) each half site contained 70% or higher similarity to RRRCWWGYYY; (ii) the fourth position must be C and the seveth position must be G; (iii) at least one of the fifth or sixth positions must be A or T; (iv) at least one base must be R in the first to third positions and at least one must be Y in the eighth to tenth positions; and (v) two or more half sites must be tandemly located within a 13-bp gap.
Electromobility shift analysis (EMSA).
p53 and TAp63γ proteins were synthesized with the TNT/T7 Quick In Vitro Translation kit (Promega). p53 and TAp63γ protein expression was confirmed and quantitated by Western blotting. DNA probes were annealed in 50 mM NaCl and 10 mM Tris buffer. Probe sequences are as follows. EMSACATGPMS, GCAGCGGGCATGCTCGGGCATGCCCACGGA; EMSACATGPMA, TCCGTGGGCATGCCCGAGCATGCCCGCTGC; EMSACATGMM3S, GCAGCGGGCATGCTGCGGCATGCACACGGA; EMSACATGMMSA, TCCGTGTGCATGCCGCAGCATGCCCGCTGC; EMSACATGMM3G1S, GCAGCGGGCATGCTGCCGGCATGCACACGGA; EMSACATGMM3G1A, TCCGTGTGCATGCCGGCAGCATGCCCGCTGC; EMSACGTGPMS, GCAGCGGGCGTGCTCGGGCGTGCCCACGGA; EMSACGTGPMA, TCCGTGGGCACGCCCGAGCACGCCCGCTGC; EMSACGTGMM3S, GCAGCGGGCGTGCTGCGGCGTGCACACGGA; EMSACGTGMMSA, TCCGTGTGCACGCCGCAGCACGCCCGCTGC; EMSACGTGMM3G1S, GCAGCGGGCGTGCTGCCGGCGTGCACACGGA; EMSACGTGMM3G1A, TCCGTGTGCACGCCGGCAGCACGCCCGCTGC; EMSACAGGMM3S, GCAGCGGGCAGGCTGCGGCAGGCACACGGA; EMSACAGGMM3A, TCCGTGTGCCTGCCGCAGCCTGCCCGCTGC; EMSACACGMM3S, GCAGCGGGCACGCTGCGGCACGCACACGGA; EMSACACGMM3A, TCCGTGTGCGTGCCGCAGCGTGCCCGCTGC; EMSACCTGMM3S, GCAGCGGGCCTGCTGCGGCCTGCACACGGA; EMSACCTGMM3A, TCCGTGTGCAGGCCGCAGCAGGCCCGCTGC; EMSAEVPLS, CTCCCAGACTGGTTGTGCAGGAGGAGGCATGAGTGTGGC; EMSAEVPLA, GCCACACTCATGCCTCCTCCTGCACAACCAGTCTGGGAG; EMSASMARCD3S, CTCGTGGGCGTGCAGATGCAAGCACAGGCC; EMSASMARCD3A, GGCCTGTGCTTGCATCTGCACGCCCACGAG.
One picomole of probe was labeled with [γ-32P]ATP (6,000 Ci/mmol; Perkin Elmer, Boston, MA) with T4 kinase (NEB, Beverly, MA). After labeling, probes were purified by NucAway Spin Columns (Ambion, Austin, TX), achieving approximately 600,000 cpm/100 fmol probe. Two microliters of in vitro-translated protein was incubated with 100 fmol of probe in 10 μl of 50 mM Tris-HCl (pH 7.5), 50 mM KCl, 5 mM dithiothreitol (DTT), 10 mM MgCl2, and 3% (vol/vol) glycerol for 20 min at room temperature followed by 20 min at 4°C. 4A4 and DO-7/BP53-12 antibodies (Neomarker, Fremont, CA) were used for competition or supershift studies. No poly(dI-dC) or other nonspecific competitors were added to the binding solution in order to obtain maximum sensitivity. No dyes were added during gel application so as to prevent disruption of weakly bound DNA-protein complexes. Samples were run on a 5% gel (29:1, acrylamide:bis-acrylamide) in 0.25× Tris-borate-EDTA (TBE) buffer at room temperature. After drying, the gel was exposed to Bio-MAX MR film (Kodak, Rochester, NY).
Chromatin immunoprecipitation (ChIP).
p53 and TAp63γ with a 2× hemagglutinin tag at the amino terminus and a 3× FLAG tag at the carboxyl terminus were subcloned into the BamHI and XhoI sites of pcDNA3.1-Hygro (Invitrogen). The tagged p53 or TAp63γ expression plasmids were transfected into DLD1 cells by calcium phosphate, and cells were harvested after 48 h. A Chromatin Immunoprecipitation kit (Upstate Cell Signaling Solutions, Waltham, MA) was used for ChIP analysis according to the manufacturer's protocol. FLAG M-2 antibody (SIGMA, St. Louis, MO) was used for immunoprecipitation. PCR consisted of 37 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 30 s using Taq polymerase (Invitrogen). The PCR primers used for ChIP were as follows. CHIPEVPLF, ACAAGTCCAAACCTTCTGTGG; CHIPEVPLR, ACTGGCTGGTCAGCTAAGTC; CHIPSMARCD3F, CTGAATCTGTGTGAGGACAACC; CHIPSMARCD3R, CTGTACACAGATGTGTCGTAGGC; CHIPp21F, GCAGTGGGGCTTAGAGTGGGG; and CHIPp21R, CAGGCTTGGAGCAGCTACAATTAC.
RESULTS
Identification of TAp63γ-induced genes by oligonucleotide microarray.
In order to identify potential p63 target genes, we performed microarray analysis on 293 cells with and without TAp63γ induction by tetracycline. In the uninduced state, no isoforms of p63 or p73 were detected by Western blot (data not shown). Thus, it is assumed that the function of ectopically expressed TAp63γ will not be blocked by other p53 family proteins, such as p63 and p73 proteins lacking the transactivation domain (ΔN). TAp63γ expression was robustly induced by the addition of tetracycline (unpublished data). Microarray analysis revealed that 129 out of 12,000 spotted genes were activated more than fourfold by TAp63γ expression (Table 1). To confirm the microarray results, we performed RT-PCR analysis for 47 genes, which demonstrated almost perfect consistency with the microarray data (unpublished data).
TABLE 1.
List of genes induced over fourfold by TAp63y in 293 inducible cellsa
| Accession no. | Gene name | Fold induction |
|---|---|---|
| NM_005689 | ABCB6 | 14.7 |
| NM_001613 | ACTA2 | 5.8 |
| NM_000024 | ADRB2 | 11.1 |
| NM_024060 | MGC5395 | 7.3 |
| NM_000693 | ALDH1A3 | 9.5 |
| NM_001635 | AMPH | 4.6 |
| NM_001630 | ANXA8 | 13.3 |
| NM_004040 | ARHB | 6.6 |
| NM_175744 | ARHC | 4.2 |
| NM_005737 | ARL | 6.1 |
| NM_021913 | AXL | 19.8 |
| NM_001197 | BIK | 8.1 |
| NM_001723 | BPAG1 | 7.4 |
| NM_000494 | BPAG2 | 11.8 |
| NM_013279 | c11orf9 | 12 |
| W28438 | c14orf78 | 17.2 |
| NM_030806 | c1orf21 | 8.9 |
| NM_007293 | C4A | 17.3 |
| NM_012121 | CDC42EP4 | 5 |
| NM_001793 | CDH3 | 8.1 |
| NM_001807 | CEL | 23.8 |
| NM_003879 | CFLAR | 8.8 |
| NM_005203 | COL13A1 | 9.2 |
| NM_001312 | CRIP2 | 27.9 |
| NM_001897 | CSPG4 | 13.9 |
| NM_020248 | CTNNBIP1 | 19 |
| NM_013993 | DDR1 | 6.5 |
| NM_007021 | DEPP | 6.6 |
| NM_004402 | DFFB | 4.2 |
| NM_004753 | DHRS3 | 8.5 |
| NM_006052 | DSCR3 | 4 |
| NM_003583 | DYRK2 | 7.3 |
| NM_004428 | EFNA1 | 15.6 |
| NM_005451 | ENIGMA | 5 |
| NM_004443 | EPHB3 | 4.9 |
| NM_001988 | EVPL | 16.9 |
| NM_004110 | FDXR | 4.5 |
| NM_005103 | FEZ1 | 10.3 |
| NM_000800 | FGF1 | 4.9 |
| NM_000141 | FGFR2 | 4 |
| NM_001451 | FOXF1 | 13.2 |
| NM_004960 | FUS | 9.3 |
| NM_001680 | FXYD2 | 4.4 |
| NM_013267 | GA | 9.5 |
| NM_000156 | GAMT | 39.6 |
| NM_006478 | GAS2L1 | 5.2 |
| NM_000820 | GAS6 | 4.4 |
| NM_002050 | GATA2 | 8.1 |
| NM_005265 | GGT1 | 5.9 |
| XM_290331 | GGT2 | 7.1 |
| NM_016235 | GPRC5 | 8.5 |
| NM_000835 | GRIN2C | 5 |
| NM_002087 | GRN | 6.4 |
| NM_007032 | HRIHFB2122 | 14.7 |
| NM_000413 | HSD17B1 | 11.1 |
| NM_005529 | HSPG2 | 9.4 |
| NM_001553 | IGFBP7 | 6.7 |
| NM_000418 | IL4R | 4 |
| NM_005541 | INPP5D | 5.7 |
| NM_014652 | IPO13 | 4.8 |
| NM_002198 | IRF1 | 4.7 |
| NM_000419 | ITGA2B | 6.7 |
| NM_002204 | ITGA3 | 10.8 |
| NM_000211 | ITGB2 | 8.2 |
| NM_025194 | ITPKC | 12.5 |
| NM_002226 | JAG2 | 4.1 |
| NM_002229 | JUNB | 9 |
| NM_002246 | KCNK3 | 10.2 |
| AB006622 | KIAA0284 | 4.2 |
| AB002301 | KIAA0303 | 13.1 |
| AB018310 | KIAA0767 | 4.5 |
| NM_014398 | LAMP3 | 4.6 |
| NM_000229 | LCAT | 14.4 |
| NM_006150 | LMO6 | 16.9 |
| NM_002392 | MDM2 | 5.1 |
| NM_006343 | MERTK | 6.4 |
| NM_004225 | MFHAS1 | 5.9 |
| NM_002430 | MN1 | 9.3 |
| M62324 | MRF1 | 5.2 |
| NM_003970 | MYOM2 | 91.9 |
| NM_005382 | NEF3 | 7.8 |
| NM_002507 | NGFR | 9.1 |
| NM_000435 | NOTCH3 | 7.1 |
| NM_014293 | NPTXR | 5.6 |
| NM_005010 | NRCAM | 5.2 |
| NM_002526 | NT5E | 27.7 |
| NM_012385 | P8 | 21.7 |
| NM_015089 | PARC | 7 |
| NM_004881 | PIG3 | 12.2 |
| NM_004864 | PLAB | 4 |
| NM_000930 | PLAT | 6.2 |
| NM_002632 | PIGF | 4 |
| NM_002688 | PNUTL1 | 9.3 |
| NM_002699 | POU3F1 | 13.1 |
| NM_002705 | PPL | 9 |
| NM_006404 | PROCR | 4.1 |
| NM_016335 | PRODH | 6.8 |
| NM_004878 | PTGES | 38.7 |
| NM_002829 | PTPN3 | 4.7 |
| NM_002852 | PTX3 | 7.3 |
| NM_014417 | PUMA | 4.3 |
| NM_014636 | RALGPS1A | 4 |
| NM_002885 | RAP1GA1 | 7.6 |
| NM_005978 | S100A2 | 25.7 |
| NM_002996 | SCYD1 | 4.4 |
| NM_002997 | SDC1 | 4.4 |
| NM_003004 | SECTM1 | 59.8 |
| NM_002639 | SERPINB5 | 6.7 |
| NM_006142 | SFN | 12.5 |
| NM_003078 | SMARCD3 | 11.2 |
| NM_003087 | SNCG | 72.1 |
| NM_003105 | SORL1 | 4.7 |
| NM_004509 | SP110 | 8.8 |
| NM_014850 | SRGAP2 | 10.2 |
| NM_000349 | STAR | 43.2 |
| NM_005819 | STX6 | 5 |
| NM_003181 | T | 5.7 |
| NM_006474 | T1A-2 | 6.7 |
| NM_000593 | TAP1 | 4.5 |
| NM_015993 | TM4SF11 | 6.3 |
| NM_003271 | TM4SF7 | 8.9 |
| NM_001252 | TNFSF7 | 4.1 |
| NM_003279 | TNNC2 | 29.2 |
| NM_016272 | TOB2 | 6.8 |
| NM_007233 | TP53AP1 | 5.2 |
| NM_003385 | VSNL1 | 37.8 |
| NM_000389 | WAF1 | 6.7 |
| NM_030761 | WNT4 | 4.6 |
| NM_007155 | ZP3 | 7.5 |
The references for known target genes are as follows: ACTA2 (4), DDR1 (33, 38), FDXR (16, 23), IL4R (40), ITGA3 (22), JAG2 (39), MDM2 (19), PIG3 (5, 36), PRODH (27), PTGES (36), PUMA (58), S100A2 (50), SCYD1 (46), SERPINB5 (61), SFN (15), TAP1 (60), TP53AP1 (49), and WAF1 (10). Italicized accession numbers indicate genes that have been previously reported as p53, p63, and/or p73 target genes.
TAp63γ specifically activates the EVPL and SMARCD3 promoters.
p53 protein generally binds to tandem repeats of RRRCWWGYYY or some other sequences and activates transcription of target genes (9, 54). To date, more than 50 genes have been identified as p53 target genes, and most of them are transcriptionally activated through binding of p53 to RRRCWWGYYY sequences (8, 31). It is also known that p63 and p73 activate their target genes by binding to RRRCWWGYYY-type sequences, similar to p53. In addition, the DNA binding domain of p63 has high similarity to that of p53. Based on these observations, we searched for p53-type response elements within an area ∼1.5 kb upstream from exon 1 on the potential TAp63γ target genes identified by microarray analysis using GenomeNet motif search and Possum with a relatively leaky parameter setting (see Materials and Methods). From this analysis we cloned the 5′-flanking region of exon 1 from 25 candidate genes into a luciferase reporter vector, pGL3-Basic, and measured the luciferase activity after cotransfection of Saos2 cells with p53 or TAp63γ. The putative response elements contained in the 25 promoter fragments are unpublished.
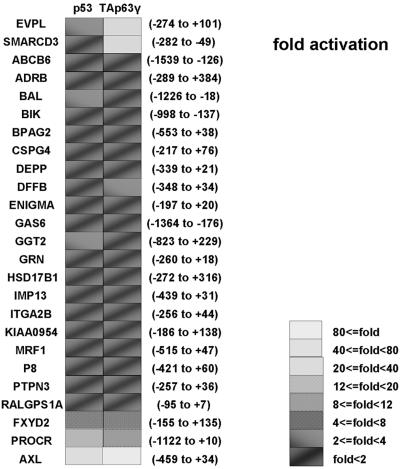
p53 and/or TAp63γ activated AXL, PROCR, EVPL, FXYD2, and SMARCD3 promoters more than fivefold (Fig. 1), while the other cloned promoters did not respond to p53 or TAp63γ. Promoters containing putative response elements with gaps between the half sites were not activated by either p53 or TAp63γ, except for EVPL (Fig. 1 and unpublished data).
FIG. 1.
Luciferase transactivation of 25 target gene promoters by p53 and/or TAp63γ. Each promoter was cloned into the pGL3-Basic plasmid and cotransfected with p53, TAp63γ, or pcDNA3.1 into Saos2 osteosarcoma cells. Data reflect fold change in light units, using readings from cells cotransfected with each promoter plasmid and pcDNA3.1 as a baseline, arbitrarily set to 1. The numbers in parentheses indicate the region of the cloned fragment.
By luciferase assay we found that the EVPL and SMARCD3 promoters were highly activated by TAp63γ (>20-fold) compared to p53 (<4-fold). The same sets of luciferase assays were performed in HCT116 (a colon cancer cell line which expresses wild-type p53 and low levels of p63 and p73) and KYSE150 (an esophageal cancer cell line which expresses mutant p53 and high levels of ΔNp63α), and similar results were obtained in these cell lines (data not shown).
In the EVPL promoter, a 376-bp fragment, −274/+101, exhibited profound luciferase activity induced by TAp63γ. This fragment was further shown to contain two distinct p63-responsive segments, one spanning from −274 to −182 and the other from −182 to −123 (Fig. 2A). Each of these segments contained tandem p53-type binding motifs and exhibited a differential response to p53 and TAp63γ. Whereas both p53 and TAp63γ activated the relative 5′ response element, which we designated RE1, to the same extent, the relative 3′ response element, RE2, only responded to TAp63γ, not to p53 (Fig. 2B). RE2 has an irregular p53-type response element structure, consisting of two half sites flanking nine intervening nucleotides. As shown in the unpublished data, it is this 9-nucleotide intervening sequence that confers p63-specific activation. It has been previously shown that the potent cis-regulatory element in skin-specific expression is located between −363 and −101 of the EVPL promoter by using primary keratinocytes (25). Since the two elements we identified, RE1 and RE2, are located within this region, one or both may be responsible for determining skin-specific EVPL expression.
In the SMARCD3 promoter, the p63-RE was determined to reside in the region between −282 and −177. Deletion of this region abolished TAp63γ-induced luciferase activity of the SMARCD3 promoter (Fig. 2C). Transcriptional activation of the EVPL and SMARCD3 promoters were also examined with other isoforms of p63. TAp63β activated both promoters to the same extent as TAp63γ, while other isoforms activated them very weakly if at all (Fig. 2D). However, although our luciferase analysis clearly showed that EVPL and SMARCD3 promoters were specifically induced by TAp63β and TAp63γ, it is possible that these genes can be activated by other p53 family members in vivo.
In order to examine in vivo binding of p53 or TAp63γ protein to the EVPL and SMARCD3 promoters, we performed chromatin immunoprecipitation (ChIP) analysis. TAp63γ precipitated both EVPL and SMARCD3 promoter fragments, whereas p53 did not precipitate either fragment (Fig. 2E). These data indicate that the EVPL and SMARCD3 promoters are specifically bound by TAp63γ, not p53.
To examine endogenous EVPL and SMARCD3 induction by p53 or TAp63γ, we performed RT-PCR analysis in several different cell systems. First, we generated p53-inducible 293 cells, and neither EVPL nor SMARCD3 induction was observed in these cells. However, we reasoned that since 293 cells were originally transformed with adenovirus, p53 function may be blocked by E1B oncoprotein, thus leading to an underestimation of p53 function. Thus, we introduced p53 or TAp63γ expression plasmids into DLD1 colon cancer cells by transient transfection. TAp63γ, but not p53, induced EVPL and SMARCD3 expression (Fig. 2F). Similar induction was observed in KYSE410 esophageal cancer and O12 head and neck cancer cell lines (data not shown). We also examined EVPL and SMARCD3 induction in some other cell lines, including Saos2 cells. However, no induction was observed in those cell lines. These data imply that expression of other transcription factors in addition to p63 may be necessary for induction of endogenous EVPL and SMARCD3 in some cell lines.
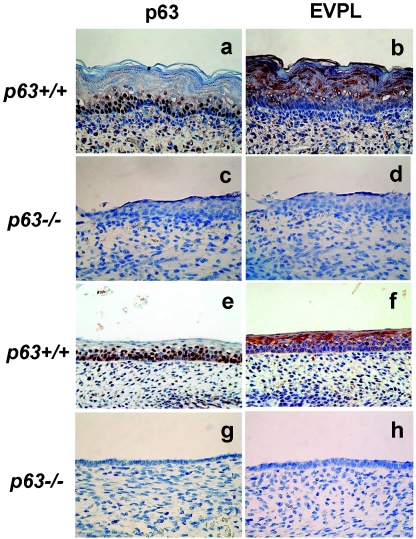
EVPL is a component of the epidermal cornified envelope which contributes to the barrier properties of the skin (47). EVPL is also a component of desmosomes and acts as an interdesmosomal scaffold (37). p63−/− mouse skin does not have stratified epithelium and only displays remnants of undifferentiated cells on the surface of the dermis (29, 57). We examined EVPL expression in p63+/+ and p63−/− mice by immunohistochemistry. EVPL was detected throughout the entire epithelium in wild-type mouse skin, especially in the differentiated and cornified cell layers of the epithelium, consistent with previous reports (24, 25, 37). However, EVPL was not detected in the single-layer epithelium on the surface of the p63−/− mouse (Fig. 3a to d). We also examined the pharynx in p63+/+ and p63−/− mice, and, consistent with previous reports, the pharyngeal epithelium of the p63−/− mouse was thinner than that of the p63+/+ mouse. Similar to our observations in the skin, EVPL exhibited high expression in the pharynx of the p63+/+ mouse but was undetectable in the p63−/− mouse (Fig. 3e to h).
FIG. 3.
Immunohistochemical staining of EVPL in p63+/+ and p63−/− mice skin and pharynx. Panels a, b, e, and f are p63+/+; panels c, d, g, and h are p63−/−. Panels a, c, e, and g were stained for p63, and panels b, d, f, and h were stained for EVPL. Panels a through d are skin; panels e through h are pharyngeal epithelium. Absence of p63 and EVPL staining are evident in both the skin and pharynx of p63 knockout mice.
In both tissues of the p63+/+ mouse, the p63 and EVPL proteins did not localize in the same cell layers. p63 protein was mostly detected in the basal layer of the stratified epithelium by 4A4 antibody, whereas EVPL was detected throughout the entire epithelium. However, this discrepancy may be due to a number of reasons. It has been shown by other investigators that TA isoforms of p63 are expressed in all layers of stratified epithelium; on the other hand, ΔNp63 is highly expressed in the basal layers (32). Although the 4A4 antibody can recognize all p63 isoforms, it is known that TAp63β and TAp63γ isoforms are very labile and that their apparent protein expression levels do not correlate with their transactivation activity (14, 34, 44). That is, short-lived p63 isoforms can be expressed and induce target genes such as EVPL but quickly become degraded, thus evading detection while EVPL is still strongly expressed. Also, since EVPL is a component of the cornified envelope, it exhibits strong expression in the surface layer of the skin. In addition, it has been shown that ΔNp63α, the most predominantly expressed isoform in squamous cells, can function as a transactivator in some experimental settings (6, 20). Thus, it is also possible that EVPL was induced in skin and pharynx by ΔNp63α. Without the availability of highly sensitive isoform-specific p63 antibodies and p63 isoform-specific knockout mice, it is presently uncertain which p63 isoforms are responsible for EVPL induction in mouse skin and pharyngeal epithelium.
TAp63γ preferably activates CGTG-containing sequences.
We observed specific features of the p63-RE half sites in the EVPL and SMARCD3 promoters which distinguish them from the classical p53-RE binding motif (RRRCWWGYYY), including (i) a G in the fifth or sixth position, within the core of the p53-RE, instead of W, and (ii) a relatively high number of mismatches in the RRR or YYY stretches. Next, we examined the contribution of the G base in the core domain and mismatches in the RRR or YYY stretches to p53- and TAp63γ-induced transactivation. Variations of p53-type response elements were cloned into the pGL3-Basic plasmid, and luciferase activity was examined after cotransfecting Saos2 cells with p53 or TAp63γ. In the case of no mismatches or a perfect match (PM) in the RRR and YYY stretches, p53 and TAp63γ similarly activated the reporter gene in almost each case, regardless of the variable core sequence (Fig. 4A). The core sequences, CCAG, CCTG, and CTCG, were activated by neither p53 nor TAp63γ, even though their complementary sequences, CTGG, CAGG, and CGAG, were strongly activated by both p53 and TAp63γ. This implies that the direction of the response element is an important factor for transactivation of p53 and TAp63γ. Substitution of C in the fourth position with G or of G in the seventh position with C in both half sites completely abrogated transactivation by p53 and TAp63γ (data not shown).
Figure 4B shows the transactivation activity with three mismatches (MM3) in the RRR and YYY stretches. Overall, activity levels were lower than in perfectly matched response elements. Moreover, the CATG-containing element was specifically activated by p53, whereas the CGTG-containing element was specifically activated by TAp63γ. After introducing a 1-nucleotide gap between the two half sites with three mismatches (MM3G1), p53 was only able to activate the CATG-containing sequence while TAp63γ only activated the CGTG-containing sequence (Fig. 4C). CACG-containing sequences (the complementary sequence of CGTG core element) were less activated by TAp63γ in both MM3 and MM3G1, emphasizing again the importance of the direction of the response element. In order to further investigate the specific activation of CGTG-containing sequences by TAp63γ, we made various mutations in the RRR and/or YYY stretches. Consistent with the data in Fig. 4, the response elements with 0, 1, and some with 2 mismatches in the RRR and/or YYY stretches activated luciferase activity similarly in response to expression of either p53 or TAp63γ (Fig. 5A). However, in response elements containing two or more mismatches, TAp63γ induced transactivation more strongly than p53. In addition to the number of mismatches, the magnitude of transactivation was also dependent on the specific nucleotide substitutions.
We also explored other putative response elements identified by the computational search, and, based on the above data, our analysis focused on the response elements lacking a gap between half sites. The response elements possessing CATG in the core were more strongly activated by p53 than by TAp63γ (Fig. 5B). On the other hand, response elements possessing a CGTG sequence in the core were equally or more strongly activated by TAp63γ than by p53. These results are consistent with the results shown previously in Fig. 4 and 5A. Some response elements we examined were not activated by either p53 or TAp63γ (unpublished data).
p53 preferentially binds CATG-containing sequences, whereas TAp63γ binds to both CATG- and CGTG-containing sequences.
To examine the mechanism of preferential induction of transactivation by TAp63γ and p53, we tested their sequence-specific DNA binding affinities by electromobility shift analysis (EMSA). Introducing mismatches or a gap into the response elements dramatically reduced their binding affinities to both p53 and TAp63γ protein (Fig. 6A). Whereas the CATG-PM probe (two tandem repeats of a p53-type RE with perfectly matched RRR and YYY stretches and CATG as its core element) was able to bind strongly to both TAp63γ and p53, CGTG-PM was able to bind strongly to TAp63γ but very weakly to p53 (Fig. 6A). Comparing lane 7 (CATG-PM with TAp63γ protein) to lane 10 (CGTG-PM with TAp63γ), the binding signal with CATG-PM was slightly stronger than that with CGTG-PM; however, the binding signal in lane 1 (CATG-PM with p53) was dramatically stronger than in lane 4 (CGTG-PM with p53). From these results, we conclude that p53 preferentially binds to CATG-containing sequences rather than CGTG-containing sequences, whereas TAp63γ can bind to both CATG- and CGTG-containing sequences with similar affinities.
FIG. 6.
EMSA (electromobility shift analysis) for p53 and TAp63γ with various oligonucleotide probes. (A) In vitro-translated wild-type p53 and wild-type TAp63γ proteins were incubated with CATG- or CGTG-containing probes and run on a gel. Two specific DNA-protein complexes, one migrating at the top of the gel and the other one in the middle, were observed. PM, perfect match in RRR and YYY stretches; MM3, three mismatches in RRR and YYY stretches; and MM3G1, three mismatches in RRR and YYY stretches and a 1-nucleotide gap between half sites. (B) Response elements containing complementary core sequences, CAGG and CCTG; CGTG and CACG bound similarly to the TAp63γ protein. (C) The SMARCD3 and EVPL-RE2 probes were incubated with in vitro-translated p53 or TAp63γ protein. Specific bands were only observed with p63 protein and not with p53. Ab, antibody.
The luciferase analysis in Fig. 4 suggested that the direction of the response element greatly affects transactivation by p53 and TAp63γ. In order to determine whether the binding affinity is similarly affected, we performed EMSA. Figure 6B shows that TAp63γ similarly bound to CAGG-MM3 and its complementary sequence, CCTG-MM3, as well as CGTG-MM3 and its complementary sequence, CACG-MM3. The discrepancy between DNA-protein binding and actual transactivation supports the idea that the binding affinity between a response element and transcription factor is necessary but not sufficient for transcriptional activation.
Specific binding to TAp63γ but not to p53 was also detected using the SMARCD3 probe, which has four mismatches, and the EVPL probe, which has seven mismatches in three incomplete half sites (Fig. 6C). The p63 antibody competed with the TAp63γ protein and probe binding. The binding signals were very weak, presumably due to the numerous mismatches.
Mutating the core elements of EVPL and SMARCD3 response elements restores transactivation by p53.
To confirm the importance of the identity of the fifth or sixth nucleotide and number of RRR and YYY mismatches in conferring p53- and p63-specific activation, we determined if the transcriptional response of the EVPL and SMARCD3 response elements to TAp63γ and p53 could be enhanced by replacing the mismatched bases in order to resemble the functional TAp63γ and p53 response elements described above. EVPL-RE2Mut7 and EVPL-RE2Mut8, which have fewer mismatches in the RRR and YYY stretches, and EVPL-RE2Mut9, in which the core element in the relative 3′ response element was restored to CATG, only enhanced TAp63γ-specific activation in EVPL-RE2 (Fig. 7A). However, mutation of at least the relative 5′ half site to CATG in the core element of EVPL-RE2 (Mut10, Mut11, and Mut12) recovered activation by both p53 and TAp63γ very effectively. Similarly, mutation to CATG also recovered p53 transactivation of SMARCD3-RE (Fig. 7B). We further examined DNA-protein binding using EVPL-RE2Mut7 and EVPL-RE2Mut11 with p53 or TAp63γ. In accordance with the luciferase results, EVPL-RE2Mut7 bound only to TAp63γ, whereas EVPL-RE2Mut11 bound to both p53 and TAp63γ (Fig. 7C). From these results, we conclude that the sequence of the core element confers specificity for transactivational induction by p53 and TAp63γ. Neither EVPL-RE2Rev nor SMARCD3-RERev, the complementary sequences of EVPL-RE2 and SMARCD3-RE, respectively, were activated by p53 or TAp63γ, reiterating the differences in transactivation potentials between complementary binding sequences.
DISCUSSION
We have shown that p63 has much higher specificity for the CGTG core sequence than p53 by using reporter analysis of mutated response elements and by DNA-protein binding assays. Our results clearly suggest that p53 and p63 can regulate different target genes by recognizing different but overlapping subsets of response elements. These differences likely represent a major reason for the functional differences between p53 and p63.
We found that TAp63γ induced high expression of many genes, and 17 out of 129 were already known to be direct target genes of p53, p63, and/or p73. Our screen for response elements was limited to the ∼1.5-kb region upstream of exon 1 in the induced genes; thus, some candidate target genes were likely missed by our analysis. Having identified and cloned five novel promoters which were activated by p63 and/or p53 in a partial survey, our microarray data likely contain many additional p63 direct target genes.
In addition to TAp63γ-inducible cells, we also analyzed TAp63α-, ΔNp63γ-, and ΔNp63α-inducible cell lines for target gene screening. Consistent with previous observations, TAp63α activated fewer genes and to a lesser degree of activation than TAp63γ, presumably due to its carboxy-terminal suppressive domain (44). Neither ΔNp63α nor ΔNp63γ activated any genes in this system (data not shown).
We also examined the transactivation abilities of TAp63β and ΔNp63β on more than 10 novel promoters that were activated by TAp63γ. TAp63β exhibited almost the same or relatively less transactivation ability than TAp63γ, and ΔNp63β showed less than 50% (mostly 10 to 20%) activity than that of TAp63γ (unpublished data). Thus, we chose to study the activity of TAp63γ most intensely, since it is the most potent inducer, and as such the genes activated by TAp63γ are most likely to represent the vast majority of p63 target genes.
In order to identify p63-specific promoters, we analyzed the promoters of 25 genes and found 5 that responded to p63 and/or p53. The cloned promoters of two genes, EVPL and SMARCD3, specifically responded to TAp63γ but not p53. We found that response elements with perfectly matched RRR and YYY stretches were similarly activated by p53 and TAp63γ, while addition of mismatches or gaps dramatically decreased transactivation activity. Remarkably, the CATG core sequence exhibited specific p53-induced transactivation activity in the presence of three mismatches in the RRR and YYY stretches and a 1-nucleotide gap between half sites. Consistent with our observations, Inga et al. previously reported that response elements containing CATG as their core binding sequence have the highest affinity for p53 binding based on their unique yeast-based assay system (18). On the other hand, we found that TAp63γ was uniquely able to activate response elements with a CGTG core sequence, three mismatches in the RRR and YYY stretches, and a 1-nucleotide gap between half sites. Based on an earlier p53-RE screen in yeast (51) and a recent interpretation (18), the frequency of the fifth position of p53-RE is 77.1% for A, 17.1% for T, and only 2.9% for G. Therefore, a G base in the fifth position is a unique feature of the p63-RE.
We constructed the reporter plasmids containing response elements used in Fig. 4 and 5 with a tandem repeat of the same core elements. However, actual binding elements consist of different combinations of core sequences. In the case of the SMARCD3 promoter, the 5′ half site of its response element has CGTG as it core and the 3′ half site has CAAG, with four mismatches in the RRR and YYY stretches. As shown in Fig. 4B, the CAAG core sequence with three flanking mismatches was more highly activated by TAp63γ than by p53. Thus, it is not surprising that the p63-RE in SMARCD3 was more strongly activated by TAp63γ. However, in the case of EVPL's 3′ p63-RE, EVPL-RE2, the 5′ half site contains CTGG as its core while the 3′ half site contains CATG. Interestingly, both CTGG and CATG were activated more by p53 than by TAp63γ (Fig. 4A and B). Indeed, upon closer examination, the mutant construct containing a direct repeat of EVPL-RE2 half sites (consisting of the 5′ half site of CTGG and the 3′ half site of CATG) was more strongly activated by p53 (unpublished data). However, by inserting 9 bases of an incomplete response element between the half sites, as found in the native EVPL-RE2, the response element exhibited specific activation by TAp63γ and not by p53. Thus, various combinations of half sites produce different p63- or p53-specific elements.
We found different binding affinities between p53 and TAp63γ to CGTG-containing sequences. The p53-RE is a palindrome sequence (RRRCWWGYYY), and p53 binds preferably to the CATG core sequence, suggesting that p53 binds more efficiently to structurally perfect bidirectional palindromes. On the other hand, p63 is able to bind to the nonpalindromic CGTG core sequence almost to the same extent as to CATG, suggesting that p63 is able to adaptively bind to both palindromic and nonpalindromic sequences. Klein et al. showed that the DNA binding domain of p63 itself could not bind to DNA without an oligomerization domain, as opposed to p53 (21). The authors speculated that this discrepancy may be due to weaker binding between p63 and p63 proteins at the DNA binding domain compared to p53-p53 binding (21). If this is the case, the p63 proteins may exhibit more binding flexibility because they are only bound to each other at the oligomerization domains. This potential difference in oligomeric binding could be one of the reasons why p53 preferably binds to palindromic sequences and p63 binds to both palindromic and nonpalindromic sequences. Crystallography analysis of p63 structure will aid in our understanding of the differential DNA binding affinities for p53 and p63.
Interestingly, our EMSA results did not perfectly match the results seen in the luciferase transactivation assay. As shown in Fig. 4 and 6, the direction of the response element greatly affects transactivation by p53 and TAp63γ but does not affect its binding ability. These results suggest that differential DNA-protein binding may not fully explain the mechanism of transactivation by p53 and TAp63γ and indicate that other factors, including the direction of the response element, likely contribute to transactivation ability. That being said, EMSA enabled us to identify the respective preferred binding sequences of p53 and TAp63γ.
Inactivation of the p63 gene in mice results in the lack of mature skin keratinocytes and defective mature epithelia and their derivatives in many other tissues, as well as other developmental abnormalities (29, 57). Our immunohistochemistry results demonstrated that EVPL was undetectable in the skin and pharynx of p63−/− mice. EVPL is a member of the plakin protein family. Plakin family members are components of hemidesmosomes, junctional complexes that contribute to the attachment of epithelial cells to the underlying basement membrane, in the epidermis (2). Genetic mutations of hemidesmosome components or autoimmunity to their components cause diseases manifested by dermo-epidermal separation, including bullous pemphigoid and epidermolysis bullosa (2). In our microarray results, we observed that two other members of the plakin family, BPAG1 and periplakin (PPL), were also induced by TAp63γ (Table 1). Recently we found that BPAG-1 was directly activated by p63 through a canonical p53-type response element which resides in the proximal region of the promoter (unpublished data). In addition to the plakin family, we also observed that BPAG2, a transmembrane component of the hemidesmosome that serves as a cell receptor connecting the cell interior to the extracellular matrix (13, 48), was induced by TAp63γ. Thus, the deficiency of mature epidermis in the p63−/− mouse could be due, at least in part, to dysregulation of hemidesmosome components in the skin.
In conclusion, our data demonstrate remarkable differences in the response elements transactivated by p53 and p63 and in their preferred binding sequences. These differential response element specificities for target gene activation may underlie, at least in part, the functional differences between various members of the p53 family.
REFERENCES
- 1.Amiel, J., G. Bougeard, C. Francannet, V. Raclin, A. Munnich, S. Lyonnet, and T. Frebourg. 2001. TP63 gene mutation in ADULT syndrome. Eur. J. Hum. Genet. 9:642-645. [DOI] [PubMed] [Google Scholar]
- 2.Borradori, L., and A. Sonnenberg. 1999. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Investig. Dermatol. 112:411-418. [DOI] [PubMed] [Google Scholar]
- 3.Celli, J., P. Duijf, B. C. Hamel, M. Bamshad, B. Kramer, A. P. Smits, R. Newbury-Ecob, R. C. Hennekam, G. Van Buggenhout, A. van Haeringen, C. G. Woods, A. J. van Essen, R. de Waal, G. Vriend, D. A. Haber, A. Yang, F. McKeon, H. G. Brunner, and H. van Bokhoven. 1999. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 99:143-153. [DOI] [PubMed] [Google Scholar]
- 4.Comer, K. A., P. A. Dennis, L. Armstrong, J. J. Catino, M. B. Kastan, and C. C. Kumar. 1998. Human smooth muscle α-actin gene is a transcriptional target of the p53 tumor suppressor protein. Oncogene 16:1299-1308. [DOI] [PubMed] [Google Scholar]
- 5.Contente, A., A. Dittmer, M. C. Koch, J. Roth, and M. Dobbelstein. 2002. A polymorphic microsatellite that mediates induction of PIG3 by p53. Nat. Genet. 30:315-320. [DOI] [PubMed] [Google Scholar]
- 6.Dohn, M., S. Zhang, and X. Chen. 2001. p63α and ΔNp63α can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 20:3193-3205. [DOI] [PubMed] [Google Scholar]
- 7.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 8.el-Deiry, W. S. 1998. Regulation of p53 downstream genes. Semin. Cancer Biol. 8:345-357. [DOI] [PubMed] [Google Scholar]
- 9.el-Deiry, W. S., S. E. Kern, J. A. Pietenpol, K. W. Kinzler, and B. Vogelstein. 1992. Definition of a consensus binding site for p53. Nat. Genet. 1:45-49. [DOI] [PubMed] [Google Scholar]
- 10.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 11.Ellisen, L. W., K. D. Ramsayer, C. M. Johannessen, A. Yang, H. Beppu, K. Minda, J. D. Oliner, F. McKeon, and D. A. Haber. 2002. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell 10:995-1005. [DOI] [PubMed] [Google Scholar]
- 12.Flores, E. R., K. Y. Tsai, D. Crowley, S. Sengupta, A. Yang, F. McKeon, and T. Jacks. 2002. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416:560-564. [DOI] [PubMed] [Google Scholar]
- 13.Fontao, L., B. Favre, S. Riou, D. Geerts, F. Jaunin, J. H. Saurat, K. J. Green, A. Sonnenberg, and L. Borradori. 2003. Interaction of the bullous pemphigoid antigen 1 (BP230) and desmoplakin with intermediate filaments is mediated by distinct sequences within their COOH terminus. Mol. Biol. Cell 14:1978-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghioni, P., F. Bolognese, P. H. Duijf, H. Van Bokhoven, R. Mantovani, and L. Guerrini. 2002. Complex transcriptional effects of p63 isoforms: identification of novel activation and repression domains. Mol. Cell. Biol. 22:8659-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermeking, H., C. Lengauer, K. Polyak, T. C. He, L. Zhang, S. Thiagalingam, K. W. Kinzler, and B. Vogelstein. 1997. 14-3-3 σ Is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1:3-11. [DOI] [PubMed] [Google Scholar]
- 16.Hwang, P. M., F. Bunz, J. Yu, C. Rago, T. A. Chan, M. P. Murphy, G. F. Kelso, R. A. Smith, K. W. Kinzler, and B. Vogelstein. 2001. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 7:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianakiev, P., M. W. Kilpatrick, I. Toudjarska, D. Basel, P. Beighton, and P. Tsipouras. 2000. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am. J. Hum. Genet. 67:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inga, A., F. Storici, T. A. Darden, and M. A. Resnick. 2002. Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence. Mol. Cell. Biol. 22:8612-8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juven, T., Y. Barak, A. Zauberman, D. L. George, and M. Oren. 1993. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene 8:3411-3416. [PubMed] [Google Scholar]
- 20.King, K. E., R. M. Ponnamperuma, T. Yamashita, T. Tokino, L. A. Lee, M. F. Young, and W. C. Weinberg. 2003. ΔNp63α functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene 22:3635-3644. [DOI] [PubMed] [Google Scholar]
- 21.Klein, C., G. Georges, K. P. Kunkele, R. Huber, R. A. Engh, and S. Hansen. 2001. High thermostability and lack of cooperative DNA binding distinguish the p63 core domain from the homologous tumor suppressor p53. J. Biol. Chem. 276:37390-37401. [DOI] [PubMed] [Google Scholar]
- 22.Kurata, S. I., T. Okuyama, M. Osada, T. Watanabe, Y. Tomimori, S. Sato, A. Iwai, T. Tsuji, Y. Ikawa, and I. Katoh. 2004. p51/p63 controls subunit α3 of the major epidermis integrin anchoring the stem cells to the niche. J. Biol. Chem. 279:50069-50077. [DOI] [PubMed] [Google Scholar]
- 23.Liu, G., and X. Chen. 2002. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene 21:7195-7204. [DOI] [PubMed] [Google Scholar]
- 24.Maatta, A., T. DiColandrea, K. Groot, and F. M. Watt. 2001. Gene targeting of envoplakin, a cytoskeletal linker protein and precursor of the epidermal cornified envelope. Mol. Cell. Biol. 21:7047-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maatta, A., C. Ruhrberg, and F. M. Watt. 2000. Structure and regulation of the envoplakin gene. J. Biol. Chem. 275:19857-19865. [DOI] [PubMed] [Google Scholar]
- 26.Malkin, D., F. P. Li, L. C. Strong, J. F. Fraumeni, Jr., C. E. Nelson, D. H. Kim, J. Kassel, M. A. Gryka, F. Z. Bischoff, M. A. Tainsky, et al. 1990. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250:1233-1238. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell, S. A., and G. E. Davis. 2000. Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc. Natl. Acad. Sci. USA 97:13009-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGrath, J. A., P. H. Duijf, V. Doetsch, A. D. Irvine, R. de Waal, K. R. Vanmolkot, V. Wessagowit, A. Kelly, D. J. Atherton, W. A. Griffiths, S. J. Orlow, A. van Haeringen, M. G. Ausems, A. Yang, F. McKeon, M. A. Bamshad, H. G. Brunner, B. C. Hamel, and H. van Bokhoven. 2001. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum. Mol. Genet. 10:221-229. [DOI] [PubMed] [Google Scholar]
- 29.Mills, A. A., B. Zheng, X. J. Wang, H. Vogel, D. R. Roop, and A. Bradley. 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398:708-713. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa, T., M. Takahashi, T. Ozaki, K. Watanabe Ki, S. Todo, H. Mizuguchi, T. Hayakawa, and A. Nakagawara. 2002. Autoinhibitory regulation of p73 by ΔNp73 to modulate cell survival and death through a p73-specific target element within the ΔNp73 promoter. Mol. Cell. Biol. 22:2575-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura, Y. 2004. Isolation of p53-target genes and their functional analysis. Cancer Sci. 95:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nylander, K., B. Vojtesek, R. Nenutil, B. Lindgren, G. Roos, W. Zhanxiang, B. Sjostrom, A. Dahlqvist, and P. J. Coates. 2002. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J. Pathol. 198:417-427. [DOI] [PubMed] [Google Scholar]
- 33.Ongusaha, P. P., J. I. Kim, L. Fang, T. W. Wong, G. D. Yancopoulos, S. A. Aaronson, and S. W. Lee. 2003. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 22:1289-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osada, M., R. Inaba, H. Shinohara, M. Hagiwara, M. Nakamura, and Y. Ikawa. 2001. Regulatory domain of protein stability of human P51/TAP63, a P53 homologue. Biochem. Biophys. Res. Commun. 283:1135-1141. [DOI] [PubMed] [Google Scholar]
- 35.Osada, M., M. Ohba, C. Kawahara, C. Ishioka, R. Kanamaru, I. Katoh, Y. Ikawa, Y. Nimura, A. Nakagawara, M. Obinata, and S. Ikawa. 1998. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat. Med. 4:839-843. [DOI] [PubMed] [Google Scholar]
- 36.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 37.Ruhrberg, C., M. A. Hajibagheri, M. Simon, T. P. Dooley, and F. M. Watt. 1996. Envoplakin, a novel precursor of the cornified envelope that has homology to desmoplakin. J. Cell Biol. 134:715-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakuma, S., H. Saya, M. Tada, M. Nakao, T. Fujiwara, J. A. Roth, Y. Sawamura, Y. Shinohe, and H. Abe. 1996. Receptor protein tyrosine kinase DDR is up-regulated by p53 protein. FEBS Lett. 398:165-169. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki, Y., S. Ishida, I. Morimoto, T. Yamashita, T. Kojima, C. Kihara, T. Tanaka, K. Imai, Y. Nakamura, and T. Tokino. 2002. The p53 family member genes are involved in the Notch signal pathway. J. Biol. Chem. 277:719-724. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki, Y., H. Mita, M. Toyota, S. Ishida, I. Morimoto, T. Yamashita, T. Tanaka, K. Imai, Y. Nakamura, and T. Tokino. 2003. Identification of the interleukin 4 receptor α gene as a direct target for p73. Cancer Res. 63:8145-8152. [PubMed] [Google Scholar]
- 41.Schmale, H., and C. Bamberger. 1997. A novel protein with strong homology to the tumor suppressor p53. Oncogene 15:1363-1367. [DOI] [PubMed] [Google Scholar]
- 42.Senoo, M., J. P. Manis, F. W. Alt, and F. McKeon. 2004. p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell 6:85-89. [DOI] [PubMed] [Google Scholar]
- 43.Senoo, M., N. Seki, M. Ohira, S. Sugano, M. Watanabe, S. Inuzuka, T. Okamoto, M. Tachibana, T. Tanaka, Y. Shinkai, and H. Kato. 1998. A second p53-related protein, p73L, with high homology to p73. Biochem. Biophys. Res. Commun. 248:603-607. [DOI] [PubMed] [Google Scholar]
- 44.Serber, Z., H. C. Lai, A. Yang, H. D. Ou, M. S. Sigal, A. E. Kelly, B. D. Darimont, P. H. Duijf, H. Van Bokhoven, F. McKeon, and V. Dotsch. 2002. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol. Cell. Biol. 22:8601-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada, A., S. Kato, K. Enjo, M. Osada, Y. Ikawa, K. Kohno, M. Obinata, R. Kanamaru, S. Ikawa, and C. Ishioka. 1999. The transcriptional activities of p53 and its homologue p51/p63: similarities and differences. Cancer Res. 59:2781-2786. [PubMed] [Google Scholar]
- 46.Shiraishi, K., S. Fukuda, T. Mori, K. Matsuda, T. Yamaguchi, C. Tanikawa, M. Ogawa, Y. Nakamura, and H. Arakawa. 2000. Identification of fractalkine, a CX3C-type chemokine, as a direct target of p53. Cancer Res. 60:3722-3726. [PubMed] [Google Scholar]
- 47.Simon, M., and H. Green. 1984. Participation of membrane-associated proteins in the formation of the cross-linked envelope of the keratinocyte. Cell 36:827-834. [DOI] [PubMed] [Google Scholar]
- 48.Stappenbeck, T. S., E. A. Bornslaeger, C. M. Corcoran, H. H. Luu, M. L. Virata, and K. J. Green. 1993. Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J. Cell Biol. 123:691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takei, Y., S. Ishikawa, T. Tokino, T. Muto, and Y. Nakamura. 1998. Isolation of a novel TP53 target gene from a colon cancer cell line carrying a highly regulated wild-type TP53 expression system. Genes Chromosomes Cancer 23:1-9. [PubMed] [Google Scholar]
- 50.Tan, M., C. W. Heizmann, K. Guan, B. W. Schafer, and Y. Sun. 1999. Transcriptional activation of the human S100A2 promoter by wild-type p53. FEBS Lett. 445:265-268. [DOI] [PubMed] [Google Scholar]
- 51.Tokino, T., S. Thiagalingam, W. S. el-Deiry, T. Waldman, K. W. Kinzler, and B. Vogelstein. 1994. p53 tagged sites from human genomic DNA. Hum. Mol. Genet. 3:1537-1542. [DOI] [PubMed] [Google Scholar]
- 52.Trink, B., K. Okami, L. Wu, V. Sriuranpong, J. Jen, and D. Sidransky. 1998. A new human p53 homologue. Nat. Med. 4:747-748. [DOI] [PubMed] [Google Scholar]
- 53.van Bokhoven, H., and H. G. Brunner. 2002. Splitting p63. Am. J. Hum. Genet. 71:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogelstein, B., and K. W. Kinzler. 1992. p53 function and dysfunction. Cell 70:523-526. [DOI] [PubMed] [Google Scholar]
- 55.Westfall, M. D., D. J. Mays, J. C. Sniezek, and J. A. Pietenpol. 2003. The ΔNp63α phosphoprotein binds the p21 and 14-3-3 σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol. Cell. Biol. 23:2264-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, A., M. Kaghad, Y. Wang, E. Gillett, M. D. Fleming, V. Dotsch, N. C. Andrews, D. Caput, and F. McKeon. 1998. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2:305-316. [DOI] [PubMed] [Google Scholar]
- 57.Yang, A., R. Schweitzer, D. Sun, M. Kaghad, N. Walker, R. T. Bronson, C. Tabin, A. Sharpe, D. Caput, C. Crum, and F. McKeon. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398:714-718. [DOI] [PubMed] [Google Scholar]
- 58.Yu, J., L. Zhang, P. M. Hwang, K. W. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673-682. [DOI] [PubMed] [Google Scholar]
- 59.Zheng, X., and X. Chen. 2001. Aquaporin 3, a glycerol and water transporter, is regulated by p73 of the p53 family. FEBS Lett. 489:4-7. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, K., J. Wang, J. Zhu, J. Jiang, J. Shou, and X. Chen. 1999. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene 18:7740-7747. [DOI] [PubMed] [Google Scholar]
- 61.Zou, Z., C. Gao, A. K. Nagaich, T. Connell, S. Saito, J. W. Moul, P. Seth, E. Appella, and S. Srivastava. 2000. p53 regulates the expression of the tumor suppressor gene maspin. J. Biol. Chem. 275:6051-6054. [DOI] [PubMed] [Google Scholar]