Abstract
We previously demonstrated that ubiquitously expressed CP2c exerts potent erythroid-specific transactivation of α-globin through an unknown mechanism. This mechanism is reported here to involve specific CP2 splice variants and protein inhibitor of activated STAT1 (PIAS1). We identify a novel murine splice isoform of CP2, CP2b, which is identical to CP2a except that it has an additional 36 amino acids encoded by an extra exon. CP2b has an erythroid cell-specific transcriptional activation domain, which requires the extra exon and can form heteromeric complexes with other CP2 isoforms, but lacks the DNA binding activity found in CP2a and CP2c. Transcriptional activation of α-globin occurred following dimerization between CP2b and CP2c in erythroid K562 and MEL cells, but this dimerization did not activate the α-globin promoter in nonerythroid 293T cells, indicating that an additional erythroid factor is missing in 293T cells. PIAS1 was confirmed as a CP2 binding protein by the yeast two-hybrid screen, and expression of CP2b, CP2c, and PIAS1 in 293T cell induced α-globin promoter activation. These results show that ubiquitously expressed CP2b exerts potent erythroid cell-specific α-globin gene expression by complexing with CP2c and PIAS1.
Erythropoiesis is the process of producing mature red blood cells (erythrocytes) during which hemoglobin, the most abundant protein in the red blood cell, is synthesized. The intact hemoglobin molecule is a spherical tetramer composed of two α chains and two β chains. Expression of these genes is known to be regulated in a cell type- and developmental stage-specific manner (9). It has been reported that this specificity is mediated by interactions between regulatory elements proximal to each globin gene and erythroid-specific and constitutively expressed transcription factors which bind to those sites (4, 5, 9, 15, 31, 47). However, the full complement of transcription factors involved in regulating globin gene transcription is not yet known.
Besides well-known erythroid transcription factors such as GATA-1 and NF-E2, CP2 family gene products are reported to be involved in α-globin gene expression. Among the six human CP2 family proteins expressed, LBP-1a/b and LBP-1c/d isoforms are produced from two different genomic loci by alternative splicing mechanisms (National Center for Biotechnology Information, Human Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/). Mouse CP2c (also known as α-CP2/CP2/LSF/UBP-1) is a homologue of human LBP-1c with 96% amino acid sequence identity (27), and CP2a (also known as NF2d9) is a mouse homologue of human LBP-1a. CP2c was initially identified as an activator of the mouse α-globin gene, which binds to the consensus DNA-binding sequence CNRG-N6-CNR(G/C) within a promoter element overlapping the CCAAT box (23, 24, 26, 27). The α-globin promoter is highly activated by the overexpressed CP2c in vitro and in vivo (24, 26). Furthermore, experimental reduction of CP2c suppressed the transcription of both α- and β-globin genes and hemoglobin synthesis during terminal differentiation of murine erythroid leukemia (MEL) cells (12). The transcriptional activity of CP2c was increased during induced differentiation of MEL cells and associated globin gene transcription, while other factors that bind to the mouse α-globin promoter showed no increase in their transcriptional activity (13, 24). It is also known that CP2c can form a heteromeric complex with an erythroid-specific partner protein, NF-E4. This complex, known as the stage selector protein, plays a role in ɛ- and γ-globin transcriptional regulation (2, 20, 52, 53). CP2c is also likely to be involved in regulation of nonglobin erythroid-specific genes. It was reported that congenital erythropoietic porphyria was caused by the mutations in GATA-1 and CP2c binding sites within the promoter of the uroporphyrinogen III synthase gene, the fourth enzyme in the heme biosynthetic pathway (43).
To gain insight into the transcriptional regulation of the murine α-globin gene by CP2c, we previously analyzed transcriptional activities of the wild type and various factor-binding site mutation forms of the α-globin promoter in erythroid and nonerythroid cells. Our findings demonstrated that CP2c binding sites on the α-globin promoter are essential for the enhanced transcription of globin genes in erythroid cells (11). This study further elucidates how CP2 family genes regulate mouse α-globin gene expression in an erythroid cell-specific manner. We identify here a novel member of the mouse CP2 family, CP2b, which is expressed from the CP2a encoding locus by a splice variation and corresponds to human LBP-1b, and show that protein inhibitor of activated STAT1 (PIAS1) physically interacts with CP2 isoforms. Both CP2b and PIAS1 along with CP2c are involved in regulation of the α-globin gene in erythroid cells. These experiments further demonstrate that the potent erythroid cell-specific transcriptional activity of CP2b/CP2c is conferred by the addition of PIAS1.
MATERIALS AND METHODS
Cell culture.
MEL cell line DS19 was cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) supplemented with 10% bovine calf serum (HyClone), 50 U/ml penicillin, and 50 μg/ml streptomycin (Gibco BRL). Terminal differentiation of MEL cells was achieved by the supplementation of a chemical inducer, hexametylene-bis-acetamide (HMBA; 5 mM). Human myelogenous leukemia cell line K562 was maintained in RPMI 1640 (Gibco BRL) supplemented with 10% heat-inactivated bovine calf serum (HyClone). For erythroid differentiation, K562 cells were treated with a chemical inducer, sodium butyrate (5 mM). Human embryonic kidney cell line 293T, human cervical carcinoma cell line HeLa, murine preadipocyte cell line 3T3-L1, murine brown adipose cell line HIB-1B, and murine embryonic fibroblast (MEF) primary cells were maintained in DMEM plus 10% fetal bovine serum (HyClone). The 129/sv-derived mouse embryonic stem cell line (mESC) was adapted to grow in the presence of leukemia inhibitory factor without feeder cells. Undifferentiated embryonic stem cells were maintained in gelatinated petri dishes in DMEM supplemented with leukemia inhibitory factor (1,000 U/ml ESGRO; Chemicon), bovine calf serum (15%), and monothioglycerol (1.5 × 10−4 M). Human mesenchymal stem (hMSC) cells derived from the human peripheral blood were maintained in DMEM supplemented with low glucose (1 mM) and fetal bovine serum (10%). Mouse mesenchymal stem cell line C3H10T1/2 (mMSC) was obtained from the American Type Culture Collection and cultured in DMEM containing 10% fetal bovine serum. Human breast cancer cell line MCF-7 was maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum.
Cloning of mouse CP2 isoform cDNAs and plasmid construction.
cDNAs of mouse CP2 isoforms were cloned from MEL cell mRNA by reverse transcription-PCR (RT-PCR). The 5′ cDNA segment of CP2a (NF2d9) was amplified by PCR using the primers CP2a1R (5′-GATGGCCTGGGTGCT CAG-3′) and CP2a2R (5′-GCTTGTGCAGCTCCATCTCC-3′), which spans nucleotides 1 to 968 of the CP2a cDNA starting from its transcription start site (37). The 3′ part was amplified by PCR using the primers CP2a3R (5′-ACTCAGCCAGCTGCCAAATC-3′) at nucleotide positions 677 to 696 and CP2a4R (5′-TCACTTCAAAATTATGTGAATACC-3′) at positions 1492 to 1515 of the CP2a cDNA. The mixture of both amplified cDNA segments was used as a template for the PCR amplification of the full-length CP2a using the primers CP2a1R and CP2a4R. To obtain the alternatively spliced cDNAs of CP2c isoforms, mRNA from MEL cells was used as a template for RT-PCR with the primers CP2c1R (5′-TGGGGCAAGGAAGGAGCC-3′) and CP2c2R (5′-GAGGGGAAGGCAGCAGCAGC-3′). CP2c1R is located at position 53 to 70 and CP2c2R corresponds to nucleotide positions 1605 to 1624 of mouse CP2c (27). All PCR products for CP2 isoforms were subcloned into the pT7 Blue vector (Novagen) and sequenced in both directions using several primers complementary to the internal cDNA regions. To construct prokaryotic and eukaryotic expression vectors for each CP2 isoform, cDNA fragments were cloned in frame with the glutathione transferase (GST) coding sequence in the pGEX vector (Amersham Pharmacia) or with the hemagglutinin (HA) tag sequence in the pCMV-HA vector (Clontech), respectively. The full-length CRTR-1 cDNA was kindly provided by P. D. Rathjen (University of Adelaide, Australia). The cDNA fragments for the N-terminal deletion form of CP2b (Δ1-42) and PIAS1 were amplified by PCR using primers (CP2b [Δ1-42], 5′-GCATTGCCCATTTTCAAGCAAG-3′ and 5′-TCACTTCAAAATTATGTGAATACC-3′; PIAS1, 5′-GGAATTCATGGCGGACAGTGCGGAAC-3′ and 5′-GCTCGAGTCAGTCCAATGAAATAATGTC-3′) and inserted in frame into the Flag-tagged pcDNA3 vector (kindly donated by J. B. Kim, Seoul National University, Seoul, South Korea). The pEF1α-LSF-DN vector (for the CP2c dominant negative) was kindly provided by Ulla Hansen (Boston University, Boston, MA). For the construction of the expression plasmids pDsRed2N1-CPb and pDsRed2N1-CPc encoding the RFP (red fluorescent protein) fusion form of CP2b and CP2c, respectively, the full-length cDNAs of mouse CP2b and CP2c were cloned into the pDsRed2N1 vector (Clontech). The entirety of all expression plasmids was confirmed by sequencing and Western blot analyses using antibodies that specifically recognize each of CP2 isoforms.
Western blotting and immunocytochemistry.
For Western blot analysis, cultured cells were harvested in cold phosphate-buffered saline and resuspended in lysis buffer (20 mM Tris-HCl, pH 7.4, 5 mM EDTA, 1 mM β-mercaptoethanol, 10% glycerol, 1% Triton X-100, 0.1 mM phenylmethylsulfonyl fluoride, 150 mM NaCl). Total lysates were centrifuged at 4°C at 12,000 rpm for 10 min, and the protein concentration of the supernatant fraction was determined by the Bradford assay according to the manufacturer's protocol (Bio-Rad). About 10 to 100 μg of total extract proteins were electrophoresed on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat dry milk in a solution of 20 mM Tris HCl (pH 7.6)-137 mM NaCl-0.1% Tween 20 and incubated with appropriate dilutions of the primary antibody at room temperature for 1 h. The excess primary antibody was removed by sequential washing, and then a 1:1,000 dilution of the appropriate secondary antibody (horseradish peroxidase conjugated) was added to the membrane at room temperature for 1 h. The anti-CP2c antibody has been previously described (12) and the anti-NF2d9 (CP2a) antibody was kindly provided by M. Negishi (National Institute of Environmental Health Sciences, National Institutes of Health). The anti-PIAS1 antibody (Santa Cruz Biotechnology) was used to detect the human and mouse PIAS1 proteins. The polyclonal anti-β-tubulin or actin antibody (Santa Cruz Biotechnology) was used as a loading control for immunoblotting of samples. The immunoreactive species on the blot were visualized by chemiluminescence using an ECL system (Amersham-Pharmacia). Quantitation of relative amounts of proteins in several cell lines was performed using an image analyzer (Bio-profile V6.0; Vilber Lourmat, France) equipped with BIO-1D analysis software according to the manufacturer's protocol. For immunocytochemistry, either the expression vector pDsRed2N1-CP2b or pDsRed2N1-CP2c or both plasmids were transiently transfected into MEL cells in the absence or presence of HMBA. MEL cells were fixed 48 or 72 h after transfection, and then α-globin or Nip3 protein endogenously expressed in transfected MEL cells was detected with the anti-hemoglobin antibody (CAPPEL/Organon Teknica, Durham, NC) or anti-Nip3-antibody (Santa Cruz Biotechnology), respectively, by incubation with the fluorescein isothiocyanate-conjugated secondary antibody. The α-globin, Nip3, and RFP-CP2b/-CP2c fusion proteins were visualized by fluorescence microscopy (Fluoview; Olympus).
Transfection and luciferase assay.
Luciferase reporter constructs containing either the wild-type or various mutant mouse α-globin promoters were created using a pGL3-TATAA vector described previously (11). K562 and MEL cells (2 × 105) were plated in 24-well dishes. A total of 400 to 600 ng of DNA including both the luciferase reporter construct and CP2 or PIAS1 expression vectors in various combinations were transiently transfected using Effectene (QIAGEN) according to the manufacturer's recommendations. 293T cells (5 × 104) were plated in 24-well dishes, and a total of 400 to 600 ng of DNA combining both reporter and CP2 or PIAS1 expression vectors was transiently transfected using the calcium phosphate or Effectene method. Cells were harvested 40 to 48 h after transfection in 100 μl of passive lysis buffer (Promega), and then 20 μl of lysate was used for luciferase assays on a Lumat LB9501 luminometer (Berthold). A dual luciferase assay system (Promega) was employed according to manufacturer's instructions, and firefly luciferase expression was normalized against Renilla luciferase. All transfection experiments were repeated at least three times independently.
Expression of GST fusion proteins and affinity chromatography.
GST fusion proteins of CP2 isoforms were expressed in Escherichia coli strain BL21(PLys). Fusion proteins were purified using glutathione-Sepharose (Amersham-Pharmacia), and their integrity was confirmed by Coomassie blue staining and Western blotting after SDS-polyacrylamide gel electrophoresis. For in vitro protein-protein interaction assays, 5 μg of GST or GST fusion proteins was incubated with 20 μl of glutathione-Sepharose beads at 4°C for 1 h. After being washed, the beads were resuspended in 100 μl of binding buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS) including the protease inhibitor cocktail (Roche), and incubated at 4°C for 2 h with the whole lysate of 293T cells transfected with HA-tagged CP2 isoform expression vectors or a control vector. After extensive washing, retained proteins were eluted by boiling in SDS protein loading buffer and analyzed by Western blotting using anti-HA (Santa Cruz Biotechnology) and anti-GST (a kind gift from C. G. Kim, Konkuk University, South Korea) antibodies.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts of both 293T and K562 cells transfected with the expression vector for either the HA-tagged CP2 isoform or a control vector were prepared as described elsewhere (23). Purified GST-CP2 isoforms or mammalian cell nuclear extracts were incubated individually or in various combinations in 25-μl reaction mixtures containing 60 mM KCl, 10 mM Tris-HCl, pH 7.9, 1 mM EDTA, 1 mM dithiothreitol, 4% glycerol, 0.1% NP-40, 1 μg of poly(dI-dC) (Pharmacia), and the 32P-labeled oligonucleotide probe. The probe DNA corresponds to CP2c consensus binding sites within the mouse α-globin promoter, 5′-GATCCCAAGTTTTACTCGGTAGAGCAAGCACAAACCAGG-3′ (the region of nucleotides −156 to −124 from the start codon). For supershift analysis, the anti-CP2c (12), anti-NF2d9, anti-PIAS1, or anti-HA antibody was coincubated in K562 or MEL cell nuclear extracts for 15 min. The reaction mixtures were separated on native 5% polyacrylamide gels, and the dried gels were autoradiographed.
ChIP assay.
A chromatin immunoprecipitation assay (ChIP) was based on a previously described protocol (48). Briefly, HMBA-induced or uninduced MEL cells were fixed with formaldehyde and then sonicated. Soluble chromatin samples were immunoprecipitated with the anti-CP2c, anti-NF2D9, anti-PIAS1, or normal rabbit immunoglobulin G (Santa Cruz Biotechnology) antibody at 4°C for 3 h. DNA was isolated from immunoprecipitated materials followed by a reversal of formaldehyde cross-linking and then subjected to PCR using mouse α-globin promoter-specific primers (forward primer, 5′-GGTTTGAGGGACTTGCTTCT-3′; reverse primer, 5′-CTGGTTGTGCTTGCTCTACC-3′), which amplify about 200-bp fragments flanking the CP2c binding element.
Transactivation and protein interaction analysis.
Protein domains involved in transcriptional activation were defined using the yeast one-hybrid assay system (Clontech). cDNA fragments encoding various parts of mouse CP2c were obtained by PCR using primer sets with added EcoRI and SalI restriction sites. Resulting PCR-amplified cDNA fragments include the full-length sequence (amino acid [aa] residues 1 to 502), aa 1 to 67, and aa 306 to 502 of the cDNA regions of CP2c. These PCR products were subcloned into the EcoRI/XhoI site of the pLexA vector in frame with the LexA-DNA binding domain (LexA BD). The full-length or N-terminal deletion forms of CP2a and CP2b were created by PCR to contain SmaI and XhoI restriction sites at their ends and then subcloned into the Klenow-blunted BamHI/XhoI site of the pLexA vector. For analysis of protein interactions, the CP2c cDNA fragment was cloned into the pB42AD vector (Clontech) in frame with the B42 polypeptide activation domain (B42AD). The resulting plasmids were transformed into the yeast EGY48 strain (p8op-LacZ) individually or in combinations. To monitor transcriptional activity or protein interactions, β-galactosidase activity was analyzed by filter lift experiments and then quantified by o-nitrophenyl-d-galactopyranoside assay as described in the yeast protocols handbook (Clontech). To confirm the identified transcriptional domain in mammalian systems, cDNA fragments for N-terminal regions of CP2 isoforms, including aa 1 to 64 of CP2a/b, aa 1 to 309 of CP2b, aa 64 to 274 of CP2b, aa 1 to 67 of CP2c, and aa 1 to 52 of CRTR-1, were inserted in frame with the GAL4 binding domain in the mammalian expression vector under the control of the cytomegalovirus (CMV) promoter (pCMV-0). The CP2b-specific exon (aa 274 to 309 of CP2b) and the CP2b N terminus plus CP2b-specific sequence (aa 1 to 64 and 274 to 309 [1-64/274-309]) were also subcloned into the pCMV-0 vector. Each vector was cotransfected into 293T and K562 cells with a luciferase reporter vector containing a GAL4 binding element. A dual luciferase assay was employed for normalizing transfection efficiency. Transfection and luciferase assays were performed as described above.
RNA preparation and semiquantitative RT-PCR.
Total RNAs were extracted from HeLa, hMSC, K562, MCF7, 293T, MEF, mMSC, mESC, MEL, 3T3-L1, and HIB-1B cells using Trizol (Invitrogen) reagent. The reverse transcription reaction was performed at 42°C for 1 h in a total volume of 10 μl containing 300 ng of RNA, 100 U of murine leukemia virus reverse transcriptase (Invitrogen), and 10 pmol of random hexamers. Half of the total RNA extracted from individual samples was used in each case for an RT-negative reaction. The resulting cDNA was used as a template for PCR with 0.2 U of AmpliTaq according to the manufacturer's recommendations (Perkin Elmer). PCR conditions were as follows: denaturing at 95°C for 5 min, followed by 26 cycles of denaturing at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, and concluding with a final extension at 72°C for 5 min. The PCR products were separated on a 5% polyacrylamide gel and visualized by ethidium bromide staining. Primer sets used in PCR were as follows: LBP-1a/b (forward primer, 5′-AAGATGGAGAAGAGAACAGCTC-3′; reverse primer, 5′-GAGCCGAATTCCATCGGCTG-3′), CP2a/b (forward primer, 5′-GCATTGCCCATTTTTAAGCAAG-3′; reverse primer, 5′-GCTTGTGCAGCTCCATCTCC-3′), LBP-1c (forward primer, 5′-GGAATTCGGTGCAGACAGAAAACAA-3′; reverse primer, 5′-TGGAGGGGGTGGTTCTGGCT-3′), CP2c (forward primer, 5′-GCATTGCCCATTTTTAAGCAAG-3′; reverse primer, 5′-TGGAGGGGGTGGTTCTGGCT-3′), human PIAS1 (forward primer, 5′-GGAATTCCACCCAGTCCATCCGGAT-3′; reverse primer, 5′-GCTCGAGATGAGAACATGTAAGGGC-3′), mouse PIAS1 (forward primer, 5′-GGAATTCATGGCGGACAGTGCGGAAC-3′; reverse primer, 5′-GCTCGAGACAAAGATTGGGTGGGAA-3′), human hypoxanthine phosphoribosyltrannsferase (hHPRT) (forward primer, 5′-GCTGGTGAAAAGGACCCCA-3′; reverse primer, 5′-AGCTCTACTAAGCAGATGGC-3′), and mouse HPRT (mHPRT) (forward primer, 5′-CACAGGACTAGAACACCTGC-3′; reverse primer, 5′-GCTGGTGAAAAGGACCTCT-3′). Quantitation of relative levels of PCR products in the linear range of the assay was performed using an image analyzer equipped with the BIO-1D analysis program. The expression level of hHPRT or mHPRT was used as an internal control.
Nucleotide sequence accession number.
The sequence of the protein CP2b has been deposited in the GenBank database under accession number AY182062.
RESULTS
Cloning of the mouse CP2b gene.
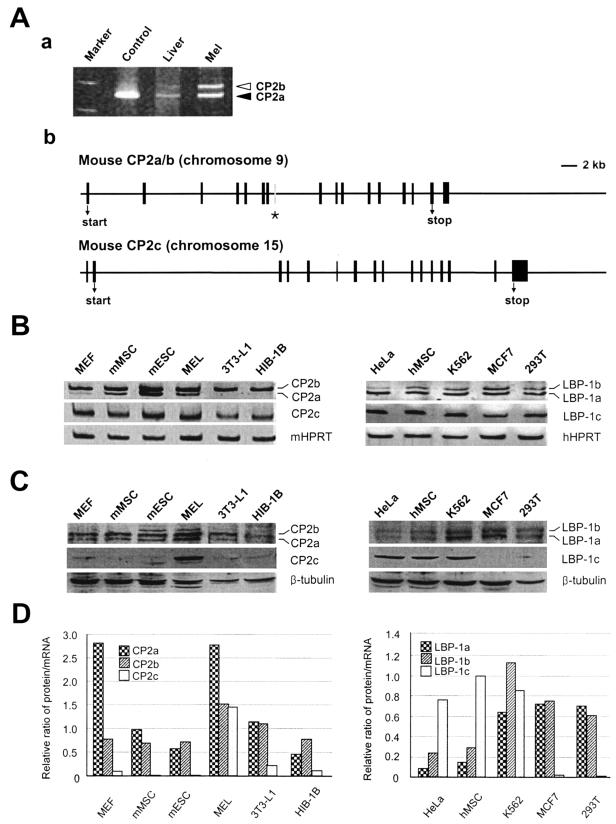
In humans, six CP2 isoforms (LBP-1a, -1b, -1c, -1d, -9, and -32) have been previously identified (19, 49), among which LBP-1a/b and LBP-1c/d isoforms are produced from two different genomic loci by alternative splicing mechanisms (Human Map Viewer). To determine whether similar isoforms are produced from mouse CP2 by alternative splicing, we performed RT-PCR using mRNA obtained from MEL cells with several primer pairs that amplify various regions of CP2a (NF2d9) and CP2c (CP2). We detected two and three different sizes of PCR products, respectively, generated by specific primer pairs for CP2a (Fig. 1A, panel a) and CP2c (data not shown). A new clone was obtained containing an additional 36-amino-acid coding region from an extra exon of the CP2a gene (Fig. 1A, panel b), which is consistent with the organization of LBP-1a/b (49). The deduced amino acid sequence of the new protein shows 92% homology to human LBP-1b, and the sequence encoded from the extra exon is identical to that of human LBP-1b (Fig. 1A, panel b, asterisk). We analyzed mRNA (Fig. 1B) and protein (Fig. 1C) levels of CP2 isoforms in several cell types, including MEF, mMSC, mESC, MEL, 3T3-L1, HIB-1B, HeLa, hMSC, K562, MCF7, and 293T cells. When the relative ratio of protein to mRNA was compared among different cell types (Fig. 1D), interestingly, CP2 isoform proteins were only detectable at significant levels in erythroid-type cells such as K562 and MEL. In most nonerythroid type cells that we investigated, the protein levels of CP2c (or LBP-1c) were almost undetectable. Although two nonerythroid-type cells, HeLa and hMSC, showed significant levels of LBP-1c, both cell types retained the relatively low levels of both LBP-1a and LBP-1b in comparison with those of other cell types (Fig. 1C and D). In contrast, the transcripts of CP2a, CP2b, and CP2c were detected by semiquantitative RT-PCR in all cell types except 3T3-L1 and HIB-1B, where the transcripts of CP2a were missing, indicating ubiquitous transcription of the CP2c gene and the CP2a/b locus (Fig. 1B). Posttranscriptional regulation may operate to influence the level of CP2 isoform proteins in a cell type specific manner.
FIG. 1.
Identification of a novel isoform of mouse CP2, CP2b, and assay of expression levels of CP2a/b/c isoforms in different cell types. (A) A new CP2 isoform, CP2b, was identified from RT-PCR of MEL cell mRNA using primers for CP2a amplification (see Materials and Methods) (a) RT-PCR analysis distinguished two different-sized transcripts, one with 830 bp of CP2a and the other with 940 bp of CP2b from mRNA samples of mouse liver and MEL cells. One single band was detected when plasmid containing CP2a cDNA was used as a template for PCR (control).(b) Structure of the genomic loci of mouse CP2 isoforms. The asterisk indicates the position of the additional exon encoding 36 amino acids present in the novel CP2b gene within the mouse CP2a/b locus. Bar, 2 kb. (B) Transcript levels of mouse CP2 and human LBP-1 isoforms were detected by semiquantitative RT-PCR in various mouse and human cell lines. (C) CP2 isoform protein levels in total cell lysates (about 100 μg of protein) from each cell type were analyzed in a 10% SDS-polyacrylamide gel by Western blotting using CP2 isoform-specific antibodies. (D) The levels of CP2 mRNAs and proteins shown in panels B and C were quantitated, and the relative ratio of protein to mRNA was compared among different cell types.
Tissue-specific CP2b-mediated α-globin gene expression.
We previously reported that reduction of CP2c suppressed the mouse α- and β-globin gene expression and hemoglobin synthesis during terminal differentiation of MEL cells (12). It also has been shown that overexpressed CP2c increased mouse α-globin gene expression in K562 cells and that CP2c binding to the mouse α-globin gene promoter was essential for this enhanced transcription of the α-globin gene in differentiating MEL cells (11). To understand the role of the newly identified isoform, CP2b, in globin gene expression, we compared the transcriptional activity of CP2b with those of other isoforms, CP2a and CP2c, using transient transcriptions. To this end, we used a human erythroid cell line (K562) in parallel with the mouse erythroid cell line (MEL). Although the studies concern the murine CP2 family, many of the transfection experiments are done in human K562 cells because the appropriate murine cells, MEL, have very low transfection efficiency (empirically less than 1%). The high-amino-acid-sequence homology between mouse and human CP2 isoforms (92% between CP2a and LBP-1a, 92% between CPb and LBP-1b, and 96% between CP2c and LBP-1c) provided confidence that K562 is a suitable model system specifically for transfection experiments, and the experimental results supported this confidence.
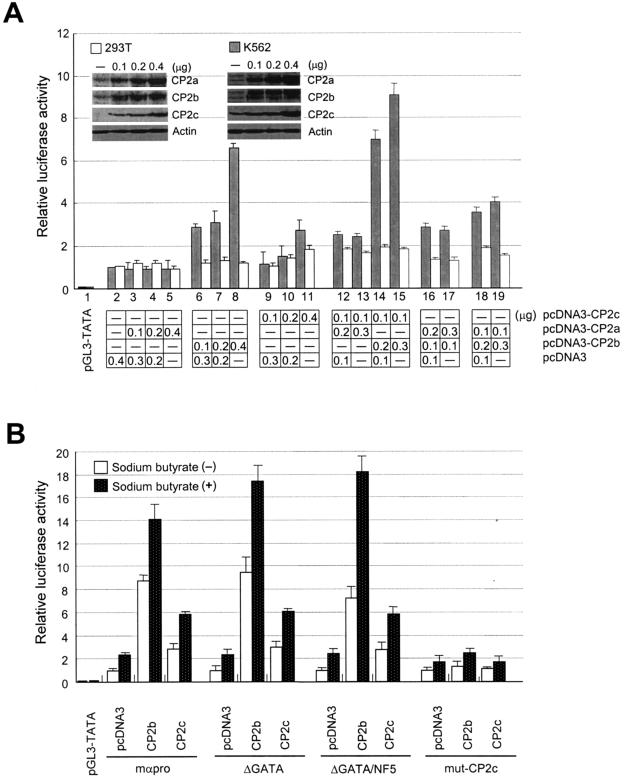
Expression plasmids for individual CP2 isoforms (pcDNA3-CP2a, pcDNA3-CP2b, and pcDNA3-CP2c) were transfected alone or in combination into either K562 or 293T cells along with a reporter plasmid encoding luciferase controlled by the mouse α-globin promoter (Fig. 2A, lanes 2 to 11). Transiently expressed CP2b induced luciferase expression by over sixfold in K562 cells. CP2c increased the luciferase activity by up to threefold from the mock-transfected (pcDNA3) cells, while the transactivation effect of CP2a was negligible in both cell types (Fig. 2A, lanes 2 to 11). Interestingly this CP2b-mediated α-globin gene expression was only observed in K562, (erythroid) cells but not in 293T (nonerythroid) cells. These results indicate that CP2b is possibly a limiting factor for the transcription of the α-globin promoter in erythroid cells. The protein levels of CP2 isoforms in the nuclear extracts from transfected and untransfected cells were measured by Western blotting to ensure that the induced luciferase activity is really due to the altered levels of CP2 proteins in the cells (Fig. 2A, inset). We also cotransfected 293T or K562 cells with CP2 isoform expression plasmids in various combinations along with a luciferase reporter plasmid controlled by the mouse α-globin promoter (Fig. 2A, lanes 12 to 19). The synergistic increment of α-globin gene expression was only detected in the cotransfected cells with both CP2b and CP2c expression plasmids, indicating a possible interaction between both proteins for synergistic activation of α-globin gene expression (Fig. 2A, lanes 14 and 15). Also, this effect was only observed in erythroid K562 cells.
FIG. 2.
α-Globin promoter activity is enhanced by CP2b. (A) CP2b-mediated transcriptional activation of the α-globin gene promoter occurs in a tissue-specific manner. Expression plasmids for each of the three CP2 isoforms (pcDNA3-CP2c, pcDNA3-CP2b, or pcDNA3-CP2a) were transiently cotransfected in various combinations along with a luciferase reporter vector driven by the mouse α-globin promoter into either K562 or 293T cells, and cell lysates were assayed for luciferase. The levels of each CP2 isoform ectopically expressed in 293T and K562 cells were detected by Western blotting (inset). (B) CP2c binding sites in the α-globin promoter are crucial for CP2b-mediated gene expression. Luciferase reporter vectors driven by the wild-type (mαpro) or mutant forms (ΔGATA, ΔGATA/NF5, or mut-CP2c) of the mouse α-globin promoter were transiently cotransfected with one of the CP2 isoform expression vectors (pcDNA3 is a negative control) into K562 cells in the absence or presence of 5 mM sodium butyrate. Average values and standard deviations were obtained from three independent experiments.
To identify the response element for the CP2b-mediated expression, we repeated the transfection experiments in K562 cells using deletion forms of the α-globin gene promoter, ΔGATA and ΔGATA/NF5, and a point mutation form, mut-CP2c (11). These mutant promoters were used for luciferase reporter gene assays by cotransfection with CP2b or CP2c expression plasmids into K562 cells. The point mutation in the CP2c binding site of the promoter resulted in the reduction of CP2b-mediated transcription activation to basal levels, whereas deletion of GATA-1 and/or NF5 binding sites had no effect (Fig. 2B). Thus, the potent CP2b-mediated activation of the mouse α-globin promoter is dependent on the CP2c binding site in the promoter, implying that CP2b binds directly or through the interaction with other CP2 isoforms to the CP2c binding site for the enhanced α-globin gene expression in erythroid cells (see below). In order to verify that the influence of CP2 isoforms on the α-globin promoter is not related to potential CP2-mediated differentiation of K562 cells, the experiments were repeated following sodium butyrate-induced K562 differentiation. Differentiation of K562 cells raised the basal expression level of α-globin promoter-driven constructs by roughly twofold, and, in turn, all responses to transfected CP2 isoforms were increased by similar twofold ratios. This indicates that the activation of the α-globin promoter in transfected K562 cells is directly induced by the ectopically expressed CP2b (or CP2c) rather than by indirect effects of CP2-mediated induction of differentiation (Fig. 2B).
CP2c is the interacting partner of CP2b for erythroid cell-specific α-globin gene expression.
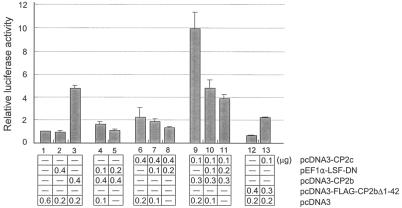
Cotransfection of K562 cells with CP2b and CP2c expression plasmids showed the synergistic increment of α-globin gene expression, suggesting the interaction between CP2b and CP2c (Fig. 2A, lanes 14 and 15). When a dominant negative form of CP2c (pEF1α-LSF-DN) (37) or the N-terminal transactivation domain deletion form of CP2b (pcDNA-FLAG-CP2b Δ1-42) was used for the reaction in K562 cells, the transcriptional activity of the reporter gene was significantly reduced in comparison to the unmutated genes (Fig. 3). These results support the hypothesis that CP2c is the CP2b binding partner for CP2b-mediated α-globin gene expression in K562 cells. Furthermore, since the synergism between CP2b and CP2c is not observed in nonerythroid 293T cells, we postulated that the CP2b-specific transcriptional activation domain confers potent K562 cell-specific transcriptional activity only when CP2b interacts with CP2c in the presence of additional K562 cell specific factor(s) or protein modification machinery (see below).
FIG. 3.
CP2b/CP2c interaction increased α-globin gene expression in erythroid cells. K562 cells were transiently cotransfected with the pGL3-mouse α-globin promoter construct along with pcDNA3 (control), pcDNA3-CP2b, pcDNA3-CP2c, pEF1α-LSF-dominant negative for CP2c, or the pcDNA3-flag-CP2bΔ1-42 vector for the CP2b N-terminal deletion form in combinations as indicated. The total amount of DNA was adjusted to 600 ng for each transfection experiment. Luciferase activities shown are averages and standard deviation of three independent experiments.
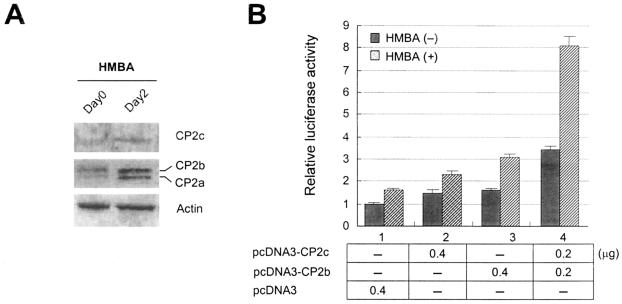
Since the α-globin gene is expressed only in erythroid cells in vivo, the synergistic interaction of CP2b and CP2c is potentially erythroid specific. We found that the expression level of CP2b protein increased during HMBA-induced terminal differentiation of MEL cells as other CP2 isoforms did (Fig. 4A). This result implies that CP2b is involved in the expression of endogenous globin genes in differentiating MEL cells. To test this hypothesis, we transfected undifferentiated or differentiated MEL cells with the CP2b (pcDNA3-CP2b) or CP2c (pcDNA3-CP2c) expression plasmid or cotransfected cells with both plasmids along with the luciferase reporter plasmid controlled by a mouse α-globin promoter. As with K562, the transcriptional synergism mediated by the CP2b/CP2c interaction was most significant in differentiated MEL cells (Fig. 4B). However, undifferentiated MEL cells also showed transcriptional activation from CP2b/CP2c transfection, indicating that CP2b/CP2c can induce α-globin gene expression without cell differentiation. To test this in vivo, we ectopically expressed CP2b and CP2c in MEL cells in the absence of HMBA and assayed for expression of the endogenous α-globin. Endogenously expressed α-globin protein was only detected when both CP2b and CP2c were overexpressed in MEL cells, while singly expressing either CP2b or CP2c was not able to induce the protein (Fig. 4C, upper panel). Nip3 is another erythroid gene expressed in terminally differentiated MEL cells without CP2c binding sites in its promoter (18). When the differentiation of MEL cells was induced, the expression of Nip3 was detected 2 days after HMBA treatment to the cells. However, overexpression of both CP2b and CP2c was not able to induce Nip3 protein in undifferentiated MEL cells (Fig. 4C, bottom panel), demonstrating that ectopically expressed CP2b/c in the absence of HMBA specifically induces α-globin gene expression rather than inducing general increases in erythroid gene expression or causing erythroid differentiation. Antibodies used to detect α-globin and Nip3 in MEL cells by immunohistochemistry (Fig. 4C) recognized a single band corresponding to each protein in Western blotting (data not shown), indicating the specificity of these antibodies to the proteins. Taken together, these results demonstrate that CP2b in conjunction with CP2c is involved in inducing endogenous α-globin gene expression in erythroid cells.
FIG. 4.
Synergistic effect of the CP2b/CP2c interaction on α-globin gene expression is detected with or without differentiation in MEL cells. (A) The expression of CP2 isoforms during HMBA-induced terminal differentiation of MEL cells. Ten micrograms of total lysate proteins prepared from differentiating MEL cells prior to or 2 days after the induction of differentiation were assayed in a 10% SDS-polyacrylamide gel by Western blotting using CP2 isoform-specific antibodies. (B) MEL cells with or without differentiation by HMBA were transiently cotransfected with the mouse α-globin promoter/luciferase construct along with pcDNA3-CP2c, pcDNA3-CP2b, or both. (C) MEL cells were transiently transfected with the expression plasmid for RFP-CP2b or RFP-CP2c fusion protein or cotransfected with both expression plasmids in the absence or presence of HMBA. Cells were fixed 48 or 72 h after transfection, and then endogenous α-globin protein or Nip3 protein was detected with a fluorescein isothiocyanate (green)-conjugated secondary antibody after incubation with the anti-hemoglobin or anti-Nip3 antibody. Fluorescence was detected by an Olympus Confocal Laser Scanning System (Fluoview; Olympus).
Both the N-terminal transcriptional activation domain and the region of aa 274 to 309 in CP2b are critical in mediating the enhanced erythroid cell-specific transcriptional activity of CP2b.
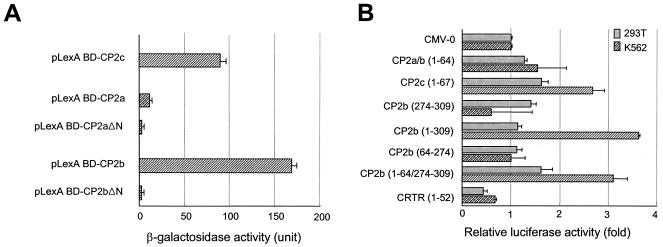
Although the CP2 isoforms are highly homologous, only CP2b mediates the strong induction of α-globin gene expression in erythroid cells. To identify any CP2b-specific domains activating transcriptional activity, we compared the transactivation domains of CP2 isoforms by employing the yeast one-hybrid system. It has been proposed that the transcriptional activation domain of CP2c might be in the C-terminal acidic region of CP2c (27). To investigate this, we subcloned cDNA fragments encoding various regions of mouse CP2c proteins in frame with the LexA BD in a yeast expression plasmid. These plasmids were cotransformed into the yeast EGY48 strain with a reporter plasmid containing the concatamerized LexA DNA-binding sites upstream of the β-galactosidase gene. We found that the N-terminal region of CP2c (aa 1 to 67) is sufficient for transcriptional activation of the reporter gene, while no activation was observed from the plasmids with other regions of CP2c (data not shown).
Considering the significant sequence homology between mouse CP2a/b and CP2c, we speculated that the N terminus of CP2a/b might be involved in transcriptional activation. To test this, we subcloned the cDNA fragments encoding the full-length or N-terminal deletion (Δ1-40) of CP2a and CP2b in frame with LexA BD into the pLexA plasmid. These plasmids were transformed into the yeast EGY48 strain, and reporter gene activities were measured. The full-length CP2b showed twofold higher transcriptional activity than CP2c, whereas CP2a displayed very weak activity (Fig. 5A). However, the N-terminal deletion of CP2b was not able to transactivate β-galactosidase (Fig. 5A), indicating that, like CP2c, the transcriptional activation domain resides in the N terminus of CP2b.
FIG. 5.
The N terminus in conjunction with the CP2b-specific extra 36 amino acids is important for the erythroid cell-specific transcriptional activation of CP2b. (A) The N-terminal region of CP2b is a putative transcriptional activation domain. The yeast one-hybrid assay of the full-length or the N-terminal (aa 1 to 40) deletion form of both CP2a and CP2b fused to LexA BD was performed. The transcriptional activity of each protein was measured by the o-nitrophenyl-d-galactopyranoside assay. (B) The strong erythroid cell-specific transcriptional activity of CP2b requires the broad N-terminal region including the CP2b-specific sequence of 36 amino acids. The mammalian expression plasmids containing the N terminus of CP2 isoforms (aa 1 to 64 or 67), the CP2b-specific sequence (aa 274 to 309), part of the CP2a/b sequence (aa 64 to 274), the N terminus of CP2b spanning the CP2b-specific sequence (aa 1 to 309), the CP2b N terminus plus CP2b-specific sequence (aa 1-64/274-309), or the N terminus of CRTR (aa 1 to 52) fused with the GAL4 DNA binding domain were transiently cotransfected into 293T or K562 cells with a luciferase reporter vector in which GAL4 binding sites are present in the promoter. A dual luciferase assay was employed and firefly luciferase expression was normalized against Renilla luciferase expression. Values are the averages and standard deviations of three independent experiments.
CP2a and CP2b are identical proteins except for the additional 36 amino acids in the middle of CP2b (Fig. 1A, panel b). Apparently, the additional sequence in CP2b renders higher transcriptional activity in CP2b. To investigate the role of the CP2b-specific 36 amino acids in transcriptional activity, reporter gene transfection assays were applied to the mammalian system. Expression vectors containing the N terminus of each CP2 isoform, the CP2b-specific sequence (aa 274 to 309), the CP2b N terminus spanning the CP2b-specific sequence (aa 1 to 309), the sequence (aa 64 to 274) of CP2b, or the CP2b N terminus plus CP2b-specific sequence (aa 1-64/274-309) fused with the GAL4 DNA binding domain were cotransfected into K562 and 293T cells along with a luciferase reporter vector linked to the GAL4 binding sites. Measurement of luciferase expression in both 293T and K562 cells showed that the N termini of CP2a/b and CP2c are responsible for their transcriptional activity, although the relative factor of increase is small in mammalian cells (Fig. 5B). The transcriptional activity of the CP2c N terminus (aa 1 to 67) was higher than that of CP2a/b. This CP2c N-terminal activity was further increased in K562 cells. The unique CP2b sequence (aa 274 to 309) showed no effect, indicating that the CP2b-specific sequence alone is not a transcriptional activation domain in K562 cells. The N terminus of CRTR-1, previously defined as a repression domain (39), showed a lower level of expression than the control, as expected. Interestingly, the sequences containing both the CP2b N terminus and CP2b-specific sequence (aa 1-64/274-309 and 1 to 309) demonstrated strongly enhanced transcriptional activity only in K562 cells. Although the sequence alone between aa 64 and 274 showed no effect (Fig. 5B), this region may participate to some extent in the full transcriptional activity exerted by CP2b (aa 1 to 309). Indeed, we observed a statistically significant difference between CP2b (aa 1 to 309) and CP2b (aa 1-64/274-309) in their transcriptional activity in K562 cells (Fig. 5B). Taken together, these results show that transacting activities of all CP2 isoforms reside in their N-terminal regions and that CP2b-specific sequence (aa 274 to 309) is involved in strong transcriptional activation of CP2b in conjunction with its N terminus.
CP2b has a weak intrinsic DNA binding activity but can interact with other CP2 isoforms.
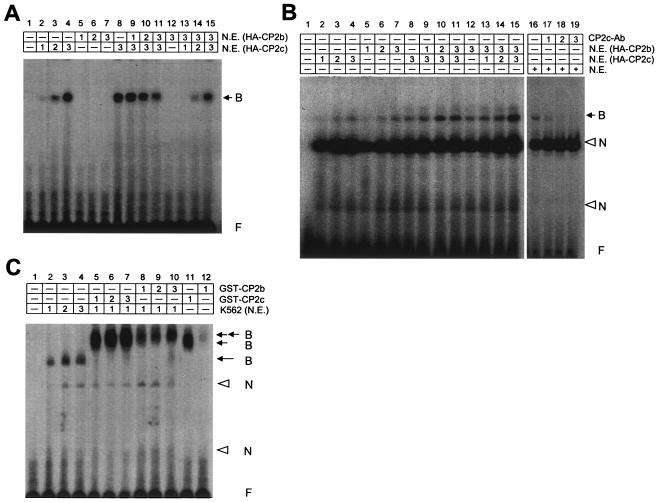
Erythroid cell-specific transcriptional activation by CP2b is mediated via the CP2c binding region in the α-globin promoter (Fig. 2B). It is also known that the stable and strong DNA binding activity of LBP-1c, the human orthologue of CP2c, is mediated by a large portion of the protein (aa 65 to 502) (42). Since the extra 36-amino-acid sequence of CP2b is located within the proposed DNA binding domain, the DNA binding activity of CP2b may not be identical to that of CP2a or CP2c. To test whether CP2b by itself binds to the target DNA, we performed EMSAs using GST-tagged recombinant CP2a, CP2b, and CP2c proteins. Purified GST-CP2 proteins were confirmed by Western blotting with CP2 isoform-specific antibodies (data not shown). An oligonucleotide encompassing the consensus CP2c binding site in the mouse α-globin promoter (24) was used as a probe for EMSA. Binding of CP2b protein to the DNA probe was barely detectable under conditions where CP2a and CP2c proteins could bind to the probe (Fig. 6A, panel a). The protein levels of each CP2 isoform added to the EMSA reaction mixture (for lanes 5, 10, and 15) were monitored by Coomassie blue-stained gel (Fig. 6A, panel b). Since CP2b activity requires the CP2c binding site (Fig. 2B), these results imply that CP2b communicates with the α-globin promoter through interaction with other CP2 isoforms which can bind the CP2c site.
FIG. 6.
CP2b has a very weak DNA binding activity but interacts with other isoforms. (A) EMSA demonstrated that recombinant GST-CP2b bound very weakly to the probe DNA, while other isoforms showed strong DNA binding activities (a). For the EMSA, increasing amounts of each recombinant CP2 isoform were incubated with the 32P-labeled DNA probe of consensus CP2 binding sites. The highest protein levels of each CP2 isoform added to the EMSA reaction mixture (lanes 5, 10, and 15) were detected on a Coomassie blue-stained gel (b). (B) CP2b modulated DNA binding activity of other isoforms. Proteins and a DNA probe were incubated either at the same time (left two panels) or GST-CP2b was added 10 min after the incubation of GST-CP2a or-CP2c protein with DNA (right two panels). (C) The yeast two-hybrid system demonstrates that both CP2a and CP2b interact with CP2c. A yeast strain EGY48 containing the reporter gene (p8op-LacZ) was transformed with plasmids as indicated. The pB42AD CP2c plasmid contains the open reading frame of the full-length CP2c protein (aa 1 to 502) in frame with the B42 polypeptide activation domain (B42AD). The pLexA CP2aΔN, pLexA CP2bΔN, and pLexA CP2c (aa 306 to 502) contain coding regions of CP2a and CP2b N-terminal (aa 1 to 40) deletion forms, and the CP2c dimerization domain fused to the LexA DNA binding domain, respectively. An empty plasmid, pLexA, was a negative control. (D) In vitro binding assay confirmed the interaction between CP2b and CP2c. Each extract from 293T cells overexpressing HA-CP2a, HA-CP2b, or HA-CP2c was incubated with GST or GST-CP2c and bound to glutathione-Sepharose. Eluted materials were electrophoresed on the 10% SDS-polyacrylamide gel, and the bound CP2 proteins were visualized by immunoblotting with HA antibody. One-tenth of the amount of extract in the binding assay was used as input extracts. Mock, cell extract transfected with the CMV-HA vector as a negative control.
To gain insight into the interaction of CP2b with other isoforms in DNA binding, we performed EMSAs in which GST-CP2b was added with GST-CP2a or GST-CP2c in the reaction mixture. Interestingly, in vitro results demonstrate that the DNA binding activity of CP2a and CP2c could be regulated by the timing of the interaction with CP2b relative to interaction with DNA. The DNA binding activity of GST-CP2a or GST-CP2c was decreased when incubated with GST-CP2b along with the DNA probe. The inhibition was dependent on the dose of CP2b, indicating that CP2b interaction with CP2a or CP2c before binding to the target DNA suppresses their DNA binding capability (Fig. 6B, left panel). When GST-CP2b was added to the reaction after a 10-min preincubation of GST-CP2a (or GST-CP2c) with the DNA probe, DNA binding activity of GST-CP2a (or GST-CP2c) was not affected by subsequently added GST-CP2b (Fig. 6B, right panel). These results suggest that erythroid cell-specific transcriptional activation of CP2b is mediated by the interaction with another CP2 isoform(s) that preoccupies the CP2c binding site in the α-globin promoter.
When interacting with DNA, CP2c binds to its binding sites as either a homodimer or part of heterodimeric complexes (49, 51). This conclusion is supported by finding that CP2a and CP2c bind to CP2c binding sites as multimers (data not shown). Since CP2b binds DNA poorly, it is likely that transcriptional activation by CP2b depends on heteromerization with other CP2 isoforms. Thus, the interaction of CP2b with other CP2 isoforms was further investigated by the yeast-two hybrid system. Yeast expression vectors containing cDNA of the N-terminal deletion form of CP2a or CP2b and the dimerization domain of CP2c fused to LexA BD (pLexA CP2aΔN, pLexA CP2bΔN, and pLexA CP2c [aa 306 to 502], respectively) and a second yeast vector containing the full-length cDNA of CP2c fused to the B42 polypeptide AD (pB42AD-CP2c) were cotransformed into the yeast EGY48 strain. To exclude false-positive β-galactosidase activity, the N-terminal transactivation domain (aa 1 to 40) deletion forms of CP2a/CP2b were used. These studies showed that CP2b could heterodimerize with CP2c (Fig. 6C). Although the interaction between CP2a and CP2b was as strong as between CP2b and CP2c (data not shown), this interaction is not likely to be important for α-globin regulation since this combination had no effect on α-globin promoter activity (Fig. 2A) and showed very weak transcriptional activity (Fig. 5). In contrast, the interaction between CP2b and CP2c resulted in synergistic activation of the α-globin promoter in K562 cells (Fig. 2A and 3). Based on these findings, it is further speculated that ectopically expressed mouse CP2b was able to form a complex with endogenous human LBP-1c to activate the α-globin promoter in K562 cells transfected with only the CP2b expression plasmid (Fig. 2A, lanes 6 to 8).
The physical interaction between CP2a or CP2b and CP2c without DNA was further verified by in vitro binding assays using GST affinity chromatography (Fig. 6D). Human 293T cells were transfected with the HA-tagged CP2a, CP2b, or CP2c expression vector. Each whole-cell lysate prepared 2 days after transfection was applied to the column bearing GST alone or a GST fusion form of full-length CP2c (GST-CP2c). Both CP2a and CP2b were retained in the GST-CP2c column but not in GST alone, indicating CP2a and CP2b can form heterodimers with CP2c in the absence of their target DNA sequences.
CP2 proteins are not sufficient for erythroid cell-type specific CP2b-mediated α-globin gene expression in nonerythroid cells.
Several experiments demonstrated that the interaction between CP2 isoforms is critical for α-globin gene regulation. Significant levels of each CP2 protein isoform are only detectable in erythroid cells among several cell types (Fig. 1C and D). In our findings, however, addition of CP2 isoforms alone or together in various combinations into 293T cells was not able to induce expression from the α-globin promoter (Fig. 2A), implying that in addition to CP2 proteins, an additional factor(s) present in K562 is needed for CP2b-mediated transcriptional activation. It is also possible that an additional component(s) present in K562 cells may affect CP2b/CP2c interaction to regulate DNA binding activity of this heteromeric complex. To investigate this possibility, we performed EMSAs using nuclear extracts from 293T or K562 cells in which HA-tagged CP2b or CP2c was ectopically expressed. In 293T cell nuclear extracts where endogenous LBP-1c (homologue of mouse CP2c) is not present, DNA binding activity of HA-CP2b was negligible (Fig. 7A, lanes 5 to 7) and that of HA-CP2c was reduced by ectopically expressing HA-CP2b in a dose-dependent manner (Fig. 7A, lanes 8 to 11), similar to the case of recombinant GST-CP2 isoforms (Fig. 6B, left panel). The CP2b inhibition of CP2c-DNA binding was overcome by excess transfected CP2c in the EMSA reaction mixture of 293T cells (Fig. 7A, lanes 12 to 15). Interestingly, in K562 cell nuclear extracts, CP2b could potentially bind to the DNA probe via the interaction with endogenous LBP-1c (Fig. 7B, lanes 5 to 7 and 12), and this DNA binding activity was greatly increased by the ectopically expressed HA-CP2c protein (Fig. 7B, lanes 8 to 11 and 13 to 15). Shifted DNA bands due to specific DNA-protein complex formation were verified by supershift analysis with the anti-CP2c antibody. Although supershifted bands are hardly detectable, disappearing shifted bands differentiate complexes containing CP2c from nonspecific bands found in K562 nuclear extracts which are not changed (Fig. 7B, lanes 16 to 19). This pattern of CP2b/CP2c-DNA binding is not expected from our findings that CP2b inhibited CP2c-DNA binding (Fig. 6B and 7A). Similar results were also observed in the coincubation of HA-CP2b and HA-CP2a from K562 cell nuclear extracts (data not shown). These results indicate that DNA binding of the CP2b/CP2c heteromeric complex in K562 cells requires some K562 cell-specific factor(s) that is missing in 293T cells.
FIG. 7.
Is CP2c critical for CP2b mediated α-globin gene expression in erythroid cells? (A) Nuclear extracts were prepared from 293T cells in which HA-CP2b or HA-CP2c was overexpressed. EMSA was performed by incubating the DNA probe with different amounts of each extract in combinations. (B) Nuclear extracts were prepared from K562 cells transfected with the HA-CP2b or HA-CP2c expression plasmid. For the EMSA, different amounts of each extract were incubated with a DNA probe (lanes 2 to 15). K562 nuclear extracts were incubated with a DNA probe in the presence or absence of anti-CP2c antibody to identify bands of the specific DNA-protein complex by generating supershifts (lanes 16 to 19). (C) Different amounts of K562 cell nuclear extracts were incubated with the DNA probe plus various amounts of recombinant GST-CP2b and GST-CP2c proteins for the EMSA. Filled arrows indicate the specific DNA-protein complexes, and open arrowheads indicate the nonspecific bands. B, bound probe; N, nonspecifically bound probe; F, free probe.
Indeed, we verified the presence of an extra component(s) that interacts with CP2b/CP2c in K562 cell nuclear extracts by EMSA using recombinant GST-CP2b and GST-CP2c. When we incubated recombinant GST-CP2b with K562 cell nuclear extracts, GST-CP2b could form DNA-protein complexes (Fig. 7C, lanes 8 to 10) as GST-CP2c did (lanes 5 to 7). GST-CP2c incubation in the EMSA reaction not including K562 nuclear extracts also showed the DNA binding activity, but the migration of this shifted band on the gel (lane 11) was clearly faster than that from samples incubated in nuclear extracts (lanes 5 to 10). Since it is known that human LBP-1c and mouse CP2c bind to consensus sequences of DNA (CP2c binding domains) as a homodimer or as a part of heterodimeric complexes (49, 51) and since the molecular size of CP2 isoforms is almost identical to each other, shifted bands on the gel would migrate approximately the same if they were all CP2 family dimers. Thus, the slowly migrating bands of samples prepared from the incubation with K562 nuclear extracts (lanes 5 to 10) are a good indication of an additional component(s) that may participate in the CP2b/CP2c interaction in erythroid K562 cells.
Is PIAS1 a potential cell-type specific coactivator for CP2b mediated α-globin gene expression?
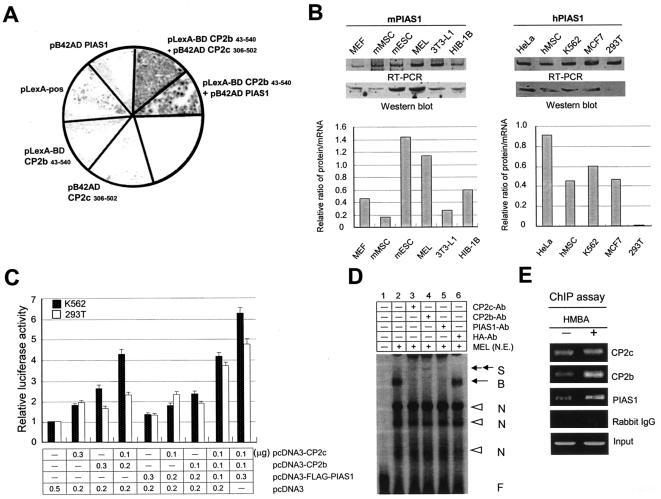
We previously demonstrated by the yeast two-hybrid system (M.-A. Park et al., unpublished data) and a phage display library screening (22) that PIAS1, SUMO-1, Ubc-9, bromodomain containing protein, TTRAP (TRAF and TNF receptor associated protein), protein 66 in the histone deacetylase complex, REST (RE-1 silencing factor), and Yin-Yang (YY1) interact with CP2c. Our preliminary data from cotransfection experiments showed that only PIAS1 among these proteins positively affects α-globin gene expression (data not shown), suggesting the potential role of PIAS1 in α-globin gene expression by interacting with CP2 isoforms. To test interaction between CP2b and PIAS1, we used the yeast two-hybrid system. For the assay, we cotransformed the EGY48 yeast strain with pLexA-BD CP2c (aa 306 to 502), pLexA-BD CP2b (aa 43 to 540), or pB42AD PIAS1 in various combinations with a reporter vector p8op-lacZ. To exclude false-positive β-galactosidase activity, the N-terminal transactivation domain deletion form of CP2b and the dimerization domain of CP2c were used. The plasmid pLexA-pos was used as a positive control. We confirmed the interaction between not only CP2b and CP2c but also CP2b and PIAS1 (Fig. 8A). CP2c binding to PIAS1 was also detected in our study (data not shown).
FIG. 8.
PIAS1 is a potential component in the CP2b/CP2c complex involved in CP2b mediated α-globin gene expression in erythroid cells. (A) PIAS1 was identified as one of the interacting proteins with CP2b by the yeast two-hybrid system. The EGY48 yeast strain was cotransformed with pLexA-BD CP2c (aa 242 to 502), pLexA-BD CP2b (aa 43 to 540), or pB42AD PIAS1 in combination with a reporter vector p8op-lacZ. The plasmid pLexA-pos was used as a positive control. (B) Endogenous levels of PIAS1 mRNA and protein in different cell types. Mouse or human HPRT was used as an internal control for RT-PCR (as described in the legend of Fig. 1B). About 100 μg of protein from total cell extract was separated on the SDS-polyacrylamide gel and analyzed by Western blotting using an anti-PIAS1 antibody (Santa Cruz Biotechnology). β-Tubulin was used as an internal control (as described in the legend of Fig. 1C). The levels of PIAS1 proteins and mRNAs shown in the upper panel were quantitated, and the relative ratio of protein to mRNA was compared among different cell types (bottom panel). (C) 293T or K562 cells were transiently cotransfected with the pGL3-luciferase reporter plasmid that harbors the mouse α-globin promoter along with the plasmids expressing CP2b, CP2c, or FLAG-PIAS1 in different combinations. The amount of DNA was adjusted to total 0.5 μg for all transfection experiments. Shown are averages and standard deviations of three independent experiments. (D) EMSA was performed by incubating the DNA probe containing the concensus CP2c binding site in the mouse α-globin promoter with nuclear extracts from MEL cells. Each antibody against CP2c, CP2b, or PIAS1 was added to the EMSA reaction mixture to identify bands of the specific DNA-protein complex by generating supershifts. The HA antibody was used as a negative control for supershift. S, supershift probe; B, bound probe; N, nonspecifically bound probe; F, free probes. (E) ChIP assay was performed using undifferentiated (without HMBA) or differentiated (with HMBA) MEL cells by formaldehyde cross-linking and subsequent immunoprecipitation of the protein-DNA complex with antibodies against CP2c, CP2b, PIAS1, or rabbit immunoglobulin G. Immunoprecipitated DNA was amplified by PCR using mouse α-globin promoter-specific primers.
When the endogenous protein level of PIAS1 was investigated in several cell types, PIAS1 was detected at significant levels in erythroid-type cells such as K562 and MEL cells and in nonerythroid cells such as HeLa, hMSC, MCF7, mESC, 3T3-L1, and HIB-1B cells as well, but it was not detected or detected only at low levels in other nonerythroid cells including 293T, MEF, and mMSC cells (Fig. 8B). PIAS1 transcripts were detected by semiquantitative RT-PCR in all cell types investigated. These results imply that PIAS1 is ubiquitously expressed, but PIAS1 protein levels are not proportional to mRNA levels across cell types, suggesting posttranscriptional regulation.
In an attempt to identify the role of PIAS1 in CP2b-mediated α-globin expression, 293T or K562 cells were transiently cotransfected with the α-globin promoter-luciferase reporter plasmid along with the plasmids expressing CP2b, CP2c, or FLAG-PIAS1 in various combinations. The addition of three proteins, CP2b, CP2c, and PIAS1, strongly induced luciferase reporter gene expression in 293T cells (Fig. 8C). This result strongly indicates that PIAS1 is the third component involved in erythroid cell-specific CP2b-mediated α-globin gene expression. In K562 cells in which endogenous PIAS1 is present, the addition of two proteins, CP2b and CP2c, was sufficient to induce α-globin gene expression, further supporting the role of PIAS1 in erythroid K562 cells (Fig. 8C). This result raised the question of whether the combination of CP2b, CP2c, and PIAS1 is sufficient to induce expression of the endogenous α-globin gene in nonerythroid cells. 293T cells were cotransfected with plasmids expressing these proteins alone or in combinations, and expression of α-globin was assayed by RT-PCR but was not detected (data not shown).
To confirm whether PIAS1 is a component of a protein-DNA complex involved in α-globin gene expression, we performed EMSAs using nuclear extracts from MEL cells, in which the oligonucleotide-containing the consensus CP2c binding site in the mouse α-globin promoter was used as a DNA probe. Indeed, the direct or indirect interaction of endogenous CP2b, CP2c, and PIAS1 with the α-globin promoter was detected by observing supershifted DNA probes when antibody for CP2b, CP2c, or PIAS1 was added to MEL cell nuclear extracts for the EMSA reaction (Fig. 8D). Moreover, the involvement of three proteins in the protein-DNA complex in MEL cells was confirmed by the ChIP assay (Fig. 8E). The interaction between endogenous proteins and the endogenous α-globin promoter was further increased in differentiated MEL cells by HMBA induction (Fig. 8E).
DISCUSSION
Identification of CP2b and PIAS1 as components involved in α-globin gene expression in erythroid cells.
In this study, we identified a novel murine CP2 isoform, CP2b, which is the mouse homologue of human LBP-1b, and also characterized its functional role in α-globin gene expression. The amino acid sequence of CP2b is identical to that of CP2a except for an additional 36 amino acids that are encoded by an extra exon inserted by alternative splicing (Fig. 1A, panel b). The amino acid sequence in the mouse CP2b-specific exon is exactly the same as that in the human homologue LBP-1b, suggesting that this conserved region is important for the function of CP2b. Indeed, the N terminus encompassing the additional 36 amino acids is responsible for potent transcriptional activation of CP2b that was not detectable in CP2a (Fig. 5). Although CP2b (or LBP-1b) is not expressed exclusively in erythroid cells, CP2b, but not other CP2 isoforms, exerts the most potent erythroid cell-specific activation of the α-globin gene promoter (Fig. 2A). This erythroid-specific transcription activation by CP2b appears to be mediated, in part, by the interaction with other CP2 isoforms such as CP2c (Fig. 3). Our in vitro data demonstrated that CP2b itself binds weakly to the CP2c consensus sequences of the α-globin gene promoter (Fig. 6A). Furthermore, forming heteromers with other CP2 isoforms, CP2b interferes with the DNA binding of other isoforms (Fig. 6B).
Since the DNA binding property of CP2b depends on the cell type used to provide nuclear extracts (Fig. 7B and C), we hypothesized that CP2b binding to the target DNA might require not only other CP2 isoforms but also additional erythroid cell-specific regulators. Our data show that PIAS1 modulates the DNA binding activity of CP2b/c complexes in vitro (Fig. 8A and C). PIAS1 was first identified as a negative regulator for cytokine signaling that represses cytokine-activated gene expression by inhibiting STAT1-DNA binding (28). PIAS1 also acts as a small ubiquitin-like-modifier (SUMO) E3 ligase for several target proteins including c-Jun, p53, lymphoid enhancer factor 1, Smad, androgen receptor, and GATA4 (34). It also has been reported that PIAS1 can act as a transcriptional activator by interacting with COUP-TF1 for human CYP11B2 gene expression in a sumoylation-independent manner (41). Although we demonstrated by extensive analysis of the cell types in this study that PIAS1 is not erythroid specific (Fig. 8B), our current hypothesis is that an erythroid cell-specific complex that regulates α-globin and possibly other erythroid genes involves the CP2b/CP2c/PIAS1 ternary complex (Fig. 8C).
Based on our findings in this study, we propose the functional role of CP2b and PIAS1 in enhanced α-globin gene expression in erythroid cells (Fig. 9). CP2b itself retains high transcriptional activity but not DNA binding activity. Thus, the involvement of CP2b in the enhanced activation of α-globin promoter in erythroid cells is via heterodimerization with CP2c (Fig. 9A). The endogenous α-globin gene is barely expressed in undifferentiated MEL cells despite measurable levels of CP2b, CP2c, and PIAS1. However, the combined overexpression of CP2b and CP2c in undifferentiated MEL cells induces α-globin gene expression (Fig. 4B and C), suggesting that sufficient levels of both CP2b and CP2c proteins in conjunction with PIAS1 are necessary to obtain full activity of α-globin promoter in erythroid cells (Fig. 4 and 8C to E). Sufficient levels of proteins are achieved by induction of erythroid cell differentiation (Fig. 4A). Thus, it is plausible that the endogenous CP2b and/or CP2c in the undifferentiated MEL cells is either nonfunctional due to protein modifications or is not at sufficient levels to attain full activity (Fig. 9B).
FIG. 9.
A model of erythroid cell-specific α-globin gene expression exerted by CP2 isoforms and their interacting protein, PIAS1. (A) Schematic diagrams illustrating functional domains (for transcriptional activation, DNA binding, and dimerization) in each CP2 isoform. (B) A proposed model depicting the involvement of CP2b/CP2c/PIAS1 in erythroid specific α-globin gene expression. Insufficient levels of proteins or protein modification may prevent full activity of the α-globin promoter in uninduced MEL cells.
CP2 family proteins and PIAS1 in erythroid cell differentiation.
The tissue- and developmental stage-specific vertebrate globin gene expression requires transcription factor binding to locus control regions and promoter regions to open chromatin structure (3, 8, 11, 33). Transcription factors including GATA-1, NF-E2, and the erythroid Kruppel-like factor as well as chromatin modifiers might be responsible for this specificity (5, 9, 15, 47). In addition to these factors, CP2 family gene products also appear to be involved in erythroid cell-specific expression (11). Specifically, the crucial role of CP2c has been emphasized in hemoglobin synthesis during erythroid terminal differentiation (12). It has also been demonstrated that reducing the level of LBP-1c and CP2c in K562 and MEL cells, respectively, suppressed the expression of globin genes (11, 25). In addition, analyses of the factor binding site mutations within the mouse α-globin gene promoter showed that ubiquitous CP2 family genes exert their roles in the regulation of globin genes in an erythroid cell-specific manner and that CP2c binding to the globin promoter is important for the up-regulation of globin genes in erythroid differentiation (11).
The synergistic interaction of CP2b and CP2c is likely to be important in α-globin gene expression in erythroid cells in vivo. Transfection of CP2b/c induced endogenous α-globin expression in MEL cells without inducing other differentiation-specific proteins. We observed that the expression level of each CP2 isoform significantly increased 2 days after the induction of terminal differentiation of MEL cells (Fig. 4A). Since terminal differentiation in MEL induces α-globin gene expression, these results imply that CP2 proteins are involved in the expression of endogenous α-globin genes in differentiating MEL cells. CP2b-mediated expression of the mouse α-globin promoter is specific to erythroid cells, in which CP2c (or LBP-1c) and PIAS1 are highly expressed, and supplementation of these two factors in addition to CP2b is sufficient for CP2b-mediated α-globin promoter expression in 293T cells. However, transfection of all three factors did not induce endogenous α-globin expression in 293T cells. This highlights the likelihood that the chromatin structure proximal to the α-globin gene and far-upstream regulatory regions (locus control regions) in nonerythoid cells such as 293T prevents the initiation of α-globin gene expression (11, 16).
CP2a-CP2b-CP2c networking in conjunction with PIAS1 in transactivation activity.
Our data suggest that CP2a, CP2b, and CP2c could combine in a variety of heterodimers. Regulation of α-globin may depend on specific combinations of nonerythroid-specific transcription factors, and the combinations formed are influenced by the levels of the interacting proteins or protein modifications. The combinatorial heterodimerization among CP2 isoforms is quite similar to other transcription factors, including the Myc-Mad-Max network, MyoD family genes, and nuclear hormone receptors. Like Max in the Myc-Mad-Max network, CP2c exists in the center of a CP2 isoform network. CP2c forms complexes that strongly activate transcription by associating with CP2b in the presence of PIAS1 but weak transcription activation complexes when bound to CP2a. In this scenario, homodimers of CP2a or CP2c could work as weak or moderate transactivating complexes, respectively. Furthermore, similar to the Myc-Mad-Max network, CP2 isoforms appear to form multiprotein complexes with other chromatin remodeling complex proteins. Myc's ability to activate transcription appears to require its association with the additional transcriptional coactivator TRRAP, which tethers the histone acetylase GCN5, whereas transcription repression by Mad proteins occurs through the assembly of a multiunit complex (Max-Mad-Sin3-N-CoR-histone deacetylase-Ski-sno complexes) (1). Indeed, PIAS1, SUMO-1, Ubc-9, bromodomain containing protein, TTRAP, and protein 66 in the histone deacetylase complex were also identified as CP2 interacting proteins in yeast two-hybrid assays (M.-A. Park et al., unpublished data) or a phage display library screening (22). Although the transcriptional activation mechanisms could be quite similar to the Myc-Mad-Max network, further studies are required to gain insight into the detailed roles of CP2 isoforms in transactivating activities.
CP2 proteins, versatile molecules expressed ubiquitously but working specifically.
CP2 family gene products are transcription factors that play important roles in various biological events including hematopoiesis, immune response, cell cycle, and neural development by regulating genes encoding globins, interleukins, and thymidylate synthase. To determine how ubiquitously expressed CP2 family genes regulate tissue-specific target genes, we have been studying the mouse α-globin promoter.
CP2c appears to be ubiquitously transcribed, but the protein is not detectable in all cell types assayed. CP2c is involved in not only erythroid differentiation but also various cellular events including immune response, cell cycle, and the pathogenesis of neuronal diseases (13, 44). LBP-1c (LSF), the human homologue of CP2c, regulates the expression of the thymidylate synthase gene which is induced at the G1-S transition in growth-stimulated cells and is essential for DNA replication and cell survival (37). CP2c/LBP-1c also regulates the expression of tissue-specific genes such as the serum amyloid A3 gene in liver (6), the interleukin-4 gene in helper T cells (10), and the αA-crystalline gene in lens cells (32). Thus, the functional involvement of ubiquitously transcribed CP2c/LBP-1c in several cellular events in a cell type-specific manner is intriguing. Although the mechanisms that regulate tissue-specific and developmental stage-specific protein expression of the CP2 family members are not yet clear, it is likely that the interaction between CP2c and cell-restricted factors could determine the specificity of target gene expression within different cellular contexts. CP2c/LBP-1c interacts with YY1 on the human immunodeficiency virus promoter to repress transcription (14, 40). In erythroid cells, CP2c interacts with an erythroid-specific factor, NF-E4, to form a stage selector protein complex that binds to and potentially activates the γ-globin promoter (52, 53). In neuronal cells, the interaction of CP2c and the neuron-specific protein Fe65 blocks cell cycle progression by down-regulating thymidylate synthase expression (7, 50). The RING polycomb group protein dinG, involved in the repression of homeotic gene expression, can interact with CP2c to repress CP2c-dependent transcription (45). The multiple isoforms of CP2 may provide further functional diversity by altering DNA binding specificity or affinity as well as dimerization properties. In mouse mesenchymal stem cells, the interaction between CP2c and CP2b synergistically increases the transcriptional activity of mBMP4 that is related to osteoblast differentiation (21). Indeed, a large number of transcription factors that control diverse cellular responses exist as multiple isoforms (17, 29, 30). CP2c may in itself possess cell-specific functions, which are conferred by cell-specific posttranslational modifications such as phosphorylation. In fact, increased DNA binding activity of phosphorylated LBP-1c has been observed in mitogenic stimulation of resting T cells (35, 46).
Surprisingly, despite data implicating involvement in the regulation of many genes, mice lacking CP2c expression showed no significant abnormality in growth and development (38). However, a CP2a knockout mouse model demonstrated that CP2a deficiency caused defective extraembryonic angiogenesis (36). In mice lacking CP2a expression, intrauterine growth was retarded at embryonic day 10.5, resulting in embryo death 1 day later. Since CP2b is also produced in the CP2a locus by alternative splicing, both isoforms will be deleted in CP2a null mice, indicating the phenotypic effects shown in null mice might not be solely due to the deficiency of CP2a.
Acknowledgments
We appreciate the help and advice of our colleagues at the Laboratory of Molecular Genetics, Department of Life Science, Hanyang University, especially J. J. Lee, H. S. Jung, H. S. Heo, S. Y. Han, J. Jeon, K. S. Choi, and I. Shin for their technical support.
This work was supported by the research fund of Hanyang University (HY-2001). J. H. Chae, Y. H. Lee, H. C. Kang, and J. H. Shin were supported by the Brain Korea 21 Project from the Ministry of Education and Human Resources of South Korea through the Research Group on Stem Cells and Early Development.
REFERENCES
- 1.Alland, L., R. Muhle, H. Hou, J. Potes, J., L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-Cor and histon deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. [DOI] [PubMed] [Google Scholar]
- 2.Amrolia, P. J., W. Gabbard, J. M. Cunningham, and S. M. Jane. 1998. Maximal activity of an erythroid-specific enhancer requires the presence of specific protein binding sites in linked promoters. J. Biol Chem. 273:13593-13598. [DOI] [PubMed] [Google Scholar]
- 3.Baron, M. H. 1997. Transcriptional control of globin gene switching during vertebrate development. Biochim. Biophys. Acta 1351:51-72. [DOI] [PubMed] [Google Scholar]
- 4.Bender, M. A., M. Bulger, J. Close, and M. Groudine. 2000. β-globin gene switching and DNase I sensitivity of the endogenous β-globin locus in mice do not require the locus control region. Mol. Cell 5:387-393. [DOI] [PubMed] [Google Scholar]
- 5.Bieker, J. J. 1998. Erythroid-specific transcription. Curr. Opin. Hematol. 5:145-150. [DOI] [PubMed] [Google Scholar]
- 6.Bing, Z., S. A. G. Reddy, Y. Ren, J. Qin, and W. S. L. Liao. 1999. Purification and characterization of the serum amyloid A3 enhancer factor. J. Biol. Chem. 274:24649-24656. [DOI] [PubMed] [Google Scholar]
- 7.Bruni, P., G. Minopoli, T. Brancaccio, M. Napolitano, R. Faraonio, N. Zambrano, U. Hansen, and T. Russo. 2002. Fe65, a ligand of the Alzheimer's beta-amyloid precursor protein, blocks cell cycle progression by down-regulating thymidylate synthase expression. J. Biol. Chem. 277:35481-35488. [DOI] [PubMed] [Google Scholar]
- 8.Cantor, A. B., and S. H. Orkin. 2002. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 21:3368-3376. [DOI] [PubMed] [Google Scholar]
- 9.Cao, A., and P. Moi. 2002. Regulation of the globin genes. Pediatr. Res. 51:415-421. [DOI] [PubMed] [Google Scholar]
- 10.Casolaro, V., A. M. Keane-Myers, S. L. Swendeman, C. Steindler, F. Zhong, M. Sheffery, S. N. Georas, and S. J. Ono. 2000. Identification and characterization of a critical CP2-binding element in the human interleukin-4 promoter. J. Biol. Chem. 275:36605-36611. [DOI] [PubMed] [Google Scholar]
- 11.Chae, J. H., and C. G. Kim. 2003. CP2 binding to the promoter is essential for the enhanced transcription of globin genes in erythroid cells. Mol. Cells 15:40-47. [PubMed] [Google Scholar]
- 12.Chae, J. H., Y. H. Lee, and C. G. Kim. 1999. Transcription factor CP2 is crucial in hemoglobin synthesis during erythroid terminal differentiation in vitro. Biochem. Biophys. Res. Commun. 263:580-583. [DOI] [PubMed] [Google Scholar]
- 13.Chae, J. H., E. J. Oh, and C. G. Kim. 1997. Analyses of transcription factor CP2 expression during development and differentiation. Korean J. Biol. Sci. 1:143-150. [Google Scholar]
- 14.Coull, J. J., F. Romerio, J. M. Sun, J. L. Volker, K. M. Galvin, J. R. Davie, Y. Shi, U. Hansen, and D. M. Margolis. 2000. The human factors YY1 and LSF repress the human immunodeficiency virus type l long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 74:6790-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu, X.-H., D.-P. Liu, and C.-C. Liang. 2002. Chromatin structure and transcriptional regulation of the β-globin locus. Exp. Cell Res. 278:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Groudine, M., R. Eisenman, R. Gelinas, and H. Weintraub. 1983. Developmental aspects of chromatin structure and gene expression. Prog. Clin. Biol. Res. 134:159-182. [PubMed] [Google Scholar]
- 17.Hastie, N. D. 2001. Life, sex, and WT1 isoforms: three amino acids can make all the difference. Cell 106:391-394. [DOI] [PubMed] [Google Scholar]
- 18.Heo, H. S., J. H. Kim, Y. J. Lee, S.-H. Kim, Y. S. Cho, and C. G. Kim. Profiling of differentially expressed genes during erythroid differentiation of MEL cells revealed by cDNA microarray. Mol. Cells, in press.
- 19.Huang, N., and W. L. Miller. 2000. Cloning of factors related to HIV-inducible LBP proteins that regulate steroidogenic factor-1-independent human placental transcription of the cholesterol side-chain cleavage enzyme, P450scc. J. Biol. Chem. 275:2852-2858. [DOI] [PubMed] [Google Scholar]
- 20.Jane, S. M., P. A. Ney, E. F. Vanin, D. L. Gumucio, and A. W. Nienhuis. 1992. Identification of a stage selector element in the human gamma-globin gene promoter that fosters preferential interaction with the 5′ HS2 enhancer when in competition with the beta-promoter. EMBO J. 11:2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, H. C., J. H. Chae, B. S. Kim, S. Y. Han, S. H. Kim, C. K. Auh, S. I. Yang, and C. G. Kim. 2004. Transcription factor CP2 is involved in activating mBMP4 in mouse mesenchymal stem cells. Mol. Cells 17:454-461. [PubMed] [Google Scholar]
- 22.Kang, H. C., B. M. Chung, J. H. Chae, S. I. Yang, C. G. Kim, and C. G. Kim. 2005. Identification and characterization of four novel peptide motifs that recognize distinct regions of the transcription factor CP2. FEBS J. 272:1265-1277. [DOI] [PubMed] [Google Scholar]
- 23.Kim, C. G., K. M. Barnhart, and M. Sheffery. 1988. Purification of multiple erythroid cell proteins that bind the promoter of the α-globin gene. Mol. Cell. Biol. 8:4270-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, C. G., S. L. Swendeman, K. M. Barnhart, and M. Sheffery. 1990. Promoter-elements and erythroid cell nuclear factors that regulate α-globin gene transcription in vitro. Mol. Cell. Biol. 10:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, H. S., E. M. Kim, J. P. Lee, C. H. Park, S. Kim, J. H. Seo, K. A. Chang, E. Yu, S. J. Jeong, Y. H. Chong, and Y. H. Suh. 2003. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3beta expression. FASEB J. 17:1951-1953. [DOI] [PubMed] [Google Scholar]
- 26.Lim, L. C., L. Fang, S. L. Swendeman, and M. Sheffery. 1993. Characterization of the molecularly cloned murine alpha-globin transcription factor CP2. J. Biol. Chem. 268:18008-18017. [PubMed] [Google Scholar]
- 27.Lim, L. C., S. L. Swendeman, and M. Sheffery. 1992. Molecular cloning of the alpha-globin transcription factor CP2. Mol. Cell. Biol. 12:828-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, B., J. Liao, X. Rao, S. A. Kushner, C. D. Chung, D. D. Chang, and K. Shuai. 1998. Inhibition of Stat1-mediated gene activation by PIAS1. Cell Biol. 95:10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez, A. J. 1998. Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu. Rev. Genet. 32:279-305. [DOI] [PubMed] [Google Scholar]
- 30.Lopez, A. J. 1995. Developmental role of transcription factor isoforms generated by alternative splicing. Dev. Biol. 172:396-411. [DOI] [PubMed] [Google Scholar]
- 31.Milot, E., J. Strouboulis, T. Trimborn, M. Wijgerde, E. de Boer, A. Langeveld, K. Tan-Un, W. Vergeer, N. Yannoutsos, F. Grosveld, and P. Fraser. 1996. Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell 87:105-114. [DOI] [PubMed] [Google Scholar]
- 32.Murata, T., M. Nitta, and K. Yasuda. 1998. Transcription factor CP2 is essential for lens-specific expression of the chicken αA-crystallin gene. Genes Cells 3:443-457. [DOI] [PubMed] [Google Scholar]
- 33.Orkin, S. H. 1995. Regulation of globin gene expression in erythroid cells. Eur. J. Biochem. 231:271-281. [DOI] [PubMed] [Google Scholar]
- 34.O'shea, J. J., and W. Watford. 2004. A peek at PIAS. Nat. Immunol. 5:875-876. [DOI] [PubMed] [Google Scholar]
- 35.Pagon, Z., J. Volker, G. M. Cooper, and U. Hansen. 2003. Mammalian transcription factor LSF is a target of ERK signaling. J. Cell. Biochem. 89:733-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parekh, V., A. McEwen, V. Barbour, Y. Takahashi, J. E. Rehg, S. M. Jane, and J. M. Cunningham. 2004. Defective extraembryonic angiogenesis in mice lacking LBP-1a, a member of the grainyhead family of transcription factors. Mol. Cell. Biol. 24:7113-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell, C. M., T. L. Rudge, Q. Zhu, L. F. Johnson, and U. Hansen. 2000. Inhibition of the mammalian transcription factor LSF induces S-phase-dependent apoptosis by downregulating thymidylate synthase expression. EMBO J. 19:4665-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramamurthy, L., V. Barbour, A. Tuckfield, D. R. Clouston, D. Topham, J. M. Cuningham, and S. M. Jane. 2001. Targeted disruption of the CP2 gene, a member of the NTF family of transcription factors. J. Biol. Chem. 276:7836-7842. [DOI] [PubMed] [Google Scholar]
- 39.Rodda, S., S. Sharma, M. Scherer, G. Chapman, and P. Rathjen. 2001. CRTR-1, a developmentally regulated transcriptional repressor related to the CP2 family of transcription factors. J. Biol. Chem. 276:3324-3332. [DOI] [PubMed] [Google Scholar]
- 40.Romerio, F., M. N. Gabriel, and D. M. Margolis. 1997. Repression of human immunodeficiency virus type 1 through the novel cooperation of human factors YY1 and LSF. J. Virol. 71:9375-9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata, H., S. Kobayashi, I. Kurihara, N. Suda, K. Yokota, A. Murai, Y. Ikeda, I. Saito, W. E. Rainey, and T. Saruta. 2004. COUP-TF and transcriptional co-regulators in adrenal steroidogenesis. Endocr. Res. 30:795-801. [DOI] [PubMed] [Google Scholar]
- 42.Shirra, M. K., and U. Hansen. 1998. LSF and NTF-1 share a conserved DNA recognition motif yet require different oligomerization states to form a stable protein-DNA complex. J. Biol. Chem. 273:19260-19268. [DOI] [PubMed] [Google Scholar]
- 43.Solis, C., G. I. Aizencang, K. H. Astrin, D. E. Bishop, and R. Desnick. 2001. Uroporphyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J. Clin. Investig. 107:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swendeman, S. L., C. Spielholz, N. A. Jenkins, D. J. Gilbert, N. G. Copeland, and M. Sheffery. 1994. Characterization of the genomic structure, chromosomal location, promoter, and development expression of the alpha-globin transcription factor CP2. J. Biol. Chem. 269:11663-11671. [PubMed] [Google Scholar]
- 45.Tuckfield, A., D. R. Clouston, T. M. Wilanowski, L.-L. Zhao, J. M. Cunningham, and S. M. Jane. 2002. Binding of the RING polycomb proteins to specific target genes in complex with the grainyhead-like family of developmental transcription factors. Mol. Cell. Biol. 22:1936-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volker, J. L., L. E. Rameh, Q. Zhu, J. DeCaprio, and U. Hansen. 1997. Mitogenic stimulation of resting T cells causes rapid phosphorylation of the transcription factor LSF and increased DNA-binding activity. Genes Dev. 11:1435-1446. [DOI] [PubMed] [Google Scholar]
- 47.Weiss, M. J., and S. H. Orkin. 1995. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl. Acad. Sci. USA 92:9623-9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye, S. K., Y. Agata, H. C. Lee, H. Kurooka, T. Kitamura, A. Shimizu, T. Honjo, and K. Ikuta. 2001. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity 15:813-823. [DOI] [PubMed] [Google Scholar]
- 49.Yoon, J. B., G. Li, and R. G. Roeder. 1994. Characterization of family of related cellular transcription factors which can modulate human immunodeficiency virus type I transcription in vitro. Mol. Cell. Bilo. 14:1776-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zambrano, N., G. Minopoli, P. Candia, and T. Russo. 1998. The Fe65 adaptor protein interacts through its PID1 domain with the transcription factor CP2/LSF/LBP1. J. Biol. Chem. 273:20128-20133. [DOI] [PubMed] [Google Scholar]
- 51.Zhong, F., S. L. Swendeman, W. Popik, P. M. Pitha, and M. Sheffery. 1994. Evidence that levels of the dimeric cellular transcription factor CP2 play little role in the activation of the HIV-1 long terminal repeat in vivo or following superinfection with herpes simplex virus type 1. J. Biol. Chem. 269:21269-21276. [PubMed] [Google Scholar]
- 52.Zhou, W., D. R. Clouston, X. Wang, L. Cerruti, J. M. Cunningham, and S. M. Jane. 2000. Induction of human fetal globin gene expression by a novel erythroid factor, NF-E4. Mol. Cell. Biol. 20:7662-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou, W., Q. Zhao, R. Sutton, H. Cumming, X. Wang, L. Cerruti, M. Hall, R. Wu, J. M. Cunningham, and S. M. Jane. 2004. The role of p22 NF-E4 in human globin gene switching. J. Biol. Chem. 279:26227-26232. [DOI] [PubMed] [Google Scholar]