Abstract
Background and Aims
Species delimitation can be challenging when analysing recently diverged species, especially those taxonomically synonymized owing to morphological similarities. We aimed to untangle the relationships between two grassland species, Petunia guarapuavensis and Petunia scheideana, exploring the dynamics of fast divergence and addressing their species delimitation.
Methods
We used a low-coverage genome sequencing and population genomic approach to distinguish species and populations between P. guarapuavensis and P. scheideana. Our analysis focused on detecting structuration, hybridization/introgression and phylogenetic patterns. We used demographic models to support species delimitation while exploring potential phylogeographical barriers influencing gene flow.
Key Results
Our findings indicated differentiation between the two species and revealed another lineage, which was phylogenetically distinct from the others and had no evidence of gene flow with them. The presence of a river acted as a phylogeographical barrier, limiting gene flow and allowing for structuration between closely related lineages. The optimal species delimitation scenario involved secondary contact between well-established lineages.
Conclusions
The rapid divergence observed in these Petunia species explains the lack of significant morphological differences, because floral diagnostic traits in species sharing pollinators tend to evolve more slowly. This study highlights the complexity of species delimitation in recently diverged groups and emphasizes the importance of genomic approaches in understanding evolutionary relationships and speciation dynamics.
Keywords: Species delimitation, population genetics, rapid divergence, structuration, river barrier, Solanaceae, subtropical highland grasslands, Petunia scheideana, Petunia guarapuavensis
INTRODUCTION
The intricate process of rapid and recent diversification is widespread throughout the tree of life and plays a crucial role in shaping biodiversity (Kozak and Wiens, 2010). This rapid divergence gives rise to a complex array of speciation mechanisms in plants, including pollinator isolation (Lagomarsino et al., 2016), progenitor-derivative speciation (Vargas et al., 2020), ecological adaptation (Hughes and Atchison, 2015), and geographical isolation followed by local adaptation (e.g. Eaton and Ree, 2013).
Species exist in multiple stages of speciation, mainly when diversification is recent (Naciri and Linder, 2020). Diversification rates and morphological evolution are not strongly correlated. Rapid diversification can occur with little morphological change and vice versa (Adams et al., 2009; Martin and Richards, 2019). This dynamic complicates the intricate species delimitation task (Fišer et al., 2018).
Species delimitation poses challenges, especially in recently diverged lineages and those undergoing adaptive radiation, particularly when they do not fit models reflecting novelties and morphological polymorphisms (Olivares et al., 2024). This complexity encompasses ongoing debates about species recognition criteria and a limited understanding of phylogenetic relationships and genetic characteristics that differentiate species (Burbrink and Ruane, 2021; Hörandl, 2022). The numerous species concepts, each emphasizing different crucial features for delimitation, have led to diverse conclusions about species boundaries, and this accounts for many groups (Carstens et al., 2013; Serrano and Ortiz, 2023).
The genus Petunia (Solanaceae) provides an ideal subject for investigating the effects of rapid divergence owing to its youth and diversity. Evolving in <2 Myr (Särkinen et al., 2013), Petunia has diversified into ~20 species, although this number remains dynamic. Defining species within Petunia presents a challenge owing to their morphological similarities, frequent hybridization events and robust ancestral polymorphism (Lorenz-Lemke et al., 2006; Longo et al., 2014; Reck-Kortmann et al., 2014; Guzmán et al., 2022; Pezzi et al., 2022; Backes et al., 2024). Furthermore, in some cases, Petunia species diverge in genetic structure with few or no morphological differences (Longo et al., 2014; Pezzi et al., 2022), which leads to a lack of diagnostic floral traits for species identification. This challenge is exemplified by Petunia guarapuavensis and Petunia scheideana, two morphologically similar species considered sister groups based on molecular markers (Ando et al., 2005; Chen et al., 2007), whereas analyses based on multiple individuals and plastid sequences (Lorenz-Lemke et al., 2010) revealed substantial divergence between them, each presenting different haplotypes. Phylogenetic analysis of the genus, combining plastid and nuclear markers (Reck-Kortmann et al., 2014), revealed that P. guarapuavensis and P. scheideana belong to distinct clades. More recently (Souza et al., 2024), based on plastid sequences and nuclear microsatellites under a population approach, observed that P. guarapuavensis and P. scheideana show genetic differences despite some polymorphism sharing. The ecological niches of the species are partly overlapped, and the elevation throughout their distribution splits the two taxa.
Petunia guarapuavensis and P. scheideana share several characteristics, such as a life cycle, herbaceous structure, melitophilous pollination and self-incompatibility (Tsukamoto et al., 1998). Moreover, they inhabit open grassy areas along pastures or roadsides and are characterized by their endemicity and rarity in the southern South American subtropical highland grasslands. According to the original description of the species, diagnostic morphological floral traits, such as corolla tube length and stigma positioning relative to the anthers, vary among populations. This clinal variation led some taxonomists to synonymize these taxa under P. scheideana (Stehmann et al., 2009). These traits are difficult to assess during fieldwork and even more so after dehydrating the material (Ando and Hashimoto, 1996).
This work aimed to untangle the intricate relationship between P. guarapuavensis and P. scheideana, contributing to a deeper understanding of the complex dynamics among fast-diverged species. Using a high-throughput genotyping method, we adopted a population genomic approach to delimit species and populations between P. guarapuavensis and P. scheideana. Our investigation addressed the following questions.
(1) How many distinct species exist under the nomenclature P. scheideana and P. guarapuavensis throughout their distribution?
(2) Are the populations of these taxa structured?
(3) Is there gene flow between populations or species?
(4) What was the evolutionary history of the species?
To answer these questions, we aimed to delimit these taxa and clarify their taxonomic status.
MATERIALS AND METHODS
Sampling, DNA extraction and sequencing
We sampled young and healthy leaves from 51 individuals, 28 P. guarapuavensis (hereafter, Pgua) and 23 P. scheideana (Psch), throughout their entire distribution (Fig. 1; Table 1). The samples were identified based on the original descriptions of the species as P. scheideana (Smith and Downs, 1964) and P. guarapuavensis (Ando and Hashimoto, 1995), and we also considered the geographical provenance of the samples. The sample size per collection site adhered to the recommendations for non-model species in population genomic analyses (Nazareno et al., 2017) and was similar to previously published studies on Petunia species (Caballero-Villalobos et al., 2021; Giudicelli et al., 2022; Guzmán et al., 2022; Pezzi et al., 2022). Vouchers were deposited at the Universidade Federal de Minas Gerais herbarium (BHCB-UFMG) in Belo Horizonte, Brazil.
Fig. 1.
The geographical distributions of Petunia guarapuavensis (Pgua) and P. scheideana (Psch).
Table 1.
Sampling information and diversity indices for the Petunia guarapuavensis and P. scheideana collection sites.
| Population ID | n | Longitude | Latitude | Voucher | Private | P | H O | H E | π | F IS |
|---|---|---|---|---|---|---|---|---|---|---|
| Pgua1 | 3 | −50.950 | −25.200 | BHCB 96562 | 20 | 0.86 | 0.23 | 0.18 | 0.21 | −0.02 |
| Pgua2 | 5 | −51.217 | −25.333 | BHCB 96577 | 43 | 0.85 | 0.23 | 0.19 | 0.22 | −0.01 |
| Pgua3 | 3 | −51.350 | −25.350 | BHCB 96581 | 14 | 0.86 | 0.21 | 0.17 | 0.21 | −0.01 |
| Pgua4 | 5 | −51.267 | −25.367 | BHCB 96579 | 58 | 0.85 | 0.24 | 0.19 | 0.22 | −0.03 |
| Pgua5 | 3 | −51.517 | −25.450 | BHCB 96599 | 12 | 0.86 | 0.23 | 0.18 | 0.22 | −0.01 |
| Pgua6 | 4 | −51.533 | −25.467 | BHCB 96606 | 22 | 0.85 | 0.22 | 0.19 | 0.22 | 0.00 |
| Pgua7 | 2 | −51.483 | −26.283 | BHCB 96623 | 4 | 0.88 | 0.20 | 0.14 | 0.20 | 0.00 |
| Pgua8 | 3 | −51.150 | −26.533 | BHCB 96642 | 3 | 0.86 | 0.21 | 0.17 | 0.21 | 0.00 |
| Psch1 | 12 | −49.300 | −26.200 | BHCB 80046 BHCB 80048 |
2544 | 0.87 | 0.19 | 0.18 | 0.19 | 0.00 |
| Psch2 | 8 | −51.044 | −26.748 | BHCB 151096 | 137 | 0.87 | 0.19 | 0.18 | 0.20 | 0.03 |
| Psch3 | 3 | −51.082 | −26.744 | BHCB 151095 | 15 | 0.87 | 0.21 | 0.16 | 0.20 | −0.02 |
Abbreviations: BHCB, voucher number at the BHCB herbarium (Universidade Federal de Minas Gerais, Belo Horizonte, Brazil); FIS, inbreeding coefficient; HE, expected heterozygosity; HO, observed heterozygosity; n, number of individuals; π, nucleotide diversity; P, polymorphic site proportion; Pgua, Petunia guarapuavensis; Psch, Petunia scheideana.
We powdered the silica-dried leaves with liquid nitrogen to extract genomic DNA following a cetyl-trimethyl ammonium bromide (CTAB; Sigma-Aldrich Chem. Co., St. Louis, MO, USA) protocol (Roy et al., 1992). We measured the DNA concentration with a Qubit Fluorometer (Thermo Fisher Scientific Co., Waltham, MA, USA) and the DNA quality with a NanoDrop DN-1000 Spectrophotometer (Thermo Fisher). Finally, we tested for the absence of nucleases using EcoRI (NEB; New England BioLabs Inc., Ipswich, MA, USA). DNA samples with 260/280 and 260/230 > 1.80 were considered high quality and used to prepare the genomic library.
We processed the DNA library with DArTseqTM (Kilian et al., 2012; Cruz et al., 2013) to reduce complexity using a method (Kilian et al., 2012) that combines PstI–MseI (NEB) enzymes and replaces a single adaptor with two adaptors. Sequencing was performed by bulking equimolar amounts of amplification products from each 96-well microtitre plate sample and using them in a c-Bot bridge PCR system (Illumina Inc., San Diego, CA, USA), followed by sequencing on the Illumina HiSeq 2500 platform (Illumina).
Filtering and variant discovery
We inspected the raw data with FASTQC v.0.11.7 (Andrews, 2010) and Multiqc (Ewels et al., 2016). Additionally, we removed barcodes and adapters, trimmed low-quality regions (lower than Q30), and discarded reads of <50 bases with FASTQ-MCF v.1.04.807 (Aronesty, 2013). Reads were mapped against the Petunia inflata v.1.0.1 reference genome (Bombarely et al., 2016) using BWA v.0.7.10-r789 (Li and Durbin, 2010) with default settings. The unmapped reads were removed, and individual SAM files were exported to a BAM format with SamTools v.1.3.1 (Danecek et al., 2021). All BAM files were merged into a unique file using the bamaddrg utility (https://github.com/ekg/bamaddrg), then sorted and indexed with SamTools.
We used FreeBayes v.1.3.6 (Garrison and Marth, 2012) to call variants by applying the following parameters: mapping quality > 30, base quality > 30 and read depth > 10. We ran VCFTOOLS v.0.1.12 (Danecek et al., 2011) to filter and retain only biallelic single nucleotide polymorphisms (SNPs) with <10 % missing data and a minimum allele frequency (--maf) of 0.04. To remove loci under linkage disequilibrium (LD), we set the minimum site distance to 100 bp (thin), keeping only one SNP per read. Outlier loci were detected using PCADAPT (Luu et al., 2017) and removed from demographic analyses. When needed, the raw VCF file was subdivided using BCFTOOLS v.1.13 (Danecek et al., 2021) and filtered again with the new set of individuals. The VCF file was converted to different formats using the PGDSPIDER v.2.1.1.2 (Lischer and Excoffier, 2012) and DartR v.2.05 (Mijangos et al., 2022) R packages to perform further analyses.
Genetic diversity and population differentiation
We used the filtered dataset, which did not include monomorphic sites, to estimate genetic diversity indices, such as nucleotide diversity (π, calculated per site), private alleles, polymorphic site proportion (P), observed (HO) and expected (HE) heterozygosity, and inbreeding coefficients (FIS), using STACKS v.2.41 (Rivera-Colón and Catchen, 2022). The pairwise fixation index (FST) was estimated in ARLEQUIN v.3.5.2.2 (Excoffier and Lischer, 2010). Finally, we performed a principal component analysis (PCA) using the gl.pcoa function in DartR.
Population structure was inferred using multivariate discriminant analysis of principal components (DAPC; Jombart et al., 2010) with the find.cluster and optim.a.score options in the ADEGENET v.2.1.3 R package (Jombart, 2008; Jombart et al., 2010) and a Bayesian model-based algorithm in STRUCTURE v.2.3.4 (Pritchard et al., 2000). We performed hierarchical analyses with ten runs per best number of groups K, up to a maximum of six, and used the admixture model with a burn-in of 125 000 steps followed by 500 000 steps. We performed STRUCTURE analyses with STRUCTURE_THREADER software (Pina-Martins et al., 2017) and summarized the results using STRUCTURE HARVESTER (Earl and von Holdt, 2012). We evaluated the most likely number of populations based on the inspection of likelihood plots and Evanno’s method (Evanno et al., 2005). We used POPHELPER (Francis, 2017) to plot the STRUCTURE graphs.
Finally, we ran a hierarchical analysis of molecular variance (AMOVA; Excoffier et al., 1992) using ARLEQUIN, considering individuals, collection sites and population groups previously suggested in the population structure analyses.
Searching for hybrids and introgression
We used the Snapclust option (Beugin et al., 2018) in ADEGENET to search for patterns of recent hybridization between samples labelled Psch (P. scheideana) and Pgua (P. guarapuavensis), and their collection sites were sequentially numbered (Fig. 1; Table 1). Using the expectation-maximization (EM) algorithm, this strategy combines quick likelihood optimization and a geometric approach to identify genetic clusters and hybrid classes. We executed Snapclust with default parameters and supplied information on hybridization coefficients for F1 and first backcross generations (hybrids.coef values of 0.5 and 0.25, respectively).
We used the HyDe package (Blischak et al., 2018) to identify hybridization based on phylogenetic invariants that emerged under the coalescent model. The admixture (γ) was estimated between two lineages, where γ = 0.5 denotes an equal contribution from each parental species or population, and values closer to zero or one indicate a more substantial contribution from one or another parent. Additionally, we used Dsuite (Malinsky et al., 2021) to perform the ABBA-BABA test and f-branch (Malinsky et al., 2018) and detected ancient and recent gene flow between lineages. We used the SNAPP tree (see Results, Fig. 8A) as a reference. The -Z argument computed z-scores for all f-branch values, displaying results using the dtools.py python script.
Fig. 8.
Evolutionary relationships between Petunia guarapuavensis and P. scheideana were inferred via SNAPP (A) and SVDQuartets (B) analyses. The estimated node age is depicted in the SNAPP, with node bars indicating the 95 % confidence intervals (CIs). The nodes with bootstraps (BS) < 95 % are marked in the SVDQuartets tree, and all the others had BS > 95 %.
We analysed ancestry painting (Runemark et al., 2018) to explore potential hybridization and introgression between identified populations. We tested different parental and hybrid combinations (Table 2), assessing the ancestry composition of putative hybrid individuals. This analysis evaluated heterozygosity levels and fixed parental alleles at sites with complete differentiation (FST = 1.0) between parental lineages. We considered alleles present in at least two individuals from each parental lineage, allowing for 10 % missing data. Fixed alleles from each parental lineage were depicted visually through ancestry painting on the putative hybrid genome. It is important to note that the genome was not phased, meaning that the alleles inherited from each parent were not distinguished.
Table 2.
Putative parental and hybrid combination for ancestral painting analysis based on population structure.
| Parental 1 | Hybrid | Parental 2 |
|---|---|---|
| Pgua1–6 | Pgua7–8 | Psch1 |
| Pgua1–6 | Pgua7–8 + Psch2–3 | Psch1 |
| Pgua1–6 | Pgua7–8 | Psch2–3 |
| Pgua1–6 | Psch2–3 | Pgua7–8 |
Evolutionary relationships
To investigate evolutionary relationships between the populations, we constructed a relationship network using the NeighborNet method in SplitsTree v.4.16 (Huson and Bryant, 2006). We recovered the phylogenetic relationships using SVDQuartets and SNAPP, with Petunia axillaris as the outgroup. We used the coalescent quartet-based approach of the SVDQuartets method (Chifman and Kubatko, 2014) implemented in PAUP* v.4a (Swofford, 2003), evaluating all possible quartets with 100 bootstrap replicates to generate a bootstrap 50 % majority-rule consensus tree.
We ran SNAPP (Bryant et al., 2012) to estimate the divergence time between lineages and rooting trees at the outgroup. Owing to the computational demands of this analysis, we created a reduced VCF file containing 13 individuals representing all lineages and the putative hybrids identified via PCA and SplitsTree. This dataset was generated using the same filtering methods described earlier that were used to select random SNPs for the analysis. As the constraint file, we used the estimated divergence between the Petunia short and long corolla tube clades based on a dated phylogeny (Särkinen et al., 2013), resulting in a log-normal prior distribution with a mean of 2.85 in real space and an s.d. of 0.16, calculated in BEAUTI using the normal distribution. Then, we used the snapp_prep.rb script (Stange et al., 2018) to prepare the input data, limiting the dataset to 1000 randomly selected SNPs and setting the chain length at 100 000 Markov chain Monte Carlo iterations. We assessed runs using TRACER v.1.6 (Rambaut et al., 2018) to examine convergence (effective sample size > 200) and tree topologies and visualized node heights using DensiTree (Bouckaert, 2010) and FigTree v.1.4.4 (https://github.com/rambaut/figtree/).
Species delimitation
To identify the species boundaries, we used DelimitR software (Smith et al., 2017; Smith and Carstens, 2020). This robust tool integrates the coalescent simulation fastsimcoal26 (fsc26; Excoffier et al., 2013) and a random forest (RF) classifier to distinguish competing demographic models. This approach involves constructing a set of models representing multiple speciation scenarios. These models are simulated under diverse demographic histories, producing folded multidimensional site frequency spectrum (mSFS) data. The data are then summarized into a binned SFS format by coarse-graining the mSFS matrix. The RF classifier is trained on these simulated data, estimating error rates and identifying the best-fitting model (Pudlo et al., 2016).
We used the predefined model set for the four maximum species (Fig. 2). To use the default models, DelimitR requires a guide tree, which we supplied following the SNAPP tree [(((0,1),2),3)], with 0 = Pgua1–6, 1 = Pgua7–8, 2 = Psch2–3 and 3 = Psch1 populations, respectively. Using this reference tree, we defined the limits between the species precisely, by investigating a wide range of demographic scenarios. We also added migration edges to test for divergence with ancestral gene flow and secondary contact (Supplementary Data Fig. S1).
Fig. 2.
DelimitR schematic scenarios. (A) Guide tree based on the SNAPP tree, accommodating a maximum of four species. (B) Potential scenarios ranging from one to four species, each involving secondary contact or divergence with gene flow, with a maximum of three migration edges. (C) The best scenario determined with a 0.91 posterior probability. 0 = Pgua1–6; 1 = Pgua7–8; 2 = Psch2–3; 3 = Psch1. Abbreviations: Pgua, Petunia guarapuavensis; Psch, Petunia scheideana. All tested scenarios can be found in Supplementary Data Fig. S1.
The guide tree delineated the relationships between putative species and the software-generated models with varying numbers of lineages. We tested models for four species (0, 1, 2 and 3), three species (0 + 1, 2 and 3), two species (0 + 1 + 2 and 3), and one species (0 + 1 + 2 + 3) (Fig. 2; Supplementary Data Fig. S1). In the initial round, we allowed for a maximum of three migration events per model, permitting gene flow between all potential lineages except Psch1, as observed in other analyses. We refined our approach based on the results from the best model in this round, including gene flow for Psch1. The mSFS was generated using EASYSFS (https://github.com/isaacovercast/easySFS; Gutenkunst et al., 2009), converting the VCF file to mSFS. We simulated 10 000 mSFS under each model, then summarized the simulated and observed mSFS by binning (four classes per population). We built an RF classifier and applied this classifier to the observed data using functions in DelimitR as described above. We used 1000 decision trees in the RF classifier owing to the many models. We recorded the out of baf (OOB) error rates, the selected model and the approximated posterior probability for the selected model. All specifications and scripts can be found in Supplementary Data Methods S1.
RESULTS
Genomic data quality and SNPs
DArTseq generated a comprehensive dataset with 84 933 340 reads. The individual read counts ranged from 499 049 to 2 200 544, averaging 1 665 359.61 reads per sample (Supplementary Data Table S1). After processing, we identified 425 638 biallelic SNPs, allowing for 10 % missing data. Following thinning per 100 bp and maf filtering, we retained a high-quality set with 18 525 SNPs (Supplementary Data Table S2). Subsequently, 2461 outlier loci were excluded, resulting in a final dataset with 16 064 potentially neutral SNPs. In some analyses involving P. axillaris as the outgroup, the VCF file included 15 739 SNPs. Additionally, we used a VCF containing 17 157 SNPs and 39 individuals in specific analyses by excluding the Psch1 population.
Genetic diversity and population differentiation
Diversity indices (Table 1) varied little among populations and species, except for the number of private alleles, with Psch1 having the highest value and Pgua7–8 displaying the lowest. The observed heterozygosity was low and did not differ from what was expected. The nucleotide diversity (π) varied between 0.19 and 0.22 among the collection sites. All sites exhibited null or low and negative inbreeding coefficients, with only Psch2 having a positive value (FIS = 0.03). The pairwise FST was low to moderate, with higher values involving all comparisons with Psch1 (Supplementary Data Tables S3 and S4).
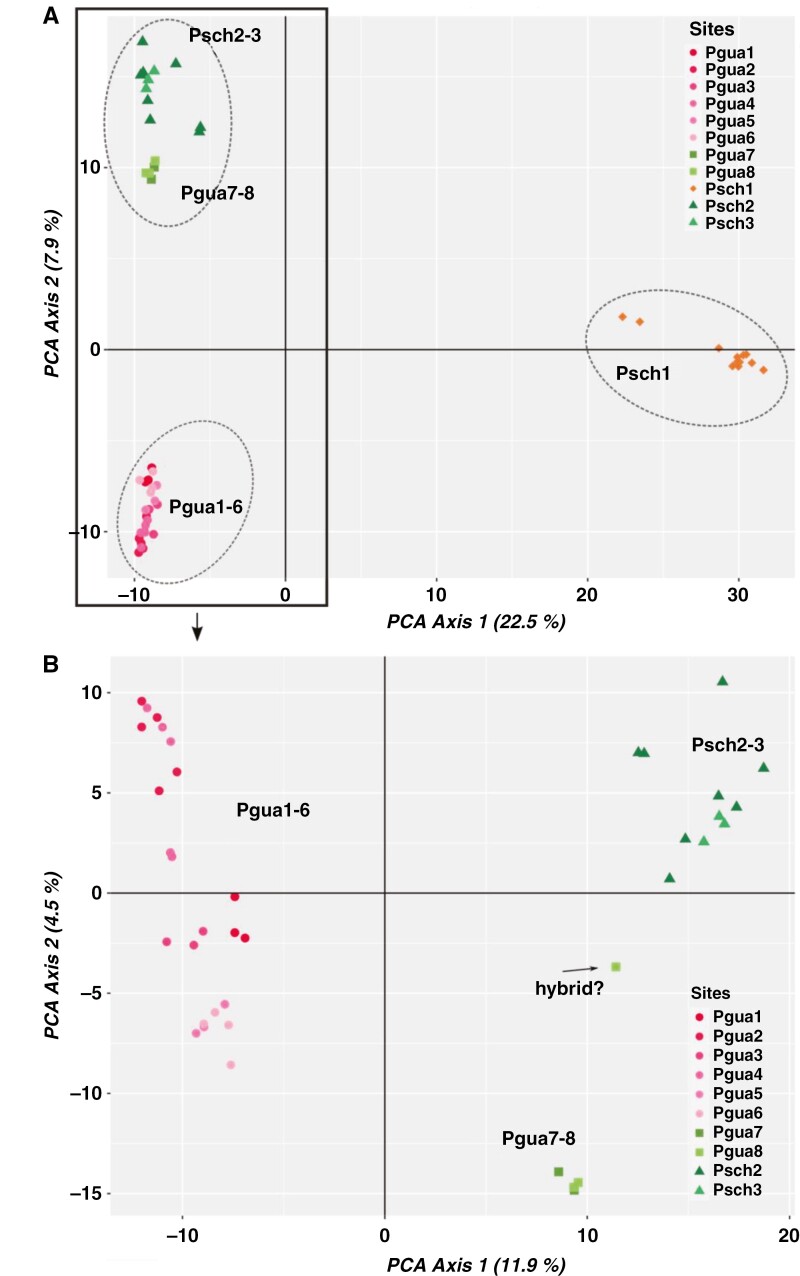
Including total sampling (Fig. 3A), the first two principal components (PCs) of the PCA clustered individuals into three distinct groups. The first cluster comprised only individuals from the Psch1 population; the second encompassed Pgua1–6 samples; and the third group included individuals from Pgua7–8 and Psch2–3. Notably, PC1 captured 22.5 % of the information, primarily differentiating Psch1 from the other collection sites. PC2 explained ~8 % of the variation and split Pgua1–6 and Pgua7–8 + Psch2–3. The analysis excluding Psch1 (Fig. 3B) kept Pgua1–6 individuals in the same group, whereas Psch2–3 and Pgua7–8 were divided. One Pgua8 individual (ID-2062318) was positioned at an intermediate distance between the Psch2–3 and Pgua7–8 groups, suggesting a hybrid state.
Fig. 3.
The principal component analysis (PCA) plot illustrates the genetic diversity among Petunia guarapuavensis and P. scheideana individuals. (A) The first two principal components (PCs), including 51 individuals and 16 064 single nucleotide polymorphisms (SNPs). Dotted lines delineate individual groups; circles in pink shades indicate Pgua1–6 collection sites; squares and green shades indicate Pgua7–8; triangles and green shades represent Psch2–3; and orange diamonds correspond to Psch1 individuals. (B) The first two PCA axes, including 39 individuals and 17 157 SNPs, excluded Psch1 collection site.
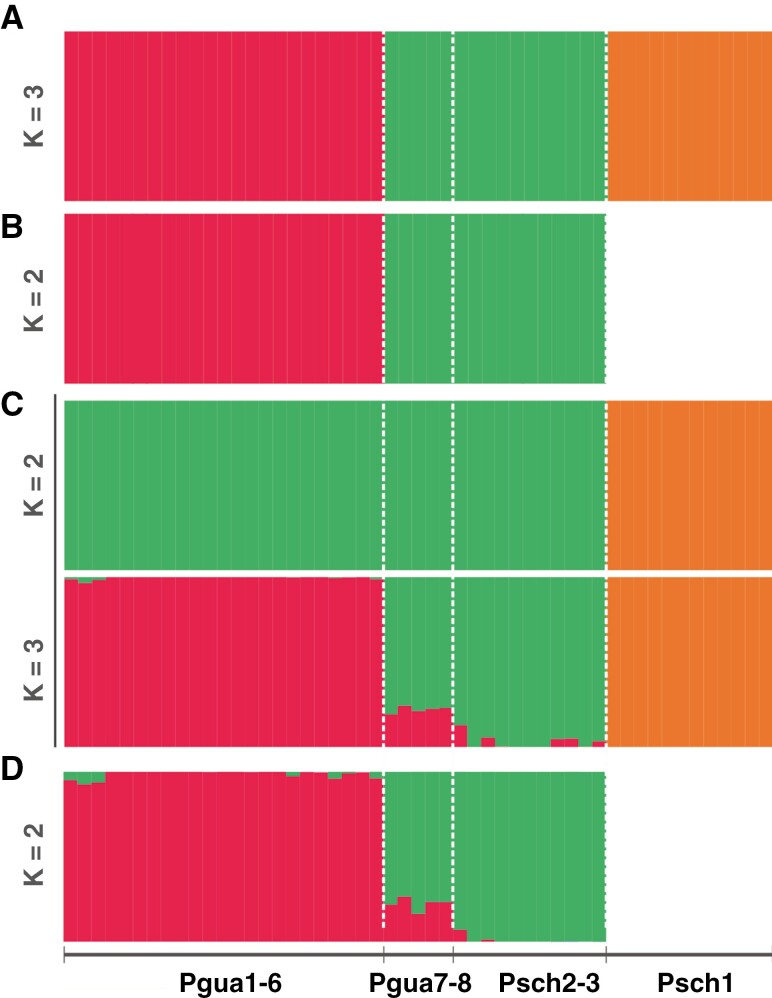
The DAPC indicated an optimal K = 3 (number of discriminant analysis [n.da] = 3, number of retained pricipal components [n.pca] = 9; Fig. 4A), mirroring the PCA groups. After excluding Psch1 individuals, two groups were observed (Fig. 4B). STRUCTURE analysis revealed the best K = 2 (Fig. 4C), indicating that Pgua1–8 and Psch2–3 share a genetic component and Psch1 has an exclusive profile. Considering the equally probable best K = 3, Pgua1–6 differed from the Psch2–3 and Pgua7–8 groups, which have some admixed individuals with Pgua1–6. After the Psch1 individuals were removed, STRUCTURE analysis recovered the division between Pgua1–6 and Pgua7–8 + Psch2–3 (Fig. 4D).
Fig. 4.
Population structure of Petunia scheideana and P. guarapuavensis. (A) DAPC best K = 3, including all individuals and lineages. (B) DAPC best K = 2 excluding the Psch1 collection site. (C) STRUCTURE analyses with the equally best K = 2 and K = 3. (D) STRUCTURE analysis with the best K = 2, excluding the Psch1 collection site.
Based on the above results, we considered Pgua1–6 and Psch1 independent populations. Although Psch2–3 and Pgua7–8 exhibited genetic patterns indicative of a unified population, they were identified as distinct species based on morphological traits and geographical distribution. In line with the taxonomic classification and guided by PCA results, we maintained the independence of these groups in some specific analyses to gain a more nuanced understanding of P. scheideana and P. guarapuavensis relationships.
The hierarchical AMOVA (Table 3) revealed that 83.58 % of the variation was attributable to differences within individuals, and 21.03 % was attributed to differences among population groups (FCT = 0.21).
Table 3.
Hierarchical AMOVA for Petunia guarapuavensis and P. scheideana populations and groups (Pgua1–6, Pgua7–8 + Psch2–3 and Psch1).
| Source of variation | d.f. | Sum of squares | Variance components | Percentage of variation | Fixation indices |
|---|---|---|---|---|---|
| Among groups | 3 | 20932.8 | 234.16 | 21.03 | 0.21 |
| Among populations within groups | 7 | 8243.64 | 65.40 | 5.87 | 0.07 |
| Among individuals within populations | 40 | 27881.5 | −116.73 | −10.48 | −0.14 |
| Within individuals | 51 | 47455.5 | 930.50 | 83.58 | 0.16 |
| Total | 101 | 104 513 | 1113.33 | – | – |
Values in bold were significant at P < 0.005.
Searching for hybrids and introgression
Snapclust consistently failed to detect recent hybrids and, instead, identified distinct groups, even when we considered isolating pairs of populations (Supplementary Data Fig. S2). In contrast, HyDe detected putative hybrids among Pgua8 individuals (Supplementary Data Table S5), with one parental component from Psch2–3 and another from all other Pgua sites. Although this might not indicate recent hybridization, it suggests introgression or gene flow between the Psch2–3 and Pgua populations. Notably, the individual identified as a putative hybrid in the PCA originated from Pgua8.
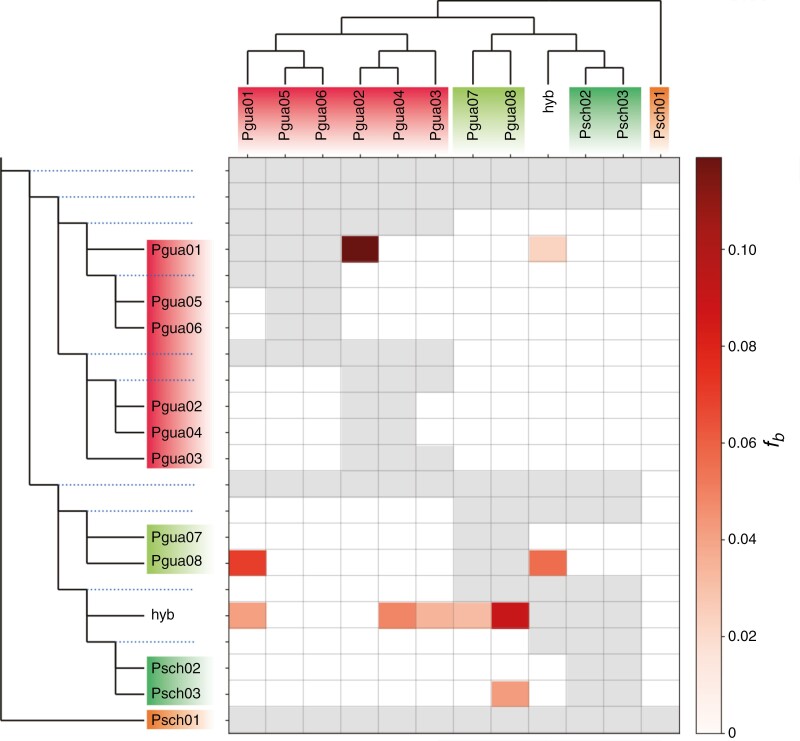
The f-branch analysis demonstrated gene flow between the Pgua1 and Pgua6 sites and between Pgua8 and Pgua1 (Fig. 5). Consistent with the results from HyDe, the putative hybrid from Pgua8 displayed components from all other Pgua populations. It revealed gene flow between Psch3 and Pgua8, supporting the notion of a well-connected group comprising Pgua1–6, Psch7–8 and Psch2–3.
Fig. 5.
Estimated gene flow footprints using the f-branch statistic. The heatmap illustrates the f-branch statistics computed in Dsuite, reflecting migration or gene flow patterns from the phylogenetic reference tree generated with SNAPP. Dotted lines in the phylogeny indicate ancestral lineages. Rows correspond to nodes in the tree, and columns indicate tips. Each cell displays the f-branch statistic between rows and columns. The grey cells indicate where comparisons cannot be made.
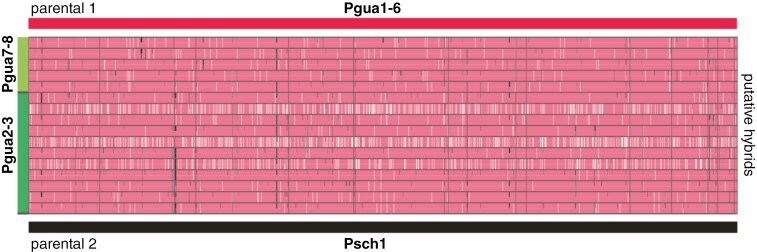
Finally, we explored various pairs of parentals and putative hybrids in the ancestral painting analysis (Fig. 6). The comparison between Psch1–6 and Psch2–3 revealed that only four sites with different alleles were fixed entirely, whereas Psch1–6 and Psch7–8 had only three sites. This result emphasized the cohesion between these groups. In the pairing, by testing Psch1–6 and Psch1 as the parental lineages, we found 2741 sites with different alleles completely fixed. Psch7–8 + Psch2–3 predominantly shared fixated alleles with Psch1–6, with minor variations highlighted in Fig. 6.
Fig. 6.
Ancestral painting analysis for putative hybridization. The scenario considered crosses between Pgua1–6 (Petunia guarapuavensis; pink) and Psch1 (Petunia scheideana; grey) and Pgua7–8 + Psch2–3 as hybrids. The horizontal bars at the top (pink) and bottom (grey) represent the 2741 sites where the parental lineages differed on fixed alleles. The remaining bars depict alleles at each site, with white indicating missing data and colours representing allele ancestry. The full bars indicate homozygosity, and the interrupted bars show heterozygous loci. The genomes were not phased.
Evolutionary relationships
The SplitsTree network (Fig. 7) revealed three main groups, in line with previous results on population structure: the Psch1, Psch1–6 and Psch7–8 + Psch2–3 groups. We also observed a sub-structuring in the clade Psch7–8 + Psch2–3, splitting individuals following their taxonomic identification and with the putative hybrid individual from Pgua8 observed in PCA positioned between subclades. The sub-structure of Psch1–6 reflected the collection site of the individuals. There were several reticulations between the main branches, which suggests gene flow among the clades. Furthermore, the analysis revealed a considerable distance between Psch1 and the remaining populations. The outgroup (P. axillaris) was equally distant from Psch1 and the combined groups Psch1–6 and Psch7–8 + Psch2–3.
Fig. 7.
Evolutionary relationships between Petunia guarapuavensis and P. scheideana individuals as observed with the SplitsTree network.
We also identified three main clades in the SNAPP analysis (Fig. 8A). The first clade included Psch1–6 individuals and was the sister group of the Psch7–8 clade. The third clade included Psch2–3 individuals. In this analysis, Psch1 grouped with the outgroup and was equally distant from the remaining Psch and Pgua as P. axillaris. The putative hybrid from Pgua8 appeared as the sister lineage of Psch7–8 populations. Psch2–3 was the first group to diverge (~0.85 Mya), followed by the divergence between the sister groups Psch1–6 and Psch7-8 (~0.60 Mya). The divergence time between P. axillaris and Psch1 and the ancestral population was 2.86 Mya, diverging from each other 2.58 Mya.
The SVDQuartets tree (Fig. 8B) produced robust bootstrap values, except for branches associated with Psch1–6. Three main clades were observed, with Psch1–6 emerging as a monophyletic group sister to the Psch7–8 + Psch2–3 clade. Psch1 formed an independent clade, exhibiting a connection with the ancestor of the remaining Pgua–Psch populations. The putative hybrid from Pgua8 was recovered as the sister lineage to Psch2–3. We rooted the tree artificially with P. axillaris, a species that with a long corolla tube. The SVDQuartets and SNAPP results, along with SplitsTree, underscored the considerable evolutionary distance between Psch1 and the remaining Psch and Pgua populations.
The relationships and incongruences observed with DensiTree (Fig. 9) indicated that the SNPs failed to delineate a single evolutionary history, mainly regarding Psch1, which sometimes appears as the sister to all other Pgua and Psch populations, and sometimes is closely related to the outgroup. The relationships between the Pgua groups Psch1–6 and Psch7–8 and the putative hybrid, which is an interspecific hybrid, are also challenging.
Fig. 9.
DensiTree comparison between species-tree inference from Petunia guarapuavensis and P. scheideana populations and phylogenetic relationships obtained with SNAPP. Petunia axillaris was used as the outgroup.
Species delimitation
The DelimitR most favourable model (Fig. 2B) considered two species. This model received 241 votes, with a posterior probability of 0.91 (Supplementary Data Table S6) and an OOB error rate of 6.56 %. The first species included Psch1–6, Psch7–8 and Psch2–3, whereas the second included Psch1 individuals only. This best model also included secondary contact and recent gene flow between these populations.
DISCUSSION
Delimiting species becomes particularly challenging when dealing with recently diverged taxa. Our study used genomic population analysis to determine the genetic diversity, population structure and evolutionary history of two Petunia species that share genetic polymorphisms and several morphological and ecological features. Despite being identified as separate species, they were posteriorly considered synonyms mainly owing to slight morphological variation. Our results, however, shed light on this complexity in a nuanced manner, not only in indicating at least two distinct species that are not necessarily as initially classified. Also, the results strongly illustrate how isolation in allopatry, changes in geographical distribution and secondary contact can lead to muddled species boundaries.
Petunia scheideana and P. guarapuavensis displayed lineage subdivisions (Table 3; Supplementary Data Table S3), which in other cases were already considered different taxa (e.g. Turchetto et al., 2014, 2015; Caballero-Villalobos et al., 2021; Guzmán et al., 2022; Pezzi et al., 2022). Such divergence among sites suggests that the differences observed between Psch1 and the other populations align with the typical distinction observed between different species of Petunia. Furthermore, Psch1, being one of the most remote populations, differed from the other samples in all subsequent analyses as the outgroup P. axillaris did.
Individuals adhering to P. scheideana (Psch2–3) and P. guarapuavensis (Pgua7–8) original descriptions were grouped in most clustering analyses, independently of the inclusion of Psch1 individuals (Figs 3 and 4). The Iguaçu River, a substantial geographical barrier in the subtropical highland grasslands, separates the distributions of Psch1–6 and Psch7–8 + Psch2–3 (Fig. 1). Several studies have highlighted the influential role of large rivers in shaping genetic variability and population structures for diverse species (e.g. Gascon et al., 2000; Godinho and Silva, 2018; Wendt et al., 2019; Kopuchian et al., 2020; Giudicelli et al., 2022; Soares and Freitas, 2024). Here, we hypothesized that the Iguaçu River contributes to the structuration observed between the Psch1–6 and Psch7–8 + Psch2–3 populations by acting as a barrier, limiting gene flow between groups.
Evidence of gene flow among the P. guarapuavensis populations revealed connectivity between them. Snapclust could not identify contemporaneous hybrids, because the analysis best identifies F1 hybrids (Beugin et al., 2018). However, HyDe, using the search of admixture with phylogenetic invariants (Blischak et al., 2018), could detect lower levels of introgression or gene flow between the lineages. HyDe showed geneflow between the individuals of Psch1–6 and Psch3 into Pgua8 (Supplementary Data Table S5). Likewise, f-branch (Fig. 5) indicated gene flow between the Psch1–6 and Psch7–8 groups despite the river separating their distributions. Although several examples confirm rivers as barriers in southern South American grasslands, in these same studies and others, these rivers do not represent fully effective barriers, with some gene flow between populations on the two riverbanks (e.g. Veroslavsky and Ubilla, 2007; Roratto et al., 2014; Giudicelli et al., 2022; Soares et al., 2023). This connectivity could stem from ancestral gene flow or polymorphisms (Guerrero and Hahn, 2017). Long-distance gene flow in Petunia is rare, with most estimates ~300 m (Turchetto et al., 2015, 2022) or less (Rodrigues et al., 2019), mainly owing to the behaviour of pollinators or seed dispersal (van der Pijl, 1982). Furthermore, Petunia species are annual (Stehmann et al., 2009), and human activities severely impact the subtropical highland grasslands (Plá et al., 2020). These ecological features could extinguish populations from one year to the next, eliminating any signal of recent step-by-step gene flow between populations or lineages.
Genetic diversity and population structure analyses indicated differentiation in at least three lineages. However, they do not discard ancestral polymorphisms shared by gene exchange between P. scheideana and P. guarapuavensis to explain populations of two species forming a homogeneous group different from their sister populations. The challenge lies in differentiating within-species population structures from among-species divergence (Sukumaran and Knowles, 2017). The robust species diagnosis depends on integrating multiple data sources and approaches, as we are doing here (Carstens et al., 2013).
Determining precise edges amid ongoing speciation, especially with recent introgression, constitutes challenges (Wiens, 2007), as observed in Petunia species. Our study revealed no gene flow or introgression between Psch1–6/Psch7–8 + Psch2–3 and Psch1 (Fig. 6), suggesting two distinct lineages probably produced by the reproductive isolation between the groups.
Based on the original description (Ando and Hashimoto, 1995), Psch1–6 individuals display morphological characteristics typical of P. guarapuavensis, whereas Psch1 is a typical P. scheideana (Smith and Down, 1964). In this context, the lineage Psch7–8 + Psch2–3 represents a cline of morphological diagnostic characters, including a hybrid detected in all molecular analyses. Clines in morphological traits can promote taxonomic questioning, leading to synonymization and grouping of all populations (Stehmann et al., 2009) in the same nominal taxon. In any case, the Psch7–8 + Psch2–3 lineage was considered independent in most analyses, and some indicated a split between Psch7–8 and Psch2–3, suggesting that the two taxa share some genetic polymorphism and morphology, which could be a consequence of interspecific gene flow. Gene exchange in Petunia species is not novel, and generally, hybridizing species maintain their integrity despite some introgression (e.g. Caballero-Villalobos et al., 2021; Pezzi et al., 2022). Furthermore, P. scheideana and P. guarapuavensis share pollination syndromes, and the same bee species have been recorded visiting their populations (Ando and Hashimoto, 1995; Tsukamoto et al., 1998).
Despite some analyses not ruling out the split between Psch1–6 and Psch7–8 + Psch2–3, suggesting a phylogeographical structure with the Iguaçu River as a barrier, the evolutionary proximity can be explained by ancestral polymorphism sharing and the short diversification time between them, as observed in other Petunia species that inhabit the highlands in southern Brazil (Lorenz-Lemke et al., 2010; Souza et al., 2022).
The evolutionary relationships indicated that Psch1 is a distinct lineage, distantly related to the other populations (Figs 7–9). The SNAPP consensus tree (Fig. 8A) identified Psch1 and P. axillaris as sister lineages positioned at the tree base, which suggests long-branch attraction (Bergsten, 2005), because there are no other species available to aid in phylogenetic relationships. The differences among SNPs in the DensiTree analysis (Fig. 9), as in each with an independent evolutionary history, emphasize the complexity of gene trees potentially differing from species trees (Doyle, 1992; Maddison, 1997; Naciri and Linder, 2015). Another possibility to explain the Psch1 evolutionary pattern could be incomplete lineage sorting (Willyard et al., 2009), frequently observed in Petunia (e.g. Pezzi et al., 2022).
The phylogenetic analysis shows no doubts about the monophyly of P. guarapuavensis or that its ancestor is the sister of the Psch2–3 lineage. Psch1–6 and Psch7–8 are closely related, and we can attribute their divergence to geographical isolation, with the Iguaçu River serving as an effective, albeit partial, phylogeographical barrier. Under this scenario, Psch2–3 could be a recently diverged lineage, independent of P. guarapuavensis, although derived from the same ancestor and for which a taxonomic treatment is still necessary, with morphology tending towards P. scheideana appearance, randomly or directed by some unknown evolutionary force.
We adopted the DelimitR approach to understand species limits, which infers species boundaries and speciation modes by conducting demographic model selection under coalescence and can allocate speciation with gene flow and secondary contact (Smith and Carstens, 2020). Our findings (Fig. 2) indicated the best scenario for speciation involving two lineages under secondary contact, one lineage corresponding to Psch1 and another comprising the remaining populations. This result did not invalidate what was discussed above. These findings reinforce the idea that P. scheideana (Psch1) and P. guarapuavensis are independent species, the latter of which is subdivided into two phylogeographical lineages (Psch1–6 and Psch7–8), with a new lineage in the process of genetic and morphological speciation (Psch2–3).
The proposed scenario probably arises from the rapid divergence between these taxa, because incipient species are typically young (Zúñiga-Reinoso and Benítez, 2015). Highland Petunia lineages were dated at ~900 kya (Lorenz-Lemke et al., 2010). The South American highland grasslands have a demographic history that allowed rapid divergence under the influence of Pleistocene climate change, which expanded grassland-adapted species during the glacial cycles. This period of climatic instability, starting ~5 Mya and intensifying between 3 and 2 Mya, facilitated the rapid diversification of endemic flora (Vasconcelos et al., 2020). The region was later fragmented by the expansion of the Araucaria forest during the warmer and moister Holocene (Iriarte and Behling, 2007), resulting in island-like grasslands surrounded by forest (Iganci et al., 2011). This isolation has driven allopatric speciation in many plant genera (Iganci et al., 2011; Backes et al., 2019; John et al., 2019), and similar patterns of diversification are observed in other highland grassland organisms (e.g. Bonatelli et al., 2014; Silva et al., 2018; Mäder and Freitas, 2019; Olivares et al., 2024). This fragmented landscape is comparable to other ecosystems, offering insights into the effects of isolation on plant gene flow (Hagen et al., 2012).
Additionally, morphological diagnostic traits in incipient species tend to evolve slowly, especially if they are selectively neutral, the population effective size is large and ancestral polymorphism is high (Wiens and Servedio, 2000; Egea et al., 2016). All these assumptions were observed here and in other Petunia species from the same and other regions (e.g. Lorenz-Lemke et al., 2010; Giudicelli et al., 2022; Guzmán et al., 2022; Pezzi et al., 2022; Soares and Freitas, 2024). Species diversification rates and morphological evolution are not significantly correlated, allowing rapid diversification with minimal morphological change and vice versa (Adams et al., 2009).
We hypothesized that the rapid divergence in Petunia would explain the lack of morphological differences between these species, similar to other species in the Solanaceae family (e.g. Bosland and Gonzalez, 2000; Vigalondo et al., 2015; Chiarini et al., 2018; Raduski and Igić, 2021). High-throughput sequencing and population genomics can play a crucial role in discerning incipient plant species (e.g. Okuyama and Kato, 2009; Shneyer and Kotseruba, 2015; Wei et al., 2021), which are characterized by morphological indistinguishability and are erroneously grouped as a single nominal taxon (Bickford et al., 2007). Our results highlighted the importance of using a hierarchical population framework to delimit species, particularly considering rapidly diverging species with weak reproductive barriers. Here, we integrated the taxonomic history, population-level analysis and environmental demography and could uncover better the complex relationships between lineages that simpler analyses might overlook. More straightforward delimitation analysis, such as reversible-jump Bayesian Markov chain Monte Carlo (rjMCMC) (i.e. Bayesian Phylogenetics and Phylogeography [BPP]; Flouri et al., 2018) could result in over-splitting of P. scheideana, but it would be unlikely that this would group part of this entity with P. guarapuavensis.
Regarding conservation, we strongly advocate for a taxonomic review of these lineages, particularly their classification; a task that is by no means simple. Defining species is pivotal, especially considering the implications for conservation efforts. Petunia guarapuavensis and P. scheideana are classified as endangered based on the International Union for Conservation of Nature criteria (data not shown), emphasizing the urgency and significance of accurate species delimitation.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: all DelimitR simulated models. Figure S2. Snapclust analysis. Table S1: GenBank accession, barcode and read information per individual in DART-seq analysis. Table S2: variant calling obtained using FreeBayes, filtered SNPs and the proportion of missing data per sample. Table S3: pairwise FST values comparing intra- and interspecific genetic structure for Petunia guarapuavensis (Pgua) and P. scheideana (Psch) collection sites. Table S4: pairwise FST comparisons considering groups observed in population structure analyses. Table S5: results of the population- and individual-level hybridization detection analyses for the non-uniform hybridization simulation using HyDe. Table S6: votes of the 1000 decision trees of DelimitR analysis for all models. Methods S1: script to run DelimitR with default scenarios in R.
ACKNOWLEDGEMENTS
We thank Dr Marcelo C. Teixeira for help with the conservation analysis.
Contributor Information
Luana S Soares, Department of Genetics, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Aureliano Bombarely, Instituto de Biologia Molecular y Celular de Plantas (IBMCP) (CSIC-UPV), Valencia, Spain.
Loreta B Freitas, Department of Genetics, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
FUNDING
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). CNPq and Programa Institucional de Internacionalização—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Print-CAPES)/Universidade Federal do Rio Grande do Sul (UFRGS) supported L.S.S.
COMPETING INTERESTS
There are no competing interests to declare.
DATA AVAILABILITY
The original unfiltered VCFs dataset is available at doi.org/10.6084/m9.figshare.25324813. Raw reads are available at https://www.ncbi.nlm.nih.gov/biosample/ under codes SAMN40212200–SAMN40212250, bioproject http://www.ncbi.nlm.nih.gov/bioproject/1082513.
LITERATURE CITED
- Adams DC, Berns CM, Kozak KH, Wiens JJ.. 2009. Are rates of species diversification correlated with rates of morphological evolution? Proceedings Biological Sciences 276: 2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Hashimoto G.. 1995. Petunia guarapuavensis (Solanaceae): a new species from planalto of Parana and Santa Catarina, Brazil. Brittonia 47: 328. [Google Scholar]
- Ando T, Hashimoto G.. 1996. A new Brazilian species of Petunia (Solanaceae) from interior Santa Catarina and Rio Grande do Sul, Brazil. Brittonia 48: 217. [Google Scholar]
- Ando T, Kokubun H, Watanabe H, et al. 2005. Phylogenetic analysis of Petunia sensu Jussieu (Solanaceae) using chloroplast DNA RFLP. Annals of Botany 96: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. 2010. FastQC: a Quality Control Tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (30 April 2024, date last accessed). [Google Scholar]
- Aronesty E. 2013. Comparison of sequencing utility programs. The Open Bioinformatics Journal 7: 1–8. [Google Scholar]
- Backes A, Mäder G, Turchetto C, et al. 2019. How diverse can rare species be on the margins of genera distribution? AoB Plants 11: plz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes A, Turchetto C, Mäder G, Segatto ALA, Bonatto SL, Freitas LB.. 2024. Shades of white: the Petunia long corolla tube clade evolutionary history. Genetics and Molecular Biology 47: e20230279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten J. 2005. A review of long‐branch attraction. Cladistics 21: 163–193. [DOI] [PubMed] [Google Scholar]
- Beugin M, Gayet T, Pontier D, Devillard S, Jombart T.. 2018. A fast likelihood solution to the genetic clustering problem. Methods in Ecology and Evolution 9: 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford D, Lohman DJ, Sodhi NS, et al. 2007. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22: 148–155. [DOI] [PubMed] [Google Scholar]
- Blischak PD, Chifman J, Wolfe AD, Kubatko LS.. 2018. HyDe: a Python package for genome-scale hybridization detection. Systematic Biology 67: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombarely A, Moser M, Amrad A, et al. 2016. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nature Plants 2: 16074. [DOI] [PubMed] [Google Scholar]
- Bonatelli IAS, Perez MF, Peterson AT, et al. 2014. Interglacial microrefugia and diversification of a cactus species complex: phylogeography and palaeodistributional reconstructions for Pilosocereus aurisetus and allies. Molecular Ecology 23: 3044–3063. [DOI] [PubMed] [Google Scholar]
- Bosland PW, Gonzalez MM.. 2000. The rediscovery of Capsicum lanceolatum (Solanaceae), and the importance of nature reserves in preserving cryptic biodiversity. Biodiversity and Conservation 9: 1391–1397. [Google Scholar]
- Bouckaert RR. 2010. DensiTree: making sense of sets of phylogenetic trees. Bioinformatics 26: 1372–1373. [DOI] [PubMed] [Google Scholar]
- Bryant D, Bouckaert R, Felsenstein J, Rosenberg NA, Roychoudhury A.. 2012. Inferring species trees directly from biallelic genetic markers: bypassing gene trees in a full coalescent analysis. Molecular Biology and Evolution 29: 1917–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbrink FT, Ruane S.. 2021. Contemporary philosophy and methods for studying speciation and delimiting species. Ichthyology & Herpetology 109: 874–894. [Google Scholar]
- Caballero-Villalobos L, Silva-Arias GA, Turchetto C, et al. 2021. Neutral and adaptive genomic variation in hybrid zones of two ecologically diverged Petunia species (Solanaceae). Botanical Journal of the Linnean Society 196: 100–122. [Google Scholar]
- Carstens BC, Pelletier TA, Reid NM, Satler JD.. 2013. How to fail at species delimitation. Molecular Ecology 22: 4369–4383. [DOI] [PubMed] [Google Scholar]
- Chen S, Matsubara K, Omori T, et al. 2007. Phylogenetic analysis of the genus Petunia (Solanaceae) based on the sequence of the Hf1 gene. Journal of Plant Research 120: 385–397. [DOI] [PubMed] [Google Scholar]
- Chiarini FE, Scaldaferro MA, Bernardello G, Acosta MC.. 2018. Cryptic genetic diversity in Solanum elaeagnifolium (Solanaceae) from South America. Australian Journal of Botany 66: 531. [Google Scholar]
- Chifman J, Kubatko L.. 2014. Quartet inference from SNP data under the coalescent model. Bioinformatics 30: 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz VMV, Kilian A, Dierig DA.. 2013. Development of DArT marker platforms and genetic diversity assessment of the U.S. collection of the new oilseed crop Lesquerella and related species. PLoS One 8: e64062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, et al. ; 1000 Genomes Project Analysis Group. 2011. The variant call format and VCFtools. Bioinformatics 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, et al. 2021. Twelve years of SAMtools and BCFtools. GigaScience 10: giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ. 1992. Gene trees and species trees: molecular systematics as one-character taxonomy. Systematic Botany 17: 144. [Google Scholar]
- Eaton DAR, Ree RH.. 2013. Inferring phylogeny and introgression using RADseq data: an example from flowering plants (Pedicularis: Orobanchaceae). Systematic Biology 62: 689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea E, David B, Choné T, Laurin B, Féral JP, Chenuil A.. 2016. Morphological and genetic analyses reveal a cryptic species complex in the echinoid Echinocardium cordatum and rule out a stabilizing selection explanation. Molecular Phylogenetics and Evolution 94: 207–220. [DOI] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J.. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, Käller M.. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL.. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM.. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Dupanloup I, Huerta-Sánchez E, Sousa VC, Foll M.. 2013. Robust demographic inference from genomic and SNP data. PLoS Genetics 9: e1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fišer C, Robinson CT, Malard F.. 2018. Cryptic species as a window into the paradigm shift of the species concept. Molecular Ecology 27: 613–635. [DOI] [PubMed] [Google Scholar]
- Flouri T, Jiao X, Rannala B, Yang Z.. 2018. Species tree inference with BPP using genomic sequences and the multispecies coalescent. Molecular Biology and Evolution 35: 2585–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RM. 2017. pophelper: an R package and web app to analyze and visualize population structure. Molecular Ecology Resources 17: 27–32. [DOI] [PubMed] [Google Scholar]
- Garrison E, Marth G.. 2012. Haplotype-based variant detection from short-read sequencing. ArXiv. doi: https://doi.org/ 10.48550/arXiv.1207.3907. [Preprint; not peer reviewed] (30 April 2024, date last accessed). [DOI] [Google Scholar]
- Gascon C, Malcolm JR, Patton JL, et al. 2000. Riverine barriers and the geographic distribution of Amazonian species. Proceedings of the National Academy of Sciences of the United States of America 97: 13672–13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli GC, Turchetto C, Guzmán-Rodriguez S, et al. 2022. Population genomics indicates microrefuges and riverine barriers for a southern South American grassland nightshade. Journal of Biogeography 49: 51–65. [Google Scholar]
- Godinho MBC, Silva FR.. 2018. The influence of riverine barriers, climate, and topography on the biogeographic regionalization of Amazonian anurans. Scientific Reports 8: 3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero RF, Hahn MW.. 2017. Speciation as a sieve for ancestral polymorphism. Molecular Ecology 26: 5362–5368. [DOI] [PubMed] [Google Scholar]
- Gutenkunst RN, Hernandez RD, Williamson SH, Bustamante CD.. 2009. Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genetics 5: e1000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Rodriguez S, Giudicelli GC, Turchetto C, Bombarely A, Freitas LB.. 2022. Neutral and outlier single nucleotide polymorphisms disentangle the evolutionary history of a coastal Solanaceae species. Molecular Ecology 31: 2847–2864. [DOI] [PubMed] [Google Scholar]
- Hagen M, Kissling WD, Rasmussen C, et al. 2012. Biodiversity, species interactions and ecological networks in a fragmented world. Advances in Ecological Research 46: 89–210. [Google Scholar]
- Hörandl E. 2022. Novel approaches for species concepts and delimitation in polyploids and hybrids. Plants (Basel, Switzerland) 11: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Atchison GW.. 2015. The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. The New Phytologist 207: 275–282. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D.. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Iganci JR, Heiden G, Miotto STS, Pennington RT.. 2011. Campos de Cima da Serra: the Brazilian Subtropical Highland Grasslands show an unexpected level of plant endemism. Botanical Journal of the Linnean Society 167: 378–393. [Google Scholar]
- Iriarte J, Behling H.. 2007. The expansion of Araucaria Forest in the southern Brazilian highlands during the last 4000 years and its implications for the development of the Taquara/Itararé Tradition. Environmental Archaeology 12: 115–127. [Google Scholar]
- John ALW, Mäder G, Fregonezi JN, Freitas LB.. 2019. Genetic diversity and population structure of naturally rare Calibrachoa species with small distribution in southern Brazil. Genetics and Molecular Biology 42: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jombart T. 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405. [DOI] [PubMed] [Google Scholar]
- Jombart T, Devillard S, Balloux F.. 2010. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian A, Wenzl P, Huttner E, et al. 2012. Diversity arrays technology: a generic genome profiling technology on open platforms. Methods in Molecular Biology (Clifton, N.J.) 888: 67–89. [DOI] [PubMed] [Google Scholar]
- Kopuchian C, Campagna L, Lijtmaer DA, et al. 2020. A test of the riverine barrier hypothesis in the largest subtropical river basin in the Neotropics. Molecular Ecology 29: 2137–2149. [DOI] [PubMed] [Google Scholar]
- Kozak KH, Wiens JJ.. 2010. Accelerated rates of climatic‐niche evolution underlie rapid species diversification. Ecology Letters 13: 1378–1389. [DOI] [PubMed] [Google Scholar]
- Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC.. 2016. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). The New Phytologist 210: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischer HEL, Excoffier L.. 2012. PGDSpider: an automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 28: 298–299. [DOI] [PubMed] [Google Scholar]
- Longo D, Lorenz-Lemke AP, Mäder G, Bonatto SL, Freitas LB.. 2014. Phylogeography of the Petunia integrifolia complex in southern Brazil. Botanical Journal of the Linnean Society 174: 199–213. [Google Scholar]
- Lorenz-Lemke AP, Mäder G, Muschner VC, et al. 2006. Diversity and natural hybridization in a highly endemic species of Petunia (Solanaceae): a molecular and ecological analysis. Molecular Ecology 15: 4487–4497. [DOI] [PubMed] [Google Scholar]
- Lorenz-Lemke AP, Togni PD, Mäder G, et al. 2010. Diversification of plant species in a subtropical region of eastern South American highlands: a phylogeographic perspective on native Petunia (Solanaceae). Molecular Ecology 19: 5240–5251. [DOI] [PubMed] [Google Scholar]
- Luu K, Bazin E, Blum MGB.. 2017. pcadapt: an R package to perform genome scans for selection based on principal component analysis. Molecular Ecology Resources 17: 67–77. [DOI] [PubMed] [Google Scholar]
- Maddison WP. 1997. Gene trees in species trees. Systematic Biology 46: 523–536. [Google Scholar]
- Mäder G, Freitas LB.. 2019. Biogeographical, ecological, and phylogenetic analyses clarifying the evolutionary history of Calibrachoa in South American grasslands. Molecular Phylogenetics and Evolution 141: 106614. [DOI] [PubMed] [Google Scholar]
- Malinsky M, Svardal H, Tyers AM, et al. 2018. Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow. Nature Ecology & Evolution 2: 1940–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky M, Matschiner M, Svardal H.. 2021. Dsuite ‐ Fast D‐statistics and related admixture evidence from VCF files. Molecular Ecology Resources 21: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Richards EJ.. 2019. The paradox behind the pattern of rapid adaptive radiation: how can the speciation process sustain itself through an early burst? Annual Review of Ecology, Evolution, and Systematics 50: 569–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijangos JL, Gruber B, Berry O, Pacioni C, Georges A.. 2022. dartR v2: an accessible genetic analysis platform for conservation, ecology and agriculture. Methods in Ecology and Evolution 13: 2150–2158. [Google Scholar]
- Naciri Y, Linder HP.. 2015. Species delimitation and relationships: the dance of the seven veils. Taxon 64: 3–16. [Google Scholar]
- Naciri Y, Linder HP.. 2020. The genetics of evolutionary radiations. Biological Reviews of the Cambridge Philosophical Society 95: 1055–1072. [DOI] [PubMed] [Google Scholar]
- Nazareno AG, Dick CW, Lohmann LG.. 2017. Wide but not impermeable: testing the riverine barrier hypothesis for an Amazonian plant species. Molecular Ecology 26: 3636–3648. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Kato M.. 2009. Unveiling cryptic species diversity of flowering plants: successful biological species identification of Asian Mitella using nuclear ribosomal DNA sequences. BMC Evolutionary Biology 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares I, Tusso S, José Sanín M, et al. 2024. Hyper-cryptic radiation of a tropical montane plant lineage. Molecular Phylogenetics and Evolution 190: 107954. [DOI] [PubMed] [Google Scholar]
- Pezzi PH, Guzmán-Rodriguez S, Giudicelli GC, Turchetto C, Bombarely A, Freitas LB.. 2022. A convoluted tale of hybridization between two Petunia species from a transitional zone in South America. Perspectives in Plant Ecology, Evolution and Systematics 56: 125688. [Google Scholar]
- Pina-Martins F, Silva DN, Fino J, Paulo OS.. 2017. Structure_threader: an improved method for automation and parallelization of programs structure, fastStructure and MavericK on multicore CPU systems. Molecular Ecology Resources 17: e268–e274. [DOI] [PubMed] [Google Scholar]
- Plá C, Külkamp J, Heiden G, Lughadha EN, Iganci JRV.. 2020. The importance of the Brazilian subtropical highland grasslands evidenced by a taxonomically verified endemic species. Phytotaxa 452: 250–267. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P.. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudlo P, Marin J-M, Estoup A, Cornuet J-M, Gautier M, Robert CP.. 2016. Reliable ABC model choice via random forests. Bioinformatics 32: 859–866. [DOI] [PubMed] [Google Scholar]
- Raduski AR, Igić B.. 2021. Biosystematic studies on the status of Solanum chilense. American Journal of Botany 108: 520–537. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA.. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Kortmann M, Silva-Arias GA, Segatto ALA, Mäder G, Bonatto SL, Freitas LB.. 2014. Multilocus phylogeny reconstruction: new insights into the evolutionary history of the genus Petunia. Molecular Phylogenetics and Evolution 81: 19–28. [DOI] [PubMed] [Google Scholar]
- Rivera-Colón AG, Catchen J.. 2022. Population genomics analysis with RAD, reprised: Stacks 2. Methods in Molecular Biology 2498: 99–149. [DOI] [PubMed] [Google Scholar]
- Rodrigues DM, Turchetto C, Lima JS, Freitas LB.. 2019. Diverse yet endangered: pollen dispersal and mating system reveal inbreeding in a narrow endemic plant. Plant Ecology & Diversity 12: 169–180. [Google Scholar]
- Roratto PA, Fernandes FA, Freitas TRO.. 2014. Phylogeography of the subterranean rodent Ctenomys torquatus: an evaluation of the riverine barrier hypothesis. Journal of Biogeography 42: 694–705. [Google Scholar]
- Roy A, Frascaria N, MacKay J, Bousquet J.. 1992. Segregating random amplified polymorphic DNAs (RAPDs) in Betula alleghaniensis. TAG. Theoretical and applied genetics. Theoretische und angewandte Genetik 85: 173–180. [DOI] [PubMed] [Google Scholar]
- Runemark A, Trier CN, Eroukhmanoff F, et al. 2018. Variation and constraints in hybrid genome formation. Nature Ecology & Evolution 2: 549–556. [DOI] [PubMed] [Google Scholar]
- Särkinen T, Bohs L, Olmstead RG, Knapp S.. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Ortiz S.. 2023. Species delimitation in a polyploid group of Iberian Jasione (Campanulaceae) unveils coherence between cryptic speciation and biogeographical regionalization. Plants (Basel, Switzerland) 12: 4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneyer VS, Kotseruba VV.. 2015. Cryptic species in plants and their detection by genetic differentiation between populations. Russian Journal of Genetics: Applied Research 5: 528–541. [Google Scholar]
- Silva GAR, Antonelli A, Lendel A, Moraes EM, Manfrin MH.. 2018. The impact of early Quaternary climate change on the diversification and population dynamics of a South American cactus species. Journal of Biogeography 45: 76–88. [Google Scholar]
- Smith ML, Carstens BC.. 2020. Process‐based species delimitation leads to identification of more biologically relevant species. Evolution 74: 216–229. [DOI] [PubMed] [Google Scholar]
- Smith LB, Downs RJ.. 1964. Notes on the Solanaceae of southern Brazil. Phytologia 10: 422–453. [Google Scholar]
- Smith ML, Ruffley M, Espíndola A, Tank DC, Sullivan J, Carstens BC.. 2017. Demographic model selection using random forests and the site frequency spectrum. Molecular Ecology 26: 4562–4573. [DOI] [PubMed] [Google Scholar]
- Soares LS, Freitas LB.. 2024. The phylogeographic journey of a plant species from lowland to highlands during the Pleistocene. Scientific Reports 14: 3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares LS, Fagundes NJR, Freitas LB.. 2023. Past climate changes and geographical barriers: the evolutionary history of a subtropical highland grassland species of Solanaceae, Petunia altiplana. Botanical Journal of the Linnean Society 201: 510–529. [Google Scholar]
- Souza A, Giudicelli GC, Teixeira MC, Turchetto C, Bonatto SL, Freitas LB.. 2022. Genetic diversity in microendemic plants from highland grasslands in southern Brazil. Botanical Journal of the Linnean Society 199: 235–251. [Google Scholar]
- Souza AC, Soares LS, Backes A, et al. 2024. Unravelling the genetic diversity and taxonomic ambiguities of endemic Petunia species from subtropical highland grasslands. Botanical Journal of the Linnean Society. doi: https://doi.org/ 10.1093/botlinnean/boae016. In press. [DOI] [Google Scholar]
- Stange M, Sánchez-Villagra MR, Salzburger W, Matschiner M.. 2018. Bayesian divergence-time estimation with genome-wide single-nucleotide polymorphism data of sea catfishes (Ariidae) supports Miocene closure of the Panamanian isthmus. Systematic Biology 67: 681–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehmann JR, Lorenz-Lemke AP, Freitas LB, Semir J.. 2009. The genus Petunia. In: Petunia. New York, NY: Springer New York, 1–28. [Google Scholar]
- Sukumaran J, Knowles LL.. 2017. Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences of the United States of America 114: 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Tsukamoto T, Ando T, Kokubun H, et al. 1998. Differentiation in the status of self-incompatibility among all natural taxa of Petunia (Solanaceae). Acta Phytotaxonomica et Geobotanica 49: 115–133. [Google Scholar]
- Turchetto C, Fagundes NJR, Segatto ALA, et al. 2014. Diversification in the South American Pampas: the genetic and morphological variation of the widespread Petunia axillaris complex (Solanaceae). Molecular Ecology 23: 374–389. [DOI] [PubMed] [Google Scholar]
- Turchetto C, Segatto ALA, Beduschi J, Bonatto SL, Freitas LB.. 2015. Genetic differentiation and hybrid identification using microsatellite markers in closely related wild species. AoB Plants 7: plv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchetto C, Segatto ALA, Lima JS, et al. 2022. So close, so far: spatial genetic structure and mating system in Petunia exserta, an endemic from a peculiar landscape in the Brazilian Pampa grasslands. Botanical Journal of the Linnean Society 199: 412–427. [Google Scholar]
- van der Pijl L. 1982. Principles of dispersal in higher plants. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Vargas OM, Goldston B, Grossenbacher DL, Kay KM.. 2020. Patterns of speciation are similar across mountainous and lowland regions for a Neotropical plant radiation (Costaceae: Costus). Evolution 74: 2644–2661. [DOI] [PubMed] [Google Scholar]
- Vasconcelos TNC, Alcantara S, Andrino CO, et al. 2020. Fast diversification through a mosaic of evolutionary histories characterizes the endemic flora of ancient Neotropical mountains. Proceedings of the Royal Society B: Biological Sciences 287: 20192933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroslavsky G, Ubilla M.. 2007. A ‘snapshot’ of the evolution of the Uruguay River (Del Plata Basin): the Salto depositional sequence (Pleistocene, Uruguay, South America). Quaternary Science Reviews 26: 2913–2923. [Google Scholar]
- Vigalondo B, Fernández-Mazuecos M, Vargas P, Sáez L.. 2015. Unmasking cryptic species: morphometric and phylogenetic analyses of the Ibero-North African Linaria incarnata complex. Botanical Journal of the Linnean Society 177: 395–417. [Google Scholar]
- Wei Z, Xia Z, Shu J, et al. 2021. Phylogeny and taxonomy on cryptic species of forked ferns of Asia. Frontiers in Plant Science 12: 748562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt EW, Silva PC, Malabarba LR, Carvalho TP.. 2019. Phylogenetic relationships and historical biogeography of Oligosarcus (Teleostei: Characidae): examining riverine landscape evolution in southeastern South America. Molecular Phylogenetics and Evolution 140: 106604. [DOI] [PubMed] [Google Scholar]
- Wiens JJ. 2007. Species delimitation: new approaches for discovering diversity. Systematic Biology 56: 875–878. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Servedio MR.. 2000. Species delimitation in systematics: inferring diagnostic differences between species. Proceedings Biological Sciences 267: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willyard A, Cronn R, Liston A.. 2009. Reticulate evolution and incomplete lineage sorting among the ponderosa pines. Molecular Phylogenetics and Evolution 52: 498–511. [DOI] [PubMed] [Google Scholar]
- Zúñiga-Reinoso A, Benítez HA.. 2015. The overrated use of the morphological cryptic species concept: an example with Nyctelia darkbeetles (Coleoptera: Tenebrionidae) using geometric morphometrics. Zoologischer Anzeiger - A Journal of Comparative Zoology 255: 47–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original unfiltered VCFs dataset is available at doi.org/10.6084/m9.figshare.25324813. Raw reads are available at https://www.ncbi.nlm.nih.gov/biosample/ under codes SAMN40212200–SAMN40212250, bioproject http://www.ncbi.nlm.nih.gov/bioproject/1082513.