Abstract
The α6β4 integrin—a laminin-5 receptor—mediates assembly of hemidesmosomes and recruitment of Shc and phosphoinositide 3-kinase through the unique cytoplasmic extension of β4. Mice carrying a targeted deletion of the signaling domain of β4 develop normally and do not display signs of skin fragility. The epidermis of these mice contains well-structured hemidesmosomes and adheres stably to the basement membrane. However, it is hypoplastic due to reduced proliferation of basal keratinocytes and undergoes wound repair at a reduced rate. Keratinocytes from β4 mutant mice undergo extensive spreading but fail to proliferate and migrate in response to epidermal growth factor (EGF) on laminin-5. EGF causes significant phosphorylation of extracellular signal-regulated kinase (ERK) and Jun N-terminal protein kinase (JNK) and phosphorylation and degradation of IκB in β4 mutant cells adhering to laminin-5. Unexpectedly, however, ERK, JNK, and NF-κB remain in the cytoplasm in β4 mutant cells on laminin-5, whereas they enter effectively into the nucleus in the same cells on fibronectin or in wild-type cells on both matrix proteins. Inhibitor studies indicate that α6β4 promotes keratinocyte proliferation and migration through its effect on NF-κB and P-JNK. These findings provide evidence that β4 signaling promotes epidermal growth and wound healing through a previously unrecognized effect on nuclear translocation of NF-κB and mitogen-activated protein kinases.
The integrins mediate cell adhesion to the extracellular matrix and transmit mechanical and chemical signals to cells (13, 23). Integrin signaling imparts a stringent control to the action of receptor tyrosine kinases (RTKs), determining the nature and direction of the cell's response to growth factors and cytokines (14, 34). In spite of considerable amounts of cell biological data, genetic evidence of the significance of integrin signaling remains scarce. In particular, it has been difficult to separate the adhesive and signaling functions of individual integrins in any model system analyzed to date.
The α6β4 integrin is a laminin-5 receptor expressed in many epithelial cells, in Schwann cells, and in endothelial cells. Integrin α6β4 signaling proceeds through Src family kinase-mediated phosphorylation of the unique cytoplasmic domain of β4, recruitment of Shc, and activation of Ras (7, 12, 31) and phosphoinositide 3-kinase (PI-3K) (48, 49). Upon dephosphorylation, the β4 tail associates with the keratin cytoskeleton, causing assembly of hemidesmosomes and, hence, strengthening adhesion to basement membranes containing laminin-5 (7, 35, 51).
The pattern of expression of α6β4 in normal and hyperproliferative skin is consistent with a role for α6β4 signaling in the control of epithelial proliferation (11). We have shown that α6β4 promotes progression through G1 and entry in S phase in keratinocytes treated with epidermal growth factor (EGF) (30). In epidermal cells, α6β4 associates with the EGF receptor (EGF-R) and Ron RTKs (32, 44). Activation of these RTKs enhances phosphorylation of β4, causing disruption of hemidesmosomes and increased keratinocyte migration and proliferation (7, 32, 44). These results suggest that these RTKs decrease the ability of α6β4 to mediate stable adhesion but increase its signaling function.
Prior genetic studies have indicated that the β1 integrins participate in epidermal growth and repair. Whereas mice lacking α3β1 display defects in epidermal adhesion and assembly of the basement membrane (8, 21), conditional ablation of all β1 integrins results in profound proliferation defects (4, 40) and aberrant wound healing (15). Despite activating the wound-related αvβ6 integrin, β1-null keratinocytes do not migrate efficiently in vitro because of defective FAK-Src-mediated remodeling of their actin cytoskeleton (41). In addition, these cells lose expression of α6β4 (40). Finally, deletion of α3 impairs keratinocyte migration in vitro (6).
Mice carrying a targeted deletion of the entire cytoplasmic domain of β4 lack hemidesmosomes and die at birth due to extensive blistering of the skin and upper gastrointestinal tract (35), precluding a definitive investigation of skin homeostasis and repair. To analyze the role of α6β4 signaling in the absence of loss of adhesion strengthening, we have recently generated mice carrying a deletion of the C-terminal, signaling segment of the β4 tail (37). We report here that these mice have intact hemidesmosomes but display defective epidermal growth and wound healing. Through studies of primary keratinocytes derived from these mice, we provide evidence that α6β4 signaling controls epidermal growth and wound healing through a previously unrecognized effect on nuclear translocation of NF-κB and P-Jun N-terminal protein kinase (P-JNK).
MATERIALS AND METHODS
Cells, antibodies, and other reagents.
Primary keratinocytes from newborn mice were grown on collagen I in EMEM.06 with 8% Chelex-treated fetal bovine serum, 2 ng/ml EGF, and 0.06 mM CaCl2 (17). We purchased rat monoclonal antibody (MAb) to β4 (346-11A) from Pharmingen; rabbit antibodies to P-extracellular signal-regulated kinase (P-ERK), P-JNK, P-Akt (S473), IκBα, and P-IκBα (S32) from Cell Signaling; rabbit antibodies to ERK2, NF-κB p65 (C-20), green fluorescent protein (GFP) (FL), and histone H3; mouse MAbs to P-ERK (T203/Y204) and P-JNK (T183/Y185); and goat antibodies to Akt from Santa Cruz; MAbs to Rac, paxillin, and Rho GDI from BD Biosciences; MAb to vinculin (hVIN-1) and rhodamine-phalloidin from Sigma; MAb to NF-κB p65 (clone 2A12A7) and sheep antibodies to JNK1 from Zymed; and fluorescein isothiocyanate (FITC)- and Cy3-conjugated affinity-purified secondary antibodies from Jackson Laboratories. The rabbit anti-α3 cyto antibody was a gift from G. Tarone. Affinity-purified rabbit antibodies to the N terminus of bullous pemphigoid antigen 2 (BPAG-2) and the LE4-6 segment of mouse laminin γ2 and MAb 121 to HD-1/plectin were previously described (18, 35, 45). Laminin-5 matrices were prepared as described previously (50). Human fibronectin and rat tail collagen I were from Sigma, and PD98059, U0126, JNK II, BAY11-7082, Y-27632, and LY294002 were from Calbiochem.
Biochemical assays.
After growth factor deprivation, keratinocytes were detached, plated on laminin-5- or anti-β4-coated plates for the indicated times, and then treated with 10 ng/ml EGF for 15 min. Cells were lysed in 50 mM HEPES, pH 7.4, 5 mM EDTA, 2 mM EGTA, 150 mM NaCl, 10% glycerol, and 1% NP-40 with protease and phosphatase inhibitors and subjected to immunoblotting or biphenylylphenyloxadiazole (PBD) pull-down assay as described previously (33). Nuclear and cytoplasmic fractions were prepared with the NE-PER kit (Pierce).
Cell proliferation, survival, and migration.
For cell proliferation, keratinocytes were synchronized in G0 by growth factor deprivation and plated on laminin-5 or fibronectin in the presence of 2 ng/ml EGF and 10 μM bromodeoxyuridine (BrdU) for 18 h. For in vivo labeling, mice were injected intravenously with 5 μM BrdU/100 g of body weight and sacrificed 1 h later. Keratinocytes and paraffin-embedded skin sections were subjected to immunofluorescent or immunohistochemical staining with anti-BrdU antibodies, using the BrdU Labeling and Detection Kit I (Roche). Retroviral infection of keratinocytes with pFB-Neo/IκB-2A or PINCO/GFP was as previously described (25). For matrix-dependent survival, keratinocytes were deprived of growth factors for 18 h, detached, and replated on laminin-5 or fibronectin in medium lacking serum and EGF. Apoptotic cell death was monitored at 4 and 18 h by using the In Situ Cell Death Detection kit (Roche). To estimate cell death in vivo, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed on paraffin-embedded skin sections. For in vitro wound assay, keratinocytes were grown until confluent on laminin-5-coated plates and deprived of growth factors for 18 h. Monolayers were scratched with a P200 tip and incubated in the presence of serum and 2 ng/ml EGF for 18 h. Wound closure was monitored by digital photography.
Immunohistochemistry, electron microscopy (EM), and immunogold labeling.
For nuclear translocation, primary keratinocytes were deprived of growth factors for 18 h, detached, plated on laminin-5-coated glass coverslips, and stimulated with 10 ng/ml EGF for 15 min. After fixation with 3.7% paraformaldehyde, they were permeabilized with 0.2% Triton X-100 and stained with the indicated antibodies. For immunofluorescent detection of hemidesmosome-like adhesions, keratinocytes were extracted with 0.2% Triton X-100 and then fixed with cold methanol as described previously (7). For immunohistochemical analysis, tissues and plugs were embedded in paraffin or snap-frozen in OCT compound (Tissue-Tek). For electron microscopy, skin samples were collected, fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, postfixed with 1% osmium tetroxide, stained en bloc with 1% uranyl-acetate, and embedded in Polybed 812. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Zeiss EM 902 or a Philips CM10 electron microscope. Immunogold labeling was performed on ultrathin cryosections as previously described (29). Preliminary experiments were conducted to identify the optimal concentration of antibodies for immunolabeling. In order to obtain highly specific labeling, we used the lowest concentration of antibodies able to give a signal (labeling of compartments known to associate with the antigen)-to-noise (labeling of compartments known not to associate with the given antigen) ratio of >5.
Wound repair.
Adult mice were anesthetized, shaved, and disinfected with 70% ethanol. Two full-thickness excisional wounds, 2 mm in diameter, were generated on either side of the dorsal midline of each mouse with a disposable biopsy punch tool (Premier, Plymouth Meeting, PA). Wounds were left uncovered and photographed daily. The wound surface remaining open at each time point was measured by using the NIH Image software. For histological analysis, complete wounds including 5 mm of adjacent normal skin were excised at 3, 4, or 5 days after injury. Cryosections across the middle of the wounds were stained with hematoxylin and eosin or the indicated antibodies.
RESULTS
The β4 substrate domain is not necessary for assembly of hemidesmosomes and epidermal adhesion in vivo.
We have recently reported that mice carrying a specific deletion of the C-terminal portion of β4 tail (henceforth termed “substrate domain,” Fig. 1A) are viable and fertile but display defective postnatal angiogenesis. Experiments on cells from these mice have provided evidence that deletion of the β4 substrate domain impairs α6β4 signaling but not adhesion to laminin-5 (37). Although the N-terminal part of the β4 tail, upstream of amino acid 1355, is sufficient for interaction with the plakin HD-1/plectin and hence for association with the keratin cytoskeleton, prior studies have suggested that the C-terminal portion of β4 tail contributes to the assembly of hemidesmosome-like adhesions in vitro by promoting recruitment of BPAG-2 and, hence, BPAG-1 (46). We thus felt it necessary to specifically examine the effect of the β4 mutation on epidermal adhesion in vivo.
FIG. 1.
Deletion of the β4 substrate domain does not affect assembly of hemidesmosomes and epidermal adhesion. (A) Structure of the wild-type β4 protein (β4 WT) and its mutant form (β4 1355T) encoded by the targeted allele. Ovals, fibronectin type III repeats; stars, tyrosinephosphorylation sites. (B) Skin sections from wild-type and mutant (1355T) mice were stained with hematoxylin and eosin (H&E), MAb 346-11A to the extracellular domain of β4, rabbit antiserum to laminin-5, MAb to HD-1, or rabbit antiserum to BPAG-2. (C) Transmission EM of the dermal-epidermal junction of wild-type and mutant skin. Bars, 0.489 μm (wild type) and 0.436 μm (1355T) (top). Immunogold labeling of BPAG-2 on ultrathin cryosections from newborn skins. Arrowheads indicate gold particles incorporated in hemidesmosomes. Bars, 0.219 μm (wild type) and 0.255 μm (1355T) (bottom). (D) Morphometric analysis of the hemidesmosomes of wild-type and mutant mice. The number of hemidesmosomes per cell profile was derived from the photographic reconstruction of 10 cell profiles per group. Morphometric data represent the means (± standard deviations) of values obtained from the analysis of 80 hemidesmosomes per group. DBL, distance from basal lamina; HBL, thickness of basal lamina. (E) Primary keratinocytes from wild-type and mutant mice were plated on laminin-5 matrix, extracted with 0.2% Triton X-100, and subjected to double staining with anti-β4 (green) and anti-HD-1 (red) (top) or anti-β4 (green) and anti-BPAG-2 (red) (bottom). WT, wild type.
Histological analysis indicated that the skin of newborn mutant mice does not display any gross abnormality, except for a slightly decreased thickness (Fig. 1B). The epidermis was tightly attached to the basement membrane and the underlying dermis. No areas of discontinuity were observed at the basement membrane junction, in accordance with the absence of skin fragility in mutant mice. In addition, an examination of skin sections from the back, ears, and tails of mutant mice of different ages up to 12 months failed to reveal any blistering. Immunofluorescent staining provided evidence that laminin-5, the mutant integrin, and the hemidesmosomal components BPAG-2 and HD-1/plectin are all regularly concentrated along the dermal-epidermal junction in mutant mice (Fig. 1B). In addition, we did not detect changes in the level of expression of fillagrin and loricrin in the granular layer and stratum corneum, respectively, in mutant mice (not shown). These results suggest that deletion of the β4 substrate domain does not affect epidermal adhesion and differentiation in vivo.
To examine the assembly of hemidesmosomes in mutant mice, we used EM. Transmission EM showed that the skin of mutant mice contains well-structured hemidesmosomes (Fig. 1C), and morphometric analysis confirmed that they are similar in number and appearance to those of wild-type skin (Fig. 1D). Furthermore, cryo-immuno-EM demonstrated that BPAG-2 is regularly incorporated in the hemidesmosomes of mutant mice (Fig. 1C). Taken together, these results provide direct evidence that the N-terminal portion of β4 tail (to amino acid 1355) is sufficient for association with the keratin cytoskeleton and assembly of adhesion-competent hemidesmosomes in vivo.
Immunofluorescence experiments were employed to examine the ability of keratinocytes from mutant mice to form hemidesmosome-like structures in vitro. As shown in Fig. 1E, the mutant keratinocytes assemble in culture hemidesmosome-like structures containing HD-1/plectin but devoid of BPAG-2. These results are in agreement with the observation that the C-terminal portion of β4 tail contributes to the recruitment of BPAG-2 and BPAG-1 to hemidesmosome-like adhesions in vitro (46).
How do we explain the apparent discrepancy between the EM results in vivo and those of immunofluorescent staining in vitro? It is possible that under physiological conditions BPAG-2 is recruited to hemidesmosomes through its binding to a basement membrane component of dermal origin. Unable to synthesize this component, cultured keratinocytes would be dependent on the C-terminal segment of β4 tail to recruit BPAG-2, and hence BPAG-1, to hemidesmosome-like adhesions. In addition, or instead, the association of BPAG-2 with the integrin α6 subunit (22) and/or with plectin (27) may be sufficient for assembly of hemidesmosomes in vivo, because this process is intrinsically more robust under these conditions than it is in vitro. Irrespective of the underlying mechanism, these findings indicate that the molecular requirements for hemidesmosome assembly are best studied in their physiological context, and deletion of the β4 signaling domain does not affect assembly of hemidesmosomes in this context.
Integrin α6β4 signaling promotes epidermal growth and repair.
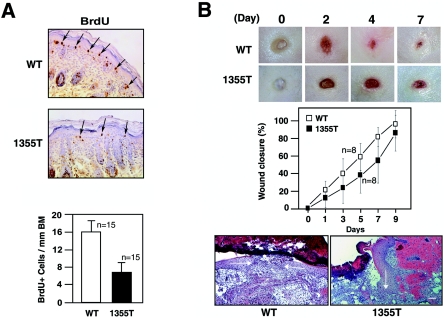
To examine epidermal proliferation in vivo, newborn mice were “pulse” labeled with BrdU for 30 min and sections of their skins were subjected to anti-BrdU staining. As shown in Fig. 2A, the skin of mutant mice contains significantly fewer BrdU-positive basal keratinocytes per linear millimeter of basement membrane (6.7 ± 2) than does that of wild-type mice (16 ± 2.5) (P < 0.01 by Student's t test). In addition, staining with MAb Ki-67, which binds to a nuclear protein expressed predominantly during the S, G2, and M phases of the cell cycle, showed that the skin of mutant mice contains fewer proliferating elements per linear millimeter of basement membrane (45.8 ± 5.42) than does that of control mice (66.2 ± 9.39) (P < 0.01). Similar results were obtained by examining the skin of adult mice (not shown). Although we had previously observed that E18.5 embryos carrying a deletion of the entire cytoplasmic domain of β4 display a defect in epidermal proliferation (35), a subsequent study had failed to detect impaired epidermal proliferation in E16.5 embryos lacking whole β4 (9). Since epidermal proliferation is not robust at E16.5, it is likely that other integrins compensate for loss of α6β4 at this embryonic stage. Taken together, our results indicate that α6β4 signaling is required for optimal proliferation of keratinocytes in newborn and adult epidermis.
FIG. 2.
The β4 substrate domain promotes epidermal growth and wound healing. (A) Wild-type and mutant (1355T) mice were injected with BrdU prior to euthanasia. Paraffin-embedded sections of their skins were subjected to immunoperoxidase staining with anti-BrdU MAb and counterstaining with hematoxylin (top). BrdU-positive cells are indicated by arrows. The graph shows the mean number of BrdU-positive cells (± standard deviation) per linear millimeter of basement membrane (BM; bottom), obtained from the analysis of 15 mice per group (n = 15). (B) Full-thickness excisional wounds were monitored by digital photography at the indicated times (top). The percentage of wound closure was estimated from the area of the wound remaining open at each time point and plotted against time. The graph shows the wound closure values (± standard deviations) obtained from the analysis of eight mice per group (n = 8) (middle). Cross sections from a 4-day-old wound from a wild-type mouse and a 5-day-old wound from a mutant mouse were stained with hematoxylin and eosin (bottom). Arrows indicate the position and direction of the advancing epithelia. Bar, 100 μm. WT, wild type.
The potential role of α6β4 signaling in epidermal survival in vivo was examined by TUNEL analysis. The results showed that the skin of wild-type and mutant mice contains a similarly small number of TUNEL-positive keratinocytes (not shown). This observation indicates that the β4 substrate domain is not necessary for keratinocyte survival in normal skin, and it suggests that the effect of inhibition of β4 signaling on epidermal growth is due to reduced cell proliferation.
To study the effect of the β4 mutation on wound healing, we generated full-thickness excisional wounds in wild-type and mutant mice and monitored wound closure over time. As shown in Fig. 2B, deletion of the β4 substrate domain decreased the rate of wound repair, while delaying final closure to a lesser degree. Histological analysis indicated that the two opposing epithelial margins had reached the midline and fused at 5 days after wounding in wild-type mice. By contrast, the advancing epithelium was still penetrating the space between the clot and the granulation tissue at the same time in mutant mice (Fig. 2B). Immunohistochemical staining showed that α6β4 is not expressed in blood vessels in the wound bed or in the fibroblasts and monocytes/macrophages of granulation tissue, suggesting that the delay in wound healing in mutant mice is not due to a defect in vascularization or function of granulation tissue (not shown). In accordance with the observation that leading-edge keratinocytes are not actively proliferating (24), we detected very few Ki-67-positive cells at the leading edge of both wild-type and mutant wounds (not shown). These observations suggest that β4 signaling promotes extension of the epidermal leading edge during wound healing through an effect on keratinocyte migration.
The β4 substrate domain opposes spreading but promotes cell migration, proliferation, and resistance to apoptosis in primary keratinocytes.
Prior studies have shown that α6β4 inhibits the ability of the α3β1 integrin to promote spreading and assembly of focal adhesions (36). Accordingly, α3β1 relocates from cell-to-cell adhesions to focal adhesions in β4-null keratinocytes plated on laminin-5 (36). Upon plating on laminin-5 in serum-free conditions, the mutant keratinocytes spread more extensively than wild-type controls (Fig. 3A). This was not a transient effect, as measurement of cell surface areas over a 2-hour time course indicated that the mutant cells spread significantly more—and increasingly better—than wild-type cells at each of the time points examined (Fig. 3B). Furthermore, indirect immunofluorescent staining showed that the mutant cells form focal adhesions and stress fibers more robustly than control cells. Finally, α3β1 localized within structures resembling focal adhesions in mutant keratinocytes but not in control cells (Fig. 3C). These results suggest that the C-terminal signaling domain of β4 tail opposes spreading and mediates the transdominant-negative effect that α6β4 exerts on α3β1.
FIG. 3.
The β4 substrate domain opposes α3β1-mediated spreading and assembly of actin filaments. (A) Wild-type (WT) and mutant (1355T) keratinocytes were plated on laminin-5 matrix and examined by phase-contrast microscopy. Representative images acquired at 30 min after plating are shown. Bar, 10 μm. (B) Quantitation of cell area during spreading of wild-type (WT) and mutant (1355T) keratinocytes upon plating on laminin-5 matrix for up to 2 h. Total cell area was calculated from the digital images. Data represent the means (± standard deviations) of 50 randomly chosen cells from three different experiments. (C) Wild-type (WT) and mutant (1355T) keratinocytes plated as above were subjected to double staining with FITC-conjugated paxillin and rhodamine-conjugated phalloidin. Bar, 5 μm. (D) Wild-type (WT) and mutant (1355T) keratinocytes plated as above were subjected to double staining with anti-α3 cyto rabbit antiserum and FITC-conjugated paxillin followed by Cy3-conjugated anti-rabbit antibody. Bar, 10 μm.
To examine the contribution of α6β4 signaling to cell migration, wild-type and mutant keratinocytes were plated on laminin-5 at confluent density and subjected to in vitro wound assay. As shown in Fig. 4A, treatment with serum and EGF stimulated wound closure by normal keratinocytes, but it exerted a very modest effect on mutant keratinocytes. Treatment with the Rho kinase inhibitor Y-27632 inhibited assembly of focal adhesions and stress fibers in mutant keratinocytes, but it did not increase the ability of β4 mutant keratinocytes to migrate in vitro, suggesting that their migratory defect is not due to increased cytoskeletal tension (not shown). In addition, the mutant keratinocytes scattered and extended lamellipodia at the onset of migration even more efficiently than did control cells, suggesting that their migratory defect is not due to defective actin cytoskeleton dynamics (not shown). We conclude that deletion of the β4 signaling domain causes a severe cell migration defect in spite of increased α3β1-dependent cell spreading. These observations suggest that α6β4 activates promigratory signals able to overcome the partial suppression of α3β1-dependent spreading.
FIG. 4.
The β4 substrate domain promotes keratinocyte migration, proliferation, and resistance to apoptosis. (A) Wild-type and mutant (1355T) keratinocytes were plated on laminin-5 and subjected to wound closure assay in the presence of serum and EGF (top). The graph shows the mean percentage of wound closure (± standard deviation) after 18 h from three different experiments (bottom). (B) Wild-type and mutant keratinocytes were synchronized in G0, plated on laminin-5 in the presence of EGF for 18 h, and subjected to double staining with anti-BrdU and 4′,6′-diamidino-2-phenylindole (DAPI) (top). The graph shows the mean percentage of BrdU+ cells (± standard deviation) from three experiments (bottom). (C) Wild-type and mutant keratinocytes were deprived of mitogens, plated on laminin-5 in the absence of growth factors for 18 h, and subjected to TUNEL and DAPI staining (top). The graph shows the mean percentage of apoptotic cells (± standard deviation) from three experiments with cells plated for the indicated times on either laminin-5 (Ln-5) or fibronectin (Fn) (bottom). WT, wild type.
To assess the role of α6β4 signaling in keratinocyte proliferation, wild-type and mutant keratinocytes were synchronized in G0, plated on laminin-5 in the presence of EGF, and subjected to BrdU incorporation assay. As shown in Fig. 4B, the mutant keratinocytes progressed through the cell cycle on laminin-5 much less efficiently than did wild-type controls. However, upon plating on collagen I in the presence of serum-derived fibronectin, they proliferated as effectively as wild-type controls (not shown). These observations indicate that the β4 substrate domain activates signals necessary for keratinocyte proliferation on laminin-5 but not other substrates.
To examine the role of α6β4 signaling in keratinocyte survival in vitro, wild-type and mutant keratinocytes were plated on laminin-5 or fibronectin, deprived of growth factors, and subjected to TUNEL analysis. As shown in Fig. 4C, the mutant keratinocytes survived growth factor deprivation on fibronectin well but underwent massive apoptosis on laminin-5 at 18 h. By contrast, only a minor fraction of wild-type cells underwent apoptosis on both substrates at 18 h of growth factor deprivation. These results indicate that the β4 substrate domain promotes activation of antiapoptotic pathways. When cultured in the presence of growth factors, the mutant keratinocytes did not display signs of apoptosis (not shown), suggesting that mitogens and possibly other survival factors can compensate for the loss of α6β4 survival signaling in vivo. Taken together, these observations indicate that the β4 substrate domain activates signaling pathways necessary for keratinocyte migration, proliferation, and resistance to stress-induced apoptosis.
The β4 substrate domain does not contribute to EGF-dependent cytoplasmic activation of ERK, phosphorylation of JNK, and degradation of IκB.
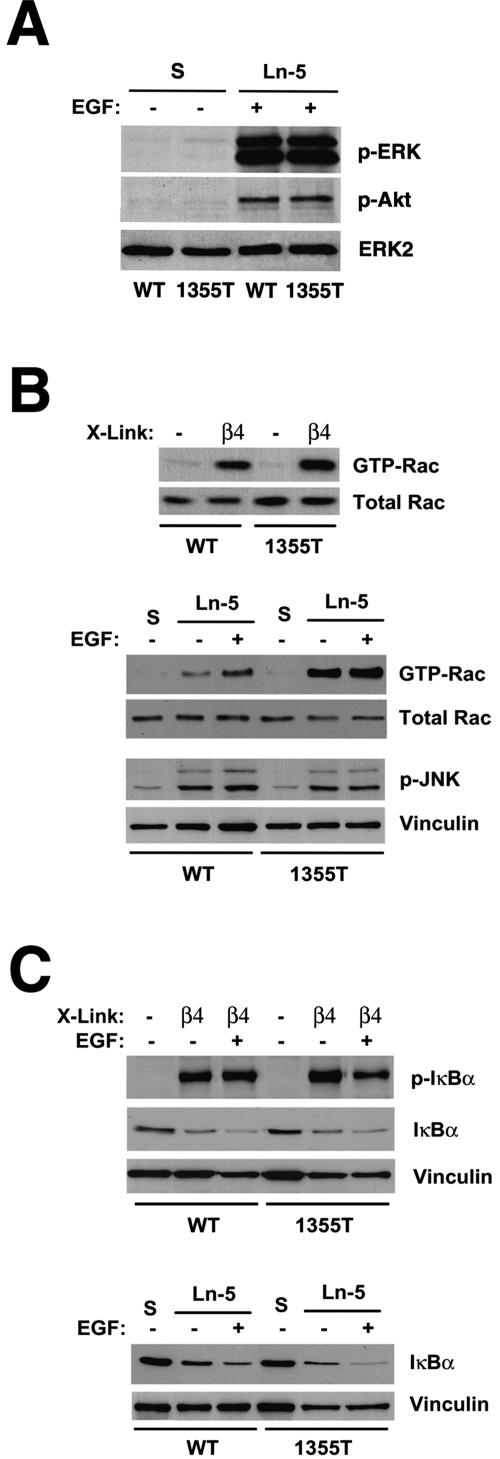
To gain insight into the mechanism by which the β4 substrate domain promotes cell migration, proliferation, and survival, we monitored the activation of various signaling pathways in wild-type and mutant keratinocytes plated on laminin-5. Adhesion to laminin-5 induced activation of ERK and Akt in wild-type but not in mutant keratinocytes (37). However, EGF stimulation caused a similarly high activation of ERK and Akt in wild-type and mutant keratinocytes adhering to laminin-5, suggesting that the β4 substrate domain is not required for EGF-R-mediated activation of ERK and PI-3K on this substrate (Fig. 5A). Together, these observations indicate that the β4 substrate domain is required for laminin-5- but not EGF-R-dependent activation of ERK and PI-3K.
FIG. 5.
The β4 substrate domain is not required for EGF-dependent phosphorylation of ERK and Akt, activation of Rac and JNK, and phosphorylation of IκB. (A) Wild-type and mutant (1355T) keratinocytes were deprived of growth factors, detached, and either kept in suspension (S) or plated on laminin-5 for 30 min and then stimulated with EGF for 10 min. Equal amounts of total proteins were probed with antibodies to activated ERK and Akt or total ERK2. (B) Growth factor-starved keratinocytes were either kept in suspension (−) or plated on dishes coated with anti-β4 (β4) for 2 h and then subjected to GST-PAK-PBD pull-down assay. Levels of active and total Rac were determined by immunoblotting with anti-Rac (top). Cells were either kept in suspension or plated on laminin-5 for 2 h with or without EGF and then subjected either to GST-PAK-PBD pull-down assay or to immunoblotting with antibodies to activated JNK or vinculin as control(bottom). (C) Growth factor-starved keratinocytes were either kept in suspension (−) or plated on dishes coated with anti-β4 (β4) for 30 min (top) or laminin-5 for 2 h (bottom) with or without EGF and then probed by immunoblotting with antibodies to phosphorylated IκBα, total IκBα, or vinculin. WT, wild type; Ln-5, laminin-5.
Glutathione S-transferase (GST)-PAK pull-down assays revealed that antibody-mediated ligation of mutant α6β4 activates Rac as efficiently as does ligation of wild-type α6β4 (Fig. 5B). Thus, although the β4 substrate domain is necessary for PI-3K-to-Akt signaling (48), it does not contribute to activation of Rac. Furthermore, addition of EGF did not enhance α6β4-mediated activation of Rac, suggesting that EGF-R signaling is not necessary for α6β4-mediated activation of Rac in keratinocytes (Fig. 5B). Interestingly, adhesion to laminin-5 activated Rac in mutant keratinocytes to a higher extent than in wild-type controls (Fig. 5B). Since α3β1 activates Rac (6, 43), this observation is consistent with the hypothesis that the β4 substrate domain mediates transdominant-negative inhibition of α3β1. In the absence of this effect, α3β1 activates Rac to a higher extent (Fig. 5B), promoting more efficient spreading on laminin-5 (Fig. 3A and B). Finally, we compared activation of JNK in wild-type and mutant keratinocytes plated on laminin-5 in the presence or absence of EGF. The results confirmed that α6β4 activates JNK (30) but excluded a role for the β4 substrate domain and the EGF-R in this process (Fig. 4B). This observation is consistent with the hypothesis that α6β4 activates JNK through Rac (30).
We next compared NF-κB signaling in wild-type and mutant keratinocytes. As shown in Fig. 5C, ligation of wild-type α6β4 was sufficient to induce phosphorylation and degradation of IκBα, which functions to retain NF-κB in the cytoplasm. This process was unimpaired in mutant keratinocytes and was not augmented by EGF (Fig. 5C). In addition, IκBα was degraded efficiently in both wild-type and mutant keratinocytes plated on laminin-5 (Fig. 5C). These observations suggest that α6β4 is able to induce the first step of NF-κB signaling, the degradation of IκB, and this process does not require the β4 substrate domain or EGF-R signaling, as observed for the activation of Rac and JNK.
Together, these results indicate that the β4 substrate domain does not contribute to activation of Rac, phosphorylation of JNK, and degradation of IκB. Therefore, the proliferative and migratory defects of mutant keratinocytes are not caused by defects in these processes.
The β4 substrate domain promotes nuclear translocation of P-ERK, P-JNK, and NF-κB.
To further examine the mechanism by which the β4 substrate domain controls cell proliferation and migration, we monitored EGF-induced nuclear translocation of P-ERK in wild-type and mutant keratinocytes plated on laminin-5. In accordance with the observation that EGF activates ERK to the same extent in wild-type and mutant keratinocytes (Fig. 5A), immunofluorescence documented a similar increase in anti-P-ERK staining in both wild-type and mutant cells plated on laminin-5 in the presence of EGF (Fig. 6A). However, whereas EGF induced entry of P-ERK into the nucleus of the majority of wild-type cells adhering to laminin-5, it did not exert this effect in mutant keratinocytes, implying that the β4 substrate domain controls EGF-induced nuclear translocation of P-ERK (Fig. 6A). This effect was specific because adhesion to fibronectin, which is mediated by the α5β1 integrin, rescued EGF-mediated nuclear translocation of P-ERK in mutant keratinocytes (not shown). Furthermore, the β4 substrate domain did not affect nuclear translocation of Akt in cells treated with EGF (Fig. 6A). To confirm this result, we performed biochemical fractionation experiments. As shown in Fig. 6B, EGF stimulation induced accumulation of ERK in the nuclear fraction of wild-type but not mutant keratinocytes plated on laminin-5. By contrast, it promoted accumulation of Akt in the nuclear fraction of both wild-type and mutant keratinocytes (Fig. 6B). These results indicate that signaling by the β4 substrate domain promotes nuclear translocation of P-ERK, but not P-Akt.
FIG. 6.
The β4 substrate domain controls nuclear translocation of p-ERK, p-JNK, and NF-κB. (A) Serum-starved wild-type and mutant (1355T) keratinocytes were plated on laminin-5 for 45 min and stimulated or not with EGF for 15 min. After fixation, the cells were stained with antibodies to P-ERK, p-Akt, P-JNK, and the p65 subunit of NF-κB and examined by confocal microscopy. The percentage of cells that had more intense staining in the nucleus than in the cytoplasm was estimated after counting of at least 1,000 cells per coverslip. The graphs show the results (± standard deviations) from three independent experiments. Bar, 10 μm. (B) Growth factor-starved keratinocytes treated as above were subjected to subcellular fractionation. Equal amounts of proteins from the cytoplasmic (C) and nuclear (N) fractions were probed with antibodies to the indicated proteins. (C) Serum-starved wild-type and mutant (1355T) keratinocytes were plated on laminin-5 matrix and subjected to wound closure assay in the presence of serum and EGF (10 ng/ml) for 2 h. They were then fixed and stained with anti-p65 antibodies and examined by confocal microscopy. Cells at the leading edge with nuclear (in wild-type cells) or cytoplasmic (in 1355T cells) NF-κB are shown by arrows. The graph shows the percentage of leading-edge cells (± standard deviation) with nuclear NF-κB. Bar, 15 μm. (D) Cross sections of wild-type and mutant (1355T) wounds shown in Fig. 2B were stained with anti-p65 antibodies and examined by confocal microscopy. Dashed white lines demarcate the leading edge of the epithelia advancing between the escar and granulation tissue in both cases. Bar, 25 μm. WT, wild type.
We next monitored nuclear translocation of the p65 subunit of NF-κB and of P-JNK. Immunofluorescent staining revealed that EGF promotes nuclear translocation of p65 and P-JNK in the large majority of wild-type keratinocytes plated on laminin-5, but it exerts this effect in only a minor fraction of mutant cells under the same conditions (Fig. 6A). Biochemical fractionation confirmed that EGF induces accumulation of JNK and p65 in the nuclear fraction of wild-type but not mutant keratinocytes plated on laminin-5 (Fig. 6B). Collectively, these results indicate that β4 signaling is necessary to induce nuclear translocation of P-ERK, P-JNK, and NF-κB but not P-Akt in cells exposed to EGF.
To examine the potential relevance of this signaling mechanism during cell migration, we examined nuclear translocation of NF-κB during keratinocyte wound closure in vitro. Wild-type and mutant keratinocytes were plated on laminin-5 at confluency and subjected to in vitro wound assay. Two hours after EGF stimulation, the cells were fixed and stained with antibodies to the p65 subunit of NF-κB. As shown in Fig. 6C, the wild-type keratinocytes located at the wound edge, but not those behind them, displayed significant nuclear accumulation of NF-κB. In contrast, the mutant keratinocytes at the wound edge did not show significant nuclear accumulation of NF-κB. These results suggest that the β4 substrate domain controls nuclear translocation of NF-κB at the onset of keratinocyte migration in vitro.
To evaluate the physiological significance of these observations, cross sections of full-thickness excisional wounds from wild-type and mutant mice were stained with anti-p65 antibodies. As shown in Fig. 6D, NF-κB accumulated in the nucleus of a significant fraction of keratinocytes at the leading edge of the advancing epithelium in wild-type wounds. By contrast, it did so in only a minor fraction of leading-edge keratinocytes in mutant wounds. These results suggest that the β4 substrate domain promotes epidermal migration in vivo by inducing nuclear translocation of NF-κB in leading-edge keratinocytes.
NF-κB and JNK promote keratinocyte proliferation and migration, and PI-3K protects them from apoptosis on laminin-5.
To identify the mechanism by which the β4 substrate domain controls keratinocyte proliferation, we plated wild-type keratinocytes on laminin-5 in the presence of specific inhibitors and used BrdU incorporation to examine their ability to progress through the cell cycle in response to EGF. As shown in Fig. 7A, the NF-κB inhibitor BAY11-7082 suppressed keratinocyte cell cycle progression, whereas the PI-3K inhibitor LY294002 and the JNK inhibitor JNK II exerted a partial effect. Notably, the MEK inhibitors PD98059 and U0126 reduced keratinocyte proliferation by a modest extent. Transduction of a retrovirus encoding the dominant-negative mutant IκB-2A, which suppresses NF-κB signaling, confirmed the role of NF-κB in keratinocyte proliferation (Fig. 7B). These results suggest that NF-κB, and to a smaller extent PI-3K and JNK, is required for keratinocyte proliferation on laminin-5. Since the EGF-R efficiently activates PI-3K but is unable to promote nuclear translocation of NF-κB and P-JNK in mutant keratinocytes plated on laminin-5 (Fig. 6), these results imply that the β4 substrate domain controls keratinocyte proliferation by promoting nuclear translocation of NF-κB and P-JNK.
FIG. 7.
NF-κB or JNK is required for keratinocyte proliferation and migration on laminin-5, whereas PI-3K mediates keratinocyte survival upon EGF withdrawal. (A) Wild-type keratinocytes were plated on laminin-5 matrix in the absence (Ctrl) or presence of inhibitors of NF-κB (12.5 μM BAY11-7082), PI-3K (10 μM LY294002), JNK (20 μM JNK II), or MEK (50 μM PD98059 or 50 μM U0126). Entry in S phase and wound closure were in response to EGF, whereas survival was in the absence of growth factors, as in Fig. 3. The graphs show the mean percentages (+ standard deviations) of BrdU-positive cells (top), wound closure (middle), or TUNEL+ cells (bottom). (B) Retroviral transduction of the IκBα superrepressor IκB-2A inhibits keratinocyte proliferation on laminin-5. Primary keratinocytes from wild-type mice were infected with viruses encoding GFP or hemagglutinin (HA)-tagged IκB-2A. The cells were synchronized in G0 and plated on laminin-5 in the presence of EGF and BrdU for 18 h. They were then subjected to double labeling with anti-BrdU (green) and anti-GFP or anti-HA (red), respectively. The graph shows the mean percentage (+ standard deviation) of BrdU-positive transduced cells per infection. At least 250 transduced cells per coverslip were counted.
To examine the mechanism by which the β4 substrate domain regulates keratinocyte migration, we examined the effect of inhibitors on keratinocyte wound closure in vitro. As shown in Fig. 7A, BAY11-7082 and, to a smaller extent, JNK II suppressed EGF-stimulated keratinocyte migration, whereas PD98059, U0126, and LY294002 did not exert a significant effect, indicating that EGF-mediated wound closure requires NF-κB and JNK. This result suggests that the β4 substrate domain promotes both cell proliferation and cell migration by promoting nuclear translocation of NF-κB and P-JNK.
Finally, we investigated the mechanism by which the β4 substrate domain promotes cell survival. Wild-type keratinocytes were plated on laminin-5, deprived of growth factors, and treated with the inhibitors. TUNEL analysis indicated that LY294002, but none of the other inhibitors, suppresses keratinocyte survival (Fig. 7A), suggesting that α6β4-mediated activation of PI-3K-to-Akt signaling is necessary for laminin-5-dependent survival.
Taken together, these observations support the hypothesis that the β4 substrate domain promotes cell proliferation and migration by inducing nuclear translocation of NF-κB and P-JNK and cell survival through activation of PI-3K.
DISCUSSION
Targeted deletion of the C-terminal segment of the β4 tail does not affect adhesion to laminin-5 and assembly of hemidesmosomes, allowing a genetic analysis of α6β4 signaling in the absence of loss of adhesion. The results of this analysis indicate that the β4 substrate domain controls laminin-5-dependent nuclear translocation of P-ERK, P-JNK, and NF-κB and, through this mechanism, plays a role in epidermal growth and wound healing. Indeed, although both β1 and αv integrins can support epidermal proliferation and migration (15, 41), the epidermis of β4 mutant mice displays a significant proliferative defect and it undergoes reepithelialization at a reduced rate, presumably because of decreased epidermal migration. In addition, the β4 mutant mice display defective postnatal angiogenesis (37). Thus, whereas the adhesive function of α6β4 is essential for epidermal stability, and its loss is incompatible with life (10, 53), signaling by the β4 substrate domain plays a role in epidermal homeostasis and repair and in angiogenesis. The observation that the β4 mutant mice are viable and fertile and do not display evident abnormalities in the absence of stress suggests that the novel signaling mechanism identified here does not contribute to embryonic development and normal adult life. Alternatively, other integrins may play redundant or compensatory roles.
We find that the β4 substrate domain performs key functions in epidermal cells. Upon plating on laminin-5, the mutant keratinocytes display poor mitogenic response to EGF, and they rapidly undergo apoptosis upon removal of the growth factor. These defects are not observed in cells plated on fibronectin, indicating that the β4 substrate domain is necessary for cell proliferation and survival on laminin-5 but not other permissive matrix substrates. In addition, keratinocytes lacking the β4 substrate domain migrate inefficiently in response to EGF, providing evidence that β4 signaling controls keratinocyte migration. Although prior studies had suggested that α6β4 activates promigratory signaling pathways in epidermal cells (43), our results are the first to provide genetic evidence that this is the case. Finally, the mutant keratinocytes show enhanced activation of Rac, spreading, and assembly of α3β1-containing focal contacts on laminin-5, suggesting that loss of the β4 substrate domain enhances the adhesive and signaling function of α3β1, which also binds to laminin-5. These results suggest that the transdominant-negative effect that α6β4 exerts on α3β1 (36) is mediated by the β4 substrate domain. In addition, they imply that the α3β1 integrin is unable to compensate for the effects of loss of β4 signaling on cell proliferation, survival, and migration. In fact, it is remarkable that the β4 mutant keratinocytes are unable to migrate efficiently, despite upregulation of α3β1, as this latter integrin clearly promotes migration on laminin-5 (6). Taken together, our observations suggest that α6β4 activates potent promigratory signals able to overcome the partial suppression of α3β1-dependent spreading.
Our conclusions stand in stark contrast with those reached recently by Sonnenberg and colleagues (42). These authors have used a K14-Cre transgene to delete β4 from the basal layer of the skin of β4 floxed mice but have obtained only a mosaic pattern of expression of Cre and, thereby, limited excision of floxed β4. They report that the β4-negative areas of the skin of these mice do not contain a decreased number of proliferating keratinocytes (42). We note, however, that it may have been very difficult to identify continuous sections of the epidermis of these mice totally lacking β4 and, hence, to perform a statistically significant analysis. In addition, it is possible that the β4-positive keratinocytes rescued the proliferation of their β4-negative neighboring cells through a paracrine mechanism. Sonnenberg and colleagues have also failed to detect a proliferation defect in immortalized, p53-negative keratinocytes lacking β4 under standard culture conditions. By contrast, we have documented a significant proliferation defect in early-passage primary keratinocytes lacking the β4 signaling domain. Notably, this defect was evident when the cells were plated on laminin-5 but not when they were plated on fibronectin. We suspect that serum-derived factors, such as fibronectin or vitronectin, or loss of p53-mediated control of the cell cycle may have allowed the β4-null immortalized keratinocytes to proliferate despite loss of α6β4 signaling. Finally, Sonnenberg and colleagues also report that their p53-negative, β4−/− keratinocytes migrate more efficiently—not less efficiently—than the β4+/+ controls (42). The apparent contrast between the migratory behavior of keratinocytes lacking α6β4 and those lacking only the β4 signaling domain is fully consistent with the model that α6β4-mediated assembly of hemidesmosomes opposes cell migration, whereas α6β4 signaling promotes it. In line with this conclusion, prior studies have indicated that the EGF-R and other RTKs enhance phosphorylation of β4, causing disruption of hemidesmosomes and increased keratinocyte migration (7, 32, 44).
What are the molecular mechanisms by which the β4 substrate domain exerts its biological function? Our biochemical studies indicate that this segment of β4 mediates activation of PI-3K-to-Akt and Ras-to-ERK signaling independently of the EGF-R, as anticipated from prior studies (30, 31, 48). Since inhibition of PI-3K, but not MEK, induces apoptosis of keratinocytes plated on laminin-5, it is likely that the β4 substrate domain protects these cells from apoptosis through PI-3K-to-Akt signaling (Fig. 8). Thus, although α6β4-mediated assembly of hemidesmosomes may impart polarity to epithelial cells and protect them from apoptotic insults (54), α6β4 promotes cell survival also by a direct signaling mechanism. We note that the β4 substrate domain is not necessary for efficient activation and nuclear translocation of P-Akt in the presence of EGF, suggesting that the antiapoptotic effect of α6β4 signaling through PI-3K may be important only when the amounts of trophic factors available to the cell are limiting. In accordance with this model, we have not detected increased apoptosis in the epidermis of mutant mice in vivo.
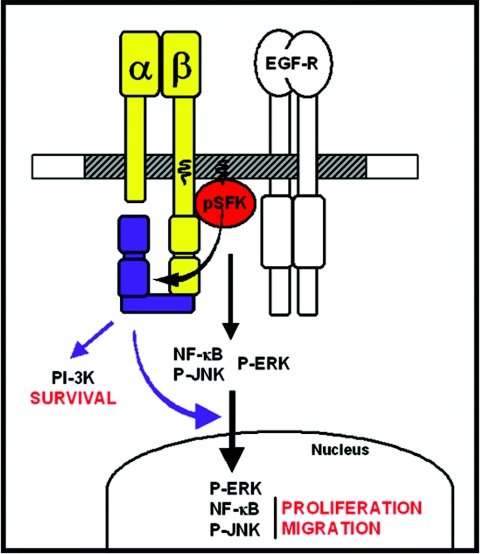
FIG. 8.
Model of integrin α6β4 signaling.
Prior studies have led to the hypothesis that the β4 substrate domain promotes carcinoma cell invasion through recruitment of PI-3K and consequent activation of Rac (48, 49). By contrast, we find that, at least in primary keratinocytes, α6β4 activates Rac and induces signaling to JNK and phosphorylation and degradation of IκB independently of the β4 substrate domain, possibly through the integrin-associated Src family kinase (32). This apparent discrepancy may reflect the existence of partially redundant signaling mechanisms activated in a cell-type-specific manner. In addition, we had proposed that the β4 substrate domain promotes epithelial cell proliferation by cooperating with the EGF-R to activate Ras-to ERK signaling (30). Yet, we now find that deletion of the β4 substrate domain does not impair activation of ERK in mutant keratinocytes treated with optimal concentrations of EGF. Thus, our genetic analysis clearly indicates that the β4 substrate domain is required for keratinocyte migration and proliferation, but it also reveals that it does not exert its function exclusively through activation of the signaling pathways previously identified through cell biological studies.
What is then the mechanism by which α6β4 promotes keratinocyte migration and proliferation? Our studies indicate that the EGF-R is unable to induce nuclear accumulation of P-ERK, P-JNK, and NF-κB, and hence presumably transcription of target genes, in mutant keratinocytes adhering to laminin-5. This striking result indicates that the β4 substrate domain is necessary for nuclear translocation of three distinct transcriptional regulators (Fig. 8). Prior studies have indicated that α5β1-mediated adhesion to fibronectin promotes nuclear accumulation of P-ERK, but not P-JNK and P-p38, and this occurs through activation of Rac (1, 2, 20). The effect of α6β4 signaling is more general, as it involves three distinct nuclear effectors, and it does not appear to be mediated by Rac, as the levels of activation of Rac are enhanced, not diminished, in β4 mutant cells. In addition, whereas the effect of fibronectin-mediated adhesion on nuclear translocation of ERK requires changes in the actin cytoskeleton consequent to cell spreading (2), the β4 mutant keratinocytes display defective nuclear accumulation of P-ERK, P-JNK, and NF-κB in spite of increased spreading on laminin-5. Also, it has been reported that the leukocyte integrin αLβ2 promotes nuclear translocation of the c-Jun coactivator JAB1 (3). However, this effect is mediated by cytohesin-1, which binds selectively to the integrin β2 cytotail (39). These observations suggest that the mechanism by which the β4 substrate domain promotes nuclear translocation of mitogen-activated protein kinases and NF-κB is novel. Future studies will be required to elucidate it.
Our results provide evidence that α6β4-dependent activation of NF-κB and JNK is necessary for keratinocyte proliferation and migration (Fig. 8). In agreement with this hypothesis, genetic studies of dorsal closure in Drosophila melanogaster and wound healing in mice have indicated that JNK signaling and AP-1-dependent transcription are crucial for epidermal migration and proliferation (26, 28, 55). In addition, Ron cooperates with α6β4 to promote NF-κB signaling and keratinocyte migration (44). In apparent contrast to our results, prior transgenic studies have indicated that overexpression of dominant-negative IκBα promotes proliferation and inhibits differentiation of basal keratinocytes, whereas overexpression of the NF-κB subunit p50 exerts the opposite effects (47). We note, however, that epidermis-specific deletion of the IκB kinase IKKβ, which mediates NF-κB signaling in the skin, does not cause cell-autonomous hyperproliferation, or impaired differentiation, but rather inhibits proliferation of keratinocytes (38). Because the EGF-R activates NF-κB (52) and NF-κB participates in the transcriptional control of cyclin D (16), transient activation of NF-κB may be required for progression of keratinocytes through the G1 phase of the cell cycle. By contrast, persistent activation of NF-κB may lead to the upregulation of genes encoding cytokines, such as tumor necrosis factor alpha and interleukin-1, and extracellular factors, such as growth inhibitory factor, which inhibit keratinocyte growth (19). In this model, the apparently contradictory effects of NF-κB signaling in the skin are explained by two temporally and kinetically distinct roles of the transcription factor. Similar considerations may apply also to the apparent discrepancy between the reported role of Ras-ERK signaling in the control of epidermal proliferation (5) and our observation that inhibition of MEK with two distinct compounds inhibits keratinocyte proliferation on laminin-5 only modestly.
In conclusion, our results provide genetic evidence that α6β4 signaling promotes epidermal growth and wound healing by controlling nuclear translocation of NF-κB and P-JNK. These findings identify a novel integrin signaling mechanism and highlight its physiological function.
Acknowledgments
We thank K. Owaribe and R. Timple for reagents, the Transgenic and Knock-out Mouse Facility and the Molecular Cytology Facility of MSKCC for help, and members of the Giancotti lab for discussions.
This work was supported by NIH awards F32 CA97886 (to S.N.N.), R37 CA58976 (to F.G.G.), GTF01018 (to C.T.), and P30 CA08748 (to MSKCC).
REFERENCES
- 1.Aplin, A. E., B. P. Hogan, J. Tomeu, and R. L. Juliano. 2002. Cell adhesion differentially regulates the nucleocytoplasmic distribution of active MAP kinases. J. Cell Sci. 115:2781-2790. [DOI] [PubMed] [Google Scholar]
- 2.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi, E., S. Denti, A. Granata, G. Bossi, J. Geginat, A. Villa, L. Rogge, and R. Pardi. 2000. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate AP-1 activity. Nature 404:617-621. [DOI] [PubMed] [Google Scholar]
- 4.Brakebusch, C., R. Grose, F. Quondamatteo, A. Ramirez, J. L. Jorcano, A. Pirro, M. Svensson, R. Herken, T. Sasaki, R. Timpl, S. Werner, and R. Fassler. 2000. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 19:3990-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, T., K. Nishida, T. Hirano, and P. A. Khavari. 2002. Gab1 and SHP-2 promote Ras/MAPK regulation of epidermal growth and differentiation. J. Cell Biol. 159:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choma, D. P., K. Pumiglia, and C. M. DiPersio. 2004. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J. Cell Sci. 117:3947-3959. [DOI] [PubMed] [Google Scholar]
- 7.Dans, M., L. Gagnoux-Palacios, P. Blaikie, S. Klein, A. Mariotti, and F. G. Giancotti. 2001. Tyrosine phosphorylation of the β4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276:1494-1502. [DOI] [PubMed] [Google Scholar]
- 8.DiPersio, C. M., K. M. Hodivala-Dilke, R. Jaenisch, J. A. Kreidberg, and R. O. Hynes. 1997. α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 137:729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiPersio, C. M., R. van der Neut, E. Georges-Labouesse, J. A. Kreidberg, A. Sonnenberg, and R. O. Hynes. 2000. α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci. 113:3051-3062. [DOI] [PubMed] [Google Scholar]
- 10.Dowling, J., Q. C. Yu, and E. Fuchs. 1996. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134:559-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs, E., J. Dowling, J. Segre, S. H. Lo, and Q. C. Yu. 1997. Integrators of epidermal growth and differentiation: distinct functions for β1 and β4 integrins. Curr. Opin. Genet. Dev. 7:672-682. [DOI] [PubMed] [Google Scholar]
- 12.Gagnoux-Palacios, L., M. Dans, W. van't Hof, A. Mariotti, A. Pepe, G. Meneguzzi, M. D. Resh, and F. G. Giancotti. 2003. Compartmentalization of integrin α6β4 signaling in lipid rafts. J. Cell Biol. 162:1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 14.Giancotti, F. G., and G. Tarone. 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19:173-206. [DOI] [PubMed] [Google Scholar]
- 15.Grose, R., C. Hutter, W. Bloch, I. Thorey, F. M. Watt, R. Fassler, C. Brakebusch, and S. Werner. 2002. A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129:2303-2315. [DOI] [PubMed] [Google Scholar]
- 16.Guttridge, D. C., C. Albanese, J. Y. Reuther, R. G. Pestell, and A. S. Baldwin, Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19:5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hager, B., J. R. Bickenbach, and P. Fleckman. 1999. Long-term culture of murine epidermal keratinocytes. J. Investig. Dermatol. 112:971-976. [DOI] [PubMed] [Google Scholar]
- 18.Hieda, Y., Y. Nishizawa, J. Uematsu, and K. Owaribe. 1992. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 116:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinata, K., A. M. Gervin, Y. J. Zhang, and P. A. Khavari. 2003. Divergent gene regulation and growth effects by NF-κB in epithelial and mesenchymal cells of human skin. Oncogene 22:1955-1964. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, E., L. Barberis, M. Brancaccio, O. Azzolino, D. Xu, J. M. Kyriakis, L. Silengo, F. G. Giancotti, G. Tarone, R. Fassler, and F. Altruda. 2002. Defective Rac-mediated proliferation and survival after targeted mutation of the β1 integrin cytodomain. J. Cell Biol. 157:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodivala-Dilke, K. M., C. M. DiPersio, J. A. Kreidberg, and R. O. Hynes. 1998. Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 142:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopkinson, S. B., S. E. Baker, and J. C. Jones. 1995. Molecular genetic studies of a human epidermal autoantigen (the 180-kD bullous pemphigoid antigen/BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and α6 integrin. J. Cell Biol. 130:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 24.Jacinto, A., A. Martinez-Arias, and P. Martin. 2001. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 3:E117-E123. [DOI] [PubMed] [Google Scholar]
- 25.Klein, S., A. R. de Fougerolles, P. Blaikie, L. Khan, A. Pepe, C. D. Green, V. Koteliansky, and F. G. Giancotti. 2002. α5β1 integrin activates an NF-κB-dependent program of gene expression important for angiogenesis and inflammation. Mol. Cell. Biol. 22:5912-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kockel, L., J. G. Homsy, and D. Bohmann. 2001. Drosophila AP-1: lessons from an invertebrate. Oncogene 20:2347-2364. [DOI] [PubMed] [Google Scholar]
- 27.Koster, J., S. van Wilpe, I. Kuikman, S. H. Litjens, and A. Sonnenberg. 2004. Role of binding of plectin to the integrin β4 subunit in the assembly of hemidesmosomes. Mol. Biol. Cell 15:1211-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, G., C. Gustafson-Brown, S. K. Hanks, K. Nason, J. M. Arbeit, K. Pogliano, R. M. Wisdom, and R. S. Johnson. 2003. c-Jun is essential for organization of the epidermal leading edge. Dev. Cell 4:865-877. [DOI] [PubMed] [Google Scholar]
- 29.Liou, W., H. J. Geuze, and J. W. Slot. 1996. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 106:41-58. [DOI] [PubMed] [Google Scholar]
- 30.Mainiero, F., C. Murgia, K. K. Wary, A. M. Curatola, A. Pepe, M. Blumemberg, J. K. Westwick, C. J. Der, and F. G. Giancotti. 1997. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16:2365-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainiero, F., A. Pepe, K. K. Wary, L. Spinardi, M. Mohammadi, J. Schlessinger, and F. G. Giancotti. 1995. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 14:4470-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariotti, A., P. A. Kedeshian, M. Dans, A. M. Curatola, L. Gagnoux-Palacios, and F. G. Giancotti. 2001. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 155:447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol. Cell 8:115-127. [DOI] [PubMed] [Google Scholar]
- 34.Miranti, C. K., and J. S. Brugge. 2002. Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4:E83-E90. [DOI] [PubMed] [Google Scholar]
- 35.Murgia, C., P. Blaikie, N. Kim, M. Dans, H. T. Petrie, and F. G. Giancotti. 1998. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J. 17:3940-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen, B. P., X. D. Ren, M. A. Schwartz, and W. G. Carter. 2001. Ligation of integrin α3β1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J. Biol. Chem. 276:43860-43870. [DOI] [PubMed] [Google Scholar]
- 37.Nikolopoulos, S. N., P. Blaikie, T. Yoshioka, W. Guo, and F. G. Giancotti. 2004. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell 6:471-483. [DOI] [PubMed] [Google Scholar]
- 38.Pasparakis, M., G. Courtois, M. Hafner, M. Schmidt-Supprian, A. Nenci, A. Toksoy, M. Krampert, M. Goebeler, R. Gillitzer, A. Israel, T. Krieg, K. Rajewsky, and I. Haase. 2002. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417:861-866. [DOI] [PubMed] [Google Scholar]
- 39.Perez, O. D., D. Mitchell, G. C. Jager, S. South, C. Murriel, J. McBride, L. A. Herzenberg, S. Kinoshita, and G. P. Nolan. 2003. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat. Immunol. 4:1083-1092. [DOI] [PubMed] [Google Scholar]
- 40.Raghavan, S., C. Bauer, G. Mundschau, Q. Li, and E. Fuchs. 2000. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150:1149-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghavan, S., A. Vaezi, and E. Fuchs. 2003. A role for αβ1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev. Cell 5:415-427. [DOI] [PubMed] [Google Scholar]
- 42.Raymond, K., M. Kreft, H. Janssen, J. Calafat, and A. Sonnenberg. 2005. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J. Cell Sci. 118:1045-1060. [DOI] [PubMed] [Google Scholar]
- 43.Russell, A. J., E. F. Fincher, L. Millman, R. Smith, V. Vela, E. A. Waterman, C. N. Dey, S. Guide, V. M. Weaver, and M. P. Marinkovich. 2003. α6β4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of α3β1 integrin. J. Cell Sci. 116:3543-3556. [DOI] [PubMed] [Google Scholar]
- 44.Santoro, M. M., G. Gaudino, and P. C. Marchisio. 2003. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell 5:257-271. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki, T., W. Gohring, K. Mann, C. Brakebusch, Y. Yamada, R. Fassler, and R. Timpl. 2001. Short arm region of laminin-5 γ2 chain: structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 314:751-763. [DOI] [PubMed] [Google Scholar]
- 46.Schaapveld, R. Q., L. Borradori, D. Geerts, M. R. van Leusden, I. Kuikman, M. G. Nievers, C. M. Niessen, R. D. Steenbergen, P. J. Snijders, and A. Sonnenberg. 1998. Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin, and involves a direct interaction between β4 and the bullous pemphigoid antigen 180. J. Cell Biol. 142:271-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seitz, C. S., Q. Lin, H. Deng, and P. A. Khavari. 1998. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc. Natl. Acad. Sci. USA 95:2307-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw, L. M. 2001. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the α6β4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell. Biol. 21:5082-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw, L. M., I. Rabinovitz, H. H. Wang, A. Toker, and A. M. Mercurio. 1997. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 91:949-960. [DOI] [PubMed] [Google Scholar]
- 50.Spinardi, L., S. Einheber, T. Cullen, T. A. Milner, and F. G. Giancotti. 1995. A recombinant tail-less integrin β4 subunit disrupts hemidesmosomes, but does not suppress α6β4-mediated cell adhesion to laminins. J. Cell Biol. 129:473-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spinardi, L., Y. L. Ren, R. Sanders, and F. G. Giancotti. 1993. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol. Biol. Cell 4:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, L., and G. Carpenter. 1998. Epidermal growth factor activation of NF-κB is mediated through IκBα degradation and intracellular free calcium. Oncogene 16:2095-2102. [DOI] [PubMed] [Google Scholar]
- 53.van der Neut, R., P. Krimpenfort, J. Calafat, C. M. Niessen, and A. Sonnenberg. 1996. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 13:366-369. [DOI] [PubMed] [Google Scholar]
- 54.Weaver, V. M., S. Lelievre, J. N. Lakins, M. A. Chrenek, J. C. Jones, F. Giancotti, Z. Werb, and M. J. Bissell. 2002. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zenz, R., H. Scheuch, P. Martin, C. Frank, R. Eferl, L. Kenner, M. Sibilia, and E. F. Wagner. 2003. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev. Cell 4:879-889. [DOI] [PubMed] [Google Scholar]