Abstract
Mast cell carboxypeptidase A (Mc-cpa) is a highly conserved secretory granule protease. The onset of expression in mast cell progenitors and lineage specificity suggest an important role for Mc-cpa in mast cells. To address the function of Mc-cpa, we generated Mc-cpa-null mice. Mc-cpa−/− mast cells lacked carboxypeptidase activity, revealing that Mc-cpa is a nonredundant enzyme. While Mc-cpa−/− peritoneal mast cells were ultrastructurally normal and synthesized normal amounts of heparin, they displayed striking histochemical and biochemical hallmarks of immature mast cells. Wild-type peritoneal mast cells had a mature phenotype characterized by differential histochemical staining with proteoglycan-reactive dyes (cells do not stain with alcian blue but stain with safranin and with berberine) and a high side scatter to forward scatter ratio by flow cytometry and were detergent resistant. In contrast, Mc-cpa−/− peritoneal mast cells, like immature bone marrow-derived cultured mast cells, stained with alcian blue normally or weakly and either did not stain with safranin and berberine or stained weakly, had a low side scatter to forward scatter ratio, and were detergent sensitive. This phenotype was partially ameliorated with age. Thus, histochemistry and flow cytometry, commonly used to measure mast cell maturation, deviated from morphology in Mc-cpa−/− mice. The Mc-cpa−/− mast cell phenotype was not associated with defects in degranulation in vitro or passive cutaneous anaphylaxis in vivo. Collectively, Mc-cpa plays a crucial role for the generation of phenotypically mature mast cells.
Mast cells are key mediators of host defense and inflammatory reactions by releasing upon activation a range of inflammatory substances which include proteases, histamine, heparin, cytokines, chemokines, and lipid mediators. Once in the extracellular space, the mediators can regulate, for instance, local blood flow, vascular permeability, smooth muscle contraction, and connective tissue remodeling (reviewed in references 6, 13, 22, and 48).
Mast cell proteases represent major protein components of secretory granules, but the role of each individual protease in mast cells remains poorly understood. The proteases are classified into a carboxypeptidase, chymases, and tryptases (reviewed in reference 16). Mast cells differ in their protease expression pattern depending on the tissue location where they reside (7, 33, 42) (reviewed in references 13 and 21). According to this classification, connective tissue mast cells (CTMC) are located primarily in the skin and at serosal sites, such as the peritoneal cavity. CTMC express, in addition to mast cell carboxypeptidase A (Mc-cpa), mouse mast cell protease 2 (Mcp-2), Mcp-4, Mcp-5, Mcp-6, and Mcp-7. CTMC are present constitutively, i.e., in the absence of an immune response involving mast cells (reviewed in reference 40). Mucosal mast cells (MMC) express a different protease spectrum, primarily Mcp-1 and Mcp-2, and their cell numbers increase upon inflammation at mucosal sites (reviewed in reference 19).
CTMC and MMC express different proteoglycans (reviewed in references 28 and 41). CTMC synthesize and store in their granules heparin, a highly sulfated glycosaminoglycan which can be specifically detected by berberine sulfate staining (8). In contrast, MMC synthesize predominantly chondroitin sulfate, but not heparin. Alcian blue (AB) and safranin (S) are commonly used histochemical dyes which stain CTMC and MMC differentially. CTMC do not stain with alcian blue but stain with safranin (AB− S+), and MMC show the reverse staining pattern (AB+ S−). This differential staining reactivity probably reflects the balance of heparin and chondroitin sulfate synthesized by CTMC and MMC, respectively (7, 14, 45).
The onset of protease expression in mast cell ontogeny differs between various mast cell proteases. Mc-cpa, a mast cell-specific gene (32), is expressed in the earliest known mast cell-committed progenitors identified in fetal blood as cells expressing high levels of c-Kit and low levels of Thy-1 and lacking FcɛRI expression (34). Mc-cpa is also, albeit weakly, expressed in immature bone marrow (BM)-derived mast cell cultures (BMMC) (5, 14, 36). In mature mast cells, Mc-cpa is abundantly expressed in CTMC but only weakly in MMC (14, 36). The genes encoding Mc-cpa are highly conserved in mice (36) and humans (31), suggesting a common but not yet known role in both species. Mc-cpa is an exopeptidase which preferentially cleaves C-terminal aliphatic amino acids. Natural substrates of Mc-cpa are unknown.
Some proteases form complexes within the mast cell prior to degranulation. Mc-cpa, a major protein component in secretory granules, is released complexed to proteoglycans upon mast cell degranulation (36). Both Mc-cpa and Mcp-5 are bound to heparin. Mc-cpa appears to depend on Mcp-5 expression because Mcp-5-deficient mast cells lack Mc-cpa protein, but not mRNA expression (16). Heparin itself is an essential component in these complexes as shown in mast cells lacking sulfated heparin (10, 17). During heparin synthesis, most of the N-acetylglucosamine residues are modified by N deacetylation and N sulfation. These modification steps are catalyzed by glucosaminyl N-deacetylase/N-sulfotransferase 2 (Ndst-2). Ndst-2-deficient mast cells lack Mcp-4, Mcp-5, Mcp-6, and mature Mc-cpa proteins (10, 15, 17). These findings strongly implicate heparin as a central assembly or storage site of protease-heparin complexes (reviewed in reference 50). As a consequence, Ndst-2−/− mast cells are poorly granulated (17), and their secretory granules are ultrastructurally enlarged and appear empty (10).
We report here that Mc-cpa−/− mast cells lack Mc-cpa protein and, concomitantly, Mcp-5 but do contain Mcp-4, and Mcp-6 proteins. On the basis of metabolic labeling with [35S]sulfate and on HNO2 degradation, heparin synthesis is unaffected by the loss of Mc-cpa. Peritoneal mast cells (PMC) develop in normal numbers in Mc-cpa−/− mice, but these mast cells have an immature phenotype akin to that of BMMC as assessed by light microscopic histochemistry. Our data imply an important constitutive, activation-independent role for a mast cell protease in normal mast cell development.
MATERIALS AND METHODS
Targeting of the Mc-cpa locus in ES cells.
Homologous sequences for the targeting vector were amplified by PCR from a 129/SvJ bacterial artificial chromosome (Incyte Genomics). The targeting vector consisted, from 5′ to 3′, of the 5′ arm (nucleotides −818 to −8 of the Mc-cpa gene [51]), an Egfp minigene (9), a loxP-flanked neomycin resistance gene (12), the 3′ arm (nucleotides +18 to +8024 of the Mc-cpa gene) (30, 32), and the herpes simplex virus thymidine kinase gene for negative selection. Embryonic stem (ES) cells (E14.1; 129/0la) were electroporated with the NotI-linearized targeting vector. Clones that had undergone homologous recombination were identified by PCR using a Mc-cpa 5′ oligonucleotide (5′-CTTTAATTCCAGCACTTGGATTTCG-3′) and an Egfp 3′-oligonucleotide (5′-CCGGACACGCTGAACTTGTGGC-3′). Homologous recombination was confirmed by Southern blotting. DNA was digested with EcoRV or with SphI and XhoI. Blots were hybridized with a radiolabeled 1.2-kb 5′ external probe (see Fig. 1A). The neo gene was excised by transient transfection with a Cre recombinase expression vector. The loss of neo was verified by G418 sensitivity and by sequencing across the remaining loxP site. ES cell clones were injected into BDF1 blastocysts, and chimeric male founder mice were crossed to C57BL/6 (B6) females.
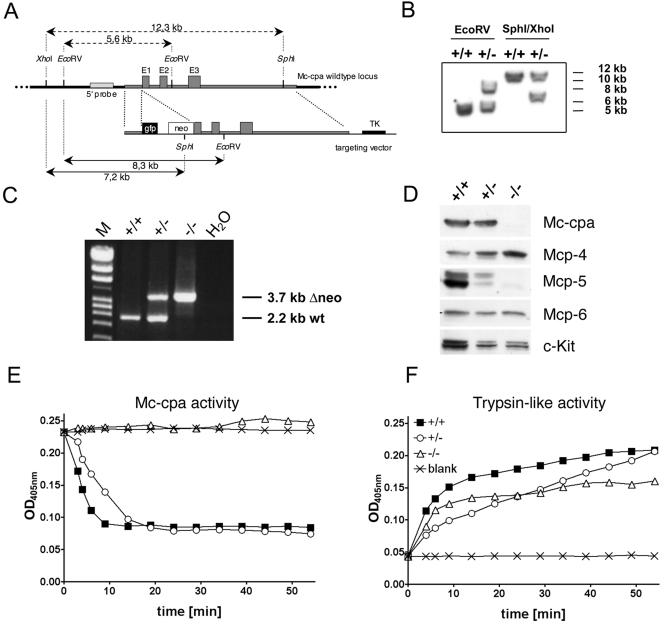
FIG. 1.
Generation of Mc-cpa−/− mice and protein and enzyme analyses of Mc-cpa−/− mast cells. (A) The targeting construct included a short 5′ arm, the enhanced green fluorescent protein gene (gfp), the neomycin resistance gene (neo), a long 3′ arm including exons 1 (from nucleotide +18 on), 2 and 3, and the thymidine kinase (TK) gene. (B) Southern blot of Mc-cpa+/+ (+/+) and Mc-cpa+/− (+/−) ES cell DNA digested with EcoRV or SphI/XhoI. The 5′ probe and expected fragment lengths are shown in panel A. (C) Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− mice were identified by single 2.2-kb PCR bands, 2.2-kb and 3.7-kb PCR bands, or single 3.7-kb PCR bands. Lane M contains molecular size markers. wt, wild type. (D) Western blot analysis of Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− mast cells shows the absence of Mc-cpa and Mcp-5 proteins in Mc-cpa−/− mast cells. Mcp-4 is upregulated, and Mcp-6 is unaltered. (E and F) Lysates of Mc-cpa+/+ (▪), Mc-cpa+/− (○), and Mc-cpa−/− (▵) peritoneal mast cells or the blank control (×) were analyzed for carboxypeptidase activity (E) and trypsin-like activity (F) by test substrates (15). Mc-cpa−/− mast cells lacked Mc-cpa activity (E). Cells from all genotypes had trypsin-like activity (F). OD405nm, optical density at 405 nm.
Mice.
Mice were maintained under specific-pathogen-free conditions. Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− mice were littermates from intercrosses of 129 × B6 F1 mice or lines of Mc-cpa+/+ and Mc-cpa−/− mice established from the fourth backcross to B6 mice. Mice were genotyped by PCR using oligonucleotides annealing upstream of the 5′ arm (5′-CTGACAGTGGCCAACTGTAAG-3′) and within the 3′ arm (5′-CTCAATGCTTTGGGTCAAGTTC-3′), yielding 2.2-kb (Mc-cpa+) and 3.7-kb (Mc-cpa−) fragments (see Fig. 1C).
Mast cell cultures.
BMMC were established by culture of BM cells at 5 × 105 cells per ml in Iscove's modified Dulbecco's medium (Gibco) supplemented with 1% interleukin 3 (IL-3) supernatant from an Il-3 gene-transfected cell line (18), and 50 ng/ml recombinant stem cell factor (R&D Systems). After 4 weeks, the vast majority of BMMC expressed c-Kit, FcɛRI, and the mast cell marker T1 (25).
Flow cytometry.
Antibodies used were allophycocyanin-labeled anti-c-Kit (2B8; Pharmingen), immunoglobulin E (IgE) (SPE-7; Sigma) followed by fluorescein isothiocyanate (FITC)-labeled anti-IgE (R35-72; Pharmingen), or FITC-labeled anti-T1 (DJ8; Morwell Diagnostics). Fcγ receptors were blocked with 0.5 mg/ml mouse IgG (Dianova) prior to staining. Cells were stained, analyzed, and sorted as described previously (47) using FACSCalibur and FACSAria instruments (Becton Dickinson). Data are displayed as dot plots using CellQuest or FACSDiva software.
Histochemistry, immunofluorescence, and determination of cell numbers.
Mast cells were cytospun (Cytospin3; Shandon) onto glass slides at 700 rpm for 5 min and stained with toluidine blue or alcian blue and safranin or with berberine (1). For berberine staining of tissue mast cells, 5-μm paraffin sections of ears fixed in Carnoy's fluid (ethanol:chloroform:acetic acid, 6:3:1) overnight and then in 100% ethanol for 8 h were dewaxed by heating the glass slides to 80°C. Remaining paraffin was removed by successive washes with xylol and ethanol. Further staining procedure was as for berberine staining on cytospins. For toluidine staining of ear sections, paraffin ear sections fixed in Carnoy's fluid and dewaxed were subjected to a descending series of ethanol solutions (96%, 80%, 70%, and 50% ethanol, and water), and subsequently stained for 10 min in 0.3% aqueous toluidine solution. After a brief wash with water, specimens were dehydrated with 96% and 99% ethanol. After a final incubation in xylol, slides were mounted with Entellan (Merck). Alcian blue and safranin staining was done on formalin-fixed paraffin sections. Dewaxing and hydrating of the specimens were done as described for toluidine staining. Slides were incubated for 15 min in alcian blue and safranin staining solution (3.6 g/liter alcian blue, 180 mg/liter safranin, 4.8 g/liter ferric ammonium sulfate in 1 M sodium acetate adjusted to pH 1.42 with HCl) and washed with water. After dehydration in tert-butanol and incubation in xylol, slides were mounted in Entellan. Antiheparin antibody staining was done on acetone-fixed 5-μm cryosections. After the sections were blocked with 0.1 mg/ml mouse IgG in phosphate-buffered saline (PBS) containing 1% bovine serum albumin, sections were incubated with ST-1 (antiheparin; IgM) (44) and then with FITC-labeled anti-IgM and allophycocyanin-labeled anti-c-Kit antibodies. Mast cells in ear skin were counted as metachromatically (toluidine) stained cells per 10 view fields (5.2 mm) beginning from the tip of the ear. c-Kit+ PMC were enumerated as c-Kit+ peritoneal exudate cells (PEC) by flow cytometry using counting beads (Caltag).
Western blots.
Total PEC were lysed in sample buffer under reducing conditions, and aliquots representing 4 × 105 PMC were subjected to 11.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer onto polyvinylidene difluoride membranes, the blots were probed with antiprotease sera followed by isotype-specific horseradish peroxidase-conjugated secondary antibodies. Membranes were developed with the ECL system (Pierce). Staining for c-Kit served as a mast cell-specific positive control. Antisera against Mc-cpa, Mcp-5, and Mcp-6 were made against mouse proteins (glutathione S-transferase fusions) in rats. The antiserum against Mcp-4 was made in rabbits against a surface-exposed peptide coupled to keyhole limpet hemocyanin (10).
Enzyme assays.
A total of 5 × 106 PEC were lysed in 470 μl PBS, 2 M NaCl, 0.5% Triton X-100. Seventy microliters of these lysates and 30 μl water were mixed with 20 μl substrate (1.8 mM), and the absorbance at 405 nm was monitored. The substrates (Bachem) were Suc-Ala-Ala-Pro-Arg-p-nitroanilide and N-(4-methoxyphenylazoformyl)-Phe-OH for trypsin-like and Mc-cpa activities, respectively (15).
Assays for mast cell functions.
PMC were treated with 2 μM ionomycin in Tyrode's buffer (10 mM HEPES, pH 7.4, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 0.1% bovine serum albumin) for 10 min at 37°C. The degree of degranulation was determined by measuring β-hexosaminidase release (27). Aliquots of supernatants and pellets from untreated and ionomycin-treated cells were extracted by three freeze-thaw cycles and incubated with the substrate p-nitrophenyl-N-acetyl-β-d-glucosamine (1 mM p-NAG [Sigma] in 0.1 M sodium citrate, pH 4.5). After 2 h, the reaction was stopped by the addition of 1 M Tris. Substrate conversion was measured by absorbance at 405 nm. The degree of degranulation was calculated as the percentage of the optical density of the supernatant divided by the optical density of the supernatant plus pellet. Total histamine content and histamine release after ionomycin treatment were determined for purified mast cells using an histamine enzyme immunoassay kit (Beckman Coulter). For passive cutaneous anaphylaxis (PCA), ears were injected intradermally with 20 ng of murine monoclonal antidinitrophenyl (anti-DNP) IgE (SPE-7) in 15 μl PBS or with PBS only. Eighteen hours later, mice were injected intravenously with 100 μg DNP-human serum albumin (HSA) in 1% Evan's blue in PBS (all reagents from Sigma). Ear photographs were taken 5 min after this antigenic challenge.
Analysis of proteoglycan synthesis and detergent lysis.
For proteoglycan analysis, 1.5 × 105 sorted PMC or 2 × 106 cultured BMMC were metabolically labeled for 18 h with 3.7 MBq/ml [35S]sulfate in sulfate-free Eagle's minimum essential medium supplemented with 10% fetal calf serum that had been dialyzed against PBS. Labeled cells were washed twice with PBS and then successively treated with 50 mM Tris-HCl (pH 7.5) containing 1% (wt/vol) Triton X-100 (fraction L1) and with 50 mM Tris-HCl (pH 7.5) containing 1% (wt/vol) Triton X-100 and 2 M KCl (fraction L2). Culture medium and cell extracts L1 and L2 (the latter after 20-fold dilution with 50 mM Tris-HCl, pH 7.0) were separately applied to DEAE Sepharose FastFlow columns (Amersham Pharmacia) equilibrated with 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl and 0.1% Triton X-100. Columns were washed with 10 volumes of equilibration buffer and subsequently with 10 volumes of 50 mM sodium acetate (pH 4.0) containing 150 mM NaCl and 0.1% Triton X-100, and total proteoglycans were eluted with 10 volumes of 50 mM sodium acetate (pH 4.0) containing 2 M NaCl. Radioactivity was determined by liquid scintillation counting of aliquots of the eluate fractions. Radioactivity-containing fractions were pooled, diluted, and applied to a 1-ml HiTrap DEAE Sepharose FastFlow column (Amersham Pharmacia) equilibrated with 50 mM sodium acetate (pH 4.0) containing 50 mM LiCl. After the column was washed with 5 ml of this buffer, a 25-ml linear gradient with a final concentration of 2.5 M LiCl was applied to sequentially elute distinct glucosaminoglycan classes. Radioactivity in the eluate fractions was determined by liquid scintillation counting. To degrade N-sulfated glycosaminoglycans (heparin and heparan sulfate), samples were subjected to HNO2 treatment at pH 1.5 as described previously (37) prior to analysis by anion-exchange chromatography.
Electron microscopy.
Ear and back skin, tongue, stomach, PEC, and sorted PMC were prepared for electron microscopy using previously reported protocols (3, 34). A total of 30 specimens from Mc-cpa+/+ and Mc-cpa+/− and Mc-cpa−/− mice were studied by electron microscopy, and all mast cells in these samples were photographed.
RESULTS
The loss of Mc-cpa protein and carboxypeptidase enzyme activity in Mc-cpa−/− mast cells.
A mutant Mc-cpa allele was generated by homologous recombination in E14 ES cells. The targeting vector (Fig. 1A) was constructed with the aims (i) to disrupt the Mc-cpa gene and (ii) to introduce the enhanced green fluorescent protein gene (Egfp) at the ATG start codon of the Mc-cpa locus (32, 51) to visualize Mc-cpa-expressing cells. Three ES cell clones showed homologous recombination at the Mc-cpa locus by Southern blotting (Fig. 1B). The lox-P-flanked neo gene was removed from the recombinant Mc-cpa locus in vitro. Mutant ES cells were injected into blastocysts, and the resulting chimeras transmitted the mutant allele through the germ line (Fig. 1C). The Egfp insertion led to a Mc-cpa-null allele (see below) which we refer to as Mc-cpa−. Consistent with the mast cell lineage-restricted expression of Mc-cpa (5, 30, 31, 34), Mc-cpa−/− mice appeared normal and healthy, and they were born at the expected Mendelian ratio.
To determine whether the introduced Mc-cpa mutation lead to a null allele, lysates from PEC (Fig. 1D to F) and BMMC (not shown) of Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− mice were tested for protease expression by Western blotting (Fig. 1D) and for enzyme activity (Fig. 1E and F). The immature Mc-cpa precursor (50 kDa) (not shown) and the mature form of Mc-cpa (36 kDa) were expressed in Mc-cpa+/+ and Mc-cpa+/− mast cells. Consistent with homozygous null alleles, Mc-cpa−/− mast cells lacked Mc-cpa protein expression (Fig. 1D). Interestingly, the expression of other secretory granule proteases was concomitantly affected. Mc-cpa−/− mast cells also lacked Mcp-5 protein (Fig. 1D) but not mRNA (not shown) expression. Mcp-4 expression was upregulated, and Mcp-6 expression was unchanged.
Next, we analyzed whether Mc-cpa is the only enzyme with carboxypeptidase specificity expressed in mast cells. Lysates from Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− peritoneal cells were incubated with test substrates. Mc-cpa+/+ and Mc-cpa+/− cell lysates cleaved a peptide substrate specific for Mc-cpa enzyme activity (15). In contrast, Mc-cpa−/− mast cells lacked this enzyme activity entirely (Fig. 1E). As a positive control, the same lysates were analyzed for cleavage of a substrate indicative of trypsin-like enzyme activity. The latter activity was present in cells from all three genotypes (Fig. 1F).
Collectively, introduction of Egfp into the Mc-cpa locus generated a null allele as shown by the absence of Mc-cpa protein and its corresponding enzyme activity. To test for Egfp expression, we developed ES cell-derived mast cells (46, 49) from targeted ES cells in vitro. Fifteen to 20% of Mc-cpa+/− ES cell-derived mast cells expressed the Egfp gene (not shown). However, when the Mc-cpa− allele was transmitted via the germ line to Mc-cpa+/− or Mc-cpa−/− mice, ex vivo-isolated mast cells did not express Egfp, a fact which has precluded the use of this mouse line to track Mc-cpa-expressing cells. Preliminary experiments indicate that expression of Egfp from this locus is prevented by DNA methylation. The molecular basis for this unexpected marker gene inactivation requires further investigation.
Phenotype, granule content, and ultrastructure of Mc-cpa−/− mast cells.
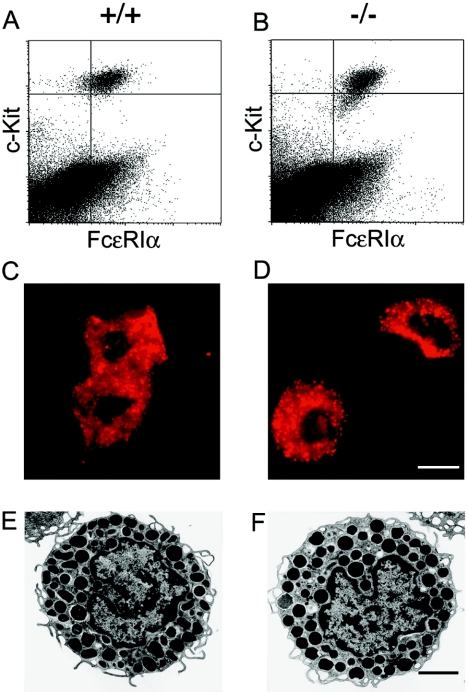
Mc-cpa expression occurs very early in mast cell ontogeny (5, 14, 34). Its expression is upregulated in CTMC which are located mostly in the skin and the peritoneal cavity. Mc-cpa expression is low in mucosal mast cells (36). Therefore, we focused our analyses on CTMC. Peritoneal mast cells, defined by their c-Kit+ FcɛRI+ phenotype, were present in both Mc-cpa+/+ (Fig. 2A) and Mc-cpa−/− (Fig. 2B) mice. Peritoneal mast cell numbers were counted by flow cytometry. Both the relative percentages of mast cells among PEC and the absolute mast cell numbers were comparable for Mc-cpa+/+ mice (1.7% ± 0.2% [mean ± standard deviation] of mast cells per total PEC; 5.1 × 104 ± 1.0 × 104 mast cells per peritoneal cavity; n = 8) and Mc-cpa−/− mice (1.3% ± 0.3%; 5.0 × 104 ± 1.1 × 104; n = 8) mice (Table 1). Sorted peritoneal mast cells from Mc-cpa+/+ and Mc-cpa−/− mice had the typical granular appearance following acridine orange staining, a dye specific for acidic granules (Fig. 2C and D). The numbers of ear skin mast cells, enumerated histologically by toluidine blue staining, were also similar in wild-type mice (156 ± 26 cells per 10 microscopic view fields, which corresponds to 5.2 mm; n = 4), and Mc-cpa−/− mice (140 ± 20 cells; n = 4) (Table 1). Hence, the loss of Mc-cpa has no impact on the size of peritoneal and skin mast cell compartments.
FIG. 2.
Mc-cpa−/− mast cells are normal with regard to staining for c-Kit and FcɛRI expression, acridine orange staining, and transmission electron microscopy. Peritoneal mast cells from Mc-cpa+/+ (+/+) (A, C, and E) and Mc-cpa−/− (−/−) (B, D, and F) mice were analyzed by flow cytometry for c-Kit and FcɛRI expression (A and B) (for relative and absolute mast cell frequencies, see Table 1), by acridine orange staining showing acidic granules (C and D), and by electron microscopy to reveal their ultrastructure (E and F). The scale bars in panel D (12 μm) and panel F (8.5 μm) apply to panels C and D and panels E and F, respectively.
TABLE 1.
Normal cell numbers but altered phenotype of connective tissue mast cells in Mc-cpa−/− micea
| Cell and genotypeb | No. of c-Kit+ PECc | PMCd (% of PEC [n]) | Histochemistry
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Cells expressing indicated phenotype (n)e
|
No. of cells/10 fields expressing indicated phenotype (n)f
|
|||||||||||
| Toluidine
|
Berberine
|
Alcian blue | Mixed | Safranin | Toluidine | Berberine
|
||||||
| Normal | Low | Normal | Low | Normal | Low | |||||||
| PMC | ||||||||||||
| +/+ | 51,000 ± 10,300 (8) | 1.7 ± 0.2 (8) | 98 | 2 | 100 | 0 | 0 | 0 | 100 (2) | |||
| −/− | 50,000 ± 10,700 (8) | 1.3 ± 0.3 (8) | 26 | 74 | 0 | 100 | 15 ± 5 (2) | 29.5 ± 0.5 (2) | 55.5 ± 5.5 (2) | |||
| Ear | ||||||||||||
| +/+ | 0 | 4.5 ± 3.5 (2) | 95.5 ± 3.5 (2) | 156 ± 26 (4) | 149 ± 21 (4) | 0 (4) | ||||||
| −/− | 1.5 ± 0.5 (2) | 16.5 ± 1.5 (2) | 81.5 ± 0.5 (2) | 140 ± 20 (4) | 91 ± 37 (4) | 6 ± 2 (4) | ||||||
Absolute numbers and relative percentages of peritoneal and ear skin mast cells were measured by cell counting and flow cytometry (PMC) and by histology (ear). Mice were 58 days old for peritoneal mast cell analysis. Mice were 51 and 65 days old for alcian and safranin staining and for toluidine staining, respectively, for ear mast cell analysis.
Peritoneal mast cells and ear skin mast cells from wild-type (Mc-cpa+/+ [+/+]) and mutant (Mc-cpa−/− [−/−]) mice.
Absolute number of c-kit+ mast cells per peritoneal exudate cell per mouse (all cells recovered from the peritoneal cavity), determined by flow cytometry.
PMC, c-Kit+ peritoneal mast cells. n, number of mice.
Distribution of normal or low-level toluidine, berberine, or alcian blue and safranin staining in all mast cells. Mixed, alcian blue and safranin. n, number of mice.
Number of cells in 10 view fields (5.2 mm skin length). n, number of mice.
Next, mast cells were examined for their ultrastructure by transmission electron microscopy (4) (Fig. 2E and F). We compared by electron microscopy a total of 2,500 mast cells from Mc-cpa+/+ (905 mast cells), Mc-cpa+/− (709 mast cells), and Mc-cpa−/− (886 mast cells) mice. The age of the mice analyzed ranged from 45 to 205 days. Cells from all genotypes contained large numbers of electron-dense mast cell granules. No morphological differences could be detected in mast cells in situ in the tongue, in ear and back skin, in the stomach, and among PEC (not shown) and in peritoneal mast cells sorted from Mc-cpa+/+ (Fig. 2E) and Mc-cpa−/− (Fig. 2F) mice. Thus, unlike the loss of Ndst-2 (10, 17), lack of Mc-cpa is not associated with a morphologically apparent phenotype.
Peritoneal mast cells of Mc-cpa−/− mice are histochemically immature.
Mature mast cells can be recognized by metachromatic staining in toluidine blue. Both Mc-cpa+/+ and Mc-cpa−/− peritoneal and skin mast cells were toluidine blue+. However, there were clear differences in the intensity of toluidine blue staining. In Mc-cpa+/+ mice, 98% of all peritoneal mast cells stained very strongly with toluidine blue (Table 1; Fig. 3H). In contrast, we observed a heterogeneous staining pattern for Mc-cpa−/− mice in which only 26% of peritoneal mast cells strongly stained with toluidine blue, the remaining mast cells exhibited low level of staining with toluidine blue (Fig. 3I and J). In the skin, comparable numbers of mast cells were recognized by toluidine blue staining in both genotypes (Table 1), however, the toluidine blue staining was weaker overall in Mc-cpa−/− mice.
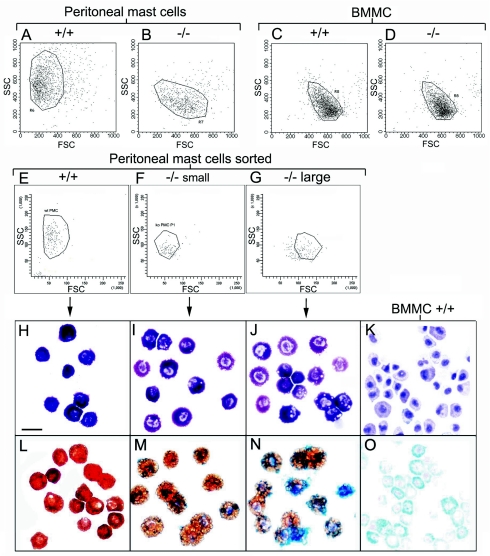
FIG. 3.
Mc-cpa−/− peritoneal mast cells resemble immature BMMC and possess a mast cell progenitor phenotype. Forward (FSC) and side scatter (SSC) were determined by flow cytometry for Mc-cpa+/+ (+/+) (A, C, and E) and Mc-cpa−/− (−/−) (B, D, F, and G) peritoneal mast cells (A, B, E, F, and G) and immature BMMC (C and D). Peritoneal mast cells from Mc-cpa−/− mice were separated by cell sorting into small (F) and large (G) subpopulations. Cytospins from the indicated peritoneal mast cell populations were stained with toluidine blue (H to J) and with alcian blue and safranin (L to N) and compared to BMMC (K and O). The scale bar in panel H (20 μm) applies to panels H to O. wt, +/+; ko, −/−.
Connective tissue and mucosal tissue mast cells can be distinguished histochemically by their staining pattern using alcian blue and safranin. CTMC are AB− S+, while MMC are AB+ S− (45). Wild-type and mutant mast cells stained very differently with these dyes. As expected, Mc-cpa+/+ peritoneal mast cells had the fully mature connective tissue phenotype (AB− S+) (Table 1; Fig. 3L). In contrast, Mc-cpa−/− peritoneal mast cells included many AB+ S−/low (did not stain with safranin or stained weakly) cells (Table 1; Fig. 3M and N). In addition to the partial reversal of the alcian blue and safranin staining, the intracellular pattern of safranin staining was altered in Mc-cpa−/− mast cells. Rather than marking discrete dense granules, as found in wild-type mast cells (Fig. 3L), safranin appeared to stain larger vacuoles in Mc-cpa−/− mast cells (Fig. 3M and N). In doubly stained cells, blue and brown areas were compartmentalized (Fig. 3N). For comparison, we stained in parallel BMMC which showed the typical immature phenotype (AB+ S−) (Fig. 3O) (11, 26). Hence, Mc-cpa−/− peritoneal mast cells are histochemically more immature than their wild-type counterpart.
Mc-cpa−/− peritoneal mast cells are immature by flow cytometric scatter.
Forward side scatter analysis showed marked differences between Mc-cpa+/+ (Fig. 3A) and Mc-cpa−/− (Fig. 3B) mast cells when each population was gated as c-Kit+ peritoneal cells. Mc-cpa+/+ mast cells had a low forward scatter, indicating relatively small size, and a high side scatter, indicating high granule content, or normal granule composition (Fig. 3A). By contrast, Mc-cpa−/− mast cells had a lower side scatter and higher forward scatter, suggesting abnormal granule content or composition and larger size. Parallel analysis of Mc-cpa+/+ BMMC (Fig. 3C) and Mc-cpa−/− BMMC (Fig. 3D), a common model of immature mast cells, demonstrated that BMMC, regardless of their genotype, had a forward side scatter comparable to that of peritoneal mast cells from Mc-cpa−/− mice.
This comparison suggested that peritoneal mast cells develop in the following order: (i) high forward scatter-low side scatter (population 1; Fig. 3G), (ii) low forward scatter-low side scatter (population 2; Fig. 3F), and (iii) low forward scatter-high side scatter (population 3; Fig. 3E). This idea could be tested more directly in Mc-cpa−/− mice in which populations 1 and 2 were very abundant (Fig. 3B). Mc-cpa−/− peritoneal mast cells were separated into populations 1 (Fig. 3G) and 2 (Fig. 3F), and each population was stained with toluidine and alcian blue plus safranin. Interestingly, population 1 was indeed enriched for cells with a very immature phenotype (AB+ S−/low) (Fig. 3N). Population 2 cells were mostly ABlow (stained weakly with alcian blue) S+ (Fig. 3M), which corresponds to a more mature CTMC phenotype. Fully mature ABlow Shigh cells were present only in wild-type mice (Fig. 3L). Thus, Mc-cpa−/− peritoneal mast cells include distinct populations of histochemically immature mast cells.
Age dependency of the immature Mc-cpa−/− peritoneal mast cell phenotype.
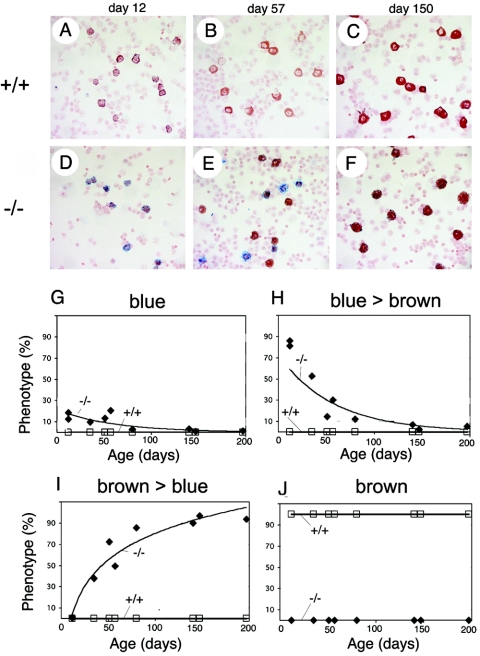
The presence of AB+ S−/low peritoneal mast cells in Mc-cpa−/− mice showed that the loss of Mc-cpa is associated with a block in the development of phenotypically normal connective tissue mast cells in vivo. Because hematopoietic processes often change over time (35, 47), we determined whether this block was age dependent. The kinetics of the alcian blue and safranin staining phenotype in the peritoneal cavity in wild-type and Mc-cpa−/− mice are shown in Fig. 4. Photomicrographs of PEC cytospins are shown for postnatal days 12, 57, and 150 (Fig. 4A to F), and all time points of analysis are summarized in Fig. 4G to J. In healthy mice, the safranin staining intensity increased with age, but all cells were AB− S+ regardless of their age (Fig. 4A to C). In contrast, in very young Mc-cpa−/− mice, all peritoneal mast cells were AB+ S−/low (Fig. 4D). The proportion of AB+ S−/low cells declined at the expense of AB− S+ with increasing age (Fig. 4D to F). However, even at 150 days of age, Mc-cpa−/− mast cells lacked the normal staining pattern (Fig. 4F). Collectively, the alcian blue and safranin staining phenotype of peritoneal mast cells in Mc-cpa−/− mice is changing over time in vivo. The “direction” of this change points from immature to more mature mast cells (1).
FIG. 4.
Age-dependent block in mast cell development in Mc-cpa−/− mice. Peritoneal cells were isolated from wild-type (Mc-cpa+/+ [+/+]) (A to C) and Mc-cpa−/− (−/−) (D to F) mice at the indicated age. Cells were cytospun and stained by alcian blue and safranin. Relative proportions of AB+++ S− (blue) (G), AB+++ S+ (blue > brown) (H), AB+ S+++ (brown > blue) (I), and AB− S+++ (brown) (J) staining (+++, strong staining; +, staining; −, no staining) are shown as a function of time.
Probing mast cells for heparin expression by berberine sulfate and antiheparin antibody.
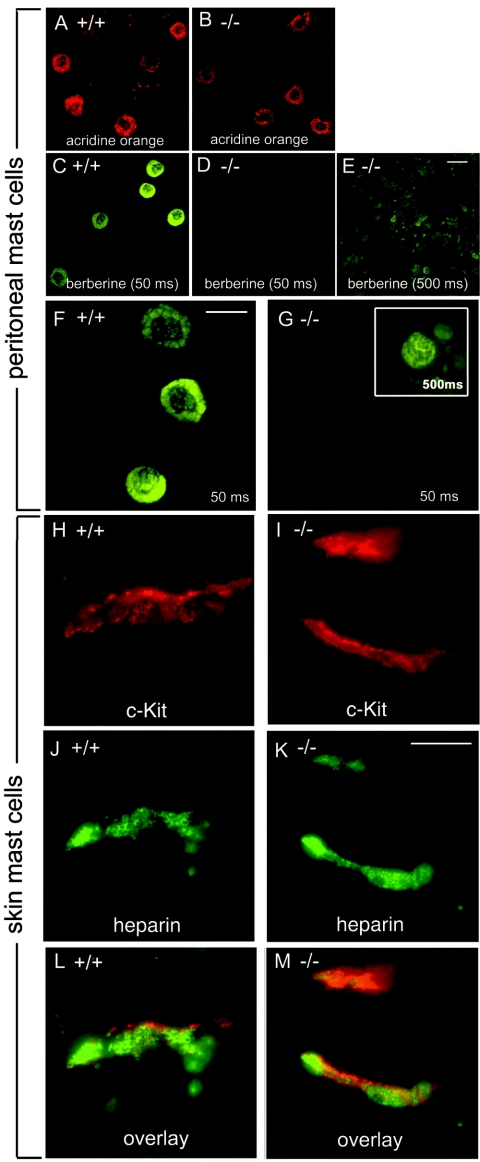
CTMC contain heparin, while MMC lack heparin (1). Heparin can be specifically stained in secretory granules with berberine sulfate which binds with high affinity to heparin, resulting in strong fluorescence (8). Mast cells from Mc-cpa+/+ and Mc-cpa−/− mice were visualized among PEC by acridine orange staining (Fig. 5A and B). Wild-type mast cells showed the typical berberine staining characterized by intensely fluorescent granules and weakly stained nuclei (Fig. 5C and F). Interestingly, Mc-cpa−/− mast cells were berberine− (Fig. 5D and G) compared to wild-type cells (Fig. 5C and F). A faint nuclear and perinuclear berberine staining of Mc-cpa−/− mast cells was detectable only after at least 10-fold-longer exposure of the film to the fluorescence (Fig. 5G, insert).
FIG. 5.
Berberine fluorescence and immunofluorescence analysis for heparin expression in Mc-cpa−/− mast cells. Peritoneal cells from wild-type (Mc-cpa+/+ [+/+]) (A, C, and F), and Mc-cpa−/− (−/−) (B, D, E, and G) mice were stained with acridine orange to visualize mast cells (A and B) and with berberine sulfate to visualize heparin-containing mast cell granules (C to G). Mc-cpa−/− peritoneal mast cells lacked berberine reactivity. Faint nuclear/perinuclear berberine fluorescence was detectable after 10-fold-longer exposure of the film to the fluorescence in Mc-cpa−/− mast cells (G). Skin sections from wild-type (H, J, and L) and Mc-cpa−/− (I, K, and M) mice were analyzed by immunofluorescence analysis using antiheparin and anti-c-Kit antibodies for the presence of cells expressing c-Kit (H and I), and heparin (J and K). Overlays are shown in panels L and M. All mast cells coexpressed c-Kit and heparin. The scale bars in panels E (20 μm), F (15 μm), and K (10 μm) apply to panels A to E, F and G, and H to M, respectively.
Because the absence of berberine fluorescence suggested that Mc-cpa−/− mast cells are devoid of heparin, we next applied a berberine-independent, heparin-specific reagent to probe mutant mast cells for the presence of heparin. To this end, we used a monoclonal antiheparin antibody which can recognize heparin-expressing cells in frozen skin sections (44). Skin mast cells were identified by staining for c-Kit (Fig. 5H and I). c-Kit+ mast cells always double labeled for antiheparin antibodies (Fig. 5J and K; overlay in Fig. 5L and M). In contrast to the berberine staining of PMC, the overall intensity of antiheparin staining was comparable in Mc-cpa+/+ and Mc-cpa−/− tissue mast cells. c-Kit was predominantly found at the cell surface (Fig. 5H and I), whereas heparin was mostly intracellular (Fig. 5L and M). Of note, the antiheparin staining pattern differed in wild-type and mutant mice. The antiheparin staining was ring shaped in Mc-cpa+/+ cells (Fig. 5J and L), whereas heparin was localized in compact dots in Mc-cpa−/− mast cells. Collectively, heparin can be detected by antiheparin antibody but not by berberine in mutant mast cells. The altered antibody staining pattern suggests that heparin storage or complex formation is altered in Mc-cpa−/− mast cells.
Heparin synthesis and secretion are independent from Mc-cpa expression.
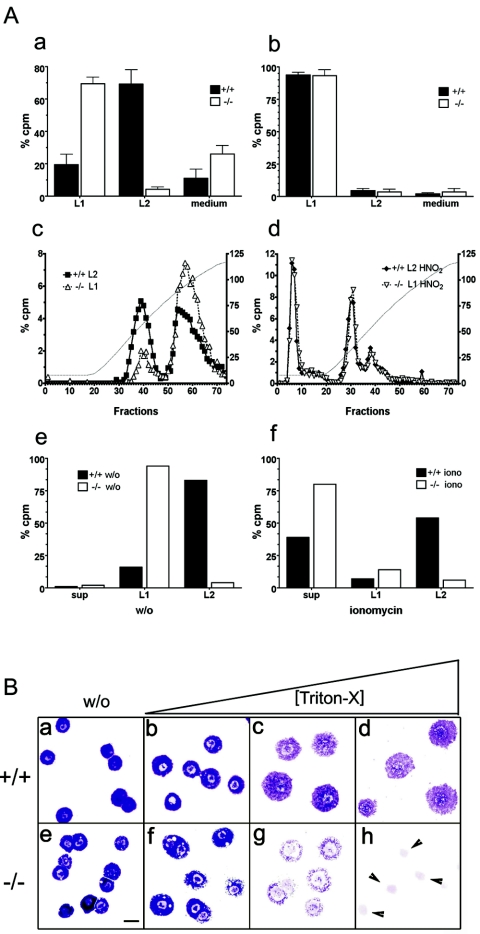
To analyze proteoglycan biosynthesis biochemically, cell sorter purified PMC were metabolically labeled with [35S]sulfate (10, 15). Labeled cells were first lysed (0.5% Triton X-100) (soluble fraction termed L1), and subsequently the insoluble material was extracted under high-salt (2 M KCl) conditions (soluble fraction termed L2) (Fig. 6A). From Mc-cpa+/+ mast cells, most 35S-labeled proteoglycans were recovered in fraction L2 (Fig. 6Aa). In contrast, most 35S-labeled proteoglycan material was already released from Mc-cpa−/− mast cells by the lysis step (L1) (Fig. 6Aa). From immature BMMC, regardless of their Mc-cpa genotype, proteoglycans were extracted within L1 (Fig. 6Ab). Thus, proteoglycan extraction is detergent resistant in fully mature peritoneal mast cells, but it is detergent sensitive in both immature BMMC and peritoneal Mc-cpa−/− mast cells.
FIG. 6.
(A) Heparin biosynthesis and secretion in Mc-cpa+/+ and Mc-cpa−/− mast cells. Sorted peritoneal (a) or cultured BM-derived (b) mast cells were metabolically labeled by overnight culture in medium containing [35S]sulfate. Percent radioactivity in counts per minute (cpm) recovered in either the lysis fraction (L1) or salt extraction fraction (L2) is shown for Mc-cpa+/+ (+/+) (solid bars) and Mc-cpa−/− (−/−) (open bars) mast cells. Proteoglycans were further fractionated by ion-exchange chromatography and LiCl gradient elution (c). The left and right peaks correspond to chondroitin sulfate and heparin, respectively (10). The heparin peaks disappeared after treatment of the material with HNO2 prior to ion-exchange chromatography (d). The left y axes in panels c and d show percent counts per minute, and the right y axes show conductivity of the LiCl gradient in milli-Siemens per centimeter. Mast cells were pulse-chase-labeled with 35S. There was no spontaneous release into the supernatant (sup), but the material was retained in fractions L1 and L2 (e). w/o, without ionomycin. Following ionomycin (iono) stimulation, mast cells released labeled proteoglycans into the supernatant (sup) (f). (B) Mc-cpa−/− mast cells are highly detergent sensitive. Peritoneal mast cells from Mc-cpa+/+ (a to d) and Mc-cpa−/− (e to h) mice were cytocentrifuged in the absence (without [w/o]) (a and e) or presence of increasing amounts of Triton X-100(0.016% [b and f], 0.02% [c and g], and 0.5% [d and h]) and stained with toluidine blue. Wild-type mast cells withstood this treatment up to 0.5% Triton X-100 (d) but Mc-cpa−/− mast cells showed “holes” at low detergent concentrations (f) and disintegrated entirely at higher concentrations (g and h). The arrowheads in panel h point to the remaining nuclei. The scale bar in panel E (20 μm) applies to panels a to h.
The vast majority of proteoglycans from Mc-cpa−/− and Mc-cpa+/+ mast cells were present in L1 and L2 fractions, respectively. These fractions were analyzed by ion-exchange chromatography and LiCl gradient elution to classify the proteoglycan types (Fig. 6Ac). Two distinct peaks emerged: one eluting at low salt concentration (Fig. 6Ac, left peaks) consistent with chondroitin sulfate, and another one eluting at high salt concentration consistent with heparin (Fig. 6Ac, right peaks) (10). For both Mc-cpa+/+ and Mc-cpa−/− mast cells, heparin peaks were identified by complete degradation with HNO2 (Fig. 6Ad). On the basis of this biochemical analysis, heparin synthesis is unaffected by the absence of Mc-cpa.
To analyze proteoglycan secretion, mast cells were pulse-labeled with [35S]sulfate and then chased, and spontaneous release (Fig. 6Ae) versus ionomycin-stimulated release (Fig. 6Af) were measured. Both Mc-cpa+/+ and Mc-cpa−/− mast cells retained their labeled proteoglycans in the cell, and again proteoglycan extraction required different conditions (L1 versus L2) (Fig. 6Ae). After ionomycin stimulation, both wild-type and mutant mast cells released the labeled proteoglycans into the supernatant; however, release was more complete in Mc-cpa−/− cells than in Mc-cpa+/+ mast cells (Fig. 6Af). These experiments demonstrate that, in the absence of Mc-cpa, the syntheses of heparin and chondroitin sulfate are normal and that heparin presumably enters the correct secretory pathway.
The rapid extraction of proteoglycans from Mc-cpa−/− mast cells by detergent only may be due to altered proteoglycan storage in the cells. However, this was unlikely, given that both synthesis and regulated secretion of heparin were normal in the mutant cells. To determine the impact of detergent on wild-type and mutant mast cells more directly, cells were cytospun in the presence of increasing amounts of Triton X-100 and stained with toluidine blue. When wild-type mast cell were subjected to this treatment, their metachromatic staining decreased and the cells flattened but did not disintegrate (Fig. 6Ba to d). In marked contrast, Mc-cpa−/− mast cells showed “holes” even at low detergent concentrations, and the cells were entirely lysed at higher concentrations (Fig. 6Be to h). Thus, Mc-cpa+/+ and Mc-cpa−/− mast cells differ in their detergent resistance. While the underlying molecular basis remains to be uncovered, it is clear that immature BMMC and Mc-cpa−/− but not Mc-cpa+/+ peritoneal mast cells are highly detergent sensitive.
Normal functions of Mc-cpa−/− mast cells.
To determine whether mast cells lacking Mc-cpa were functionally impaired, we tested several mast cell functions in vitro and in vivo. First, the capacity of mutant mast cells for degranulation was measured by hexosaminidase release after stimulation with ionomycin. This stimulus was chosen because PMC, regardless of their Mc-cpa genotype, were refractory to FcɛRI-mediated stimulation in vitro. The magnitudes of hexosaminidase release were comparable in Mc-cpa+/+ and Mc-cpa−/− mast cells (Table 2). Second, we determined histamine content and release. Both were similar in Mc-cpa+/+ and Mc-cpa−/− mast cells (Table 2).
TABLE 2.
Hexosaminidase and histamine release from wild-type and mutant mast cellsa
| Genotypeb | % β-Hexosaminidase release (n)c
|
Histamine
|
|||
|---|---|---|---|---|---|
| Total amt (nmol/cell) (n)d | % Release (n)c
|
||||
| w/o | Ionomycin | w/o | Ionomycin | ||
| +/+ | 4.6 ± 2.4 (3) | 80.0 ± 5.1 (3) | 123.7 ± 3.1 (3) | 3.3 | 92.6 |
| −/− | 5.7 ± 1.0 (3) | 82.1 ± 10.6 (3) | 115.0 ± 22.6 (3) | 4.3 | 95.2 |
Cell sorter-purified mast cells from Mc-cpa+/+ and Mc-cpa−/− mice were left untreated or stimulated with ionomycin. Hexosaminidase and histamine were measured by colorimetric assay and by enzyme-linked immunosorbent assay, respectively.
+/+, Mc-cpa+/+; −/−, Mc-cpa−/−.
Release into the supernatant with or without ionomycin stimulation (w/o). Percentages of the release of hexosaminidase and histamine were calculated relative to the total hexosaminidase and histamine contents. n, number of mice.
The total amount of histamine was determined by comparison to a histamine standard curve. n, number of mice.
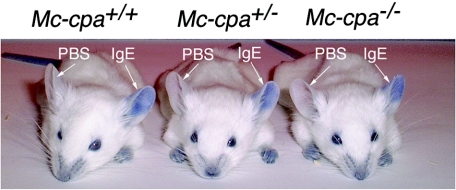
An important in vivo mast cell function, the immediate hypersensitivity reaction, was analyzed by local passive cutaneous anaphylaxis. Ears were injected intradermally with anti-DNP IgE or, as a negative control, with PBS. On the next day, DNP-HSA together with Evan's blue was injected intravenously. Within 5 min after this antigenic challenge, the mast cell-dependent PCA increased the vascular permeability, causing local dye extravasation in IgE- but not in PBS-injected skin (Fig. 7). We analyzed PCA in mice of all three genotypes (Mc-cpa+/+ [n = 5], Mc-cpa+/− [n = 5], and Mc-cpa−/− [n = 5]). The onset and magnitude of dye extravasation were similar in all genotypes (Fig. 7). Collectively, Mc-cpa−/− mast cells displayed hallmarks of immaturity but showed normal functions in these in vitro and in vivo assays.
FIG. 7.
Mc-cpa−/− mast cells mount a normal passive cutaneous anaphylaxis reaction. Mc-cpa+/+, Mc-cpa+/−, and Mc-cpa−/− mice were injected intradermally into the left and right ears with hapten (DNP)-specific IgE or PBS, respectively. On the next day, mice were challenged by intravenous injection of hapten-carrier (DNP-HSA) together with Evan's blue. The PCA reaction is evident by extravasation of the dye in IgE-injected but not in control PBS-injected ears. Mice of all genotypes mounted indistinguishable PCA reactions. One representative mouse of five mice for each genotype are shown.
DISCUSSION
Mc-cpa and mast cell ontogeny.
Developing mast cells diverge at an unknown branch into two sublineages, connective tissue and mucosal mast cells. The onset of Mc-cpa expression occurs at the mast cell progenitor stage (34), and Mc-cpa expression increases as immature mast cells mature into CTMC but not MMC (36). The loss of Mc-cpa changes the phenotype of CTMC towards an immature mast cell phenotype, demonstrating that Mc-cpa expression is not only associated with CTMC maturation but is required for the generation of fully mature peritoneal mast cells as defined by classical histochemistry. This phenotype points to an important intracellular function for Mc-cpa in CTMC development.
Mc-cpa and proteoglycan syntheses.
Mc-cpa−/− mast cells and BMMC share several hallmarks of immature mast cells. The pattern of alcian blue and safranin binding to mast cell granules distinguishes BMMC and CTMC. On the basis of their reactivity with these dyes, Mc-cpa−/− CTMC appear to be at an intermediate stage between immature BMMC and mature Mc-cpa+/+ CTMC. It has been assumed that the correlation between mast cell maturation and histochemical phenotype is indicative of the proteoglycan type which is predominantly expressed in BMMC (chondroitin sulfate) and CTMC (heparin) (1, 14, 45). Previous experiments using total peritoneal exudate cells which include, in addition to mast cells, lymphocytes and macrophages, had suggested that the source of chondroitin sulfate among peritoneal exudate cells is non-mast cells (10). In contrast, we show that proteoglycan synthesis by highly purified peritoneal masts was not limited to heparin but also included chondroitin sulfate. Despite the fact that wild-type and Mc-cpa−/− CTMC synthesized chondroitin sulfate and heparin, they differed in their histochemical phenotype. Thus, alcian blue and safranin staining does not faithfully reflect whether chondroitin sulfate or heparin are synthesized.
Is Mc-cpa involved in heparin sorting?
The majority of Mc-cpa protein forms a high-molecular-weight complex with proteoglycans inside mast cells (36). This complex formation is probably due to the fact that Mc-cpa and heparin are highly positively and negatively charged, respectively. Formation of Mc-cpa-proteoglycan complexes could occur as soon as both come into proximity, e.g., in the Golgi apparatus where the complex is sorted into the secretory granules (39). Carboxypeptidase E is an example of a carboxypeptidase which functions as a sorting receptor for secretory pathway proteins, including prohormones, in neuroendocrine pituitary cells (2). The close association of Mc-cpa and heparin raised the possibility that sorting of heparin into the secretory pathway is a Mc-cpa-dependent process. However, we have shown that Mc-cpa−/− CTMC can secrete heparin in a regulated manner, ruling out an obligatory requirement for Mc-cpa (or Mcp-5) expression for heparin sorting.
Interdependence of Mc-cpa and Mcp-5 expression.
Ndst-2-deficient mast cells lack sulfated heparin and fail to express Mcp-4, Mcp-5, Mcp-6, and mature Mc-cpa protein (10, 15, 17). Thus, heparin synthesis is essential for protease protein expression or stability. Mc-cpa and Mcp-5 are highly dependent on each other, because Mcp-5-null mice lack Mc-cpa protein (43) and Mc-cpa-null mice lack Mcp-5 protein (this paper). The absence of Mcp-5 protein in Mc-cpa−/− mast cells is not due to the absence of transcription, because Mcp-5 mRNA was expressed normally (not shown). In contrast to Ndst-2-deficient mast cells, Mcp-6 expression was unaffected and Mcp-4 expression was even upregulated in Mc-cpa−/− mast cells (Fig. 1). While the molecular mechanism underlying these strong alterations remains to be found, it is clear that mast cell proteases depend not only on heparin but also upon each other in order to be expressed in their physiological stoichiometry. In the case of Mc-cpa and Mcp-5, it is likely that these two proteins form a direct complex.
Proteoglycans and mast cell morphology.
Histochemical parameters have been widely used to assess mast cell maturation. In vivo, PMC change their phenotype from AB+ S− (immature) in 1-week-old rats to AB− S+ (mature) in 3-week-old rats (1). In mast cell cultures, high doses of Kit ligand can induce a histochemical shift from AB+ S− towards AB− S+ cells (14, 45). Comparison of mice lacking Ndst-2 (10, 17), Mcp-5 (43), or Mc-cpa (this paper) shows that Ndst-2, but not Mcp-5 or Mc-cpa, is required for the generation of morphologically normal mast cell granules. With regard to the Mc-cpa−/− mice, it is surprising that mutant mast cells showed aberrantly strong alcian blue and weak safranin staining (Fig. 3 and 4) yet normal ultrastructural mast cell morphology (Fig. 2). Aliquots of the mast cells sorted for subsequent electron microscopy analysis were analyzed by alcian blue and safranin staining and showed the phenotypic distribution shown in Table 1. We have examined the ultrastructure of a large number of mast cells by electron microscopy (2,500 cells), and there was no greater morphological heterogeneity in Mc-cpa−/− mast cells than in Mc-cpa+/+ mast cells. In this case, a prominent histochemical mast cell phenotype does not translate into ultrastructural alterations.
Mc-cpa and detergent sensitivity.
One striking aspect of the Mc-cpa−/− mast cell phenotype is the differential detergent sensitivity comparing wild-type (resistant), Mc-cpa−/− (sensitive) peritoneal mast cells and BMMC (sensitive). We noticed this difference initially by the fact that Mc-cpa−/− but not Mc-cpa+/+ PMC released labeled macromolecular material following detergent lysis (Fig. 6). Pulse-chase experiments strongly suggest that proteoglycan synthesis and routing into the regulated secretory pathway occur independently from Mc-cpa expression. However, the integrity of the mutant mast cells appears to be affected dramatically as shown by detergent titration (Fig. 6). Cell membranes are composed of detergent-resistant and detergent-sensitive material (38). How the absence of Mc-cpa causes the detergent-sensitive phenotype remains to be determined.
Age-dependent phenotype of Mc-cpa−/− peritoneal mast cells.
With increasing age, Mc-cpa−/− PMC acquired a more mature, but never a completely normal histochemical phenotype (Fig. 4). The peritoneal cavity is colonized by mast cells early in life (1), and there is very little turnover of peritoneal mast cells in the absence of inflammation. In fact, six weeks following BM-transplantation into lethally irradiated mice, only 2% of PMC were replaced by donor stem cell-derived mast cells (C. Waskow and H.-R. Rodewald, unpublished). Therefore, it is likely that the phenotypically distinct mast cells from young and old Mc-cpa−/− mice are precursor and product within the peritoneal cavity.
Role of Mc-cpa in mast cell function.
We have uncovered an intracellular requirement for Mc-cpa in mast cell maturation. Despite this immature phenotype, Mc-cpa−/− mast cells were normal with regard to mediator degranulation in vitro and PCA in vivo. These data imply that Mc-cpa is not required for immediate mast cell responses. However, Mc-cpa may be involved in delayed allergic responses and in mast cell-mediated inflammation. Physiological substrates of Mc-cpa are presently unknown. Several functions for Mc-cpa have been proposed. For instance, Mc-cpa can process angiotensin in combination with Mcp-4 in vitro (20). Pancreatic carboxypeptidase A catalyzes leukotriene conversion (29) which may also occur via Mc-cpa in mast cells. A role for Mc-cpa in endothelin-1 degradation has been suggested in one report (24) but not in another report (23). It remains to be determined whether Mc-cpa plays a role in these pathways in vivo. Experiments addressing these questions using Mc-cpa−/− mice are under way.
Acknowledgments
We thank Tilman Borggrefe, Claudia Waskow, and Gunnar Pejler for discussions, Peter Conradt for cell sorting, and the Animal Facility staff for their care in maintenance of mouse colonies.
This work was supported by Deutsche Forschungsgemeinschaft grant DFG-RO754/2-1 to H.-R.R., in part by a Ph.D. stipend from F. Hoffmann-La Roche, Basel, Switzerland, to T.B.F., and by NIH grant AI33372 to A.M.D.
REFERENCES
- 1.Chen, X. J., and L. Enerback. 1999. Immature peritoneal mast cells in neonatal rats express the CTMC phenotype, as well as functional IgE receptors. APMIS 107:957-965. [DOI] [PubMed] [Google Scholar]
- 2.Cool, D. R., E. Normant, F. Shen, H. C. Chen, L. Pannell, Y. Zhang, and Y. P. Loh. 1997. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88:73-83. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak, A. M. 1987. Procedural guide to specimen handling for the ultrastructural pathology service laboratory. J. Electron Microsc. Tech. 6:255-301. [Google Scholar]
- 4.Dvorak, A. M., R. A. Seder, W. E. Paul, E. S. Morgan, and S. J. Galli. 1994. Effects of interleukin-3 with or without the c-kit ligand, stem cell factor, on the survival and cytoplasmic granule formation of mouse basophils and mast cells in vitro. Am. J. Pathol. 144:160-170. [PMC free article] [PubMed] [Google Scholar]
- 5.Eklund, K. K., N. Ghildyal, K. F. Austen, D. S. Friend, V. Schiller, and R. L. Stevens. 1994. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J. Exp. Med. 180:67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enerback, L. 1997. The differentiation and maturation of inflammatory cells involved in the allergic response: mast cells and basophils. Allergy 52:4-10. [DOI] [PubMed] [Google Scholar]
- 7.Enerback, L. 1966. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol. Microbiol. Scand. 66:303-312. [DOI] [PubMed] [Google Scholar]
- 8.Enerbäck, L. 1974. Berberine sulphate binding to mast cell polyanions: a cytofluometric method for the quantification of heparin. Histochemistry 42:301-313. [DOI] [PubMed] [Google Scholar]
- 9.Fehling, H. J., G. Lacaud, A. Kubo, M. Kennedy, S. Robertson, G. Keller, and V. Kouskoff. 2003. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130:4217-4227. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg, E., G. Pejler, M. Ringvall, C. Lunderius, B. Tomasini-Johansson, M. Kusche-Gullberg, I. Eriksson, J. Ledin, L. Hellman, and L. Kjellen. 1999. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400:773-776. [DOI] [PubMed] [Google Scholar]
- 11.Galli, S. J., A. M. Dvorak, J. A. Marcum, T. Ishizaka, G. Nabel, H. Der Simonian, K. Pyne, J. M. Goldin, R. D. Rosenberg, H. Cantor, and H. F. Dvorak. 1982. Mast cell clones: a model for the analysis of cellular maturation. J. Cell Biol. 95:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, H., Y. R. Zou, and K. Rajewsky. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73:1155-1164. [DOI] [PubMed] [Google Scholar]
- 13.Gurish, M. F., and K. F. Austen. 2001. The diverse roles of mast cells. J. Exp. Med. 194:F1-F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurish, M. F., N. Ghildyal, H. P. McNeil, K. F. Austen, S. Gillis, and R. L. Stevens. 1992. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J. Exp. Med. 175:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henningsson, F., J. Ledin, C. Lunderius, M. Wilen, L. Hellman, and G. Pejler. 2002. Altered storage of proteases in mast cells from mice lacking heparin: a possible role for heparin in carboxypeptidase A processing. Biol. Chem. 383:793-801. [DOI] [PubMed] [Google Scholar]
- 16.Huang, C., A. Sali, and R. L. Stevens. 1998. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 18:169-183. [DOI] [PubMed] [Google Scholar]
- 17.Humphries, D. E., G. W. Wong, D. S. Friend, M. F. Gurish, W. T. Qiu, C. Huang, A. H. Sharpe, and R. L. Stevens. 1999. Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400:769-772. [DOI] [PubMed] [Google Scholar]
- 18.Karasuyama, H., and F. Melchers. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 18:97-104. [DOI] [PubMed] [Google Scholar]
- 19.Knight, P. A., S. H. Wright, E. M. Thornten, J. Brown, and H. R. P. Miller. 2000. Expression, function, and regulation of mast cell granule chymases during mucosal allergic responses, p. 257-273. In G. Morone, L. M. Lichtenstein, and S. J. Galli (ed.), Mast cells and basophils. Academic Press, Ltd., London, England.
- 20.Lundequist, A., E. Tchougounova, M. Abrink, and G. Pejler. 2004. Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II. J. Biol. Chem. 279:32339-32344. [DOI] [PubMed] [Google Scholar]
- 21.Lunderius, C., Z. Xiang, G. Nilsson, and L. Hellman. 2000. Murine mast cell lines as indicators of early events in mast cell and basophil development. Eur. J. Immunol. 30:3396-3402. [DOI] [PubMed] [Google Scholar]
- 22.Marone, G., S. J. Galli, and Y. Kitamura. 2002. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 23:425-427. [DOI] [PubMed] [Google Scholar]
- 23.Maurer, M., J. Wedemeyer, M. Metz, A. M. Piliponsky, K. Weller, D. Chatterjea, D. E. Clouthier, M. M. Yanagisawa, M. Tsai, and S. J. Galli. 2004. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 432:512-516. [DOI] [PubMed] [Google Scholar]
- 24.Metsarinne, K. P., P. Vehmaan-Kreula, P. T. Kovanen, O. Saijonmaa, M. Baumann, Y. Wang, T. Nyman, F. Y. Fyhrquist, and K. K. Eklund. 2002. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted peptide. Arterioscler. Thromb. Vasc. Biol. 22:268-273. [DOI] [PubMed] [Google Scholar]
- 25.Moritz, D. R., H. R. Rodewald, J. Gheyselinck, and R. Klemenz. 1998. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 161:4866-4874. [PubMed] [Google Scholar]
- 26.Nabel, G., S. J. Galli, A. M. Dvorak, H. F. Dvorak, and H. Cantor. 1981. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature 291:332-334. [DOI] [PubMed] [Google Scholar]
- 27.Nishizumi, H., and T. Yamamoto. 1997. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 158:2350-2355. [PubMed] [Google Scholar]
- 28.Prydz, K., and K. T. Dalen. 2000. Synthesis and sorting of proteoglycans. J. Cell Sci. 113:193-205. [DOI] [PubMed] [Google Scholar]
- 29.Reddanna, P., K. S. Prabhu, J. Whelan, and C. C. Reddy. 2003. Carboxypeptidase A-catalyzed direct conversion of leukotriene C4 to leukotriene F4. Arch. Biochem. Biophys. 413:158-163. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds, D. S., D. S. Gurley, and K. F. Austen. 1992. Cloning and characterization of the novel gene for mast cell carboxypeptidase A. J. Clin. Investig. 89:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds, D. S., D. S. Gurley, R. L. Stevens, D. J. Sugarbaker, K. F. Austen, and W. E. Serafin. 1989. Cloning of cDNAs that encode human mast cell carboxypeptidase A, and comparison of the protein with mouse mast cell carboxypeptidase A and rat pancreatic carboxypeptidases. Proc. Natl. Acad. Sci. USA 86:9480-9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds, D. S., R. L. Stevens, D. S. Gurley, W. S. Lane, K. F. Austen, and W. E. Serafin. 1989. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J. Biol. Chem. 264:20094-20099. [PubMed] [Google Scholar]
- 33.Reynolds, D. S., R. L. Stevens, W. S. Lane, M. H. Carr, K. F. Austen, and W. E. Serafin. 1990. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc. Natl. Acad. Sci. USA 87:3230-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodewald, H.-R., M. Dessing, A. M. Dvorak, and S. J. Galli. 1996. Identification of a committed precursor for the mast cell lineage. Science 271:818-822. [DOI] [PubMed] [Google Scholar]
- 35.Rodewald, H.-R., and H. J. Fehling. 1998. Molecular and cellular events in early thymocyte development. Adv. Immunol. 69:1-112. [DOI] [PubMed] [Google Scholar]
- 36.Serafin, W. E., E. T. Dayton, P. M. Gravallese, K. F. Austen, and R. L. Stevens. 1987. Carboxypeptidase A in mouse mast cells. Identification, characterization, and use as a differentiation marker. J. Immunol. 139:3771-3776. [PubMed] [Google Scholar]
- 37.Shively, J. E., and H. E. Conrad. 1976. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry 15:3932-3942. [DOI] [PubMed] [Google Scholar]
- 38.Simons, K., and W. L. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269-295. [DOI] [PubMed] [Google Scholar]
- 39.Springman, E. B., M. M. Dikov, and W. E. Serafin. 1995. Mast cell procarboxypeptidase A. Molecular modeling and biochemical characterization of its processing within secretory granules. J. Biol. Chem. 270:1300-1307. [DOI] [PubMed] [Google Scholar]
- 40.Stevens, R. L. 2000. Human and mouse mast cell tryptases, p. 235-256. In G. Morone, L. M. Lichtenstein, and S. J. Galli (ed.), Mast cells and basophils. Academic Press, Ltd., London, England.
- 41.Stevens, R. L. 1989. Mast cell proteoglycans. Prog. Clin. Biol. Res. 297:131-143. [PubMed] [Google Scholar]
- 42.Stevens, R. L., D. S. Friend, H. P. McNeil, V. Schiller, N. Ghildyal, and K. F. Austen. 1994. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc. Natl. Acad. Sci. USA 91:128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens, R. L., D. Qui, H. P. McNeil, D. S. Friend, J. E. Hunt, K. F. Austen, and J. Zhang. 1996. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) cannot store carboxypeptidase A in their granules. FASEB J. 10:1307. [Google Scholar]
- 44.Straus, A. H., L. R. Travassos, and H. K. Takahashi. 1992. A monoclonal antibody (ST-1) directed to the native heparin chain. Anal. Biochem. 201:1-8. [DOI] [PubMed] [Google Scholar]
- 45.Tsai, M., T. Takeishi, H. Thompson, K. E. Langley, K. M. Zsebo, D. D. Metcalfe, E. N. Geissler, and S. J. Galli. 1991. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc. Natl. Acad. Sci. USA 88:6382-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai, M., J. Wedemeyer, S. Ganiatsas, S. Y. Tam, L. I. Zon, and S. J. Galli. 2000. In vivo immunological function of mast cells derived from embryonic stem cells: an approach for the rapid analysis of even embryonic lethal mutations in adult mice in vivo. Proc. Natl. Acad. Sci. USA 97:9186-9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waskow, C., S. Paul, C. Haller, M. Gassmann, and H. R. Rodewald. 2002. Viable c-Kit (W/W) mutants reveal pivotal role for c-Kit in the maintenance of lymphopoiesis. Immunity 17:277-288. [DOI] [PubMed] [Google Scholar]
- 48.Wedemeyer, J., M. Tsai, and S. J. Galli. 2000. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 12:624-631. [DOI] [PubMed] [Google Scholar]
- 49.Wiles, M. V., and G. Keller. 1991. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development 111:259-267. [DOI] [PubMed] [Google Scholar]
- 50.Zehnder, J. L., and S. J. Galli. 1999. Mast-cell heparin demystified. Nature 400:714-715. [DOI] [PubMed] [Google Scholar]
- 51.Zon, L. I., M. F. Gurish, R. L. Stevens, C. Mather, D. S. Reynolds, K. F. Austen, and S. H. Orkin. 1991. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J. Biol. Chem. 266:22948-22953. [PubMed] [Google Scholar]